Abstract
Purpose of review
To provide a general update on recent developments in the immunobiology of IL-33 and IL-33-targeted immune cells. We also discuss emerging concepts regarding the potential role IL-33 appears to play in altering alloimmune responses mediating host-versus-graft and graft-versus-host alloresponses.
Recent findings
Stromal cells and leukocytes display regulated expression of IL-33 and may actively or passively secrete this pleotropic cytokine. ILC2 and a large proportion of tissue resident Treg express membrane-bound ST2, the IL-33 receptor. While Treg are appreciated suppressors of the inflammatory function of immune cells, both ILC2s and tissue resident Treg could play key roles in tissue repair and homeostasis. The functions of IL-33 in transplantation are poorly understood. However, like other disease models, the functions of IL-33 in alloimmunity appear to be quite pleiotropic. IL-33 is associated with immune regulation and graft protection in cardiac transplant settings. Yet, it is highly pro-inflammatory and stimulates lethal graft-versus-host disease (GVHD) through its capacity to stimulate Type 1 immunity.
Summary
Intensive studies on IL-33/ST2 signaling pathways and ST2+ cell populations in solid organ and cell transplantation are warranted. A better understanding of this important pathway will provide promising therapeutic targets controlling pathogenic alloimmune responses, as well as potentially facilitating the function of regulatory and reparative immune cells post-transplantation.
Keywords: IL-33, ST2, ILC2, Treg, transplantation
INTRODUCTION
IL-33 was identified as an IL-1 family cytokine in 2005 and it was rapidly demonstrated that recombinant IL-33 protein injections produced potent Type 2 immune responses [1]. As to be expected from these early observations, a role for endogenous IL-33 as a mediator of Type 2 immune pathologies helminth immunity and fibrotic disease has been soundly established [2]. Yet, convincing recent studies have also demonstrated the capacity of IL-33 to fuel the expansion of regulatory T cells (Treg) and support Treg stability during inflammation [3]. Paradoxically, pro-inflammatory stimuli induces the expression of the IL-33 receptor, Suppressor of Tumorigenicity 2 (ST2), on CD4+ T helper Type 1 cells and cytotoxic CD8+ T cells, which also allows IL-33 to potentiate Type 1 response [4-7], particularly in development of acute graft-versus-host disease (GVHD) after allogeneic hematopoietic cell transplantation (AlloHCT) [8]. Thus, in the short period since its discovery, a complex picture has emerged where intrinsic IL-33 properties allow it to promote Type 1, Type 2, and regulatory responses. Now significant gaps in our knowledge regarding the mechanism by which this pleotropic molecule mediates its functions must be filled. Given the constitutive expression of IL-33 in fibroblasts, epithelium, and endothelium and its potent immune modulating capacity [3], IL-33 is most likely a ubiquitous and crucial modulator of alloimmune after transplantation. However, the role of IL-33 in alloimmunity has only been minimally investigated and remains poorly understood. This review details the limited studies completed to date defining the roles of IL-33 in alloimmunity following solid organ transplantation and AlloHCT. Also, the recent demonstration that IL-33 can target ST2+ tissue-resident Treg cells and type 2 innate lymphoid cells (ILC2) were significant observations in immunology. Given their co-localization with IL-33 in the periphery, significant attention has been placed on defining if these potential vanguard cells play key roles in maintaining tissue homeostasis and sensing and repairing tissue injuries [3]. Thus, we will also describe how we envision the recent advances in our understanding of the impact of IL-33 on Treg cells and ILC2 may impact outcomes after transplantation.
IL-33 AND ITS RECEPTOR
We have reviewed the basics of the molecular biology of IL-33 and its receptor complex elsewhere [2, 6]. In brief, like IL-1β or IL-18, IL-33 is synthesized with a typical IL-1 family 12-stranded β-trefoil C-terminal cytokine domain, but it also has a unique N-terminal nuclear localization sequence and chromatin-binding motif [1, 6]. Given this N-terminal domain and lack of a secretion signaling sequence, IL-33 is found in the nucleus, primarily of epithelial cells and endothelial cells, but also in the fibroblasts of lymphoid and non-lymphoid organs [4, 9-11]. Pro-inflammatory stimuli, such as TLR ligands [12], IL-3 and -4 [13], TNFα and IL-1β [14], as well as viral and bacterial infections [4, 15, 16], greatly augment IL-33 expression in these locations. Hypertrophic and mechanical stress can profoundly increase IL-33 levels in fibroblasts [17, 18]. While exact mechanisms enabling IL-33 secretion from viable cells are lacking, when IL-33 is released through tissue damage or necrotic-cell death pathways [19], it is fully functional and able to induce ST2-mediated signaling [20]. Such characteristics have led IL-33 to be classified as an “alarmin”, or endogenous factor that is released from damaged tissue to activate pro-inflammatory innate immune responses [4, 21-23]. Interestingly, a recent study revealed that mast cell-related proteases cleave IL-33 into three mature forms (IL-3395–270, IL-33107–270 and IL-33109–270), which may be especially potent at inducing immune cell activation [24]. Through the use of transgenic mice allowing cell-specific deletion of IL-33, it was recently demonstrated that IL-33 upregulation in the endothelium and subsequent secretion from these cells is crucial to the systemic inflammatory state associated with hypertension [25]. Interestingly, a recent study shows that human and mouse red blood cells also store IL-33, which is also expressed by hematopoietic progenitors [26]. While mechanisms of for secretion and processing of IL-33 are still emerging, these above observations all support the concept that IL-33 is a ubiquitous immune modulator.
The IL-33 receptor consists of the membrane-bound IL-33-binding protein, ST2, and the subsequently recruited IL-1RAcP, a signaling component shared with other IL-1 family members. ST2 is constitutively expressed by immune cells, which secrete type 2 cytokines, such as effector Th2 cells, mast cells, eosinophils, basophils and ILC2 [6]. Recent studies also revealed that a subset of Treg cells in both lymphoid and peripheral tissues express ST2 [27-31]. Pro-inflammatory stimuli can also induce the expression of functional IL-33 receptor on cytotoxic CD8+ T cells [4, 30] and Th1 cells [32]. In general, the IL-33/ST2 signaling pathways appear similar to those of closely related TLRs and IL-1 family members, thus the unique biological effects of IL-33 are most accounted for by timed regulation of ST2 expression on diverse immune cell or a synchronized patterns of ST2+ immune cell recruitment to sites of tissue damage and infection. This concept in IL-33 immunobiology was recently described by Locksley and colleagues as part of an organizational framework they put forth to account for the emerging roles for IL-33 in (1) tissue homeostasis, (2) amplification of Type 2 and regulatory immune responses mediating parasite immunity and tissue repair, and (3) conversion to support of Type 1 immune responses and systemic inflammation [3].
CONTROLLING THE BURN - BENEFICIAL ATTRIBUTES OF IL-33 AFTER TRANSPLANTATION
As IL-33 is expressed by cells of solid organs [6, 9-11], its release through cell damage following surgical ischemia/reperfusion injury or alloimmune responses would allow it to target any local cells that express ST2 [6]. Even though it often described as “alarmin”, and Type 1- and Type 2-mediating roles for IL-33 have been well established, to date there is no direct evidence that IL-33 is a potent mediator of allo-rejection in solid organ transplantation. To the contrary, 3 independent studies showed exogenous recombinant IL-33 treatment protected murine heart allografts from both acute [27, 33] and chronic [34] rejections. Mechanistically, we demonstrated that ST2 expression and Treg cells are both indispensible for this protective role [27].
IL-33-mediated Treg expansion
This observation in rodent heart transplantation models is compatible with the model put forth by Locksley and colleagues where ST2+ Treg targeted by IL-33 limit inflammation to support tissue homeostasis and repair. Unlike other alarmins, such as HMGB1 or ATP, which cause dendritic cell (DC) production of IL-12p70 and DC promotion of Type 1 immunity [35, 36], we find that IL-33 does not induce DC production of IL-12p70, nor support Th1 responses [30]. Instead, IL-33 has been repeatedly found to endow DC with the capacity for polarization of naïve CD4+ T cell into Th2 effectors secreting IL-5 [30, 37, 38]. Interestingly, we identified a crucial role for IL-33-stimulation of myeloid cell IL-2 production that supported the selective expansion of ST2+ Treg over other CD4+ T cell subsets [30]. Specifically, we demonstrated IL-33 induces the secretion of IL-2 by DC, which were required to mediate ST2+ Treg expansion by IL-33 [30]. Treg are well appreciated to be a specialized lineage of T lymphocytes that are of fundamental importance for immunological tolerance by suppressing T cell response to self, commensal, environmental and dietary antigens. In rodent models, increasing the frequency of Treg relative to alloreactive effector T cells facilitates transplant tolerance. As such, preferential in vivo expansion of Treg or adoptive transfer of ex vivo expanded Treg are viewed as one of the more promising immunotherapies to reduce the need for chronic immunosuppression after transplantation [39]. It will be important to better establish in rodent models if the in vivo Treg expanding capacity of IL-33 can be harnessed to support transplant tolerance. Likewise, it is crucial to define if capacity of IL-33 to support the ex vivo expansion of mouse Treg [29, 30, 40] is translatable to efforts to expand human Treg for adoptive transfer.
IL-33 support of reparative tissue-resident immune cells - Treg
Non-lymphoid tissue-resident CD4+ Foxp3+ Treg cells have recently been described in skeletal muscle [28], intestine [29], fat [40, 41], lung [31], and skin [42]. Tissue-resident Treg cells are thought to modulate immunological aspects, such as T cell activity and myeloid cell maintenance in non-lymphoid tissues, as well as shape non-immunological processes such as tissue repair after physical[28] and infectious injuries [31]. Notably, these non-lymphoid tissue-resident Treg cells are enriched in those expressing ST2 [28, 29, 40-42] relative to Treg of the lymphoid tissues [30]. Like ST2+ Treg of the lymphoid tissues, the impact of IL-33 on tissue-resident ST2+ Treg is only starting to be characterized.
In the colon, ~60% Treg cells express ST2, which are composed of both peripherally induced Treg and thymus-derived Treg cells. IL-33 increases Foxp3 and ST2 expression in induced Treg cells by phosphorylating and recruiting GATA3 to Foxp3 promoter and Il1rl1 enhancer, respectively, and recruiting RNA polymerase II to the promoters of both [29]. With respect to ST2+ thymic-derived Treg cells, IL-33 upregulates the expression of ST2, Foxp3 and activation and proliferation markers in Treg cells. Importantly, IL-33 maintains Foxp3 expression of Treg cells and their suppressor function in a chronic inflammatory environment in the colon [29]. An intriguing study by Vasanthakumar et al. [40], which investigated the Treg cells in visceral adipose tissue (VAT), found most of which co-express ST2 and Blimp-1, a transcription factor characterizing effector Treg cells. Although TCR activation, co-stimulation and IL-2 in culture induces robust Treg expansion, they revealed that IL-33 further promotes expansion of the ST2+ Treg cells in a MyD88-dependent manner. Additional examinations suggest a role for IL-33 beyond ST2+ VAT-Treg proliferation, as it also maintains their identity by sustaining Foxp3, GATA3 and PPAR-γ expression [40]. Another study found that Treg cells generated early in life (day 0-10) express a higher level of ST2 and are more suppressive compared to those generated after that period. This study also indicates that ST2+ Treg cells may persist through out the life of the mouse [43]. However, the relationship between ST2+ Treg of lymphoid and non-lymphoid tissues is far from clear and key questions surrounding ST2 expression by Treg are yet to be answered. In particular it will be important to address if all Treg cells can become ST2+ or if only a specific subpopulation is able to regulate expression of the IL-33-receptor. If it is the later, identification of the stimuli that drive expression of functional ST2 on Treg will be of critical importance.
Tissue-resident Treg cells are thought to modulate immunological aspects, such as T cell activity and myeloid cell maintenance in non-lymphoid tissues, and nonimmunological processes such as tissue repair after physical [28] and infectious [31] injuries. Foxp3+ Treg were recently demonstrated to potentiate skeletal muscle repair through their expression of the growth factor amphiregulin (Areg) [28]. While these early studies did not test if Areg on Treg was the mediator of skeletal repair, they did demonstrate that Areg administration after Treg depletion supported muscle regeneration [28]. IL-33 signaling induces Areg expression by ST2+ ILC2 [44] and a recent study demonstrated a critical role for Treg cells in lung tissue repair during infection is mediated by Treg production of Areg in response to IL-33. This was independent of TCR-mediated suppressor function [31]. It will be important to establish how ST2+ Treg expression of Areg is critical to their capacity to direct heart transplant protection [27] or repair. Likewise, if local IL-33 in the transplant could support tissue-resident Treg proliferation needs to be established. A recent study by Morita et al. revealed that IL-33 causes mast cells, like DC [30] to support local Treg expansion via production of IL-2 [45]. How these findings relate to the studies of Bromberg and colleagues that demonstrate appropriate Treg migration from the periphery to lymphoid organs is critical for the induction of transplant tolerance will need to be established [46]. Their studies suggest that Treg migrate through the allograft, where they are activated to locally suppress inflammation and alloantigen acquisition by inflammatory DC. Treg then migrate to the lymph nodes (LNs), where they can also suppress alloantigen-specific T cell priming during interactions with tolerogenic DC [47, 48]. Given that IL-33 can be found in both transplant tissues and the LNs, defining how IL-33 impacts on this pattern of leukocyte migration and these critical interactions will be of significant interest.
IL-33 support of reparative tissue-resident immune cells – ILC2
The study of ILC subsets has been advancing rapidly during the past 5 years, which has reshaped the framework of immunology. ILCs, like all other lymphocytes, develop from a common lymphoid progenitor (CLP) and two downstream precursors expressing either Id2 [49] or promyelocytic leukemia zinc finger (PLZF) [50]. Certain transcription factors, including retinoic-acid-receptor-related orphan nuclear receptor alpha (RORα) [51], T cell factor 1 (TCF1) [52], and GATA3 that is crucial for development of all IL-7Rα+ ILCs [53], are key to ILC2 differentiation and function. Characterized by their membrane-bound ST2 in addition to IL-7Rα (CD127), IL-2Rα (CD25) and Thy1 (CD90) that are shared by all ILCs, ILC2 exist mainly in barrier tissues, such as the lung [51, 54-57], intestine [58], skin [59], as well as adipose tissue [60, 61]. Consistent with their anatomical localizations, ILC2 play an important role in anti-parasitic immunity by increasing the production of mucus. Mechanistically, ILC2 secrete type 2 cytokines including IL-4, IL-5, IL-9 and IL-13 in response to IL-25, IL-33 [58, 61] or thymic stromal lymphopoietin (TSLP) [62]. Like ST2+ Treg cells, ILC2 also possess a tissue repair function by IL-33-driven Areg production during viral infection in the lung [44]. Lastly, ILC2 have been shown to be metabolic-relevant and pathogenic in a wide range of type 2 inflammation-associated diseases as reviewed elsewhere [63]. Given their immunological properties, ILC2 are anticipated to shape allograft rejection and repair, especially in organs such as the lung and the intestine that are rich in ILC.
FUELING THE FIRE - IL-33 AS A DRIVER OF ALLOIMMUNITY
AlloHCT is a promising experimental therapy to promote donor-specific tolerance and recent clinical trials support its applicability. Still the risk of GVHD, a potentially fatal complication that results from donor immune cell destruction of allogeneic recipients tissues, precludes routine use of AlloHCT for tolerance induction [64]. Recipient conditioning, usually consisting of chemotherapy treatment and total body irradiation (TBI), is required to eliminate recipient’s lymphocytes and allow for engraftment of donor stem cells [4, 64-67]. Such conditioning regimens are harmful to the rapidly dividing cells of the barrier tissues, especially the epithelial cells of gastrointestinal mucosa. Epithelial damage generates a pro-inflammatory environment in the gut rife with commensal bacteria-derived TLR ligands that stimulate pro-inflammatory cytokine production by local myeloid antigen presenting cells, such as DC and macrophages [68]. This sequence of events fosters the alloimmune responses causing GVHD [64] and may even stand in the way of transplant (Tx) tolerance [69].
IL-33 promotes acute GVHD
We have recently revealed that IL-33 is elevated in both rodent and patient GVHD-target barrier tissues following conditioning with TBI or chemotherapy, as well as during GVHD [8]. Although how IL-33 is released remains unclear, we provided 3 independent pieces of evidence supporting the concept that IL-33 released from recipient tissues after TBI promotes alloimmunity and lethal acute GVHD [8]. First, delivery of an IL-33 antagonist after AlloHCT rescued the majority of animals in a lethal acute GVHD model. Second, inflammation associated with AlloHCT increases ST2 on T cells of both mouse and humans. This expression of ST2 is critical to alloimmune responses, as transfer of ST2-deficient donor T cells failed to induce lethal GVHD. Finally, IL-33 deficiency in the host non-hematopoietic cells decreases GVHD severity. The absence of IL-33 stimulation of ST2+ donor T cells resulted in significantly decreased Type 1 immune responses [8]. These findings also fall nicely into the model proposed by Locksley, where pathogen-associated molecular patterns found on commensals or pathogens breaching barrier tissues induce pro-inflammatory cytokine secretion via DC activation, which mediates the expression of ST2 on Th1, CTL and NK cells. This then “converts” IL-33 activities towards Type 1 responses aiming to kill pathogens. Thus, moving forward it will be critical to test this hypothesis and precisely establish why IL-33 facilitates Type 1 alloimmunity contributing to GVHD instead of supporting Type 2 or regulatory responses in this model. Likewise, it will be critical to establish how IL-33 “conversion” induced by other alarmins or pathogen-induced signals impacts on solid organ outcomes.
A role for IL-33 in inflammatory death pathways
As mentioned, IL-33 localizes to the nucleus and lacks a classic secretion sequence. Thus, undefined mechanisms must exist that enable IL-33 to reach extracellular spaces where it can shape immune responses. Secretion of IL-33 from viable cells has been observed [25, 70, 71], but the bias in the field is that IL-33 release is predominantly from damaged or dying cells [4, 6, 19]. Apoptotic cell death is typically viewed as an immunologically silent form of cell death, whereas unregulated necrotic cell death resulting from extraordinary stimuli, such as trauma or temperature extremes, is described as pro-inflammatory [72]. Apoptosis is a caspase-dependent process and it was described early on that IL-33 is cleaved into inactive forms by pro-apoptotic caspases [6]. Recently several new caspase-independent, regulated cell death pathways have been identified [72]. A predominating, but unproven, view is that biologically active IL-33 is released mainly during necroptosis, a form of regulated necrosis that is mediated by receptor-interacting protein kinase 3 (RIP3) [3, 72]. Due to the universal occurrence of necrotic cell death during I/R injury and allograft rejection and the proinflammatory effect of necrosis, it is not unexpected that blocking necroptosis may protect graft survival. A recent study testing this found that Ripk3−/− donor hearts survived longer than WT allografts when recipients received short-term rapamycin [73]. However, the authors did not provide direct evidence to show it was the lack of necroptosis or release of IL-33 that was mediating the graft-protective effect. Support for inflammation resulting from necroptosis-mediated release of IL-33 was generated in a model where the removal of an upstream inhibitor of RIP3 resulted in IL-33 release and lethal systemic inflammation [19]. Yet, exciting data from Yatim et al. [74], however, using ingenious transgenic mice where dying cells utilize only necroptosis (presumably releasing IL-33 and other alarmins) or apoptosis, suggest that these pathways are both poorly immunogenic [74]. Specifically, the capacity of apoptotic or necroptotic cells to stimulate DC to promote a CD8 response was equally poor unless the NF-KB signaling pathway was activated in the dying cells [74]. Thus, the context of how a cell dies is of immunological importance and how outcomes are shaped by IL-33 released by the various cell death pathways activated by recipient conditioning, alloimmunity, or I/R injury after transplant surgery will be important to determine.
CONCLUSION
The presence of non-self material, particularly pathogens, stimulates allograft rejection and impedes tolerance induction [69, 75]. Yet, the role self-derived immunomodulatory molecules, such as alarmins and cytokines, play in shaping alloimmunity and tolerance is poorly understood and complicated by observed pleiotropic functions. Cytokines are typically categorized as either “pro-inflammatory”, or promoting rejection, or “regulatory”, and supporting allograft survival and transplant tolerance [76]. Still, pro-inflammatory cytokines like IFN-γ [77], IL-2 [78], IL-4 [79], are also necessary for tolerance induction [76]. Likewise, the most studied alarmin, high mobility group box 1, also exhibits both pro-inflammatory [35, 80] and tolerogenic properties [81]. These publications and recent studies of IL-33, suggest we may need to alter our thinking about self-derived immunomodulatory stimuli in alloimmunity and protocol for tolerance induction. Specifically, it appears that cytokines and alarmins are not intrinsically pro-inflammatory or regulatory, but instead possess mechanisms to contribute to both immunological outcomes. IL-33, classified as both a cytokine and alarmin, has emerged as an ideal molecule to study an immunologically relevant molecule possessing opposing functions (See Figure 1). We expect future studies will continue to reveal its capacity to promote alloimmunity via its amplification of Type 1 and Type 2 immune responses. However, the potent capacity of IL-33 to stimulate regulatory and reparative cells, particularly Treg and ILCs in the tissues, suggests IL-33 may be harnessed to control acute and chronic rejection. Obviously, our goal should be to establish the means to counter the pro-inflammatory capacities of IL-33, but commandeer its regulatory capacity for the benefit of transplant recipients.
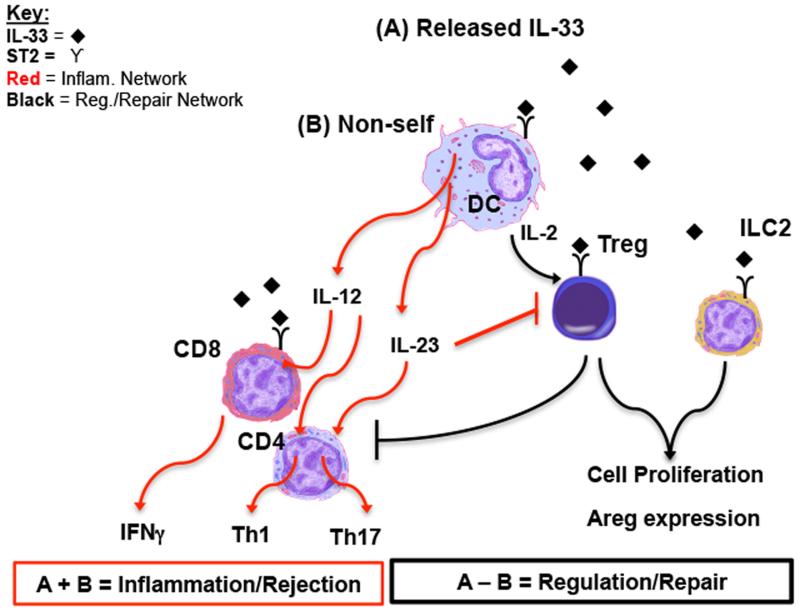
Figure 1. A model accounting for the dichotic function of IL-33 in alloimmunity.
(A) IL-33 is released by secretion from stressed cells (17,18, 25) or cell death/damage [19], where it can target ST2+ immune cells, including DC, Treg, and ILC2. In the absence of (B) non-self molecule (i.e. bacteria or alloantigen [69, 75], IL-33 drives a regulation and reparative pathway. In this pathway DC are stimulated to secrete IL-2, which works with IL-33 to support Treg proliferation [30]. Expansion of the Treg pool would be expected to suppress inflammation and related T cell response. Likewise, IL-33 stimulation of both ILC2 and Treg induces their expression of Areg, which can facilitate tissue repair [3, 31]. In the presence of non-self, IL-33 can contribute to a pro-inflammatory pathway. Specifically, DC are stimulated to secrete IL-12, which promotes a Th1 response, but also up-regulates ST2 on CD8. CD8 expression of ST2 allows IL-33 to augment their production of Type 1 cytokines [8]. Likewise, IL-23 directly counteracts the IL-33-driven function of ST2+ Treg [29].
KEY POINTS.
IL-33 can act on ILC2 cells and ST2+ Treg cells to promote tissue repair and homeostasis.
IL-33 plays an immunoregulatory role in heart transplant.
IL-33 is detrimental after AlloHCT by promoting Type 1 immune responses and lethal acute GVHD.
ACKNOWLEDGEMENT
We thank Carla Forsythe for excellent administrative support in the generation of this manuscript.
FINANCIAL SUPPORT AND SPONSORSHIP
Support of H.R.T. has been derived by grants from the National Institutes of Health/National Heart Lung and Blood Institute (NHLBI; R01HL122489, R00HL097155), Roche Organ Transplant Research Foundation (Grant Award #5891142), and American Heart Association (14GRNT2040004), as well as an AST/Pfizer Basic Science Faculty Development Grant. Q.L. has been supported by a grant from National Natural Science Foundation of China (81401318).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
*of special interest
- [1].Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- [2].Lott JM, Sumpter TL, Turnquist HR. New dog and new tricks: evolving roles for IL-33 in type 2 immunity. J Leukoc Biol. 2015;97:1037–1048. doi: 10.1189/jlb.3RI1214-595R. [DOI] [PubMed] [Google Scholar]
- *[3].Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity. 2015;42:1005–1019. doi: 10.1016/j.immuni.2015.06.006. Comprehensive review providing a framework to interpret and test the role of IL-33 in systemic and local immune responses.
- [4].Bonilla WV, Frohlich A, Senn K, et al. The alarmin interleukin-33 drives protective antiviral CD8(+) T cell responses. Science. 2012;335:984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- [5].Yang Q, Li G, Zhu Y, et al. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8+ T cells. Eur J Immunol. 2011;41:3351–3360. doi: 10.1002/eji.201141629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liu Q, Turnquist HR. Implications for Interleukin-33 in solid organ transplantation. Cytokine. 2013;62:183–194. doi: 10.1016/j.cyto.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gao X, Wang X, Yang Q, et al. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8+ T and NK cells. J Immunol. 2015;194:438–445. doi: 10.4049/jimmunol.1401344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[8].Reichenbach DK, Schwarze V, Matta BM, et al. The IL-33/ST2 axis augments effector T cell responses during acute GVHD. Blood. 2015 doi: 10.1182/blood-2014-10-606830. This is the first report to demonstrated that endogenous IL-33 is upregulated and released from GVHD-target tissue post-TBI, where its dominant function was to drive lethal alloreactive donor T cells responses.
- [9].Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? Plos One. 2008;3 doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kuchler AM, Pollheimer J, Balogh J, et al. Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am J Pathol. 2008;173:1229–1242. doi: 10.2353/ajpath.2008.080014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pichery M, Mirey E, Mercier P, et al. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188:3488–3495. doi: 10.4049/jimmunol.1101977. [DOI] [PubMed] [Google Scholar]
- [12].Talabot-Ayer D, Calo N, Vigne S, et al. The mouse interleukin (Il)33 gene is expressed in a cell type- and stimulus-dependent manner from two alternative promoters. J Leukoc Biol. 2012;91:119–125. doi: 10.1189/jlb.0811425. [DOI] [PubMed] [Google Scholar]
- [13].Zhao WH, Hu ZQ. Up-regulation of IL-33 expression in various types of murine cells by IL-3 and IL-4. Cytokine. 2012;58:267–273. doi: 10.1016/j.cyto.2012.01.019. [DOI] [PubMed] [Google Scholar]
- [14].Xu D, Jiang HR, Kewin P, et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci U S A. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Polumuri SK, Jayakar GG, Shirey KA, et al. Transcriptional regulation of murine IL-33 by TLR and non-TLR agonists. J Immunol. 2012;189:50–60. doi: 10.4049/jimmunol.1003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hazlett LD, McClellan SA, Barrett RP, et al. IL-33 shifts macrophage polarization, promoting resistance against Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2010;51:1524–1532. doi: 10.1167/iovs.09-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sanada S, Hakuno D, Higgins LJ, et al. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kakkar R, Hei H, Dobner S, Lee RT. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J Biol Chem. 2012;287:6941–6948. doi: 10.1074/jbc.M111.298703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rickard JA, O’Donnell JA, Evans JM, et al. RIPK1 Regulates RIPK3-MLKL-Driven Systemic Inflammation and Emergency Hematopoiesis. Cell. 2014;157:1175–1188. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- [20].Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Haraldsen G, Balogh J, Pollheimer J, et al. Interleukin-33 - cytokine of dual function or novel alarmin? Trends Immunol. 2009;30:227–233. doi: 10.1016/j.it.2009.03.003. [DOI] [PubMed] [Google Scholar]
- [22].Oboki K, Ohno T, Kajiwara N, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A. 2010;107:18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chan JK, Roth J, Oppenheim JJ, et al. Alarmins: awaiting a clinical response. J Clin Invest. 2012;122:2711–2719. doi: 10.1172/JCI62423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lefrancais E, Duval A, Mirey E, et al. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci U S A. 2014;111:15502–15507. doi: 10.1073/pnas.1410700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen WY, Hong J, Gannon J, et al. Myocardial pressure overload induces systemic inflammation through endothelial cell IL-33. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1424236112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wei J, Zhao J, Schrott V, et al. Red Blood Cells Store and Release Interleukin-33. J Investig Med. 2015;63:806–810. doi: 10.1097/JIM.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Turnquist HR, Zhao Z, Rosborough BR, et al. IL-33 expands suppressive CD11b+ Gr-1(int) and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. J Immunol. 2011;187:4598–4610. doi: 10.4049/jimmunol.1100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Burzyn D, Kuswanto W, Kolodin D, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Schiering C, Krausgruber T, Chomka A, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Matta BM, Lott JM, Mathews LR, et al. IL-33 Is an Unconventional Alarmin That Stimulates IL-2 Secretion by Dendritic Cells To Selectively Expand IL-33R/ST2+ Regulatory T Cells. J Immunol. 2014;193:4010–4020. doi: 10.4049/jimmunol.1400481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Arpaia N, Green JA, Moltedo B, et al. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Baumann C, Bonilla WV, Frohlich A, et al. T-bet- and STAT4-dependent IL-33 receptor expression directly promotes antiviral Th1 cell responses. Proc Natl Acad Sci U S A. 2015;112:4056–4061. doi: 10.1073/pnas.1418549112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yin H, Li XY, Jin XB, et al. IL-33 prolongs murine cardiac allograft survival through induction of TH2-type immune deviation. Transplantation. 2010;89:1189–1197. doi: 10.1097/TP.0b013e3181d720af. [DOI] [PubMed] [Google Scholar]
- [34].Brunner SM, Schiechl G, Falk W, et al. Interleukin-33 prolongs allograft survival during chronic cardiac rejection. Transpl Int. 2011;24:1027–1039. doi: 10.1111/j.1432-2277.2011.01306.x. [DOI] [PubMed] [Google Scholar]
- [35].Yang D, Postnikov YV, Li Y, et al. High-mobility group nucleosome-binding protein 1 acts as an alarmin and is critical for lipopolysaccharide-induced immune responses. J Exp Med. 2012;209:157–171. doi: 10.1084/jem.20101354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wilkin F, Duhant X, Bruyns C, et al. The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J Immunol. 2001;166:7172–7177. doi: 10.4049/jimmunol.166.12.7172. [DOI] [PubMed] [Google Scholar]
- [37].Rank MA, Kobayashi T, Kozaki H, et al. IL-33-activated dendritic cells induce an atypical TH2-type response. J Allergy Clin Immunol. 2009;123:1047–1054. doi: 10.1016/j.jaci.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Besnard AG, Togbe D, Guillou N, et al. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol. 2011;41:1675–1686. doi: 10.1002/eji.201041033. [DOI] [PubMed] [Google Scholar]
- [39].McDonald-Hyman C, Turka LA, Blazar BR. Advances and challenges in immunotherapy for solid organ and hematopoietic stem cell transplantation. Sci Transl Med. 2015;7:280rv282. doi: 10.1126/scitranslmed.aaa6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[40].Vasanthakumar A, Moro K, Xin A, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. 2015;16:276–285. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- *[41].Kolodin D, van Panhuys N, Li C, et al. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 2015;21:543–557. doi: 10.1016/j.cmet.2015.03.005. These two papers nicely describe the development, accumulation, and maintenance of adipose tissue resident ST2+ Treg cells, which may play a role in systemic metabolism.
- [42].MacDonald KG, Dawson NA, Huang Q, et al. Regulatory T cells produce profibrotic cytokines in the skin of patients with systemic sclerosis. J Allergy Clin Immunol. 2015;135:946–e949. doi: 10.1016/j.jaci.2014.12.1932. [DOI] [PubMed] [Google Scholar]
- [43].Yang S, Fujikado N, Kolodin D, et al. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015;348:589–594. doi: 10.1126/science.aaa7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Monticelli LA, Sonnenberg GF, Abt MC, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Morita H, Arae K, Unno H, et al. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation by Promoting Regulatory T Cell Numbers. Immunity. 2015;43:175–186. doi: 10.1016/j.immuni.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ding Y, Xu J, Bromberg JS. Regulatory T cell migration during an immune response. Trends Immunol. 2012;33:174–180. doi: 10.1016/j.it.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhang N, Schroppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Warren KJ, Iwami D, Harris DG, et al. Laminins affect T cell trafficking and allograft fate. J Clin Invest. 2014;124:2204–2218. doi: 10.1172/JCI73683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[49].Klose CS, Flach M, Mohle L, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- *[50].Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. doi: 10.1038/nature13047. This report and ref 49 provide critical insight on the common progenitors of all ILC.
- [51].Halim TY, MacLaren A, Romanish MT, et al. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37:463–474. doi: 10.1016/j.immuni.2012.06.012. [DOI] [PubMed] [Google Scholar]
- [52].Yang Q, Monticelli LA, Saenz SA, et al. T cell factor 1 is required for group 2 innate lymphoid cell generation. Immunity. 2013;38:694–704. doi: 10.1016/j.immuni.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yagi R, Zhong C, Northrup DL, et al. The transcription factor GATA3 is critical for the development of all IL-7Ralpha-expressing innate lymphoid cells. Immunity. 2014;40:378–388. doi: 10.1016/j.immuni.2014.01.012. This paper and ref 51 and 52 identified transcription factors critical to ILC2 development.
- [54].Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- [55].Chang YJ, Kim HY, Albacker LA, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Nussbaum JC, Van Dyken SJ, von Moltke J, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Halim TY, Steer CA, Matha L, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Roediger B, Kyle R, Yip KH, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Brestoff JR, Kim BS, Saenz SA, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Moro K, Yamada T, Tanabe M, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- [62].Mjosberg J, Bernink J, Golebski K, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- *[63].Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348:aaa6566. doi: 10.1126/science.aaa6566. Excellent review of innate lymphoid cell immunobiology.
- [64].Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12:443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sachs DH, Sykes M, Kawai T, Cosimi AB. Immuno-intervention for the induction of transplantation tolerance through mixed chimerism. Semin Immunol. 2011;23:165–173. doi: 10.1016/j.smim.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Leventhal J, Abecassis M, Miller J, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4:124ra128. doi: 10.1126/scitranslmed.3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Strober S. Path to clinical transplantation tolerance and prevention of graft-versus-host disease. Immunol Res. 2014;58:240–248. doi: 10.1007/s12026-014-8502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hauer-Jensen M, Denham JW, Andreyev HJ. Radiation enteropathy--pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol. 2014;11:470–479. doi: 10.1038/nrgastro.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chong AS, Alegre ML. The impact of infection and tissue damage in solid-organ transplantation. Nat Rev Immunol. 2012;12:459–471. doi: 10.1038/nri3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Byers DE, Alexander-Brett J, Patel AC, et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest. 2013;123:3967–3982. doi: 10.1172/JCI65570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Tjota MY, Williams JW, Lu T, et al. IL-33-dependent induction of allergic lung inflammation by FcgammaRIII signaling. J Clin Invest. 2013;123:2287–2297. doi: 10.1172/JCI63802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Linkermann A, Stockwell BR, Krautwald S, Anders HJ. Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat Rev Immunol. 2014;14:759–767. doi: 10.1038/nri3743. [DOI] [PubMed] [Google Scholar]
- [73].Pavlosky A, Lau A, Su Y, et al. RIPK3-Mediated Necroptosis Regulates Cardiac Allograft Rejection. Am J Transplant. 2014;14:1778–1790. doi: 10.1111/ajt.12779. [DOI] [PubMed] [Google Scholar]
- [74].Yatim N, Jusforgues-Saklani H, Orozco S, et al. RIPK1 and NF-kappaB signaling in dying cells determines cross-priming of CD8+ T cells. Science. 2015;350:328–334. doi: 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Oberbarnscheidt MH, Zeng Q, Li Q, et al. Non-self recognition by monocytes initiates allograft rejection. J Clin Invest. 2014;124:3579–3589. doi: 10.1172/JCI74370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Walsh PT, Strom TB, Turka LA. Routes to transplant tolerance versus rejection; the role of cytokines. Immunity. 2004;20:121–131. doi: 10.1016/s1074-7613(04)00024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Konieczny BT, Dai Z, Elwood ET, et al. IFN-gamma is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T cell costimulation pathways. J Immunol. 1998;160:2059–2064. [PubMed] [Google Scholar]
- [78].Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- [79].Inoue Y, Konieczny BT, Wagener ME, et al. Failure to induce neonatal tolerance in mice that lack both IL-4 and IL-13 but not in those that lack IL-4 alone. J Immunol. 2001;167:1125–1128. doi: 10.4049/jimmunol.167.2.1125. [DOI] [PubMed] [Google Scholar]
- [80].Zou H, Yang Y, Gao M, et al. HMGB1 is involved in chronic rejection of cardiac allograft via promoting inflammatory-like mDCs. Am J Transplant. 2014;14:1765–1777. doi: 10.1111/ajt.12781. [DOI] [PubMed] [Google Scholar]
- [81].Kazama H, Ricci JE, Herndon JM, et al. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]