Abstract
N 6-(2-Hydroxy-3-buten-1-yl)-2′-deoxyadenosine (N6-HB-dA I) and N6,N6-(2,3-dihydroxybutan-1,4-diyl)-2′-deoxyadenosine (N6,N6-DHB-dA) are exocyclic DNA adducts formed upon alkylation of the N6 position of adenine in DNA by epoxide metabolites of 1,3-butadiene (BD), a common industrial and environmental chemical classified as a human and animal carcinogen. Since the N6-H atom of adenine is required for Watson-Crick hydrogen bonding with thymine, N6-alkylation can prevent adenine from normal pairing with thymine, potentially compromising the accuracy of DNA replication. To evaluate the ability of BD-derived N6-alkyladenine lesions to induce mutations, synthetic oligodeoxynucleotides containing site-specific (S)-N6-HB-dA I and (R,R)-N6,N6-DHB-dA adducts were subjected to in vitro translesion synthesis in the presence of human DNA polymerases β, η, ι, and κ. While (S)-N6-HB-dA I was readily bypassed by all four enzymes, only polymerases η and κ were able to carry out DNA synthesis past (R,R)-N6,N6-DHB-dA. Steady-state kinetic analyses indicated that all four DNA polymerases preferentially incorporated the correct base (T) opposite (S)-N6-HB-dA I. In contrast, hPol β was completely blocked by (R,R)-N6,N6-DHB-dA, while hPol η and κ inserted A, G, C, or T opposite the adduct with similar frequency. HPLC-ESI-MS/MS analysis of primer extension products confirmed that while translesion synthesis past (S)-N6-HB-dA I was mostly error-free, replication of DNA containing (R,R)-N6,N6-DHB-dA induced significant numbers of A, C, and G insertions and small deletions. These results indicate that singly substituted (S)-N6-HB-dA I lesions are not miscoding, but exocyclic (R,R)-N6,N6-DHB-dA adducts are strongly mispairing, probably due to their inability to form stable Watson-Crick pairs with dT.
Keywords: DNA damage, DNA polymerase, mutagenesis mechanism, nucleic acid chemistry, enzyme kinetics, mass spectrometry (MS)
INTRODUCTION
Genomic DNA is under continuous attack by reactive species present in our diet, our environment, and generated endogenously as a result of oxidative stress, immune response, and normal cellular metabolism.1,2 The resulting structurally modified DNA nucleobases (DNA adducts) can compromise the accuracy of DNA replication, leading to heritable genetic damage. Mutations in genes responsible for genomic stability, signal transduction, and cell death can lead to the initiation of cancer.3
Many carcinogen-induced DNA adducts block the progression of replicative DNA polymerases (Pols) α, δ, and ε as a result of their steric bulk, their effects on DNA structure, and their inability to form standard Watson-Crick base pairs.4 A specialized group of translesion synthesis (TLS) polymerases including hPols η, ι, κ, and ζ are recruited to stalled replication forks; this is followed by polymerase switching during which the replicative polymerases are replaced by the TLS polymerases. 5,6 These TLS polymerases are characterized by open and flexible active sites which can accommodate the bulky DNA adducts, allowing for replication to continue in the presence of nucleobase damage.5–10 For example, Pol η accurately bypasses thymine dimers, thereby suppressing the mutagenic effects of UV-induced DNA damage, while Pol κ bypasses oxidation-induced thymine glycol and N2-guanine adducts induced by benzo[a]pyrene diolepoxide.11,12 Following lesion bypass, another polymerase switching occurs, and replicative DNA polymerases take over again, completing chromosomal replication. Although lesion bypass polymerases alleviate the genotoxicity of bulky DNA lesions, they generally have lower fidelity compared to replicative DNA polymerases as a result of the relaxed active site geometry and the lack of exonuclease proofreading.13,14 These increased error rates during replication may lead to miscoding and mutations, potentially contributing to multi-stage carcinogenesis.15
One important carcinogen ubiquitously present in cigarette smoke, cooking fires, and urban air is 1,3-butadiene (BD).16,17 BD is a major industrial chemical used in the manufacture of rubber and plastics, with annual US production of 9 metric tons.18 BD induces tumors at multiple organ sites of laboratory mice and rats19,20 and increases the risk of leukemia in occupationally exposed workers.21 BD is metabolically activated to 3,4-epoxy-1-butene (EB)22,23 and 1,2,3,4-diepoxybutane (DEB).24 Both EB and DEB are directly acting genotoxic agents that can induce large numbers of mutations at G:C and A:T base pairs and deletions.25,26 DEB induced A→T transversions and partial deletions, while EB caused G→A point mutations and A→T transversions at the hprt of TK6 human lymphoblastoid cells exposed to the BD epoxides.27 In vivo studies in B6C3F1 laci transgenic mice exposed to BD showed that BD induced A→G transitions and A→T transversions.27
EB and DEB react with DNA to form a range of DNA adducts including adenine and guanine monoadducts,28,29 DNA-DNA cross links,30–32 and exocyclic DNA adducts.33,34 Among nucleobase adducts induced by BD epoxides, deoxyadenosine adducts are of significant interest because they are likely to contribute to A→T, A→C, and A→G mutations observed upon exposure to BD and its epoxides.25,27 EB modifies the exocyclic amine group of adenine to form N6-(2-hydroxy-3-buten-1-yl)- 2′-deoxyadenosine (N6-HB-dA I) and N6-(1-hydroxy-3-buten-2-yl)-2′-deoxyadenosine (N6-HB-dA II) (Scheme 1).35–38 N6-HB-dA I and N6-HB-dA II adducts have been observed in DNA isolated from several tissues of laboratory mice and rats exposed to BD by inhalation.39,40 EB can sequentially react at two sites within the adenine heterocycle to form exocyclic deoxyadenosine lesions: N6,N6-(2,3-dihydroxybutan-1,4-diyl)-2′-deoxyadenosine (N6,N6-DHB-dA), N6-(2-hydroxy-3-hydroxymethylpropan-1,3-diyl)-2′-deoxy-adenosine (1,N6-γ HMHP-dA), and 1,N6-(1-hydroxymethyl-2-hydroxypropan-1,3-diyl)-2′-deoxyadenosine (1,N6-α HMHP-dA) (Scheme 1).32,39–42 1,N6-HMHP-dA and N6,N6-DHB-dA adducts were found in calf thymus DNA treated with DEB,33 while 1,N6-HMHP-dA adducts were also detected in DNA of mice exposed to BD.41 While it has been reported that N6-HB-dA II adducts are not mispairing in Escherichia coli,43 polymerase bypass past N6-HB-dA I and N6,N6-DHB-dA lesions has not yet been examined.
Scheme 1.
Formation of BD-induced N6-deoxyadenosine adducts N6-HB-dA, N6,N6-DHB-dA, and 1,N6-HMHP-dA from 3,4-epoxy-1-butene (EB) and 1,2,3,4-diepoxybutane (DEB), respectively.
The primary goal of the this study was to investigate the influence of N6-HB-dA I and N6,N6-DHB-dA adducts on DNA replication by human DNA polymerases, with an ultimate goal of establishing the mechanisms of BD-induced A → T, A→C, and A→G mutations and deletions. Primer extension studies were conducted using synthetic DNA templates containing site specific (S)-N6-HB-dA I and (R,R)-N6,N6-DHB-dA in the presence of recombinant human Pol β, Pol κ, Pol η, and Pol ι. Our results reveal significant differences between the outcomes of replication past N6-HB-dA I and N6,N6-DHB-dA lesions. While N6-HB-dA I was not miscoding, N6,N6-DHB-dA induced large numbers of misincorporations and deletions, probably as a result of its inability to form a Watson-Crick base pair with dT.
EXPERIMENTAL PROCEDURES
Materials
Full-length recombinant human polymerase κ (hPol κ) used for gel electrophoresis experiments was purchased from Enzymax (Lexington, KY). Human Pol β was obtained from Trevigen (Gaithersburg, MD). Recombinant human DNA polymerases hPol η (amino acids 1–437), hPol ι (amino acids 1–420), and hPol κ (amino acids 19–526) (active core enzymes) were expressed and purified according to previously published procedures.44–46 T4 polynucleotide kinase (T4-PNK) and E. coli uracil DNA glycosylase (UDG) were purchased from New England Biolabs (Beverly, MA). [γ-32P]ATP was purchased from Perkin-Elmer Life Sciences (Boston, MA). Acrylamide/bis-acrylamide solution (40% 19:1, w/w) and Bio-spin columns were obtained from Bio-Rad laboratories (Hercules, CA). All other chemicals were purchased either from Sigma-Aldrich or Fisher Scientific.
Oligonucleotide Synthesis, Labeling, and Annealing
Synthetic 18-mer oligodeoxynucleotides (5′-TCATXGAATCCTTCCCCC-3′) containing 6-chloropurine at position X were prepared by standard solid phase synthesis and coupled with (S)-N-Fmoc-1-aminobut-3-en-2-ol47 and (R,R)-pyrrolidine-3,4-diol48 to yield the corresponding strands containing site- and stereospecific (S)-N6-HB-dA I and (R,R)-N6,N6-DHB-dA adducts. Detailed methodologies for oligodeoxynucleotide synthesis have been previously reported.47,48 The corresponding unmodified 18-mer template containing native dA (5′-TCATAGAAT CCTTCCCCC-3′), 13-mer primers (5′-GGGGGAAGGATTC-3′ and 5′-GGGGGAAGGAUTC-3′) and a 9-mer primer (5′-GGGGGAAGG-3′) were purchased from Integrated DNA Technologies (Coralville, IA). All DNA oligomers were purified by semi-preparative HPLC, characterized by HPLC-ESI− MS/MS, and quantified by UV spectroscopy.47,48 The 13-mer primer (5′-GGGGGAAGGATTC-3′) and the 9-mer primer (5′-GGGGGAAGG-3′) were radiolabeled and subsequently annealed to the corresponding 18-mer templates to obtain primer-template complexes for in vitro replication studies (Scheme 2).
Scheme 2.
Oligonucleotide sequences employed in this study.
Primer Extension Assays
Primer extension studies were performed using the previously published methods,49 with a few modifications. For standing start experiments, 32P-endlabeled 13-mer:18-mer primer-template complexes (Scheme 2A) containing unmodified dA, (S)-N6-HB-dA I, or (R,R)-N6,N6-DHB-dA at the 14th position of the template strand (50 nM) were incubated at 37 °C in the presence of individual human DNA polymerases (hPol β, 12.5 nM; hPol η, 10 nM; hPol ι, 20 nM; hPol κ, 5 nM) in a buffer containing 50 mM Tris-HCl (pH 7.8), 50 mM NaCl, 5 mM dithiothreitol, 100 μg/ml BSA, and 10% glycerol (v/v). Primer extension reactions were initiated by the addition of dNTP mix (500 μM) and MgCl2 (5 mM). Aliquots of the reaction mixture (4 μL) were taken at 0, 5, 15, 30, 45, and 60 min and quenched with stop solution (95% formamide (w/v), 10 mM EDTA, 0.03% bromophenol blue (w/v), 0.03% xylene cyanol (w/v), 36 μL). “Running start” reactions were conducted analogously using 32P-endlabeled 9-mer primer/template duplexes (50 nM, Scheme 2B) in the presence of higher enzyme concentrations (hPol β, 25 nM; hPol η, 25 nM; hPol κ, 12.5 nM). Primer extension products were resolved by gel electrophoresis using a 20% (w/v) denaturing polyacrylamide gel containing 7 M urea. The radioactive primer extension products were visualized on a GE Typhoon FLA 7000 phosphorimager (GE Healthcare Life Sciences, Piscataway, NJ).
Steady-state Kinetic Analyses
The kinetics of incorporation of various nucleotides opposite (S)-N6-HB-dA I or (R,R)-N6,N6-DHB-dA were determined by conducting polymerization reactions in the presence of increasing concentrations of individual dNTPs (0–800 μM) for different time periods (0–180 min). DNA polymerase concentrations used were: hPol β, 12.5 nM; hPol η, 10 nM; hPol ι, 20 nM; hPol κ, 2.5–3.5 nM; the DNA concentration was 50 nM. The radioactive product bands were visualized on a GE Typhoon FLA7000 phosphorimager and quantified using ImageQuant software (GE Healthcare Life Sciences, Piscataway, NJ). Nonlinear regression analysis (one-site hyperbolic fits in Prism (GraphPad, La Jolla, CA)) was employed to determine the steady-state kinetic parameters for nucleotide incorporation.
HPLC-ESI-MS/MS Analysis of Primer Extension Products from DNA Polymerase Reactions
Uracil-containing primer/template complexes (100 pmol) (Scheme 2C) were incubated with hPol η or hPol κ (40 pmol) in a buffer containing 50 mM Tris-HCl (pH 7.8), 5% glycerol (v/v), 5 mM dithiothreitol, 5 mM MgCl2, 100 μg/mL bovine serum albumin and 1 mM each of the four dNTPs at 37 °C for 5 hours. Excess dNTPs were removed by size-exclusion using Bio-Spin 6 columns (Bio-Rad, Hercules, CA), and appropriate buffers were added to restore the concentrations in the filtrate to 50 mM Tris-HCl (pH 7.8), 5 mM dithiothreitol and 1 mM EDTA. The mixture was incubated with uracil DNA glycosylase (UDG) (6 units, 37 °C for 6 h), and the resulting abasic sites were cleaved with hot piperidine (0.25 M, 95 °C for 1 h) to reduce the size of primer extension products for sequencing by HPLC-ESI-MS/MS.49 The final reaction mixture was dried under vacuum and reconstituted in 25 μL of 15 mM ammonium acetate buffer containing a 14-mer oligonucleotide used as an internal standard for mass spectrometry (5′p-CTTCACGAGCCCCC-3′, 40 pmol). Primer extension products were resolved on a Agilent Zorbax SB 300 C18 (0.5 mm × 150 mm, 5 μm) column using an Eksigent HPLC system (Eksigent, Dublin, CA) coupled to a Thermo LTQ Orbitrap Velos mass spectrometer (ThermoFisher Scientific, Waltham, MA).49 Relative quantification and MS/MS sequencing of primer extension products were conducted as described previously.49
RESULTS
Primer Extension under Standing Start and Running Start Conditions
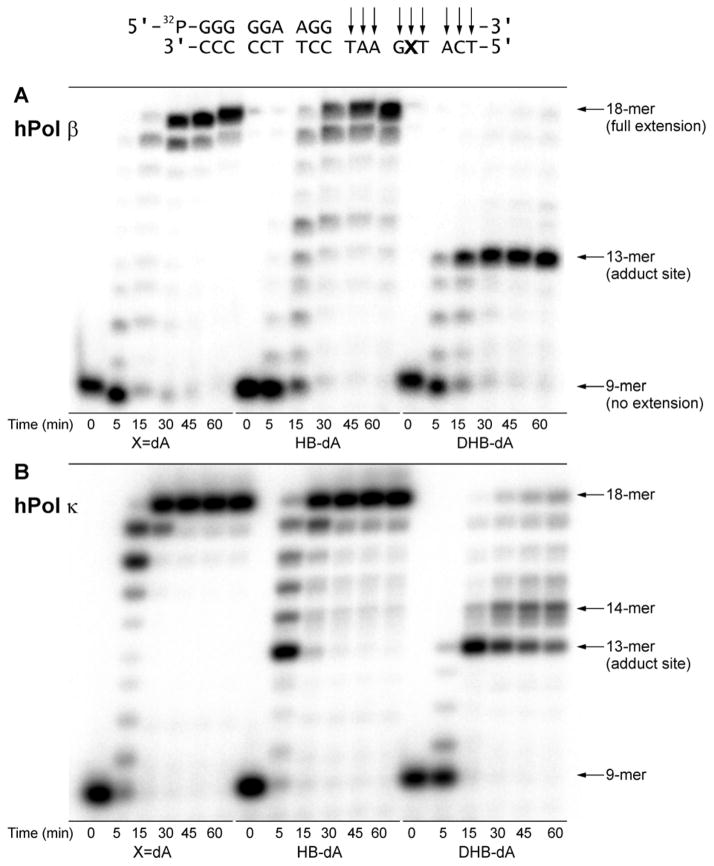
“Standing start” experiments were conducted with 13-mer primer/18-mer template complexes, where the primer 3′ terminus was positioned immediately upstream from the adducted base (Scheme 2A). Control experiments with the unmodified template showed that under these experimental conditions, hPol β, hPol κ, and hPol η produced full length 18-mer products (Figures 1A, 2A and 2B, first panel), while hPol ι reactions generated primarily 14–16-mer products (Figure 1B, first panel). The presence of (S)-N6-HB-dA I in the template strand did not prevent hPol β, hPol κ or hPol η from extending the primer to the terminus to yield 18-mer products (Figures 1A, 2A, 2B, second panels). However, the efficiency of hPol β, hPol κ, and hPol η-mediated DNA synthesis was reduced in the presence of (S)-N6-HB-dA, as evidenced by the observation of incomplete primer extension products (14–17-mers in the second panels of Figures 1A, 2A, and 2B). hPol ι formed 14–16-mer products, as was the case for the control template (Figure 1B, second panel). These results suggest that hPol ι -dependent TLS pathways would require the participation of an additional TLS polymerase that could extend from the nucleotide inserted by Pol ι.
Figure 1.
Standing start primer extension past (S)-N6-HB-dA I and (R,R)-N6,N6-DHB-dA adducts by hPol β (A), hPol ι (B), hPol κ (C) and hPol η (D). 32P-13-mer primer/18-mer template complexes containing the adduct or unmodified dA at the 14th position of the template (50 nM) were incubated in the presence of hPol β (12.5 nM), hPol ι (20 nM), hPol κ (5 nM), or hPol η (10 nM) in the presence of all four dNTPs. The reactions were quenched at designated time points (0–60 minutes), and the products were analyzed by 20% (w/v) PAGE and visualized by phosphorimaging.
Figure 2.
Running start bypass of (S)-N6-HB-dA I and (R,R)-N6,N6-DHB-dA in the presence of hPol β (A) and hPol κ (B). Primer/template complexes (50 nM) were incubated with hPol β (25 nM) and hPol κ (12.5 nM) in the presence of all 4 dNTPs. The reactions were stopped by adding quench solution at specific time points (0–60 minutes). The radiolabeled extension products were separated using 20% (w/v) PAGE and visualized by phosphorimaging. Similar results for hPol η are shown in Supporting Information Figure S1.
Polymerase bypass of (R,R)-N6,N6-DHB-dA was significantly less efficient. DNA synthesis by hPol β was completely blocked by this lesion (Figure 1A, third and fourth panels), while hPol ι incorporated a single base opposite the adduct but did not extend the primer any further, even at increased enzyme concentrations (Figure 1B). Under standard conditions, primer extension by hPol κ was stalled after single nucleotide incorporation opposite (R,R)-N6,N6-DHB-dA to produce 14-mer products (Figure 1C, third panel), but full extension was achieved at increased enzyme concentrations (Figure 1C, fourth panel). Similarly, hPol η was able to bypass (R,R)-N6,N6-DHB-dA to form 18-mer full extension products, but only when polymerase concentrations were increased significantly (Figure 1D). These observations indicate that the rate of postlesion synthesis is slower than the rate of single nucleotide incorporation opposite the damaged base.
To determine whether polymerase bypass of BD-dA adducts is more efficient under running start conditions, primer extension were repeated with -5 primers (Scheme 2B). As was the case in standing start experiments, hPol β, hPol κ, and hPol η were able to extend the primer past (S)-N6-HB-dA I and completely to the terminus (central panels of Figures 2A, 2B, and S1). In contrast, the presence of (R,R)-N6,N6-DHB-dA led to replication stalling at the site of damage and the formation of 13–14-mer incomplete extension products (right panels in Figures 2A, 2B, and S1) due to inefficient postlesion synthesis.
Possible Co-operativity of TLS Polymerases During Replication through (R,R)-N6,N6-DHB-dA Lesions
To determine whether TLS polymerases can potentially work together to help bypass strongly blocking (R,R)-N6,N6-DHB-dA adducts, additional primer extension experiments were conducted in the presence of both hPol ι and hPol η. As shown in Figure 1B, hPol ι incorporated a single nucleotide opposite (R,R)-N6,N6-DHB-dA but was unable to catalyze further primer extension. When the incomplete extension products from hPol ι reactions were incubated with hPol η, the primer was extended completely to the terminus to form18-mer products (Figure S2A). Full extension was also achieved when hPol ι and hPol η were added simultaneously (Lane 3 in Figure S2B). The amounts of 18-mer full extension products generated following extension reactions with both enzymes (12%, Lane 3) were higher than in control experiments where extension reactions were performed with hPol η alone (8%, Lane 2) or when extension products from reactions performed with hPol ι and hPol η alone were combined following protein inactivation (2%, lane 4). These observations provide preliminary evidence that hPol ι mediated TLS pathway past N6,N6-DHB-dA lesions requires Pol η for extending from the nucleotide inserted by Pol ι opposite the adduct.
Steady-state Kinetic Analysis of Incorporation of Individual dNTPs Opposite the BD-dA Lesions
In order to investigate the fidelity of nucleotide incorporation opposite (R,R)-N6,N6-DHB-dA and (S)-N6-HB-dA I, primer extension experiments were repeated in the presence of individual dNTPs (0–800 μM). The kinetics of nucleotide insertion was evaluated by conducting polymerization experiments in the presence of increasing concentrations of each nucleotide for specified time periods (0–180 min). Steady-state kinetic parameters (kcat and Km) for nucleotide incorporation, catalytic specificity constants (kcat/Km) and the misinsertion frequencies for nucleotide insertion (f) were determined as described previously.49,50
hPol β was less efficient at incorporating the correct nucleotide (dTMP) opposite (S)-N6-HB-dA I as compared to unmodified dA, as reflected by the 20-fold decrease in the kcat/Km values (Table 1). No kinetic analysis was possible for dTMP incorporation opposite (R,R)-N6,N6-DHB-dA by this polymerase, as product formation was too low to be quantified. In the case of hPol ι, dTMP incorporation opposite (S)-N6-HB-dA I and (R,R)-N6,N6-DHB-dA was 5- and 33- fold less efficient than opposite unmodified dA, respectively. As shown in Table 1, the kcat values for these reactions were similar, while the Km values were markedly increased in the presence of either BD-dA lesion.
Table 1.
Steady-state kinetic parameters for incorporation of individual nucleotides opposite dA, (S)-N6-HB-dA I and (R,R)-N6,N6-DHB-dA lesions by the four human DNA polymerases hPol κ, η, ι and β. f = misincorporation frequency = (kcat/Km)incorrect dNTP/(kcat/Km)correct dNTP (dTTP). Fold decrease in efficiency calculated as (kcat/Km) dNTP/(kcat/Km) dTTP opposite X=dA.
| Polymerase | Template | dNTP | kcat (min−1) | Km (μM) | kcat/Km (μM−1 min−1) | f | Fold decrease efficiency |
|---|---|---|---|---|---|---|---|
| hPol β | dA | T | 2.6 ± 0.5 | 28 ± 7 | 0.09 | 1 | 1 |
| N6-HB-dA I | T | 2.4 ± 0.4 | 511 ± 140 | 4.8 × 10−3 | 1 | 19 | |
| N6,N6-DHB-dA | T | NA | NA | NA | NA | NA | |
|
| |||||||
| hPol ι | dA | T | 1.0 ± 0.1 | 21 ± 2 | 0.05 | 1 | 1 |
| N6-HB-dA I | T | 0.88 ± 0.06 | 83 ± 13 | 0.01 | 1 | 5 | |
| N6,N6-DHB-dA | T | 0.74 ± 0.08 | 481 ± 117 | 1.5 × 10−3 | 1 | 33 | |
|
| |||||||
| hPol κ | dA | T | 5.7 ± 0.6 | 5.5 ± 1.8 | 1.0 | 1 | 1 |
| A | 0.87 ± 0.05 | 355 ± 45 | 2.4 × 10−3 | 2.4 × 10−3 | 430 | ||
| G | 4.3 ± 0.7 | 197 ± 18 | 0.02 | 0.02 | 52 | ||
|
| |||||||
| N6-HB-dA I | T | 5.2 ± 0.5 | 14 ± 3 | 0.38 | 1 | 3 | |
| A | 0.8 ± 0.02 | 153 ± 12 | 5.4 × 10−3 | 0.01 | 190 | ||
| G | 1.0 ± 0.08 | 180 ± 35 | 5.4 × 10−3 | 0.01 | 190 | ||
| C | 0.08 ± 0.01 | 15 ± 7 | 5.5 × 10−3 | 0.01 | 190 | ||
|
| |||||||
| N6,N6-DHB-dA | T | 0.35 ± 0.04 | 153 ± 37 | 2.3 × 10−3 | 1 | 450 | |
| A | 0.35 ± 0.03 | 286 ± 67 | 1.2 × 10−3 | 0.53 | 870 | ||
| G | 0.45 ± 0.06 | 455 ± 119 | 1.0 × 10−3 | 0.43 | 1040 | ||
| C | 0.29 ± 0.03 | 170 ± 38 | 1.7 × 10−3 | 0.76 | 610 | ||
|
| |||||||
| hPol η | dA | T | 2.4 ± 0.19 | 1.8 ± 0.53 | 1.3 | 1 | 1 |
| A | 0.60 ± 0.02 | 51 ± 7 | 0.01 | 8.8 × 10−3 | 130 | ||
| G | 0.52 ± 0.07 | 85 ± 27 | 6.0 × 10−3 | 4.6 × 10−3 | 220 | ||
|
| |||||||
| N6-HB-dA I | T | 2.60 ± 0.19 | 31 ± 7 | 0.08 | 1 | 16 | |
| A | 0.15 ± 0.02 | 112 ± 31 | 1.4 × 10−3 | 0.02 | 940 | ||
| G | 0.07 ± 0.01 | 177 ± 36 | 0.4 × 10−3 | 4.6 × 10−3 | 3300 | ||
| C | 0.25 ± 0.02 | 178 ± 30 | 1.4 × 10−3 | 0.02 | 940 | ||
|
| |||||||
| N6,N6-DHB-dA | T | 0.13 ± 0.02 | 79 ± 17 | 1.7 × 10−3 | 1 | 780 | |
| A | 0.12 ± 0.01 | 72 ± 14 | 1.6 × 10−3 | 0.98 | 830 | ||
| G | 0.31 ± 0.02 | 115 ± 19 | 2.7 × 10−3 | 1.6 | 490 | ||
| C | 0.25 ± 0.02 | 140 ± 32 | 1.8 × 10−3 | 1.1 | 730 | ||
hPol κ preferentially incorporated dTMP opposite dA in the unmodified template, with dAMP and dGMP added 50–400 fold less efficiently (Table 1). Similarly, hPol κ preferentially inserted the correct nucleotide (dTMP) opposite (S)-N6-HB-dA I, although nucleotide incorporation was 3-fold less efficient as compared to the unmodified template (kcat/Km = 0.38 vs 1.0 μM−1 min−1, see Table 1). The efficiency of addition of incorrect nucleotides (dCMP, dAMP, and dGMP) opposite (S)-N6-HB-dA I was 100-fold lower (Table 1), suggesting that this adduct retains the ability to form correct Watson-Crick base pair with dT. In contrast, hPol κ catalyzed the incorporation of all four nucleotides opposite (R,R)-N6,N6-DHB with similar efficiency (kcat/Km = 2.3 (T), 1.2 (A), 1.1 (G), and 1.7 × 10−3 μM−1 min−1 (C), Table 1). Nucleotide incorporation opposite (R,R)-N6,N6-DHB-dA by hPol κ- was ~ 450–1040 fold less efficient than opposite unmodified dA (Table 1), indicating that hPol κ has a low tolerance for this adduct.
Similar kinetic experiments were conducted with hPol η. We found that the efficiency of error-free replication opposite (S)-N6-HB-dA I was ~60-fold higher than that of dAMP or dCMP and ~200-fold higher than that of dGMP (Table 1). In contrast, the preference order of hPol η–mediated nucleotide insertion opposite (R,R)-N6,N6-DHB-dA by hPol η was G > T ~ A ~ C, with ~1.6-fold higher efficiency for dGMP incorporation as compared to the other three bases. kcat/Km values for insertion of all four dNTPs opposite (R,R)-N6,N6-DHB-dA were similar for both hPol η and hPol κ (Table 1). Overall, these results indicate that translesion synthesis past (S)-N6-HB-dA I is quite efficient and is essentially error-free. In contrast, the presence of (R,R)-N6,N6-DHB-dA in the template strand creates a strong replication block and introduces polymerase errors.
HPLC-ESI-MS/MS Analysis of Primer Extension Products Formed by hPol η and hPol κ
HPLC-ESI-MS/MS sequencing of hPol κ and hPol η-mediated primer extension products was used to confirm the results of gel electrophoresis based experiments (Table 1, Figs 1–2) and to identify additional primer extension products not detectable by gel electrophoresis (e.g. deletion products). In brief, polymerase primers were constructed to contain a uracil residue three bases upstream from the lesion site (Scheme 2C). Following in vitro replication in the presence of human polymerases, primer extension products were cleaved with UDG/piperidine to yield 5-, 6-, or 7-mer products, which could be readily sequenced by tandem mass spectrometry.49,50 The relative abundance of each primer extension product was calculated by comparing their HPLC-ESI−-MS peak areas to that of a 14-mer internal standard, while their identity was established by MS/MS sequencing on an Orbitrap Velos mass spectrometer.49
HPLC-ESI-MS/MS analysis of primer extension products generated using the (S)-N6-HB-dA I-containing template and hPol κ revealed oligodeoxynucleotide products at m/z 825.1, 1078.7, 1086.2, and 1090.7, corresponding to 5′-p TCTTATGA-3′, 5′-p TCCATGA-3′, 5′-p TCTATGA-3′, and 5′-p TCAATGA-3′, respectively. The error-free extension product (5′-p TCTATGA-3′) accounted for 83% of the total extension products (Scheme 3). The relative contributions of the three additional extension products, 5′-p TCTTATGA-3′, 5′-p TCCATGA-3′, and 5′-p TCAATGA-3′, were 6, 3, and 8 %, respectively (Scheme 3). These results are consistent with the gel electrophoresis data shown in Figures 1 and 2 and Table 1, indicating that the correct nucleotide (dTMP) is preferentially inserted opposite (S)-N6-HB-dA I.
Scheme 3.
Percentages of primer extension products formed upon in vitro primer extension of (S)-N6-HB-dA I and (R,R)-N6,N6-DHB-dA containing DNA templates by hPol κ.
In contrast, HPLC-ESI-MS/MS sequencing of primer extension products originating from hPol κ catalyzed replication of the (R,R)-N6,N6-DHB-dA containing template resulted in a complex mixture of products, including those with m/z 777.6, 922.1, 934.1, 942.1, 1078.7, 1086.2, 1090.7 and 1098.7, which correspond to 5′-p TC__TGA-3′, 5′-p TCC_TGA-3′, 5′-p TCA_TGA-3′, 5′-p TCG_TGA-3′, 5′-p TCCATGA-3′, 5′-p TCTATGA-3′, 5′-p TCAATGA-3′, and 5′-p TCGATGA-3′, respectively (Figure 3, Scheme 3). The products formed by misinsertion of A opposite the lesion (5′-p TCAATGA-3′) accounted for 20% of the total products (Scheme 3). Approximately 38% of total polymerization products corresponded to -1 and -2 deletions. 5′-p TCA_TGA-3′ and 5′-p TC__TGA-3′ were the major deletion products, corresponding to 18 and 9% of total products, respectively (Scheme 3). The product of error-free extension (5′-p TCTATGA-3′) accounted for only 37% of total extension products.
Figure 3.
Extracted ion chromatograms of primer extension products formed upon in vitro primer extension opposite 18-mer template containing (R,R)-N6,N6-DHB-dA by hPol κ. 13-mer dU primer/18-mer template duplex (100 pmol) containing (R,R)-N6,N6-DHB-dA was incubated with hPol κ (40 pmol) and a mixture of all four dNTPs in a reaction buffer for 5 hours. Excess dNTPs were removed by size exclusion chromatography, and the primer was cleaved by UDG followed by piperidine treatment. The cleaved extension products were separated and detected using capillary HPLC-ESI−-MS/MS as described.
Parallel reactions were conducted for hPol η. We found that in vitro replication of the (S)-N6-HB-dA I containing template produced only 5′-p TCTATGA-3′ (error free, 92%) and 5′-p TCCATGA-3′ (8%) (Scheme 4). In contrast, hPol η catalyzed bypass of (R,R)-N6,N6-DHB-dA generated seven different oligomers (Scheme 4). The major product (5′-p TCCATGA-3′) was formed by misinsertion of C opposite (R,R)-N6,N6-DHB-dA (46%). Error-free product (5′-p TCTATGA-3′) accounted for 19% of the total extension products, while 20% corresponded to the T → A transversion product (5′-p TCAATGA-3′). Deletion products accounted for 6% of in vitro replication products past (R,R)-N6,N6-DHB-dA by hPol η (Scheme 4).
Scheme 4.
Relative percentages of primer extension products formed upon in vitro primer extension of (S)-N6-HB-dA I and (R,R)-N6,N6-DHB-dA containing DNA templates by hPol η.
Nucleotide sequences of the primer extension products were determined from their MS/MS spectra. Good sequence coverage of a-B and w ion series was observed, allowing for accurate identification. For example, the MS/MS spectrum of m/z 1078.7 showed product ions at m/z 1059.3 and 1276.3, corresponding to the a4-B4 and w4 ions for 5′-p TCCATGA-3′, respectively (Figure 4C). MS/MS spectra of the major primer extension products (5′-p TCTATGA-3′, 5′-p TCAATGA-3′, 5′-p TCCATGA-3′, and 5′-p TCA_TGA-3′) are shown in Figure 4. Overall, the HPLC-ESI-MS/MS results confirmed that polymerase bypass of (S)-N6-HB-dA I by human polymerases is mainly error-free, while (R,R)-N6,N6-DHB-dA adducts are highly mispairing, resulting in predicted A→G transitions, A→T and A→C transversions, and deletions (Schemes 3 and 4). In general, HPLC-ESI−-MS/MS results were consistent with kinetic results shown in Table 1, although several differences have been noted. This is not surprising because the steady-state kinetic experiments shown in Table 1 characterize the kinetics of single nucleotide incorporation opposite a lesion, while the formation of full-length extension products detected by HPLC-ESI−-MS/MS requires post-lesion synthesis steps. As discussed above, these extension steps can be rate-limiting, affecting the identity of the products detected by mass spectrometry.
Figure 4.
MS/MS spectra of the major products formed upon in vitro primer extension opposite 18-mer template containing (R,R)-N6,N6-DHB-dA by hPol κ and hPol η. (A) CID spectra of 5′-p TCTATGA-3′, the major product of hPol κ incubation. (B) CID spectra of 5′-p TCAATGA-3′, the second major product in both the reaction mixtures (C) CID spectra of 5′-p TCCATGA-3′, the major product of hPol η catalyzed primer extension. (D) CID spectra of 5′-p TCA_TGA-3′, -1 deletion product that accounted for 18% of replication products following incubation with hPol κ.
DISCUSSION
BD and its epoxide metabolites induce A→G, A→T, and A→C mutations,25,27,51–54 but the structural origins of these genetic changes remain to be established. BD gives rise to a large range of DNA adducts including 1-hydroxy-3-buten-2-yl and 2,3,4-trihydrobybut-1-yl monoadducts, exocyclic lesions, DNA-DNA cross-links, and DNA-peptide and DNA-protein conjugates. Polymerase bypass of synthetic DNA templates containing N6-THB-adenine monoadducts showed that these adducts induced very low levels of A → G and A → C base substitutions (< 0.3%).43 N1-Trihydroxybutyl-dI and N1-(1-hydroxy-3-buten-2-yl)-dI lesions originating from deamination of the corresponding N1-dA adducts and 1,4-bis-(2′-deoxyadenosin-N6-yl)-butane-2,3-diol (bis-N6A-BD) cross-links were strongly mispairing,43,55 but have not yet been detected in vivo. Glutathione-derived S-[4-(N6)-deoxyadenosinyl)-2,3-dihydroxybutyl]glutathione induced A→G mutations.56,57 N6-HB-dA II adducts (Scheme 1) were not miscoding in either in vitro and in vivo assays.43
Recent studies in our laboratory identified several novel exocyclic adducts of DEB, including N6,N6-(2,3-dihydroxybutan-1,4-diyl)-2′-deoxyadenosine (N6,N6-DHB-dA), N6-(2-hydroxy-3-hydroxymethyl-propan-1,3-diyl)-2′-deoxy-adenosine (1,N6-γ HMHP-dA), and 1,N6-(1-hydroxymethyl-2-hydroxypropan-1,3-diyl)-2′-deoxyadenosine (1,N6-α HMHP-dA). Primer extension past (R,S)-1,N6-γ HMHP-dA in the presence of human lesion bypass Pols η and κ gave rise to large numbers of A and G insertions (corresponding to A→T and A→C transversions) and deletions.49 In the present study, in vitro translesion synthesis studies were extended to another exocyclic adduct of DEB ((R,R)-N6,N6-DHB-dA). To our knowledge, this is the first example of an exocyclic bis-N6,N6-dA DNA adduct, and therefore its effects on DNA replication were of significant interest. For comparison, the corresponding monoalkylated (S)-N6-HB-dA I adducts were also included in the study.
Our primer extension studies included recombinant human DNA polymerases β, η, ι, and κ. Among these, hPol β belongs to the X family of DNA Polymerases and is characterized by relatively high fidelity.15 Polymerases η, ι, and κ are Y-family polymerases involved in translesion synthesis. These three enzymes are structurally distinct and exhibit different adduct specificities.10 For example, bulky O6-[4-oxo-4-(3-pyridyl)butyl] guanine (O6-POB-G) adducts are bypassed by hPol η, but not by hPol ι and κ.58 Bulky anti-N2-BPDE (7,8-dihydroxy-anti-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene) adducts are bypassed by hPol κ.12 (R,S)-1,N6-γ HMHP-dA adducts examined in our earlier work were bypassed by all three polymerases, but with different efficiency and fidelity.49 Additionally, S-[4-(N6-deoxyadenosinyl)-2,3-dihydroxybutyl] glutathione (N6dA-(OH)2 butyl-GSH) and N6 -(2,3,4-trihydroxybutyl) deoxyadenosine (N6-THB-dA) adducts were also bypassed by hpols η, ι, and κ.57 In the present work, we were interested in evaluating whether human TLS polymerases η, ι, and κ have the ability to bypass (S)-N6-HB-dA I and (R,R)-N6,N6-DHB-dA adducts induced by BD in an error-free or error-prone manner.
We found that all four polymerases examined (β, η, ι, and κ) catalyzed error-free replication past monosubstituted N6-HB-dA I lesions (Figures 1–2), with the correct nucleobase (dT) incorporated preferentially opposite the lesion (Table 1). Among the three TLS polymerases evaluated, the efficiency of primer extension past N6-HB-dA I was hPol κ > hPol η > hPol ι (Figure 1), while the efficiency of incorporation of correct base (T) opposite N6-HB-dA I was hPol κ > hPol ι > hPol η (Table 1). HPLC-ESI−-MS/MS sequencing of primer extension products confirmed that replication past N6-HB-dA I was largely error free (Schemes 3 and 4). These results are consistent with previous studies for structurally analogous adducts such as N6-HB-dA II, N6-THB-dA, and N6-dA adducts of styrene oxide.43,59 Additionally, hpols η, ι, and κ, Pol T7, and Dpo4 were able to bypass N6-THB-dA adduct in an efficient and error-free manner.57
Among all N6-adenine adducts derived from epoxide metabolites of BD (Scheme 1), N6-HB-dA I and II are the least mutagenic, probably because they retain one of the N6-hydrogens, allowing for correct hydrogen bonding with dT. Structural studies of (S)-N6-HB-dA I in 5′-d(C1G2G3A4C5 Y6A7 G8A9 A10G11)-3′:5′-d(C12T13T14C15T16T17G18T19C20C21C22)-3′ using high field NMR (Y6 = (S)-N6-HB-dA I)60 have revealed that the adduct is accommodated in the major grove of the DNA, maintaining standard Watson-Crick base pairing with T17.60 Although (S)-N6-HB-dA I (Y6) did not stack well with its 5′ neighbor (C5), it stacked normally with its 3′ neighbor (A7), thereby leading to only minor perturbations in the overall DNA structure. Structurally analogous N6-THB-dA and N6-dA adducts are also readily accommodated in the DNA major grove, maintaining Watson-Crick base pairing.61–63
In contrast, none of the DNA polymerases examined in this study were able to catalyze efficient replication past (R,R)-N6,N6-DHB-dA (Table 1). (R,R)-N6,N6-DHB-dA adducts completely blocked DNA replication by hPol β (Figures 1–2) and required high concentrations of hPol η and κ to achieve lesion bypass. However, our experiments shown in Figure S2 provide preliminary evidence that hPol ι and hPol η may act cooperatively to allow for bypass of (R,R)-N6,N6-DHB-dA lesions, with hPol ι acting as an inserter polymerase and hPol η catalyzing post-lesion synthesis.
Steady-state kinetic studies revealed that hPol η and κ incorporated both correct and incorrect nucleotides opposite N6,N6-DHB-dA (Table 1). The efficiency of insertion of correct base (T) opposite N6,N6-DHB-dA by hPol η and κ was reduced by ~400- to 800-fold as compared to unmodified dA (Table 1). Similar decreases in efficiency (up to 1000-fold) have been previously reported for the incorporation of dTMP opposite 1,N6-ethenodeoxyadenosine by hPol κ.64 The order of efficiency for incorporation of correct base (T) opposite (R,R)-N6,N6-DHB-dA was hPol ι > hPol κ > hPol η (Table 1). HPLC-ESI−-MS/MS sequencing of the extension products generated in the presence of hPols η and κ identified a number of misincorporation and -1 and -2 deletion products (Schemes 3 and 4). If these events also take place in vivo, they would result in A→G, A→T, A→C, and frameshift mutations.
An NMR structure of (R,R)-N6,N6-DHB-dA in the 5′-d(C1G2G3A4C5Y6A7G8A9A10G11)-3′ : 5′-d(C12T13T14C15 T16T17G18T19C20C21C22)-3′ duplex where Y6 = (R,R)-N6,N6-DHB-dA adduct opposite T17 reveals that the adduct was accommodated in the major grove of DNA, with the 3,4- dihydroxypyrrolidine (DHB) moiety adopting an out-of-plane rotation about the C6-N6 bond.65 The adduct maintained an anti conformation about the glycosydic bond. The DHB moiety was out of plane, and the complementary T17 remained stacked within the duplex, forming a single hydrogen between the N1-imine nitrogen of (R,R)-N6,N6-DHB-dA and the N3H imino proton of T17.65 There was no hydrogen bonding between the N6 of (R,R)-N6,N6-DHB-dA and the O4 atom of the complementary thymine, leading to decreased thermal stability of the duplex. Furthermore, there was no evidence for the presence of any other hydrogen bonding between the hydroxyl groups of DHB and the complementary T17. The presence of an N6,N6-DHB substituent on adenine led to the loss of base stacking interactions with the 5′ neighboring C5 and interrupted the base stacking interaction between the complementary T17 and 3′ neighbor G18. Because N6,N6-DHB-dA is able to form only one hydrogen bond with dT, TLS polymerases cannot discriminate between individual dNTPs, leading to incorporation of any of the four dNTPs opposite this lesion. This is consistent with studies by Zhang et al. showing that double alkylation of the exocyclic amino group of N2,N2-dimethyl G results in mispairing with dA, dT, and dG.66 Interestingly, hpol κ and Pol T7 misincorporated dC opposite bulky N6dA-(OH)2 butyl-GSH adducts, while the polymerase bypass by hpols η and ι opposite this lesion was error-free.57
By comparison with (R,S)-1,N6-γ HMHP-dA investigated in our earlier study,49 (R,R)-N6,N6-DHB-dA represents a stronger block to DNA replication. If bypassed, both 1,N6-γ HMHP-dA and N6,N6-DHB-dA adducts can direct the incorporation of G, A, or T in the presence of hPols η and κ, but can be replicated in an error-free manner by hPol ι (Table 1). It should be noted, however, that hPol ι incorporated only a single T opposite the exocyclic DEB-dA lesions, requiring another polymerase to complete primer extension.49 Our preliminary experiments (Figure S2) suggest that hPol ι may collaborate with hpol κ or hPol η to allow for error-free bypass of (R,R)-N6,N6-DHB-dA in vivo. However, further studies incorporating additional protein factors (e.g. proliferating cell nuclear antigen, PCNA), are needed to establish whether such polymerase switching takes place in cells.67
In summary, our primer extension results indicate that monoalkylated (S)-N6-HB-dA I adducts are not mispairing and are unlikely to be responsible for the toxicity and mutations observed following exposure to BD. In contrast, doubly alkylated (R,R)-N6,N6-DHB-dA lesions are strongly blocking and are capable of inducing A→G transitions, A→T and A→C transversions, and small deletions. Therefore, if formed in vivo, (R,R)-N6,N6-DHB-dA may contribute to BD-mediated toxicity and mutations, along with previously characterized 1,N6-γ HMHP-dA and S-[4-(N6-deoxyadenosinyl)-2,3-dihydroxybutyl] glutathione adducts.49,57 Future structural studies of ternary complexes involving specific DNA polymerases and primer-template duplexes containing BD-dA adducts are required to improve our understanding of the mechanisms of lesion bypass.
Supplementary Material
Acknowledgments
Funding:
HPLC-ESI-MS/MS studies were conducted at the Bioanalytical Core Facility of the University of Minnesota Masonic Cancer Center, which is supported by National Institutes of Health Grant CA-077598. The primary author of this paper was financially supported by University of Minnesota Graduate School Doctoral Dissertation Fellowship. This research was supported by NIH grants R01 CA-100670 (NT) and R01 ES-010375 (FPG).
We thank Dr. Leena Maddukuri (University of Arkansas for Medical Sciences), Dr. Sarah Shuck (Vanderbilt University), and Dr. Colin Campbell (University of Minnesota) for their help in designing the polymerase bypass experiments described in this paper. We are also thankful to Dr. Peter Villalta (University of Minnesota, Mass Spectrometry Core) for his help and advice during HPLC-ESI-MS/MS method development and Bob Carlson for his assistance in preparing the graphics for this paper.
ABBREVIATIONS
- 1,N6-α HMHP-dA
1,N6-(1-hydroxymethyl-2-hydroxypropan-1,3-diyl)-2′-deoxyadenosine
- 1N6-γ HMHP-dA
N6-(2-hydroxy-3-hydroxymethylpropan-1,3-diyl)-2′-deoxyadenosine
- BD
1,3-butadiene
- DEB
1,2,3,4-diepoxybutane
- EB
3,4-epoxy-1-butene
- hPol
human Polymerase
- N6N6-DHB-dA
N6,N6-(2,3-dihydroxybutan-1,4-diyl)-2′-deoxyadenosine
- N6-HB-dA I
N6-(2-hydroxy-3-buten-1-yl)-2′-deoxyadenosine
- N6-HB-dA II
N6-(1-hydroxy-3-buten-2-yl)-2′-deoxyadenosine
- T4-PNK
T4 Polynucleotide kinase
- TLS Polymerases
translesion synthesis Polymerases
- UDG
uracil DNA glycosylase
Footnotes
Supporting Information available: Extension of 32P-end labeled 9-mer primer annealed to 18-mer template containing site specific dA, (S)-N6-HB-dA I, or (R,R)-N6,N6-DHB-dA by hpol η under running-start conditions; and co-operativity between human TLS polymerases hpol ι and hpol η during the bypass of the (R,R)-N6,N6-DHB-dA lesion. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.De Bont R, van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19:169–185. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- 2.Hakem R. DNA-damage repair; the good, the bad, and the ugly. EMBO J. 2008;27:589–605. doi: 10.1038/emboj.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 4.Friedberg EC, Lehmann AR, Fuchs RP. Trading places: how do DNA polymerases switch during translesion DNA synthesis? Mol Cell. 2005;18:499–505. doi: 10.1016/j.molcel.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Lehmann AR, Niimi A, Ogi T, Brown S, Sabbioneda S, Wing JF, Kannouche PL, Green CM. Translesion synthesis: Y-family polymerases and the polymerase switch. DNA Repair (Amst) 2007;6:891–899. doi: 10.1016/j.dnarep.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Prakash S, Prakash L. Translesion DNA synthesis in eukaryotes: a one- or two-polymerase affair. Genes Dev. 2002;16:1872–1883. doi: 10.1101/gad.1009802. [DOI] [PubMed] [Google Scholar]
- 7.Burgers PM, Koonin EV, Bruford E, Blanco L, Burtis KC, Christman MF, Copeland WC, Friedberg EC, Hanaoka F, Hinkle DC, Lawrence CW, Nakanishi M, Ohmori H, Prakash L, Prakash S, Reynaud CA, Sugino A, Todo T, Wang Z, Weill JC, Woodgate R. Eukaryotic DNA polymerases: proposal for a revised nomenclature. J Biol Chem. 2001;276:43487–43490. doi: 10.1074/jbc.R100056200. [DOI] [PubMed] [Google Scholar]
- 8.Waters LS, Minesinger BK, Wiltrout ME, D’Souza S, Woodruff RV, Walker GC. Eukaryotic translesion polymerases and their roles and regulation in DNA damage tolerance. Microbiol Mol Biol Rev. 2009;73:134–154. doi: 10.1128/MMBR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohmori H, Friedberg EC, Fuchs RP, Goodman MF, Hanaoka F, Hinkle D, Kunkel TA, Lawrence CW, Livneh Z, Nohmi T, Prakash L, Prakash S, Todo T, Walker GC, Wang Z, Woodgate R. The Y-family of DNA polymerases. Mol Cell. 2001;8:7–8. doi: 10.1016/s1097-2765(01)00278-7. [DOI] [PubMed] [Google Scholar]
- 10.Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu Rev Biochem. 2005;74:317–353. doi: 10.1146/annurev.biochem.74.082803.133250. [DOI] [PubMed] [Google Scholar]
- 11.Fischhaber PL, Gerlach VL, Feaver WJ, Hatahet Z, Wallace SS, Friedberg EC. Human DNA polymerase kappa bypasses and extends beyond thymine glycols during translesion synthesis in vitro, preferentially incorporating correct nucleotides. J Biol Chem. 2002;277:37604–37611. doi: 10.1074/jbc.M206027200. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki N, Ohashi E, Kolbanovskiy A, Geacintov NE, Grollman AP, Ohmori H, Shibutani S. Translesion synthesis by human DNA polymerase kappa on a DNA template containing a single stereoisomer of dG-(+)- or dG-(−)-anti-N(2)-BPDE (7,8-dihydroxy-anti-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene) Biochemistry. 2002;41:6100–6106. doi: 10.1021/bi020049c. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda T, Bebenek K, Masutani C, Hanaoka F, Kunkel TA. Low fidelity DNA synthesis by human DNA polymerase-eta. Nature. 2000;404:1011–1013. doi: 10.1038/35010014. [DOI] [PubMed] [Google Scholar]
- 14.Tissier A, McDonald JP, Frank EG, Woodgate R. polι, a remarkably error-prone human DNA polymerase. Genes Dev. 2000;14:1642–1650. [PMC free article] [PubMed] [Google Scholar]
- 15.McCulloch SD, Kunkel TA. The fidelity of DNA synthesis by eukaryotic replicative and translesion synthesis polymerases. Cell Res. 2008;18:148–161. doi: 10.1038/cr.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunnemann KD, Kagan MR, Cox JE, Hoffmann D. Analysis of 1,3-butadiene and other selected gas-phase components in cigarette mainstream and sidestream smoke by gas chromatography-mass selective detection. Carcinogenesis. 1990;11:1863–1868. doi: 10.1093/carcin/11.10.1863. [DOI] [PubMed] [Google Scholar]
- 17.Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control. 2003;12:424–430. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White WC. Butadiene production process overview. Chem Biol Interact. 2007;166:10–14. doi: 10.1016/j.cbi.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Melnick RL, Huff J, Chou BJ, Miller RA. Carcinogenicity of 1,3-butadiene in C57BL/6 x C3H F1 mice at low exposure concentrations. Cancer Res. 1990;50:6592–6599. [PubMed] [Google Scholar]
- 20.Owen PE, Glaister JR, Gaunt IF, Pullinger DH. Inhalation toxicity studies with 1,3-butadiene. 3 Two year toxicity/carcinogenicity study in rats. Am Ind Hyg Assoc J. 1987;48:407–413. doi: 10.1080/15298668791384959. [DOI] [PubMed] [Google Scholar]
- 21.National Toxicology Program. 1,3-Butadiene. Rep Carcinog. 2011;12:75–77. [PubMed] [Google Scholar]
- 22.Duescher RJ, Elfarra AA. Human liver microsomes are efficient catalysts of 1,3-butadiene oxidation: evidence for major roles by cytochromes P450 2A6 and 2E1. Arch Biochem Biophys. 1994;311:342–349. doi: 10.1006/abbi.1994.1246. [DOI] [PubMed] [Google Scholar]
- 23.Csanady GA, Guengerich FP, Bond JA. Comparison of the biotransformation of 1,3-butadiene and its metabolite, butadiene monoepoxide, by hepatic and pulmonary tissues from humans, rats and mice. Carcinogenesis. 1992;13:1143–1153. doi: 10.1093/carcin/13.7.1143. [DOI] [PubMed] [Google Scholar]
- 24.Krause RJ, Elfarra AA. Oxidation of butadiene monoxide to meso- and (+/−)-diepoxybutane by cDNA-expressed human cytochrome P450s and by mouse, rat, and human liver microsomes: evidence for preferential hydration of meso-diepoxybutane in rat and human liver microsomes. Arch Biochem Biophys. 1997;337:176–184. doi: 10.1006/abbi.1996.9781. [DOI] [PubMed] [Google Scholar]
- 25.Cochrane JE, Skopek TR. Mutagenicity of butadiene and its epoxide metabolites: I. Mutagenic potential of 1,2-epoxybutene, 1,2,3,4-diepoxybutane and 3,4-epoxy-1,2-butanediol in cultured human lymphoblasts. Carcinogenesis. 1994;15:713–717. doi: 10.1093/carcin/15.4.713. [DOI] [PubMed] [Google Scholar]
- 26.Cochrane JE, Skopek TR. Mutagenicity of butadiene and its epoxide metabolites: II. Mutational spectra of butadiene, 1,2-epoxybutene and diepoxybutane at the hprt locus in splenic T cells from exposed B6C3F1 mice. Carcinogenesis. 1994;15:719–723. doi: 10.1093/carcin/15.4.719. [DOI] [PubMed] [Google Scholar]
- 27.Recio L, Steen AM, Pluta LJ, Meyer KG, Saranko CJ. Mutational spectrum of 1,3-butadiene and metabolites 1,2-epoxybutene and 1,2,3,4-diepoxybutane to assess mutagenic mechanisms. Chem Biol Interact. 2001;135–136:325–341. doi: 10.1016/s0009-2797(01)00220-4. [DOI] [PubMed] [Google Scholar]
- 28.Himmelstein MW, Acquavella JF, Recio L, Medinsky MA, Bond JA. Toxicology and epidemiology of 1,3-butadiene. Crit Rev Toxicol. 1997;27:1–108. doi: 10.3109/10408449709037482. [DOI] [PubMed] [Google Scholar]
- 29.Kirman CR, Albertini RA, Gargas ML. 1,3-Butadiene: III. Assessing carcinogenic modes of action. Crit Rev Toxicol. 2010;40(Suppl 1):74–92. doi: 10.3109/10408444.2010.507183. [DOI] [PubMed] [Google Scholar]
- 30.Park S, Hodge J, Anderson C, Tretyakova N. Guanine-adenine DNA cross-linking by 1,2,3,4-diepoxybutane: potential basis for biological activity. Chem Res Toxicol. 2004;17:1638–1651. doi: 10.1021/tx0498206. [DOI] [PubMed] [Google Scholar]
- 31.Park S, Tretyakova N. Structural characterization of the major DNA-DNA cross-link of 1,2,3,4-diepoxybutane. Chem Res Toxicol. 2004;17:129–136. doi: 10.1021/tx0342058. [DOI] [PubMed] [Google Scholar]
- 32.Park S, Anderson C, Loeber R, Seetharaman M, Jones R, Tretyakova N. Interstrand and intrastrand DNA-DNA cross-linking by 1,2,3,4-diepoxybutane: role of stereochemistry. J Am Chem Soc. 2005;127:14355–14365. doi: 10.1021/ja051979x. [DOI] [PubMed] [Google Scholar]
- 33.Seneviratne U, Antsypovich S, Goggin M, Dorr DQ, Guza R, Moser A, Thompson C, York DM, Tretyakova N. Exocyclic deoxyadenosine adducts of 1,2,3,4-diepoxybutane: synthesis, structural elucidation, and mechanistic studies. Chem Res Toxicol. 2010;23:118–133. doi: 10.1021/tx900312e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang XY, Elfarra AA. Identification and characterization of a series of nucleoside adducts formed by the reaction of 2′-deoxyguanosine and 1,2,3,4-diepoxybutane under physiological conditions. Chem Res Toxicol. 2003;16:1606–1615. doi: 10.1021/tx0341355. [DOI] [PubMed] [Google Scholar]
- 35.Tretyakova N, Lin Y, Sangaiah R, Upton PB, Swenberg JA. Identification and quantitation of DNA adducts from calf thymus DNA exposed to 3,4-epoxy-1-butene. Carcinogenesis. 1997;18:137–147. doi: 10.1093/carcin/18.1.137. [DOI] [PubMed] [Google Scholar]
- 36.Tretyakova NY, Chiang SY, Walker VE, Swenberg JA. Quantitative analysis of 1,3-butadiene-induced DNA adducts in vivo and in vitro using liquid chromatography electrospray ionization tandem mass spectrometry. J Mass Spectrom. 1998;33:363–376. doi: 10.1002/(SICI)1096-9888(199804)33:4<363::AID-JMS643>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 37.Selzer RR, Elfarra AA. In vitro reactions of butadiene monoxide with single- and double-stranded DNA: characterization and quantitation of several purine and pyrimidine adducts. Carcinogenesis. 1999;20:285–292. doi: 10.1093/carcin/20.2.285. [DOI] [PubMed] [Google Scholar]
- 38.Koivisto P, Kostiainen R, Kilpelainen I, Steinby K, Peltonen K. Preparation, characterization and 32P-postlabeling of butadiene monoepoxide N6-adenine adducts. Carcinogenesis. 1995;16:2999–3007. doi: 10.1093/carcin/16.12.2999. [DOI] [PubMed] [Google Scholar]
- 39.Koivisto P, Adler ID, Sorsa M, Peltonen K. Inhalation exposure of rats and mice to 1,3-butadiene induces N6-adenine adducts of epoxybutene detected by 32P-postlabeling and HPLC. Environ Health Perspect. 1996;104(Suppl 3):655–657. doi: 10.1289/ehp.96104s3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koivisto P, Adler ID, Pacchierotti F, Peltonen K. Regio and stereospecific DNA adduct formation in mouse lung at N6 and N7 position of adenine and guanine after 1,3 butadiene inhalation exposure. Biomarkers. 1998;3:385–397. doi: 10.1080/135475098231039. [DOI] [PubMed] [Google Scholar]
- 41.Goggin M, Seneviratne U, Swenberg JA, Walker VE, Tretyakova N. Column switching HPLC-ESI+-MS/MS methods for quantitative analysis of exocyclic dA adducts in the DNA of laboratory animals exposed to 1,3-butadiene. Chem Res Toxicol. 2010;23:808–812. doi: 10.1021/tx900439w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goggin M, Sangaraju D, Walker VE, Wickliffe J, Swenberg JA, Tretyakova N. Persistence and repair of bifunctional DNA adducts in tissues of laboratory animals exposed to 1,3-butadiene by inhalation. Chem Res Toxicol. 2011;24:809–817. doi: 10.1021/tx200009b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carmical JR, Nechev LV, Harris CM, Harris TM, Lloyd RS. Mutagenic potential of adenine N6 adducts of monoepoxide and diolepoxide derivatives of butadiene. Environ Mol Mutagen. 2000;35:48–56. [PubMed] [Google Scholar]
- 44.Irimia A, Eoff RL, Guengerich FP, Egli M. Structural and functional elucidation of the mechanism promoting error-prone synthesis by human DNA polymerase kappa opposite the 7,8-dihydro-8-oxo-2′-deoxyguanosine adduct. J Biol Chem. 2009;284:22467–22480. doi: 10.1074/jbc.M109.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pence MG, Choi JY, Egli M, Guengerich FP. Structural basis for proficient incorporation of dTTP opposite O6-methylguanine by human DNA polymerase ι. J Biol Chem. 2010;285:40666–40672. doi: 10.1074/jbc.M110.183665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi JY, Guengerich FP. Adduct size limits efficient and error-free bypass across bulky N2-guanine DNA lesions by human DNA polymerase η. J Mol Biol. 2005;352:72–90. doi: 10.1016/j.jmb.2005.06.079. [DOI] [PubMed] [Google Scholar]
- 47.Quirk-Dorr D, Murphy K, Tretyakova N. Synthesis of DNA oligodeoxynucleotides containing structurally defined N6-(2-hydroxy-3-buten-1-yl)-adenine adducts of 3,4-epoxy-1-butene. Chem Biol Interact. 2007;166:104–111. doi: 10.1016/j.cbi.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Seneviratne U, Antsypovich S, Dorr DQ, Dissanayake T, Kotapati S, Tretyakova N. DNA oligomers containing site-specific and stereospecific exocyclic deoxyadenosine adducts of 1,2,3,4-diepoxybutane: synthesis, characterization, and effects on DNA structure. Chem Res Toxicol. 2010;23:1556–1567. doi: 10.1021/tx100146v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotapati S, Maddukuri L, Wickramaratne S, Seneviratne U, Goggin M, Pence MG, Villalta P, Guengerich FP, Marnett L, Tretyakova N. Translesion synthesis across 1, N6-(2-Hydroxy-3-Hydroxymethylpropan-1,3-Diyl)-2′-Deoxyadenosine (1,N6-γ-HMHP-dA) Adducts by Human and Archbacterial DNA Polymerases. J Biol Chem. 2012;287:38800–38811. doi: 10.1074/jbc.M112.396788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maddukuri L, Eoff RL, Choi JY, Rizzo CJ, Guengerich FP, Marnett LJ. In vitro bypass of the major malondialdehyde- and base propenal-derived DNA adduct by human Y-family DNA polymerases kappa, iota, and Rev1. Biochemistry. 2010;49:8415–8424. doi: 10.1021/bi1009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Recio L, Sisk S, Meyer K, Pluta L, Bond JA. Mutagenicity and mutational spectra of 1,3-butadiene in the bone marrow of B6C3F1 lacI transgenic mice. Toxicology. 1996;113:106–111. doi: 10.1016/0300-483x(96)03434-8. [DOI] [PubMed] [Google Scholar]
- 52.Recio L, Meyer KG, Pluta LJ, Moss OR, Saranko CJ. Assessment of 1,3-butadiene mutagenicity in the bone marrow of B6C3F1 lacI transgenic mice (Big Blue): a review of mutational spectrum and lacI mutant frequency after a 5-day 625 ppm 1,3-butadiene exposure. Environ Mol Mutagen. 1996;28:424–429. doi: 10.1002/(SICI)1098-2280(1996)28:4<424::AID-EM18>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 53.Recio L, Pluta LJ, Meyer KG. The in vivo mutagenicity and mutational spectrum at the lacI transgene recovered from the spleens of B6C3F1 lacI transgenic mice following a 4-week inhalation exposure to 1,3-butadiene. Mutat Res. 1998;401:99–110. doi: 10.1016/s0027-5107(97)00319-9. [DOI] [PubMed] [Google Scholar]
- 54.Steen AM, Meyer KG, Recio L. Analysis of hprt mutations occurring in human TK6 lymphoblastoid cells following exposure to 1,2,3,4-diepoxybutane. Mutagenesis. 1997;12:61–67. doi: 10.1093/mutage/12.2.61. [DOI] [PubMed] [Google Scholar]
- 55.Kanuri M, Nechev LV, Tamura PJ, Harris CM, Harris TM, Lloyd RS. Mutagenic spectrum of butadiene-derived N1-deoxyinosine adducts and N6,N6-deoxyadenosine intrastrand cross-links in mammalian cells. Chem Res Toxicol. 2002;15:1572–1580. doi: 10.1021/tx025591g. [DOI] [PubMed] [Google Scholar]
- 56.Cho SH, Guengerich FP. Conjugation of butadiene diepoxide with glutathione yields DNA adducts in vitro and in vivo. Chem Res Toxicol. 2012;25:706–712. doi: 10.1021/tx200471x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho SH, Guengerich FP. Replication past the butadiene diepoxide-derived DNA adduct S-[4-(N6-deoxyadenosinyl)-2,3-dihydroxybutyl]glutathione by DNA polymerases. Chem Res Toxicol. 2013;26:1005–1013. doi: 10.1021/tx400145e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi JY, Chowdhury G, Zang H, Angel KC, Vu CC, Peterson LA, Guengerich FP. Translesion synthesis across O6-alkylguanine DNA adducts by recombinant human DNA polymerases. J Biol Chem. 2006;281:38244–38256. doi: 10.1074/jbc.M608369200. [DOI] [PubMed] [Google Scholar]
- 59.Kanuri M, Finneman J, Harris CM, Harris TM, Lloyd RS. Efficient nonmutagenic replication bypass of DNAs containing beta-adducts of styrene oxide at adenine N6. Environ Mol Mutagen. 2001;38:357–360. doi: 10.1002/em.10030. [DOI] [PubMed] [Google Scholar]
- 60.Kowal EA, Wickramaratne S, Kotapati S, Turo M, Tretyakova N, Stone MP. Major groove orientation of the (2S)-N6-(2-hydroxy-3-buten-1-yl)-2′-deoxyadenosine DNA adduct induced by 1,2-epoxy-3-butene. Chem Res Toxicol. 2014;27:1675–1686. doi: 10.1021/tx500159w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Merritt WK, Scholdberg TA, Nechev LV, Harris TM, Harris CM, Lloyd RS, Stone MP. Stereospecific structural perturbations arising from adenine N6 butadiene triol adducts in duplex DNA. Chem Res Toxicol. 2004;17:1007–1019. doi: 10.1021/tx049908j. [DOI] [PubMed] [Google Scholar]
- 62.Scholdberg TA, Nechev LV, Merritt WK, Harris TM, Harris CM, Lloyd RS, Stone MP. Structure of a site specific major groove (2S,3S)-N6-(2,3,4-trihydroxybutyl)-2′-deoxyadenosyl DNA adduct of butadiene diol epoxide. Chem Res Toxicol. 2004;17:717–730. doi: 10.1021/tx034271+. [DOI] [PubMed] [Google Scholar]
- 63.Stone MP, Feng BB. Sequence and stereospecific consequences of major groove α-(N6-adenyl)-styrene oxide adducts in an oligodeoxynucleotide containing the human N-ras codon 61 sequence. Magn Chem Res. 1996;34:S105–S114. [Google Scholar]
- 64.Levine RL, Miller H, Grollman A, Ohashi E, Ohmori H, Masutani C, Hanaoka F, Moriya M. Translesion DNA synthesis catalyzed by human pol eta and pol kappa across 1, N6-ethenodeoxyadenosine. J Biol Chem. 2001;276:18717–18721. doi: 10.1074/jbc.M102158200. [DOI] [PubMed] [Google Scholar]
- 65.Kowal EA, Seneviratne U, Wickramaratne S, Doherty KE, Cao X, Tretyakova N, Stone MP. Structures of exocyclic R,R- and S,S-N6,N6-(2,3-Dihydroxybutan-1,4-Diyl)-2′-Deoxyadenosine Adducts Induced by 1,2,3,4-Diepoxybutane. Chem Res Toxicol. 2014;27:805–817. doi: 10.1021/tx400472p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang H, Eoff RL, Kozekov ID, Rizzo CJ, Egli M, Guengerich FP. Structure-function relationships in miscoding by Sulfolobus solfataricus DNA polymerase Dpo4: guanine N2,N2-dimethyl substitution produces inactive and miscoding polymerase complexes. J Biol Chem. 2009;284:17687–17699. doi: 10.1074/jbc.M109014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mailand N, Gibbs-Seymour I, Bekker-Jensen S. Regulation of PCNA-protein interactions for genome stability. Nat Rev Mol Cell Biol. 2013;14:269–282. doi: 10.1038/nrm3562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.