Highlights
-
•
ERN, Pe, startle reflex, and parietal asymmetry were measured in young children.
-
•
Reduced ERN was related to a larger startle and greater right parietal activity.
-
•
Age predicted smaller startle, larger ERN, and better behavioral performance.
-
•
Age did not moderate the association between ERN and startle.
-
•
Age did not moderate the association between ERN and parietal asymmetry.
Keywords: Cognitive control, Defensive reactivity, Young children, Error-related negativity, Startle, Parietal asymmetry
Abstract
Interactions between cognitive control and affective processes, such as defensive reactivity, are intimately involved in healthy and unhealthy human development. However, cognitive control and defensive reactivity processes are often studied in isolation and rarely examined in early childhood. To address these gaps, we examined the relationships between multiple neurophysiological measures of cognitive control and defensive reactivity in young children. Specifically, we assessed two event-related potentials thought to index cognitive control processes – the error-related negativity (ERN) and error positivity (Pe) – measured across two tasks, and two markers of defensive reactivity processes – startle reflex and resting parietal asymmetry – in a sample of 3- to 7-year old children. Results revealed that measures of cognitive control and defensive reactivity were related such that evidence of poor cognitive control (smaller ERN) was associated with high defensive reactivity (larger startle and greater right relative to left parietal activity). The strength of associations between the ERN and measures of defensive reactivity did not vary by age, providing evidence that poor cognitive control relates to greater defensive reactivity across early childhood years.
1. Introduction
It has been long acknowledged that interactions between emotional and cognitive processes are integral to healthy and unhealthy human development (Gray, 2004). Individual differences in cognitive control are thought to reflect variations in neural systems for regulating behavior and affect whereas variation in defensive reactivity represents differences in responsiveness of the brain's negative-valence system. In particular, researchers have been interested in how top-down cognitive control processes govern bottom-up processes such as defensive reactivity (Moser et al., 2015). Evidence from adults and adolescents indicate that prefrontal cortex regions involved in cognitive control processes are functionally linked to emotion centers of the brain (i.e., the amygdala; Monk, 2008, Siegle et al., 2007) such that prefrontal control regions down-regulate activation of emotion generation regions (Hare et al., 2008, Ochsner and Gross, 2005). Individuals for whom this functional connection is effective tend to engage in adaptive self-regulation skills whereas individuals with poor prefrontal-mediated cognitive control tend to have difficulty regulating affective processes and therefore experience more emotional problems (Casey et al., 2010, Muris et al., 2007, Oldehinkel et al., 2007).
The capacity to engage cognitive control processes begins to emerge in early childhood and continues to develop into late adolescence (Eisenberg et al., 2009, Rothbart et al., 2007). Changes in cognitive control during this developmental period have important implications for understanding how these top-down processes ultimately interact with bottom-up affective processes. As cognitive control and defensive reactivity processes co-develop over time, their interaction contributes to a wide range of functional outcomes. However, measures of cognitive control and defensive reactivity are often studied in isolation. Studies that have explored their interaction (Muris et al., 2007, Oldehinkel et al., 2007) have used parent report questionnaires, which tap behaviors that are considerably down-stream from the neurobiological mechanisms involved in these systems early in development.
Toward this end, the current study had two primary aims. The first was to examine the relationship between cognitive control and defensive reactivity at the neurophysiological level in children. The second was to examine whether age, as a proxy for developmental status, moderated the association between these indices of cognitive control and defensive reactivity.
1.1. Cognitive control
We selected the error-related negativity (ERN) and error positivity (Pe) as neurophysiological indices of interest because of evidence for their construct validity as measures of cognitive control in adults (e.g., Yeung and Summerfield, 2012) and evidence that both markers can be elicited in children as young as 3 years (Grammer et al., 2014). The ERN appears as a negative deflection at frontocentral electrodes within approximately 100 ms of an error. It has been identified as a robust marker of processes related to error correction or suppression (Gehring et al., 2012, Yeung and Summerfield, 2012). The Pe follows the ERN, is maximal at centroparietal sites between 200 and 400 ms following an error, and is thought to reflect conscious error detection (Hughes and Yeung, 2011, Nieuwenhuis et al., 2001). The morphology and scalp distribution of the ERN and Pe in children appear similar to those of adults (Arbel and Donchin, 2011); however, whereas the ERN is reported to increase with age, the Pe tends to be quite stable over time.
1.2. Defensive reactivity
We selected the startle reflex and resting parietal asymmetry based on evidence these are among the best-replicated physiological correlates of defensive reactivity (e.g., Bradley et al., 2001, Heller and Nitschke, 1998, Sabatinelli et al., 2005). Research in animals (e.g., Davis et al., 2008, LeDoux and Schiller, 2009) and humans (e.g., Sabatinelli et al., 2005) has demonstrated that the startle reflex activates the brain's fear-defense circuit, instantiated by the amygdala, and is enhanced when individuals are exposed to threatening stimuli. Eliciting the startle reflex in young children has been inconsistent due to methodological challenges selecting age-appropriate stimuli. In order to address these challenges, Quevedo et al. (2010) developed a task using age-appropriate film clips that successfully elicited the startle in children aged 3–9 years and adults (M age = 22.16 years). It is unclear whether the startle relates to neurophysiological measures of cognitive control.
Greater right relative to left parietal activity in adults has also been identified as a reliable indicator of defensive reactivity given its associations with vigilance and anxious arousal (Bruder et al., 1997, Compton et al., 2003, Heller et al., 1997, Heller and Nitschke, 1998, Metzger et al., 2004). Similar correlates of parietal asymmetry have been observed in childhood such as enhanced right-lateralizated parietal activity in children who exhibit high fear-proneness (e.g., McManis et al., 2002, Shankman et al., 2005, Shankman et al., 2011). There is also evidence that right frontal asymmetry is associated with negative affect and withdrawal-related behaviors (e.g., Davidson, 1992, Davidson and Tomarken, 1989), constructs that overlap with defensive reactivity. However, more recent evidence suggests that increased emotional arousal may be specific to parietal asymmetry rather than frontal asymmetry in early childhood (Shankman et al., 2005, Shankman et al., 2011). Therefore, parietal asymmetry may be a clearer marker of defensive reactivity during this developmental period, and thus we focus on parietal asymmetry in this report.
1.3. Associations between measures of cognitive control and defensive reactivity
Understanding the development of cognitive control and defensive reactivity will require studying the relationship between these processes rather than each in isolation. Few studies have explicitly tested the relationship between markers of cognitive control and defensive reactivity, and all have been conducted in adults. For example, Hajcak and Foti (2008) reported that enlarged ERN was associated with increased startle, but others have failed to replicate this result (Lewis and Pitts, 2015), and re-analysis of the original findings indicated they were driven by a single outlier (Moser et al., 2014).
There is much debate regarding the relationship between the ERN and measures of defensive reactivity, as some propose that enlarged ERN in anxiety reflects cognitive inefficiency (Moser et al., 2013) whereas others suggest enlarged ERN is an index of defensive reactivity (Proudfit et al., 2013). Recent findings have indicated that the ERN is actually smaller in young anxious children (Meyer et al., 2012, Torpey et al., 2013). Meyer and colleagues (2012) found that a smaller ERN was related to higher levels of parent-reported anxiety, but only in the younger children of the sample. Similarly, Torpey et al. (2013) found that a smaller ERN characterized young children who displayed fearful behaviors. Others have reported that an enlarged ERN at age 6 predicts onset of an anxiety disorder 3 years later (Meyer et al., 2015). Thus, how the ERN – conceptualized as a marker of cognitive control – relates to defensive reactivity measures in youngsters is currently unclear.
There are no investigations of associations between cognitive control markers and parietal resting asymmetry, and none on the association between the Pe and physiological markers of defensive reactivity. In terms of the association between Pe and self-reported correlates of defensive processes, some have shown a smaller Pe (Hajcak et al., 2004, Moser et al., 2012) and others a larger Pe (Weinberg et al., 2010) correlated with greater negative emotion. In older children, a larger Pe is related to higher obsessive – compulsive symptoms (Santesso et al., 2006). Findings are therefore likewise equivocal as to how the Pe relates to markers of defensive reactivity.
1.4. The current study and hypotheses
In the current study we measured the ERN, Pe, startle response, and right parietal asymmetry to advance our understanding of the relationship between cognitive control and defensive reactivity in young children. We expected that a smaller ERN would be related to a larger startle response to negative-valenced stimuli given findings in young children that a smaller ERN is associated with higher levels of fear and anxiety (e.g., Meyer et al., 2012, Torpey et al., 2013). Similarly, we expected that a smaller ERN would be associated with greater right parietal activity. We anticipated that a larger Pe would be associated with a larger startle reflex and greater right parietal activity given previous findings that larger Pe is related to greater anxiety symptoms in older children (Santesso et al., 2006).
With regards to the second aim, we expected age to moderate the relationship between cognitive control and defensive reactivity measures. Specifically, we hypothesized that the association between poor cognitive control and high defensive reactivity would be stronger in younger children as compared to older children in the sample based on previously reviewed studies that observed a smaller ERN in fearful youth, but only in very young children (Meyer et al., 2012, Torpey et al., 2013).
2. Method
2.1. Participants
Subjects between the ages of 3 and 7 years were drawn from a larger investigation of child temperament among community children (N = 277), the aim of which was to examine change in temperament traits across early to middle childhood, and associations between traits and familial risk for psychopathology. A subset of 96 participants (M age = 6.00 years, SD = 1.21; 46 females and 50 males) was selected to complete the neurophysiological portion of the study that is the focus of this report. We have previously reported on associations between some measures used in this paper (i.e., startle reflex, Flanker ERN, and parietal asymmetry), along with a behavioral measure of child defensive reactivity, in a pilot demonstration using a small subset of children included in this report (N ≤ 17; Moser et al., 2015). Children were eligible to complete the neurophysiological portion of the study if they had no known history of epilepsy, head trauma resulting in a loss of consciousness for more than five minutes, or any hearing, visual, or physical disabilities that could cause difficulties understanding and/or using a computer. In addition, their scores on an experimenter-report measure of effortful control (Gagne et al., 2011, Vroman et al., 2014) completed by the experimenter who conducted the child's laboratory temperament assessment was Z ≥ −1.65. We thus excluded children who scored the lowest on effortful control in a prior lab visit and were unlikely to be cooperative with tasks requiring an extended period of quiet sitting. The Michigan State University institutional review board approved all procedures, and participants received a gift card for their participation.
Parents of eligible children were provided a detailed description of the study and were invited to complete the neurophysiological portion of the study. When families arrived to the laboratory, a research assistant provided an overview of the study's procedures to parents and their children. After providing written consent, an experimenter guided the child through each step of the physiological recording set up and electrode application. The parent was permitted to stay in the room to observe the setup after which parents waited in a separate observation room where they could view a live camera feed of their child completing tasks. The experimenter was present in the testing room throughout the tasks, but sat behind the child out of view. The experimenter provided feedback and encouragement to complete the task between task blocks depending on the child's accuracy (see below for description).
Of the 96 children who participated in neurophysiological tasks, 8 were excluded from analyses due to poor quality EEG recordings across all tasks. Of the 88 children who had data from the resting asymmetry task, 9 were excluded due to errors in data collection, leaving a sample of 79 (41 females, 38 males). For each ERN task, data from participants who committed errors on more than 35% of trials and/or committed fewer than 6 errors (Olvet and Hajcak, 2009a) were excluded from that task (6 from the Go/No-Go task, and 23 from the Flanker task). In total, 56 participants (28 females, 28 males) were included in analyses for the Go/No-Go task, and 43 participants (25 females, 18 males) were included in analyses for the Flanker task. Of the 50 participants who participated in the startle paradigm, 2 refused to complete the task and 2 were excluded due to technical errors, leaving a final sample of 46 (24 females, 22 males). On average, children used in the current analyses were 6.03 years old (SD = 1.20; range = 3.15–7.99).
2.2. Cognitive control tasks
2.2.1. Go/No-Go task
Children completed a picture version of the Go/No-Go task developed by Lamm et al. (2014) that has been used with other samples of young children to investigate the ERN and Pe (Grammer et al., 2014). Procedures for the current study were identical to those of Grammer et al. Children were asked to help a zookeeper capture animals that had escaped from their cages by pressing the spacebar quickly and accurately to each animal (Go stimuli). They were presented with images of three orangutans that were helping the zookeeper and therefore did not need to be put back in their cages; children were instructed to withhold pressing the spacebar when they saw one of these orangutans (No-Go stimuli). On each trial, a colorful zoo animal was presented at a central location on the computer monitor. Before the stimulus was presented, a fixation cross appeared on the screen for 750 ms. The intertrial interval (ITI) was set to 500 ms.
The task began with a practice block consisting of 12 trials (9 Go trials and 3 No-Go trials). The practice block was repeated until the child demonstrated an understanding of the task. Then, children completed 8 blocks that consisted of 40 trials each (30 Go trials and 10 No-Go trials), totaling to 320 trials and lasting approximately 20 minutes. Novel sets of animal images (Go stimuli) balanced for animal size, color, and type were used in each block.
2.2.2. Flanker task
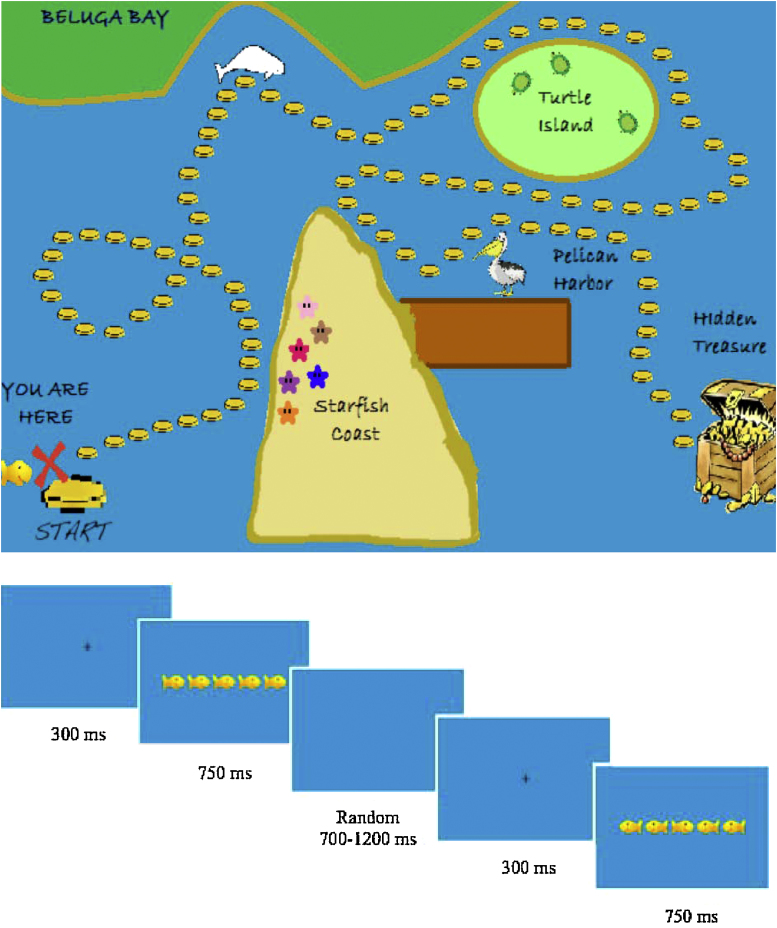
A developmentally appropriate version of the Flanker task adapted from Rueda et al. (2004) was administered to participants. Flanker stimuli consisted of 5 yellow cartoon fish swimming to the left or right on a blue background (see Fig. 1). The child was instructed to focus on responding to the swimming direction of the middle fish/central target stimulus while ignoring the flanking fish stimuli. The task began with a practice block of 20 trials, including 5 congruent left trials (all fish facing to the left), 5 congruent right trials (all fish facing to the right), 5 incongruent left trials (middle fish facing left and flanking fish facing right), and 5 incongruent right trials (middle fish facing right and flanking fish facing left). The practice block was repeated until the child understood the task. After the practice block, children completed 7 blocks that consisted of 20 trials (5 of each trial type as in the practice block), for a total of 140 trials lasting approximately 15 minutes. A fixation cross appeared before each stimulus, which remained on the screen for 750 ms. ITI varied randomly between 700 and 1200 ms.
Fig. 1.
Top: Treasure map slide on the Flanker task. Bottom: Sample trials of the Flanker task.
2.3. Defensive reactivity tasks
2.3.1. Startle paradigm
Traditional methods used to elicit startle responses in adults such as the International Affective Picture System (e.g., Bradley et al., 2001) contain content inappropriate for younger children. However, more recent methods suggest that emotion-eliciting video clips are effective for eliciting startle in young children (Quevedo et al., 2010). Children in the present study viewed 12 age-appropriate video clips (4 pleasant, 4 unpleasant, 4 neutral) ranging in duration from 0.82 to 1.35 minutes (M = 1.18, SD = 0.14). A blue screen was presented between each clip for 10-s intervals (i.e., ITI). Preceding the set of 12 clips, a neutral film clip with a nature scene lasting 1 min was used for startle habituation. White noise bursts (set at 95 dB; near-instantaneous rise time) were presented binaurally at varying points throughout the task during the habituation clip, video viewing, and 10-s rests in between videos (i.e., ITI), to elicit a startle eye blink response recorded from two electrodes under the left eye.
2.3.2. Resting parietal asymmetry
Resting EEG was recorded during four 1-min intervals during which children were instructed to sit in a relaxed, seated position while either looking at an outline of a spaceship or closing their eyes (adapted from Fox et al., 1995). The intervals alternated between having the child's eyes open and eyes closed and the order of eyes closed vs. eyes open first was counterbalanced across children.
2.4. Neurophysiological recording and data reduction
All EEG recordings were taken from 64 Ag-AgCl electrodes using the Active Two Biosemi System (BioSemi, Amsterdam, The Netherlands). For EEG data acquisition, electrodes were placed in a stretch-lycra cap according to the 10/20 system with two additional electrodes placed on the left and right mastoids. Electrooculogram activity from eye movements and blinks were recorded at FP1 and three additional electrodes placed 1 cm from the pupil, one placed directly beneath the left pupil and the remaining two placed on the left and right outer canthi. In accordance with BioSemi's design specifications, the Common Mode Sense active electrode and Driven Right Leg passive electrode served as the reference during data acquisition. All EEG signals were digitized with a sampling rate of 512 Hz using ActiView software (BioSemi).
EMG activity was recorded from two Ag-AgCl electrodes placed over the orbicularis oculi (one electrode directly under the left pupil and the second electrode placed to the right of the electrode beneath the pupil). EMG signals were digitized with a sampling rate of 1024 Hz, bandpass filtered from 30 to 300 Hz, and amplified at 20 K. Offline analyses, described for each task below, were performed using BrainVision Analyzer 2 (BrainProducts, Gilching, Germany).
EEG data were re-referenced to the numeric mean of the mastoids and band-pass filtered with cutoffs of 0.1 and 30 Hz (12 dB/oct rolloff). All trials were also corrected for eye movements and blinks using the method developed by Gratton et al. (1983). A computer-based algorithm was used to detect physiological artifacts such that individual trials were rejected if there was a voltage step greater than 50 μV between sampling points, a voltage difference of more than 200 μV within a trial, or a maximum voltage difference less than 0.5 μV within a trial. For the Go/No-Go and Flanker tasks, trials with reaction times occurring outside of a 200–1300 millisecond window were also removed from analyses.
2.4.1. Cognitive control measures
Based on visual inspection of the grand average ERNs and previous published reports of the ERN in young samples (e.g., Grammer et al., 2014), the ERN and Pe in the Go/No-Go task were quantified using average amplitude measures relative to a −200 to 0 ms pre-response baseline along five midline electrode sites (Fz, FCz, Cz, CPz, and Pz). The ERN from the Go/No-Go task was defined as the average amplitude in the 0–100 ms post-response time on incorrect-response trials whereas the Pe was defined as the average amplitude in a 300–500 ms post-response time on incorrect-response trials. The ERN in the Flanker task was observed to peak earlier than the Go/No-Go ERN. Specifically, the ERN in the Flanker task was observed shortly after the response in the grand average waveform (ERN grand average peak identified at 11 ms). Based on the peak ERN observed in the grand average waveform, the ERN in the Flanker task was defined as the average amplitude on incorrect-response trials in the time window 50 ms before and after this peak (−39 to 61 ms) relative to a −150 to −50 ms pre-response baseline1. The Pe in the Flanker task was defined as the average amplitude in a 300–500 ms post-response time on incorrect-response trials.
2.4.2. Defensive reactivity measures
Startle responses were coded based on criteria outlined by Blumenthal et al. (2005) and have been used in a similar age sample by Quevedo and colleagues (2010). Coding parameters are described in Supplementary materials. As is common for startle, magnitude values included responses with near-zero amplitudes and a positively skewed distribution, with skewness coefficients ranging from 2.01 to 2.89, and kurtosis coefficients ranging from 4.54 to 9.02. Therefore, startle magnitudes were transformed into t-scores and then log-transformed.
Relative activity within the alpha frequency band between right and left recording sites was extracted as parietal alpha asymmetry scores (Shankman et al., 2011). Each subject's data were re-referenced off-line to both the average of the mastoids (heretofore called the mastoid reference) and the average of EEG sites (heretofore called the average reference). Results were reported using both mastoid and average reference schemes to address potential sources of error variance (Davidson, 1988). Each 1-min EEG block was divided into 2-s epochs. A fast Fourier transform (FFT) was applied to all artifact-free epochs, after the data had been weighted with a Hamming window that tapered the distal 10% of each epoch. The alpha range was defined as 7.5–11.5 Hz based on previous studies investigating alpha asymmetry in younger children (Lopez-Duran et al., 2012, Marshall et al., 2002) and alpha asymmetry scores were computed by taking the difference of natural log transformed power scores (Gasser et al., 1982) for PO3/4 and PO7/8 symmetrical right and left electrode sites. Given that EEG power values tend to be positively skewed, natural log transformations have become a conventional data processing procedure in studies of alpha asymmetry. For consistency with previous research, asymmetry scores were computed such that the left natural log transformed score was always subtracted from the right natural log transformed score (i.e., ln[Right Site] − ln[Left Site]). Given the inverse relationship between alpha power and activity, lower alpha asymmetry values indicated relatively greater right activity compared to left (i.e., relatively greater left alpha).
3. Results
3.1. Cognitive control measures
3.1.1. Behavioral performance
Behavioral performance on the Go/No-Go and Flanker tasks are presented in Table 1. Behavioral performance measures across the Go/No-Go and Flanker task were positively associated (overall accuracy r = 0.47, p = .006; RT on error trials r = 0.32, p = 0.07; RT on correct trials r = 0.71, p < .001). As expected, children were significantly faster in responding on error No-Go trials relative to correct Go trials (t(54) = 15.82, p < .001, d = 2.13) and on error relative to correct trials in the Flanker task (t(42) = 11.35, p < .001, d = 1.73).
Table 1.
Behavioral performance on the Go/No-Go task (N = 55) and Flanker task (N = 43).
| Mean | SD | Range | |
|---|---|---|---|
| Go/No-Go task | |||
| Error No-Go trials | 28.16 | 9.44 | 13–49 |
| Correct Go trials | 203.64 | 30.30 | 112–237 |
| Percent error on No-Go trials | 35.78 | 12.24 | 16.00–63.00 |
| Percent error on Go trials | 4.70 | 5.26 | 0.00–22.00 |
| Total accuracy (%) | 87.5 | 5.16 | 71.49–95.41 |
| Reaction time error (ms) | 466.44 | 60.88 | 325.50–610.71 |
| Reaction time correct (ms) | 566.86 | 70.01 | 441.30–750.31 |
| Flanker task | |||
| Error trials | 17.88 | 12.43 | 6–68 |
| Correct trials | 113.37 | 44.21 | 50–221 |
| Total accuracy (%) | 82.24 | 7.67 | 64.75–95.00 |
| Reaction time error (ms) | 482.50 | 64.70 | 346.76–597.88 |
| Reaction time correct (ms) | 557.60 | 46.42 | 461.14–660.92 |
Post-error slowing, or the increase in reaction time after an error, relative to corrects, was evaluated using a one-factor (trial type: post-error vs. post-correct) repeated-measures ANOVA. In the Go/No-Go task, reaction time on hit trials was slower after errors than corrects, although the effect was not statistically significant (F(1, 54) = 2.39, p = 0.13, ). A similar pattern was found in the Flanker task (F(1, 42) = 1.35, p = 0.25, ).
3.1.2. ERN
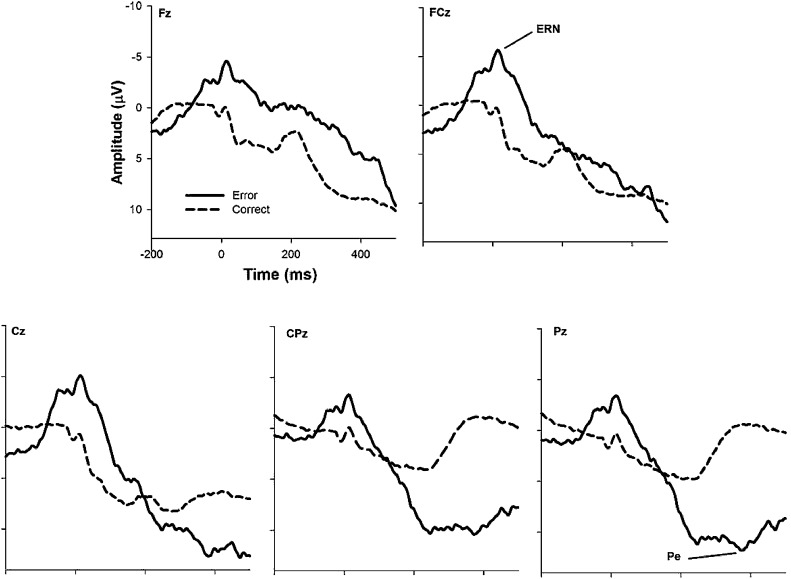
The response-locked waveforms from the Go/No-Go task can be seen in Fig. 2 and descriptive statistics in Table 2. In the Go/No-Go task, there was greater negativity on error No-Go trials compared to correct Go trials (F(1, 55) = 68.46, p < .001, ). This effect varied by (site X trial type interaction, F(4, 220) = 18.21, p < .001, ), indicating a larger error vs. correct difference at frontocentral recording sites. Given that the ERN and mean difference in amplitude between error and error trials (ΔERN) was largest at FCz compared to Pz (t(55) = 3.01, p = .004, d = 0.42; t(55) = 5.08, p < .001, d = 0.68), further analyses focused on FCz. As such, associations between the ERN and behavioral performance (see Table 3) revealed that a larger, or more negative, ERN was related to higher accuracy and faster reaction time on errors.
Fig. 2.
Grand-average ERP waveforms elicited during the Go/No-Go task. Time 0 represents response onset.
Table 2.
Mean (SD) ERN, CRN, and Pe voltage amplitudes (μV) for Go/No-Go and Flanker tasks across five midline sites.
| Components | Fz | FCz | Cz | CPz | Pz |
|---|---|---|---|---|---|
| Go/No-Go ERN | −2.96 (5.23) | −3.32 (5.33) | −2.51 (4.94) | −0.90 (4.09) | −1.16 (4.21) |
| Go/No-Go CRN | 2.49 (2.88) | 3.43 (3.12) | 3.86 (3.13) | 1.50 (3.19) | 2.11 (3.31) |
| Go/No-Go ΔERN | −5.45 (6.10) | −6.75 (5.96) | −6.37 (5.31) | −2.39 (4.60) | −3.28 (4.43) |
| Go/No-Go Pe | 4.44 (8.11) | 8.25 (8.37) | 11.5 (7.83) | 8.95 (8.41) | 10.1 (7.98) |
| Go/No-Go Pe correct | 8.95 (5.06) | 9.31 (4.94) | 6.80 (4.50) | −0.77 (5.79) | −0.02 (4.43) |
| Go/No-Go ΔPe | −4.50 (7.74) | −1.06 (8.41) | 4.70 (8.31) | 9.72 (8.88) | 10.1 (7.88) |
| Flanker ERN | −1.29 (4.39) | −1.54 (4.91) | −1.31 (4.67) | 0.36 (4.47) | 0.18 (4.90) |
| Flanker CRN | 0.71 (3.39) | 0.21 (3.40) | 0.96 (3.45) | 1.24 (3.65) | 1.30 (3.92) |
| Flanker ΔERN | −1.99 (4.82) | −1.75 (5.31) | −2.27 (5.18) | −0.88 (5.29) | −1.12 (5.14) |
| Flanker Pe | 1.28 (8.02) | 3.93 (7.64) | 5.27 (7.39) | 5.22 (7.34) | 4.25 (8.00) |
| Flanker Pe correct | 0.15 (5.32) | −2.39 (5.40) | −6.19 (6.01) | −9.71 (5.77) | −10.6 (5.73) |
| Flanker ΔPe | 1.14 (8.73) | 6.32 (8.49) | 11.5 (9.46) | 14.9 (9.14) | 14.9 (9.64) |
Note. ERN, error-related negativity; CRN, correct-response negativity, the voltage amplitude on correct trials identified in the same time window as the ERN; ΔERN, difference between the ERN and CRN; Pe, error positivity; ΔPe, difference between the Pe and Pe correct.
Table 3.
Bivariate correlations between ERP measures and behavioral performance in Go/No-Go task (N = 55).
| Variable | FCz |
Pz |
||||
|---|---|---|---|---|---|---|
| ERN | CRN | ΔERN | Pe | Pe correct | ΔPe | |
| # Error No-Go trials | 0.10 | −0.30* | 0.24 | −0.14 | −0.09 | −0.08 |
| # Correct Go trials | −0.31* | 0.02 | −0.29* | −0.19 | −0.29* | 0.01 |
| % Error on No-Go trials | 0.15 | −0.33* | 0.30* | −0.16 | −0.09 | −0.10 |
| % Error on Go trials | −0.36** | −0.02 | −0.31* | −0.04 | −0.30* | 0.16 |
| Total accuracy | −0.40** | 0.17 | −0.45** | 0.02 | −0.23† | 0.18 |
| RT error on No-Go trials | 0.38** | 0.09 | 0.29* | 0.23† | 0.22 | 0.09 |
| RT correct on Go trials | 0.19 | 0.09 | 0.12 | 0.38** | 0.29* | 0.19 |
Note.
p ≤ 0.10.
p ≤ 0.05.
p ≤ 0.01.
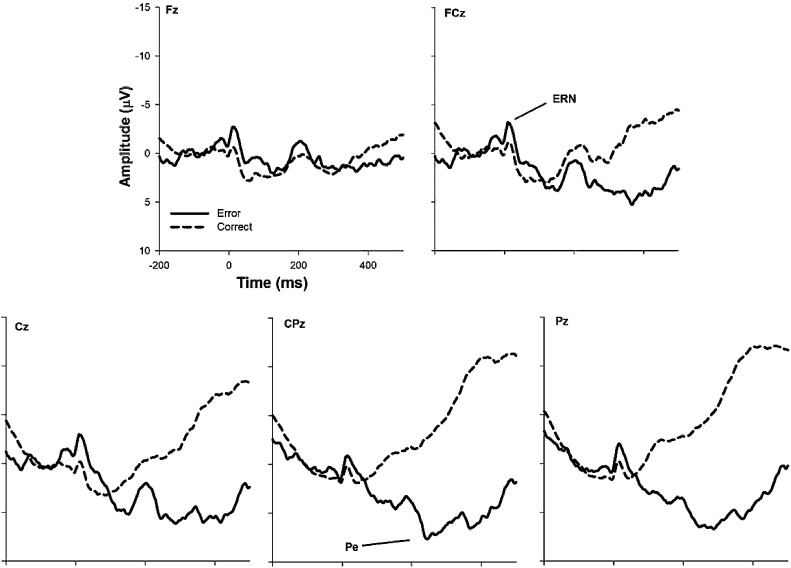
The response-locked waveforms from the Flanker task can be seen in Fig. 3 and descriptive statistics are in Table 2. There were main effects of both trial type (F(1, 42) = 5.56, p = 0.02, ) and electrode site (F(4, 168) = 4.96, p = .001, ), indicating greater negativity on error trials and at frontocentral recording sites. However, there was no interaction between trial type and electrode site (F(4, 168) = 1.77, p = 0.14, ). The ERN and ΔERN were, however, numerically largest at FCz and thus further analyses focus on this site. Associations between the ERN and behavioral performance measures (see Table 4) revealed that a larger ΔERN was related to faster reaction times on error and correct trials.
Fig. 3.
Grand-average ERP waveforms elicited during the Flanker task. Time 0 represents response onset.
Table 4.
Bivariate correlations between ERP measures and behavioral performance in Flanker task (N = 43).
| Variable | FCz |
CPz |
||||
|---|---|---|---|---|---|---|
| ERN | CRN | ΔERN | Pe | Pe correct | ΔPe | |
| # Error trials | −0.11 | −0.18 | 0.01 | −0.15 | 0.10 | −0.18 |
| # Correct trials | −0.03 | −0.04 | −0.01 | 0.11 | 0.27† | −0.08 |
| Total accuracy | 0.15 | 0.28 | 0.03 | 0.30* | −0.03 | 0.26† |
| Reaction time error | 0.29† | −0.19 | 0.39** | −0.25 | 0.12 | −0.28† |
| Reaction time correct | 0.22 | −0.16 | 0.30* | −0.11 | 0.11 | −0.16 |
Note.
p ≤ 0.10.
p ≤ 0.05.
p ≤ 0.01.
3.1.3. Pe
Presence of the Pe at centro-parietal sites as assessed by the Go/No-Go task can be observed in Fig. 2. Descriptive statistics of the Pe can be found in Table 2. Results indicated main effects of trial type (F(1, 55) = 15.85, p < .001, ), site (F(4, 220) = 21.3, p < .001, ), and significant interaction between the two (F(4, 220) = 108.97, p < .001, ), suggesting greater positivity on error trials at posterior sites. The Pe and ΔPe were numerically largest at Pz, and therefore Pe analyses focused on the Pz electrode site. Associations between the Pe and behavioral performance (see Table 3) indicated a larger, or more positive, Pe was associated with slower reaction time on correct trials. Presence of the Pe at centro-parietal sites in the Flanker task is shown in Fig. 3 (see Table 2 for descriptive statistics). Results indicated a main effect of trial type (F(1, 42) = 61.3, p < .001, ), site (F(4, 168) = 22.4, p < .001, ), and significant interaction between the two (F(4, 168) = 75.5, p < .001, ). Together, these results indicate greater positivity on trials occurring at more posterior sites. The ΔPe did not differ between CPz and Pz (t(42) = 0.58, p = 0.57; d = 0.08), but the Pe was more positive at CPz than at Pz (t(42) = 1.98, p = .05; d = 0.30). Therefore, analyses of the Flanker Pe were conducted using CPz.2 A moderate association between Pe and higher overall accuracy was observed (see Table 4).
3.2. Defensive reactivity measures
3.2.1. Startle
Descriptive statistics of startle magnitudes are presented in Table 5. A one-factor (valence: neutral vs. positive vs. negative) ANOVA suggested a main effect of video valence (F(2, 88) = 4.48, p = 0.01, ). Follow-up analyses indicated that the startle magnitude on negative-valenced video clips was significantly larger compared to positive video clips (t(45) = 2.87, p = .006; d = 0.42), but comparable to neutral video clips (t(44) = 0.37, p = 0.71; d = 0.06). Children exhibited an inhibited startle response to positive video clips relative to neutral stimuli (t(44) = 2.22, p = 0.03; d = 0.33). Analyses described below focus on startle magnitude elicited on negative-valenced video clips, as a marker of defensive reactivity.
Table 5.
Mean (SD) startle response magnitudes (μV) to neutral-, positive-, and negative-valenced video clips (N = 30).
| Mean | SD | Range | |
|---|---|---|---|
| Neutral startle | 0.59 | 0.52 | −1.23 to 1.50 |
| Positive startle | 0.43 | 0.47 | −0.62 to 1.43 |
| Negative startle | 0.62 | 0.52 | −0.47 to 1.64 |
| ITI startle | 0.32 | 0.43 | −0.31 to 1.37 |
3.3. Associations between measures of cognitive control and defensive reactivity
3.3.1. Between ERN/Pe and startle (see Table 6, Table 7)
Table 6.
Bivariate correlations between startle magnitude and ERP measures from Go/No-Go and Flanker tasks.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Go/No-Go ERN | ||||||||||||
| 2. Go/No-Go CRN | 0.08 | |||||||||||
| 3. Go/No-Go ΔERN | 0.85** | −0.45** | ||||||||||
| 4. Go/No-Go Pe | 0.01 | 0.31* | −0.15 | |||||||||
| 5. Go/No-Go Pe correct | 0.06 | 0.28* | −0.10 | 0.39** | ||||||||
| 6. Go/No-Go ΔPe | −0.03 | 0.11 | −0.08 | 0.76** | −0.31* | |||||||
| 7. Flanker ERN | 0.16 | 0.15 | 0.04 | 0.32† | 0.14 | 0.26 | ||||||
| 8. Flanker CRN | 0.16 | 0.09 | 0.07 | 0.14 | 0.24 | −0.01 | 0.22 | |||||
| 9. Flanker ΔERN | 0.06 | 0.09 | 0.00 | 0.21 | −0.01 | 0.25 | 0.78** | −0.44** | ||||
| 10. Flanker Pe | −0.12 | −0.17 | 0.01 | 0.28 | 0.18 | 0.19 | 0.25 | 0.46** | −0.06 | |||
| 11. Flanker Pe correct | 0.14 | −0.23 | 0.27 | −0.07 | 0.08 | −0.13 | 0.12 | 0.16 | 0.01 | 0.04 | ||
| 12. Flanker ΔPe | −0.19 | −0.01 | −0.15 | 0.29 | 0.11 | 0.25 | 0.13 | 0.27† | −0.05 | 0.78** | −0.60** | |
| 13. Negative Startle | 0.35* | 0.13 | 0.25 | 0.03 | 0.19 | −0.10 | 0.42* | 0.30 | 0.19 | −0.05 | 0.17 | −0.15 |
Note. Go/No-Go and Flanker n = 30; Go/No-Go and startle n = 41; Flanker and startle n = 24.
p ≤ 0.10.
p ≤ 0.05.
p ≤ 0.01.
Table 7.
Bivariate correlations between ERN and startle with alpha asymmetry scores using average and mastoid reference schemes.
| Variable | Average reference |
Mastoid reference |
||
|---|---|---|---|---|
| PO34 | PO78 | PO34 | PO78 | |
| Go/No-Go ERN | −0.21 | −0.03 | −0.14 | −0.04 |
| Go/No-Go CRN | 0.09 | −0.01 | 0.07 | 0.01 |
| Go/No-Go ΔERN | −0.24† | −0.02 | −0.16 | −0.04 |
| Go/No-Go Pe | 0.07 | 0.01 | 0.05 | −0.04 |
| Go/No-Go Pe correct | 0.22 | 0.00 | 0.22 | 0.03 |
| Go/No-Go ΔPe | −0.08 | 0.01 | −0.10 | −0.06 |
| Flanker ERN | −0.14 | −0.15 | −0.16 | −0.15 |
| Flanker CRN | 0.25 | 0.30† | 0.23 | 0.28† |
| Flanker ΔERN | −0.29† | −0.33* | −0.30† | −0.32† |
| Flanker Pe | 0.01 | 0.09 | 0.01 | 0.07 |
| Flanker Pe correct | −0.04 | −0.08 | −0.06 | −0.09 |
| Flanker ΔPe | 0.04 | 0.12 | 0.05 | 0.12 |
| Negative startle | −0.02 | −0.15 | 0.08 | −0.07 |
| Mean | 0.11 | 0.36 | 0.10 | 0.32 |
| SD | 0.29 | 0.41 | 0.24 | 0.39 |
Note. Alpha asymmetry scores were calculated by ln[Right Site] − ln[Left Site]. N = 53 for Go/No-Go task and alpha asymmetry scores. N = 38 for fish flanker task and alpha asymmetry scores. N = 88 for all sites for mean and standard deviation.
p ≤ 0.10.
p ≤ 0.05.
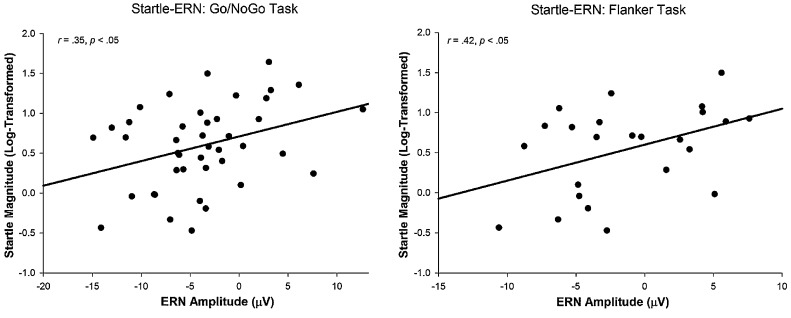
Consistent with our hypothesis, the ERN as measured by Go/No-Go and Flanker tasks was positively associated with startle magnitude on negative-valenced video clips, indicating that smaller ERN was significantly associated with greater startle magnitude.3 The scatterplot of these relationships can be seen in Fig. 4. A linear regression analysis was conducted to test the degree to which unique variances of the ERN measured by each task predicted startle magnitude. When both Go/No-Go ERN and Flanker ERN were included in the regression model, results indicated that unique variance associated with the Flanker ERN predicted startle magnitude (b = 0.05, SEb = 0.02, β = 0.48, p = 0.03), whereas the Go/No-Go ERN no longer predicted startle (b = 0.00, SEb = 0.03, β = 0.01, p = 0.95). In terms of Pe, Pe components were not associated with startle.
Fig. 4.
Scatterplots of the associations between startle magnitude on negative-valenced stimuli and the ERN as assessed by the Go/No-Go Task (left) and Flanker task (right).
3.3.2. Between ERN/Pe and asymmetry
Descriptive statistics for the parietal asymmetry are presented in Table 7. Associations between parietal asymmetry and cognitive control measures were similar across average and mastoid reference schemes. Consistent with our hypothesis, a smaller ΔERN as measured by the Flanker task was associated with greater relative right than left parietal activity at PO7/8. A similar (but smaller) association was observed between the ERN in the Go/No-Go task and right parietal activity at PO3/4. In contrast, the Pe was unrelated to parietal asymmetry.
3.4. The role of age in the association between cognitive control and defensive reactivity
3.4.1. Age-related differences in measures of cognitive control
Regression analyses revealed that younger age (in months) was associated with worse overall accuracy on Flanker and Go/No-Go tasks (see Table 8). Younger age also predicted faster reaction times on both error and correct trials in both tasks. Consistent with previous research, younger age predicted a smaller ERN and ΔERN as measured by the Flanker task, even after accounting for behavioral performance (see Table 8). However, in contrast to the moderate-to-large associations between children's age and behavioral performance, these effects were quite small, explaining only 9–12% of the variance in the ERN. Age did not significantly predict variance in Pe or ΔPe as measured by the Flanker task. In the Go/No-Go task, age was not a significant predictor of the ERN or ΔERN whereas older age predicted a larger Pe.
Table 8.
Results of regression analyses quantifying associations between behavioral performance, ERP components, and age.
| Flanker task | Age B(SE) |
Accuracy B(SE) |
R2 | Go/No-Go task | Age B(SE) |
Accuracy B(SE) |
R2 |
|---|---|---|---|---|---|---|---|
| Behavior | Behavior | ||||||
| # Error trials | −0.01 (0.15) | 0.00 | # Error No-Go trials | 0.03 (0.08) | 0.00 | ||
| # Correct trials | 1.18 (0.49)* | 0.12 | # Correct Go trials | 1.68 (0.23)*** | 0.50 | ||
| Total accuracy | 0.002 (0.001)** | 0.10 | Total accuracy | 0.001 (0.00)** | 0.16 | ||
| RT error | −6.16 (1.43)*** | 0.29 | RT error on No-Go trials | −2.24 (0.49)*** | 0.53 | ||
| RT correct | −4.82 (0.98)*** | 0.35 | RT correct on Go trials | −3.56 (0.51)*** | 0.47 | ||
| % Error on No-Go trials | 0.00 (0.00) | 0.00 | |||||
| % Error on Go trials | −0.002 (0.00)*** | 0.34 | |||||
| Neurophysiology | Neurophysiology | ||||||
| ERN | −0.13 (0.06)* | 14.4 (8.61) | 0.12 | ERN | −0.02 (0.06) | −39.2 (16.0)* | 0.16 |
| CRN | 0.01 (0.04) | 6.39 (6.26) | 0.03 | CRN | −0.02 (0.04) | 13.1 (10.1) | 0.03 |
| ΔERN | −0.14 (0.07)* | 8.14 (9.50) | 0.09 | ΔERN | 0.00 (0.07) | −52.4 (17.6)** | 0.45 |
| Pe CPz | −0.07 (0.09) | 29.9 (13.0)* | 0.08 | Pe Pz | −0.20 (0.10)* | 33.0 (25.1) | 0.08 |
| Pe correct CPz | −0.01 (0.08) | −1.77 (10.8) | 0.10 | Pe correct Pz | −0.04 (0.07) | −18.0 (17.2) | 0.06 |
| ΔPe CPz | −0.06 (0.12) | 29.7 (16.4)† | 0.08 | ΔPe Pz | −0.16 (0.09)† | 51.0 (24.7)* | 0.29 |
Note.
p ≤ 0.10.
p ≤ 0.05.
p ≤ 0.01.
p < 0.001.
3.4.2. Age-related differences in defensive reactivity measures
Greater startle magnitude on negative-valenced video clips was associated with younger age (b = −0.01, SEb = 0.01, β = −0.36, p = 0.01). Associations between age and alpha asymmetry scores were non-significant and comparable across both reference schemes (bs = −1 × 10−3 to 6 × 10−3, SEbs = 1 × 10−3 to 2 × 10−3, βs = −0.05 to 0.00, ps = 0.68 to 0.98).
3.4.3. Moderating effects of age
To test our hypothesis, age was tested as a moderator of the association between the ERN and startle magnitude on negative-valenced stimuli. Separate hierarchical regression analyses were conducted for the ERN measured by the Go/No-Go and the ERN measured by the Flanker task. All predictors were centered on the mean and interaction terms were computed between age and the ERN measured by each task. Age and ERN were entered in the first step of the regression, and the interaction term entered in the second step. Results indicated that age (b = −0.01, SEb = 0.01, β = −0.34, p = 0.03) and the ERN measured by the Go/No-Go task (b = 0.02 SEb = 0.01, β = 0.27, p = 0.07) were significant predictors of startle magnitude. However, age did not moderate the association between the ERN measured by the Go/No-Go and startle magnitude (b = 1.26 × 10−4, SEb = 1 × 10−3, β = 0.02, p = 0.90). While only marginally significant, the ERN measured by the Flanker task predicted startle magnitude (b = 0.04, SEb = 0.02, β = 0.37, p = 0.09), whereas age did not (b = −0.01, SEb = 0.01, β = −0.18, p = 0.38), once the two variables were considered together in the analysis. Again, age did not moderate the association between the ERN measured by the Flanker and startle magnitude (b = −1 × 10−3, SEb = 2 × 10−3, β = −0.15, p = 0.51).
Hierarchical regression analyses were also conducted to test the moderating role of age in the association between the ERN and parietal asymmetry. All predictors were centered on the mean and interaction terms were computed between age and the ERN measured by each task. Age and ERN were entered in the first step of the regression, and the interaction term entered in the second step. Results indicated similar results across electrode site and reference scheme suggesting that age (bs = −1 × 10−3, SEbs = 1 × 10−3, βs = −0.19 to −0.13, ps = 0.19 to 0.38) and ERN as measured by the Go/No-Go task did not significantly predict parietal asymmetry scores (bs = −0.01, SEbs = 0.01, βs = −0.19 to −0.06, ps = 0.10 to 0.67). Age did not moderate the association between ERN and parietal asymmetry (bs = 1 × 10−3, SEbs = 1 × 10−3, βs = −2 × 10−3 to 0.14, ps = 0.34 to 1.00). In terms of the Flanker task, results indicated that age was a marginally significant predictor of parietal asymmetry scores (bs = −0.01, SEbs = 0.01, βs = −0.34 to −0.27, ps = 0.04 to 0.11) while the ERN did not predict parietal asymmetry scores (bs = −0.02 to −0.01, SEbs = 0.01 to 0.02, βs = −0.32 to −0.20, ps = 0.18 to 0.24). Age did not moderate the association between ERN and parietal asymmetry (bs = −2 × 10−3, SEbs = 1 × 10−3, βs = −0.19 to 0.09, ps = 0.31 to 0.66).
4. Discussion
The primary aim of the current study was to examine the relationship between cognitive control and defensive reactivity at the level of physiological and neural activity in young children. A secondary aim was to evaluate the degree to which age moderated the association between indices of cognitive control (ERN/Pe) and defensive reactivity (startle/parietal asymmetry). Two key findings emerged. First, results indicated that measures of cognitive control and defensive reactivity were associated such that evidence of poor cognitive control (smaller ERN) was associated with high defensive reactivity (larger startle response and greater right relative to left parietal activity). Second, age did not moderate the relationship between these measures, suggesting that the strength of the association between poor cognitive control and high defensive reactivity does not differ by age for children between 3 and 7 years. The implications and significance of these findings are discussed in detail below.
4.1. Relationship between measures of cognitive control and defensive reactivity
The development of the capacity to engage in cognitive control begins in early preschool years and continues into late adolescence and has important implications for understanding how these top-down processes ultimately interact with affective, bottom-up processes (Eisenberg et al., 2009, Rothbart et al., 2007). Our findings highlighted the importance of understanding the link between cognitive control and defensive reactivity processes in young children. We found that a smaller ERN across both tasks was associated with a larger startle response to negative stimuli. A small-to-moderate association between a smaller ΔERN and greater relative right than left parietal activity was also found. However, the Pe was unrelated to either startle or resting parietal asymmetry, which indicates that the association between defensive reactivity and cognitive control processes was specific to those indexed by the ERN.
The negative association between cognitive control and defensive reactivity is entirely consistent with the notion of top-down emotion regulation observed in adolescence and adults (Hare et al., 2008, Ochsner and Gross, 2005). That is, children who exhibited poor cognitive control (smaller ERN) also showed higher defensive reactivity (larger startle and greater right relative to left parietal activity). This is in keeping with findings from adolescents and adults suggesting that regions of prefrontal cortex (ACC, dorsolateral prefrontal cortex) have inhibitory functional connections with emotional hubs such as the amygdala (Monk, 2008, Siegle et al., 2007). Our results suggest this pattern emerges in early childhood.
4.2. Role of age
In terms of age-related differences in measures of cognitive control, younger age predicted poorer behavioral performance. This is consistent with the developmental literature, which primarily consists of behavioral data, indicating poorer self-regulation and related cognitive control skills in early childhood. Younger age also predicted a smaller ERN and ΔERN as measured by the Flanker task even after accounting for behavioral performance. These findings are consistent with previous studies suggesting that the ERN is reduced in younger children (e.g., Davies et al., 2004, Kim et al., 2007). However age effects on ERN were much smaller than relations between age and behavioral performance. Further research is necessary to identify the interplay and possibly unique developmental trajectories of behavioral vs. neural efficiency. Such work will help clarify which process develops first (e.g., the neural framework develops to facilitate behavioral performance, or behavioral proficiency shapes neural responses). It is important to keep in mind that chronological age is merely a proxy for developmental processes, so an important agenda for future research will be to identify and test more precise mechanisms of biological maturation and experience that may drive the development of neurophysiological processes underlying cognitive control and defensive reactivity.
In terms of the development of defensive reactivity, age predicted significant variation in startle magnitude such that greater startle magnitude on negative-valenced video clips was associated with younger age. This finding is consistent with previous literature indicating that larger startle magnitudes on neutral-valenced videos were associated with younger age, a trend that has been reported in older populations (e.g., Ellwanger et al., 2003, Ludewig et al., 2003; but see Quevedo et al., 2010). Age marginally predicted parietal asymmetry scores when the ERN as measured by the Flanker task was included in the regression analysis such that older age was associated with increases in right relative to left parietal activity, suggesting that the ERN accounted for some of the variance in parietal asymmetry scores not explained by age.
Lastly, moderation analyses suggested that age did not moderate the associations between the ERN and startle, suggesting that the strength of association across these measures did not vary by age. This finding is consistent with the notion that poor cognitive control may be related to higher defensive reactivity during this developmental period between 3 and 7 years of age.
4.3. Limitations and future directions
The present results should be considered in light of several limitations. First, there is currently no consensus as to which task is most appropriate for eliciting these neural markers of cognitive control and defensive reactivity. This lack of consensus has resulted in hundreds of different paradigms used in both adult and child studies, making across-study comparisons difficult. To address this limitation, the present study used both existing and adapted paradigms from the current developmental literature. It is likely that future studies using a similar approach – i.e., having multiple tasks to assess multiple underlying constructs – will be most informative.
Secondly, task instructions varied by participant to ensure an adequate number of trials for ERP analysis, and may have influenced behavioral performance measures such as post-error slowing. For example, children who were not committing enough errors were instructed to respond more quickly; this may have influenced post-error adjustments. Third, the Go/No-Go and Flanker tasks were not counterbalanced and the children completed the Flanker following the Go/No-Go task. The Flanker task was more difficult, as accuracy was generally lower and had a wider range than the Go/No-Go task. It is possible that its difficulty and completion later in the laboratory visit could have introduced more measurement error if there were systematic differences in the order of task presentation (i.e., children were more fatigued or distracted following the Go/No-Go task).
Fourth, in terms of subject selection, an experimenter-report measure of effortful control was used to exclude children who were unlikely to be cooperative with tasks requiring an extended period of quiet sitting. Our exclusion of children who fell more than 1.65 standard deviations below the mean on this measure suggests that our findings may not be as generalizable to children who may have been unlikely to cooperate with tasks and may experience notable difficulties with cognitive control. However, this selection criteria excluded less than 1 percent of the entire sample, thus it is likely that our sample still reflects a representative distribution of skills related to cognitive control.
In addition to these limitations, it is curious that we found relationships between cognitive control and defensive reactivity measures despite finding that our indices of cognitive control were not correlated and our measures of defensive reactivity were unrelated. There seem at least three possible explanations for the lack of convergence within measures of cognitive control and defensive reactivity.
First, as previously alluded to, it is possible that the internal reliability of these measures in young children is less stable. There is some evidence that the ERN and Pe may be less reliable in younger children (Davies et al., 2004). A closer examination of the internal reliability of these tasks is important to understanding the psychometric properties of individual difference measures derived from these widely used paradigms. Unfortunately, no such studies have been conducted in this age range, and limit the extent to which the present results can be attributed to differences in the posited stability of these measures in young children compared to adults.
Second, there are methodological differences (e.g., task stimuli, response requirements) between the two cognitive control tasks and defensive reactivity paradigms. Recent work indicates that even slight variations to response and stimulus properties can influence cognitive control measures (cf. Grutzmann et al., 2014, Plant and Quinlan, 2013, Schroder et al., 2012). The notion that task differences can reduce correlations is consistent with a recent study that compared ERN across picture-word and Flanker tasks (Foti et al., 2013), and found lower convergence (r = .45) as compared to Riesel et al. (r = .65) and Meyer et al. (r = .61) studies that reported higher convergence using the Flanker, Go/No-Go, and Stroop tasks. In terms of methodological differences between the two defensive reactivity paradigms, they differ substantially in their mode of tapping defensive reactivity insofar parietal asymmetry indexes tonic levels of relative right vs. left parietal activation while participants are not engaged in processing complex stimuli and as startle indexes immediate responses to specific emotionally evocative probes.
Third, it is possible the processes tapped by these tasks may not yet be as fully integrated in children this young as they are in older children and adults. In terms of the Go/No-Go and Flanker tasks, the design of each task suggests that the Go/No-Go task may better capture inhibitory control processes while the Flanker task may be more sensitive to attentional control processes. Attentional control refers to the ability to effectively allocate one's attention in a flexible manner while inhibitory control refers to the capacity to regulate and resist prepotent behavioral impulses or responses. Indeed, recent reviews indicate that attentional and inhibitory control mechanisms may develop over different time courses (see Diamond, 2013 for a review). Children are explicitly instructed in the Go/No-Go task to purposefully inhibit their prepotent impulse to respond on No-Go trials whereas children are asked to pay attention to the middle stimulus in the Flanker task, thereby taxing their ability to ignore flanking stimuli and maintain attentional focus. In terms of the defensive reactivity measures, the primary structures and areas from which these indices are thought to originate are quite different. Research in both animals (e.g., Davis et al., 2008, LeDoux and Schiller, 2009) and humans (e.g., Sabatinelli et al., 2005) has consistently demonstrated that the startle reflex activates the brain's fear-defense circuit, mediated by the amygdala. In contrast, parietal resting asymmetry measures baseline resting brain activity. Therefore, it is possible that the connections between the neural networks associated with these two neurophysiological markers are still developing in children of this age range. However, this hypothesis would need to be tested more definitely with future longitudinal studies to determine whether differences in neurophysiological markers lag behind behavioral markers of defensive reactivity, as this is unclear in the developmental literature.
Despite the lack of association between the neural indices of cognitive control, it is important to note that behavioral measures across tasks demonstrated strong relationships (rs > .70), suggesting that the two tasks were tapping similar mechanisms at the level of overt behavioral responses. This indicates differential convergent validity across cognitive control tasks depending on level of analysis, with ERPs being less closely tethered than behavior.
Given the lack of convergence within measures of cognitive control and defensive reactivity, the associations across constructs need to be interpreted with caution. That is, if these tasks exhibited poor reliability, we would be less likely to uncover significant findings. Indeed, the interpretability of findings from the present study and existing investigations using these measures is dependent on its reliability, so it is critical for future examinations to assess the internal reliability of these measures in early childhood to affirm the validity of using these measures to assess individual differences. However, reliability analyses have only recently begun to find their place in psychophysiological research. Thus, future studies will need to consider such psychometric issues in interpreting findings in young children. Yet it is important to note that the possibility of poor internal reliability of these measures would only explain the lack of association between the ERN/Pe across tasks and startle/parietal asymmetry; it would not explain our primary finding of the associations between the ERN and startle/parietal asymmetry given that unreliability reduces rather than inflates the number of significant correlations (Schmitt, 1996). In other words, increasing the reliability of these measures would hypothetically strengthen the association between measures of cognitive control and defensive reactivity.
Another important future direction is to understand the shared and unique variances in startle reflexes elicited across different affective contexts. Supplemental analyses suggested that the magnitude of association between startle to neutral stimuli and the ERN were smaller compared to that of startle to negative stimuli, but similar in effect size. In contrast, the magnitude of association between startle to positive-stimuli and the ERN were even smaller and non-significant. These results suggest that individual differences in startle response to negative stimuli may be most sensitive to individual differences in the ERN as compared to the startle to neutral and positive stimuli. However, they also suggest that startle magnitude elicited across negative and neutral contexts might tap similar defensive processes that relate to a smaller ERN. Such an interpretation dovetails with research showing that neutral or ambiguous, stimuli also elicit defensive processes (e.g., Richards, 2004, Whalen, 1998). For example, Quevedo et al. (the only other paper to report affective startle using videos in youth) showed that a larger overall startle was associated with anxiety symptoms in this age range. Future research should continue to investigate associations between defensive reactivity elicited in several affective contexts and cognitive control across development.
Lastly, the cross-sectional nature of our age analyses precluded any clear-cut developmental inferences. Longitudinal data are needed to examine the developmental changes and individual variation in these neurophysiological measures during early childhood. Importantly, the primary implication of our findings is that future studies investigating cognitive control and/or defensive reactivity processes should incorporate multiple measures within these constructs of interest. The use of multiple assessments may help delineate the type of top-down control that is related to regulating defensive reactivity processes. More specifically, the two cognitive control processes implicated in the regulation of defensive reactivity include: (1) monitoring performance and identifying the need for increased control; and (2) engaging in control responses that maximize goal-directed behavior (Shackman et al., 2011, Shenhav et al., 2013). Given that individual differences in the ability to engage in these cognitive control processes develop rapidly during early childhood (Diamond, 2013), it is critical that future research examines how these changes interact with ongoing changes in defensive reactivity processes to better understand the underlying dynamics of these major neural networks.
4.4. Conclusion
Despite these limitations, our study helps begin to shed light on the processes that underlie the association between cognitive control and defensive reactivity in young children. Despite low convergence within measures of cognitive control and defensive reactivity, results demonstrated moderate relationships between measures of cognitive control and defensive reactivity. Specifically, results were consistent with the notion that poorer cognitive control relates to increased defensive responsiveness. These results provide the first neurophysiological evidence that an association between reduced top-down control and greater defensive reactivity may be observed among young children. Together, these findings suggest that cognitive control and defensive reactivity are multifaceted constructs in early childhood and understanding their integration across development is of great importance (Gray, 2004).
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgement
We thank Jennie Grammer and colleagues for sharing data processing procedures and the Go/No-go task.
Footnotes
Flanker ERN analyses were also conducted using the average amplitude between 0 and 100 ms, which is identical to how the Go/No-Go ERN was scored, and results did not differ from those presented in the manuscript.
Pe analyses for the Flanker task were also conducted at Pz and results revealed comparable results to those conducted at CPz, and are available upon request.
Compared to startle to negative clips (r = 0.35, p = 0.03 for Go/No-Go; r = 0.42, p = 0.04 for Flanker), the magnitude of associations between startle to neutral clips and the ERN were smaller (r = 0.31, p = 0.05 for Go/No-Go; r = 0.36, p = 0.09 for Flanker), but similar in effect size. Correlations between positive-valenced startle and ERN were smaller still, and non-significant (r = 0.20, p = 0.21 for Go/No-Go; r = 0.29, p = 0.17 for Flanker).
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2015.09.001.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Arbel Y., Donchin E. When a child errs: the ERN and the Pe complex in children. Psychophysiology. 2011;48(1):55–63. doi: 10.1111/j.1469-8986.2010.01042.x. [DOI] [PubMed] [Google Scholar]
- Blumenthal T.D., Cuthbert B.N., Filion D.L., Hackley S., Lipp O.V., Van Boxtel A. Committee report: guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42(1):1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bradley M.M., Codispoti M., Cuthbert B.N., Lang P.J. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1(3):276. [PubMed] [Google Scholar]
- Bruder G.E., Fong R., Tenke C.E., Leite P., Towey J.P., Stewart J.E., Quitkin F.M. Regional brain asymmetries in major depression with or without an anxiety disorder: a quantitative electroencephalographic study. Biol. Psychiatry. 1997;41(9):939–948. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Levita L., Libby V., Pattwell S.S., Ruberry E.J., Soliman F., Somerville L.H. The storm and stress of adolescence: insights from human imaging and mouse genetics. Dev. Psychobiol. 2010;52:225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton R.J., Banich M.T., Mohanty A., Milham M.P., Herrington J., Miller G.A., Heller W. Paying attention to emotion. Cogn. Affect. Behav. Neurosci. 2003;3(2):81–96. doi: 10.3758/cabn.3.2.81. [DOI] [PubMed] [Google Scholar]
- Davidson R.J. EEG measures of cerebral asymmetry: conceptual and methodological issues. Int. J. Neurosci. 1988;39(1–2):71–89. doi: 10.3109/00207458808985694. [DOI] [PubMed] [Google Scholar]
- Davidson R.J. Anterior cerebral asymmetry and the nature of emotion. Brain Cogn. 1992;20(1):125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Davidson R.J., Tomarken A.J. vol. 3. 1989. Laterality and emotion: an electrophysiological approach; pp. 419–441. (Handbook of Neuropsychology). [Google Scholar]
- Davies P.L., Segalowitz S.J., Gavin W.J. Development of response-monitoring ERPs in 7- to 25-year-olds. Dev. Neuropsychol. 2004;25(3):355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Davis M., Antoniadis E.A., Amaral D.G., Winslow J.T. Acoustic startle reflex in rhesus monkeys: a review. Rev. Neurosci. 2008;19(2–3):171–186. doi: 10.1515/revneuro.2008.19.2-3.171. [DOI] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N., Valiente C., Spinrad T.L., Cumberland A., Liew J., Reiser M., Losoya S.H. Longitudinal relations of children's effortful control, impulsivity, and negative emotionality to their externalizing, internalizing, and co-occurring behavior problems. Dev. Psychol. 2009;45(4):988–1008. doi: 10.1037/a0016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwanger J., Geyer M.A., Braff D.L. The relationship of age to prepulse inhibition and habituation of the acoustic startle response. Biol. Psychol. 2003;62(3):175–195. doi: 10.1016/s0301-0511(02)00126-6. [DOI] [PubMed] [Google Scholar]
- Foti D., Kotov R., Hajcak G. Psychometric considerations in using error-related brain activity as a biomarker in psychotic disorders. J. Abnorm. Psychol. 2013;122:520–531. doi: 10.1037/a0032618. [DOI] [PubMed] [Google Scholar]
- Fox N.A., Rubin K.H., Calkins S.D., Marshall T.R., Coplan R.J., Porges S.W., Stewart S. Frontal activation asymmetry and social competence at four years of age. Child Dev. 1995;66(6):1770–1784. [PubMed] [Google Scholar]
- Gagne J.R., Van Hulle C.A., Aksan N., Essex M.J., Goldsmith H.H. Deriving childhood temperament measures from emotion-eliciting behavioral episodes: scale construction and initial validation. Psychol. Assess. 2011;23(2):337. doi: 10.1037/a0021746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser T., Bächer P., Möcks J. Transformations towards the normal distribution of broad band spectral parameters of the EEG. Electroencephalogr. Clin. Neurophysiol. 1982;53(1):119–124. doi: 10.1016/0013-4694(82)90112-2. [DOI] [PubMed] [Google Scholar]
- Gehring W.J., Liu Y., Orr J.M., Carp J. The error-related negativity (ERN/Ne) In: Luck S.J., Kappenman E., editors. Oxford Handbook of Event-Related Potential Components. Oxford University Press; New York: 2012. pp. 231–291. [Google Scholar]
- Grammer J.K., Carrasco M., Gehring W.J., Morrison F.J. Age-related changes in error processing in young children: a school-based investigation. Dev. Cogn. Neurosci. 2014;9:93–105. doi: 10.1016/j.dcn.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G., Coles M.G., Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gray J.R. Integration of emotion and cognitive control. Curr. Direct. Psychol. Sci. 2004;13(2):46–48. [Google Scholar]
- Grutzmann R., Endrass T., Klawohn J., Kathmann N. Response accuracy rating modulates ERN and Pe amplitudes. Biol. Psychol. 2014;96:1–7. doi: 10.1016/j.biopsycho.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Foti D. Errors are aversive defensive motivation and the error-related negativity. Psychol. Sci. 2008;19(2):103–108. doi: 10.1111/j.1467-9280.2008.02053.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G., McDonald N., Simons R.F. Error-related psychophysiology and negative affect. Brain Cogn. 2004;56(2):189–197. doi: 10.1016/j.bandc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W., Nitschke J.B. The puzzle of regional brain activity in depression and anxiety: the importance of subtypes and comorbidity. Cogn. Emot. 1998;12:421–447. [Google Scholar]
- Heller W., Nitschke J.B., Etienne M.A., Miller G.A. Patterns of regional brain activity differentiate types of anxiety. J. Abnorm. Psychol. 1997;106:376–385. doi: 10.1037//0021-843x.106.3.376. [DOI] [PubMed] [Google Scholar]
- Hughes G., Yeung N. Dissociable correlates of response conflict and error awareness in error-related brain activity. Neuropsychologia. 2011;49(3):405–415. doi: 10.1016/j.neuropsychologia.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.Y., Iwaki N., Imashioya H., Uno H., Fujita T. Error-related negativity in a visual go/no-go task: children vs. adults. Dev. Neuropsychol. 2007;31(2):181–191. doi: 10.1080/87565640701190775. [DOI] [PubMed] [Google Scholar]
- Lamm C., Walker O.L., Degnan K.A., Henderson H.A., Pine D.S., McDermott J.M., Fox N.A. Cognitive control moderates early childhood temperament in predicting social behavior in 7-year-old children: an ERP study. Dev. Sci. 2014;17(5):667–681. doi: 10.1111/desc.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J.E., Schiller D. What animal fear models have taught u s about human amygdala function? In: Whalen P.J., Phelps E.A., editors. The Human Amygdala. Guilford; New York, NY: 2009. pp. 43–60. [Google Scholar]
- Lewis M., Pitts M. 2015, July 16. Replication of Hajcak & Foti (2008, PS, Study 1) Retrieved from osf.io/73pnd. [Google Scholar]
- Lopez-Duran N.L., Nusslock R., George C., Kovacs M. Frontal EEG asymmetry moderates the effects of stressful life events on internalizing symptoms in children at familial risk for depression. Psychophysiology. 2012;49(4):510–521. doi: 10.1111/j.1469-8986.2011.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig K., Ludewig S., Seitz A., Obrist M., Geyer M.A., Vollenweider F.X. The acoustic startle reflex and its modulation: effects of age and gender in humans. Biol. Psychol. 2003;63(3):311–323. doi: 10.1016/s0301-0511(03)00074-7. [DOI] [PubMed] [Google Scholar]
- Marshall P.J., Bar-Haim Y., Fox N.A. Development of the EEG from 5 months to 4 years of age. Clin. Neurophysiol. 2002;113(8):1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- McManis M.H., Kagan J., Snidman N.C., Woodward S.A. EEG asymmetry, power, and temperament in children. Dev. Psychobiol. 2002;41(2):169–177. doi: 10.1002/dev.10053. [DOI] [PubMed] [Google Scholar]
- Metzger L.J., Paige S.R., Carson M.A., Lasko N.B., Paulus L.A., Pitman R.K., Orr S.P. PTSD arousal and depression symptoms associated with increased right-sided parietal EEG asymmetry. J. Abnorm. Psychol. 2004;113(2):324–329. doi: 10.1037/0021-843X.113.2.324. [DOI] [PubMed] [Google Scholar]
- Meyer A., Hajcak G., Torpey-Newman D.C., Kujawa A., Klein D.N. Enhanced error-related brain activity in children predicts the onset of anxiety disorders between the ages of 6 and 9. J. Abnorm. Psychol. 2015;124(2):266. doi: 10.1037/abn0000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A., Weinberg A., Klein D.N., Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: evidence from 8 to 13 year-olds. Dev. Cogn. Neurosci. 2012;2(1):152–161. doi: 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C.S. The development of emotion-related neural circuitry in health and psychopathology. Dev. Psychopathol. 2008;20:1231–1250. doi: 10.1017/S095457940800059X. [DOI] [PubMed] [Google Scholar]
- Moser J.S., Durbin C.E., Patrick C.J., Schmidt N.B. Combining neural and behavioral indicators in the assessment of internalizing psychopathology in children and adolescents. J. Clin. Child Adolesc. Psychol. 2015;44(2):329–340. doi: 10.1080/15374416.2013.865191. [DOI] [PubMed] [Google Scholar]
- Moser J.S., Moran T.P., Jendrusina A.A. Parsing relationships between dimensions of anxiety and action monitoring brain potentials in female undergraduates. Psychophysiology. 2012;49(1):3–10. doi: 10.1111/j.1469-8986.2011.01279.x. [DOI] [PubMed] [Google Scholar]
- Moser J.S., Moran T.P., Schroder H.S., Donnellan M.B., Yeung N. On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Front. Hum. Neurosci. 2013;7:466. doi: 10.3389/fnhum.2013.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser J.S., Moran T.P., Schroder H.S., Donnellan M.B., Yeung N. The case for compensatory processes in the relationship between anxiety and error monitoring: a reply to Proudfit, Inzlicht, and Mennin. Front. Hum. Neurosci. 2014;8:64. doi: 10.3389/fnhum.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P., Meesters C., Blijlevens P. Self-reported reactive and regulative temperament in early adolescence: relations to internalizing and externalizing problem behavior and “Big Three” personality factors. J. Adolesc. 2007;30:1035–1049. doi: 10.1016/j.adolescence.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S., Ridderinkhof K.R., Blom J., Band G.P., Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38(5):752–760. [PubMed] [Google Scholar]
- Oldehinkel A., Hartman C., Ferdinand R., Verhulst F., Ormel J. Effortful control as modifier of the association between negative emotionality and adolescents' mental health problems. Dev. Psychopathol. 2007;30(5):523–539. doi: 10.1017/S0954579407070253. Retrieved from http://journals.cambridge.org/abstract_s0954579407070253. [DOI] [PubMed] [Google Scholar]
- Olvet D.M., Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009;46(5):957–961. doi: 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Plant R.R., Quinlan P.T. Could millisecond timing errors in commonly used equipment be a cause of replication failure in some neuroscience studies? Cogn. Affect. Behav. Neurosci. 2013;13:598–614. doi: 10.3758/s13415-013-0166-6. [DOI] [PubMed] [Google Scholar]
- Proudfit G.H., Inzlicht M., Mennin D.S. Anxiety and error monitoring: the importance of motivation and emotion. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo K., Smith T., Donzella B., Schunk E., Gunnar M. The startle response: developmental effects and a paradigm for children and adults. Dev. Psychobiol. 2010;52(1):78–89. doi: 10.1002/dev.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A. Cognition, Emotion, and Psychopathology: Theoretical, Empirical, and Clinical Directions. Cambridge University Press; 2004. Anxiety and the resolution of ambiguity; pp. 130–148. [Google Scholar]
- Rothbart M.K., Sheese B.E., Posner M.I. Executive attention and effortful control: linking temperament, brain networks, and genes. Child Dev. Perspect. 2007;1(1):2–7. [Google Scholar]
- Rueda M.R., Posner M.I., Rothbart M.K., Davis-Stober C.P. Development of the time course for processing conflict: an event-related potentials study with 4 year olds and adults. BMC Neurosci. 2004;5(1):39. doi: 10.1186/1471-2202-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D., Bradley M.M., Fitzsimmons J.R., Lang P.J. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24(4):1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Santesso D.L., Segalowitz S.J., Schmidt L.A. Error-related electrocortical responses are enhanced in children with obsessive–compulsive behaviors. Dev. Neuropsychol. 2006;29(3):431–445. doi: 10.1207/s15326942dn2903_3. [DOI] [PubMed] [Google Scholar]
- Schmitt N. Uses and abuses of coefficient alpha. Psychol. Assess. 1996;8(4):350–353. [Google Scholar]
- Schroder H.S., Moran T.P., Moser J.S., Altmann E.M. When the rules are reversed: action-monitoring consequences of reversing stimulus-response mappings. Cogn. Affect. Behav. Neurosci. 2012;12:629–643. doi: 10.3758/s13415-012-0105-y. [DOI] [PubMed] [Google Scholar]
- Shackman A., Salomons T., Slagter H., Fox A., Winter J., Davidson R. The integration of negative affect, pain, and cognitive control in the cingulate cortex. Nat. Rev. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman S.A., Klein D.N., Torpey D.C., Olino T.M., Dyson M.W., Kim J., Tenke C.E. Do positive and negative temperament traits interact in predicting risk for depression? A resting EEG study of 329 preschoolers. Dev. Psychopathol. 2011;23(02):551–562. doi: 10.1017/S0954579411000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman S.A., Tenke C.E., Bruder G.E., Durbin C.E., Hayden E.P., Klein D.N. Low positive emotionality in young children: association with EEG asymmetry. Dev. Psychopathol. 2005;17(01):85–98. doi: 10.1017/s0954579405050054. [DOI] [PubMed] [Google Scholar]
- Shenhav A., Botvinick M.M., Cohen J.D. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79(2):217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle G.J., Thompson W., Carter C.S., Steinhauer S.R., Thase M.E. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol. Psychiatry. 2007;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Torpey D.C., Hajcak G., Kim J., Kujawa A.J., Dyson M.W., Olino T.M., Klein D.N. Error-related brain activity in young children: associations with parental anxiety and child temperamental negative emotionality. J. Child Psychol. Psychiatry. 2013;54(8):854–862. doi: 10.1111/jcpp.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vroman L.N., Lo S.L., Durbin C.E. Structure and convergent validity of children's temperament traits as assessed by experimenter ratings of child behavior. J. Res. Person. 2014;52:6–12. [Google Scholar]
- Weinberg A., Olvet D.M., Hajcak G. Increased error-related brain activity in generalized anxiety disorder. Biol. Psychology. 2010;85(3):472–480. doi: 10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Whalen P.J. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Curr. Direct. Psychol. Sci. 1998:177–188. [Google Scholar]
- Yeung N., Summerfield C. Metacognition in human decision-making: confidence and error monitoring. Philos. Trans. R. Soc. B: Biol. Sci. 2012;367(1594):1310–1321. doi: 10.1098/rstb.2011.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.