Abstract
Glyptosternoid fishes (Siluriformes), one of the three broad fish lineages (the two other are schizothoracines and Triplophysa), have a limited distribution in the rivers in the Tibetan Plateau and peripheral regions. To investigate the genetic mechanisms underlying adaptation to the Tibetan Plateau in several fish species from gradient altitudes, a total of 20,659,183–37,166,756 sequence reads from six species of catfish were generated by Illumina sequencing, resulting in six assemblies. Analysis of the 1,656 orthologs among the six assembled catfish unigene sets provided consistent evidence for genome-wide accelerated evolution in the three glyptosternoid lineages living at high altitudes. A large number of genes refer to functional categories related to hypoxia and energy metabolism exhibited rapid evolution in the glyptosternoid lineages relative to yellowhead catfish living in plains areas. Genes showing signatures of rapid evolution and positive selection in the glyptosternoid lineages were also enriched in functions associated with energy metabolism and hypoxia. Our analyses provide novel insights into highland adaptation in fishes and can serve as a foundation for future studies aiming to identify candidate genes underlying the genetic basis of adaptation in Tibetan fishes.
Keywords: Tibetan Plateau, adaption, gradient altitudes, comprehensive transcriptome, glyptosternoid fishes, accelerated genic evolution
The mechanisms underlying organismal adaptation to high-altitude hypoxia have recently become of great interest (Gou et al. 2014). The Tibetan Plateau (the ‘‘Roof of the World’’) is the highest plateau on Earth, with an average elevation of more than 4000 m. The plateau covers more than 2,500,000 km2 of plateaus and mountains in central Asia and is surrounded by towering mountain ranges. Despite its inhospitable environment, various adaptive responses that may be responsible for highland adaptation have been identified in several species, including Tibetans (Beall et al. 2010; Bigham et al. 2010; Simonson et al. 2010; Yi et al. 2010; Peng et al. 2011), yak (Qiu et al. 2012), Tibetan antelope (Ge et al. 2013), Tibetan wild boar (M. Li et al. 2013), ground tit (Qu et al. 2013), Tibetan mastiff (Gou et al. 2014; Li et al. 2014), and a schizothoracine fish (Yang et al. 2015). Among these adaptive processes, genes exhibiting signs of positive selection and expansion were significantly enriched in hypoxia and energy metabolism pathways. However, almost all previous genome-wide studies were performed on endothermic terrestrial vertebrates. Little is known about the genomic basis of the adaptation of fishes to highland regions, with the exception of the schizothoracine fishes, which only include one species from a high-altitude environment (Yang et al. 2015). Therefore, investigating the mechanism of adaptation to the conditions of the Tibetan Plateau in several fishes from gradient altitudes may provide novel insights.
Glyptosternoid fishes (Sisoridae, Siluriformes), one of the three broad fish lineages (the two other are schizothoracines and Triplophysa), exhibit a limited distribution in the rivers of the Tibetan Plateau and peripheral regions. These fishes provide an excellent model system in which to study how fishes adapt to high-altitude habitats. They form a lineage that is distributed in the rivers around the Tibetan Plateau and eastern Himalayas [e.g., the Yaluzangbujiang (Brahmaputra River), Irrawaddy, Nujiang (Upper Salween), Lancangjiang (Upper Mekong River), Jinshajiang (Upper Yangtze), Yuanjiang (Red River), Nanpanjiang (Upper Pearl River), and the Brahmaputra basin] (Guo et al. 2005) and the downstream portions of these rivers in India, Burma, Thailand, Laos, Bangladesh, and Vietnam. The glyptosternoid fishes are extremely well adapted to the rapidly flowing water environment and possess a series of adaptive structures, including a depressed body and head, horizontally inserted pectoral and ventral fins, and an adhesive apparatus on their paired fins, which are suited for this environment (Guo et al. 2005; Peng et al. 2006). The fishes (Glyptosternon maculatum, Pareuchiloglanis sinensis, and Pareuchiloglanis macrotrema) studied here are freshwater catfish that belong to the Glyptosternoid fishes that inhabit the cold waters of the highlands. Glyptosternon maculatum, the base of the Glyptosternoid fishes, inhabits shallow, rocky rivers with a moderate current, where it feeds on invertebrates. It occurs only in the middle-reach of the Yaluzangbu River in Tibet, and in the Brahmaputra River in India. Pareuchiloglanis sinensis and Pareuchiloglanis macrotrema are found only in the Jinshajiang (upper Yangtze) and Yuanjiang (Red River), respectively.
The objective of this study was to undertake a comparative transcriptome-wide search for genes that might be involved in adaptation to high-elevation environments and to identify their associated functions in six catfish from gradient altitudes. We sampled liver tissues of G. maculatum, P. sinensis, P. macrotrema, Amur catfish (Silurus asotus), yellowhead catfish (Pelteobagrus fulvidraco), and Synodontis nigriventris and sequenced their transcriptomes using an Illumina sequencing platform. We identified genes showing strong signs of positive selection by comparing their transcriptomes. The functional and phenotypic outcomes of these candidate genes were inferred by annotation based on genomic resources of a variety of model vertebrate species. We report a list of candidate genes that are highly likely to be involved in high-elevation adaptation processes.
Materials and Methods
Ethics statement
The methods involving animals in this study were carried out in accordance with the Laboratory Animal Management Principles of China. All experimental protocols were approved by the Ethics Committee of the Institute of Hydrobiology, Chinese Academy of Sciences.
Fish sampling
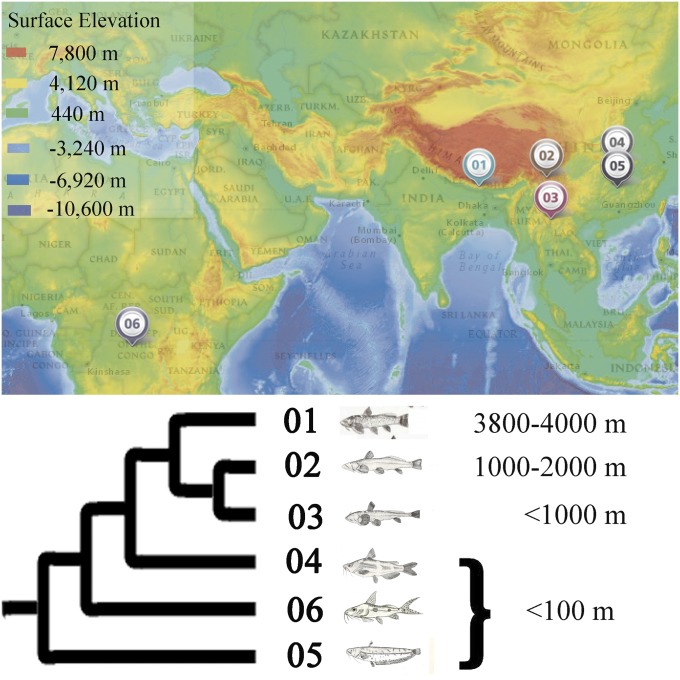
We sampled three glyptosternoid fishes, including G. maculatum from the Yaruzampbo River in Tibet (3800–4000 m), P. sinensis from Daduhe River in Sichuan (1000–2000 m), and P. macrotrema from the Yuan River in Yunnan (<1000 m), as well as three species from low altitude (details in Figure 1, and Supporting Information, Table S1). Samples were taken back to the laboratory, where each species of fish were stocked into one aquarium. All aquaria were maintained under a natural photoperiod, water temperature fluctuated from 10° to 15°, and pH ranged from 6.5 to 7.5. Dissolved oxygen concentration remained higher than 5 mg/L, and ammonia-nitrogen was lower than 0.01 mg/L. The acclimation study was conducted for 48 hr and all fish were starved. Then three individuals of each species were chosen and anesthetized with MS-222 (100 mg/L, Sigma Chemical Company, St Louis, MO), and liver tissues immediately collected. The tissues were flash frozen in liquid nitrogen and placed in a −80° freezer prior to processing for total RNA isolation. For each species, liver tissues from three individuals were mixed.
Figure 1.
Altitude distribution of six catfish species. Map from National Geographic’s MapMaker Interactive on April 13, 2015 (http://education.nationalgeographic.com/education/mapping/interactive-map/?ar_a=1); the lower of Surface Elevation was added. Details on the species are provided in Table S1. The tree inferred using 1656 orthologous gene with the Bayesian inference (BI) and maximum likelihood (ML) method is shown below the map.
RNA isolation and sequencing
We sequenced the liver tissue transcriptomes to access a large number of diverse transcripts because the liver is a highly complex organ with a complex transcriptome (Shackel et al. 2002, 2006). Total RNA was isolated using TRIzol reagent (Life Technologies Corp., Carlsbad, CA) according to the manufacturer’s protocols, and cleaned up using the RNeasy mini kit (Qiagen, Valencia, CA). RNA samples were quantified with the 2100 Bioanalyzer (Agilent Technologies). mRNA was enriched using beads with oligo (dT) and fragmented using fragmentation buffer. The first cDNA chain was synthesized using random hexamers, and the second chain was synthesized with buffer, dNTPs, RNase H, and DNA polymerase I. After the cDNA was purified using Agencourt AMPure XP (Beckman Coulter Inc., Atlanta, GA), the samples were blunt-end repaired and ligated with poly(A) and adapter sequences. Then, sizes were selected using Agencourt AMPure XP (Beckman Coulter Inc., Atlanta, GA). Each of the libraries was amplified by PCR. The libraries were quantified by RT-PCR, and transcriptome sequencing was performed on an Illumina HiSequation 2500 platform. Short sequence reads of paired end (PE) 100 bp were generated. All of the data were deposited into the NCBI Sequence Read Archive database (SRA run accession numbers are provided in Table S1).
De novo assembly and gene functional annotation
These reads were then filtered, and defective reads were removed for each species. High-quality sequencing reads and appropriate assembly methods are essential for obtaining a reliable de novo assembly, which serves as the foundation of all subsequent analyses (Robertson et al. 2010; Surget-Groba and Montoya-Burgos 2010). First, quality control checks were conducted on the raw sequence data using FastQC (http://www.bioinformatics.bbsrc.ac.uk/projects/fastqc). The adapter sequences and sites with lower qualities (Phred score < 20) reads were trimmed using the Cutadapt (Martin 2011). All subsequent analyses were based on these cleaned reads. De novo sequence assembly was performed using Trinity (Grabherr et al. 2011) software designed for the assembly of short read sequences with default parameters. Only contigs with lengths greater than 200 bp were used for further analysis. To lower the redundancy in the dataset, low-coverage artifacts or redundancies were removed using the CD-HIT-EST program (Li and Godzik 2006) with an identity threshold of 95%.
To annotate the assembled unigenes, we downloaded the zebrafish (Danio rerio) protein dataset from the Ensembl database (http://useast.ensembl.org, release 78), and then used BLASTx searches to map the unigenes to the proteins with an E-value cutoff of 1e–10. Putative functions for the assembled unigenes were assigned by the Blast2GO suite (Gotz et al. 2008) using BLASTx against the nonredundant (nr) database with a conservative E-value cutoff of 1e–5.
Orthology determination
We used HaMStR (version v.13.2.3) to infer orthology (Ebersberger et al. 2009), which in turn used usearch (http://drive5.com/usearch), GeneWise (Birney et al. 2004), and HMMER (Eddy 2011) to search the combined assembly data for protein sequences matching a set of “customl” orthologs. The “customl” orthologs in our case consisted of a database of eight genomes representing Ostariophysi fishes: Cypriniformes, D. rerio (DANIO); Tetraodontiformes, Takifugu rubripes (TFUGU); Beloniformes, Oryzias latipes (MEDAK); Perciformes, Oreochromis niloticus (ONILE); Cyprinodontiformes, Xiphophorus maculatus (SWORD); Gasterosteiformes, Gasterosteus aculeatus (STIKLE); Tetraodontiformes, Tetraodon nigroviridis (TETRA); and Gadiformes, Gadus morhua (ANCOD). If the unigenes were assigned to different “custom” orthology sequences, the longest unigene was chosen.
One-to-one orthologs between six catfish and zebrafish were determined using the reciprocal BLAST best-hit method with an E-value cutoff of 1 × 10−10. Then, putative single copy orthologs among six catfish were obtained. The longest transcript was chosen for genes with multiple transcripts. Each orthologous gene set was aligned using GeneWise (Birney et al. 2004), and trimmed using Gblocks (Castresana 2000) with the parameter “–t = c.” We further deleted all gaps and “N” from the alignments to reduce the effect of ambiguous bases on the inference of positive selection. After the deletion process, trimmed alignments shorter than 150 bp (50 codons) were discarded from subsequent analyses.
Substitution rate estimation and selection analyses
To estimate the lineage-specific evolutionary rates for each branch of the six species, the Codeml program in the PAML package (Yang 2007) was run with the free-ratio model (model = 1) for each ortholog, a concatenation of all alignments of the orthologs, and 1000 concatenated alignments constructed from 10 randomly chosen orthologs. Parameters including dN, dS, Ka/Ks, N*dN, and S*dS values were obtained for each branch, and genes were discarded if N*dN or S*dS < 1 or dS > 1 according to the method described in a previous study (Goodman et al. 2009). We used the branch model to identify fast-evolving genes, with the null model assuming that all branches have been evolving at the same rate and the alternative model allowing foreground branches to evolve under a different rate. The likelihood ratio test (LTR) with df = 1 was used to discriminate between alternative models for each ortholog in the gene set. Multiple testing was corrected by applying the false discovery rate method (FDR) implemented in R (Storey and Tibshirani 2003). We considered the genes to be evolving with a significantly faster rate in the foreground branch if the FDR-adjusted P value was less than 0.05, and a higher ω value was detected in the foreground branch than the background branches. To detect positive selection on a few codons along specific lineages, we used the optimized branch-site model (Zhang et al. 2005) following the author’s recommendations. A likelihood ratio test was used to compare a model that allowed sites to be under positive selection in the foreground branch with the null model, in which sites could evolve neutrally and under purifying selection. The p-values were computed based on the chi-square statistic adjusted by the FDR method; genes with adjusted P values < 0.05 were treated as candidates for positive selection. Gene ontology (GO) functional enrichment analyses for both fast-evolving genes and positively selected genes were performed by DAVID (Dennis et al. 2003).
Data availability
The sequencing reads were deposited in the short read archive (SRA) of GenBank (accession numbers listed in Table S1).
Results
Illumina sequencing, de novo assembly, and sequence validation
A total of 20,659,183–37,166,756 sequence reads were generated from the six catfish by Illumina sequencing (for detailed information, see Table S2). First, we filtered these reads and removed the defective reads for each species. High-quality sequence reads and the use of appropriate assembly methods are essential to obtaining reliable de novo assembly, which is the foundation for all other analyses (Surget-Groba and Montoya-Burgos 2010; Robertson et al. 2010; Garg et al. 2011). In total, six raw assemblies were obtained for the six catfish and further merged by integrating sequence overlaps and eliminating redundancies for each species. Detailed information on the total length of the final set of assemblies and unigene numbers is summarized in Table S3; the length distribution of all unigenes is shown in Figure S1.
We used gene coverage and transcript sequence quality to assess how well the assembled sequences represented the actual transcriptome population. The transcriptome gene coverage was judged by comparing it with the sequence information available for G. maculatum. All 13 mitochondrial protein-coding genes in the NCBI database were present in full length in our assembled transcripts. Next, we compared our assembled scaffolds with the zebrafish transcriptome (ENSEMBL Zv9) and found that 36,832 out of 48,435 (76.04%) zebrafish transcripts had matches in the assembled contigs. At the same time, 14,828 reciprocal best-hit BLAST matches with the zebrafish transcriptome were identified using an E-value of 1e–5. Transcriptome quality was assessed by comparing the mitochondrial protein-coding genes found in the assembled sequences to the mitochondrion sequence in GenBank (NC_021597). A total of 11,409 nucleotide identities were observed out of the 11,398 bp (99.9%) total nucleotide length of the contig to the coding mitochondrial sequences in the BLAST matches, suggesting very good transcriptome sequence quality. The observed 0.1% sequence difference might be due to high intraspecific genetic variability. The same assembly quality was found for the other five catfish species.
Gene annotation
We used several complementary approaches to annotate the assembled unigenes. First, a BLASTx search against zebrafish proteins returned an average of 25.5–49.3% catfish unigenes with significant hits to zebrafish genes (Table S4 and Figure S2). The average percentages of unigenes with BLAST hits are similar to previous de novo transcriptome studies of nonmodel organisms (Guo et al. 2013; Yang et al. 2015). Unigenes without significant hits may consist of orphan genes, noncoding RNAs, untranslated transcripts, or misassembled transcripts. Second, we used Blast2GO with the GO annotation database to assign their putative functions (Figure S3). Finally, clusters of orthologous groups of proteins (COGs) and the nonredundant (NR) databases were used to further annotate these unigenes and to produce good results for putative proteins (Table S5).
Identification of putative orthologs
To better understand the evolutionary dynamics of glyptosternoid fish in response to the highland environment, putative orthologs among six catfish were determined using two methods. First, the Hmmer search was used to identify putative orthologs among the six species (Bazinet et al. 2013). A total of 708 putative orthologs were identified by comparing all six transcript sets; a total of 170 was retained after alignment and trimming for quality control (see Materials and Methods).
To the reciprocal BLAST best-hit method, a total of 1656 orthologs ranging from 150 to 7155 bp were retained after alignment and trimming for quality control; these orthologs were used in the subsequent evolutionary analysis. Despite the fact that the lengths of the orthologs were shorter after trimming, the shapes of their length distributions were generally similar (Figure S4).
Accelerated evolution in the glyptosternoid lineage
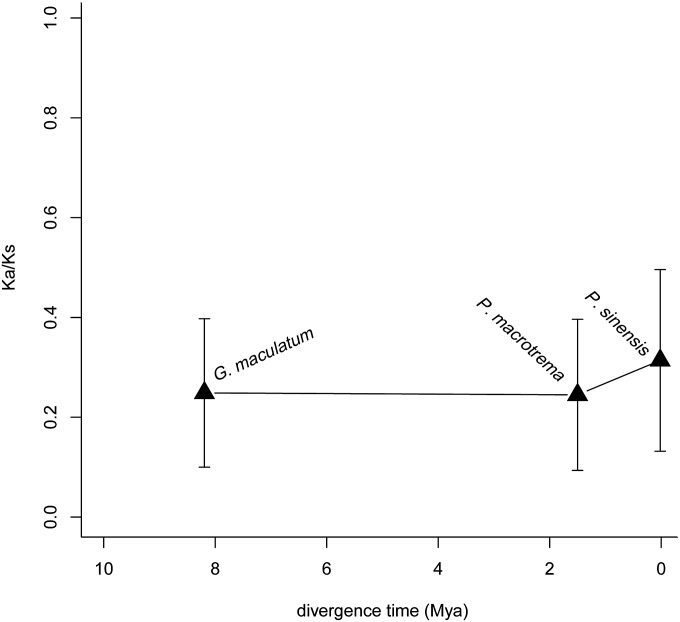
First, to compare the overall difference in selective constraints in the different branches at the gene level, each orthologous gene was evaluated for substitution rates (i.e., Ka, Ks, and Ka/Ks) using the species tree (inferred using the BI and ML method) (Figure 2A). The free-ratio model (M1 model) in PAML was used, which allows for an independent Ka/Ks ratio for each branch. Indeed, by examining the Ka/Ks ratios for 1656 orthologous genes in the glyptosternoid lineage (Figure 2B), we found that 480 genes had higher Ka/Ks values in all three glyptosternoid fish lineages. Then, we calculated the Ka/Ks ratio for each branch in a concatenated alignment of all 1656 orthologs and 1000 concatenated alignments constructed from 10 randomly chosen orthologs and found that both datasets also exhibited a significantly higher Ka/Ks ratio for all of the living glyptosternoid lineage fish branches compared to the other catfish branches (P < 2.2 × 10−16, Figure 2, C and D). The Ka/Ks of P. sinensis was highest within the glyptosternoid lineage (0.3139, Figure 3). Therefore, comparison of the Ka/Ks ratios indicated accelerated evolution in glyptosternoid fish after their split from the yellowhead catfish.
Figure 2.
Phylogenetic tree used in this study (A), and branch-specific Ka/Ks ratios obtained from different datasets (B, C, D). Gray and black arrows in (A) indicate decreased or increased terminal Ka/Ks ratios, respectively, compared with the ancestral branch. The Ka/Ks ratios for terminal branches were estimated from each ortholog (B), all concatenated orthologs (C), and 1000 concatenated alignments constructed from 10 randomly chosen orthologs (D).
Figure 3.
Ka/Ks ratios of the three living glyptosternoid lineages.
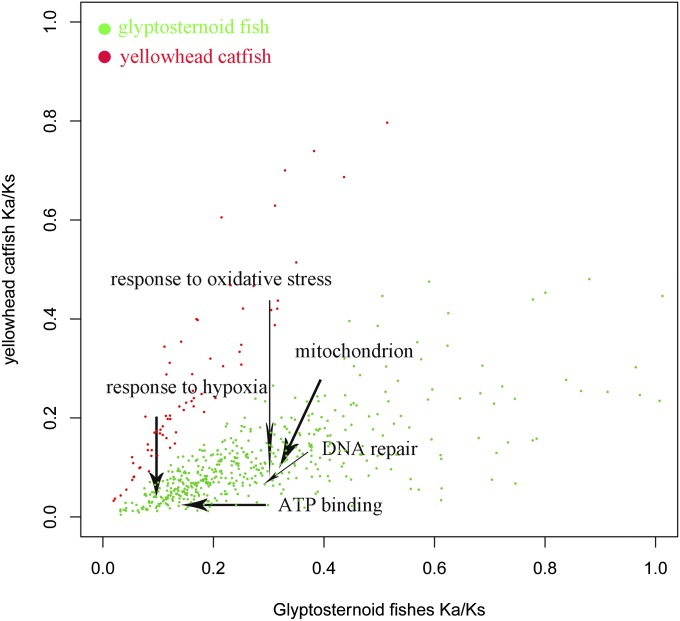
A number of GO categories were involved in the 480 genes in the glyptosternoid lineage that underwent rapid evolution compared to the yellowhead catfish. For example, genes associated with energy metabolism, hypoxia response, and DNA repair showed significantly accelerated evolution in glyptosternoid fish compared to the yellowhead catfish. Categories that underwent rapid evolution compared to the zebrafish included “response to hypoxia,” “response to oxidative stress,” “oxidoreductase activity,” “mitochondrion,” “ATP binding,” “GTPase activity,” and “DNA repair” (Figure 4 and Table S6).
Figure 4.
Scatter plot of the mean Ka/Ks ratios for each gene ontology (GO) category in glyptosternoid and yellowhead catfish. GO categories with significantly higher mean Ka/Ks ratios in glyptosternoid (green) and yellowhead catfish (red) are highlighted.
Fast-evolving and positively selected genes
To detect genes that might have evolved due to lineage-specific adaptation, two types of gene sets were compiled: 1) fast-evolving genes (FEGs) that exhibited a significantly higher Ka/Ks ratio in specific lineages compared with other lineages, and 2) positively selected genes (PSGs) that were influenced by positive selection only on a few codons along a particular lineage (see Materials and Methods). In total, we identified 121–178 FEGs in living glyptosternoid fish, 63 FEGs in yellowhead catfish and 58–244 PSGs in living glyptosternoid fish, and 48 PSGs in yellowhead catfish (Table S7 and Table S8). This finding suggests that the living glyptosternoid fish lineages have higher numbers of FEGs and PEGs compared to yellowhead catfish. Functional enrichment analysis showed that the FEGs identified in each glyptosternoid lineage were significantly enriched for genes involved in energy metabolism and oxidation-related functions, including “ATP binding,” “mitochondrial part,” “GTPase regulator activity,” “ATPase activity,” and “Acyl-CoA oxidase/dehydrogenase,” whereas FEGs detected in yellowhead catfish were generally enriched in functions involved in structure components (Table S9).
To identify genes that might directly contribute to the adaptation to high altitude, we used the 244 PSGs in G. maculatum as the candidate genes, then combined two approaches to annotate all of the candidate genes according to their functional roles. First, we compared our candidate genes (PSGs) to an a priori list proposed by Zhang et al. (2014), which included 1351 putative hypoxia-related genes. Second, we used the functional annotated information for each PSG to identify the genes associated with the hypoxia response reported in previous experimental studies. In total, we identified 13 candidate PSGs in glyptosternoids that may be involved in the hypoxia response: Slc2a8, Igfbp7, C2, Cp, Ndc1, Hspa5, Ttr, Gapdh, Prmt5, Srebf1, Perp, Map3k14, and Fam162a (Table 1).
Table 1. Positively selected genes involved in the hypoxia response in Glyptosternon maculatum.
| Gene ID | Gene Name | Description |
|---|---|---|
| ENSDARG00000020344 | Slc2a8 | Solute carrier family 2 (facilitated glucose transporter), member 8 |
| ENSDARG00000017389 | Igfbp7 | Insulin-like growth factor binding protein 7 |
| ENSDARG00000019772 | C2 | Complement component 2 |
| ENSDARG00000010312 | Cp | Ceruloplasmin (ferroxidase) |
| ENSDARG00000021120 | Ndc1 | NDC1 transmembrane nucleoporin |
| ENSDARG00000004665 | Hspa5 | Heat shock 70 kDa protein 5 (glucose-regulated protein, 78 kDa) |
| ENSDARG00000037191 | Ttr | Transthyretin |
| ENSDARG00000043457 | Gapdh | Glyceraldehyde-3-phosphate dehydrogenase |
| ENSDARG00000079605 | Prmt5 | Protein arginine methyltransferase 5 |
| ENSDARG00000067607 | Srebf1 | Sterol regulatory element binding transcription factor 1 |
| ENSDARG00000063572 | Perp | PERP, TP53 apoptosis effector |
| ENSDARG00000063344 | Fam162a | Family with sequence similarity 162, member A |
| ENSDARG00000074060 | Map3k14 | Mitogen-activated protein kinase kinase kinase 14 |
Discussion
The glyptosternoid fishes distributed in the rivers of the Tibetan Plateau and peripheral regions are extremely well adapted to high altitude. These fishes are distributed across wide altitudes that range from several hundred to more than 4000 m. Thus, these species serve as an ideal model system in which to investigate genetic adaption to high altitude. However, no genome sequencing data are available, even at the order level (Siluriformes). Transcriptome sequencing represents an effective and accessible approach to initiating comparative genomic analyses on nonmodel organisms when genome sequencing data are not available because transcriptomes contain a large number of protein-coding genes that are most likely enriched for targets of natural selection. Over the past few years, comparative genomics has been widely employed as a tool to understand the genetic basis of many fundamental evolutionary questions, including adaptation (Jones et al. 2012; Axelsson et al. 2013; Zhao et al. 2013; Koch 2014; Zhang et al. 2014), speciation (Harr and Price 2012; Soria-Carrasco et al. 2014; Van Leuven et al. 2014) and genetic variation (Guo et al. 2012; Aflitos et al. 2014; Joseph et al. 2015). Here, we generated and annotated the first comprehensive transcriptome resource for three glyptosternoid fishes that are endemic to the Tibetan Plateau, and show many unique traits that have enabled their adaptation to highland environments compared to three other catfish species from the plains using RNA-seq technology. We generated 1656 pairwise orthologous genes between zebrafish, which served as an important basis for comparative genomic studies of adaptation in fishes. Therefore, the transcriptome resources produced by our study are useful for understanding the genetic makeup of fishes at high altitudes, and provide a foundation for further studies to identify candidate genes underlying the adaptation of fishes to the Tibetan Plateau. The glyptosternoid fishes (Siluriformes) represent one of the three broad fish lineages (including the schizothoracines and Triplophysa) commonly found on the Tibetan Plateau. Previous research focused on the high-altitude adaptation of the schizothoracine fish (Cyprinidae) based on the mitochondrial genome (Y. Li et al. 2013) and comprehensive transcriptome (Yang et al. 2015) of only one species. Our results were similar to the results of the schizothoracine fish study (Yang et al. 2015). It suggested that accelerated evolution occurred in glyptosternoid fishes living in high-altitude environments after their split from yellowhead catfish. The evolutionary rate of P. sinensis was highest within the glyptosternoid fish lineage, followed by G. maculatum and P. macrotrema. G. maculatum, the basal group of glyptosternoid fishes, is distributed widely in Brahmaputra drainages. This species was found to originate in the Late Miocene age (c. 8.2 Ma), whereas P. macrotrema separated from P. longicauda 1.5 MYA ago. P. sinensis is very young; this lineage formed during the Late Pleistocene (ca. 0.018 Ma) and is distributed in the Upper Yangtze River. This divergence time was inferred from 12 mitochondrial protein coding genes from 22 glyptosternoid fishes (unpublished data). Orogenic development in northern Tibet has affected the fauna, including fish and pikas (Yu et al. 2000; Ruber et al. 2004). With the uplift of the Tibetan Plateau, Chinese glyptosternoid fish speciation exploded. At the same time, P. sinensis may have experienced a more accelerated evolution facilitated by the Upper Yangtze River environment.
The most extreme challenge for species living in high-altitude environments is the low oxygen supply (Beall 2007). To identify the potential genes directly involved in hypoxia, we focused on the function of positively selected genes in the glyptosternoid fish lineage. We identified several interesting candidate genes that may be involved in the response to hypoxia. Among them, 13 genes were found to most likely be involved in the adaptive process to high-elevation environments, particularly genes associated with the response to hypoxia and oxygen binding. For example, solute carrier family 2 (facilitated glucose transporter) member 8 (SCL1A8) belongs to the solute carrier 2A family, which includes intracellular glucose transporters regulated by hypoxia-inducible factor-1α (HIF-1α) (Saxena et al. 2007; Shao et al. 2014). Insulin-like growth factor-binding protein-7, encoded by the Igfbp7 gene, is involved in the regulation of the availability of insulin-like growth factors (IGFs) in tissues and modulating IGF binding to its receptors, and may suppress the stimulatory effect of vascular endothelial growth factor (VEGFA) (Tamura et al. 2014). Ceruloplasmin (Cp), a copper protein with a potent ferroxidase activity that converts Fe2+ to Fe3+ in the presence of molecular oxygen, is a ferroxidase that is important in the regulation of both systemic and intracellular iron levels. Cp-stimulated iron release was absolutely dependent on the presence of apotransferrin and hypoxia (Sarkar et al. 2003). Cp has a critical role in iron metabolism in the brain and retina, and alters intracellular iron-regulated proteins and pathways, including ferritin, transferrin receptor, glutamate and hypoxia-inducible factor-1a, through the Cp-induced nuclear translocation of the hypoxia-inducible factor-1 (HIF-1) subunit HIF-1a (Harned et al. 2012). NDC1, a crucial membrane-integral nucleoporin of metazoan nuclear pore complexes, is required for nuclear pore complex assembly (Mansfeld et al. 2006); moreover, cytoplasmic–nuclear transport of HIF-1α occurs through the nuclear pore (Nagara et al. 2013). Transthyretin (TTR), the thyroid hormone-binding protein, is critical for adaptation to hypoxia; the expression of this protein is upregulated in low-oxygen environments (Patel et al. 2012). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a multifunctional enzyme that is overexpressed in many tumors and is induced by hypoxia in normal and malignant cells. The degree to which hypoxia transcriptionally activates GAPDH is cell-type specific (Lu et al. 2002). The GAPDH promoter region contains a hypoxia responsive element (HRE) consisting of a HIF-1 consensus binding site plus adjacent sequence (Graven et al. 1999). Protein arginine methyltransferase 5 (PRMT5) is a novel regulator of HIF-1- and HIF-2-mediated responses. Hypoxia-inducible factors (HIF-1 and HIF-2) are essential mediators for the adaptive transcriptional response of cells and tissues to low-oxygen conditions. PRMT1 is a repressor of both HIF-1 and HIF-2. The cellular depletion of PRMT1 by small interfering RNA targeting led to increased HIF transcriptional activity. This activation was the result of enhanced HIF-α subunit transcription that allowed for increased HIF-α subunit availability. Sterol regulatory element binding transcription factor 1 (SREBF1) encodes a transcription factor that binds to the sterol regulatory element-1 (SRE1), which is a decamer flanking the low-density lipoprotein receptor gene and other genes involved in sterol biosynthesis. Sterol regulatory element binding proteins (SREBPs) are hypoxic transcription factors required for adaptation to low-oxygen environments (Todd et al. 2006; Bien and Espenshade 2010). The candidate genes are potentially involved in hypoxia pathways, but none are shared with previously reported genes in other fishes (Yang et al. 2015). This observation suggests that catfish at high altitudes may have employed a different genic toolkit to adapt to the extreme environment of the Tibetan Plateau. Moreover, several experimental studies revealed that amino acid variants in the alpha- and/or beta-globin genes can undoubtedly change Hb-O2 affinity in high-altitude species, including deer mice (Storz et al. 2009, 2010; Natarajan et al. 2013) and Andean hummingbirds (Projecto-Garcia et al. 2013). Although the genetic basis of hypoxia tolerance has yet to be fully elucidated in some vertebrate species, evidence from a number of birds, mammals, and amphibians suggests that modifications of hemoglobin (Hb) function may often play a key role in mediating an adaptive response to high altitude hypoxia (Storz 2007; Storz and Moriyama 2008). However, evidence about the mutations to adaptation to the Tibetan Plateau in Tibetan fishes was unclear. Therefore, the genes that display signatures of positive selection will serve as a baseline for further investigations that aim to understand high-elevation adaptation at both the molecular and phenotypic levels. A deep understanding of the adaptation to the Tibetan Plateau in Tibetan fishes can only be achieved by experimental and functional genomics in future. It also needs to be further confirmed by population genomics studies in the future.
Acknowledgments
This work was supported by the Pilot projects (Grant No. XDB13020100).
Author contributions: X.M. contributed to the sampling, molecular experiments, data analysis, and writing of the manuscript. W.D. and J.K. contributed to the molecular experiments and sampling. L.Y. participated in the analysis of the results. S.H. contributed to the research design and writing of the manuscript. All authors read and approved the final manuscript.
Footnotes
Supporting information is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.115.024448/-/DC1
Sequence data from this article have been deposited with the GenBank Data Library under accession nos. SRR1994393, SRR1994396, SRR1994404, SRR1994459, SRR1994457, and SRR1994461.
Communicating editor: W. S. Davidson
Literature Cited
- Aflitos S., Schijlen E., de Jong H., de Ridder D., Smit S., et al. , 2014. Exploring genetic variation in the tomato (Solanum section Lycopersicon) clade by whole-genome sequencing. Plant J. 80: 136–148. [DOI] [PubMed] [Google Scholar]
- Axelsson E., Ratnakumar A., Arendt M.-L., Maqbool K., Webster M. T., et al. , 2013. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 495: 360–364. [DOI] [PubMed] [Google Scholar]
- Bazinet A. L., Cummings M. P., Mitter K. T., Mitter C. W., 2013. Can RNA-Seq resolve the rapid radiation of advanced moths and butterflies (Hexapoda: Lepidoptera: Apoditrysia)? An exploratory study. PLoS One 8: e82615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall C. M., 2007. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc. Natl. Acad. Sci. USA 104: 8655–8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall C. M., Cavalleri G. L., Deng L., Elston R. C., Gao Y., et al. , 2010. Natural selection on EPAS1 (HIF2 alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc. Natl. Acad. Sci. USA 107: 11459–11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien C. M., Espenshade P. J., 2010. Sterol regulatory element binding proteins in fungi: hypoxic transcription factors linked to pathogenesis. Eukaryot. Cell 9: 352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigham A., Bauchet M., Pinto D., Mao X., Akey J. M., et al. , 2010. Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet. 6: e1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birney E., Clamp M., Durbin R., 2004. GeneWise and genomewise. Genome Res. 14: 988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castresana J., 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17: 540–552. [DOI] [PubMed] [Google Scholar]
- Dennis G., Jr, Sherman B. T., Hosack D. A., Yang J., Gao W., et al. , 2003. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 4: P3. [PubMed] [Google Scholar]
- Ebersberger I., Strauss S., von Haeseler A., 2009. HaMStR: Profile hidden Markov model based search for orthologs in ESTs. BMC Evol. Biol. 9: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy S. R., 2011. Accelerated profile HMM searches. PLOS Comput. Biol. 7: e1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R., Patel R. K., Tyagi A. K., Jain M., 2011. De novo assembly of chickpea transcriptome using short reads for gene discovery and marker identification. DNA Res. 18: 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge R.-L., Cai Q., Shen Y.-Y., San A., Ma L., et al. , 2013. Draft genome sequence of the Tibetan antelope. Nat. Commun. 4: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M., Sterner K. N., Islam M., Uddin M., Sherwood C. C., et al. , 2009. Phylogenomic analyses reveal convergent patterns of adaptive evolution in elephant and human ancestries. Proc. Natl. Acad. Sci. USA 106: 20824–20829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz S., Garcia-Gomez J. M., Terol J., Williams T. D., Nagaraj S. H., et al. , 2008. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 36: 3420–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou X., Wang Z., Li N., Qiu F., Xu Z., et al. , 2014. Whole-genome sequencing of six dog breeds from continuous altitudes reveals adaptation to high-altitude hypoxia. Genome Res. 24: 1308–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabherr M. G., Haas B. J., Yassour M., Levin J. Z., Thompson D. A., et al. , 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29: 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graven K. K., Yu Q., Pan D., Roncarati J. S., Farber H. W., 1999. Identification of an oxygen responsive enhancer element in the glyceraldehyde-3-phosphate dehydrogenase gene. Biochim. Biophys. Acta 1447: 208–218. [DOI] [PubMed] [Google Scholar]
- Guo B., Zou M., Wagner A., 2012. Pervasive indels and their evolutionary dynamics after the fish-specific genome duplication. Mol. Biol. Evol. 29: 3005–3022. [DOI] [PubMed] [Google Scholar]
- Guo B., Chain F. J. J., Bornberg-Bauer E., Leder E. H., Merila J., 2013. Genomic divergence between nine- and three-spined sticklebacks. BMC Genomics 14: 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., He S., Zhang Y., 2005. Phylogeny and biogeography of Chinese sisorid catfishes re-examined using mitochondrial cytochrome b and 16S rRNA gene sequences. Mol. Phylogenet. Evol. 35: 344–362. [DOI] [PubMed] [Google Scholar]
- Harned J., Ferrell J., Nagar S., Goralska M., Fleisher L. N., et al. , 2012. Ceruloplasmin alters intracellular iron regulated proteins and pathways: ferritin, transferrin receptor, glutamate and hypoxia-inducible factor-1 alpha. Exp. Eye Res. 97: 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harr B., Price T., 2012. Speciation: clash of the genomes. Curr. Biol. 22: R1044–R1046. [DOI] [PubMed] [Google Scholar]
- Jones F. C., Grabherr M. G., Chan Y. F., Russell P., Mauceli E., et al. , 2012. The genomic basis of adaptive evolution in threespine sticklebacks. Nature 484: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B., Corwin J. A., Kliebenstein D. J., 2015. Genetic variation in the nuclear and organellar genomes modulates stochastic variation in the metabolome, growth, and defense. PLoS Genet. 11: e1004779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch L., 2014. Genome evolution: evolutionary insights from comparative bear genomics. Nat. Rev. Genet. 15: 442–443. [DOI] [PubMed] [Google Scholar]
- Li M., Tian S., Jin L., Zhou G., Li Y., et al. , 2013. Genomic analyses identify distinct patterns of selection in domesticated pigs and Tibetan wild boars. Nat. Genet. 45: 1431–1438. [DOI] [PubMed] [Google Scholar]
- Li W., Godzik A., 2006. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22: 1658–1659. [DOI] [PubMed] [Google Scholar]
- Li Y., Ren Z., Shedlock A. M., Wu J., Sang L., et al. , 2013. High altitude adaptation of the schizothoracine fishes (Cyprinidae) revealed by the mitochondrial genome analyses. Gene 517: 169–178. [DOI] [PubMed] [Google Scholar]
- Li Y., Wu D.-D., Boyko A. R., Wang G.-D., Wu S.-F., et al. , 2014. Population variation revealed high-altitude adaptation of Tibetan mastiffs. Mol. Biol. Evol. 31: 1200–1205. [DOI] [PubMed] [Google Scholar]
- Lu S., Gu X., Hoestje S., Epner D. E., 2002. Identification of an additional hypoxia responsive element in the glyceraldehyde-3-phosphate dehydrogenase gene promoter. Biochim. Biophys. Acta 1574:152–156. [DOI] [PubMed] [Google Scholar]
- Mansfeld J., Guttinger S., Hawryluk-Gara L. A., Pante N., Mall M., et al. , 2006. The conserved transmembrane nucleoporin NDC1 is required for nuclear pore complex assembly in vertebrate cells. Mol. Cell 22: 93–103. [DOI] [PubMed] [Google Scholar]
- Martin M., 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 17: 10–12. [Google Scholar]
- Nagara Y., Tateishi T., Yamasaki R., Hayashi S., Kawamura M., et al. , 2013. Impaired cytoplasmic-nuclear transport of hypoxia-inducible factor-1 alpha in amyotrophic lateral sclerosis. Brain Pathol. 23: 534–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C., Inoguchi N., Weber R. E., Fago A., Moriyama H., et al. , 2013. Epistasis among adaptive mutations in deer mouse hemoglobin. Science 340: 1324–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J., Landers K. A., Mortimer R. H., Richard K., 2012. Expression and uptake of the thyroxine-binding protein transthyretin is regulated by oxygen in primary trophoblast placental cells. J. Endocrinol. 212: 159–167. [DOI] [PubMed] [Google Scholar]
- Peng Y., Yang Z., Zhang H., Cui C., Qi X., et al. , 2011. Genetic variations in Tibetan populations and high-altitude adaptation at the Himalayas. Mol. Biol. Evol. 28: 1075–1081. [DOI] [PubMed] [Google Scholar]
- Peng Z., Ho S. Y., Zhang Y., He S., 2006. Uplift of the Tibetan plateau: evidence from divergence times of glyptosternoid catfishes. Mol. Phylogenet. Evol. 39: 568–572. [DOI] [PubMed] [Google Scholar]
- Projecto-Garcia J., Natarajan C., Moriyama H., Weber R. E., Fago A., et al. , 2013. Repeated elevational transitions in hemoglobin function during the evolution of Andean hummingbirds. Proc. Natl. Acad. Sci. USA 110: 20669–20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Q., Zhang G., Ma T., Qian W., Wang J., et al. , 2012. The yak genome and adaptation to life at high altitude. Nat. Genet. 44: 946–949. [DOI] [PubMed] [Google Scholar]
- Qu Y., Zhao H., Han N., Zhou G., Song G., et al. , 2013. Ground tit genome reveals avian adaptation to living at high altitudes in the Tibetan plateau. Nat. Commun. 4: 2071. [DOI] [PubMed] [Google Scholar]
- Robertson G., Schein J., Chiu R., Corbett R., Field M., et al. , 2010. De novo assembly and analysis of RNA-seq data. Nat. Methods 7: 909–912. [DOI] [PubMed] [Google Scholar]
- Ruber L., Britz R., Kullander S. O., Zardoya R., 2004. Evolutionary and biogeographic patterns of the Badidae (Teleostei: Perciformes) inferred from mitochondrial and nuclear DNA sequence data. Mol. Phylogenet. Evol. 32: 1010–1022. [DOI] [PubMed] [Google Scholar]
- Sarkar J., Seshadri V., Tripoulas N. A., Ketterer M. E., Fox P. L., 2003. Role of ceruloplasmin in macrophage iron efflux during hypoxia. J. Biol. Chem. 278: 44018–44024. [DOI] [PubMed] [Google Scholar]
- Saxena V., Orgill D., Kohane I., 2007. A set of genes previously implicated in the hypoxia response might be an important modulator in the rat ear tissue response to mechanical stretch. BMC Genomics 8: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackel N. A., Gorrell M. D., McCaughan G. W., 2002. Gene array analysis and the liver. Hepatology 36: 1313–1325. [DOI] [PubMed] [Google Scholar]
- Shackel N. A., Seth D., Haber P. S., Gorrell M. D., McCaughan G. W., 2006. The hepatic transcriptome in human liver disease. Comp. Hepatol. 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Wellman T. L., Lounsbury K. M., Zhao F.-Q., 2014. Differential regulation of GLUT1 and GLUT8 expression by hypoxia in mammary epithelial cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307: R237–R247. [DOI] [PubMed] [Google Scholar]
- Simonson T. S., Yang Y., Huff C. D., Yun H., Qin G., et al. , 2010. Genetic evidence for high-altitude adaptation in Tibet. Science 329: 72–75. [DOI] [PubMed] [Google Scholar]
- Soria-Carrasco V., Gompert Z., Comeault A. A., Farkas T. E., Parchman T. L., et al. , 2014. Stick insect genomes reveal natural selection’s role in parallel speciation. Science 344: 738–742. [DOI] [PubMed] [Google Scholar]
- Storey J. D., Tibshirani R., 2003. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 100: 9440–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., 2007. Hemoglobin function and physiological adaptation to hypoxia in high-altitude mammals. J. Mammal. 88: 24–31. [Google Scholar]
- Storz J. F., Moriyama H., 2008. Mechanisms of hemoglobin adaptation to high altitude hypoxia. High Alt. Med. Biol. 9: 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Runck A. M., Sabatino S. J., Kelly J. K., Ferrand N., et al. , 2009. Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc. Natl. Acad. Sci. USA 106: 14450–14455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Runck A. M., Moriyama H., Weber R. E., Fago A., 2010. Genetic differences in hemoglobin function between highland and lowland deer mice. J. Exp. Biol. 213: 2565–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget-Groba Y., Montoya-Burgos J. I., 2010. Optimization of de novo transcriptome assembly from next-generation sequencing data. Genome Res. 20: 1432–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Yoshie M., Hashimoto K., Tachikawa E., 2014. Inhibitory effect of insulin-like growth factor-binding protein-7 (IGFBP7) on in vitro angiogenesis of vascular endothelial cells in the rat corpus luteum. J. Reprod. Dev. 60: 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd B. L., Stewart E. V., Burg J. S., Hughes A. L., Espenshade P. J., 2006. Sterol regulatory element binding protein is a principal regulator of anaerobic gene expression in fission yeast. Mol. Cell. Biol. 26: 2817–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leuven J. T., Meister R. C., Simon C., McCutcheon J. P., 2014. Sympatric speciation in a bacterial endosymbiont results in two genomes with the functionality of one. Cell 158: 1270–1280. [DOI] [PubMed] [Google Scholar]
- Yang L., Wang Y., Zhang Z., He S., 2015. Comprehensive transcriptome analysis reveals accelerated genic evolution in a Tibet fish, Gymnodiptychus pachycheilus. Genome Biol. Evol. 7: 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., 2007. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24: 1586–1591. [DOI] [PubMed] [Google Scholar]
- Yi X., Liang Y., Huerta-Sanchez E., Jin X., Cuo Z. X., et al. , 2010. Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329: 75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N., Zheng C. L., Zhang Y. P., Li W. H., 2000. Molecular systematics of pikas (genus Ochotona) inferred from mitochondrial DNA sequences. Mol. Phylogenet. Evol. 16: 85–95. [DOI] [PubMed] [Google Scholar]
- Zhang G., Li C., Li Q., Li B., Larkin D. M., et al. , 2014. Comparative genomics reveals insights into avian genome evolution and adaptation. Science 346: 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Z., Nielsen R., Yang Z. H., 2005. Evaluation of an improved branch-site likelihood method for detecting positive selection at the molecular level. Mol. Biol. Evol. 22: 2472–2479. [DOI] [PubMed] [Google Scholar]
- Zhao S., Zheng P., Dong S., Zhan X., Wu Q., et al. , 2013. Whole-genome sequencing of giant pandas provides insights into demographic history and local adaptation. Nat. Genet. 45: 67–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequencing reads were deposited in the short read archive (SRA) of GenBank (accession numbers listed in Table S1).