Abstract
The dung of herbivores, the natural habitat of the model mushroom Coprinopsis cinerea, is a nutrient-rich but also very competitive environment for a saprophytic fungus. We showed previously that C. cinerea expresses constitutive, tissue-specific armories against antagonists such as animal predators and bacterial competitors. In order to dissect the inducible armories against such antagonists, we sequenced the poly(A)-positive transcriptome of C. cinerea vegetative mycelium upon challenge with fungivorous and bacterivorous nematodes, Gram-negative and Gram-positive bacteria and mechanical damage. As a response to the fungivorous nematode Aphelenchus avenae, C. cinerea was found to specifically induce the transcription of several genes encoding previously characterized nematotoxic lectins. In addition, a previously not characterized gene encoding a cytoplasmic protein with several predicted Ricin B-fold domains, was found to be strongly upregulated under this condition. Functional analysis of the recombinant protein revealed a high toxicity toward the bacterivorous nematode Caenorhabditis elegans. Challenge of the mycelium with A. avenae also lead to the induction of several genes encoding putative antibacterial proteins. Some of these genes were also induced upon challenge of the mycelium with the bacteria Escherichia coli and Bacillus subtilis. These results suggest that fungi have the ability to induce specific innate defense responses similar to plants and animals.
Keywords: basidiomycete, fungal defense, RNA sequencing, CCTX2, transcriptomics
Interactions between organisms can be either beneficial or detrimental for the partners involved. In order to avoid the detrimental effects caused by antagonistic organisms, in particular multicellular organisms have evolved sophisticated defense strategies, comprising mechanisms to recognize the presence of other organisms, and to distinguish between self and nonself (Zhang et al. 2010; Zipfel 2008), as well as the production of defense molecules, such as proteins (Bleuler-Martinez et al. 2011; Gallo and Hooper 2012; Vandenborre et al. 2011), RNAs (Liu et al. 2012), peptides (Walton et al. 2010), and secondary metabolites (Engel et al. 2002; Rohlfs and Churchill 2011; Spiteller 2008). It has been hypothesized that such defense systems originally evolved to prevent the fusion of somatic conspecifics that were genetically different (Muller and Muller 2003; Srivastava et al. 2010). Cytoplasmic and transmembrane pattern recognition receptors (PRRs) specifically recognizing conserved microbe- (MAMPs) or damage- (DAMPs) associated molecular patterns have been described and characterized in many animals including cnidarians (Bosch 2013), annelids (Skanta et al. 2013), mollusks (Yoshino et al. 2008), arthropods (Wang and Ligoxygakis 2006), and chordates (Hopkins and Sriskandan 2005). Plants also recognize MAMPs and DAMPs using PRRs, and share several other innate defense mechanisms with animals, including the production of reactive oxygen (Gleason et al. 2011; Nathan and Cunningham-Bussel 2013; Liu et al. 2010) and nitrogen (Prior et al. 2009; Nurnberger et al. 2004) species as well as the biosynthesis of toxic proteins (Vandenborre et al. 2011), antimicrobial peptides (Benko-Iseppon et al. 2010; Tennessen 2005), and secondary metabolites (Bednarek 2012). The signaling pathways involved in animal and plant defense responses are conserved (Pedley and Martin 2005), and often lead to differential gene expression, suggesting that innate defense systems are an ancient and widespread trait that appeared very early in evolution. Accordingly, fungi are expected to also deploy innate defense mechanisms but, to date, not much is known about these mechanisms.
A main aspect of defense is the ability of an organism to distinguish between self and nonself. Fungi are known to distinguish between compatible or noncompatible cells of their own kind by their mating type system (Bidard et al. 2013; Hall et al. 2010) or by a mechanism referred to as vegetative heterokaryon incompatibility (HI) (Bidard et al. 2013; Hutchison et al. 2009). The latter mechanism has been well characterized in the filamentous ascomycetes Podospora anserina and Neurospora crassa, and involves the recognition of noncompatible hyphae via cytoplasmic proteins resembling PRRs, and containing HET and STAND domains, which leads to an extensive transcriptional response, including the induction of genes encoding toxins (Bidard et al. 2013; Hutchison et al. 2009). Little is known about the recognition of antagonists, including competitors, predators and parasites, by fungi, and the subsequent fungal responses affecting the interaction of the fungi with these organisms. In Aspergillus nidulans, it was shown that the master regulator of secondary metabolite production, LaeA, is responsible for the resistance of this fungus to predation by larvae of the fruit fly Drosophila melanogaster (Caballero Ortiz et al. 2013). In agreement with these results, challenge of the vegetative mycelium with fungivorous collembola induced the formation of fruiting bodies, and the synthesis of toxic secondary metabolites, suggesting that A. nidulans is able to respond to its predator by mounting an effective defense response (Caballero Ortiz et al. 2013; Doll et al. 2013). Similarly, A. fumigatus responded to the presence of actinomycetous bacteria by producing antibacterial polyketides (Schroeckh et al. 2009). This response of the fungus depended on direct physical interaction between the bacterial and fungal filaments and on the acetylation of histones (Nutzmann et al. 2011). Finally, analysis of the transcriptional response of the plant pathogenic fungus Magnaporthe oryzae to the bacterial antagonist Lysobacter enzymogenes allowed the identification of a class of potential antibacterial defense effector proteins (Mathioni et al. 2013).
We have recently shown that the coprophile model mushroom Coprinopsis cinerea transcribes a broad array of genes encoding putative defense proteins against bacterial competitors and animal predators constitutively in a tissue-specific manner (Plaza et al. 2014; Essig et al. 2014). In addition, the biosynthesis of two nematotoxic defense proteins, CGL1 and CGL2, was shown to be induced in the vegetative mycelium of C. cinerea upon challenge with the predatory nematode Aphelenchus avenae (Bleuler-Martinez et al. 2011). The specificity and the extent of this fungal defense response remained unclear, however. In order to resolve these issues, we assessed the transcriptional response of the vegetative mycelium of C. cinerea to nematode predation and bacterial coculture at a genome-wide level. The results of this study show that several loci encoding nematotoxic and potentially bactericidal proteins are specifically induced in response to nematode predation and bacterial cocultivation, respectively.
Materials and Methods
Strains and general cultivation conditions
Escherichia coli DH5α was used for cloning and plasmid amplification. E. coli strain BL21 (DE3) was used for protein expression and biotoxicity assays, and strain OP50 was used for maintenance of Caenorhabditis elegans. For these purposes, E. coli was cultivated on LB or NGM medium as described (Stiernagle 2006). E. coli strain Nissle 1917, and Bacillus subtilis strain 168 were used for challenging C. cinerea and cultivated as described below. C. elegans wild-type strain Bristol type (N2) was obtained from Caenorhabditis Genetics Center (CGC) and cultivated on NGM plates preseeded with E. coli OP50 as described (Stiernagle 2006). The fungivorous nematode A. avenae (a kind gift from Prof. Richard Sikora, University of Bonn, Germany) was propagated at 20° on vegetative mycelium of Agaricus bisporus pregrown on PDA plates (Difco). Vegetative mycelia of C. cinerea strain Okayama7 (O7; monokaryon) and A43mutB43mut (AmBm; homodikaryon), used for the challenge experiments and gene/cDNA cloning, respectively, were cultivated on YMG agar (0.4% yeast extract, 1% malt extract, 50 mM glucose, 1.5% agar) plates at 37°. Cultivation of primordia of strain AmBm was performed as described previously (Kues 2000).
C. cinerea challenge experiment
B. subtilis 168 and E. coli Nissle 1917 were cultivated from frozen stocks for 18 hr at 37° on YMG plates. Single colonies were inoculated in 2.5 ml YMG broth (0.4% yeast extract, 1% malt extract, 50 mM glucose), and cultivated to stationary phase at 37° under constant shaking. Bacteria were then washed twice in sterile-filtered PBS (135 mM NaCl, 2.5 mM KCl, 10 mM Na2HPO4, and 17.5 mM KH2PO4; pH 7.4) and their OD600 was adjusted with PBS to 0.0005 before use. The fungal-feeding nematodes were cultivated as described above, and harvested by the Baermann funnel method (Staniland 1954). Bacterivorous C. elegans N2 was cultivated as described above and harvested by washing the plates with PBS. After harvesting, all nematodes were transferred to water agar plates supplemented with 200 µg/ml G418, 50 µg/ml Nystatin and 100 µg/ml Ampicillin, and incubated for 48 hr to eliminate all the residual bacteria or fungi. Before use, nematodes were suspended and washed twice in sterile-filtered PBS and adjusted to a density of 2500 worms/ml. Individual single colonies of C. cinerea O7 vegetative mycelium were cultivated on separate 30-ml YMG agar plates covered with sterile cellophane discs at 37° in the dark for 96 hr. The other organisms were applied in 200 µl sterile-filtered PBS to the individual mycelial colonies and incubated for 72 hr at 24°: ca. 500 mixed-stage A. avenae; ca. 500 mixed-stage C. elegans; OD600: 0.0005 B. subtilis 168, or OD600: 0.0005 E. coli Nissle 1917. To mimic the tissue damage inflicted on the hyphae by the fungivorous nematode, the C. cinerea O7 mycelial colony was cut with a sterile scalpel every 24 hr for 72 hr. As negative control, 200 µl sterile-filtered buffer (PBS) was applied to the mycelial colony. The individual challenge experiments were performed in triplicate. Immediately after the challenge, the individual mycelial colonies were harvested and flash frozen using liquid nitrogen in separate tubes and stored at –80°.
Extraction of total RNA
The frozen mycelium was lyophilized in a VirTis Freezemobile for 18 hr. Lyophilized tissue (20 mg/replicate/treatment) was lysed in three FastPrep FP120 homogenization steps of 45 sec at 4.5, 5.5 and 6.5 m/sec in the presence of 250 mg 0.5-mm glass beads, while cooling the samples for 5 min on ice between the steps. RNA was extracted from the lysed tissue using 1 ml Qiazol (Qiagen), and 0.2 ml chloroform (ReagentPlus, Sigma-Aldrich). The mixture was centrifuged at 12,000 × g for 15 min at 4°, and RNA from the upper aqueous phase was washed incolumn using the RNeasy Lipid Tissue Mini Kit (Qiagen), and eluted in 60 µl RNase-free water. Concentration and quality of the purified RNA was determined with a Qubit (1.0) fluorometer (Life Technologies), and a Bioanalyzer 2100 (Agilent), respectively. Samples with a 260/280 nm ratio of 1.8–2.1, a 28S/18S ratio of 1.5–2 and no signs of rRNA degradation were used for library construction.
Illumina HiSeq 2000 library construction and sequencing
A TruSeq Stranded mRNA Sample Prep Kit (Illumina) was used to construct expression libraries (three biological replicates per treatment). Briefly, total RNA (1 μg) from each biological replicate was poly(A)-enriched, and then reverse-transcribed into double-stranded cDNA in the presence of Actinomycin during first-strand synthesis. Double-stranded cDNA was fragmented, end-repaired, and adenylated before being ligated to TruSeq adapters and selectively enriched by PCR. Concentration and quality of the enriched libraries were assessed using Qubit (1.0) fluorometer and LabChip GX (PerkinElmer), showing an average fragment size of 260 bp. Libraries were normalized to 10 nM with 10 mM Tris-HCl pH 8.5 supplemented with 0.1% Tween 20. In addition, TruSeq PE Cluster Kit v3-cBot-HS (Illumina) was used to generate clusters from 10 pM pooled normalized libraries. Paired-end sequencing was performed on an Illumina HiSeq 2000 at 2 X 101 bp or single-end 100 bp using the TruSeq SBS Kit v3-HS (Illumina).
Validation by qRT-PCR
RNA-seq results were validated by qRT-PCR. Single-stranded cDNA from three (A. avenae challenge or nonchallenge control) or two (scalpel-damaged mycelia) biological replicates was synthesized using Transcriptor Universal cDNA Master (Roche) from 2 µg total RNA. qRT-PCR reactions (20 µl) were mixed in three technical replicates per primer set and sample, containing 900 nM forward and reverse primers designed to span exon–exon junctions (Supporting Information, Table S1), 10 µl 2X FastStart Universal SYBR Green Master (Rox, Roche), and 1 ng/µl cDNA template. qRT-PCR was performed in a Rotor-Gene 3000 (Corbett Life Science) with the following thermal profile: a hold step at 95° for 15 min, followed by 40 cycles of 95° for 15 sec, 62° for 30 sec, and 72° for 30 sec. In order to control the specificity of amplification, the reaction was concluded with a melting curve analysis ramping from 55° to 99° in steps of 1° every 5 sec. PCR efficiencies and cycle thresholds were obtained using LinRegPCR 12, and differential expression ratios were calculated by the CT difference formula (Schefe et al. 2006). Tubulin beta chain (CC1G_04743) was used as a housekeeping normalizer. In addition, water or 1 ng/µl RNA was included as a negative control reaction. To further validate the significance of the RNA-seq-derived differential expression analysis, the constitutive expression of an array of housekeeping loci commonly used in qRT-PCR normalization (Ferreira and Cronje 2012; Huggett et al. 2005; Langnaese et al. 2008; Silveira et al. 2009; Wan et al. 2011) was verified in the sequencing datasets after library size normalization.
Bioinformatic analysis
To obtain gene counts, reads from each RNAseq library were first trimmed to a specific length to ensure that reads with a low quality 3′ end still aligned. To determine the trimming length, 50,000 reads were randomly selected from the sequence data set, then trimmed to varying lengths and aligned to the reference genome (Stajich et al. 2010). Next, mapping rates were compared between the different trim lengths. The trim length with the highest percentage of mapping was selected as the trim length for all reads from each library. Following trimming, all reads were aligned to the reference transcriptome using the Burrows-Wheeler Aligner (Li and Durbin 2009) (seed length = 25, maximum hits = 1). The genome of C. cinerea strain O7 that was used as a reference is available for download from http://genome.jgi.doe.gov/Copci1/Copci1.home.html (Coprinopsis_cinerea.transcripts.fasta). Using the alignment, the number of reads that aligned to each gene for each sample separately, were counted (in-house script), and used to produce the counts matrix. Only reads that aligned uniquely and on the reverse strand of the reference were counted. Illumina mapped reads were deposited in the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress/) under the accession number E-MTAB-2763. To determine the percentage of loci showing baseline expression, five reads/locus were taken as a minimal threshold. Reads per kilobase of transcript per million mapped reads (RPKM) were calculated for every locus in order to scale-normalize all the samples according to library and transcript size. For this, the number of reads for a locus in a library was divided by the total size of the library in mega-reads (millions of reads of the library that were mapped to the reference genome) multiplied by the length of the transcript encoded by the locus in kilobases (thousands of bases). Fold change per locus and a Welch’s t-test comparing the different challenge and control libraries were computed using normalized sense-RPKMs (Table S2). One advantage of using a higher number of biological replicates per treatment than the two necessary to apply EdgeR’s exact test (Robinson et al. 2010), was the possibility of using Welch’s t-test for pairwise comparison. Volcano plots comparing every treatment with the negative control were constructed. A Welch’s t-test p-value ≤ 0.05 [–log10 (p-value) > 1.3] and fold change ≥ 4 [log2 (fold change) ≥ 2] were the criteria established to classify a locus as significantly induced in a treatment. Nonetheless, for comparison with Welch’s, we applied an EdgeR’s exact test to our dataset containing raw read-counts after estimating normalization factors (calcNormFactors), common dispersion (estimateCommonDisp), and tagwise dispersion (estimateTagwiseDisp). Thereafter, a table containing logFC, logCPM, p-value, and FDR for all the loci was retrieved from EdgeR (Robinson et al. 2010). The number of differentially expressed genes based on false discovery rate (FDR) ≤ 0.05, or the combination of FDR ≤ 0.05 and a logFC ≤ –2 or logFC ≥ 2 was estimated. When the number of genes showing significant differential expression derived from the three types of analyses [FDR ≤ 0.05 only, FDR ≤ 0.05 combined with logFC and Welch’s p-value ≤ 0.05 combined with log2 (fold change)] was compared, a combination of Welch’s p-value ≤ 0.05 and log2 (fold change) > 2 or < –2 provided the smallest number of differentially expressed genes, thus proving to be more stringent than the two estimations derived from the EdgeR analysis package (Table S3). These stringent differential expression criteria were used henceforth. Venn diagrams were constructed to identify commonly regulated loci among treatments (http://bioinformatics.psb.ugent.be/webtools/Venn/). Functional annotation based on sequence similarity for loci found to be significantly induced was deduced by PSI-BLAST (Altschul et al. 1997) and PHYRE2 using standard parameters (Kelley et al. 2015). SignalP 4.1 (Petersen et al. 2011) and TMHMM v. 2.0 (Sonnhammer et al. 1998) were used to predict the presence of secretion signal and transmembrane helices in differentially expressed loci.
Cloning and heterologous expression of CCTX2
Total RNA extraction and cDNA synthesis from C. cinerea AmBm were performed as described previously (Plaza et al. 2014). In brief, cDNA was synthesized using cDNA transcriptor universal (Roche) from 2 µg of total RNA following the manufacturer’s instructions. The coding region of CCTX2 (CC1G_10077) was amplified from cDNA using primers 10077forNdeI (GGAGTCGGCATATGGCTCTCAACGAAGGTG) and 10077revNotI (GAATAGCGGCCGCCTACAACTCGGAGTGCTTG). The PCR product was cloned into the pET24b expression vector (Novagen) using the NdeI and NotI restriction sites. Cloning of CGL2 into pET24b was described previously (Walti et al. 2008). For protein expression, the plasmid was transformed into E. coli BL21 (DE3). Transformants were cultivated in LB medium containing 50 µg/ml kanamycin to an OD600 = 0.7 and protein expression was induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 20° for 16 hr. Solubility of the protein was tested as previously described (Kunzler et al. 2010).
Nematotoxicity assay
Nematotoxicity of CCTX2 was determined by feeding the recombinant E. coli expressing CCTX2 to C. elegans as described (Kunzler et al. 2010; Sabotic et al. 2012). Briefly, a liquid toxicity assay was performed using 20–30 synchronized L1 larvae. The larvae were incubated for 2 days at 20° with an E. coli BL21 culture at OD600 of 2, expressing CCTX2, the positive control CGL2 (Butschi et al. 2010) or harboring the ’empty’ vector plasmid pET24b. Toxicity was assessed by counting larvae developed into L4 stage or adulthood. The assay was done in quadruplicate. Welch’s t-test was performed to validate statistical significance of the differences observed.
Protein expression analysis in C. cinerea
For protein expression analysis, vegetative mycelium of C. cinerea O7 was challenged with A. avenae and C. elegans as described above for RNA sequencing analysis. Whole cell protein extracts of the respective samples were prepared as described in (Bleuler-Martinez et al. 2011). Induction of CGL2 and CCTX2 in the mycelial samples was verified by immunoblotting using protein-specific polyclonal antisera (1:5000). The antiserum against CGL2 has been described previously (Boulianne et al. 2000). The antiserum against the purified recombinant CCTX2 was raised in rabbits by Seramun Diagnostica GmBH (Berlin, Germany). Expression of CCTX2 was performed as described above, and purification of the protein was achieved by affinity chromatography on a Sepharose column.
Data availability
Reference for data available in public repository: Illumina mapped reads were deposited in the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress/) under the accession number E-MTAB-2763.
Results
General features of the analyzed C. cinerea transcriptomes
Illumina RNA-seq stranded libraries were constructed and sequenced in biological triplicates for mycelial colonies of the monokaryotic C. cinerea strain Okayama7 (O7) challenged with bacterivorous (C. elegans N2) and fungivorous nematodes (A. avenae), gram-positive (B. subtilis 168), and gram-negative bacteria (E. coli Nissle 1917), mechanical damage or the application of buffer (negative control). Sequencing of these libraries showed that 82–84% of the suggested 13,393 open reading frames (ORF) in the genome of C. cinerea were significantly transcribed (more than five reads mapping to the ORF). A total 753 million sense and antisense reads (100 bases each) were mapped to the 37.5 Mb genome of C. cinerea O7, accounting for a sequencing output of nearly 75 billion bases (2000 times the genome size) (Table 1 and Table S2). Illumina mapped reads were deposited in the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress/) under the accession number E-MTAB-2763.
Table 1. General features of Coprinopsis cinerea mycelia exposed to different biotic and abiotic stress conditions.
| Mapped Reads (Sense + Antisense) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Replicate 1a | % of Totalb | Replicate 2a | % of Totalb | Replicate 3a | % of Totalb | Total Mapped | Mean % of Loci Transcribed |
| O7 + PBS | 3.31E+07 | 81.1 | 4.14E+07 | 78.6 | 4.54E+07 | 83.1 | 1.20E+08 | 82.3 |
| O7 + A. avenae | 2.51E+07 | 53.7 | 4.90E+07 | 82.6 | 3.87E+07 | 75.5 | 1.13E+08 | 82.1 |
| O7 + C. elegans | 3.14E+07 | 77.1 | 5.28E+07 | 78.9 | 4.31E+07 | 83.0 | 1.27E+08 | 82.4 |
| O7 + mechanical damage | 2.40E+07 | 80.5 | 5.14E+07 | 80.5 | 4.08E+07 | 82.8 | 1.16E+08 | 82.6 |
| O7 + E. coli | 5.53E+07 | 79.4 | 4.77E+07 | 79.5 | 5.09E+07 | 82.0 | 1.54E+08 | 82.5 |
| O7 + B. subtilis | 4.86E+07 | 81.0 | 3.78E+07 | 83.1 | 3.63E+07 | 83.0 | 1.23E+08 | 83.3 |
| Total no. of mapped reads | 7.53E+08c | |||||||
| Approximate read length | 100 bases | |||||||
| Approximate total output | 75 Gigabases | |||||||
Biological replicates.
Percentage of the total number of sequenced reads that was mapped to the reference genome.
Total number of mapped reads: 7.53E+08; Approximate read length: 100 bases; Approximate total sequence output: 75 Gbases.
Identification of a novel nematotoxic protein based on the analysis of the differential transcriptome of C. cinerea challenged with the fungivorous nematode A. avenae
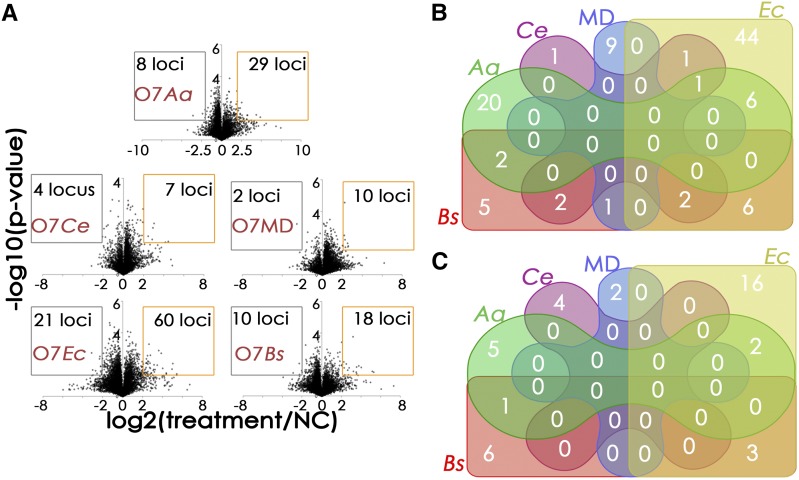
One of the goals of this study was the identification of novel defense effectors of C. cinerea against predatory nematodes based on the analysis of the set of C. cinerea genes upregulated upon challenge with these organisms. To achieve this goal, vegetative mycelium of C. cinerea strain O7 was challenged with the fungivorous nematode A. avenae for 72 hr, and the poly(A)-positive transcriptome of the challenged mycelial colonies was analyzed by RNA-seq and compared to the transcriptome of the buffer control. Genes displaying p-values ≤ 0.05 and expression fold changes (treatment/negative control or negative control/treatment) ≥ 4 were considered to be significantly upregulated or downregulated. This analysis led to the identification of eight C. cinerea genes being significantly downregulated and 29 genes being significantly upregulated upon challenge with A. avenae (Figure 1A, Table 2, and Table 3). Transcription of some of these genes responded exclusively to the presence of A. avenae, whereas the transcription of some was influenced also by the other biotic stresses applied in this study (Figure 1, B and C, Table 2, and Table 3) (see below).
Figure 1.
Challenge of Coprinopsis cinerea vegetative mycelium by Aphelenchus avenae predation and bacterial cocultivation induce treatment-specific defense responses at the transcriptional level. (A) Volcano plots showing the genome-wide differential expression analysis of Coprinopsis cinerea O7 challenged with the fungivorous nematode A. avenae (O7Aa), the bacterivorous nematode Caenorhabditis elegans N2 (O7Ce), Escherichia coli Nissle 1917 (O7Ec) and Bacillus subtilis 168 (O7Bs) relative to the application of buffer as negative control (NC). As additional treatment, supposedly mimicking the mechanical damage of hyphae inflicted by fungivore feeding, Coprinopsis cinerea vegetative mycelium was repeatedly cut with a scalpel (O7MD). Genes showing log2 (treatment/NC) ≥ 2 or ≤ –2, and –log10 (Welch's t-test-derived p-values calculated from three biological replicates) ≥ 1.3 were considered to be significantly upregulated (orange boxes) or downregulated (gray boxes). (B) Venn’s diagram computed for genes significantly upregulated by the fungivorous nematode A. avenae (Aa), the bacterivorous nematode Caenorhabditis elegans (Ce), the scalpel-inflicted hyphal damage (MD), E. coli (Ec) or B. subtilis (Bs). (C) Venn’s diagram computed for genes significantly downregulated by A. avenae (Aa), Caenorhabditis elegans (Ce), the scalpel-inflicted hyphal damage (MD), E. coli (Ec) or B. subtilis (Bs).
Table 2. Coprinopsis cinerea Okayama7 protein-encoding genes significantly upregulated in response to the applied treatments.
| Treatment/Locus | PSI-Blast/PHYRE2 Prediction | SignalP | TMHMM | Pfam Cross-Reference | Aa/NC | Ce/NC | MD/NC | Ec/NC | Bs/NC |
|---|---|---|---|---|---|---|---|---|---|
| A. avenae (Aa) | |||||||||
| CC1G_01501 | CBM-containing secreted protein | Y | 0 | 4.1 | — | — | — | — | |
| CC1G_02104 | Peroxidase | Y | 0 | PF11895; PF00141 | 5.4 | — | — | — | — |
| CC1G_02355 | Hypothetical secreted protein | Y | 0 | 4.4 | — | — | — | — | |
| CC1G_02622 | Ankyrin-repeat protein | N | 0 | PF12796 | 5.2 | — | — | — | — |
| CC1G_03076 | GH24 lysozyme | Y | 0 | PF00959 | 5.2 | — | — | — | — |
| CC1G_04169 | WSC domain-containing secreted protein | Y | 0 | PF00734; PF09362 | 5.2 | — | — | — | — |
| CC1G_05003 | CGL1 galectin | N | 0 | PF00337 | 5 | — | — | — | — |
| CC1G_05005 | CGL2 galectin | N | 0 | PF00337 | 4.1 | — | — | — | — |
| CC1G_05809 | Glycosyl hydrolase family 18 protein | Y | 0 | 5.8 | — | — | — | — | |
| CC1G_06698 | RTA1-like protein | N | 7 | PF04479 | 20.4 | — | — | — | — |
| CC1G_08057 | Wnt-like secreted protein | Y | 0 | 4.4 | — | — | — | — | |
| CC1G_08593 | Hypothetical secreted protein | Y | 0 | 6.2 | — | — | — | — | |
| CC1G_09966 | NmrA-family protein | N | 0 | PF05368 | 4.9 | — | — | — | — |
| CC1G_10077 | Ricin B-fold domain-containing protein | N | 0 | PF14200 | 4.9 | — | — | — | — |
| CC1G_10726 | Hypothetical secreted protein | Y | 0 | 28.4 | — | — | — | — | |
| CC1G_11792 | Hypothetical membrane protein | N | 1 | 5 | — | — | — | — | |
| CC1G_11847 | Defensin-related protein | Y | 0 | 9.4 | — | — | — | — | |
| CC1G_12246 | Hypothetical transmembrane protein | N | 7 | 6.8 | — | — | — | — | |
| CC1G_14365 | Hypothetical protein | N | 0 | 6.1 | — | — | — | — | |
| CC1G_14558 | Hypothetical protein | N | 0 | 5.6 | — | — | — | — | |
| C. elegans (Ce) | |||||||||
| CC1G_04017 | Hypothetical protein | N | 0 | — | 5.8 | — | — | — | |
| Mechanical damage (MD) | |||||||||
| CC1G_02583 | Membrane-anchored glycosylhydrolase | N | 1 | — | — | 6 | — | — | |
| CC1G_03098 | Ras-like GTPase | N | 0 | PF01926 | — | — | 4.8 | — | — |
| CC1G_03514 | Hypothetical protein | N | 0 | — | — | 4.3 | — | — | |
| CC1G_09359 | Hypothetical protein | N | 0 | — | — | 4.9 | — | — | |
| CC1G_10157 | Hypothetical protein | N | 0 | — | — | 5 | — | — | |
| CC1G_11387 | Cyclopropane-fatty-acyl-phospholipid synthase esynthase | N | 0 | PF02353 | — | — | 4.5 | — | — |
| CC1G_11620 | Hypothetical protein | N | 0 | — | — | 4.6 | — | — | |
| CC1G_12367 | Hypothetical transmembrane protein | N | 7 | — | — | 5.7 | — | — | |
| CC1G_15356 | Hypothetical protein | N | 0 | — | — | 5.9 | — | — | |
| E. coli (Ec) | |||||||||
| CC1G_00122 | Cytochrome P450 | N | 0 | PF00067 | — | — | — | 4.1 | — |
| CC1G_01525 | Transaldolase | N | 0 | PF00923 | — | — | — | 4.5 | — |
| CC1G_02052 | YCII-related domain protein | N | 0 | PF03795 | — | — | — | 5.6 | — |
| CC1G_02062 | N-alpha-acetyltransferase 60-like | N | 0 | PF00583 | — | — | — | 9.4 | — |
| CC1G_02166 | Hypothetical membrane protein | N | 1 | — | — | — | 9.3 | — | |
| CC1G_02345 | Malate synthase | N | 0 | PF01274 | — | — | — | 5.7 | — |
| CC1G_02382 | Lipolytic enzyme | Y | 0 | PF00657 | — | — | — | 5.4 | — |
| CC1G_02862 | Snoal-like polyketide cyclase family protein | Y | 0 | — | — | — | 6.5 | — | |
| CC1G_02908 | Alcohol dehydrogenase | N | 0 | PF08240; PF00107 | — | — | — | 4.8 | — |
| CC1G_03339 | Fasciclin-domain containing secreted protein | Y | 0 | PF02469 | — | — | — | 4.8 | — |
| CC1G_03442 | Endonuclease/exonuclease/phosphatase | Y | 0 | PF03372 | — | — | — | 5.1 | — |
| CC1G_03541 | Hypothetical secreted protein | Y | 0 | — | — | — | 16.7 | — | |
| CC1G_04927 | Hypothetical protein | N | 3 | — | — | — | 5.9 | — | |
| CC1G_05515 | FAD-dependent oxidoreductase | Y | 0 | PF01266 | — | — | — | 5.5 | — |
| CC1G_05607 | Ig-domain containing secreted protein | Y | 0 | — | — | — | 5.1 | — | |
| CC1G_05864 | LolT-1-like PLP-dependent aminotransferase | N | 0 | PF00266 | — | — | — | 7.1 | — |
| CC1G_05914 | Ammonium transporter | N | 11 | PF00909 | — | — | — | 32 | — |
| CC1G_06488 | Urea transporter | N | 15 | PF00474 | — | — | — | 5.5 | — |
| CC1G_06620 | Isocitrate lyase | N | 0 | PF00463 | — | — | — | 4.5 | — |
| CC1G_06972 | NAD(P)H-dependent dehydrogenase | N | 0 | PF07992 | — | — | — | 4.1 | — |
| CC1G_07061 | Hypothetical protein | N | 0 | — | — | — | 4.8 | — | |
| CC1G_07630 | MAPEG protein | N | 0 | PF01124 | — | — | — | 4.1 | — |
| CC1G_07735 | Mitochondrial carrier/telomere-binding protein | N | 0 | PF00153; PF10451 | — | — | — | 5 | — |
| CC1G_08094 | High affinity methionine permease | N | 12 | PF13520 | — | — | — | 6 | — |
| CC1G_08394 | Hypothetical protein | N | 0 | — | — | — | 8.5 | — | |
| CC1G_08758 | Hypothetical secreted protein | Y | 0 | — | — | — | 4.3 | — | |
| CC1G_08822 | WSC domain-containing protein | Y | 0 | PF01822 | — | — | — | 4.1 | — |
| CC1G_08888 | Acyl-coA carboxylate coA-transferase | N | 0 | PF13336; PF02550 | — | — | — | 9.1 | — |
| CC1G_11310 | Pria protein | Y | 0 | — | — | — | 5.6 | — | |
| CC1G_11374 | PLAC8-domain-containing protein | N | 0 | PF04749 | — | — | — | 4.3 | — |
| CC1G_11786 | Hypothetical transmembrane protein | N | 4 | — | — | — | 6.1 | — | |
| CC1G_12758 | Acyl-coA N-acyltransferase | N | 0 | PF13302 | — | — | — | 20.3 | — |
| CC1G_12964 | Citrate synthase | N | 0 | PF00285 | — | — | — | 4.1 | — |
| CC1G_12996 | Ricin B-fold domain-containing protein | Y | 0 | PF00652 | — | — | — | 4.3 | — |
| CC1G_13124 | Ammonium transporter | N | 9 | PF00909 | — | — | — | 7.9 | — |
| CC1G_13213 | Glycerophosphoryl diester phosphodiesterase | Y | 0 | PF03009 | — | — | — | 12.2 | — |
| CC1G_13246 | Hypothetical transmembrane protein | N | 5 | — | — | — | 6.2 | — | |
| CC1G_13826 | Hypothetical transmembrane protein | N | 4 | — | — | — | 7.4 | — | |
| CC1G_14125 | Mitochondrial carrier | N | 3 | PF00153 | — | — | — | 5.1 | — |
| CC1G_14829 | Glutathione-S-transferase | N | 0 | PF00043; PF13417 | — | — | — | 8.4 | — |
| CC1G_15187 | Hypothetical protein | N | 0 | — | — | — | 4 | — | |
| CC1G_15681 | Hypothetical protein | N | 0 | — | — | — | 8.3 | — | |
| CC1G_15703 | Cytochrome p450 | N | 0 | PF00067 | — | — | — | 4.7 | — |
| CC1G_08300 | Hydrophobin | N | 1 | — | — | — | 10.5 | — | |
| B. subtilis (Bs) | |||||||||
| CC1G_03042 | GH24 lysozyme | Y | 0 | PF00959 | — | — | — | — | 5.6 |
| CC1G_05798 | CBM-containing secreted protein | Y | 0 | — | — | — | — | 4.2 | |
| CC1G_09605 | Hypothetical protein | N | 0 | — | — | — | — | 8.9 | |
| CC1G_10004 | Hypothetical secreted protein | Y | 0 | — | — | — | — | 5.1 | |
| CC1G_08311 | Monocarboxylate permease | N | 11 | PF07690 | — | — | — | — | 4.7 |
| Ec+Bs | |||||||||
| CC1G_00718 | Hypothetical secreted protein | Y | 1 | — | — | — | 6.6 | 6.4 | |
| CC1G_05600 | Hypothetical secreted protein | Y | 0 | — | — | — | 10.9 | 11.7 | |
| CC1G_08056 | Wnt-like secreted protein | Y | 0 | — | — | — | 20.6 | 15.9 | |
| CC1G_08433 | Hypothetical protein | N | 0 | — | — | — | 42.8 | 11.1 | |
| CC1G_09365 | Triacylglycerol lipase | Y | 0 | PF01083 | — | — | — | 6.8 | 11.5 |
| CC1G_14477 | GH24 lysozyme | N | 0 | PF00959 | — | — | — | 6.9 | 6.2 |
| Aa+Ec | |||||||||
| CC1G_02441 | Hypothetical protein | Y | 0 | 6.6 | — | — | 5.2 | — | |
| CC1G_04734 | Med17 domain-containing protein | Y | 0 | 4.5 | — | — | 5.7 | — | |
| CC1G_07582 | Hypothetical secreted protein | Y | 0 | 8.9 | — | — | 5.1 | — | |
| CC1G_09529 | Hypothetical secreted protein | Y | 0 | 5 | — | — | 5.7 | — | |
| CC1G_10384 | O-Methylsterigmatocystin oxidoreductase | N | 0 | PF00067 | 4.6 | — | — | 6.7 | — |
| CC1G_06684 | LysM domain-containing protein | Y | 0 | PF01476 | 4.2 | — | — | 15.4 | — |
| Ce+Ec | |||||||||
| CC1G_02581 | Hypothetical membrane protein | Y | 2 | — | 8 | — | 31.1 | — | |
| Aa+Bs | |||||||||
| CC1G_05472 | Wnt-like secreted protein | Y | 0 | 9 | — | — | — | 6.7 | |
| CC1G_03047 | GH24 lysozyme | N | 0 | PF00959 | 7 | — | — | — | 4.8 |
| Ce+Bs | |||||||||
| CC1G_08818 | Hypothetical secreted protein | Y | 0 | — | 17.7 | — | — | 38.1 | |
| CC1G_13803 | Hypothetical protein | N | 0 | — | 7.2 | — | — | 12.7 | |
| Aa+Ce+Ec | |||||||||
| CC1G_13818 | MFS general substrate transporter | Y | 9 | 9.1 | 5.4 | — | 16.1 | — | |
| MD+Bs | |||||||||
| CC1G_15139 | Metalloprotease | Y | 0 | PF05572 | — | — | 4 | 13.4 | |
| Ce+Ec+Bs | |||||||||
| CC1G_01042 | PAP2 superfamily protein | Y | 0 | — | 21.7 | — | 42.9 | 35 | |
| CC1G_05219 | KapM protein | Y | 0 | — | 7.5 | — | 14.5 | 9.3 | |
As thresholds of significant differential expression, fold (treatment/negative control) ≥ 4 and Welch’s t-test p-value ≤ 0.05 (from three biological replicates per treatment) were used. Presence (Y) or absence (N) of secretion signal was computed with SignalP 4.1. Number of transmembrane helices was predicted using TMHMM v. 2.0 in the proteins encoded by differentially expressed genes. Pfam cross-reference IDs are shown when available.
Table 3. Coprinopsis cinerea Okayama7 protein-encoding genes significantly downregulated in response to the applied treatments.
| Treatment/Locus | PSI-Blast/PHYRE2 Prediction | SignalP | TMHMM | Pfam Cross-Reference | NC/Aa | NC/Ce | NC/MD | NC/Ec | NC/Bs |
|---|---|---|---|---|---|---|---|---|---|
| A. avenae (Aa) | |||||||||
| CC1G_09526 | Endoglucanase-4 | Y | 0 | PF03443 | 4.1 | — | — | — | — |
| CC1G_09644 | Alpha-galactosidase | N | 0 | 5.8 | — | — | — | — | |
| CC1G_13605 | Hypothetical protein | N | 0 | 20.5 | — | — | — | — | |
| CC1G_13985 | Hypothetical protein | N | 0 | 6.7 | — | — | — | — | |
| CC1G_14464 | Reverse transcriptase/ribonuclease H | N | 0 | 6.2 | — | — | — | — | |
| C. elegans (Ce) | |||||||||
| CC1G_02106 | Hypothetical protein | Y | 0 | — | 6.7 | — | — | — | |
| CC1G_09458 | Extracellular tungstate binding | N | 0 | — | 4.1 | — | — | — | |
| CC1G_13558 | Hypothetical protein | Y | 0 | — | 5.1 | — | — | — | |
| CC1G_15088 | Hypothetical protein | N | 0 | — | 5.4 | — | — | — | |
| Mechanical damage (MD) | |||||||||
| CC1G_08269 | Hypothetical protein | Y | 0 | PF14273 | — | — | 4.6 | — | — |
| CC1G_08983 | Hypothetical protein | N | 7 | — | — | 6.7 | — | — | |
| E. Coli (ec) | |||||||||
| CC1G_01035 | Hypothetical protein | N | 0 | — | — | — | 8.8 | — | |
| CC1G_01577 | Glycosyl hydrolase family 62 protein | Y | 0 | PF00734; PF03664 | — | — | — | 6.7 | — |
| CC1G_01879 | RNA recognition motif 2 partial | N | 0 | PF04059 | — | — | — | 4.1 | — |
| CC1G_02999 | Hypothetical protein | N | 0 | — | — | — | 4.2 | — | |
| CC1G_06017 | Tyrosinase central domain-containing protein | Y | 0 | PF00264 | — | — | — | 5.3 | — |
| CC1G_08259 | Leucine-rich repeat domain protein | N | 0 | PF12937 | — | — | — | 16.5 | — |
| CC1G_10148 | Hypothetical protein | N | 0 | — | — | — | 4.1 | — | |
| CC1G_10470 | Tyrosinase central domain-containing protein | Y | 0 | PF00264 | — | — | — | 8 | — |
| CC1G_10494 | Hypothetical protein | N | 5 | — | — | — | 4.7 | — | |
| CC1G_11188 | ycaC protein | N | 2 | PF00857 | — | — | — | 8.1 | — |
| CC1G_11580 | Hypothetical protein | N | 0 | — | — | — | 4.5 | — | |
| CC1G_12408 | Hypothetical protein | N | 0 | — | — | — | 4.2 | — | |
| CC1G_12509 | Galactose mutarotase-like protein | N | 0 | — | — | — | 4.4 | — | |
| CC1G_14013 | Cytochrome P450 | N | 1 | PF00067 | — | — | — | 4.5 | — |
| CC1G_14014 | O-Methylsterigmatocystin oxidoreductase | N | 0 | PF00067 | — | — | — | 4.6 | — |
| CC1G_14164 | AlphaN-acetylglucosamine transferase | N | 1 | — | — | — | 4.8 | — | |
| B. Subtilis (bs) | |||||||||
| CC1G_01487 | Hypothetical protein | N | 0 | — | — | — | — | 6.9 | |
| CC1G_04915 | Hypothetical protein | N | 4 | — | — | — | — | 9 | |
| CC1G_05337 | Hypothetical protein | N | 7 | — | — | — | — | 6.4 | |
| CC1G_05968 | Hypothetical protein | N | 1 | — | — | — | — | 5.6 | |
| CC1G_08393 | Hypothetical protein | Y | 1 | — | — | — | — | 6.2 | |
| CC1G_14873 | Hypothetical protein | N | 0 | — | — | — | — | 4.4 | |
| Ec+bs | |||||||||
| CC1G_03158 | Hypothetical protein | Y | 3 | — | — | — | 5.9 | 7.4 | |
| CC1G_09799 | AgaK1 protein kinase | N | 0 | PF00069 | — | — | — | 5.9 | 7.1 |
| CC1G_10006 | Tyrosinase | N | 0 | PF00264 | — | — | — | 4.3 | 4.1 |
| Aa+ec | |||||||||
| CC1G_06907 | WD40 domain-containing protein | N | 1 | 5.8 | — | — | 6.8 | — | |
| CC1G_15644 | Hypothetical protein | Y | 0 | 10.2 | — | — | 13.6 | — | |
| Aa+bs | |||||||||
| CC1G_15616 | Extracellular tungstate binding | N | 0 | 4.9 | — | — | — | 6.2 | |
As thresholds of significant differential expression, fold (negative control/treatment) ≥ 4 and Welch’s t-test p-value ≤ 0.05 (from three biological replicates per treatment) were used. Presence (Y) or absence (N) of secretion signal was computed with SignalP 4.1. Number of transmembrane helices was predicted using TMHMM v. 2.0 in the proteins encoded by differentially expressed genes. Pfam cross-reference IDs are shown when available.
In order to validate the RNA-seq data of the A. avenae challenge, the expression of a selection of genes showing statistically significant induction in response to A. avenae was confirmed by qRT-PCR (Figure S1) and immunoblotting (Figure S2). As an additional validation of the RNA-seq data, the expression of several reported housekeeping loci (Ferreira and Cronje 2012; Huggett et al. 2005; Langnaese et al. 2008; Silveira et al. 2009; Wan et al. 2011) was not changed by any of the treatments applied to C. cinerea (Table S4).
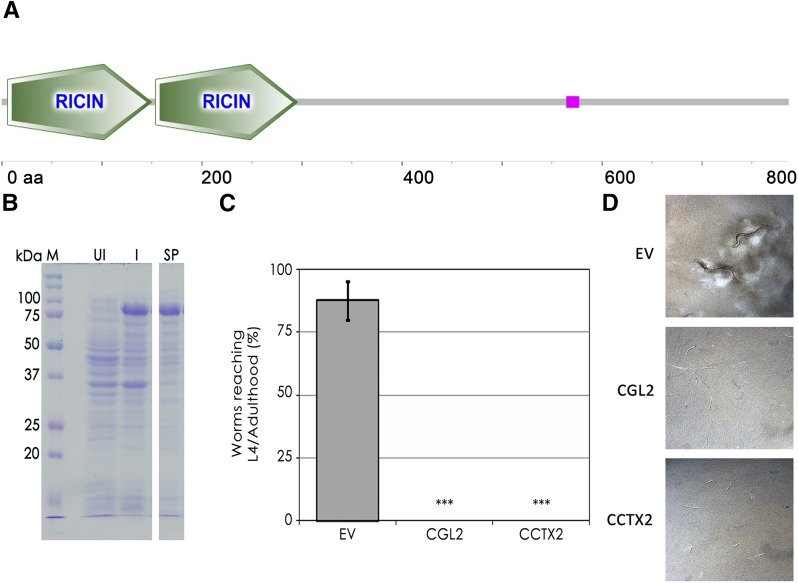
Among the set of genes that was specifically upregulated by A. avenae predation, CC1G_10726 and CC1G_06698 were the most highly induced, with fold changes of 20 and 28, respectively. Whereas the hypothetical protein encoded by CC1G_10726 did not, other than a consensus signal peptide for classical secretion (SignalP), show any sequence or structural similarity to any other functionally characterized protein in the currently available databases, CC1G_06698 codes for a seven-transmembrane domain protein that is homologous to the Rta1p protein of Saccharomyces cerevisiae. This protein may have a potential detoxifying function since it confers resistance of S. cerevisiae toward 7-aminocholesterol (Soustre et al. 1996). Consistent with previous results (Bleuler-Martinez et al. 2011), the two paralogous genes CC1G_05003 and CC1G_05005 coding for the nematotoxic galectins CGL1 and CGL2 (Butschi et al. 2010), respectively, were found to be specifically induced by A. avenae (Table 2 and Figure 1A). The set of C. cinerea genes specifically and significantly induced by A. avenae included the hitherto uncharacterized gene CC1G_10077 coding for a predicted cytoplasmic protein containing two putative RicinB-fold domains (Figure 1A, Figure 2A, and Table 2). In order to assess whether this protein, hereafter termed CCTX2 (C.cinerea toxin 2), was toxic to nematodes, the cDNA of CCTX2 was cloned and expressed in the cytoplasm of E. coli BL21. The heterologous expression of the cDNA yielded a soluble recombinant protein of the expected molecular weight of 89 kDa (Figure 2B). C. elegans L1 larvae were fed with E. coli expressing CCTX2, and the development of these larvae was compared to larvae fed with E. coli containing an ’empty’ vector control or expressing the previously characterized C. cinerea defense lectin CGL2 (Butschi et al. 2010). The protein CCTX2 was found to significantly impair the development of C. elegans larvae by arresting the worms at the L1 larval stage (Figure 2, C and D). These results suggest that CCTX2, together with the nematotoxic galectins CGL1 and CGL2, is expressed as a defense effector against nematodes feeding on C. cinerea vegetative mycelium.
Figure 2.
CC1G_10077-encoded CCTX2 is nematotoxic. (A) Schematic domain representation of CCTX2 predicting two Ricin-type beta-trefoil domains potentially involved in carbohydrate binding. (B) Expression of soluble CCTX2 in E. coli. The cDNA of CC1G_10077 was cloned, and CCTX2 was expressed in the cytoplasm of E. coli BL21(DE3). Extracts of uninduced (UI) and (I) cells, as well as soluble proteins from induced cells (SP), were run on SDS-PAGE and stained with Coomassie Brilliant Blue. The predicted molecular weight of CCTX2 is 89 kDa. (C) Nematotoxicity of CCTX2. CCTX2-expressing bacteria and control bacteria containing ’empty’ vector (EV), or expressing the previously characterized nematotoxic lectin CGL2, were fed for 48 hr to L1 larvae of Caenorhabditis elegans N2 in order to assess the toxicity of CCTX2 toward nematodes. A Welch’s t-test was computed to test the significance of the differences observed between treatments and the ’empty’ vector control. ***p-value ≤ 0.001. Bars represent the standard deviation calculated for four biological replicates. (D) Phase contrast micrographs of Caenorhabditis elegans N2 fed with E. coli BL21 containing ’empty’ vector (EV), or E. coli expressing CGL2 or CCTX2.
Considerable degree of specificity between differential transcriptomes of C. cinerea challenged with different types of abiotic and biotic stress
In order to evaluate the specificity of the transcriptional response of C. cinerea to the fungivorous nematode A. avenae, we determined the differential transcriptome of C. cinerea O7 vegetative mycelium upon challenge with the bacterivorous nematode C. elegans (Figure 1A, Table 2, and Table 3). In general, only a very low number of C. cinerea genes was upregulated or downregulated in response to C. elegans compared to the buffer control (Figure 1A). Consistent with our previous results (Bleuler-Martinez et al. 2011), this treatment did not induce the expression of any of the genes upregulated in response to A. avenae, suggesting that the mere presence of live nematodes is not sufficient to trigger the transcription of loci encoding defense effector proteins against this type of antagonist in C. cinerea (Figure 1B and Table 2). Fungivorous nematodes feed on fungal hyphae using a specialized feeding apparatus (stomatostylet), which resembles the needle of a syringe and allows the nematode to pierce the cell wall and membrane of fungal hyphae and suck out their contents (Ragsdale et al. 2008). In order to mimic this type of cell hyphal damage upon nematode predation, C. cinerea vegetative mycelium was repeatedly cut with a sterile scalpel (see Materials and Methods). The expression of nematotoxic lectins was not induced by this treatment (Figure 1B and Table 2), demonstrating that hyphal damage and resulting cytoplasmic leakage are not sufficient to trigger the nematotoxic response of C. cinerea to predation by A. avenae.
In order to compare the transcriptional responses of C. cinerea vegetative mycelium to animal predation and bacterial competition, the fungus was cocultivated with the Gram-positive bacterium B. subtilis 168 and the Gram-negative bacterium E. coli Nissle 1917. In the presence of B. subtilis, 28 genes of C. cinerea were found to be differentially expressed, with 18 genes being upregulated and 10 genes being downregulated (Figure 1A). Challenge of C. cinerea with E. coli resulted in a total of 81 differentially expressed genes including 60 significantly induced and 21 significantly repressed (Figure 1A). A comparison of the different sets of genes showing differential expression in response to the various types of biotic stress applied, revealed that the number of genes induced or repressed by more than one type of biotic stress was low, and that the transcriptional response of most genes was rather stress-specific (Figure 1, B and C, Table 2, and Table 3). Interestingly, the gene sets contain different members of the same gene family, which apparently differ in their responsiveness to biotic stress. One of these gene families encodes a set of homologous proteins containing a phage lysozyme domain of the glycosylhydrolase family 24 (GH24). Of this gene family, CC1G_03042 and CC1G_03076 were specifically induced by B. subtilis and A. avenae, respectively, whereas CC1G_03047 was upregulated in response to both B. subtilis and A. avenae, and CC1G_14477 was induced by both B. subtilis and E. coli (Table 2). Other examples of proteins that are encoded by paralogous genes and differed in the regulation of their biosynthesis in response to different types of biotic stress, are two secreted proteins containing a structurally predicted carbohydrate-binding module (CBM) (encoded by CC1G_01501 induced by A. avenae and CC1G_05798 induced by B. subtilis) and three secreted, cysteine-rich proteins with structural similarity to Wnt signaling proteins (Chu et al. 2013) (encoded by CC1G_08057 induced by A. avenae, CC1G_08056 induced by E. coli and B. subtilis and CC1G_05472 induced by A. avenae and B. subtilis) (Table 2).
Discussion
Similar to plants (Spoel and Dong 2012), fungi are nonmotile organism lacking an adaptive immune system and specialized immune cells. Accordingly, both plants and fungi have evolved inducible innate defense systems that allow them to repel predators (Howe and Jander 2008; Caballero Ortiz et al. 2013; Bleuler-Martinez et al. 2011). In contrast to the well-characterized plant innate defense system, only scarce information about the fungal defense system is available.
Previous reports suggested that both filamentous asco- and basidio-mycetes are able to induce specific responses to biotic stresses (Bleuler-Martinez et al. 2011; Caballero Ortiz et al. 2013; Nutzmann et al. 2011). The specificity and the extent of this response at a genome-wide level was not investigated, however. Our results show that C. cinerea has the ability to discriminate between different abiotic (mechanical damage) and biotic stimuli, and respond to these stimuli by the induction or repression of specific gene sets. The increased production of cytoplasmic nematotoxic proteins (CGL1, CGL2, CCTX2) and secreted, potentially antibacterial proteins (family of putative phage lysozymes and/or defensin-related proteins) upon challenge of C. cinerea with the fungivorous nematode A. avenae and bacteria, respectively, suggests that fungi are able not only to distinguish between different antagonists but also to respond to these antagonists by inducing appropriate defense proteins.
It should be noted, however, that this general conclusion of our results is based on the postulated antibacterial activity of the upregulated family of phage lysozyme (GH24) domain-containing proteins, which still has to be demonstrated. Among the family members identified in this study, CC1G_03042 was specifically induced by the Gram-positive bacterium B. subtilis, while CC1G_14477 was upregulated in the presence of either B. subtilis or the Gram-negative bacterium E. coli. Surprisingly, two of the genes (CC1G_03076 and CC1G_03047) were also induced in mycelia challenged with A. avenae. As a possible explanation, this induction by a nematode may be due to contamination of A. avenae with bacteria. Arguments against this hypothesis are that the nematode was passaged over antibiotic-containing agar plates, and that challenge with C. elegans, which is raised on bacteria, did not lead to induction of these genes. Alternatively, the induction of antibacterial proteins by fungivorous nematodes may protect the hyphae from opportunistic bacteria feeding on the nutrient-rich cytoplasm leaking from the hyphae as a consequence of damage inflicted by nematode feeding.
Lysozymes are enzymes hydrolyzing the β-1,4-glycosidic bond linking monomers of N-acetylmuramic acid and N-acetylglucosamine in the bacterial peptidoglycan (Van Herreweghe and Michiels 2012). Lysozyme induction has been observed in coelomocytes from the earthworm Eisenia andrei exposed to E. coli (Joskova et al. 2009). Furthermore, the C-type lysozyme MgCLYZ from the mussel Mytilus galloprovincialis was shown to be have lytic activity against the Gram-negative bacteria Vibrio anguillarum, Enterobacter cloacae, Pseudomonas putida, Proteus mirabilis and B. aquimaris (Wang et al. 2013), indicating that some lysozymes are part of the innate defense response against Gram-negative microorganisms. Intriguingly, lysozymes of the GH24 family are only found in fungi, bacteria and bacteriophages, suggesting that the acquisition of these defense genes by fungi could be derived from a past horizontal gene transfer (HGT) event (Keeling and Palmer 2008). Accordingly, Metcalf et al. (2014) recently presented evidence for HGT of lysozymes of the GH25 family from bacteria to eukaryotes including fungi. A thorough biochemical analysis of these enzymes is needed to determine their role in fungal defense.
In contrast to the genes coding for potential antibacterial proteins, none of the genes coding for nematotoxic proteins was induced upon challenge of C. cinerea with bacteria. The specific induction of lectin-encoding genes CGL1 (CC1G_05003) and CGL2 (CC1G_05005) upon nematode predation in C. cinerea is a nice internal control of the results of this study since these lectins were previously shown to be toxic to the bacterivorous nematode C. elegans (Butschi et al. 2010), and to be induced upon challenge with A. avenae (Bleuler-Martinez et al. 2011). Interestingly, these genes are, in addition to upregulation upon nematode grazing, constitutively upregulated during fruiting body formation (Plaza et al. 2014; Boulianne et al. 2000). This dual regulation probably ensures constitutive protection of the reproductive organ and on-demand protection of the vegetative mycelium of the fungus.
One of the main results of this study is the identification of the novel nematotoxic protein CCTX2 based on its induction upon challenge with A. avenae. The protein contains several predicted Ricin B-fold domains shown to be present in lectins and protease inhibitors displaying nematotoxic or entomotoxic activity (Sabotic et al. 2012; Schubert et al. 2012). Accordingly, CCTX2 was shown to inhibit the development of C. elegans larvae, indicating that this protein acts as an effector in an inducible defense response against nematodes grazing on vegetative hyphae of C. cinerea. Induction of lectin- and toxin-encoding genes has also been observed during HI reactions between ascomycetes like Podospora and Neurospora (Bidard et al. 2013; Hutchison et al. 2009). The latter reactions were shown to be triggered by protein–protein interactions in the merged cytoplasm, and were proposed to have originally evolved as a recognition mechanism to detect pathogens (Paoletti and Saupe 2009). Despite the similarities with regard to target genes, no differential expression of C. cinerea HET and STAND domain-containing proteins was observed upon any of the treatments applied. These results suggest that HI and defense may use different regulatory pathways.
In conclusion, RNA-seq of C. cinerea challenged with fungivorous nematodes and bacteria showed that the induction of genes encoding nematotoxic lectins and potential antibacterial proteins is part of the transcriptional response of C. cinerea against predators and bacterial competitors. The specificity of this response suggests that C. cinerea has the ability to discriminate between different kinds of stimuli, and to regulate its gene expression accordingly. Furthermore, the Ricin B fold-containing protein CCTX2, induced by A. avenae challenge, was shown to be toxic to C. elegans larvae, demonstrating that this locus belongs to a transcriptional defense program triggered in C. cinerea upon nematode attack. This study supports previous reports (Bleuler-Martinez et al. 2011) suggesting the existence of elements (specific recognition and response) of inducible innate defense systems of plants and animals in C. cinerea and possibly multicellular fungi in general.
Acknowledgments
We thank Markus Aebi for his continuing interest, helpful discussions, and critical reading of the manuscript. This project was supported by the Swiss National Science Foundation Grant 31003A_130671. Illumina libraries from C. cinerea Okayama 7 were sequenced as part of the DOE Joint Genome Institute’s Community Sequencing Program ’Functional genomics in the model mushroom Coprinopsis cinerea’. The work conducted by the U.S. Department of Energy Joint Genome Institute is supported by the Office of Science of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.
Footnotes
Supporting information is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.115.023069/-/DC1
Communicating editor: A. M. Dudley
Literature Cited
- Altschul S. F., Madden T. L., Schaffer A. A., Zhang J., Zhang Z., et al. , 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25(17): 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek P., 2012. Chemical warfare or modulators of defence responses—the function of secondary metabolites in plant immunity. Curr. Opin. Plant Biol. 15(4): 407–414. [DOI] [PubMed] [Google Scholar]
- Benko-Iseppon A. M., Galdino S. L., Calsa T., Jr, Kido E. A., Tossi A., et al. , 2010. Overview on plant antimicrobial peptides. Curr. Protein Pept. Sci. 11(3): 181–188. [DOI] [PubMed] [Google Scholar]
- Bidard F., Clave C., Saupe S. J., 2013. The transcriptional response to nonself in the fungus Podospora anserina. G3 (Bethesda) 3(6): 1015–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler-Martinez S., Butschi A., Garbani M., Walti M. A., Wohlschlager T., et al. , 2011. A lectin-mediated resistance of higher fungi against predators and parasites. Mol. Ecol. 20(14): 3056–3070. [DOI] [PubMed] [Google Scholar]
- Bosch T. C., 2013. Cnidarian-microbe interactions and the origin of innate immunity in metazoans. Annu. Rev. Microbiol. 67: 499–518. [DOI] [PubMed] [Google Scholar]
- Boulianne R. P., Liu Y., Aebi M., Lu B. C., Kues U., 2000. Fruiting body development in Coprinus cinereus: regulated expression of two galectins secreted by a non-classical pathway. Microbiology 146(Pt 8): 1841–1853. [DOI] [PubMed] [Google Scholar]
- Butschi A., Titz A., Walti M. A., Olieric V., Paschinger K., et al. , 2010. Caenorhabditis elegans N-glycan core beta-galactoside confers sensitivity towards nematotoxic fungal galectin CGL2. PLoS Pathog. 6(1): e1000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero Ortiz S., Trienens M., Rohlfs M., 2013. Induced fungal resistance to insect grazing: reciprocal fitness consequences and fungal gene expression in the Drosophila–Aspergillus model system. PLoS One 8(8): e74951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M. L., Ahn V. E., Choi H. J., Daniels D. L., Nusse R., et al. , 2013. Structural studies of Wnts and identification of an LRP6 binding site. Structure 21(7): 1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll K., Chatterjee S., Scheu S., Karlovsky P., Rohlfs M., 2013 Fungal metabolic plasticity and sexual development mediate induced resistance to arthropod fungivory. Proc. Biol. Sci. 280: 20131219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel S., Jensen P. R., Fenical W., 2002. Chemical ecology of marine microbial defense. J. Chem. Ecol. 28(10): 1971–1985. [DOI] [PubMed] [Google Scholar]
- Essig A., Hofmann D., Munch D., Gayathri S., Kunzler M., et al. , 2014. Copsin, a novel peptide-based fungal antibiotic interfering with the peptidoglycan synthesis. J. Biol. Chem. 289(50): 34953–34964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira E., Cronje M. J., 2012. Selection of suitable reference genes for quantitative real-time PCR in apoptosis-induced MCF-7 breast cancer cells. Mol. Biotechnol. 50(2): 121–128. [DOI] [PubMed] [Google Scholar]
- Gallo R. L., Hooper L. V., 2012. Epithelial antimicrobial defence of the skin and intestine. Nat. Rev. Immunol. 12(7): 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason C., Huang S., Thatcher L. F., Foley R. C., Anderson C. R., et al. , 2011. Mitochondrial complex II has a key role in mitochondrial-derived reactive oxygen species influence on plant stress gene regulation and defense. Proc. Natl. Acad. Sci. USA 108(26): 10768–10773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C., Welch J., Kowbel D. J., Glass N. L., 2010. Evolution and diversity of a fungal self/nonself recognition locus. PLoS One 5(11): e14055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins P. A., Sriskandan S., 2005. Mammalian Toll-like receptors: to immunity and beyond. Clin. Exp. Immunol. 140(3): 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe G. A., Jander G., 2008. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 59: 41–66. [DOI] [PubMed] [Google Scholar]
- Huggett J., Dheda K., Bustin S., Zumla A., 2005. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 6(4): 279–284. [DOI] [PubMed] [Google Scholar]
- Hutchison E., Brown S., Tian C., Glass N. L., 2009. Transcriptional profiling and functional analysis of heterokaryon incompatibility in Neurospora crassa reveals that reactive oxygen species, but not metacaspases, are associated with programmed cell death. Microbiology 155(Pt 12): 3957–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joskova R., Silerova M., Prochazkova P., Bilej M., 2009. Identification and cloning of an invertebrate-type lysozyme from Eisenia andrei. Dev. Comp. Immunol. 33(8): 932–938. [DOI] [PubMed] [Google Scholar]
- Keeling P. J., Palmer J. D., 2008. Horizontal gene transfer in eukaryotic evolution. Nat. Rev. Genet. 9(8): 605–618. [DOI] [PubMed] [Google Scholar]
- Kelley L. A., Mezulis S., Yates C. M., Wass M. N., Sternberg M. J., 2015. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10(6): 845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kues U., 2000. Life history and developmental processes in the basidiomycete Coprinus cinereus. Microbiol. Mol. Biol. Rev. 64(2): 316–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzler M., Bleuler-Martinez S., Butschi A., Garbani M., Luthy P., et al. , 2010. Biotoxicity assays for fruiting body lectins and other cytoplasmic proteins. Methods Enzymol. 480: 141–150. [DOI] [PubMed] [Google Scholar]
- Langnaese K., John R., Schweizer H., Ebmeyer U., Keilhoff G., 2008. Selection of reference genes for quantitative real-time PCR in a rat asphyxial cardiac arrest model. BMC Mol. Biol. 9: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Durbin R., 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25(14): 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Wang X., Wang H. D., Wu J., Ren J., et al. , 2012. Escherichia coli noncoding RNAs can affect gene expression and physiology of Caenorhabditis elegans. Nat. Commun. 3: 1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Williams C. E., Nemacheck J. A., Wang H., Subramanyam S., et al. , 2010. Reactive oxygen species are involved in plant defense against a gall midge. Plant Physiol. 152(2): 985–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathioni S. M., Patel N., Riddick B., Sweigard J. A., Czymmek K. J., et al. , 2013. Transcriptomics of the rice blast fungus Magnaporthe oryzae in response to the bacterial antagonist Lysobacter enzymogenes reveals candidate fungal defense response genes. PLoS One 8(10): e76487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf J. A., Funkhouser-Jones L. J., Brileya K., Reysenbach A. L., Bordenstein S. R., 2014. Antibacterial gene transfer across the tree of life. eLife 3 10.7554/eLife.04266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. E., Muller I. M., 2003. Origin of the metazoan immune system: identification of the molecules and their functions in sponges. Integr. Comp. Biol. 43(2): 281–292. [DOI] [PubMed] [Google Scholar]
- Nathan C., Cunningham-Bussel A., 2013. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 13(5): 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger T., Brunner F., Kemmerling B., Piater L., 2004. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol. Rev. 198: 249–266. [DOI] [PubMed] [Google Scholar]
- Nutzmann H. W., Reyes-Dominguez Y., Scherlach K., Schroeckh V., Horn F., et al. , 2011. Bacteria-induced natural product formation in the fungus Aspergillus nidulans requires Saga/Ada-mediated histone acetylation. Proc. Natl. Acad. Sci. USA 108(34): 14282–14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti M., Saupe S. J., 2009. Fungal incompatibility: evolutionary origin in pathogen defense? BioEssays 31(11): 1201–1210. [DOI] [PubMed] [Google Scholar]
- Pedley K. F., Martin G. B., 2005. Role of mitogen-activated protein kinases in plant immunity. Curr. Opin. Plant Biol. 8(5): 541–547. [DOI] [PubMed] [Google Scholar]
- Petersen T. N., Brunak S., von Heijne G., Nielsen H., 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8(10): 785–786. [DOI] [PubMed] [Google Scholar]
- Plaza D. F., Lin C. W., van der Velden N. S., Aebi M., Kunzler M., 2014. Comparative transcriptomics of the model mushroom Coprinopsis cinerea reveals tissue-specific armories and a conserved circuitry for sexual development. BMC Genomics 15: 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior K., Hautefort I., Hinton J. C., Richardson D. J., Rowley G., 2009. All stressed out. Salmonella pathogenesis and reactive nitrogen species. Adv. Microb. Physiol. 56: 1–28. [DOI] [PubMed] [Google Scholar]
- Ragsdale E. J., Crum J., Ellisman M. H., Baldwin J. G., 2008. Three-dimensional reconstruction of the stomatostylet and anterior epidermis in the nematode Aphelenchus avenae (Nematoda: Aphelenchidae) with implications for the evolution of plant parasitism. J. Morphol. 269(10): 1181–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. D., McCarthy D. J., Smyth G. K., 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26(1): 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfs M., Churchill A. C., 2011. Fungal secondary metabolites as modulators of interactions with insects and other arthropods. Fungal Genet. Biol. 48(1): 23–34. [DOI] [PubMed] [Google Scholar]
- Sabotic J., Bleuler-Martinez S., Renko M., Avanzo Caglic P., Kallert S., et al. , 2012. Structural basis of trypsin inhibition and entomotoxicity of cospin, serine protease inhibitor involved in defense of Coprinopsis cinerea fruiting bodies. J. Biol. Chem. 287(6): 3898–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schefe J. H., Lehmann K. E., Buschmann I. R., Unger T., Funke-Kaiser H., 2006. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression’s CT difference” formula. J Mol Med (Berl) 84(11): 901–910. [DOI] [PubMed] [Google Scholar]
- Schroeckh V., Scherlach K., Nutzmann H. W., Shelest E., Schmidt-Heck W., et al. , 2009. Intimate bacterial-fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 106(34): 14558–14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Bleuler-Martinez S., Butschi A., Walti M. A., Egloff P., et al. , 2012. Plasticity of the beta-trefoil protein fold in the recognition and control of invertebrate predators and parasites by a fungal defence system. PLoS Pathog. 8(5): e1002706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira E. D., Alves-Ferreira M., Guimaraes L. A., da Silva F. R., Carneiro V. T., 2009. Selection of reference genes for quantitative real-time PCR expression studies in the apomictic and sexual grass Brachiaria brizantha. BMC Plant Biol. 9: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skanta F., Roubalova R., Dvorak J., Prochazkova P., Bilej M., 2013. Molecular cloning and expression of TLR in the Eisenia andrei earthworm. Dev. Comp. Immunol. 41(4): 694–702. [DOI] [PubMed] [Google Scholar]
- Sonnhammer E. L., von Heijne G., Krogh A., 1998. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 6: 175–182. [PubMed] [Google Scholar]
- Soustre I., Letourneux Y., Karst F., 1996. Characterization of the Saccharomyces cerevisiae RTA1 gene involved in 7-aminocholesterol resistance. Curr. Genet. 30(2): 121–125. [DOI] [PubMed] [Google Scholar]
- Spiteller P., 2008. Chemical defence strategies of higher fungi. Chemistry 14(30): 9100–9110. [DOI] [PubMed] [Google Scholar]
- Spoel S. H., Dong X., 2012. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 12(2): 89–100. [DOI] [PubMed] [Google Scholar]
- Srivastava M., Simakov O., Chapman J., Fahey B., Gauthier M. E., et al. , 2010. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature 466(7307): 720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stajich J. E., Wilke S. K., Ahren D., Au C. H., Birren B. W., et al. , 2010. Insights into evolution of multicellular fungi from the assembled chromosomes of the mushroom Coprinopsis cinerea (Coprinus cinereus). Proc. Natl. Acad. Sci. USA 107(26): 11889–11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staniland L. N., 1954. A modification of the Baermann funnel technique for the collection of nematodes from plant material. J. Helminthol. 28(1–2): 115–117. [DOI] [PubMed] [Google Scholar]
- Stiernagle T., 2006. Maintenance of C. elegans. (February 11, 2006), WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.101.1, http//www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennessen J. A., 2005. Molecular evolution of animal antimicrobial peptides: widespread moderate positive selection. J. Evol. Biol. 18(6): 1387–1394. [DOI] [PubMed] [Google Scholar]
- Van Herreweghe J. M., Michiels C. W., 2012. Invertebrate lysozymes: diversity and distribution, molecular mechanism and in vivo function. J. Biosci. 37(2): 327–348. [DOI] [PubMed] [Google Scholar]
- Vandenborre G., Smagghe G., Van Damme E. J., 2011. Plant lectins as defense proteins against phytophagous insects. Phytochemistry 72(13): 1538–1550. [DOI] [PubMed] [Google Scholar]
- Walti M. A., Walser P. J., Thore S., Grunler A., Bednar M., et al. , 2008. Structural basis for chitotetraose coordination by CGL3, a novel galectin-related protein from Coprinopsis cinerea. J. Mol. Biol. 379(1): 146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton J. D., Hallen-Adams H. E., Luo H., 2010. Ribosomal biosynthesis of the cyclic peptide toxins of Amanita mushrooms. Biopolymers 94(5): 659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H., Yuan W., Ruan M., Ye Q., Wang R., et al. , 2011. Identification of reference genes for reverse transcription quantitative real-time PCR normalization in pepper (Capsicum annuum L.). Biochem. Biophys. Res. Commun. 416(1–2): 24–30. [DOI] [PubMed] [Google Scholar]
- Wang L., Ligoxygakis P., 2006. Pathogen recognition and signalling in the Drosophila innate immune response. Immunobiology 211(4): 251–261. [DOI] [PubMed] [Google Scholar]
- Wang Q., Wang C., Mu C., Wu H., Zhang L., et al. , 2013. A novel C-type lysozyme from Mytilus galloprovincialis: insight into innate immunity and molecular evolution of invertebrate C-type lysozymes. PLoS One 8(6): e67469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino T. P., Dinguirard N., Kunert J., Hokke C. H., 2008. Molecular and functional characterization of a tandem-repeat galectin from the freshwater snail Biomphalaria glabrata, intermediate host of the human blood fluke Schistosoma mansoni. Gene 411(1–2): 46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Zmasek C. M., Godzik A., 2010. Domain architecture evolution of pattern-recognition receptors. Immunogenetics 62(5): 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C., 2008. Pattern-recognition receptors in plant innate immunity. Curr. Opin. Immunol. 20(1): 10–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Reference for data available in public repository: Illumina mapped reads were deposited in the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress/) under the accession number E-MTAB-2763.