Abstract
Purpose
To examine the expression of putative limbal epithelial stem cell (LESC) markers and wound healing rates in primary healthy and diabetic human limbal epithelial cells (LECs) cultured on different substrata.
Methods
Primary limbal epithelial cells were isolated from human autopsy corneas and discarded corneoscleral rims with dispase II treatment. LECs were cultured in EpiLife medium on human amniotic membrane (AM) denuded with mild alkali treatment, on plastic dishes and on glass slides coated with a mixture of human fibronectin, collagen type IV, and laminin (FCL). Cultured LECs were fixed in p-formaldehyde or methanol, and the expression of the putative LESC markers ΔNp63α, PAX6, and ABCG2 and keratins K12, K15, and K17 was examined with immunostaining. Wound healing was evaluated in scratch wound assay in LECs cultured on FCL-coated plates 20 h after wounding.
Results
LECs cultured on denuded AM expressed ΔNp63α, PAX6 (both showed nuclear staining), K15, K17 (cytoskeleton staining), and ABCG2 (cytoplasmic and/or plasma membrane staining). LECs cultured on FCL-coated slides also expressed these markers, whereas no expression was detected for differentiated corneal epithelial cell marker K12. Decreased expression of LESC markers was observed in diabetic LECs compared to healthy LECs cultured on the FCL-coated slides. This reduction was most prominent for K15 and K17. Diabetic LECs were found to heal scratch wounds slower than healthy cells in accordance with previous results in corneal organ cultures.
Conclusions
Healthy human LECs cultured either on AM or FCL-coated slides preserved LESC marker expression. The observed reduction in LESC marker expression and slower wound healing in cultured diabetic LECs are in line with our earlier reports and may account for diabetic LESC dysfunction and clinically observed impaired corneal epithelial wound healing.
Introduction
Corneal blindness is the second most common devastating eye disorder, affecting more than 6 million people worldwide [1,2]. In pathological conditions associated with diabetes mellitus (DM), the cornea is affected by such complications as neurotrophic corneal ulcers, loss of corneal sensation, keratitis, and a characteristic epithelial dystrophy called diabetic keratopathy [3-8]. The diabetic cornea displays reduced numbers of hemidesmosomes [3,4], edema [9,10], altered growth factor signaling [5], basement membrane abnormalities [11], and delayed wound healing leading to persistent epithelial defects [12,13].
In humans, corneal epithelial wound healing and renewal mainly depend on stem cells that reside in the basal epithelial layer of the corneoscleral junction (the limbus) [14-19]. Defects of these limbal epithelial stem cells (LESCs) may have serious adverse effects on corneal function such as conjunctival in-growth and neovascularization of the corneal stroma, which often lead to corneal opacity and vision loss [20-22]. Dysfunction of the limbal niche and its resident LESCs could be responsible for various abnormalities in the diabetic corneal epithelium due to their role in epithelial renewal and wound healing.
Previously, we found that overexpression of hepatocyte growth factor (HGF) receptor tyrosine kinase c-met and/or silencing of matrix metalloproteinase-10 (MMP-10) and cathepsin F in organ-cultured diabetic corneas could normalize epithelial marker expression, as well as wound healing time [23-25]. Additionally, we examined various putative stem cell markers in ex vivo diabetic and healthy limbal epithelia and showed that the immunostaining patterns of several stem cell markers were altered in the diabetic limbus [26], which could potentially be corrected with gene therapy [24,25]. These data suggest that the limbal compartment may play an important role in diabetic corneal alterations that can be ameliorated with gene therapy.
Therefore, it is important to confirm whether cultured limbal epithelial cells preserve stem marker expression abnormalities described earlier in organ-cultured corneas and thus can be used to study diabetes-associated corneal alterations. If this assumption proved to be correct, cultured diabetic cells could be used for gene therapy for potential transplantation to diseased corneas in cases of severe diabetic LESC dysfunction exacerbated by diabetic corneal neuropathy [8,13]. To this end, we examined limbal epithelial stem marker expression and wound healing in primary healthy and diabetic LECs cultured on different substrata. We found that human LECs cultured either on denuded human amniotic membrane (AM) or extracellular matrix-coated dishes preserved LESC marker expression patterns in healthy and diabetic cells. Reduced LESC marker expression and wound healing rates were observed in the cultured diabetic LECs. This is in agreement with our earlier reports on alterations in human diabetic corneas, which may account for diabetic LESC dysfunction and impaired corneal epithelial wound healing.
Methods
LEC isolation
Primary LEC cultures were prepared from human healthy and diabetic postmortem corneas and whole globes purchased from the National Disease Research Interchange that operates under National Institutes of Health oversight (IRB exempt protocol EX-1055). The study adhered to the Declaration of Helsinki and the ARVO statement on human subjects. Discarded healthy donor corneoscleral rims were provided by Drs. Rabinowitz and Maguen after corneal transplantation, under an approved Cedars-Sinai Medical Center IRB protocol (Pro00019393). Amniotic membranes were obtained from human placentas, after written informed consent from the prospective mothers, upon their Cesarean section deliveries, under an approved IRB protocol (Pro00019230).
Healthy LEC cultures were prepared from four donor corneas or corneoscleral rims (age range from 54 to 86 years), whereas diabetic LEC cultures were prepared from three donors with type II diabetes and one donor with type I diabetes (age range from 54 to 81 years). Donor characteristics are detailed in Table 1.
Table 1. Donor characteristics.
| Case number | Age, years | Sex | DM type | Duration, years | Cause of death |
|---|---|---|---|---|---|
| NL 13–10 |
82 |
F |
|
|
Cardiopulmonary arrest |
| NL 14–01 |
86 |
F |
|
|
Chronic obstructive pulmonary disease |
| NL 14–02 |
65 |
M |
|
|
Gunshot wound |
| NL 15–03 |
54 |
M |
|
|
Gunshot wound |
| DR 13–15 |
54 |
M |
IDDM |
46 |
Acute renal failure |
| DM 14–06 |
72 |
M |
NIDDM |
30 |
End stage renal disease |
| DM 14–41 |
67 |
M |
NIDDM |
21 |
Myocardial infarction |
| DM 15–04 | 81 | M | NIDDM | 20 | Congestive heart and renal failure |
Primary limbal epithelial cells were isolated from the excised human corneas and corneoscleral rims after dispase II treatment (2.4 U/ml, Roche Life Science, Indianapolis, IN) for 2 h at 37 °C [27]. Before the enzymatic treatments, the corneal endothelium and remnants of the adhering iris were removed with a sterile cotton swab. The limbal epithelial cells eased off the rims were dissociated with 0.25% trypsin – 0.02% EDTA (Thermo Fisher Scientific, Waltham, MA) for 30 min at room temperature [27,28]. LECs were cultured in EpiLife medium with 60 μM Ca2+ containing N2, B27, and human keratinocyte growth supplement (HKGS; Thermo Fisher Scientific), and 10 ng/ml epidermal growth factor (EGF). Cells were seeded on human amniotic membrane (AM) denuded by brief rubbing of the epithelial side in 0.25 N NaOH [28], and on plastic dishes or glass slides coated with a mixture of human fibronectin (BD Biosciences, San Diego, CA), collagen type IV, and laminin (FCL; both from Sigma-Aldrich, St. Louis, MO) at 0.5–1 μg/cm2 [29].
Immunostaining
Individual LEC cultures from three healthy and three diabetic corneas and corneoscleral rims were fixed in p-formaldehyde (1%, 10 min at 4 °C) followed by permeabilization in 0.25% Triton X-100 for 5 min at room temperature or in ice-cold methanol (10 min at 4 °C). The expression of putative LESC markers ΔNp63α and PAX6, keratins K12, K15, and K17, and ATP-binding cassette transporter ABCG2 was examined with immunostaining. Additionally, cultures were stained for differentiated corneal epithelial marker K12. The following primary antibodies were used: goat anti-ΔNp63α (sc-8609), goat anti-K12 (sc-17099), mouse anti-K15 (sc-47697), mouse anti-K17 (sc-58726; all from Santa Cruz Biotechnology, Dallas, TX), mouse anti-ABCG2 (MAB4155, Millipore, Billerica, MA), and rabbit anti-PAX6 (PRB-278P, BioLegend, San Diego, CA). Secondary antibodies conjugated either with fluorescein isothiocyanate (FITC) or tetramethylrhodamine (TRITC) were from Jackson ImmunoResearch Laboratories (West Grove, PA). Cell nuclei were visualized with 4',6-diamidino-2-phenylindole (DAPI) counterstaining.
Wound healing assay
Scratch wounds were created in four healthy and four diabetic subconfluent LEC cultures using the 200-μl pipette tip, after which the dislodged cells were washed out with fresh medium. The scratch wound experiments were performed in triplicate. Cultures in basal EpiLife without supplements or growth factors healed slowly. To stimulate healing, 1 or 4 ng/ml EGF was added to the medium. EGF was chosen because signaling through its receptor is a major mediator of corneal epithelial wound healing [30,31]. The doses were chosen to make scratch wounds in the healthy cultures heal at 20 h for approximately 90%. Twenty hours later, wound healing was quantified by measuring the wound areas on digitized photographs using ImageJ software. The results of nine experiments were analyzed with the Student t test using Prism5 statistical software (GraphPad, San Diego, CA), with p<0.05 considered to be significant.
Results
Stem cell marker expression in LECs cultured on denuded AM
The AM is thought to be similar in structure and composition to the limbal epithelial niche basement membrane and is non-immunogenic [32,33]. For these reasons, denuded AM has been widely used for culture, expansion, and transplantation of LESCs [34-42]. To grow LECs for immunostaining analyses, we used a simple and effective method of denuding human AM with mild alkaline treatment that we have recently developed [28].
Most cells in healthy LEC cultures on AM were positive for several putative stem cell markers similar to the data obtained for limbal immunostaining of organ-cultured human corneas. Representative staining patterns for ΔNp63α, K17, and ABCG2 are shown in Figure 1. Comparison of marker patterns between healthy and diabetic LEC cultures on AM revealed a consistent decrease in staining of diabetic cells, as shown for ΔNp63α (Figure 2). This was also consistent with such a decrease observed in the ex vivo and in the organ-cultured corneas [24-26]. Differentiated corneal epithelial marker K12 was not found either in healthy or diabetic cultures.
Figure 1.
Expression of putative stem cell markers in healthy limbal epithelial cell cultures on amniotic membrane. The healthy (NL) cells are generally positive for ΔNp63α (A, B) and K17 (C, D). Many cells are also positive for ABCG2 (E, F). The right panels have 4',6-diamidino-2-phenylindole (DAPI) nuclear counterstaining. As ΔNp63α is also nuclear, the corresponding DAPI panel is shown separately. Bars=20 μm.
Figure 2.
Decreased expression of putative stem cell marker ΔNp63α in diabetic as compared to healthy limbal epithelial cell cultures on amniotic membrane. Images in A and B represent healthy (NL) and diabetic (DM) LEC and were obtained using the same exposure time. C and D show overlay with 4',6-diamidino-2-phenylindole (DAPI) nuclear counterstaining of A and B, respectively. Bar=20 μm.
Stem cell marker expression in healthy and diabetic LECs cultured on FCL-coated slides
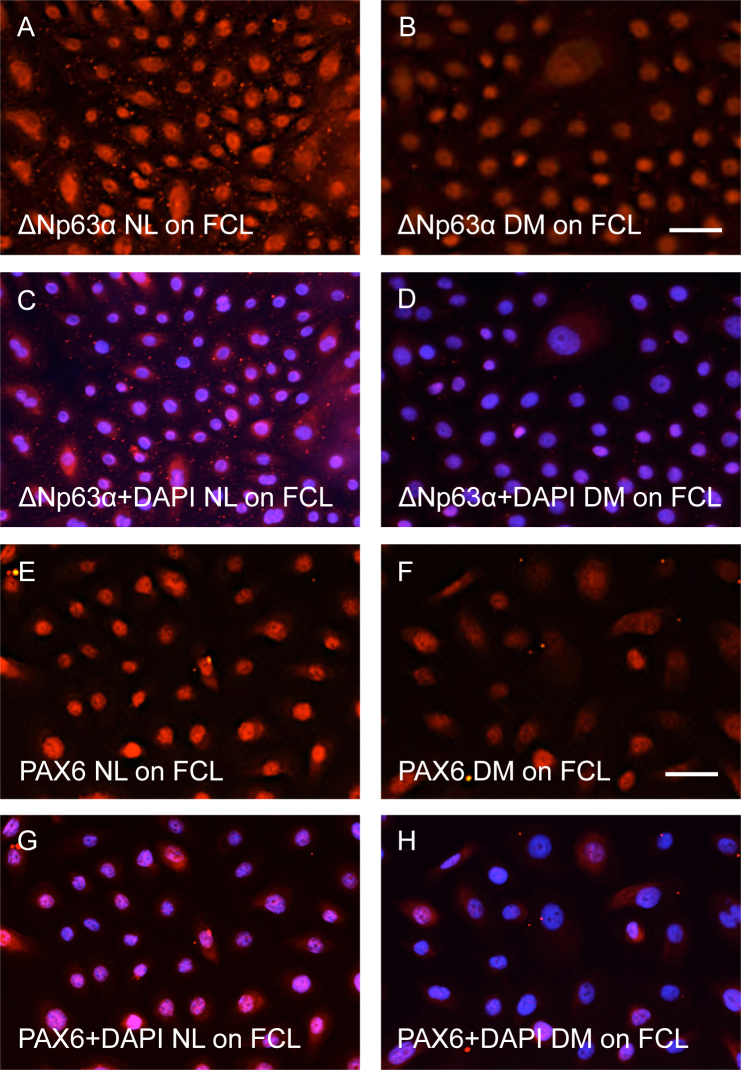
Another substratum tested for LEC growth was glass coated with basement membrane proteins found in the corneal epithelial basement membrane [43-45], that is, FCL. Similar to the results obtained for the LECs cultured on human AM, we observed positive and fairly strong immunostaining for ΔNp63α in the nuclei of healthy cells (Figure 3A), which was consistently weaker in diabetic cells (Figure 3B). The comparisons were made for healthy and diabetic cells immunostained in the same experiment, with images taken at the same exposure time. Essentially the same data were obtained for another marker, PAX6, which is also the main transcription factor defining eye development (Figure 3E,F). Other putative LESC markers, including membrane transporter ABCG2 (Figure 4) and intermediate filament components K15 and K17 (Figure 5), also showed marked reduction in staining intensity in the diabetic LECs compared to healthy LECs. K12 was not observed similar to the cultures on AM. Therefore, decreased stem cell marker staining previously observed in diabetic corneas persisted in cultured diabetic limbal cells.
Figure 3.
Expression of ΔNp63α and PAX6. Both markers are mainly localized in the nuclei of limbal epithelial cells (LECs) cultured on fibronectin, collagen type IV, and laminin (FCL)-coated slides. The staining is reduced in diabetic (DM) LECs compared to the healthy (NL) LECs. Images in A and B, or in E and F, were obtained using the same exposure time. C, D, same pictures as in A and B, and G, H are the same as E and F, respectively, but with 4',6-diamidino-2-phenylindole (DAPI) nuclear counterstain. Bars=20 μm.
Figure 4.
Stem cell marker ABCG2 expression is decreased in the cytoplasm of diabetic limbal epithelial cells as compared to their healthy counterparts. Images in A and B represent healthy (NL) and diabetic (DM) LEC and were obtained using the same exposure time. C, D, same pictures as in A and B, respectively, but with 4',6-diamidino-2-phenylindole (DAPI) nuclear counterstain. Cells were cultured on fibronectin, collagen type IV, and laminin (FCL). Bar=20 μm.
Figure 5.
Both keratins 15 and K17 are expressed at substantially lower levels in limbal epithelial cells isolated from diabetic corneas than from healthy corneas. Immunostaining for K15 (A, B) and K17 (E, F) is decreased in diabetic (DM) LEC as compared to the healthy (NL) ones. Images in A and B, or in E and F were obtained using the same exposure time. C, D, same pictures as in A and B, and G, H are the same as E and F, respectively, but with 4',6-diamidino-2-phenylindole (DAPI) nuclear counterstain. Cells were cultured on fibronectin, collagen type IV, and laminin (FCL). Bars=20 μm.
Wound healing in primary LECs cultured from healthy and diabetic corneas
To evaluate whether cell migration was affected in cultured primary diabetic LECs, scratch wound assay was performed using cells cultured on FCL-coated plates. To increase healing, basal EpiLife medium without growth factors was supplemented with 1 or 4 ng/ml EGF that is known to stimulate corneal cell migration. At both EGF concentrations, the rate of the wound closure of diabetic cells was decreased by 26% and 15% (at 1 and 4 ng/mL EGF, respectively) compared to their healthy counterparts (Figure 6). In both conditions, this difference was significant.
Figure 6.
Wound healing rate is decreased in diabetic limbal epithelial cells compared to healthy ones as revealed by scratch wound assay in the presence of 1 and 4 ng/ml epidermal growth factor. A: Wound healing in the presence of 1 and 4 ng/ml EGF was evaluated by measuring wound areas for healthy (NL) and diabetic (DM) LEC using ImageJ software and presented as percentage of wound area for healthy (NL) and diabetic (DM) LEC using ImageJ software and presented as percentage of wound area coverage ± standard error of mean. * p<0.05; *** p<0.0001 versus healthy controls. B: Representative images of healthy and diabetic limbal epithelial cells (LECs) after 20 h of wound healing in the medium with 1 ng/ml epidermal growth factor (EGF). Vertical lines demarcate the borders of the original wound. Bar=500 µm.
Discussion
Therapeutic applications of stem cells currently comprise a particularly active field of research that could potentially transform medical practice. Adult stem cells have specific characteristics such as self-renewal and unlimited proliferative capacity, as well as an ability to differentiate into various specialized cell types [46,47]. Significant success in this area was achieved in part due to the development of methods for enriching and cultivating tissue stem cells based on the expression of specific surface antigens and the lack of terminal differentiation markers [48-51].
In the human cornea, epithelial stem cells are located at the basal epithelial cell layer within the limbus, the narrow region between the opaque sclera and the clear cornea [14,18,19,52]. These cells are located in a specialized niche known as the palisades of Vogt in humans, which may possess morphological and functional features crucial for maintaining stemness, protecting stem cells from environmental stresses, and conveying access to molecular signals from the adjacent vasculature [53,54]. Together with adjacent stromal cells, the limbal basement membrane that has a special composition different from the central cornea [43-45] may play a significant role in the maintenance of stem cells.
As part of the AM, the human amniotic epithelial basement membrane is quite similar to the limbal basement membrane in composition [28,32,33]. In addition, the AM is antiangiogenic, antibacterial, and barely immunogenic, contains important growth factors, and promotes wound healing [35-38,55-57]. The AM is used clinically for LESC transplantation either intact AM or more frequently after the removal of amniotic epithelial cells (denuded AM) for better LESC adhesion [42]. The denuded AM enhances LESC proliferation and clonogenicity [34,36,38-41]. Recently, we developed a simple reproducible method for denuding the AM based on the ability of mild alkaline solutions to dissolve cells [58,59]. This method proved to be suitable for culturing LESCs and limbal-derived induced pluripotent stem cells [28].
We have previously shown that diabetic human organ-cultured corneas preserved alterations of specific marker expression and wound healing observed in ex vivo diabetic corneas [60]. Diabetic limbal epithelial cells also had altered patterns of putative stem cell marker expression ex vivo and in organ-cultured corneas [24-26]. Gene therapy with downregulation of MMP-10 and cathepsin F, and upregulation of HGF receptor c-met proto-oncogene was able to normalize diabetic corneal wound healing and diabetic and stem cell marker expression, which shows potential for improving corneal function affected in diabetes [24-26]. In severe cases of diabetic retinopathy with significantly compromised LESCs resulting in, for example, recurrent erosions or ulcers, it might be beneficial to transplant healthy (allogeneic) or gene therapy-normalized (autologous) limbal cells to provide functional stem cells capable of normal renewal of the corneal epithelium. This would necessitate expansion of LECs in culture on appropriate substrata and subsequent transplantation onto affected corneas. To this end, it was important to determine whether cultured LECs would be similar to their ex vivo counterparts in terms of stem cell marker expression and wound healing.
The analysis of healthy and diabetic LESC-enriched cultures performed here constitutes a novel approach compared to our previous work with whole corneas, as cultured LECs are often used for transplantation to diseased corneas [20,41,42]. The validation of diabetic LECs as dysfunctional was important for the purposes of subsequent gene therapy and the possibility of transplantation in severe cases of disease. We have performed this validation and found for the first time that primary cultured human LECs preserve the expression of putative stem cell markers ΔNp63α, PAX6, and ABCG2 and keratins K15 and K17, previously shown to be expressed by limbal stem cells in ex vivo corneas and in corneal organ cultures [24-26]. Another novel finding was that the diabetic LECs on the AM continued to display decreased immunostaining for these markers. The same reduced LESC marker expression was observed in LECs cultured on FCL-coated slides, making it possible to further study wound healing on this substratum. Similar to the whole corneas, LECs from diabetic donors displayed about 20% slower wound healing compared to healthy cells. These alterations may account for diabetic LESC dysfunction leading to impaired corneal epithelial wound healing.
Transplantation of culture-expanded LECs enriched in LESCs has been used clinically in several countries in cases of LESC deficiency [61,62]. The cells are usually transplanted on fibrin glue or human AM. This method could potentially be used to correct LESC abnormalities in severe cases of advanced diabetic keratopathy as well. The present data suggest that just activating the growth of diabetic LECs by culture expansion would not normalize their phenotype, possibly due to metabolic epigenetic memory [63]. Gene therapy, which has been successful in whole corneas [23-26], could also be advantageous for cultured diabetic stem cell normalization and subsequent autologous transplantation on AM. Experiments are under way to correct diabetic abnormalities in cultured diabetic LECs using specific gene therapy.
Acknowledgments
The study was supported by NIH (R01EY013431 and R01EY023429) and a grant from the Board of Governors Regenerative Medicine Institute, Cedars-Sinai Medical Center.
References
- 1.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: A global perspective. Bull World Health Organ. 2001;79:214–21. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11285665&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 2.Sinha R, Sharma N, Vajpayee RB. Corneal blindness – present status: A look at the leading causes of and current treatment success with blindness worldwide. Cataract Refract Surg Today. 2005; 59–61. [Google Scholar]
- 3.Cavallerano J. Ocular manifestations of diabetes mellitus. Optom Clin. 1992;2:93–116. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1504481&dopt=Abstract [PubMed] [Google Scholar]
- 4.Saini JS, Khandalavla B. Corneal epithelial fragility in diabetes mellitus. Can J Ophthalmol. 1995;30:142–6. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7627899&dopt=Abstract [PubMed] [Google Scholar]
- 5.Sánchez-Thorin JC. The cornea in diabetes mellitus. Int Ophthalmol Clin. 1998;38:19–36. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9604736&dopt=Abstract [PubMed] [Google Scholar]
- 6.Negi A, Vernon SA. An overview of the eye in diabetes. J R Soc Med. 2003;96:266–72. doi: 10.1258/jrsm.96.6.266. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12782689&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito J, Enoki M, Hara M, Morishige N, Chikama T, Nishida T. Correlation of corneal sensation, but not of basal or reflex tear secretion, with the stage of diabetic retinopathy. Cornea. 2003;22:15–8. doi: 10.1097/00003226-200301000-00004. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12502941&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 8.Bikbova G, Oshitari T, Tawada A, Yamamoto S. Corneal changes in diabetes mellitus. Curr Diabetes Rev. 2012;8:294–302. doi: 10.2174/157339912800840479. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22587515&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 9.Gekka M, Miyata K, Nagai Y, Nemoto S, Sameshima T, Tanabe T, Maruoka S, Nakahara M, Kato S, Amano S. Corneal epithelial barrier function in diabetic patients. Cornea. 2004;23:35–7. doi: 10.1097/00003226-200401000-00006. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14701955&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 10.Ben Osman N, Jeddi A, Sebai L, Zghal I, Kaoueche M, Gaigi S, Ayed S. The cornea of diabetics. J Fr Ophtalmol. 1995;18:120–3. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7738303&dopt=Abstract [PubMed] [Google Scholar]
- 11.Ljubimov AV, Huang ZS, Huang GH, Burgeson RE, Gullberg D, Miner JH, Ninomiya Y, Sado Y, Kenney MC. Human corneal epithelial basement membrane and integrin alterations in diabetes and diabetic retinopathy. J Histochem Cytochem. 1998;46:1033–41. doi: 10.1177/002215549804600907. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9705969&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 12.Inoue K, Kato S, Ohara C, Numaga J, Amano S, Oshika T. Ocular and systemic factors relevant to diabetic keratoepitheliopathy. Cornea. 2001;20:798–801. doi: 10.1097/00003226-200111000-00004. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11685054&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 13.Calvo-Maroto AM, Perez-Cambrodí RJ, Albarán-Diego C, Pons A, Cerviño A. Optical quality of the diabetic eye: a review. Eye (Lond) 2014;28:1271–80. doi: 10.1038/eye.2014.176. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25125072&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2424919&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotsarelis G, Cheng SZ, Dong G, Sun T-T, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–9. doi: 10.1016/0092-8674(89)90958-6. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2702690&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 16.Tseng SC. Concept and application of limbal stem cells. Eye (Lond) 1989;3:141–57. doi: 10.1038/eye.1989.22. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2695347&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 17.Joe AW, Yeung SN. Concise review: identifying limbal stem cells: classical concepts and new challenges. Stem Cells Transl Med. 2014;3:318–22. doi: 10.5966/sctm.2013-0137. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24327757&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Girolamo N. Moving epithelia: Tracking the fate of mammalian limbal epithelial stem cells. Prog Retin Eye Res. 2015;48:203–25. doi: 10.1016/j.preteyeres.2015.04.002. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25916944&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 19.Amitai-Lange A, Altshuler A, Bubley J, Dbayat N, Tiosano B, Shalom-Feuerstein R. Lineage tracing of stem and progenitor cells of the murine corneal epithelium. Stem Cells. 2015;33:230–9. doi: 10.1002/stem.1840. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25187087&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 20.Pellegrini G, Rama P, De Luca M. Vision from the right stem. Trends Mol Med. 2011;17:1–7. doi: 10.1016/j.molmed.2010.10.003. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21075055&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 21.Hatch KM, Dana R. The structure and function of the limbal stem cell and the disease states associated with limbal stem cell deficiency. Int Ophthalmol Clin. 2009;49:43–52. doi: 10.1097/IIO.0b013e3181924e54. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19125063&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Kinoshita S. New hopes and strategies for the treatment of severe ocular surface disease. Curr Opin Ophthalmol. 2011;22:274–8. doi: 10.1097/ICU.0b013e3283477d4d. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21537182&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 23.Saghizadeh M, Kramerov AA, Yu FS, Castro MG, Ljubimov AV. Normalization of wound healing and diabetic markers in organ cultured human diabetic corneas by adenoviral delivery of c-met gene. Invest Ophthalmol Vis Sci. 2010;51:1970–80. doi: 10.1167/iovs.09-4569. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19933191&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saghizadeh M, Epifantseva I, Hemmati DM, Ghiam CA, Brunken WJ, Ljubimov AV. Enhanced wound healing, kinase and stem cell marker expression in diabetic organ-cultured human corneas upon MMP-10 and cathepsin F gene silencing. Invest Ophthalmol Vis Sci. 2013;54:8172–80. doi: 10.1167/iovs.13-13233. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24255036&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saghizadeh M, Dib CM, Brunken WJ, Ljubimov AV. Normalization of wound healing and stem cell marker patterns in organ-cultured human diabetic corneas by gene therapy of limbal cells. Exp Eye Res. 2014;129:66–73. doi: 10.1016/j.exer.2014.10.022. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25446319&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saghizadeh M, Soleymani S, Harounian A, Bhakta B, Troyanovsky SM, Brunken WJ, Pellegrini G, Ljubimov AV. Alterations of epithelial stem cell marker patterns in human diabetic corneas and effects of c-met gene therapy. Mol Vis. 2011; 17:2177–90 <http://www.molvis.org/molvis/v17/a236>. [PMC free article] [PubMed]
- 27.Nakatsu MN, Ding Z, Ng MY, Truong TT, Yu F, Deng SX. Wnt/β-catenin signaling regulates proliferation of human cornea epithelial stem/progenitor cells. Invest Ophthalmol Vis Sci. 2011;52:4734–41. doi: 10.1167/iovs.10-6486. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21357396&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saghizadeh M, Winkler MA, Kramerov AA, Hemmati DM, Ghiam CA, Dimitrijevich SD, Sareen D, Ornelas L, Ghiasi H, Brunken WJ, Maguen E, Rabinowitz YS, Svendsen CN, Jirsova K, Ljubimov AV. A simple alkaline method for decellularizing human amniotic membrane for cell culture. PLoS ONE. 2013;8:e79632. doi: 10.1371/journal.pone.0079632. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24236148&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blazejewska EA, Schlötzer-Schrehardt U, Zenkel M, Bachmann B, Chankiewitz E, Jacobi C, Kruse FE. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells. 2009;27:642–52. doi: 10.1634/stemcells.2008-0721. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19074417&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu KP, Li Y, Ljubimov AV, Yu FS. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes. 2009;58:1077–85. doi: 10.2337/db08-0997. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19188434&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res. 2015;49:17–45. doi: 10.1016/j.preteyeres.2015.07.002. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26197361&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea. 1999;18:73–9. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9894941&dopt=Abstract [PubMed] [Google Scholar]
- 33.Dietrich-Ntoukas T, Hofmann-Rummelt C, Kruse FE, Schlötzer-Schrehardt U. Comparative analysis of the basement membrane composition of the human limbus epithelium and amniotic membrane epithelium. Cornea. 2012;31:564–9. doi: 10.1097/ICO.0b013e3182254b78. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22382594&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 34.Riau AK, Beuerman RW, Lim LS, Mehta JS. Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials. 2010;31:216–25. doi: 10.1016/j.biomaterials.2009.09.034. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19781769&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 35.Schulze U, Hampel U, Sel S, Goecke TW, Thäle V, Garreis F, Paulsen F. Fresh and cryopreserved amniotic membrane secrete the trefoil factor family peptide 3 that is well known to promote wound healing. Histochem Cell Biol. 2012;138:243–50. doi: 10.1007/s00418-012-0943-2. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22476621&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 36.Zhang T, Yam GH, Riau AK, Poh R, Allen JC, Peh GS, Beuerman RW, Tan DT, Mehta JS. The effect of amniotic membrane de-epithelialization method on its biological properties and ability to promote limbal epithelial cell culture. Invest Ophthalmol Vis Sci. 2013;54:3072–81. doi: 10.1167/iovs.12-10805. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23580491&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 37.Kubo M, Sonoda Y, Muramatsu R, Usui M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci. 2001;42:1539–46. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11381058&dopt=Abstract [PubMed] [Google Scholar]
- 38.Baradaran-Rafii A, Aghayan HR, Arjmand B, Javadi MA. Amniotic membrane transplantation. Iran J Ophthalmic Res. 2007;2:58–75. [Google Scholar]
- 39.Li W, Hayashida Y, He H, Kuo CL, Tseng SC. The fate of limbal epithelial progenitor cells during explant culture on intact amniotic membrane. Invest Ophthalmol Vis Sci. 2007;48:605–13. doi: 10.1167/iovs.06-0514. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17251456&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sudha B, Sitalakshmi G, Iyer GK, Krishnakumar S. Putative stem cell markers in limbal epithelial cells cultured on intact & denuded human amniotic membrane. Indian J Med Res. 2008;128:149–56. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19001678&dopt=Abstract [PubMed] [Google Scholar]
- 41.Shortt AJ, Secker GA, Lomas RJ, Wilshaw SP, Kearney JN, Tuft SJ, Daniels JT. The effect of amniotic membrane preparation method on its ability to serve as a substrate for the ex-vivo expansion of limbal epithelial cells. Biomaterials. 2009;30:1056–65. doi: 10.1016/j.biomaterials.2008.10.048. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19019426&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 42.Vazirani J, Basu S, Kenia H, Ali MH, Kacham S, Mariappan I, Sangwan V. Unilateral partial limbal stem cell deficiency: contralateral versus ipsilateral autologous cultivated limbal epithelial transplantation. Am J Ophthalmol. 2014;157:584–90. doi: 10.1016/j.ajo.2013.11.011. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24269851&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 43.Ljubimov AV, Burgeson RE, Butkowski RJ, Michael AF, Sun TT, Kenney MC. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest. 1995;72:461–73. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7723285&dopt=Abstract [PubMed] [Google Scholar]
- 44.Schlötzer-Schrehardt U, Dietrich T, Saito K, Sorokin L, Sasaki T, Paulsson M, Kruse FE. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp Eye Res. 2007;85:845–60. doi: 10.1016/j.exer.2007.08.020. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17927980&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 45.Kabosova A, Azar DT, Bannikov GA, Campbell KP, Durbeej M, Ghohestani RF, Jones JC, Kenney MC, Koch M, Ninomiya Y, Patton BL, Paulsson M, Sado Y, Sage EH, Sasaki T, Sorokin LM, Steiner-Champliaud MF, Sun TT, Sundarraj N, Timpl R, Virtanen I, Ljubimov AV. Compositional differences between infant and adult human corneal basement membranes. Invest Ophthalmol Vis Sci. 2007;48:4989–99. doi: 10.1167/iovs.07-0654. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17962449&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verga Falzacappa MV, Ronchini C, Reavie LB, Pelicci PG. Regulation of self-renewal in normal and cancer stem cells. FEBS J. 2012;279:3559–72. doi: 10.1111/j.1742-4658.2012.08727.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22846222&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 47.Furusawa C, Kaneko K. A dynamical-systems view of stem cell biology. Science. 2012;338:215–7. doi: 10.1126/science.1224311. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23066073&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 48.Mohsen-Kanson T, Hafner AL, Wdziekonski B, Villageois P, Chignon-Sicard B, Dani C. Expression of cell surface markers during self-renewal and differentiation of human adipose-derived stem cells. Biochem Biophys Res Commun. 2013;430:871–5. doi: 10.1016/j.bbrc.2012.12.079. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23268339&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 49.Wray H, Mackenzie IC, Storey A, Navsaria H. α6 Integrin and CD44 enrich for a primary keratinocyte population that displays resistance to UV-induced apoptosis. PLoS ONE. 2012;7:e46968. doi: 10.1371/journal.pone.0046968. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23071680&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–24. doi: 10.1016/0092-8674(93)90251-k. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8500165&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 51.Chen S, Lewallen M, Xie T. Adhesion in the stem cell niche: biological roles and regulation. Development. 2013;140:255–65. doi: 10.1242/dev.083139. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23250203&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.West JD, Dorà NJ, Collinson JM. Evaluating alternative stem cell hypotheses for adult corneal epithelial maintenance. World J Stem Cells. 2015;7:281–99. doi: 10.4252/wjsc.v7.i2.281. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25815115&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan CK, Pham LN, Chinn C, Spee C, Ryan SJ, Akhurst RJ, Hinton DR. Mouse strain-dependent heterogeneity of resting limbal vasculature. Invest Ophthalmol Vis Sci. 2004;45:441–7. doi: 10.1167/iovs.03-0869. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14744883&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 54.Ecoiffier T, Yuen D, Chen L. Differential distribution of blood and lymphatic vessels in the murine cornea. Invest Ophthalmol Vis Sci. 2010;51:2436–40. doi: 10.1167/iovs.09-4505. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20019372&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu J, Sheha H, Fu Y, Liang L, Tseng SC. Update on amniotic membrane transplantation. Expert Rev Ophthalmol. 2010;5:645–61. doi: 10.1586/eop.10.63. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21436959&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamhane A, Vajpayee RB, Biswas NR, Pandey RM, Sharma N, Titiyal JS, Tandon R. Evaluation of amniotic membrane transplantation as an adjunct to medical therapy as compared with medical therapy alone in acute ocular burns. Ophthalmology. 2005;112:1963–9. doi: 10.1016/j.ophtha.2005.05.022. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16198422&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 57.Gomes JA, Romano A, Santos MS, Dua HS. Amniotic membrane use in ophthalmology. Curr Opin Ophthalmol. 2005;16:233–40. doi: 10.1097/01.icu.0000172827.31985.3a. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16000896&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 58.Ljubimov AV, Martel N, Yamasaki H. Response of cultured rat liver epithelial cell lines to tumour-promoting phorbol esters. Exp Cell Res. 1985;156:311–26. doi: 10.1016/0014-4827(85)90540-3. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3967682&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 59.Vivek Kumar PR, Cheriyan VD, Seshadri M. Could a strong alkali deproteinization replace the standard lysis step in alkaline single cell gel electrophoresis (comet) assay (pH > 13)? Mutat Res. 2009;678:65–70. doi: 10.1016/j.mrgentox.2009.06.007. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19563911&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 60.Kabosova A, Kramerov AA, Aoki AM, Murphy G, Zieske JD, Ljubimov AV. Human diabetic corneas preserve wound healing, basement membrane, integrin and MMP-10 differences from normal corneas in organ culture. Exp Eye Res. 2003;77:211–7. doi: 10.1016/s0014-4835(03)00111-8. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12873452&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pellegrini G, Rama P, Di Rocco A, Panaras A, De Luca M. Concise review: hurdles in a successful example of limbal stem cell-based regenerative medicine. Stem Cells. 2014;32:26–34. doi: 10.1002/stem.1517. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24038592&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 62.Sehic A, Utheim ØA, Ommundsen K, Utheim TP. Pre-clinical cell-based therapy for limbal stem cell deficiency. J Funct Biomater. 2015;6:863–88. doi: 10.3390/jfb6030863. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26343740&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kowluru RA, Kowluru A, Mishra M, Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2015;48:40–61. doi: 10.1016/j.preteyeres.2015.05.001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25975734&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]