Abstract
Background. Despite the high prevalence of herpes simplex virus type 2 (HSV-2) in sub-Saharan Africa, the natural history of infection among Africans is not well characterized. We evaluated the frequency of genital HSV shedding in HIV-seropositive and HIV-seronegative men and women in Uganda.
Methods. Ninety-three HSV-2–seropositive Ugandan adults collected anogenital swab specimens for HSV DNA quantification by polymerase chain reaction 3 times daily for 6 weeks.
Results. HSV-2 was detected from 2484 of 11 283 swab specimens collected (22%), with a median quantity of 4.3 log10 HSV copies/mL (range, 2.2–8.9 log10 HSV copies/mL). Genital lesions were reported on 749 of 3875 days (19%), and subclinical HSV shedding was detected from 1480 of 9113 swab specimens (16%) collected on days without lesions. Men had higher rates of total HSV shedding (relative risk [RR], 2.0 [95% confidence interval {CI}, 1.3–2.9]; P < .001); subclinical shedding (RR, 1.7 [95% CI, 1.1–2.7]; P = .01), and genital lesions (RR, 2.1 [95% CI, 1.2–3.4]; P = .005), compared with women. No differences in shedding rates or lesion frequency were observed based on HIV serostatus.
Conclusions. HSV-2 shedding frequency and quantity are high among HSV-2–seropositive adults in sub-Saharan Africa, including persons with and those without HIV infection. Shedding rates were particularly high among men, which may contribute to the high prevalence of HSV-2 and early acquisition among African women.
Keywords: genital herpes, human herpesvirus-2, HSV-2, viral shedding, Africa
Herpes simplex virus type 2 (HSV-2) is the major cause of genital herpes and among the most frequent sexually transmitted pathogens worldwide. The incidence and prevalence of HSV-2 infection is greatest in sub-Saharan Africa (SSA), where up to 50% of incident human immunodeficiency virus type 1 (HIV) infections are attributable to HSV [1–6]. HSV-2 establishes lifelong infection and is characterized by periods of intermittent reactivation that can be associated with clinical lesions or with subclinical, asymptomatic shedding. Studies of HSV-2 shedding in cohorts in the United States detected HSV by polymerase chain reaction (PCR) in genital samples on 9%–40% of days sampled [7–11], with higher rates observed with more frequent sampling [12, 13]. Approximately 50% of the samples with detectable HSV-2 represent subclinical reactivation, which is important in the transmission of genital herpes to sex partners [14, 15] and, along with clinical reactivation, is associated with the infiltration of CD4+ T cells, which serve as targets for HIV infection in the genital tissue [16].
Despite the high prevalence of HSV-2 in SSA, the natural history of the infection among Africans has not been well characterized. Importantly, several lines of evidence suggest that HSV-2 infection in Africa may differ from that in the United States. For example, the number of HSV DNA copies in genital secretions is higher in African samples, compared with samples from the United States and South America [17]. The efficacy of acyclovir in suppressing HSV shedding may also be attenuated in African women [18]. Differences in the virulence of viral strains of African origin have been described in animal models of HSV-2 infection, as have genetic polymorphisms in a variety of host response genes [19, 20]. Finally, the high rates of HIV infection in SSA may influence the natural history of HSV-2, owing to interactions between these infections.
Because populations in SSA have the highest prevalence of HSV-2 infection and are most likely to benefit from novel interventional and prevention trials, including studies of candidate HSV-2 vaccines, there is a need to assess viral shedding in carefully characterized cohorts in this region. To address this gap, we sought to evaluate the frequency and correlates of genital HSV shedding in HIV-seropositive and HIV-seronegative men and women in Uganda.
METHODS
Study Participants
We enrolled adults ≥18 years of age who resided in Kampala, Uganda, and were referred by clinicians from the Mulago Hospital sexually transmitted diseases clinic and 4 community HIV clinics between June 2011 and January 2012. Entry criteria included HSV-2 seropositivity; HIV-seropositive persons were eligible if they were not receiving antiretroviral therapy (ART). Pregnancy, receipt of anti-HSV drugs (including acyclovir, valacyclovir, and ganciclovir), and receipt of immunosuppressive therapies (including oral steroids) were exclusion criteria. Because HSV antibody assays are not used in clinical practice in Uganda, almost all participants had a clinical history of genital herpes. However, recruitment was not focused on persons with severe disease. We sought to enroll nearly equal numbers of HIV-infected and HIV-uninfected participants. All participants provided written informed consent, and institutional review boards approved all study protocols.
Procedures
An experienced, dedicated study clinician trained participants to obtain genital swab specimens for detection of HSV at home 3 times daily for 42 consecutive days, as described previously [11, 21, 22]. Participants were provided kits with polyethylene terephthalate swabs and prelabeled vials with PCR medium for swab collection at home, and they were asked to collect swab specimens in the morning, at midday, and at bedtime. Women swabbed the cervicovaginal, vulvar, and perianal areas, and men swabbed the penile and perianal area; genital lesions, if present, were included in the area swabbed. Participants also received a diary card and were taught to record clinical signs and symptoms of genital herpes on a daily basis.
At enrollment, participants completed a standardized medical history and physical examination. At weekly clinic visits, participants returned swab samples collected at home and reviewed the diary with study staff; they also completed an interim medical history and received a brief physical examination at each visit. HIV-seropositive participants provided blood for CD4+ T-cell count and plasma HIV RNA testing at enrollment, and those not already in care were referred to local HIV clinics for management according to Ugandan Ministry of Health guidelines.
Laboratory Methods
HSV-2 antibody status was initially determined using the Focus HerpeSelect enzyme-linked immunosorbent assay, with a positive test result defined as index of at least 3.5, and subsequently confirmed by HSV Western blotting at the University of Washington [23]. Swab samples were evaluated for HSV DNA with a real-time, quantitative, fluorescent polymerase-chain reaction (PCR) assay (TaqMan) at the University of Washington virology laboratory [24–26]. Samples with ≥150 copies/mL of HSV DNA were considered positive [24].
HIV serology was determined by a commercial enzyme-linked immunoassay, CD4+ T-cell counts were measured by flow cytometry, and HIV RNA levels were measured using real-time reverse-transcription PCR (detection level, 100 HIV RNA copies/mL) at the Makerere University–Johns Hopkins University Core Laboratory in Kampala, Uganda.
Definitions
The HSV shedding rate was defined as the number of swab specimens with HSV detected divided by the total number of swab specimens. The genital lesion rate was defined as the number of days with participant-reported genital lesions consistent with HSV infection divided by the total number of days with diary information. Subclinical shedding rates were calculated as for the total HSV shedding rate, excluding swab specimens collected on days genital lesions were observed. Episodes of shedding were defined as the presence of ≥1 swab specimen with a positive PCR result and at least 2 consecutive swab samples with negative HSV PCR results before and after the episode [12, 13]. Episodes began at the midpoint between the time the first sample in the episode was collected and the time the previous sample was collected and ended at the midpoint between the time the last sample in the episode was detected and the time the subsequent sample was collected. Because of missing data, the exact duration of the episode was not always known. In these cases, the midpoint between a minimum value (which assumes that missing data were HSV negative) and a maximum value (which assumes that missing data were HSV positive) of the episode duration was used to impute episode duration. For episodes that occurred at the beginning or end of the study, we considered episode duration to be censored at the minimum duration. The quantity of shedding was measured by the number of HSV copies, as defined by the log10 number of HSV DNA copies/mL of PCR buffer in swab specimens with HSV detected.
Statistical Analysis
Outcomes of interest included HSV shedding rate, subclinical shedding rate, genital lesion rate, episode frequency and duration, total HSV copy number, and subclinical HSV copy number. We examined sex, age, age at sexual debut, history of genital herpes symptoms, years since first genital herpes symptoms, and HIV status as potential risk factors. Age was modeled as a continuous variable; age at sexual debut was dichotomized at the median (17 years) to avoid the influence of outliers on model estimates. The time in years since first appearance of genital herpes symptoms was categorized on the basis of previous studies [27]. Other independent variables evaluated included circumcision status for men, hormonal contraception and menses status for women, and CD4+ T-cell count and plasma HIV RNA for HIV seropositive participants. We explored best-fit groupings for CD4+ T-cell count and plasma HIV RNA level parameterization. CD4+ T-cell count was dichotomized at 350 cells/mm3, a common cutoff for ART initiation in Uganda; plasma HIV RNA load was dichotomized at 10 000 copies/mL on the basis of prior studies indicating that this level of viral replication is associated with a longer HSV shedding duration [13, 28]. Risk factors for total shedding, subclinical shedding, and lesion rates were evaluated using generalized estimating equations (GEE) with Poisson distribution and log link to account for within-person correlation. GEE with Gaussian distribution and identity link were used to assess risk factors for HSV log10 copy number outcomes. Multivariable models were constructed on the basis of the purposeful variable selection method, using entry criteria of P < .25 from univariate analysis and, for retention in the multivariable model, a P value of < .10 or the presence of confounding that resulted in >20% change in estimates [29]. Summaries of episode duration used survival analysis to account for episodes with censored durations. Episode duration was compared by HIV status, using marginal Cox models with robust standard errors to account for correlation among episodes from the same person. To evaluate the potential impact on our findings of HIV-seropositive participants with undetectable plasma HIV RNA and presumed unreported ART use [30], we conducted sensitivity analyses for each model by excluding HIV-infected participants with undetectable plasma RNA. Two-sided P values of <.05 were considered statistically significant. Analyses were completed using SAS, version 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
Characteristics of Study Participants
Among 197 persons screened, 93 were enrolled in the study. The predominant reason for ineligibility was lack of HSV-2 antibody (n = 85). One excluded participant had atypical HSV-2 detected by Western blot, with no HSV-2 shedding during the study; 8 were positive for HSV-1 only by Western blot, with no HSV-2 shedding during the study; 5 did not collect any swab specimens at home; 4 reported receiving ART; and 1 woman was pregnant. The median age of participants was 36 years (range, 18–67 years); 31 (33%) were men, and nearly all were Ugandan (Table 1). Fifty-four participants (58%) were infected with HIV, with a median CD4+ T-cell count of 502 cells/mm3 (interquartile range [IQR], 291–681 cells/mm3) and a median HIV RNA level of 4.8 log10 copies/mL (IQR, 4.0–5.2 log10 copies/mL) among those with detectable virus. Seventeen HIV-seropositive participants had undetectable HIV RNA levels despite self-reporting no history of ART.
Table 1.
Demographic and Clinical Characteristics of the Study Cohort
| Characteristic | Total Cohort (n = 93) | HIV-Seronegative Group |
HIV-Seropositive Group |
||
|---|---|---|---|---|---|
| Men (n = 11) | Women (n = 28) | Men (n = 20) | Women (n = 34) | ||
| Age, y | 36 (18–67) | 30 (18–48) | 28 (19–58) | 40 (26–67) | 39 (25–56) |
| Male sex | 31 (33) | 11 (100) | … | 20 (100) | … |
| Ugandan nationality | 90 (97) | 10 (91) | 28 (100) | 19 (95) | 33 (97) |
| HIV seropositivity | 54 (58) | … | … | 20 (100) | 34 (100) |
| CD4+ T-cell count, cells/mm3, median (IQR)a | 502 (291–681) | … | … | 292 (245–535) | 582 (442–724) |
| HIV RNA load, log10 copies/mL, median (IQR)a,b | 4.8 (4.0–5.2) | … | … | 5.0 (4.5–5.2) | 4.7 (3.7–5.1) |
| Male circumcision | 10 (32) | 4 (36) | … | 6 (30) | … |
| Hormonal contraception | 26 (42) | … | 15 (54) | … | 11 (32) |
| Age at first sex, y | 17 (10–25) | 17 (14–25) | 17 (13–23) | 18 (10–25) | 17 (13–21) |
| HSV antibody status | |||||
| HSV type 2 only | 4 (4) | 1 (9) | 0 (0) | 0 (0) | 3 (9) |
| HSV types 1 and 2 | 89 (96) | 10 (91) | 28 (100) | 20 (100) | 31 (91) |
| History of genital herpes symptoms | 80 (86) | 9 (82) | 23 (82) | 17 (85) | 31 (91) |
| Time from first genital herpes episode to enrollment, yc | 2.5 (0.1–36.6) | 2.2 (0.3–16.8) | 1.7 (0.1–34.6) | 5.0 (0.1–26.6) | 2.0 (0.1–36.6) |
Data are median value (range) or no. (%) of subjects, unless otherwise indicated.
Abbreviations: HIV, human immunodeficiency virus type 1; HSV, herpes simplex virus; IQR, interquartile range.
a Among HIV-seropositive subjects.
b Measured only for those with detectable plasma HIV RNA.
c Among those reporting a history of genital herpes symptoms.
Eighty-nine participants (96%) were HSV-2 and HSV-1 seropositive, while 4 (4%) were HSV-2 seropositive only. The median age at sexual debut was 17 years (range, 10–25 years). Twenty-six women (42%) reported using hormonal contraception, and 10 (32%) men were circumcised. Thirty-four participants (37%) reported a history of symptoms of oral herpes; 80 (86%) reported a history of symptoms of genital herpes, with a median time between the first clinical episode and study enrollment of 2.5 years (range, 0.1–36.6 years).
The study participants collected a median of 125 of 126 anticipated genital swab specimens (range, 19–132 swab specimens) over a median of 43 days (range, 7–45 days). A total of 11 283 genital swab specimens collected on 3869 days were included in the analysis; all 3 intended samples were collected on 3657 days (94%).
HSV Genital Shedding
HSV was detected at least once among 87 participants (94%). The overall genital HSV shedding rate from this 3-times-daily sampling study was 22% (2484 of 11 283 swab specimens); on 30% of days (1169 of 3869) at least one of the swab specimens was positive (Table 2). HSV was detected on 46% of days among HIV-seropositive men, on 41% of days among HIV-seronegative men, on 26% of days among HIV-seropositive women, and on 20% of days among HIV-seronegative women. To determine whether 3-times-daily sampling detected more rapidly cleared subclinical episodes than daily sampling, as shown in US studies [12, 13], we evaluated once-daily sampling by selecting only the first sample per day from each participant. Eighty-two participants (88%) had HSV detected at least once, and only 21% of days had HSV detected, illustrating that many episodes of subclinical shedding last <24 hours.
Table 2.
Genital Herpes Simplex Virus (HSV) Shedding and Lesion Outcomes in the Study Cohort
| HSV Outcome | Total Cohort (n = 93) | HIV-Seronegative Group |
HIV-Seropositive Group |
||
|---|---|---|---|---|---|
| Men (n = 11) | Women (n = 28) | Men (n = 20) | Women (n = 34) | ||
| HSV detection frequency | |||||
| Swabs per participant, no. | 125 (19–132) | 125 (124–132) | 126 (71–127) | 124 (19–129) | 125 (43–127) |
| Days sampled per participant, no. | 43 (7–45) | 43 (42–45) | 43 (24–43) | 43 (7–44) | 43 (15–44) |
| Participants with ≥1 swab with HSV detected | 87 (94) | 10 (91) | 26 (93) | 20 (100) | 31 (91) |
| Swabs with HSV detected | 2484 (22) | 418 (30) | 463 (13) | 811 (35) | 792 (19) |
| Days with HSV detected | 1169 (30) | 194 (41) | 236 (20) | 370 (46) | 369 (26) |
| Swabs with subclinical HSV detected | 1480 (16) | 263 (22) | 322 (11) | 343 (23) | 552 (16) |
| Days with subclinical HSV detected | 758 (24) | 133 (33) | 175 (17) | 183 (36) | 267 (22) |
| Lesion frequency | |||||
| Days with lesions reported | 749 (19) | 74 (16) | 171 (14) | 292 (36) | 212 (15) |
| Shedding episodes | |||||
| Episode rate per 30 d | |||||
| All episodes | 2.8 (0–10.4) | 4.2 (0–6.3) | 2.1 (0–7.6) | 4.2 (0.7–8.7) | 2.1 (0–10.4) |
| Subclinical episodes | 1.7 (0–8.3) | 2.8 (0–4.2) | 1.4 (0–7.1) | 2.1 (0–4.8) | 1.4 (0–8.3) |
| Episodes with ≥1 lesion-day | 0.7 (0–5.4) | 0.7 (0–2.8) | 0 (0–3.5) | 0.7 (0–5.4) | 0 (0–4.2) |
| Episode duration, h, median (IQR) | |||||
| All episodes | 22.5 (8.5–64.8) | 16.3 (8.5–64.4) | 15.6 (8.3–55.0) | 28.9 (12.9–123.9) | 19.9 (8.4–55.0) |
| Subclinical episodes | 14.7 (8.0–32.8) | 9.2 (8.0–24.5) | 10.4 (8.0–34.5) | 21.3 (8.6–32.2) | 15.3 (8.0–39.1) |
| Episodes with ≥1 lesion–day, no. | 100.6 (16.4–182.3) | 184.1 (16.5–272.0) | 47.4 (8.5–112.7) | 143.7 (25.3–175.9) | 55.2 (16.1–185.3) |
| HSV load, log10 copies/mLa | |||||
| Overall | 4.3 (2.2–8.9) | 4.4 (2.2–8.9) | 3.9 (2.2–8.5) | 4.9 (2.2–8.6) | 4.2 (2.2–8.2) |
| Subclinical episodes | 3.7 (2.2–8.7) | 3.6 (2.2–8.7) | 3.6 (2.2–8.5) | 3.6 (2.2–8.4) | 3.9 (2.2–7.8) |
| Lesional | 5.3 (2.2–8.9) | 5.6 (2.2–8.9) | 4.5 (2.3–8.5) | 6.3 (2.2–8.6) | 4.7 (2.2–8.2) |
Data are median value (range) or no. (%) of subjects, unless otherwise indicated.
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
a Among samples with HSV detected.
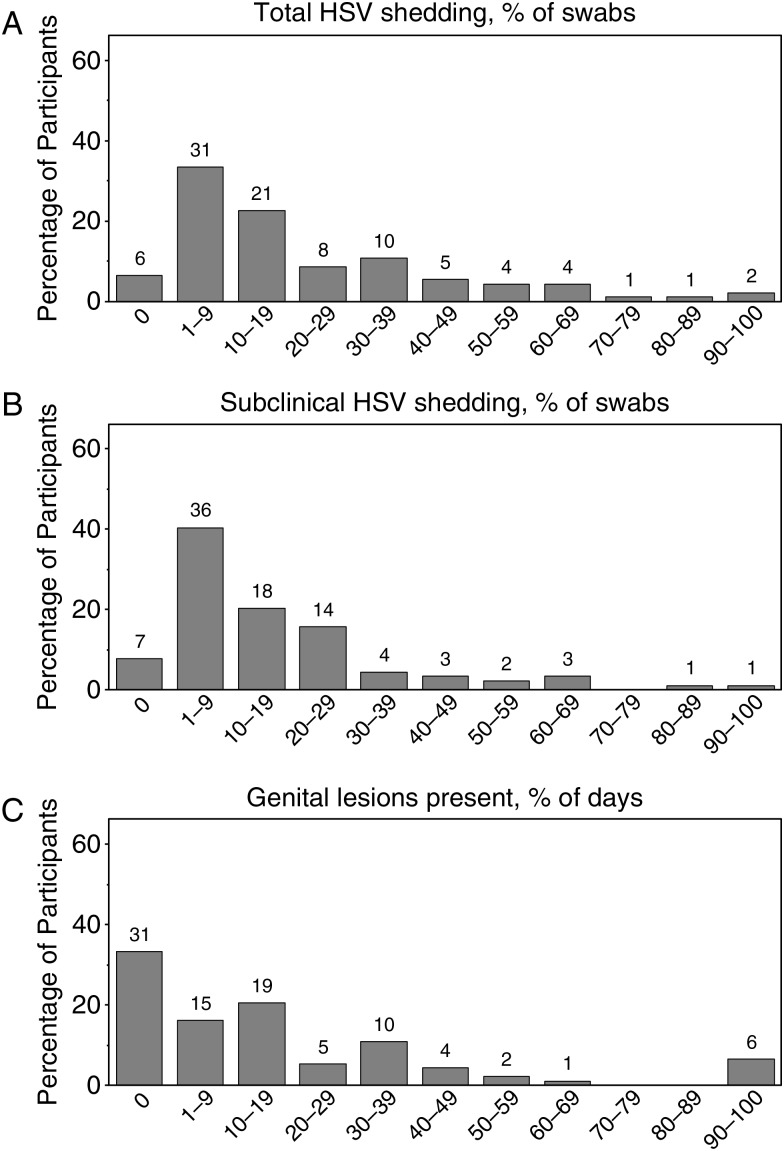
Diurnal variation in shedding frequency was not observed during the 3 periods (Supplementary Figure 1). However, the frequency of viral shedding varied widely: 6 people did not shed during the study, 31 shed on 1% to <10% of the days, 21 shed on 10% to <20%, 18 shed on 20% to <40%, 13 shed on 40% to <70%, and 4 shed on ≥70% (Figure 1).
Figure 1.
Distribution of participants by the percentage of swab specimens associated with total herpes simplex virus (HSV) shedding (A), the percentage of swab specimens associated with subclinical HSV shedding (B), and the percentage of days with genital lesions (C). The numbers above the bars refer to the absolute number of participants in each group.
Men had higher rates of total HSV shedding, compared with women (relative risk [RR], 2.0 [95% confidence interval {CI}, 1.3–2.9]; P < .001) in univariate analysis. Among HIV-infected participants, CD4+ T-cell count of <350 cells/µL (RR, 1.7 [95% CI, 1.0–2.7]; P = .04) and HIV plasma RNA load of >10 000 copies/mL (RR, 1.7 [95% CI, 1.0–2.8]; P = .04) were associated with increased shedding in univariate analysis. No multivariate model was identified for total HSV shedding because no model had >1 significant variable. No other factors, including HIV status, age, age at first sex, history of genital herpes symptoms, years since first genital herpes symptoms, male circumcision, menses, or use of hormonal birth control, were found to be significantly associated with total HSV shedding (Table 3).
Table 3.
Risk Factors for Herpes Simplex Virus (HSV) Genital Shedding or Lesion Frequency
| Risk Factor | Total HSV Shedding |
Subclinical HSV Shedding |
Genital Lesions |
|||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Unadjusted |
Unadjusted |
Adjusteda |
|||||
| RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | |
| HIV status (seropositive vs seronegative) | 1.4 (.9–2.1) | .14 | 1.2 (.8–1.9) | .41 | 1.6 (.9–2.7) | .12 | … | |
| Sex (men vs women) | 2.0 (1.3–2.9) | <.001 | 1.7 (1.1–2.7) | .01 | 2.0 (1.2–3.4) | .009 | 2.1 (1.2–3.4) | .005 |
| Age (per y increase) | 1.0 (.98–1.02) | .79 | 1.0 (.97–1.02) | .56 | 1.0 (.98–1.03) | .66 | … | |
| Age at first sex, y | ||||||||
| 10–17 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | … | ||||
| 18–25 | 1.1 (.8–1.7) | .54 | 0.9 (.6–1.5) | .77 | 1.0 (.6–1.7) | .97 | … | |
| History of genital herpes symptoms | 1.3 (.7–2.3) | .35 | 1.1 (.6–2.0) | .76 | 3.7 (1.4–9.5) | .007 | 3.8 (1.5–9.8) | .005 |
| Time since first genital herpes symptoms, y | ||||||||
| <1 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | … | ||||
| 1–9 | 1.4 (.8–2.5) | .25 | 1.4 (.7–2.5) | .33 | 2.2 (1.0–5.0) | .06 | … | |
| ≥10 | 1.1 (.5–2.3) | .79 | 1.1 (.5–2.3) | .89 | 1.4 (.6–3.3) | .46 | … | |
| Unknown | 1.0 (.6–1.9) | .89 | 1.0 (.5–2.1) | .91 | 2.0 (.8–5.1) | .14 | … | |
| No history of genital herpes | 0.9 (.5–1.8) | .75 | 1.0 (.5–2.1) | .90 | 0.5 (.1–1.5) | .21 | … | |
| Male circumcision | 1.2 (.7–2.1) | .52 | 1.3 (.7–2.3) | .36 | 0.8 (.3–1.9) | .58 | … | |
| Hormonal birth controlb | 1.1 (.6–2.0) | .79 | 1.0 (.5–1.9) | .97 | 0.9 (.5–1.7) | .75 | … | |
| Menses | 0.9 (.6–1.2) | .47 | 0.9 (.6–1.2) | .51 | 1.0 (.6–1.5) | .95 | … | |
| CD4+ T-cell count (<350 vs ≥350 cells/mm3)c | 1.7 (1.0–2.7) | .04 | 1.5 (.8–2.7) | .17 | 1.6 (.8–3.1) | .15 | … | |
| HIV RNA load (>104 vs ≤104 log10 copies/mL)c | 1.7 (1.0–2.8) | .04 | 1.5 (.8–2.9) | .21 | 1.6 (.9–3.0) | .13 | … | |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; RR, relative risk.
a Adjusted for sex and history of genital herpes symptoms.
b Among women only. The reference group includes those using no birth control and those using birth control other than hormonal agents.
c For HIV-seropositive subjects only.
Subclinical HSV Genital Shedding
The overall percentage of genital swab specimens with HSV collected on days without genital lesions was 16% (1480 of 9113); the percentage of days with subclinical HSV shedding was 24% (758 of 3121; Table 2). HSV subclinical shedding occurred at least once for 82 participants (92%). The percentage of days with subclinical shedding varied widely by participant (Figure 1). Overall, 65% of days with genital HSV shedding occurred on days on which no lesions were reported.
Men had higher rates of subclinical shedding, compared with women (RR, 1.7 [95% CI, 1.1–2.7]; P = .01). No other variables were associated with subclinical shedding in univariate analysis (Table 3).
Genital Lesions
The overall percentage of days with genital lesions reported was 19% (749 of 3875; Table 2). Sixty-two participants (67%) reported at least 1 day with a genital lesion; the percentage of days with genital lesions varied by participant (Figure 1). HSV was detected on 55% of days with lesions reported (411 of 748 days with lesions and HSV PCR results).
Men had higher rates of genital lesions, compared with women (RR, 2.0 [95% CI, 1.2–3.4]; P = .009) in univariate analysis and after adjustment for history of genital herpes symptoms (RR, 2.1 [95% CI, 1.2–3.4]; P = .005). History of genital herpes symptoms was also associated with increased rates of genital lesions (RR, 3.7 [95% CI, 1.4–9.5]; P = .007) in univariate analysis and after adjustment for sex (RR, 3.8 [95% CI, 1.5–9.8]; P = .005). No other factors were significantly associated with lesion frequency (Table 3).
Episode Frequency and Duration
The median frequency of shedding episodes over a 30-day period for the entire cohort was 2.8 episodes (range, 0–10.4 episodes) of HSV shedding, 1.7 episodes (range, 0–8.3 episodes) of subclinical shedding, and 0.7 episodes (range, 0–5.4 episodes) with at least 1 day of genital lesions. Of 398 total episodes, duration was known exactly for 334 (84%). The median duration of any HSV shedding episode was approximately 22.5 hours (IQR, 8.5–64.8 hours); the median episode duration was highest among HIV-seropositive men (28.9 hours [IQR, 12.9–123.9 hours]), followed by HIV-seropositive women (19.9 hours [IQR, 8.4–55.0 hours]), HIV-seronegative men (16.3 hours [IQR, 8.5–64.4 hours]), and HIV-seronegative women (15.6 hours [IQR, 8.3–55.0 hours]). The median subclinical episode length was 14.7 hours (IQR, 8.0–32.8 hours); the median subclinical episode duration was highest among HIV-seropositive men (21.3 hours [IQR, 8.6–32.2 hours]), followed by HIV-seropositive women (15.3 hours [IQR, 8.0–39.1 hours]), HIV-seronegative men (9.2 hours [IQR, 8.0–24.5 hours]), and HIV-seronegative women (10.4 hours [IQR, 8.0–34.5 hours]). The median duration of episodes with at least 1 day of genital lesions was 100.6 hours (IQR, 16.4–182.3 hours). Episode duration did not differ significantly by HIV status in unadjusted or age-adjusted analyses.
HSV DNA Copy Number
Of the 2484 genital swab specimens in which HSV-2 was detected, the median quantity was 4.3 log10 copies/mL (range, 2.2–8.9 log10 copies/mL). Presence of lesions was the only variable significantly associated with HSV copy number. Median quantity on days with lesions was 5.3 log10 copies/mL (range, 2.2–8.9 log10 copies/mL) and on days without lesions was 3.7log10 copies/mL (range, 2.2–8.7 log10 copies/mL; log10 change, 0.8 [95% CI, .5, 1.2]; P < .001). Similar results were observed in sensitivity analyses that excluded HIV-positive participants with undetectable HIV plasma RNA, although that analysis suggested that participants with CD4+ T-cell counts of <350 cells/µL had higher HSV copy numbers (log10 change, 0.5 [95% CI, −.02 to 1.0]; P = .06) when adjusting for presence of lesions.
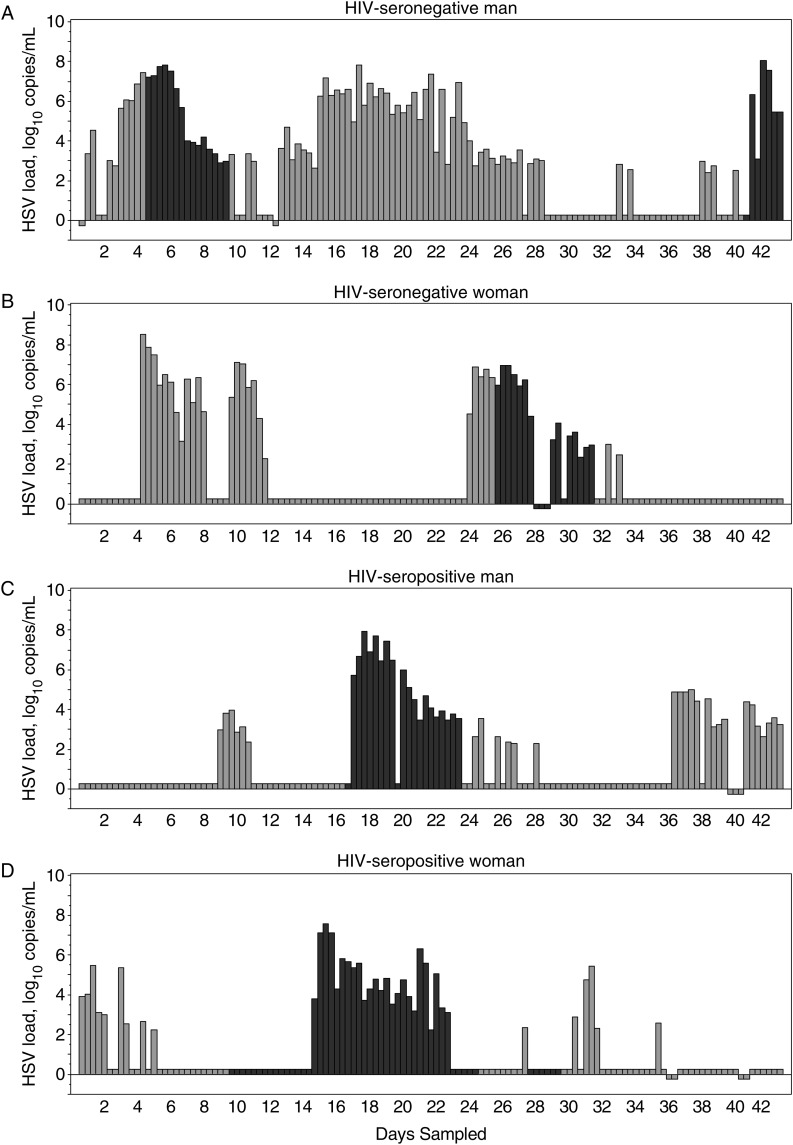
Of the 1480 genital swab specimens in which HSV-2 was detected on days without lesions, the quantity of HSV was lower among men who were circumcised, compared with those who were uncircumcised (log10 change, −0.6 [95% CI, −1.0, −.1]; P = .02). No other factors were associated with HSV copy number in univariate analysis. Representative HSV-2 shedding and copy number patterns for HIV-positive and HIV-negative men and women are shown in Figure 2.
Figure 2.
Representative herpes simplex virus type 2 (HSV-2) shedding patterns for 4 participants, by sex and human immunodeficiency virus (HIV) status. Black bars indicate lesions present, gray bars indicate no lesions present, and negative values indicate missing samples. A, Data for a 29-year-old HIV-seronegative man. B, Data for a 57-year-old HIV-seronegative woman. C, Data for a 33-year-old HIV-seropositive man with a CD4+ T-cell count of 273 cells/mm3 and a plasma HIV RNA load of 149 955 copies/mL. D, Data for a 49-year-old HIV-seropositive woman with a CD4+ T-cell count of 52 cells/mm3 and a plasma HIV RNA load of 290 575 copies/mL.
To evaluate the potential impact on our findings of presumed unreported ART use by HIV-seropositive participants with undetectable plasma HIV RNA [30], we conducted sensitivity analyses for each model by excluding the 17 HIV-infected participants without detectable plasma RNA. In general, the sensitivity analyses yielded results similar to those observed in the full cohort, with minor changes in point estimates and P values for some variables (Supplementary Table 4 and Supplementary Data).
DISCUSSION
To our knowledge, our study is the first to rigorously characterize viral shedding in HSV-2–seropositive Africans, revealing several important observations about the natural history of genital herpes in SSA. Genital HSV-2 was detected frequently in our cohort, with rates of total and subclinical HSV-2 shedding comparable to those in many US studies [11–13, 27, 28, 31]. Of note, men had significantly higher rates of total and subclinical shedding than women, but the differences by HIV status were smaller.
Finding higher HSV-2 shedding rates among men is in contrast to US-based studies that have shown that genital HSV shedding rates among immunocompetent women are approximately 40% higher than among immunocompetent men [24, 32]. The frequency of genital lesions among men in our study was greater than that among women, which likely contributed to higher overall shedding rates among men. The HIV-infected men in our study generally had lower CD4+ T-cell counts than the HIV-infected women, which may also have contributed to the higher HSV shedding rates observed among these men. Our finding that subclinical HSV-2 copy number is lower in circumcised men, compared with uncircumcised men, is intriguing. Circumcision has been shown in multiple studies to protect men against HSV-2 acquisition [33–36], and one study has shown a transiently increased rate of HSV reactivation in the penis following circumcision [37]. However, the data on HSV shedding by circumcision status are limited [38]. This finding may have implications for increased transmission of HSV-2 in SSA, where a greater proportion of men are uncircumcised, compared with the United States. Thus, male circumcision may also provide a benefit for male-to-female HSV-2 transmission, potentially impacting HSV-2 epidemiology if it is provided to a critical number of men in SSA.
Importantly, we did not observe differences in HSV-2 shedding rates or copy number based on HIV status, although our numbers within subsets of participants were small. US studies have observed that episodes of HSV reactivation are longer in persons with HIV infection, especially those with advanced immunosuppression [13, 28]. We also observed a trend toward longer episode length among HIV-seropositive men and women in our study. The HIV-infected participants in our study were only moderately immunosuppressed, however, and these observations may differ in persons with more marked immunosuppression.
We found that some of our HIV-infected participants had undetectable plasma HIV RNA despite a self-report of not taking ART. Based on studies that have identified ART in plasma samples of up to half of participants with undetectable HIV loads who reported no ART use [30], we assumed unreported ART use among our participants with undetectable HIV RNA. Sensitivity analyses excluding these participants did not appreciably alter the observed total or subclinical HSV-2 shedding rates, suggesting that ART regimens do not directly influence HSV-2 reactivation [39]. Studies of oral tenofovir similarly did not reduce HSV shedding in HIV/HSV-2 coinfected adults in Canada [40].
Our study mostly included persons with a history of symptomatic genital herpes (86% of participants), which may in part be due to enrolling patients referred from sexually transmitted disease and HIV clinics; as a result, our findings may not be generalizable to those with serologic evidence of HSV-2 infection only. Further, we only observed participants for a 6-week-long snapshot in the natural history of disease; however, participants collected genital swab specimens 3-times daily with extremely high adherence, so our findings are likely representative of the period observed. Similar to findings in the United States, the use of 3-times-daily sampling rather than once-daily sampling allowed us to capture short shedding episodes in this cohort.
In summary, our study demonstrates that HSV-2 shedding frequency, lesion frequency, and HSV-2 shedding quantity are high among HSV-2–seropositive adults in SSA, including persons with and those without HIV infection. These findings underscore the need for broad-based implementation of traditional genital herpes management strategies in SSA, including long-term antiviral medications and consistent condom use. In particular, the high shedding rates observed among men likely contribute to the high incidence of HSV-2 in Africa and underscore that men remain important targets of interventions to reduce HSV transmission. Our study also highlights the potential benefits of novel prevention interventions, including an HSV-2 vaccine, which would not only reduce the morbidity and mortality related to HSV-2 infection but could also help curtail the HIV epidemic in SSA.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the study participants and the study team, whose commitment made this study possible.
Disclaimer. The funding sources played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Financial support. This work was supported by the National Institutes of Health (NIH) (grants K23 CA-150931, A1-03073,1 and K24 A1-071113).
Potential conflicts of interest. A. W. has received grants from the NIH, Agenus, Genocea, Gilead, and Vical and has been a consultant for Aicuris, Merck, and Amgen. L. C. is on the scientific advisory board for and holds stock (<1% of the company) in Immune Design Corp and is a coinventor listed on several patents involving potential HSV vaccine development. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Abu-Raddad LJ, Magaret AS, Celum C et al. . Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS One 2008; 3:e2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Farrell N. Increasing prevalence of genital herpes in developing countries: implications for heterosexual HIV transmission and STI control programmes. Sex Transm Infect 1999; 75:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wald A. Synergistic interactions between herpes simplex virus type-2 and human immunodeficiency virus epidemics. Herpes 2004; 11:70–6. [PubMed] [Google Scholar]
- 4.Biraro S, Kamali A, White R et al. . Effect of HSV-2 on population-level trends in HIV incidence in Uganda between 1990 and 2007. Trop Med Int Health 2013; 18:1257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 2006; 20:73–83. [DOI] [PubMed] [Google Scholar]
- 6.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis 2002; 185:45–52. [DOI] [PubMed] [Google Scholar]
- 7.Gupta R, Wald A, Krantz E et al. . Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis 2004; 190:1374–81. [DOI] [PubMed] [Google Scholar]
- 8.Fife K, Warren T, Ferrera D et al. . Effect of valacyclovir on viral shedding in immunocompetent patients with recurrent herpes simplex virus 2 genital herpes: a US-based randomized, double-blind, placebo-controlled clinical trial. Mayo Clin Proc 2006; 81:1321–7. [DOI] [PubMed] [Google Scholar]
- 9.Leone P, Warren T, Hamed K, Fife K, Wald A. Famciclovir reduces viral mucosal shedding in HSV-seropositive persons. Sex Transm Dis 2007; 34:900–7. [DOI] [PubMed] [Google Scholar]
- 10.Mark K, Corey L, Meng T et al. . Topical resiquimod 0.01% gel decreases herpes simplex virus type 2 genital shedding: a randomized, controlled trial. J Infect Dis 2007; 195:1324–31. [DOI] [PubMed] [Google Scholar]
- 11.Wald A, Corey L, Cone R, Hobson A, Davis G, Zeh J. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J Clin Invest 1997; 99:1092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mark KE, Wald A, Magaret AS et al. . Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis 2008; 198:1141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mark KE, Wald A, Magaret AS et al. . Rapidly cleared episodes of oral and anogenital herpes simplex virus shedding in HIV-infected adults. J Acquir Immune Defic Syndr 2010; 54:482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mertz GJ, Benedetti J, Ashley R, Selke SA, Corey L. Risk factors for the sexual transmission of genital herpes. Ann Intern Med 1992; 116:197–202. [DOI] [PubMed] [Google Scholar]
- 15.Schiffer JT, Abu-Raddad L, Mark KE et al. . Frequent release of low amounts of herpes simplex virus from neurons: results of a mathematical model. Sci Transl Med 2009; 1:7ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston C, Zhu J, Jing L et al. . Virologic and immunologic evidence of multifocal genital herpes simplex virus 2 infection. J Virol 2014; 88:4921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs J, Celum C, Wang J et al. . Clinical and virologic efficacy of herpes simplex virus type 2 suppression by acyclovir in a multicontinent clinical trial. J Infect Dis 2010; 201:1164–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Celum C, Wald A, Hughes J et al. . Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371:2109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudek TE, Torres-Lopez E, Crumpacker C, Knipe DM. Evidence for differences in immunologic and pathogenesis properties of herpes simplex virus 2 strains from the United States and South Africa. J Infect Dis 2011; 203:1434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman RM, Lamers SL, Weiner B et al. . Genome sequencing and analysis of geographically diverse clinical isolates of herpes simplex virus 2. J Virol 2015; 89:8219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med 1995; 333:770–5. [DOI] [PubMed] [Google Scholar]
- 22.Wald A, Zeh J, Barnum G, Davis LG, Corey L. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med 1996; 124:8–15. [DOI] [PubMed] [Google Scholar]
- 23.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol 1988; 26:662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wald A, Huang ML, Carrell D, Selke S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis 2003; 188:1345–51. [DOI] [PubMed] [Google Scholar]
- 25.Ryncarz AJ, Goddard J, Wald A, Huang ML, Roizman B, Corey L. Development of a high-throughput quantitative assay for detecting herpes simplex virus DNA in clinical samples. J Clin Microbiol 1999; 37:1941–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jerome KR, Huang ML, Wald A, Selke S, Corey L. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol 2002; 40:2609–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phipps W, Saracino M, Magaret A et al. . Persistent genital herpes simplex virus-2 shedding years following the first clinical episode. J Infect Dis 2011; 203:180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schacker T, Zeh J, Hu HL, Hill E, Corey L. Frequency of symptomatic and asymptomatic herpes simplex virus type 2 reactivations among human immunodeficiency virus-infected men. J Infect Dis 1998; 178:1616–22. [DOI] [PubMed] [Google Scholar]
- 29.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med 2008; 3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahle EM, Kashuba A, Baeten JM et al. . Unreported antiretroviral use by HIV-1-infected participants enrolling in a prospective research study. J Acquir Immune Defic Syndr 2014; 65:e90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tronstein E, Johnston C, Huang ML et al. . Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 2011; 305:1441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wald A, Zeh J, Selke S et al. . Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med 2000; 342:844–50. [DOI] [PubMed] [Google Scholar]
- 33.Mehta SD, Moses S, Parker CB, Agot K, Maclean I, Bailey RC. Circumcision status and incident herpes simplex virus type 2 infection, genital ulcer disease, and HIV infection. AIDS 2012; 26:1141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okuku HS, Sanders EJ, Nyiro J et al. . Factors associated with herpes simplex virus type 2 incidence in a cohort of human immunodeficiency virus type 1-seronegative Kenyan men and women reporting high-risk sexual behavior. Sex Transm Dis 2011; 38:837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sobngwi-Tambekou J, Taljaard D, Lissouba P et al. . Effect of HSV-2 serostatus on acquisition of HIV by young men: results of a longitudinal study in Orange Farm, South Africa. J Infect Dis 2009; 199:958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tobian AA, Charvat B, Ssempijja V et al. . Factors associated with the prevalence and incidence of herpes simplex virus type 2 infection among men in Rakai, Uganda. J Infect Dis 2009; 199:945–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grabowski MK, Kigozi G, Gray RH et al. . Herpes simples Virus type 2 shedding from male circumcision wounds in Rakai, Uganda. J Infect Dis 2015; 212:1613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Wagoner NJ, Geisler WM, Sizemore JM Jr, Whitley R, Hook EW III. Herpes simplex virus in African American heterosexual males: the roles of age and male circumcision. Sex Transm Dis 2010; 37:217–22. [DOI] [PubMed] [Google Scholar]
- 39.Posavad CM, Wald A, Kuntz S et al. . Frequent reactivation of herpes simplex virus among HIV-1-infected patients treated with highly active antiretroviral therapy. J Infect Dis 2004; 190:693–6. [DOI] [PubMed] [Google Scholar]
- 40.Tan DH, Kaul R, Raboud JM, Walmsley SL. No impact of oral tenofovir disoproxil fumarate on herpes simplex virus shedding in HIV-infected adults. AIDS 2011; 25:207–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.