Abstract
Background
Core temperature patterns in patients warmed with forced-air remain poorly characterized. Also unknown is the extent to which transient and mild intraoperative hypothermia contributes to adverse outcomes in broad populations.
Methods
We evaluated esophageal (core) temperatures in 58,814 adults having surgery lasting >60 min who were warmed with forced air. Independent associations between hypothermic exposure and transfusion requirement and duration of hospitalization was evaluated.
Results
In every percentile subgroup, core temperature decreased during the first hour and subsequently increased. The mean lowest core temperature during the first hour was 35.7 ± 0.6°C. Sixty-four percent of the patients reached a core temperature threshold of <36°C 45 min after induction; 29% reached a core temperature threshold of <35.5°C. Nearly half the patients had continuous core temperatures <36°C for more than an hour, and 20% of the patients were <35.5°C for more than an hour. Twenty percent of patients had continuous core temperatures <36°C for more than 2 h, and 8% of the patients were below 35.5°C for more than 2 h. Hypothermia was independently associated with both transfusion and duration of hospitalization, although prolongation of hospitalization was small.
Conclusions
Even in actively warmed patients, hypothermia is routine in the first hour of anesthesia. Thereafter, average core temperatures progressively increase. Nonetheless, intraoperative hypothermia was common, and often prolonged. Hypothermia was associated with increased transfusion requirement which is consistent with numerous randomized trials.
Introduction
Intraoperative core hypothermia causes serious complications including coagulopathy,1 surgical wound infections,2 and perhaps myocardial complications.3 It also decreases drug metabolism,4 prolongs recovery,5 and provokes thermal discomfort.6 It is thus now standard-of-care to warm surgical patients. Various guidelines, including the Surgical Care Improvement Project (SCIP-10) and National Institute of Health and Clinical Excellence (NICE), suggest that patients should be normothermic, defined as a core temperature of at least 36°C at the end of surgery.
Forced air remains by far the most common warming approach. Forced air markedly reduces cutaneous heat loss7,8; consequently, most warmed patients are normothermic by the end of surgery.2 But core-to-peripheral redistribution of body heat precipitously reduces core temperature in the hour after induction of anesthesia,9,10 even in actively warmed patients.2,11 Most patients thus at least initially experience some intraoperative hypothermia. Intraoperative core temperature patterns in patients warmed with forced air remain poorly characterized.
While randomized trials are considered the highest level of clinical evidence, they unsurprisingly target at-risk patients. For example, infection trials targeted colorectal surgery patients2,12 and the largest coagulation studies were conducted in patients having hip arthroplasties.13,14 Trials thus often lack generalizability. The extent to which hypothermia trial results apply to broad surgical populations thus remains unknown.
A second limitation of published hypothermia trials is that most compared forced-air warming to routine care which, at the time, was usually just passive insulation. Consequently, temperature differences between the groups were usually 1.5–2.0°C at the end of surgery — far more than is now typical. Whether smaller amounts of hypothermia, also worsen important outcomes remains unknown.
A third issue is that final intraoperative core temperature thus poorly characterizes the U-shaped hypothermic exposure that usually results from current thermal management. Time-weighted averages, which incorporate temperatures from throughout surgery, would better characterize current temperature patterns.
And finally, we need to consider that most hypothermia trials date from the 1990s. Fortunately, the intervening decades have seen substantial practice improvement. For example, blood conservation is now routine; minimally invasive surgery causes less blood loss; and transfusion thresholds are generally lower. As another example, the only major study evaluating the effect of hypothermia on hospital length-of-stay dates to 1996,2 a period when colectomy patients typically stayed in the hospital 2 weeks. Whether these and similar results still apply remains unknown.
Each of these limitations of existing results can, to an extent, be addressed through analysis of large current data sets. The Cleveland Clinic Perioperative Health Documentation System includes intraoperative core temperature and accurately characterizes transfusion requirement and hospital length-of-stay. Initially, we therefore evaluated core temperature in a large cohort of actively warmed noncardiac surgical patients. Thereafter, we used these registry data to test the hypothesis that hypothermic exposure in degree.hours below a threshold of 37°C is associated with increased intraoperative red blood cell transfusion requirement and duration of hospitalization.
Materials and Methods
With Cleveland Clinic Institutional Review Board (Cleveland, Ohio) approval, we extracted data on 143,157 adults having non-cardiac surgery at Cleveland Clinic between April 1, 2005 and February 15, 2013. Only the most recent visit for each patient was used for analysis. We included patients in whom core temperature was measured in the esophagus. Virtually all surgical patients are warmed with forced air (Bair Hugger, 3M, St. Paul, MN); generally, active warming begins after draping. Prewarming was not used. Ambient temperature in preoperative holding areas at the Clinic is generally maintained near 23°C; operating rooms are typically maintained at 20–21°C, but can be as low as 18–19°C in some rooms. Only two of ≈50 relevant operating rooms are equipped with laminar flow.
We excluded operations in which the duration of anesthesia was less than 60 minutes (induction to emergence), as coded in the electronic anesthesia record. Induction was when induction doses of general anesthetics were given; emergence was less precisely defined, but generally when clinicians began preparing patients for emergence. We also excluded patients in whom there was less than 30 min of core temperature monitoring, in whom monitoring was disrupted for more than 30 min, or in whom core temperature monitoring started more than 45 min after induction of anesthesia.
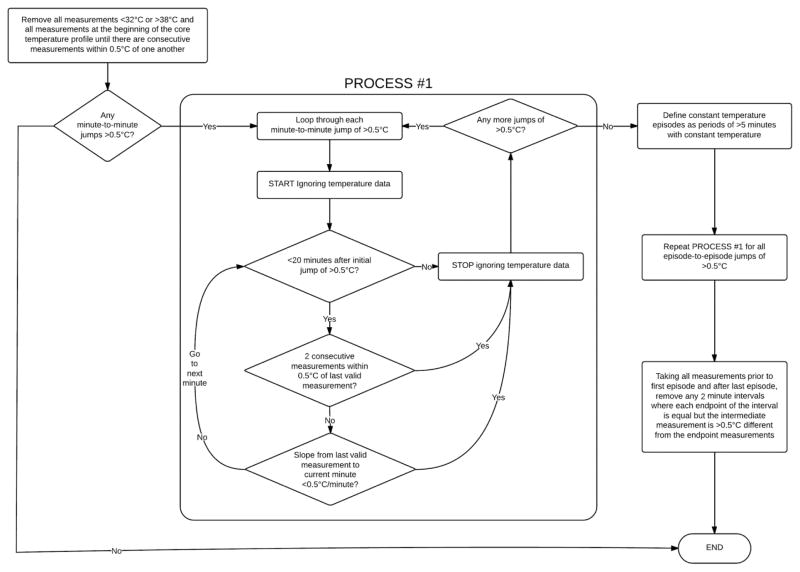
Artifactual data were removed from each patient’s core temperature profile according to the algorithm depicted in figure 1. After artifact removal, temperature profiles were then smoothed using a Gaussian kernel smoothing algorithm; this is similar to a “sliding window” (or moving average), except that instead of taking the simple average of measurements within the window, a weighted average is taken where the weights are drawn from a Gaussian curve according to the horizontal distance from the desired estimate. The ‘ksmooth’ function within the ‘sm’ library for R statistical software Version 3.0.0 * was used to produce the smoothed estimates,15 using a bandwidth parameter of 30 minutes to define the width of the Gaussian kernel (specifically, the standard deviation of the Gaussian kernel is 0.25 times the selected bandwidth).
Fig. 1.
Flow chart indicating the artifact removal algorithm for intraoperative core temperature measurements.
Restricted cubic spline regression curves characterized the distribution of core temperature measurements over postinduction time; separate curves were estimated for the median, 1st, and 3rd quartiles; 1st and 9th deciles; and 5th and 95th percentile of the core temperatures. Curves were fit using quantile regression.16
The incidence of hypothermia – defined according to progressive core-temperature thresholds of <36.0°C, <35.5°C, and <35.0°C – was plotted as a function of postinduction time to evaluate core-to-peripheral redistribution of body heat. Pointwise 95% confidence intervals were estimated for each of these three incidence functions using normal approximation theory for proportions. Nominal confidence interval width was set to three standard errors to better enforce the 95% confidence level in the presence of multiple simultaneous estimates.
For our primary outcome analysis of red blood cell transfusion (coded as a binary outcome) and hospital length of stay, we removed from consideration patients not admitted on the same day as their surgery. Also, patients with missing baseline hemoglobin, baseline platelets, and/or body mass index were excluded. Furthermore, for the analysis of duration of hospitalization, we removed ambulatory surgery patients.
For both outcomes, we characterized the primary hypothermia exposure using an “area under the threshold” measure defined as the size of the region above the core-temperature-versus-time curve but below a horizontal line at 37.0°C. We analyzed the independent association between area under the 37.0°C threshold and intraoperative erythrocyte transfusion using multivariable logistic regression. Likewise, we analyzed the independent association between area under the 37.0°C threshold and duration of hospitalization using multivariable linear regression. A sensitivity analysis for transfusion, in which we excluded massively transfused patients (defined as receiving four or more units), was performed. Duration of hospitalization was transformed to approximate normality using the logarithmic transformation; patients who died in the hospital were assigned a duration of hospitalization equal to the maximum observed value among patients discharged alive, which was 477 days.
For each model, we represented the adjusted relationship between area under the 37.0°C threshold and outcome using cubic splines. Chi-squared tests were used to test whether or not there was an independent association between area under the 37.0°C threshold and outcome. A curve of predicted probability of transfusion versus area under the 37.0°C threshold for an “at risk” reference population (of patients >55 yr old with body mass index <25 kg/m2, preoperative hemoglobin <1.4 g/dL, and duration of surgery >4 h) was visualized, as was a curve of predicted geometric mean duration of hospitalization versus area under the 37.0°C threshold for all inpatients included in the analysis of duration of hospitalization.
Both models adjusted for year, type, and duration of surgery, body mass index, age, preoperative platelet count, preoperative hemoglobin, estimated blood loss, and individual anesthesiologist, as well as the Elixhauser comorbidities17 (see table 1 for a listing of these comorbidities). Principal type of surgery was characterized according to the U.S. Agency for Healthcare Research and Quality’s Clinical Classifications Software for International Classification of Diseases and Injuries, version 9, Clinical Modification procedure codes. Type of surgery and anesthesiologist categories with insufficient cell sizes were aggregated into respective all-purpose “other” categories; specifically, the bottom 10% of cases were aggregated for each of the two variables. Still, type of surgery and anesthesiologist each were represented by too many individual levels to reliably model using standard regression adjustment. Thus, we created surrogate measures for each analysis to represent potential confounding effects of each of these factors.
Table 1.
Baseline Characteristics for 45,866 Patients Included in the Analysis of the Association between Hypothermia and Transfusion
| Factor | Area Under the 37°C Threshold - Quartile-Based Groups | |||
|---|---|---|---|---|
|
First Quartile ≤1.2°C·hr (N = 11,474) |
Second Quartile 1.2°C·hr – 2.2°C·hr (N = 11,461) |
Third Quartile 2.2°C·hr – 3.6°C·hr (N = 11,469) |
Fourth Quartile >3.6°C·hr (N = 11,462) |
|
| Outpatient Surgery | 26 | 19 | 10 | 3 |
| Estimated Blood Loss (cc) | 48 [10, 100] | 50 [20, 150] | 100 [50, 250] | 200 [100, 400] |
| Year of Surgery | 2009 [2007, 2011] | 2009 [2008, 2011] | 2009 [2007, 2011] | 2009 [2007, 2011] |
| Preoperative Hemoglobin (UNITS) | 13.5 [12.4, 14.5] | 13.7 [12.7, 14.7] | 13.8 [12.7, 14.8] | 13.8 [12.7, 14.8] |
| Preoperative Platelets (UNITS) | 255 [211, 306] | 247 [205, 295] | 245 [203, 290] | 240 [200, 287] |
| Duration of Surgery (min) | 137 [104, 191] | 168 [135, 219] | 212 [174, 266] | 289 [238, 355] |
| Body Mass Index (kg/m^2) | 28 [24, 34] | 28 [24, 33] | 28 [24, 32] | 27 [24, 32] |
| Age (years) | 53 [41, 64] | 56 [45, 67] | 58 [47, 68] | 60 [50, 69] |
| Elixhauser Comorbidities | ||||
| Congestive Heart Failure | 3 | 2 | 2 | 3 |
| Valvular Disease | 2 | 3 | 3 | 3 |
| Pulmonary Circulation Disorders | 1 | 1 | 1 | 1 |
| Peripheral Vascular Disease | 2 | 3 | 4 | 6 |
| Hypertension (Uncomplicated) | 38 | 40 | 43 | 45 |
| Hypertension (Complicated) | 3 | 3 | 3 | 4 |
| Paralysis | 1 | 1 | 1 | 1 |
| Other Neurological Disorders | 5 | 6 | 6 | 7 |
| Chronic Pulmonary Disease | 12 | 11 | 11 | 11 |
| Diabetes Without Chronic Complications | 13 | 12 | 13 | 11 |
| Diabetes With Chronic Complications | 2 | 2 | 2 | 2 |
| Hypothyroidism | 11 | 11 | 11 | 11 |
| Renal Failure | 4 | 3 | 4 | 5 |
| Liver Disease | 3 | 3 | 3 | 2 |
| Chronic Peptic Ulcer Disease | 0 | 0 | 0 | 0 |
| HIV and AIDS | 0 | 0 | 0 | 0 |
| Lymphoma | 1 | 1 | 1 | 1 |
| Metastatic Cancer | 5 | 5 | 5 | 6 |
| Solid Tumor Without Metastasis | 10 | 12 | 17 | 26 |
| Rheumatoid arthritis/Collagen Vascular Diseases | 3 | 3 | 2 | 3 |
| Coagulation Deficiency | 1 | 2 | 2 | 3 |
| Obesity | 20 | 17 | 17 | 15 |
| Weight Loss | 2 | 2 | 2 | 3 |
| Fluid and Electrolyte Disorders | 6 | 6 | 8 | 13 |
| Blood Loss Anemia | 1 | 1 | 1 | 2 |
| Deficiency Anemias | 6 | 5 | 5 | 6 |
| Alcohol Abuse | 1 | 1 | 1 | 1 |
| Drug Abuse | 0 | 0 | 0 | 1 |
| Psychoses | 2 | 2 | 2 | 2 |
| Depression | 12 | 11 | 11 | 9 |
AIDS = acquired immunodeficiency syndrome; HIV = human immunodeficiency virus infection.
Summary statistics presented as either a percentage or median [first and third quartiles].
For the analysis of transfusion, we adjusted for the type-of-surgery-specific mean area below the 37.0°C threshold and the type-of-surgery-specific transfusion rate, as well as the same two measures specific to each anesthesiologist. The same was done for the analysis of duration of hospitalization, although the mean duration of hospitalization for each factor level was used instead of the transfusion rate.
A nominal Type I error rate of 0.025 was used to restrict the Type I error rate to 5% for the simultaneous analysis of two outcomes. R statistical software version 2.15.2 for 64-bit Unix operating system (The R Foundation for Statistical Computing, Vienna, Austria) was used for all analyses.
Results
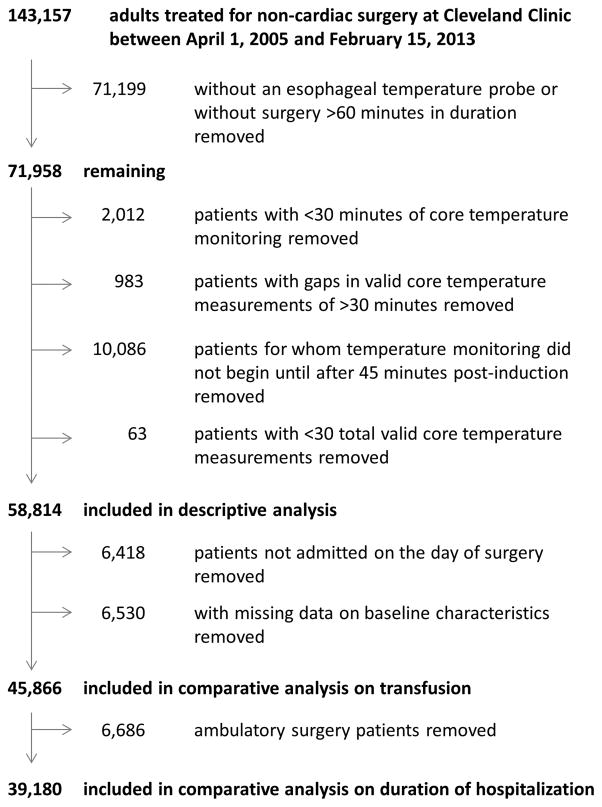
Among 143,157 patients considered for our study, 58,814 met criteria for inclusion in the descriptive analysis (fig. 2). Procedures were diverse (table 2).
Fig. 2.
Study flow diagram.
Table 2.
Top 20 Procedures Among 58,814 Patients Meeting Study Inclusion Criteria
| Hysterectomy; abdominal and vaginal | 6.1% |
| Other OR lower GI therapeutic procedures | 5.6% |
| Colorectal resection | 5.5% |
| Nephrectomy; partial or complete | 4.7% |
| Laminectomy; excision intervertebral disc | 4.5% |
| Open prostatectomy | 4.3% |
| Spinal fusion | 4.1% |
| Thyroidectomy; partial or complete | 3.5% |
| Other OR gastrointestinal therapeutic procedures | 3.4% |
| Other therapeutic endocrine procedures | 3.3% |
| Incision and excision of CNS | 2.5% |
| Cholecystectomy and common duct exploration | 2.5% |
| Other hernia repair | 2.5% |
| Other OR therapeutic nervous system procedures | 2.3% |
| Other OR therapeutic procedures of urinary tract | 2.2% |
| Other OR therapeutic procedures; female organs | 2.1% |
| Other OR therapeutic procedures on skin and breast | 2.0% |
| Other OR upper GI therapeutic procedures | 2.0% |
| Hip replacement; total and partial | 1.7% |
| Arthroplasty knee | 1.6% |
| < OTHER > | 33.7% |
CNS = central nervous system; GI = gastrointestinal; OR = operating room.
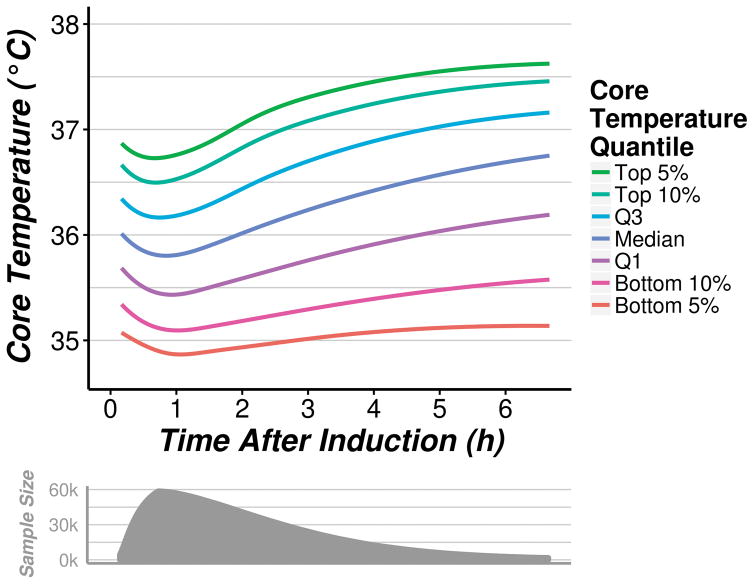
Figure 3 displays the distribution of core temperature as a function of time after induction; generally, core temperature decreased during the first hour of anesthesia and subsequently increased for the duration of surgery. Median core temperature was about 35.8°C an hour after induction. Core temperature in the first quartile of patients was about 35.5°C after an hour, but was less than 35°C in more than 5% of the patients. The mean lowest core temperature during the first hour of anesthesia was 35.7 ± 0.6°C.
Fig. 3.
Distribution of core temperature as a function of time after induction among 58,814 patients.
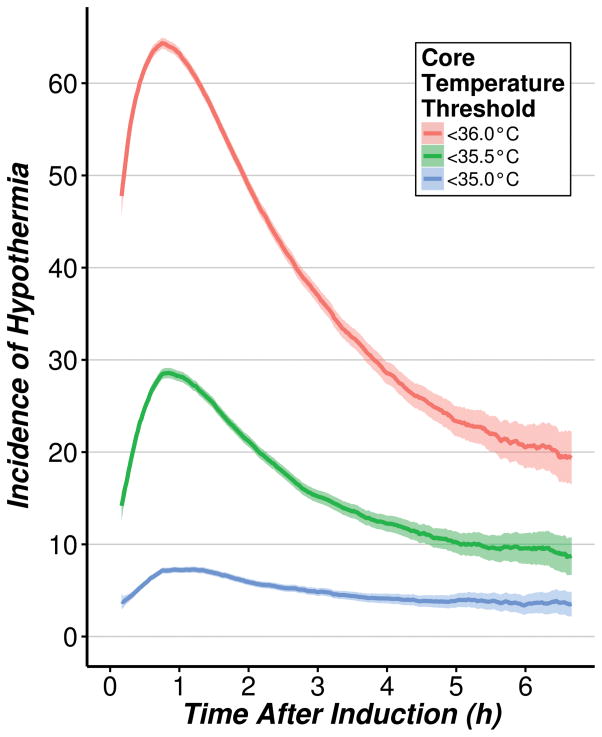
Figure 4 shows the incidence of hypothermia under various core-temperature thresholds. At 45 min after induction, 64.4% [95% confidence interval: 63.8%, 64.9%] of the patients reached a core temperature threshold of <36°C; 28.9% [28.0%, 29.1%] of the patients reached a core temperature threshold of <35.5°C at 51 min after induction; and 7.3% [6.9%, 7.6%] of the patients reached a core temperature threshold of <35°C at 71 min after induction. Even after 6 h of anesthesia, about 20% of patients had core temperatures <36°C, 9% were <35.5°C, and 4% were <35°C.
Fig. 4.
Incidence of hypothermia as a function of time after induction, under progressive core temperature thresholds defining hypothermia.
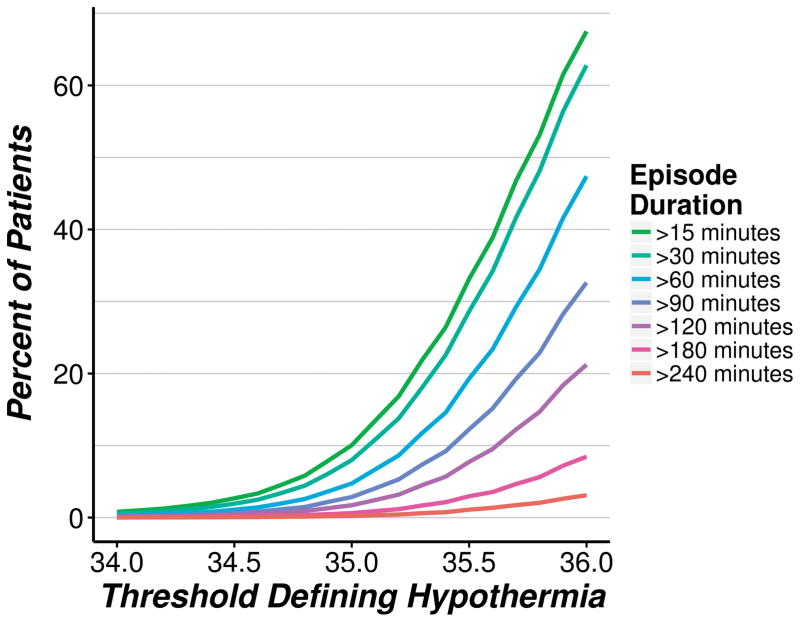
Figure 5 shows the incidence of hypothermic episodes of varying duration (>15 min, >30 min, >60 min, etc.) as a function of progressive core temperature thresholds. Nearly half the patients had continuous core temperatures <36°C for more than an hour, and 20% of the patients were below 35.5°C for more than an hour (teal line in figure, 3rd from top). 20% of patients had continuous core temperatures <36°C for more than 2 h, and 8% of the patients were below 35.5°C for more than 2 h (pink line in figure, 4th from top).
Fig. 5.
Incidence of (any) hypothermic episodes during the case, according to progressive core temperature thresholds defining hypothermia.
Mean core temperature over the duration of anesthesia in the entire population was 36.0 ± 0.6°C; final intraoperative core temperature averaged 36.3 ± 0.5°C (882 patients had missing end-of-case temperatures). While hypothermic incidences tended to vary across procedure categories, end-of-case temperatures were consistently above 36.0°C (table 3).
Table 3.
Distribution of Primary Procedure in the Sample, Along with Incidence of Hypothermia under Varying Core Temperature Thresholds and End-of-Case Temperatures
| Procedure Category | N (%) of all patients | N (%) with Hypothermia
|
Median [Q1, Q3] End-of-Case Temperature |
||
|---|---|---|---|---|---|
| <36.0°C | <35.5°C | <35.0°C | |||
| Operations on the digestive system | 16,525 (28.1%) | 4,468 (27.0%) | 1,078 (6.5%) | 148 (0.9%) | 36.3 [36.0, 36.6] |
| Operations on the musculoskeletal system | 8,272 (14.1%) | 2,407 (29.1%) | 764 (9.2%) | 173 (2.1%) | 36.2 [36.0, 36.6] |
| Operations on the female genital organs | 6,637 (11.3%) | 2,153 (32.4%) | 531 (8.0%) | 73 (1.1%) | 36.2 [36.0, 36.5] |
| Operations on the nervous system | 6,466 (11.0%) | 2,146 (33.2%) | 590 (9.1%) | 129 (2.0%) | 36.2 [36.0, 36.6] |
| Operations on the urinary system | 6,162 (10.5%) | 23,73 (38.5%) | 716 (11.6%) | 111 (1.8%) | 36.2 [36.0, 36.5] |
| Operations on the endocrine system | 3,692 (6.3%) | 607 (16.4%) | 123 (3.3%) | 16 (0.4%) | 36.4 [36.1, 36.8] |
| Operations on the male genital organs | 3,253 (5.5%) | 984 (30.2%) | 256 (7.9%) | 41 (1.3%) | 36.2 [36.0, 36.5] |
| Operations on the integumentary system | 3,236 (5.5%) | 1,025 (31.7%) | 262 (8.1%) | 56 (1.7%) | 36.2 [36.0, 36.6] |
| Operations on the cardiovascular system | 2,789 (4.7%) | 933 (33.5%) | 274 (9.8%) | 60 (2.2%) | 36.1 [35.8, 36.4] |
| Operations on the hemic and lymphatic system | 891 (1.5%) | 225 (25.3%) | 66 (7.4%) | 10 (1.1%) | 36.3 [36.0, 36.6] |
| Operations on the nose; mouth; and pharynx | 389 (0.7%) | 83 (21.3%) | 25 (6.4%) | 6 (1.5%) | 36.4 [36.1, 36.8] |
| Miscellaneous diagnostic and therapeutic procedures | 237 (0.4%) | 64 (27.0%) | 18 (7.6%) | 1 (0.4%) | 36.3 [36.0, 36.6] |
| Operations on the ear | 109 (0.2%) | 10 (9.2%) | 1 (0.9%) | 0 (0.0%) | 36.4 [36.1, 36.7] |
| Obstetrical procedures | 63 (0.1%) | 15 (23.8%) | 4 (6.3%) | 0 (0.0%) | 36.3 [36.0, 36.7] |
| Operations on the eye | 48 (0.1%) | 4 (8.3%) | 1 (2.1%) | 0 (0.0%) | 36.4 [36.2, 36.8] |
| Operations on the respiratory system | 45 (0.1%) | 15 (33.3%) | 8 (17.8%) | 5 (11.1%) | 36.3 [36.0, 36.6] |
After removing patients not admitted on the day of surgery and patients with missing data on covariates, 45,866 remaining patients were analyzed for association between the area under the threshold hypothermic exposure and transfusion.
The overall distribution of area under the 37°C threshold was log-normal in nature (see histograms in fig. 6 and 7), with a median [Q1, Q3] of 2.2 [1.2, 3.6] degree·hours. Most patients had at least 1 degree·hour below the 37°C threshold. Patient characteristics and surgical procedures for these patients are presented according to quartiles of this area below the threshold metric in table 1.
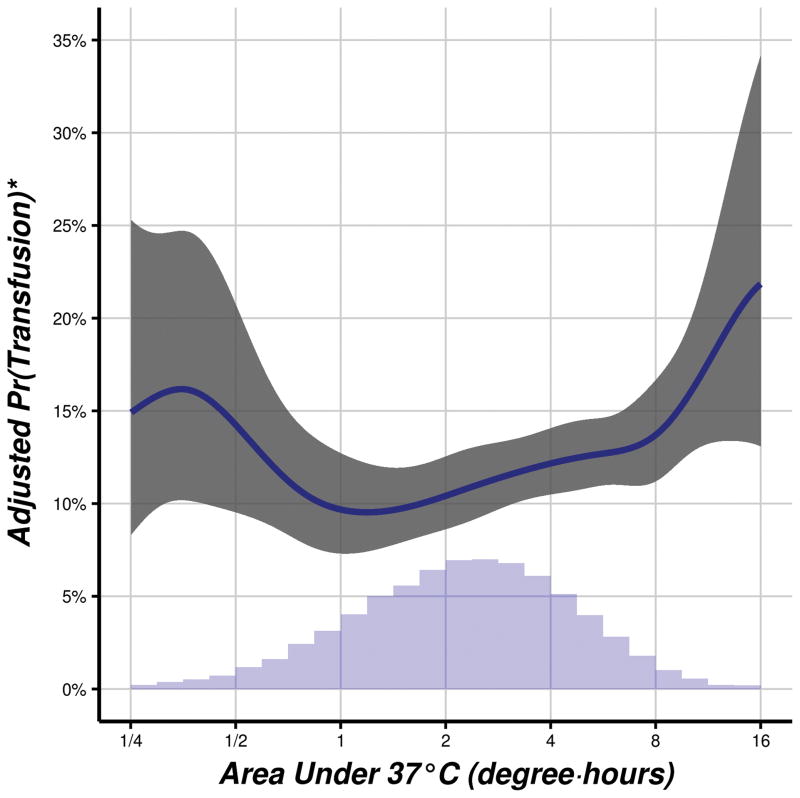
Fig. 6.
Adjusted probability of transfusion estimates versus integrated area above the core-temperature-versus-time curve and below a threshold of 37°C, Estimates adjusted to an “at risk” reference population defined by age >55 yr, body mass index <25 kg/m2, preoperative hemoglobin <1.4 g/dL, and duration of surgery >4 h. Shaded regions represent pointwise, Bonferroni-adjusted (for simultaneous analysis on two outcomes) 95% confidence intervals. Regression model based on 45,866 patients who were admitted on the day of surgery and who had esophageal temperature monitoring.
*adjusted for year, type, and duration of surgery, body mass index, age, preoperative platelet count, preoperative hemoglobin, estimated blood loss, and individual anesthesiologist, as well as the Elixhauser comorbidities16 (see table 2 for a listing of these comorbidities).
Pr = probability.
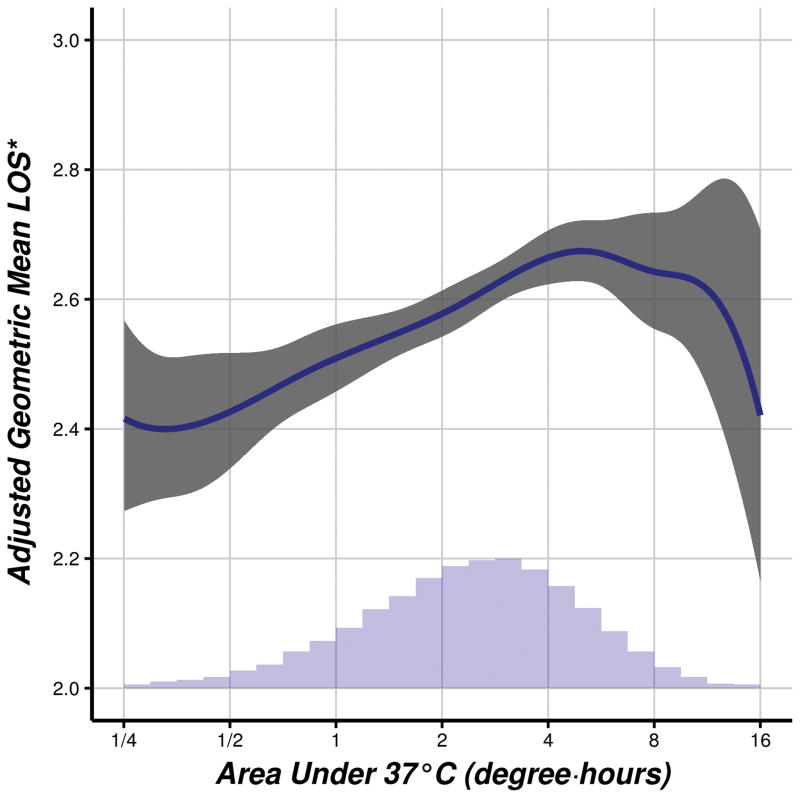
Fig. 7.
Adjusted estimates of geometric mean duration of hospitalization versus integrated area above the core-temperature-versus-time curve and below a threshold of 37°C, for 39,180 hospital in-patients who were admitted on the day of surgery and who had intraoperative esophageal temperature monitoring. Shaded regions represent pointwise, Bonferroni-adjusted (for simultaneous analysis on two outcomes) 95% confidence intervals.
*adjusted for year, type, and duration of surgery, body mass index, age, preoperative platelet count, preoperative hemoglobin, estimated blood loss, and individual anesthesiologist, as well as the Elixhauser comorbidities16 (see table 2 for a listing of these comorbidities).
LOS = length of stay.
Overall, 2,251/45,866 patients (4.6%) were transfused. Based on our multivariable logistic regression model (which had a C-statistic of 0.98), we found a significant association between area under 37°C and transfusion (P = 0.018, significant after the Bonferroni correction). Odds ratios relative to a reference value of 1 degree·hour are presented in table 4. Generally speaking, transfusion was increasingly likely as area under the 37°C threshold increased to more than 4 degree·hours, with an odds ratio [pointwise 95% confidence interval] estimate of 1.48 [1.03, 2.13] for transfusion comparing patients with 8 degree·hours to patients with 1 degree·hour. Predicted probabilities of transfusion for an “at risk” reference population of patients >55 yr old with body mass index <25 kg/m2, preoperative hemoglobin <1.4 g/dL, and duration of surgery >4 h are given in figure 6. Results of our sensitivity analysis excluding massively transfused patients (n = 429; 0.9%) were similar to that of the primary analysis.
Table 4.
Association between Intraoperative Hypothermia and Outcomes Transfusion requirement and Duration of Hospitalization
| Area under 37°C (degree·hours) | Adjusted# Odds Ratio [Pointwise 95% CI] for Intraoperative Erythrocyte Transfusion (N = 45,866) | Adjusted# Ratio of Geometric Mean Duration of Hospitalization [Pointwise 95% CI] (N = 39,180) |
|---|---|---|
| 0.25 | 1.34 [1.04, 1.73] | 0.96 [0.90, 1.03] |
| 0.50 | 1.10 [0.98, 1.24] | 0.97 [0.93, 1.01] |
| 1.00 | (reference) | (reference) |
| 2.00 | 1.00 [0.89, 1.13] | 1.03 [1.00, 1.05] |
| 4.00 | 1.12 [0.91, 1.39] | 1.06 [1.03, 1.09] |
| 8.00 | 1.41 [1.08, 1.84] | 1.05 [1.01, 1.10] |
| 16.00 | 2.02 [1.30, 3.14] | 0.96 [0.86, 1.08] |
Estimates adjusted for year, type, and duration of surgery, body mass index, age, preoperative platelet count, preoperative hemoglobin, estimated blood loss, and individual anesthesiologist, as well as the Elixhauser comorbidities, which are listed in table 1.
CI = confidence interval.
For the analysis of association between area-under-the-threshold and duration of hospitalization, we further removed 6,686 ambulatory surgery patients (fig. 2). Median [first quartile, third quartile] duration of hospitalization was 3 [1, 4] days. Based on our multivariable linear regression model (R2 = 0.40), we found a significant association between area under the 37°C threshold and geometric mean duration of hospitalization (P < 0.001), although the strength of the association was of questionable clinical importance: the ratio of geometric mean estimates for various values of area under the 37°C threshold (compared to a reference value of 1 degree·hour; see table 4) are all modest, and a plot of predicted mean duration of hospitalization versus area under the 37°C threshold (fig. 7) reveals estimates only ranging from 2.4 days to approximately 2.7 days.
Discussion
Core temperature represents temperature of highly perfused tissues, mostly the trunk and head, representing about half the body mass. It is the considered the best single temperature and is the primary determinant of thermoregulatory responses.18 In contrast, the peripheral thermal compartment (mostly the arms and legs) is typically 2–4°C less than core temperature.19,20 The gradient between core and peripheral temperatures is determined by the thermal environment and thermoregulatory vasomotion. Induction of general10 or neuraxial9 anesthesia causes vasodilation which promotes heat flow from core to peripheral tissues. This redistribution of body heat is the primary cause of hypothermia during the first hour of anesthesia even in actively warmed patients.2,11
The magnitude of redistribution hypothermia is defined by the reduction in core temperature during the initial hour of anesthesia. We were unable to precisely determine the amount of redistribution since accurate preoperative temperatures were unavailable — although virtually all patients are normothermic before induction of anesthesia. Typically, core temperatures are about 36.5°C for first-start cases (near the circadian nadir), rising to 37.5°C in the late afternoon and early evening.21 Furthermore, esophageal temperature monitoring did not necessarily start immediately after induction. Nonetheless, mean core temperatures decreased during the initial hour of anesthesia, reaching a nadir of 35.7 ± 0.6°C. It is thus likely that the magnitude of redistribution hypothermia was about 1°C which is similar to previously reports, as was absolute core temperature after an hour of anesthesia.22–25
Intraoperative forced air did not prevent redistribution hypothermia which is consistent with previous reports2,11 and the fact that it results from a large internal flow of heat from core to peripheral tissues. In contrast, it is well established that prewarming reduces redistribution hypothermia22,26,27 by warming peripheral tissues to nearly core temperature.28 Without a thermal gradient, the second Law of Thermodynamics specifies that there can be no flow of heat — and thus no redistribution hypothermia.
As expected, redistribution reduced core temperature during the initial hour of anesthesia. Previous work shows that unwarmed surgical patients continue to become hypothermic until they become cold enough to trigger thermoregulatory vasoconstriction, typically at about 34.5°C,29–31 which prevents further hypothermia by constraining metabolic heat to the core thermal compartment.20 For example, final intraoperative core temperatures are typically about 34.5°C in unwarmed patients having open abdominal surgery.2 The pattern in our actively warmed patients differed: after the initial hour of anesthesia, core temperature progressively increased throughout surgery. Consequently, 91% of the patients had core temperatures ≥36°C at the end of anesthesia.
Because core temperatures progressively increased after the initial hour of anesthesia (when redistribution was complete), patients having longer operations were more likely to be normothermic at the end of surgery. Although counterintuitive, it is thus more difficult to end with normothermia in shorter than longer cases. Prewarming is thus most important for short cases, and essential if normothermia is to be maintained throughout surgery. While prewarming has a relatively small effect on core temperature, prewarming would presumably have prevented hypothermia in at least some of the patients who experienced prolonged periods of intraoperative hypothermia.
Normal body temperature averages 37°C. Nonetheless, an intraoperative core temperature of 36°C is widely considered “normothermic” and existing guidelines suggest a final core temperature >36°C. Published trials in regards to perioperative hypothermia and adverse outcomes were based on final intraoperative temperatures in patients assigned to either active warming or passive insulation, which in most trials resulted in a core temperature difference of 1–2°C at the end of surgery. And while some hypothermia-induced complications probably are based on final temperature (i.e., thermal comfort, shivering, adrenergic stress), others such as blood loss are based on instantaneous tissue temperature and thus presumably accrue throughout surgery.
It is difficult or impossible to determine from available hypothermia trials exactly which temperature ranges as well as which duration of time in a certain temperature range are most associated with adverse outcomes. It thus seems important to consider intraoperative temperature patterns rather than just final intraoperative temperature. Our analysis extends previous work in considering the magnitude of intraoperative hypothermia, defined in terms of integrated °C.hours within various temperature ranges, which allows us to identify time and depth of hypothermia associated with clinically important worsened outcomes.
Hypothermia trials generally have good internal validity. They were also largely restricted to specific at-risk patient populations. For example, most wound infection studies were performed in patients having colon-rectal surgery and most blood loss and transfusion studies included only orthopedic or cardiac surgical patients. How generalizable this data might be remains unclear. We therefore included all noncardiac surgical patients into our analysis.
An important distinction is that essentially all patients at the Cleveland Clinic are actively warmed whereas the “control” groups in most hypothermia outcome trials were only provided with passive insulation. But even with forced-air warming, intraoperative core temperatures are often less than 36°C. For example, 20% of our warmed patients had a core temperature less than 35.5°C for at least an hour (i.e., 0.5°C.hour for a 36°C threshold); 5% of our actively warmed patients were a °C.hour below 35°C. Whether these lesser amounts of hypothermia affect outcome remains unknown.
Transfusion requirements progressively increased from 1 to 8 °C.hour below 37°C. That hypothermia impairs platelet function32 and the enzymes of the coagulation cascade33 is well established, as is the relationship between hypothermia and blood loss.1 Furthermore, numerous randomized trials, summarized in a meta-analysis,1 show that hypothermia increases transfusion requirements. Specifically, core temperatures around 35.5°C at the end of surgery significantly increased the relative risk for transfusion by approximately 22% (CI 3–37%). Our registry analysis extends previous work by including a broad noncardiac surgery population rather than generally being restricted to procedures known for blood loss.
The other outcome we evaluated was hospital length-of-stay which was significantly prolonged, but not by a clinically meaningful amount (i.e., from ≈2.4 to ≈2.7 days in the range from 0.5 to 4°C.hour below 37°C. In contrast, the single major trial evaluating the duration of hospitalization which observed a 20% prolongation in patients who were 2°C hypothermic at the end of surgery.2 Although integrated core temperature was not determined in that study,2 the difference between the groups was probably well over 4°C.hour. Sparse available data thus suggest that moderate degrees of hypothermia have little effect on the duration of hospitalization, but that substantial amounts may produce clinically important prolongations.
Active warming is not yet a worldwide standard-of-care. It is thus likely that a substantial fraction of the roughly 240 million patients having noncardiac surgery each year reach core temperatures that increase transfusion requirements and prolong hospitalization. This cost to the healthcare system surely exceeds the now-modest price of active warming.
Our analysis was restricted to two major hypothermic complications: transfusion requirement and hospital length-of-stay. Other major outcomes demonstrated in randomized trials include surgical wound infection and morbid myocardial outcomes. They were not included here simply because neither is reliably included in our registry.
There is a temperature gradient within the esophagus. Probes inserted insufficiently far may thus be cooled by respiratory gases in the adjacent trachea. While our routine practice is to insert the probes about 40 cm which is enough,34 we cannot determine how often probes were only proximally inserted. Similarly, we have no way of determining the extent to which temperatures monitored at less reliable sites might have been inadvertently coded as esophageal temperatures in our electronic record. Either factor would result in artifactually low temperatures. However, we show that low esophageal temperatures are significantly associated with transfusion requirement and the duration of hospitalization. If artifact contributed substantially to low apparent temperatures, there is no reason to believe that they would be associated with hypothermic complications. That they were thus suggests that recorded low temperatures indeed represented patient hypothermia.
Our analysis is restricted to a single center. Results will differ in other centers to the extent that they use different preoperative and intraoperative ambient temperatures, more laminar flow, have shorter or longer cases, or a different case-type mix. Results will also differ to the extent that other centers use more or less effective active warming. We restricted analysis to patients in whom temperature monitoring was coded as esophageal in our electronic anesthesia record. While our routine is to start forced-air warming after draping, it is possible that warming was delayed in some patients.
As with any retrospective analysis, we present associations, which should not be considered evidence of causality. But in this case, causality has already been demonstrated in randomized trials. Our results are more-or-less consistent with randomized results and extend previous work by addressing important issues specifically suited to a registry analysis: 1) generalizability; 2) the smaller magnitude of hypothermia that is now common in actively warmed patients; 3) the distinction between final core temperature and transient intraoperative hypothermia; and, 4) practice changes in the decades since most randomized trials were conducted.
In summary, core temperature decreased during the first hour and subsequently increased in every percentile subgroup. More than half the patients had core temperatures below 36°C within the first hour of anesthesia, and nearly a third had core temperatures below 35.5°C during this period. Even in actively warmed patients, redistribution thus contributes to hypothermia in the first hour of anesthesia. Thereafter, core temperature progressively increased. Nonetheless, intraoperative hypothermia was common, and often prolonged. For example, nearly half the patients had continuous core temperatures <36°C for more than an hour, and 20% of the patients were <35.5°C for more than an hour. Mean core temperature over the duration of anesthesia, 36.0 ± 0.6°C, was thus lower than final intraoperative core temperature which averaged 36.3 ± 0.5°C. Our outcome analysis differs from previous reports in considering hypothermic exposure throughout surgery, not just to final intraoperative temperature and that it includes a large variety of surgical procedures. While hypothermia significantly increased both transfusion requirements and duration of hospitalization, only the increase in transfusions was clinically important. Additional randomized trials are needed to evaluate outcomes of very mild hypothermia (i.e., between 35 and 36°C) and whether maintaining even higher temperatures (i.e., between 36 and 37.5°C) are helpful.
Acknowledgments
Funded by 3M (St. Paul, Minnesota). Dr. Dalton’s effort was supported by the Clinical and Translational Science Collaborative of Cleveland, KL2TR000440 from the National Center for Advancing Translational Sciences component of the National Institutes of Health and National Institutes of Health (Bethesda, Maryland) roadmap for Medical Research.
Footnotes
Bowman A W, Azzalini A: R package ‘sm’: nonparametric smoothing methods (version 2.2-5) 2013. http://cran.r-project.org/web/packages/sm/index.html. Last accessed August 1, 2014.
Drs. Kurz and Sessler serve on advisory boards for 3M; all fees are donated to charity. All other authors have no competing interests.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
References
- 1.Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement: A meta-analysis. Anesthesiology. 2008;108:71–7. doi: 10.1097/01.anes.0000296719.73450.52. [DOI] [PubMed] [Google Scholar]
- 2.Kurz A, Sessler DI, Lenhardt RA. Study of wound infections and temperature group: Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209–15. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 3.Frank SM, Fleisher LA, Breslow MJ, Higgins MS, Olson KF, Kelly S, Beattie C. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events: A randomized clinical trial. JAMA. 1997;277:1127–34. [PubMed] [Google Scholar]
- 4.Leslie K, Sessler DI, Bjorksten AR, Moayeri A. Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesth Analg. 1995;80:1007–14. doi: 10.1097/00000539-199505000-00027. [DOI] [PubMed] [Google Scholar]
- 5.Lenhardt R, Marker E, Goll V, Tschernich H, Kurz A, Sessler DI, Narzt E, Lackner F. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology. 1997;87:1318–23. doi: 10.1097/00000542-199712000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Kurz A, Sessler DI, Narzt E, Bakar A, Lenhardt R, Huemer G. Postoperative hemodynamic and thermoregulatory consequences of intraoperative core hypothermia. J Clin Anesth. 1995;7:359–66. doi: 10.1016/0952-8180(95)00028-g. [DOI] [PubMed] [Google Scholar]
- 7.Sessler DI, Moayeri A. Skin-surface warming: Heat flux and central temperature. Anesthesiology. 1990;73:218–24. [PubMed] [Google Scholar]
- 8.Giesbrecht GG, Ducharme MB, McGuire JP. Comparison of forced-air patient warming systems for perioperative use. Anesthesiology. 1994;80:671–9. doi: 10.1097/00000542-199403000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Matsukawa T, Sessler DI, Christensen R, Ozaki M, Schroeder M. Heat flow and distribution during epidural anesthesia. Anesthesiology. 1995;83:961–7. doi: 10.1097/00000542-199511000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Matsukawa T, Sessler DI, Sessler AM, Schroeder M, Ozaki M, Kurz A, Cheng C. Heat flow and distribution during induction of general anesthesia. Anesthesiology. 1995;82:662–73. doi: 10.1097/00000542-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Hynson J, Sessler DI. Intraoperative warming therapies: A comparison of three devices. J Clin Anesth. 1992;4:194–9. doi: 10.1016/0952-8180(92)90064-8. [DOI] [PubMed] [Google Scholar]
- 12.Melling AC, Ali B, Scott EM, Leaper DJ. Effects of preoperative warming on the incidence of wound infection after clean surgery: A randomised controlled trial. Lancet. 2001;358:876–80. doi: 10.1016/S0140-6736(01)06071-8. [DOI] [PubMed] [Google Scholar]
- 13.Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild intraoperative hypothermia increases blood loss and allogeneic transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289–92. doi: 10.1016/s0140-6736(96)90466-3. [DOI] [PubMed] [Google Scholar]
- 14.Winkler M, Akça O, Birkenberg B, Hetz H, Scheck T, Arkilic CF, Kabon B, Marker E, Grubl A, Czepan R, Greher M, Goll V, Gottsauner-Wolf F, Kurz A, Sessler DI. Aggressive warming reduces blood loss during hip arthroplasty. Anesth Analg. 2000;91:978–84. doi: 10.1097/00000539-200010000-00039. [DOI] [PubMed] [Google Scholar]
- 15.Bowman AW. Applied smoothing techniques for data analysis: The kernel approach with S-Plus illustrations. Oxford: New York, Clarendon: Oxford University Press; 1997. [Google Scholar]
- 16.Koenker R, Bassett G., Jr Regression quantiles. Econometrica. 1978;46:33–50. [Google Scholar]
- 17.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Lenhardt R, Greif R, Sessler DI, Laciny S, Rajek A, Bastanmehr H. Relative contribution of skin and core temperatures to vasoconstriction and shivering thresholds during isoflurane anesthesia. Anesthesiology. 1999;91:422–9. doi: 10.1097/00000542-199908000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Plattner O, Ikeda T, Sessler DI, Christensen R, Turakhia M. Postanesthetic vasoconstriction slows postanesthetic peripheral-to-core transfer of cutaneous heat, thereby isolating the core thermal compartment. Anesth Analg. 1997;85:899–906. doi: 10.1097/00000539-199710000-00034. [DOI] [PubMed] [Google Scholar]
- 20.Kurz A, Sessler DI, Christensen R, Dechert M. Heat balance and distribution during the core-temperature plateau in anesthetized humans. Anesthesiology. 1995;83:491–9. doi: 10.1097/00000542-199509000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Sessler DI, Lee KA, McGuire J. Isoflurane anesthesia and circadian temperature cycles. Anesthesiology. 1991;75:985–9. doi: 10.1097/00000542-199112000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Just B, Trévien V, Delva E, Lienhart A. Prevention of intraoperative hypothermia by preoperative skin-surface warming. Anesthesiology. 1993;79:214–8. doi: 10.1097/00000542-199308000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda T, Kazama T, Sessler DI, Toriyama S, Niwa K, Shimada C, Sato S. Induction of anesthesia with ketamine reduces the magnitude of redistribution hypothermia. Anesth Analg. 2001;93:934–8. doi: 10.1097/00000539-200110000-00027. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda T, Ozaki M, Sessler DI, Kazama T, Ikeda K, Sata S. Intraoperative phenylephrine infusion decreases the magnitude of redistribution hypothermia. Anesth Analg. 1999;89:462–5. doi: 10.1097/00000539-199908000-00040. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda T, Sessler DI, Ikeda T, Sessler DI, Kikura M, Kazama T, Ikeda K, Sato S. Less core hypothermia when anesthesia is induced with inhaled sevoflurane than intravenous propofol. Anesth Analg. 1999;88:921–4. doi: 10.1097/00000539-199904000-00044. [DOI] [PubMed] [Google Scholar]
- 26.Camus Y, Celva E, Sessler DI, Lienhart A. Pre-induction skin-surface warming minimizes intraoperative core hypothermia. J Clin Anesth. 1995;7:384–8. doi: 10.1016/0952-8180(95)00051-i. [DOI] [PubMed] [Google Scholar]
- 27.Hynson JM, Sessler DI, Moayeri A, McGuire J, Schroeder M. The effects of pre-induction warming on temperature and blood pressure during propofol/nitrous oxide anesthesia. Anesthesiology. 1993;79:219–28. doi: 10.1097/00000542-199308000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Sessler DI, Schroeder M, Merrifield B, Matsukawa T, Cheng C. Optimal duration and temperature of pre-warming. Anesthesiology. 1995;82:674–81. doi: 10.1097/00000542-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Xiong J, Kurz A, Sessler DI, Plattner O, Christensen R, Dechert M, Ikeda T. Isoflurane produces marked and non-linear decreases in the vasoconstriction and shivering thresholds. Anesthesiology. 1996;85:240–5. doi: 10.1097/00000542-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Matsukawa T, Kurz A, Sessler DI, Bjorksten AR, Merrifield B, Cheng C. Propofol linearly reduces the vasoconstriction and shivering thresholds. Anesthesiology. 1995;82:1169–80. doi: 10.1097/00000542-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Annadata RS, Sessler DI, Tayefeh F, Kurz A, Dechert M. Desflurane slightly increases the sweating threshold, but produces marked, non-linear decreases in the vasoconstriction and shivering thresholds. Anesthesiology. 1995;83:1205–11. doi: 10.1097/00000542-199512000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Valeri CR, Khabbaz K, Khuri SF, Marquardt C, Ragno G, Feingold H, Gray AD, Axford T. Effect of skin temperature on platelet function in patients undergoing extracorporeal bypass. J Thorac Cardiovasc Surg. 1992;104:108–16. [PubMed] [Google Scholar]
- 33.Rohrer MJ, Natale AM. Effect of hypothermia on the coagulation cascade. Crit Care Med. 1992;20:1402–5. doi: 10.1097/00003246-199210000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Whitby JD, Dunkin LJ. Temperature differences in the oesophagus: Preliminary study. Br J Anaesth. 1968;40:991–5. doi: 10.1093/bja/40.12.991. [DOI] [PubMed] [Google Scholar]