Abstract
Crimean-Congo hemorrhagic fever (CCHF) is a widely distributed, tick-borne viral disease. Humans are the only species known to develop illness after CCHF virus (CCHFV) infection, characterized by a nonspecific febrile illness that can progress to severe, often fatal, hemorrhagic disease. A variety of animals may serve as asymptomatic reservoirs of CCHFV in an endemic cycle of transmission. Seroepidemiological studies have been instrumental in elucidating CCHFV reservoirs and in determining endemic foci of viral transmission. Herein, we review over 50 years of CCHFV seroepidemiological studies in domestic and wild animals. This review highlights the role of livestock in the maintenance and transmission of CCHFV, and provides a detailed summary of seroepidemiological studies of wild animal species, reflecting their relative roles in CCHFV ecology.
Introduction
Crimean-Congo hemorrhagic fever virus (CCHFV), a nairovirus of the Bunyaviridae family, is the causative agent of a severe human hemorrhagic fever disease characterized by fever, weakness, myalgia, and hemorrhagic signs [1]. Clinical disease is restricted to humans and is fatal in 3%–30% of cases. Crimean-Congo hemorrhagic fever (CCHF) has been described over a wide geographic area including Asia, Africa, and Europe. The natural vector and reservoir has been identified as Hyalomma spp. ticks, and the distribution of human cases closely mirrors vector distribution. CCHFV is transmitted to humans by the bite of an infected tick, contact with patients during the acute phase of illness, or by contact with blood or tissues of viremic animals. Early diagnosis is critical for patient support and for preventing spread of infection through well-documented human-to-human transmission [2]. Ribavirin has been used extensively as an antiviral treatment, but remains controversial [3,4].
In general, CCHFV circulates in nature in unnoticed enzootic tick–vertebrate–tick cycles. Asymptomatic CCHFV infection has been reported in numerous vertebrate species and appears to be pervasive in both wild and domestic animals [5]. Asymptomatic viremia lasting up to 7–15 days has been described in several vertebrate animal species [6–8], and CCHFV has been isolated from livestock and small mammals. An extensive amount of research has been conducted on CCHFV reservoir species and their respective roles in virus maintenance and transmission. Seroepidemiological studies comprise the majority of this research, elucidating reservoir species and virus circulation. CCHFV serosurveillance has relied on a variety of techniques, including virus neutralization assays [9,10], reverse passive hemagglutination inhibition (RPHI) assays [11–13], immunodiffusion assays such as agar gel diffusion precipitation (AGDP) [14,15], complement fixation (CF) assays [9,16–18], indirect immunofluorescence assays (IFA) [19–23], indirect or sandwich enzyme-linked immunoassays (ELISA) [23–27], and competitive ELISA (CELISA) [28].
Several groups have published reports of detailed serosurveys conducted recently in various countries, including Albania, Iran, Sudan, and India. However, numerous studies investigating serological evidence of CCHFV in animal species were performed decades ago, are difficult to obtain, and are often published in non-English languages. Animal serosurvey data have been examined and discussed in CCHFV reviews [1,6], but no literature currently exists cohesively presenting current and past reports of the presence or absence of CCHFV antibodies in domestic and wild animals. Virus emergence and reemergence continue to be key topics of national and international health security. As with other hemorrhagic fever viruses, the potential introduction of CCHFV into new geographic areas [29–31] should be considered and requires appropriate knowledge of virus ecology, transmission dynamics, and competent reservoir hosts and vectors.
Herein, we provide a detailed summary of the extensive seroepidemiological CCHFV studies performed internationally in both domestic and wild animals (Fig 1). This report serves as an important resource in discussion of the role of animals in CCHFV maintenance and transmission to humans. The information provided specifically aids in understanding the global impact of CCHFV and clarifying the roles of domestic and wild animals in putative expansion of CCHFV endemic regions.
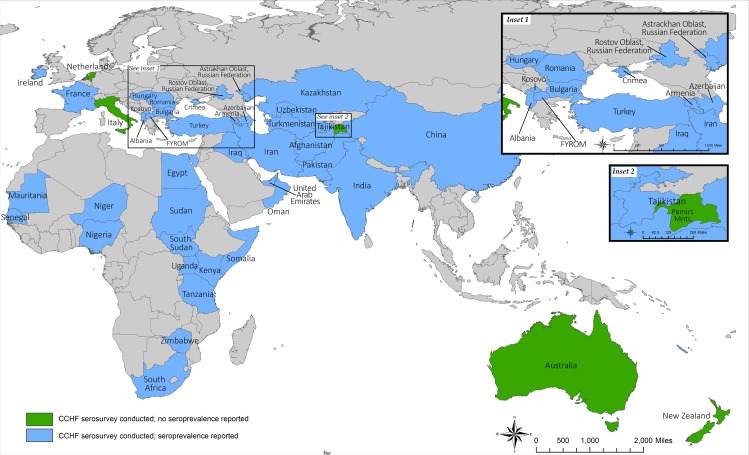
Fig 1. Geographic summary of countries represented in CCHFV seroepidemiological surveys.
Countries with evidence of seroprevalence in animals represented in blue, countries with absence of seroprevalence represented in green, and countries without reported serosurveys represented in grey.
Domestic Animals
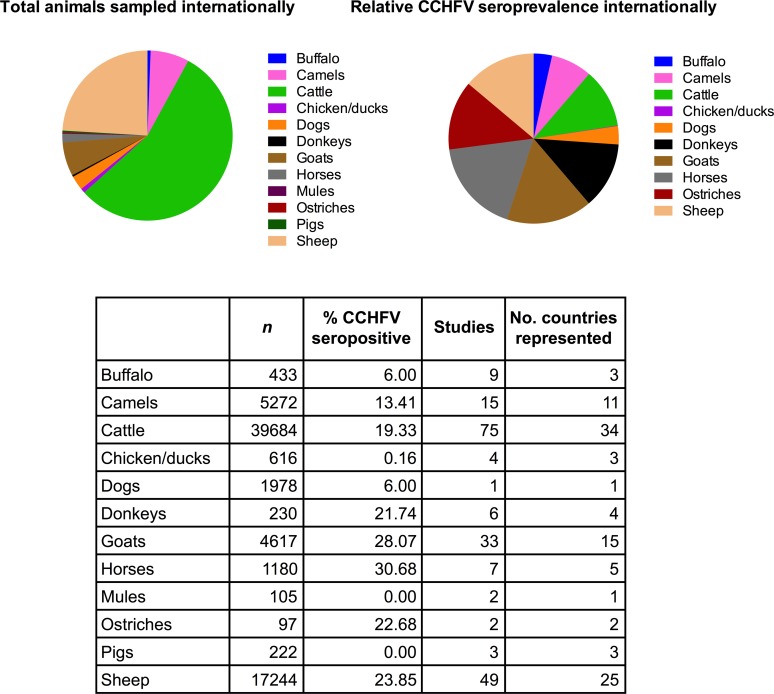
Seroepidemiological studies in endemic regions indicate that various domestic and peri-domestic animals could be asymptomatically infected with CCHFV. Detection of CCHFV antibodies in domestic animals has been important in providing initial evidence of circulating virus and in localizing CCHFV foci and increased risk for human infection [6,32,33]. A wide spectrum of domestic animal species has been investigated internationally, including cattle, sheep, goats, horses, pigs, dogs, and chickens (Table 1). Other domestic species investigated include buffalo, camels, and ostriches. Examples of high seroprevalence in domestic animals include 79.1% seropositive cattle (Afghanistan) [34], 75.0% sheep (Afghanistan) [34], 66.0% goats (Turkey) [10], 58.8% horses (Iraq) [35], and 39.5% donkeys (Tajikistan) [36]. High seroprevalence has also been reported in camels; the highest (excluding the 1/1 animal found positive in Pakistan) percentage of seropositive camels was reported in Kenya at 26% (n = 499). The largest reported sample size of a single species comprised almost 9,000 cattle tested in South Africa [37]. The role of cattle, sheep, and other large vertebrates in CCHFV ecology is reflected in the relative levels of species-specific CCHFV antibody prevalence reported internationally (Fig 2). Among studies that indicate sample size, cattle are the most often studied (75 studies), followed by sheep (49 studies) and goats (33 studies). Data on cattle and sheep have also been reported from the largest number of countries (34 and 25, respectively) (Table 1). Reports of other species are more limited; seroprevalence in domestic dogs, for example, was only reported in one study based on samples obtained in South Africa and Zimbabwe [13].
Table 1. Crimean-Congo hemorrhagic fever virus (CCHFV) seroprevalence in domestic animals.
| Animal | Country (Region) of Specimen Origin | Seroprevalence | Assay | Reference | |
|---|---|---|---|---|---|
| n | % | ||||
| Buffalo | Egypt | 47 | 0 | CF | [38] |
| Egypt (central) | 153 | 0 | IgG ELISA | [39] | |
| India | 2 | 0 | AGDP | [18] | |
| India (Jammu and Kashmir) | 23 | 0 | CF | [40] | |
| India (Jammu and Kashmir) | 14 | 0 | AGDP | [40] | |
| India | 3 | 0 | IgG ELISA | [41] | |
| India (Maharashtra, Rajasthan) | 46 | 2.2 | IgG ELISA | [42] | |
| India (Ahmadabad) | 123 | 19.5 | IgG ELISA | [42] | |
| Pakistan | 22 | 4.5 | CF | [43] | |
| Camels | China (Tarim, Junggar, and Turpan-Hami Basins) | 10 | 40 | RPHI | [44]* |
| Egypt | 34 | 8.8 | CF | [38] | |
| Egypt (central) | 10 | 0 | IgG ELISA | [39] | |
| India (Jammu and Kashmir) | 3 | 0 | CF | [40] | |
| India (Jammu and Kashmir) | 3 | 0 | AGDP | [40] | |
| Iran | 99 | 19.1 | AGDP | [45] | |
| Iran | 157 | 0 | AGDP | [9] in [6] | |
| Iraq | 99 | 23.2 | CF | [35] | |
| Kenya | 499 | 26 | AGDP, IFA | [20] | |
| Niger | 353 | 13.6 | IgG ELISA | [21] | |
| Oman | 109 | 16 | IgG ELISA | [46] | |
| Pakistan | 1 | 100 | IgG ELISA | [47] | |
| Russia (Astrakhan Oblast) | NR | 1.4 | AGDP | [48] in [6] | |
| Sudan | 3802 | 12 | AGDP, IFA | [20] | |
| Sudan | 13 | 7.7 | IgG ELISA | [47] | |
| United Arab Emirates | 80 | 6.3 | IgG ELISA | [47] | |
| Cattle | Afghanistan | 230 | 5.6 | AGDP | [18,49] |
| Afghanistan (Engil District) | 92 | 79.1 | IgG ELISA | [34] | |
| Albania | 14 | 0 | IgG ELISA | [50] | |
| Albania (ten regions surveyed) | 337 | 4.74 | IgG ELISA | [51] | |
| Albania (Berat) | 50 | 4 | IgG ELISA | [52] | |
| Albania (Gjirokastra) | 50 | 2.1 | IgG ELISA | [53] | |
| Albania (Kolonje) | 54 | 7.4 | IgG ELISA | [52] | |
| Albania (Kukes) | 11 | 0 | IgG ELISA | [53] | |
| Albania (Rreshen) | 40 | 2.6 | IgG ELISA | [53] | |
| Armenia | 1373 | 4.2 | AGDP | [54] | |
| Azerbaijan (Sabirabad and Saatly) | 651 | 16.2 | AGDP | [55] | |
| Azerbaijan (Sal’yany) | 142 | 11.9 | AGDP | [55] | |
| Azerbaijan (Pushkino) | 38 | 10.1 | AGDP | [55] | |
| Azerbaijan (Apsheron) | 102 | 11.0 | AGDP | [55] | |
| Azerbaijan (Divichin) | 161 | 3.1 | AGDP | [55] | |
| Azerbaijan (Lenkoran’) | 238 | 3.8 | AGDP | [55] | |
| Azerbaijan (Sabirabad) | 454 | 4.2 | AGDP | [55] | |
| Azerbaijan (Saatly) | 424 | 4.7 | AGDP | [55] | |
| Bulgaria | 1756 | 33.2 | AGDP | [56] | |
| Bulgaria (Municipality of Aytos) | 127 | 71 | IgG ELISA | [27] | |
| Bulgaria | 1775 | 7.89 | IFA | [22] | |
| Egypt | 43 | 0 | CF | [38] | |
| Egypt | 200 | 0 | AGDP, IFA | [20] | |
| Egypt (central) | 161 | 0.6 | IgG ELISA | [39] | |
| Germany | 78 | 0 | RPHI | [57] | |
| Hungary | 687 | 0.9 | AGDP | [58] | |
| Hungary (Hajdú-Bihar) | 161 | 0 | AGDP | [59] | |
| India | 22 | 0 | AGDP | [15] | |
| India | 25 | 0 | AGDP | [18] | |
| India | 12 | 0 | AGDP | [18] | |
| India | 711 | 12.1 | IgG ELISA | [60] | |
| India | 32 | 43.8 | IgG ELISA | [41] | |
| India (northern West Bengal) | 5 | 0 | IgG ELISA | [42] | |
| India (Ahmadabad) | 74 | 4.1 | IgG ELISA | [42] | |
| India (Jammu and Kashmir) | 66 | 0 | CF | [40] | |
| India (Jammu and Kashmir) | 55 | 0 | AGDP | [40] | |
| Iran | 100 | 19 | AGDP | [45] | |
| Iran | 130 | 18 | AGDP | [9] | |
| Iran | 876 | 5.9 | ELISA | [61] | |
| Iran (Ardabil Province) | 10 | 30 | IgG ELISA | [62] | |
| Iran (Isfahan Province) | 15 | 20 | IgG ELISA | [63] | |
| Iran | 1091 | 25.0 | IgG ELISA | [64] | |
| Iraq | 411 | 29.3 | CF | [35] | |
| Iraq (Basrah, southern Iraq) | 48 | 37 | IgG ELISA | [65] | |
| Ireland | 54 | 1.9 | RPHI | [57] | |
| Italy | 50 | 0 | RPHI | [57] | |
| Kazakhstan | 842 | 0.7 | AGDP | [66] | |
| Kenya/Uganda | 93 | 76.3 | AGDP | [45] | |
| Kosovo | 353 | 18.4 | IgG ELISA | [67] | |
| Niger | 1201 | 46 | IgG ELISA | [21] | |
| Nigeria | 1164 | 25.7 | AGDP | [68] | |
| Oman | 27 | 4 | IgG ELISA | [46] | |
| Pakistan | 45 | 2.2 | CF | [43] | |
| Pakistan | 1 | 0 | IgG ELISA | [47] | |
| Pamirs | 189 | 0 | AGDP | [36] | |
| Republic of Macedonia | 158 | 14.6 | IgG ELISA | [24] | |
| Russia (Astrakhan Oblast) | NR | 5.1 | CF, AGDP | [69] | |
| Russia (Rostov Oblast) | 430 | 23.0 | AGDP | [70] | |
| Russia (Rostov Oblast) | 355 | 2.8 | AGDP | [71] | |
| Russia (Rostov Oblast) | 2155 | 0.5–17.0 | AGDP | [72] | |
| Senegal | 1269 | 6.1 | AGDP | [73] | |
| Somalia | 16 | 6.3 | IgG ELISA | [47] | |
| South Africa | 8667 | 28 | RPHI | [37] | |
| South Africa | 6128 | 26.5 | RPHI | [74] | |
| Sudan (North Kurdufan State) | 299 | 7.0 | IgG ELISA | [75] | |
| Sudan (East Darfur State) | 282 | 19.14 | IgG ELISA | [26] | |
| Tajikistan (FRM Tajik SSR, northern) | 184 | 0 | AGDP | [36] | |
| Tajikistan (FRM Tajik SSR) | 1585 | 1.1 | AGDP | [36] | |
| Tajikistan (FRM Tajik SSR) | 775 | 1.1 | AGDP | [76] | |
| Turkmenistan (FRM Turkmen SSR, Ashkhada region) | 199 | 3.5 | AGDP | [32] | |
| Turkmenistan (FRM Turkmen SSR, Geok-Tepe region) | 29 | 31 | AGDP | [32] | |
| Tanzania (central zone: Mpwapwa) | 166 | 0.6 | AGDP | [6] | |
| Tanzania (northern zone: Longido, Monduli, Tengeru) | 256 | 7.4 | AGDP | [6] | |
| Tanzania (Sukumaland) | 209 | 4.8 | AGDP | [6] | |
| Tanzania (Lake Victoria coastal region) | 417 | 6.3 | AGDP | [6] | |
| Turkey (Marmara region) | 201 | 13 | IgG ELISA | [10] | |
| The Netherlands | 7 | 0 | IgG ELISA | [47] | |
| Uganda | 104 | 36.5 | AGDP | [77] | |
| United Arab Emirates | 34 | 0 | IgG ELISA | [47] | |
| Zimbabwe | 763 | 45 | RPHI | [37] | |
| Chickens | Kosovo | 8 | 0 | IgG ELISA | [67] |
| Tajikistan (FRM Tajik SSR) | 136 | 0 | CF, AGDP | [76] | |
| Chickens/ducks† | Kazakhstan | 428 | 0.2 | AGDP | [66] |
| Dogs | South Africa, Zimbabwe | 1978 | 6 | RPHI | [13] |
| Donkeys | Azerbaijan (Sal’yany) | 69 | 18.8 | AGDP | [55] |
| Bulgaria | 103 | 17.4 | AGDP | [56] | |
| Bulgaria (Municipality of Aytos) | 8 | 50 | IgG ELISA | [27] | |
| India (Jammu and Kashmir) | 6 | 0 | CF | [40] | |
| India (Jammu and Kashmir) | 6 | 0 | AGDP | [40] | |
| Tajikistan (FRM Tajik SSR) | 38 | 39.5 | AGDP | [36] | |
| Ducks | Tajikistan (FRM Tajik SSR) | 44 | 0 | CF, AGDP | [76] |
| Goats | Afghanistan | 233 | 9 | AGDP | [18,49] |
| Albania | 10 | 20 | IgG ELISA | [50] | |
| Bulgaria | 411 | 62.3 | AGDP | [56] | |
| Bulgaria (Municipality of Aytos) | 15 | 60 | IgG ELISA | [27] | |
| India | 1 | 0 | IgG ELISA | [47] | |
| India | 117 | 9.4 | AGDP | [15] | |
| India | 45 | 40 | AGDP | [15] | |
| India | 186 | 16.1 | AGDP | [18] | |
| India | 279 | 41.2 | IgG ELISA | [60] | |
| India | 28 | 46.4 | IgG ELISA | [41] | |
| India (Maharashtra, northern West Bengal, Rajasthan) | 146 | 2.1 | IgG ELISA | [42] | |
| India (Ahmadabad) | 76 | 30.3 | IgG ELISA | [42] | |
| India (Jammu and Kashmir) | 75 | 0 | CF | [40] | |
| India (Jammu and Kashmir) | 35 | 0 | AGDP | [40] | |
| Iran | 135 | 36 | AGDP | [9] | |
| Iran | 5 | 40 | IgG ELISA | [47] | |
| Iran (Ardabil Province) | 3 | 33.3 | IgG ELISA | [62] | |
| Iran (Khorasan Province) | 150 | 46 | NR | In [63] | |
| Iran (Isfahan Province) | 21 | 9.5 | IgG ELISA | [63] | |
| Iran | 987 | 24.8 | IgG ELISA | [64] | |
| Iraq | 562 | 49.6 | CF | [35] | |
| Kosovo | 10 | 10 | IgG ELISA | [67] | |
| Mauritania | 27 | 11.1 | IgG ELISA | [78] | |
| Mauritania | 27 | 0 | IgM ELISA | [78] | |
| Niger | 224 | 4.9 | IgG ELISA | [21] | |
| Oman | 146 | 14 | IgG ELISA | [46] | |
| Pakistan | 48 | 0 | CF | [43] | |
| Pakistan | 1 | 0 | IgG ELISA | [47] | |
| Somalia | 14 | 21.4 | IgG ELISA | [47] | |
| Sudan | 356 | 3.9 | RPHI | [57] | |
| Turkey | 76 | 0 | RPHI | [57] | |
| Turkey (Marmara region) | 147 | 66.0 | IgG ELISA | [10] | |
| United Arab Emirates | 21 | 0 | IgG ELISA | [47] | |
| Goats/sheep† | Iran | 201 | 45 | AGDP | [45] |
| Kazakhstan | 832 | 0.4 | AGDP | [66] | |
| Tajikistan (FRM Tajik SSR, central) | 107 | 0.9 | AGDP | [36] | |
| Tajikistan (FRM Tajik SSR) | 326 | 1.5 | AGDP | [76] | |
| Turkmenistan (FRM Turkmen SSR) | 663 | 11.3 | AGDP | [32] | |
| Horses | Bulgaria | 536 | 39 | AGDP | [56] |
| Hungary (Hajdú-Bihar) | 8 | 0 | AGDP | [59] | |
| India | 282 | 1.1 | AGDP | [18] | |
| India (Jammu and Kashmir) | 16 | 0 | CF | [40] | |
| India (Jammu and Kashmir) | 15 | 0 | AGDP | [40] | |
| Iraq | 252 | 58.8 | CF | [35] | |
| Russia (Astrakhan Oblast) | NR | 3.1 | CF, AGDP | [69] | |
| Russia (Rostov Oblast) | NR | Pos | AGDP | [70] | |
| Tajikistan (FRM Tajik SSR) | 71 | 2.8 | AGDP | [76] | |
| Misc. small ruminants/livestock† | Iran (Isfahan Province) | NR | 56 | NR | In [63] |
| Kosovo (excluding sheep) | NR | 14 | IgG ELISA | [79] | |
| Niger | 418 | 10.3 | IgG ELISA | [21] | |
| Senegal | 1269 | 6.1 | AGDP | [73] | |
| Misc. domestic animals† | India | 40 | 2.5 | AGDP | [18] |
| India | 139 | 7.9 | AGDP | [18] | |
| Mules | India (Jammu and Kashmir) | 64 | 0 | CF | [40] |
| India (Jammu and Kashmir) | 41 | 0 | AGDP | [40] | |
| Ostriches | Iran | 5 | 20 | IgG ELISA | [80] |
| South Africa | 92 | 23.9 | RPHI | [17] | |
| Pigs | Egypt | 46 | 0 | CF | [38] |
| India (Maharashtra) | 25 | 0 | IgG ELISA | [42] | |
| Russia (Rostov Oblast) | 151 | 0 | AGDP | [72] | |
| Sheep | Afghanistan (Engil District) | 40 | 75.0 | IgG ELISA | [34] |
| Azerbaijan (Sabirabad and Saatly) | 91 | 16.2 | AGDP | [55] | |
| Azerbaijan (Pushkino) | 89 | 6.7 | AGDP | [55] | |
| Australia | 30 | 0 | IgG ELISA | [47] | |
| Bulgaria | 1190 | 32.9 | AGDP | [56] | |
| Bulgaria (Municipality of Aytos) | 242 | 74 | IgG ELISA | [27] | |
| China (Tarim, Junggar, and Turpan-Hami basins) | 3640 | 12.6 | RPHI | [44]* | |
| Egypt | 52 | 23.1 | CF | [38] | |
| Egypt | 400 | 0 | AGDP, IFA | [20] | |
| Egypt (central) | 174 | 0 | IgG ELISA | [39] | |
| Greece (Kastoria) | 40 | 25.0 | IgG ELISA | [81] | |
| Hungary | 48 | 31.3 | AGDP | [58] | |
| India | 13 | 7.7 | AGDP | [15] | |
| India | 136 | 0 | AGDP | [18] | |
| India | 149 | 0.7 | AGDP | [18] | |
| India | 236 | 32.6 | IgG ELISA | [60] | |
| India | 19 | 47.4 | IgG ELISA | [41] | |
| India (Maharashtra, Rajasthan) | 17 | 35.3 | IgG ELISA | [42] | |
| India (Ahmadabad) | 32 | 50 | IgG ELISA | [42] | |
| India (Jammu and Kashmir) | 38 | 0 | CF | [40] | |
| India (Jammu and Kashmir) | 12 | 0 | AGDP | [40] | |
| Iran | 728 | 38 | AGDP | [9] | |
| Iran | 2 | 0 | IgG ELISA | [47] | |
| Iran (Ardabil Province) | 43 | 41.9 | IgG ELISA | [62] | |
| Iran (Mazandaran Province) | 270 | 3.7 | IgG ELISA | [82] | |
| Iran (Khorasan Province) | 298 | 77.5 | NR | In [63] | |
| Iran (Isfahan Province) | 286 | 12.6 | IgG ELISA | [63] | |
| Iran | 2447 | 58.7 | IgG ELISA | [64] | |
| Iraq | 769 | 57.6 | CF | [35] | |
| Iraq (Basrah, southern Iraq) | 74 | 20 | IgG ELISA | [65] | |
| Kosovo | 30 | 10 | IgG ELISA | [67] | |
| Kosovo | NR | 32.6 | IgG ELISA | [79] | |
| Mauritania | 70 | 20 | IgG ELISA | [78] | |
| Mauritania | 70 | 0 | IgM ELISA | [78] | |
| New Zealand | 67 | 0 | RPHI | [57] | |
| Niger | 271 | 3 | IgG ELISA | [21] | |
| Oman | 34 | 3 | IgG ELISA | [46] | |
| Pakistan | 46 | 0 | CF | [43] | |
| Pamirs | 266 | 0 | AGDP | [36] | |
| Romania (Tulcea, northern Dobrogea) | 471 | 27.8 | IgG ELISA | [33] | |
| Russia (Astrakhan Oblast) | NR | 0.3 | CF, AGDP | [69] | |
| Senegal | 942 | 10.4 | IgG ELISA | [83] | |
| Somalia | 12 | 50 | IgG ELISA | [47] | |
| Somalia | 28 | 0 | RPHI | [57] | |
| Sudan | 1972 | 4.3 | RPHI | [57] | |
| Tajikistan | 614 | 2.6 | AGDP | [36] | |
| Tajikistan (northern) | 379 | 0 | AGDP | [36] | |
| Tajikistan (central) | 82 | 4.9 | AGDP | [36] | |
| Turkey | 95 | 3.2 | RPHI | [57] | |
| Turkey (Marmara region) | 160 | 31.8 | IgG ELISA | [10] | |
| United Arab Emirates | 30 | 0 | IgG ELISA | [47] | |
NR, not reported; FRM, formerly; SSR, Socialist Soviet Republic; AGDP, agar gel diffusion precipitation; CF, complement fixation; ELISA, enzyme linked immunosorbent assay; IFA, immunofluorescence assay; Pos, seropositivity reported; RPHI, reverse passive hemagglutination inhibition assay.
*Personal communication with Drs. Zhihong Hu and Yujiang Zhang for species breakdown of sample count
†Sample numbers and results not differentiated by animal.
Fig 2. Total international CCHFV seroprevalence reported in domestic animals by species.
Seroprevalence determined by sum of seropositive animals over the sum of total animals, sampled internationally. Studies that did not report sample numbers or differentiate between types of animal were excluded.
Domestic animal species are often implicated in CCHFV transmission when human CCHF cases are detected. Sheep have been recognized as very important CCHFV reservoirs in certain endemic regions, and have been epidemiologically linked to human cases on several occasions [64,79,84,85]. In Uzbekistan, three CCHF cases were described in persons involved in the handling of tissue from a cow [86]. Similarly, the first patient in an epizootic of CCHFV in Mauritania became ill shortly after butchering a goat [78]. As such, increased CCHFV IgG seropositivity in livestock often parallels reports of CCHF cases in humans with exposure to livestock (e.g., slaughterers, butchers, and farmers), particularly in those who handle blood and organs from infected livestock [34,87–92]. Conversely, negative seroprevalence results in domestic animal samples reflect either low-level transmission or the absence of CCHFV in those geographic areas. Thus, no evidence of seroprevalence in domestic animals was found in samples from Germany, Italy, the Netherlands, Australia, or New Zealand, all countries with no CCHFV cases reported to date [57].
The tick–vertebrate–tick cycle of CCHFV maintenance is reflected in relative tick abundance and associated animal seroprevalence. Cattle heavily infested with ticks were more likely to be CCHFV seropositive [26,75], and vector control to reduce the tick burden was associated with decreased seroprevalence [75]. Cattle are noted as the most sensitive indicator of low-level CCHFV circulation because they tend to be highly infested with Hyalomma spp. ticks, the numbers of which can be ten times higher than those found on small ruminants [93]. In Iran, following detection of human CCHFV cases in Kurdistan Province in 2007, ticks were collected from cattle, sheep, and goats. Of the collected ticks, 5.6% (5/90) were positive by reverse transcription PCR for CCHFV, and four of the five positive ticks were collected from cattle [94]. While there appears to be an association between the presence of infected ticks and detection of seropositive animals [95], viral RNA in attached ticks does not directly indicate seropositivity in host species, and vice versa: infected ticks have been found on seronegative animals and uninfected ticks on seropositive animals.
Abiotic variation by season, country, and region is reported in CCHFV seroprevalence studies. Studies in Turkmenistan (then Turkmen Soviet Socialist Republic [SSR]) reported an increase in CCHFV seropositive domestic animal species during the summer season, and found large variations between regions and individual farms (seropositivity range 5.9%–32%) [32]. Geographic variation of CCHFV seroprevalence in domestic animals within a single country has also been reported in several studies [10,51,61,82]. Longitudinal studies in Russia (Rostov Oblast) demonstrated considerable variation when repeated sampling was performed in the same location. These studies reported September as the optimum period for detecting precipitating antibodies in this area, with a notable decrease in seroprevalence in the winter–spring period [71]. In support of the recognized endemic transmission cycle of CCHF, variation in seroprevalence is often associated with competent vector distribution, host preference of competent tick vectors, and tick load on a particular animal species. Anti-CCHFV antibody prevalence is highest in biotopes where Hyalomma spp. ticks often predominate. Sustained endemic transmission is found only where Hyalomma spp. ticks are present, and epizootic transmission occurs during periods of increased abundance of these ticks [96]. In the hyperendemic CCHFV region in Turkey, the overall tick infestation rate of livestock was 61.2%; 63.1% of cattle and 56.9% of sheep were infested with one or more tick. The dominant species infesting both cattle and sheep was Hyalomma marginatum [97].
A subset of biotic factors determining domestic animal CCHFV seroprevalence were investigated in Senegalese sheep by Wilson et al., who reported that the sex of the animal did not affect antibody prevalence [83]. Other factors, including increasing age, are consistently associated with higher seroprevalence in domestic animals [26,27,61,75,82]. Age likely reflects repeated exposure potential, as described by Adam et al., who found that calves started to get infected after the age of two, the age at which they are released to pasture for grazing and, thus, are more likely to be exposed to infected ticks [75]. Breed may also play a role: in Sudan, cross-bred cattle were 37 times more likely to be seropositive than endogenous breeds [75]. Further insight into the dynamics of infection in domestic species was provided by a longitudinal serosurvey conducted by Zeller et al. in Senegal from 1989 to 1992 [95]. Investigators collected ticks feeding on two cows and 12 goats, and obtained paired blood samples three times per month. Seropositive animals infested with infected ticks had even higher anti-CCHFV IgG antibody titers than seropositive animals without ticks, supporting the occurrence of reinfection in domestic species. The persistence of anti-CCHFV IgM antibodies in naturally infected animals was found to be 1–2 months [95].
Studies in companion animals are very limited and, thus, difficult to broadly interpret. Antibodies to CCHFV were reported in 6% (n = 1978) of dogs in South Africa and Zimbabwe [13]. In another study, in association with human CCHF cases in Mauritania in 2003, feeding ticks were collected from livestock and dogs. A proportion (five of 56 tested) of Rhipicephalus evertsi evertsi ticks collected from sheep were found to be CCHFV positive by reverse transcription PCR, but none of the five Rhipicephalus sanguineus ticks collected from dogs were positive [78]. While vector competence and host preference may indicate the risk of natural infection and transmission in companion animal species in the absence of serological data, broadly translating vector data to risk of exposure remains complex, as tick data is not always consistent and is influenced by many factors unrelated to the host. For example, CCHFV has been isolated from Rhipicephalus spp. ticks [98]. However, R. sanguineus (brown dog ticks) have been reported as positive or negative for CCHFV depending on the study [44,99]. Additional data on companion animals and associated vector species will aid in more clearly evaluating the role of companion animals in the ecology of CCHFV.
Wild Animals
The seroepidemiological reports of CCHFV in wild animals reviewed herein comprise almost 7,000 samples from over 175 avian, mammalian, and reptilian species (Table 2). Considerable seroprevalence was consistently reported in hares (3%–22%), buffalo (10%–20%), and rhinoceroses (40%–68%). Of the species investigated, those with low reported seroprevalence include elephants (single animal), marmots (no evidence), all non-human primate species (no evidence), and all insectivore rodent species (no evidence). While anti-CCHFV antibodies were not detected in Insectivora rodents, several seropositive hedgehogs (Erinaceus europaeus, Hemiechinus auritus) have been reported, and a substantial tick load of up to 40 larval and nymphal H. marginatum ticks has been described on hedgehog hosts during the peak season of immature tick activity [6,100]. However, the role of hedgehogs in enzootic maintenance appears to be variable by species. H. auritus develop viremia during experimental infection [101] and are considered a natural CCHFV reservoir by serving as a source of CCHFV for feeding ticks. In contrast, in the same study, experimental infection in the European hedgehog (E. europaeus) did not produce detectable viremia, suggesting reduced susceptibility to infection or more efficient viral clearance.
Table 2. CCHFV seroprevalence in wild animals.
| Class | Order | Common name | Scientific name | Country (Region) of Specimen Origin | Seroprevalence | Test | Reference | |
|---|---|---|---|---|---|---|---|---|
| n | % | |||||||
| N/A | N/A | Misc. wild animals | N/A | East Africa (Kenya, Uganda) | 162 | 1.9 | AGDP | [45] |
| Aves | Anseriformes | Common teal, Eurasian teal | Anas crecca | Turkmenistan (FRM Turkmen SSR, Gasan-Kuli region) | 1 | 0 | AGDP | [32] |
| Aves | Anseriformes | Red-billed teal | Anas erythrorhyncha | South Africa | 9 | 0 | RPHI | [17] |
| Aves | Anseriformes | Yellow-billed duck | Anas undulata | South Africa | 91 | 0 | RPHI | [17] |
| Aves | Apodiformes | Little swift | Apus barbatus | South Africa | 15 | 0 | RPHI | [17] |
| Aves | Charadriiformes | Caspian long-legged plover | Charadrius spp. | Turkmenistan (FRM Turkmen SSR, Gasan-Kuli region) | 11 | 0 | AGDP | [32] |
| Aves | Charadriiformes | Eurasian woodcock | Scolopax rusticola | Turkmenistan (FRM Turkmen SSR, Gasan-Kuli region) | 1 | 0 | AGDP | [32] |
| Aves | Charadriiformes | Gull | Larus spp. | Albania (Kukes) | 6 | 0 | IgG ELISA | [50] |
| Aves | Charadriiformes | Grey plover | Pluvialis squatarola | Turkmenistan (FRM Turkmen SSR, Gasan-Kuli region) | 2 | 0 | AGDP | [32] |
| Aves | Charadriiformes | Red-backed sandpiper, dunlin | Erolia alpina, Calidris alpina | Turkmenistan (FRM Turkmen SSR, Gasan-Kuli region) | 4 | 0 | AGDP | [32] |
| Aves | Charadriiformes | Redshank | Tringa spp. | Turkmenistan (FRM Turkmen SSR, Gasan-Kuli region) | 5 | 0 | AGDP | [32] |
| Aves | Charadriiformes | Sanderling | Calidris alba | Turkmenistan (FRM Turkmen SSR, Gasan-Kuli region) | 4 | 0 | AGDP | [32] |
| Aves | Charadriiformes | Snowy plover | Charadrius nivosus | Turkmenistan (FRM Turkmen SSR, Gasan-Kuli region) | 10 | 0 | AGDP | [32] |
| Aves | Ciconiiformes | Abdim’s stork | Ciconia abdimii | South Africa | 7 | 0 | RPHI | [17] |
| Aves | Columbiformes | Eurasian collared dove | Streptopelia decaocto | Albania (Kukes) | 6 | 0 | IgG ELISA | [50] |
| Aves | Columbiformes | European turtle dove | Streptopelia turtur | Albania (Kukes) | 1 | 0 | IgG ELISA | [50] |
| Aves | Columbiformes | Laughing dove | Stigmatopelia senegalensis | South Africa | 14 | 0 | RPHI | [17] |
| Aves | Columbiformes | Rock dove | Columba livia | Albania (Kukes) | 6 | 0 | IgG ELISA | [50] |
| Aves | Columbiformes | Pigeons/doves | Columba spp. | Albania (Kukes) | 5 | 0 | IgG ELISA | [50] |
| Aves | Galliformes | Helmeted guineafowl | Numida meleagris | South Africa | 37 | 5‡ | CELISA | [28] |
| Aves | Galliformes | Rock partridge | Alectoris graeca | Albania (Kukes) | 3 | 0 | IgG ELISA | [50] |
| Aves | Gruiformes | Common moorhen | Gallinula chloropus meridionalis | South Africa | 13 | 0 | RPHI | [17] |
| Aves | Misc. species | n/a | n/a | South Africa | 32 | 0 | RPHI | [17] |
| Aves | Passeriformes | Cape sparrow | Passer melanurus melanurus | South Africa | 5 | 0 | RPHI | [17] |
| Aves | Passeriformes | Eurasian magpie | Pica pica | Russia (Rostov Oblast) | NR | 1 animal | AGDP, IHI | [102] in [6] |
| Aves | Passeriformes | Eurasian tree sparrow | Passer montanus | Albania (Kukes) | 1 | 0 | IgG ELISA | [50] |
| Aves | Passeriformes | Common starling | Sturnus vulgaris | Albania (Kukes) | 1 | 0 | IgG ELISA | [50] |
| Aves | Passeriformes | Hooded crow | Corvus corone cornix | Albania (Kukes) | 5 | 0 | IgG ELISA | [50] |
| Aves | Passeriformes | House sparrow | Passer domesticus | Albania (Kukes) | 5 | 0 | IgG ELISA | [50] |
| Aves | Passeriformes | South masked weaver | Ploceus velatus inustus | South Africa | 16 | 0 | RPHI | [17] |
| Aves | Passeriformes | Red bishop | Euplectes orix | South Africa | 110 | 0 | RPHI | [17] |
| Aves | Passeriformes | Red-billed quelea | Quelea quelea | South Africa | 95 | 0 | RPHI | [17] |
| Aves | Passeriformes | True thrushes | Turdus spp. | Albania (Kukes) | 1 | 0 | IgG ELISA | [50] |
| Aves | Passeriformes | Typical warblers | Sylvia spp. | Albania (Kukes) | 1 | 0 | IgG ELISA | [50] |
| Aves | Passeriformes | Woodchat shrike | Lanius senator | Albania (Kukes) | 1 | 0 | IgG ELISA | [50] |
| Aves | Pelecaniformes | Cattle egret | Bubulcus ibis | South Africa | 39 | 0 | RPHI | [17] |
| Aves | Pelecaniformes | African sacred ibis | Threskiornis aethiopicus aethiopicus | South Africa | 14 | 0 | RPHI | [17] |
| Aves | Struthioniformes | Ostrich | Struthio camelus | South Africa | 9 | 0 | CELISA | [28] |
| Mammalia | Artiodactyla | Black wildebeest | Connochaetes gnou | South Africa, Zimbabwe | 30 | 0 | RPHI | [13] |
| Mammalia | Artiodactyla | Blesbok | Damaliscus dorcas | South Africa, Zimbabwe | 23 | 8.7 | RPHI | [13] |
| Mammalia | Artiodactyla | Blue wildebeest | Connochaetes taurinus | South Africa, Zimbabwe | 51 | 0 | RPHI | [13] |
| Mammalia | Artiodactyla | Blue wildebeest | Connochaetes taurinus | South Africa | 31 | 0 | CELISA | [28] |
| Mammalia | Artiodactyla | Bushbuck | Tragelaphus scriptus | South Africa, Zimbabwe | 8 | 0 | RPHI | [13] |
| Mammalia | Artiodactyla | Bushbuck | Tragelaphus scriptus | South Africa | 1 | 0 | CELISA | [28] |
| Mammalia | Artiodactyla | Red river hog | Potamochoerus porcus | South Africa, Zimbabwe | 3 | 0 | RPHI | [13] |
| Mammalia | Artiodactyla | Duiker | Sylvicapra grimmia | South Africa, Zimbabwe | 12 | 8.3 | RPHI | [13] |
| Mammalia | Artiodactyla | Duiker | Sylvicapra grimmia | South Africa | 1 | 0 | CELISA | [28] |
| Mammalia | Artiodactyla | Common eland | Taurotragus oryx | South Africa, Zimbabwe | 127 | 46 | RPHI | [13] |
| Mammalia | Artiodactyla | Gemsbok | Oryx gazella | South Africa, Zimbabwe | 13 | 46.2 | RPHI | [13] |
| Mammalia | Artiodactyla | Giraffe | Giraffa camelopardalis | South Africa, Zimbabwe | 3 | 100 | RPHI | [13] |
| Mammalia | Artiodactyla | Giraffe | Giraffa camelopardalis | South Africa | 44 | 23 | CELISA | [28] |
| Mammalia | Artiodactyla | Grey rhebok | Pelea capreolus | South Africa, Zimbabwe | 1 | 0 | RPHI | [13] |
| Mammalia | Artiodactyla | Cape grysbok | Raphicerus melanotis | South Africa, Zimbabwe | 13 | 0 | RPHI | [13] |
| Mammalia | Artiodactyla | Lichtenstein’s hartebeest | Sigmoceros lichtensteinii | South Africa | 1 | 0 | CELISA | [28] |
| Mammalia | Artiodactyla | Hippopotamus | Hippopotamus amphibius | South Africa, Zimbabwe | 6 | 0 | RPHI | [13] |
| Mammalia | Artiodactyla | Hippopotamus | Hippopotamus amphibius | South Africa | 15 | 0 | CELISA | [28] |
| Mammalia | Artiodactyla | Impala | Aepyceros melampus | South Africa, Zimbabwe | 211 | 1.4 | RPHI | [13] |
| Mammalia | Artiodactyla | Impala | Aepyceros melampus | South Africa | 47 | 11 | CELISA | [28] |
| Mammalia | Artiodactyla | Klipspringer | Oreotragus oreotragus | South Africa, Zimbabwe | 1 | 0 | RPHI | [13] |
| Mammalia | Artiodactyla | Greater kudu | Tragelaphus strepsiceros | South Africa, Zimbabwe | 78 | 21.8 | RPHI | [13] |
| Mammalia | Artiodactyla | Greater kudu | Tragelaphus strepsiceros | South Africa | 4 | 50 | CELISA | [28] |
| Mammalia | Artiodactyla | Mountain reedbuck | Redunca fulvorufula | South Africa, Zimbabwe | 3 | 33.3 | RPHI | [13] |
| Mammalia | Artiodactyla | Nyala | Tragelaphus angasii | South Africa, Zimbabwe | 5 | 40 | RPHI | [13] |
| Mammalia | Artiodactyla | Nyala | Tragelaphus angasii | South Africa | 1 | 0 | CELISA | [28] |
| Mammalia | Artiodactyla | Red hartebeest | Alcelaphus buselaphus | South Africa, Zimbabwe | 6 | 16.7 | RPHI | [13] |
| Mammalia | Artiodactyla | Southern reedbuck | Redunca arundinum | South Africa, Zimbabwe | 24 | 4.2 | RPHI | [13] |
| Mammalia | Artiodactyla | Roan antelope | Hippotragus equinus | South Africa, Zimbabwe | 2 | 0 | RPHI | [13] |
| Mammalia | Artiodactyla | Roan antelope | Hippotragus equinus | South Africa | 8 | 0 | CELISA | [28] |
| Mammalia | Artiodactyla | Sable antelope | Hippotragus niger | South Africa | 49 | 6 | CELISA | [28] |
| Mammalia | Artiodactyla | Sable antelope | Hippotragus niger | South Africa, Zimbabwe | 28 | 32.1 | RPHI | [13] |
| Mammalia | Artiodactyla | Springbok | Antidorcas marsupialis | South Africa, Zimbabwe | 69 | 1.4 | RPHI | [13] |
| Mammalia | Artiodactyla | Steenbok | Raphicerus campestris | South Africa, Zimbabwe | 12 | 0 | RPHI | [13] |
| Mammalia | Artiodactyla | Suni | Neotragus moschatus | South Africa | 4 | 0 | CELISA | [28] |
| Mammalia | Artiodactyla | Common tsessebe | Damaliscus lunatus | South Africa | 2 | 0 | CELISA | [28] |
| Mammalia | Artiodactyla | Common tsessebe | Damaliscus lunatus | South Africa, Zimbabwe | 1 | 0 | RPHI | [13] |
| Mammalia | Artiodactyla | Warthog | Phacochoerus aethiopicus | South Africa, Zimbabwe | 40 | 5 | RPHI | [13] |
| Mammalia | Artiodactyla | Warthog | Phacochoerus aethiopicus | South Africa | 21 | 0 | CELISA | [28] |
| Mammalia | Artiodactyla | Waterbuck | Kobus ellipsiprymnus | South Africa, Zimbabwe | 9 | 44.4 | RPHI | [13] |
| Mammalia | Carnivora | Aardwolf | Proteles cristatus | South Africa, Zimbabwe | 4 | 0 | RPHI | [13] |
| Mammalia | Carnivora | Banded mongoose | Mungos mungo | South Africa, Zimbabwe | 1 | 0 | RPHI | [13] |
| Mammalia | Carnivora | Bat-eared fox | Otocyon megalotis | South Africa, Zimbabwe | 10 | 0 | RPHI | [13] |
| Mammalia | Carnivora | Black-backed jackal | Canis mesomelas | South Africa, Zimbabwe | 6 | 0 | RPHI | [13] |
| Mammalia | Carnivora | Cape fox | Vulpes chama | South Africa, Zimbabwe | 1 | 0 | RPHI | [13] |
| Mammalia | Carnivora | Cape grey mongoose | Herpestes pulverulentus | South Africa, Zimbabwe | 1 | 0 | RPHI | [13] |
| Mammalia | Carnivora | Caracal | Felis caracal | South Africa, Zimbabwe | 17 | 0 | RPHI | [13] |
| Mammalia | Carnivora | Cheetah | Acinonyx jubatus | South Africa, Zimbabwe | 1 | 0 | RPHI | [13] |
| Mammalia | Carnivora | Cheetah | Acinonyx jubatus | South Africa | 14 | 0 | CELISA | [28] |
| Mammalia | Carnivora | Clawless otter | Aonyx capensis | South Africa, Zimbabwe | 1 | 0 | RPHI | [13] |
| Mammalia | Carnivora | Fox | Vulpes spp. | Tajikistan (FRM Tajik SSR) | 5 | 0 | CF, AGDP | [76] |
| Mammalia | Carnivora | Common genet/small-spotted genet | Genetta genetta | South Africa | 1 | 0 | CELISA | [28] |
| Mammalia | Carnivora | Common genet/small-spotted genet | Genetta genetta | South Africa, Zimbabwe | 10 | 0 | RPHI | [13] |
| Mammalia | Carnivora | Genet | Genetta g. senegalensis | Senegal | NR | Positive | [73] in [6] | |
| Mammalia | Carnivora | Honey badger | Mellivora capensis | South Africa, Zimbabwe | 1 | 0 | RPHI | [13] |
| Mammalia | Carnivora | Leopard | Panthera pardus | South Africa, Zimbabwe | 1 | 0 | RPHI | [13] |
| Mammalia | Carnivora | Leopard | Panthera pardus | South Africa | 6 | 0 | CELISA | [28] |
| Mammalia | Carnivora | African lion | Panthera leo | South Africa | 116 | 0 | CELISA | [28] |
| Mammalia | Carnivora | Red fox | Vulpes vulpes | Russia (Rostov Oblast) | 5 | 40 | IHI (neg by AGDP) | [102] in [6] |
| Mammalia | Carnivora | Red fox | Vulpes vulpes | Turkmenistan | NR | Positive | NR | [103] in [6] |
| Mammalia | Carnivora | Pallas’s cat | Felis manul (now Otocolobus manul) | Turkmenistan | NR | Positive | NR | [103] in [6] |
| Mammalia | Carnivora | Small spotted cat | Felis nigripes | South Africa, Zimbabwe | 1 | 0 | RPHI | [13] |
| Mammalia | Carnivora | Striped polecat | Ictonyx striatus | South Africa, Zimbabwe | 5 | 0 | RPHI | [13] |
| Mammalia | Carnivora | Suricate | Suricata suricatta | South Africa, Zimbabwe | 3 | 33.3 | RPHI | [13] |
| Mammalia | Carnivora | Water mongoose | Atilax paludinosus | South Africa, Zimbabwe | 1 | 0 | RPHI | [13] |
| Mammalia | Carnivora | African wildcat | Felis lybica | South Africa, Zimbabwe | 3 | 0 | RPHI | [13] |
| Mammalia | Carnivora | African wild dog | Lycaon pictus | South Africa | 62 | 5 | CELISA | [28] |
| Mammalia | Carnivora | Yellow mongoose | Cynictis penicillata | South Africa, Zimbabwe | 7 | 0 | RPHI | [13] |
| Mammalia | Cetartiodactyla | African buffalo | Syncerus caffer | South Africa, Zimbabwe | 287 | 20 | RPHI | [13] |
| Mammalia | Cetartiodactyla | African buffalo | Syncerus caffer | South Africa | 312 | 10 | CELISA | [28] |
| Mammalia | Chiroptera | Bats | Misc. spp. | France | 19 | 15.3 | AGDP | [104] |
| Mammalia | Chiroptera | Large mouse-eared bat | Myotis blythii omari | Iran | NR | Positive | AGDP | [9] |
| Mammalia | Chiroptera | Common noctule | Nyctalus noctula | Iran | NR | Positive | AGDP | [9] |
| Mammalia | Erinaceomorpha | Hedgehog | Misc spp. | Tajikistan (FRM Tajik SSR) | 4 | 0 | CF, AGDP | [76] |
| Mammalia | Erinaceomorpha | Long-eared hedgehog | Hemiechinus auritus | Turkmenistan (FRM Turkmen SSR) | NR | Positive | AGDP | [103]; [105] in [6] |
| Mammalia | Erinaceomorpha | South African hedgehog | Erinaceus frontalis | South Africa, Zimbabwe | 8 | 0 | RPHI | [13] |
| Mammalia | Hyracoidea | Rock hyrax | Procavia capensis | South Africa, Zimbabwe | 19 | 0 | RPHI | [13] |
| Mammalia | Insectivora | Dark-footed forest shrew | Myosorex cafer | South Africa, Zimbabwe | 2 | 0 | RPHI | [13] |
| Mammalia | Insectivora | Elephant shrew | Elephantulus spp. | South Africa, Zimbabwe | 112 | 0 | RPHI | [13] |
| Mammalia | Insectivora | Musk shrew | Crocidura spp. | South Africa, Zimbabwe | 23 | 0 | RPHI | [13] |
| Mammalia | Insectivora | Round-eared elephant shrew | Macroscelides proboscideus | South Africa, Zimbabwe | 31 | 0 | RPHI | [13] |
| Mammalia | Lagomorpha | Cape hare | Lepus capensis | South Africa, Zimbabwe | 62 | 22.6 | RPHI | [13] |
| Mammalia | Lagomorpha | Cape hare | Lepus capensis | Turkmenistan | NR | Positive | CF, AGDP | [103] in [6] |
| Mammalia | Lagomorpha | European hare | Lepus europaeus | Russia (Rostov Oblast) | 20 | 20 | IHI (neg by AGDP) | [102] in [6] |
| Mammalia | Lagomorpha | European hare | Lepus europaeus | Hungary | 198 | 6 | IgG ELISA, IFA | [23] |
| Mammalia | Lagomorpha | Greater red rock hare | Pronolagus crassicaudatus | South Africa, Zimbabwe | 13 | 0 | RPHI | [13] |
| Mammalia | Lagomorpha | Hare | Lepus spp. | South Africa, Zimbabwe | 49 | 14.3 | RPHI | [13] |
| Mammalia | Lagomorpha | Hare | Lepus spp. | South Africa | 63 | 0 | CELISA | [28] |
| Mammalia | Lagomorpha | Hare | Lepus spp. | Albania (Kukes) | 4 | 0 | IgG ELISA | [50] |
| Mammalia | Lagomorpha | Hare | Lepus spp. | Bulgaria | 33 | 3 | AGDP | [56] |
| Mammalia | Lagomorpha | Hare | Lepus spp. | Iran | NR | Positive | NR | [106] in [6] |
| Mammalia | Lagomorpha | Jameson’s red rock hare | Pronolagus radensis | South Africa, Zimbabwe | 4 | 0 | RPHI | [13] |
| Mammalia | Lagomorpha | Red rock hare | Pronolagus spp. | South Africa, Zimbabwe | 9 | 0 | RPHI | [13] |
| Mammalia | Lagomorpha | Scrub hare | Lepus saxatilis | South Africa, Zimbabwe | 131 | 14.5 | RPHI | [13] |
| Mammalia | Lagomorpha | Smith’s red rock hare | Pronolagus rupestris | South Africa, Zimbabwe | 25 | 0 | RPHI | [13] |
| Mammalia | Perissodactyla | Black rhinoceros | Diceros bicornis | South Africa | 5 | 40 | CELISA | [28] |
| Mammalia | Perissodactyla | Black rhinoceros | Diceros bicornis | South Africa, Zimbabwe | 5 | 60 | RPHI | [13] |
| Mammalia | Perissodactyla | Burchell’s zebra | Equus burchelli | South Africa, Zimbabwe | 93 | 17 | RPHI | [13] |
| Mammalia | Perissodactyla | White rhinoceros | Ceratotherium simum | South Africa, Zimbabwe | 8 | 50 | RPHI | [13] |
| Mammalia | Perissodactyla | White rhinoceros | Ceratotherium simum | South Africa | 31 | 68 | CELISA | [28] |
| Mammalia | Perissodactyla | Zebra | Equus burchelli | South Africa | 28 | 7 | CELISA | [28] |
| Mammalia | Primata | Chacma baboon | Papio ursinus | Kenya | 226 | 0 | AGDP | [77] |
| Mammalia | Primata | Chacma baboon | Papio ursinus | South Africa | 21 | 0 | CELISA | [28] |
| Mammalia | Primata | Chacma baboon | Papio ursinus | South Africa, Zimbabwe | 289 | 0 | RPHI | [13] |
| Mammalia | Primata | Vervet monkey | Cercopithecus pygerythrus | South Africa, Zimbabwe | 233 | 0 | RPHI | [13] |
| Mammalia | Primata | Vervet monkey | Cercopithecus pygerythrus | South Africa | 1 | 0 | CELISA | [28] |
| Mammalia | Proboscidea | African bush elephant | Loxodonta africana | South Africa, Zimbabwe | 211 | 0.5 | RHPI | [13] |
| Mammalia | Proboscidea | African bush elephant | Loxodonta africana | South Africa | 23 | 0 | CELISA | [28] |
| Mammalia | Rodentia | African marsh rat | Dasymys incomtus | South Africa, Zimbabwe | 1 | 0 | RPHI | [13] |
| Mammalia | Rodentia | Angoni vlei rat | Otomys angoniensis | South Africa, Zimbabwe | 1 | 0 | RPHI | [13] |
| Mammalia | Rodentia | Brown rat | Rattus norvegicus | South Africa, Zimbabwe | 6 | 0 | RPHI | [13] |
| Mammalia | Rodentia | Brown rat | Rattus norvegicus | Pakistan | 9 | 22.2 | CF | [43] |
| Mammalia | Rodentia | Karoo bush rat | Otomys unisulcatus | South Africa, Zimbabwe | 52 | 0 | RPHI | [13] |
| Mammalia | Rodentia | Bushveld gerbil | Tatera leucogaster | South Africa, Zimbabwe | 61 | 9.8 | RPHI | [13] |
| Mammalia | Rodentia | Cape ground squirrel | Xerus inauris | South Africa, Zimbabwe | 37 | 2.7 | RPHI | [13] |
| Mammalia | Rodentia | Coypu | Myocastor coypus | Tajikistan (FRM Tajik SSR) | 156 | 0 | CF, AGDP | [36,76] |
| Mammalia | Rodentia | Gerbil | Meriones crassus | Iran | NR | Positive | AGDP | [9] |
| Mammalia | Rodentia | Great gerbil | Rhombomys opimus | Turkmenistan (FRM Turkmen SSR, Bakharden region) | 18 | 0 | AGDP | [32] |
| Mammalia | Rodentia | Highveld gerbil | Tatera brantsii | South Africa, Zimbabwe | 224 | 2.2 | RPHI | [13] |
| Mammalia | Rodentia | House mouse | Mus musculus | South Africa, Zimbabwe | 11 | 0 | RPHI | [13] |
| Mammalia | Rodentia | House rat | Rattus rattus | South Africa, Zimbabwe | 40 | 0 | RPHI | [13] |
| Mammalia | Rodentia | House rat | Rattus rattus | Pakistan | 54 | 1.9 | CF | [43] |
| Mammalia | Rodentia | Indian bush rat | Golunda ellioti | Pakistan | 1 | 0 | CF | [43] |
| Mammalia | Rodentia | Indian bush rat | Golunda ellioti | Tajikistan (FRM Tajik SSR) | 16 | 0 | CF, AGDP | [36,76] |
| Mammalia | Rodentia | Indian desert jird | Meriones hurrianae | Pakistan | 33 | 9 | CF | [43] |
| Mammalia | Rodentia | Indian gerbil | Tatera indica | Pakistan | 47 | 19 | CF | [43] |
| Mammalia | Rodentia | Indian palm squirrel | Funambulus pennanti | Pakistan | 2 | 0 | CF | [43] |
| Mammalia | Rodentia | Lesser bandicoot rat | Bandicota bengalensis | Pakistan | 2 | 0 | CF | [43] |
| Mammalia | Rodentia | Libyan jird (red-tailed) | Meriones libycus | Tajikistan (FRM Tajik SSR) | 4 | 0 | CF, AGDP | [76] |
| Mammalia | Rodentia | Long-clawed ground squirrel | Spermophilopsis leptodactylus | Turkmenistan (FRM Turkmen SSR, Bakharden region) | 1 | 0 | AGDP | [32] |
| Mammalia | Rodentia | Long-tailed marmot | Marmota caudata | Tajikistan (FRM Tajik SSR, Murgab region) | 288 | 0 | AGDP | [36] |
| Mammalia | Rodentia | Long-tailed marmot | Marmota caudata | Tajikistan (FRM Tajik SSR, central) | 275 | 0 | AGDP | [36] |
| Mammalia | Rodentia | Misc. rodents | Iraq | 35 | 14.2 | CF | [35] | |
| Mammalia | Rodentia | Misc. rodents | Iran | 175 | 2.9 | AGDP | [45] | |
| Mammalia | Rodentia | Multimammate mouse | Mastomys spp. (coucha, natalensis) | South Africa, Zimbabwe | 245 | 0.3 | RPHI | [13] |
| Mammalia | Rodentia | Muskrat | Ondatra zibethicus | Tajikistan (FRM Tajik SSR, northern) | 35 | 0 | CF, AGDP | [36,76] |
| Mammalia | Rodentia | Namaqua gerbil | Desmodillus auricularis | South Africa, Zimbabwe | 58 | 0 | RPHI | [13] |
| Mammalia | Rodentia | Namaqua rock rat | Aethomys namaquensis | South Africa, Zimbabwe | 95 | 1.1 | RPHI | [13] |
| Mammalia | Rodentia | Cape porcupine | Hystrix africaeaustralis | Tajikistan (FRM Tajik SSR) | 1 | 0 | CF, AGDP | [76] |
| Mammalia | Rodentia | Cape porcupine | Hystrix africaeaustralis | South Africa, Zimbabwe | 8 | 12.5 | RPHI | [13] |
| Mammalia | Rodentia | Cape porcupine | Hystrix africaeaustralis | South Africa | 2 | 0 | CELISA | [28] |
| Mammalia | Rodentia | South African pouched mouse | Saccostomus campestris | South Africa, Zimbabwe | 3 | 0 | RPHI | [13] |
| Mammalia | Rodentia | South African springhare | Pedetes capensis | South Africa, Zimbabwe | 33 | 12.1 | RPHI | [13] |
| Mammalia | Rodentia | Pygmy mouse | Mus minutoides | South Africa, Zimbabwe | 8 | 0 | RPHI | [13] |
| Mammalia | Rodentia | Red veld rat | Aethomys chrysophilus | South Africa, Zimbabwe | 35 | 0 | RPHI | [13] |
| Mammalia | Rodentia | Short-tailed bandicoot rat | Nesokia indica | Pakistan | 7 | 0 | CF | [43] |
| Mammalia | Rodentia | Small five-toed jerboa | Allactaga spp. | Tajikistan (FRM Tajik SSR) | 2 | 0 | CF, AGDP | [76] |
| Mammalia | Rodentia | Griselda’s striped grass mouse | Lemniscomys griselda | South Africa, Zimbabwe | 5 | 0 | RPHI | [13] |
| Mammalia | Rodentia | Soft-furred rat | Rattus (Millardia) meltada | Pakistan | 2 | 0 | CF | [43] |
| Mammalia | Rodentia | Four-striped grass mouse | Rhabdomys pumilio | South Africa, Zimbabwe | 344 | 0.6 | RPHI | [13] |
| Mammalia | Rodentia | Acacia rat | Thallomys paedulcus | South Africa, Zimbabwe | 2 | 0 | RPHI | [13] |
| Mammalia | Rodentia | Turkestan rat | Rattus pyctoris | Tajikistan (FRM Tajik SSR) | 8 | 0 | CF, AGDP | [76] |
| Mammalia | Rodentia | Vlei rat | Otomys irroratus | South Africa, Zimbabwe | 36 | 0 | RPHI | [13] |
| Reptilia | Squamata | Blunt-nosed viper | Macrovipera lebetina | Tajikistan | 1 | 0 | CF, AGDP | [76] |
| Reptilia | Squamata | European legless lizard (sheltopusik) | Pseudopus apodus | Tajikistan (FRM Tajik SSR) | 4 (or 5) | 0 | CF, AGDP | [36,76] |
| Reptilia | Testudinata | Horsfield’s tortoise | Testudo horsfieldii | Tajikistan | 60 | 1.6%‡ | AGDP | [6,36] |
‡ Only known report of seropositive result in taxonomic order.
AGDP, agar gel diffusion precipitation; CELISA, competitive ELISA; CF, antibody complement fixation; IHI, indirect hemagglutination inhibition test; N/A, not applicable; NR, not reported; RPHI, reverse passive hemagglutination-inhibition assay; FRM, formerly; SSR, Soviet Socialist Republic.
Two reports have found antibodies to CCHFV in representatives of the mammalian order Chiroptera. Using the AGDP test with antigens prepared from CCHFV strains isolated in then-Soviet republics, antibodies were detected in blood sera from two bats in France, from an area bordering with Spain [104]. The species sampled were not specified, and this remains the only report of CCHFV seroprevalence in France. One additional study in northern Iran reported evidence by AGDP in Chiroptera species, in the sera of the greater mouse-eared bat and the common noctule [9]. While these reports appear to be the only evidence of CCHFV infection in bats, recent investigations into bat viruses suggest that there are other species of nairoviruses circulating in bat populations. Using modern sequencing techniques, the first bat nairovirus was identified in French insectivorous bat specimens [107], and a novel nairovirus was isolated from Zambian bats [108].
In reptiles, anti-CCHFV antibodies were detected in one Horsfield’s tortoise (Testudo horsfieldii) trapped in early June in Bul’yoni-Bolo winter camp in the Dangara region of Tajikistan [36]. There are several conflicting reports as to the total sample size of the study, ranging from four to 209 tortoises [6,36]; reported seroprevalence is based on the most detailed report provided by T.P. Pak [36]. Other limited investigations of reptile samples did not yield any evidence of antibodies to CCHFV [36,76]. However, a recent report detected CCHFV in Hyalomma aegyptium [109], the tortoise tick, suggesting that tortoises may be similar to certain bird species (discussed below), in which infected ticks are commonly found feeding on the animal, and CCHFV transmission to ticks may occur even in the absence of detectable antibodies in the host.
Birds
Many bird species are important hosts for Hyalomma ticks and can transport ticks over long distances [110,111]. The transport of CCHFV-infected ticks by birds is a current topic of concern regarding regional spread of the virus [29,112]. Historical studies found birds associated with cattle pastures to be important in feeding immature tick stages, and that rooks (Corvus frugilegus) were particularly important; an increase in CCHF cases was associated with increased rook populations [113]. However, CCHFV infection and the presence or absence of an antibody response in avian species remains unclear. The majority of serosurveys of wild avian species report no serological evidence of CCHFV infection in birds, despite investigation of numerous species and substantial sample pools (Table 2). This absence of viremia is interesting, as some species support large numbers of CCHFV-infected ticks [6,69]. This observation has been supported by experimental infection; the red-billed hornbill (Tockus erythrorhynchus) was found to replicate CCHFV without detectable viremia and was able to infect immature Hyalomma rufipes ticks [114,115]. However, another experimental infection study of mostly ground-feeding birds suggested that anti-CCHFV antibodies may be produced following infection; blue-helmeted guineafowl (Numida meleagris), for example, developed low-level viremia followed by a transient antibody response [17]. Studies on Anseriformes and Galliformes species are also conflicting. In pathogenicity studies, experimentally infected domestic chickens were found to be refractory to CCHFV infection [17]. However, a 0.2% CCHFV seroprevalence in chickens and ducks (n = 428) was reported in Kazakhstan [66].
The absence of detectable anti-CCHFV antibodies in birds may reflect limitations in assay sensitivity. Most of the serological surveys on birds in the former USSR were based on the AGDP test [6], and several studies have shown that the AGDP test is less sensitive than the RPHI or IFA tests for detection of CCHFV antibodies [13,17,37]. More recent investigations, however, suggest that past reports accurately reflect the absence of antibody production, and that most species of birds do not appear to develop viremia. An investigation by Shepherd et al. on the sera of 460 birds of 37 species failed to detect antibodies to CCHFV [17]. However, the absence of antibody production is not universal to all bird species. Ostriches appear to be an exception amongst avian species in harboring and possibly transmitting CCHFV to humans. In the above-mentioned studies by Shepherd et al., anti-CCHFV antibodies were found in 22/92 (23.9%) ostriches (Struthio camelus). Of note, antibodies were detected in 6/9 (66.6%) ostriches in association with a human CCHF case in a worker who became ill after slaughtering ostriches on a farm in South Africa [17]. Additionally, 1/5 (20%) ostriches tested in association with four CCHF cases in workers from two ostrich farms in Iran were also found to be positive for CCHFV IgG [80]. Experimental infection has shown that viremia in ostriches is very short in duration [116].
CCHFV Isolation from Animals
Experimental studies suggest that many animal species develop a transient viremia, and thus may play a role in transmitting CCHFV to ticks in nature. However, reports of CCHFV isolation from animals are limited. CCHFV has been isolated from a febrile cow in Kenya, cattle and a goat in a Nigerian abattoir, a goat placed as a sentinel for arboviruses in Senegal, European hares in Crimea, and a hedgehog in Nigeria (Table 3). Further supporting serological data, in an extensive study in endemic foci in Russia (Astrakhan Oblast), no virus was isolated from over 350 bird specimens representing 35 species.
Table 3. CCHFV isolation from domestic and wild animals.
| Common name | Scientific name | Country of Origin | No. Isolates | Reference |
|---|---|---|---|---|
| Cattle | Bos spp. | Kenya (Nakaru) | 1 | [18] |
| Nigeria | 4 | [117] | ||
| European hare | Lepus europaeus | Ukraine (Crimea) | 3 | [49] |
| Goat | Capra spp. | Nigeria | 1 | [117] |
| Senegal (Bandia Forest) | 1 | [6,18] | ||
| Hedgehog | Hemiechinus auritus | Crimea | 0/17 | [118] |
| Erinaceus albiventris | Nigeria | 1 | [117] | |
| Misc. birds | Russia (Astrakhan Oblast) | 0/360 | In [6] |
The paucity of CCHFV isolates from animals likely reflects a relatively brief viremic period and difficulty in identifying infected animals due to absent or mild clinical disease [119–121]. The majority of reported CCHFV isolations are from ticks or human case-patients. This is a result of an increased relative likelihood of isolation and, in turn, a preference for tick and human case specimens for isolation attempts. However, inability to isolate CCHFV from vertebrate animals does not necessarily indicate a lack of infection in these animals, and does not rule them out as potential CCHFV hosts capable of spreading disease to humans.
Discussion
A large amount of research investigating the role of animals in transmission and maintenance of CCHFV was performed beginning in the late 1960s and 1970s. This work was instrumental in identifying mammalian species, particularly livestock, as critical in the maintenance of CCHFV and as sources of human exposure. The knowledge gained from these studies has also been important in developing prevention and control strategies such as the use of acaricides on livestock in endemic regions. Recently, numerous studies have provided additional information on known reservoir species and provided country-specific information on animal species with notable roles in CCHFV maintenance.
The reports summarized herein must be considered broadly and examined for trends and not specifics due to several factors. Reported seroprevalence may be biased by sample size, seasonality, and diversity in sampling sites, since if one animal is seropositive, additional positive animals are likely to be found in that location at that time. In addition, these reports used a variety of serological assays. There are caveats to interpretation of individual assay results [12], and direct comparison of results from a variety of assays is confounded by variation in assay sensitivity and specificity. Several groups have performed direct comparisons of the reported serological assays [20,36,69,122]; however, results of the comparisons themselves will vary depending on the conditions of the specific assay and the species investigated. Also, several iterations of the same format of serological tests have been used over the years, making generalized statements about their relative reliability challenging. Comparison of serological techniques for use in animals has been performed for other zoonotic viral hemorrhagic fevers [123]. For CCHFV, the merits and pitfalls of several of the serological assays were reviewed by Hoogstraal [6], who advises that most earlier seroepidemiological results be regarded as suggestive of CCHFV seropositivity but not as positive proof.
Overall, serological detection methods have improved over time. Technological advances, including the advent of ELISA assays, allow detection of low amounts of infectious virus or of inactivated antigen and antibodies to CCHFV, and have been shown to be more sensitive, specific, rapid, and reproducible than CF, IFA, RPHI, or AGDP [124]. ELISAs are generally considered the preferred method of serological investigation for CCHFV. However, sandwich ELISA techniques cannot be applied successfully to all species [28], necessitating further advances in testing, including a CELISA that was validated during an extensive CCHFV serological survey in South Africa [28]. Of note, species-specific validations of ELISAs have been performed; Qing et al. evaluated a recombinant nucleoprotein-based system for IgG detection in sheep sera [125], and Mertens et al. developed an ELISA for CCHFV IgG antibodies in bovine sera, showing it to have >98% diagnostic sensitivity and specificity [24].
Finally, there is also the potential for cross-reactivity with other related nairoviruses such as Dugbe virus, Nairobi sheep disease, and Qalyub viruses [20,25]. Antibodies to other nairoviruses may exist independently or in conjunction with CCHFV-specific antibodies. Thus, reports of seroprevalence in areas not previously identified to have CCHFV transmission would benefit from additional surveillance, such as tick studies, to help support novel identification of CCHFV foci.
Irrespective of the nuances of serological assay interpretation and incongruity, the data from the studies summarized here, importantly, indicate broad areas with endemic transmission and highlight reservoir species with the highest potential to affect public health. Some species may serve as direct sources of viral transmission (e.g., viremic livestock, ostriches), whereas others aid principally in maintaining high levels of CCHFV endemicity (e.g., hares). These data also highlight species that could present a risk but have not previously been implicated in human cases, such as camels that are replacing cattle use in certain regions due to climate change [126].
With extensive areas of endemic transmission, the issue of CCHFV importation via animal hosts, ticks, or human cases is a critical concern. Importation of livestock was highlighted in a 1994–1995 CCHFV outbreak in the United Arab Emirates; CCHFV sequences from the patients of this outbreak were identical or closely related to those from three Hyalomma spp. ticks obtained from livestock recently imported from Somalia [127]. It is not clear, however, whether the imported animals were infected at the time of importation or more susceptible to infection upon arrival. Williams et al. [46] reported higher seroprevalence in imported sheep and goats than in indigenous animals, which was attributed to increased susceptibility of naïve animals and virus circulation within the quarantine areas. A subset of the sheep sampled was from Western Australia, a region in which no CCHFV-competent vectors have been reported. The majority of imported animals surveyed from Australia had been in Oman for more than 30 days and, although reported as tick-free upon entry, had high levels of Hyalomma spp. infestation at the time of sampling, providing opportunity for CCHFV exposure. Importation of human cases has also occurred. To date, four human cases of CCHF have been imported into a non-endemic country: in 2004, a case was imported into France from Senegal [128]; in 2009, a US soldier entered Germany from Afghanistan; in 2012, an infected person arrived in the United Kingdom from Afghanistan; and in 2014, another came into the UK from Bulgaria. Other unconfirmed reports include a suspected case imported to the UK from Zimbabwe in 1997 and into Germany from Bulgaria in 2001 [129].
CCHFV is widely distributed, circulates in numerous vertebrate species, and can be transmitted to humans in several ways. Serosurveillance of animals will continue to be an essential tool for monitoring levels of endemic transmission and for investigating areas where CCHFV is not known to circulate. The importance of timely assessment of the potential role of domestic and wildlife species in disease introduction and emerging disease response is very important in the case of CCHFV. Our report summarizes data from international studies investigating the presence of antibodies to CCHFV in domestic and wild animals. We provide comprehensive species-specific information and highlight the appropriate literature serving as a critical resource in future discussion of putative importation and extension of known CCHFV endemicity.
Key Learning Points
Anti-CCHFV antibodies are detected in a wide spectrum of domestic and wild animals from many countries.
Cattle, followed by sheep and goats, have been investigated in the largest number of seroepidemiological studies.
Despite a high tick burden in many avian species, anti-CCHFV antibodies have not been detected in birds, with the exception of guinea fowl and ostriches.
Epidemiological evidence and serological data show that handling livestock species (i.e., cattle, sheep, goats, ostriches) can serve as a source of disease transmission to humans.
CCHFV seroepidemiological data in animals is an indicator of potential disease foci.
Top Five Papers
Causey OR, Kemp GE, Madbouly MH, David-West TS. Congo virus from domestic livestock, African hedgehog, and arthropods in Nigeria. Am J Trop Med Hyg. 1970;19(5): 846–50.
Hoogstraal H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol. 1979;15(4): 307–417.
Donets M, Rezapkin G, Ivanov A, Tkachenko E. Immunosorbent assays for diagnosis of Crimean-Congo hemorrhagic fever (CCHF). Am J Trop Med Hyg. 1982;31: 156–62.
Shepherd A, Swanepoel R, Leman P, Shepherd SP. Field and laboratory investigation of Crimean-Congo haemorrhagic fever virus (Nairovirus, family Bunyaviridae) infection in birds. Trans R Soc Trop Med Hyg. 1987;81: 1004–7.
Shepherd AJ, Swanepoel R, Shepherd SP, McGillivray GM, Searle LA. Antibody to Crimean-Congo hemorrhagic fever virus in wild mammals from southern Africa. Am J Trop Med Hyg. 1987;36(1): 133–42. http://www.ncbi.nlm.nih.gov/pubmed/3101526
Acknowledgments
The authors would like to thank Tatyana Klimova for critical editing of the manuscript and Elizabeth Ervin for assistance with figures. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding Statement
This work was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC [to J.R.S.], and by the National Institutes of Health Loan Repayment Award [to J.R.S.]. This work was also supported by CDC and CDC foundation project funded by NIAID grant R01AI109008 [to E.B.]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 2013;100: 159–89. 10.1016/j.antiviral.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 2.Ergonul O. Crimean-Congo hemorrhagic fever virus: new outbreaks, new discoveries. Curr Opin Virol. Elsevier B.V.; 2012;2: 215–20. 10.1016/j.coviro.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 3.Ceylan B, Calica A, Ak O, Akkoyunlu Y, Turhan V. Ribavirin is not effective against Crimean-Congo hemorrhagic fever: Observations from the Turkish experience. Int J Infect Dis. International Society for Infectious Diseases; 2013;17: e799–e801. 10.1016/j.ijid.2013.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arda B, Aciduman A, Johnston JC. A randomised controlled trial of ribavirin in Crimean Congo haemorrhagic fever: ethical considerations. J Med Ethics. 2012;38: 117–120. 10.1136/medethics-2011-100107 [DOI] [PubMed] [Google Scholar]
- 5.Nalca A, Whitehouse CA. Crimean-Congo hemorrhagic fever virus infection among animals In: Ergonul O, Whitehouse C, editors. Crimean–Congo Hemorrhagic Fever: A Global Perspective. Dordrecht, Netherlands: Springer; 2007. pp. 155–165. [Google Scholar]
- 6.Hoogstraal H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol. 1979;15: 307–417. [DOI] [PubMed] [Google Scholar]
- 7.Whitehouse CA. Crimean-Congo hemorrhagic fever. Antiviral Res. 2004;64: 145–60. 10.1016/j.antiviral.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 8.Ergönül O. Crimean-Congo haemorrhagic fever. Lancet Infect Dis. 2006;6: 203–14. 10.1016/S1473-3099(06)70435-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saidi S, Casals J, Faghih MA, Faghih AA. Crimean hemorrhagic fever-Congo (CHF-C) virus antibodies in man, and in domestic and small mammals, in Iran. Am J Trop Med Hyg. 1975;24: 353–357. [DOI] [PubMed] [Google Scholar]
- 10.Tuncer P, Yesilbag K, Alpay G, Dincer E, Girisgin AO, Aydin L, et al. Crimean-Congo Hemorrhagic Fever infection in domestic animals in Marmara region, Western Turkey. Ankara Univ Vet Fak Derg. 2014;61: 49–53. [Google Scholar]
- 11.Gaidamovich S, Klisenko G, Shanoyan N, Obukhova V, Melnikova E. Indirect hemagglutination for diagnosis of Crimean hemorrhagic fever. Intervirology. 1974;2: 181–185. [DOI] [PubMed] [Google Scholar]
- 12.Swanepoel R, Struthers JK, McGillivray GM. Reversed passive hemagglutination and inhibition with Rift Valley fever and Crimean-Congo hemorrhagic fever viruses. Am J Trop Med Hyg. 1983;32: 610–617. [DOI] [PubMed] [Google Scholar]
- 13.Shepherd AJ, Swanepoel R, Shepherd SP, McGillivray GM, Searle LA. Antibody to Crimean-Congo hemorrhagic fever virus in wild mammals from southern Africa. Am J Trop Med Hyg. 1987;36: 133–42. http://www.ncbi.nlm.nih.gov/pubmed/3101526 [DOI] [PubMed] [Google Scholar]
- 14.Chumakov M, Butenko A, Zavodona T, Tkachenko E, Rubin S, Smirnova S. Antigenic relationships between Crimean hemorrhagic fever virus strains isolated from different geographical regions (NAMRU-T853). Mater 16 Nauch Sess Inst Polio Virus Entsef. 1969; 151–152. [Google Scholar]
- 15.Shanmugam J, Smirova S, Chumakov M. Detection of antibodies to CHF-Congo viruses in human and dometic animal blood sera in India. Tr Inst Polio Virus Entsef. 1973;21: 149–152. [Google Scholar]
- 16.Berezin V, Chumakov M, Reshetnikov I, Zgurskaya G. Study of the role of birds in the ecology of Crimean hemorrhagic fever virus. 1971; 94–95.
- 17.Shepherd A, Swanepoel R, Leman P, Shepherd SP. Field and laboratory investigation of Crimean-Congo haemorrhagic fever virus (Nairovirus, family Bunyaviridae) infection in birds. Trans R Soc Trop Med Hyg. 1987;81: 1004–1007. [DOI] [PubMed] [Google Scholar]
- 18.Shanmugam J, Smirnova S, Chamakov M. Presence of Antibody to Arboviruses of the Crimean Haemorrhagic Fever-Congo (CHF-Congo) Group in Human Beings and Domestic Animals in India. Indian J Med Res. 1976;64: 1403–1413. [PubMed] [Google Scholar]
- 19.Saluzzo JF, Digoutte JP, Camicas JL, Chauvancy G. Crimean-Congo haemorrhagic fever and Rift Valley fever in south-eastern Mauritania. Lancet. 1985;1: 116 http://www.ncbi.nlm.nih.gov/pubmed/2857020 [DOI] [PubMed] [Google Scholar]
- 20.Morrill JC, Soliman AK, Imam IZ, Botros BA, Moussa MI, Watts DM. Serological evidence of Crimean-Congo haemorrhagic fever viral infection among camels imported into Egypt. J Trop Med Hyg. 1990;93: 201–204. [PubMed] [Google Scholar]
- 21.Mariner JC, Morrill J, Ksiazek TG. Antibodies to hemorrhagic fever viruses in domestic livestock in Niger: Rift Valley fever and Crimean-Congo hemorrhagic fever. Am J Trop Med Hyg. 1995;53: 217–21. http://www.ncbi.nlm.nih.gov/pubmed/7573699 [DOI] [PubMed] [Google Scholar]
- 22.Gergova I, Kamarinchev B. Comparison of the prevalence of Crimean-Congo hemorrhagic fever virus in endemic and non-endemic Bulgarian locations. J Vector Borne Dis. 2013;50: 265–70. http://www.ncbi.nlm.nih.gov/pubmed/24499848 [PubMed] [Google Scholar]
- 23.Németh V, Oldal M, Egyed L, Gyuranecz M, Erdélyi K, Kvell K, et al. Serologic evidence of Crimean-Congo hemorrhagic fever virus infection in Hungary. Vector Borne Zoonotic Dis. 2013;13: 270–2. 10.1089/vbz.2012.1011 [DOI] [PubMed] [Google Scholar]
- 24.Mertens M, Vatansever Z, Mrenoshki S, Krstevski K, Stefanovska J, Djadjovski I, et al. Circulation of Crimean-Congo Hemorrhagic Fever Virus in the Former Yugoslav Republic of Macedonia Revealed by Screening of Cattle Sera Using a Novel Enzyme-linked Immunosorbent Assay. PLoS Negl Trop Dis. 2015;9: e0003519 10.1371/journal.pntd.0003519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guilherme JM, Gonella-Legall C, Legall F, Nakoume E, Vincent J. Seroprevalence of five arboviruses in Zebu cattle in the Central African Republic. Trans R Soc Trop Med Hyg. 1996;90: 31–33. 10.1016/S0035-9203(96)90468-X [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim AM, Adam I a., Osman BT, Aradaib IE. Epidemiological survey of Crimean Congo hemorrhagic fever virus in cattle in East Darfur State, Sudan. Ticks Tick Borne Dis. Elsevier GmbH.; 2015;6: 439–444. 10.1016/j.ttbdis.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 27.Barthel R, Mohareb E, Younan R, Gladnishka T, Kalvatchev N, Moemen A, et al. Seroprevalance of Crimean–Congo haemorrhagic fever in Bulgarian livestock. Biotechnol Biotechnol Equip. Taylor & Francis; 2014;28: 540–542. 10.1080/13102818.2014.931685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burt FJ, Swanepoel R, Braack LE. Enzyme-linked immunosorbent assays for the detection of antibody to Crimean-Congo haemorrhagic fever virus in the sera of livestock and wild vertebrates. Epidemiol Infect. 1993;111: 547–557. 10.1017/S0950268800057277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palomar AM, Portillo A, Santibanez P, Mazuelas D, Arizaga J, Crespo A, et al. Crimean-Congo Hemorrhagic Fever Virus in Ticks from Migratory Birds, Morocco. Emerg Infect Dis. 2013;19: 260–263. 10.3201/eid1902.121193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindeborg M, Barboutis C, Ehrenborg C, Fransson T, Jaenson TGT, Lindgren P- E, et al. Migratory Birds, Ticks, and Crimean-Congo Hemorrhagic Fever Virus. Emerg Infect Dis. 2012;18 10.3201/eid1812.120718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Estrada-Peña A, Palomar A, Santibáñez P, Sánchez N, Habela M, Portillo A, et al. Crimean-Congo Hemorrhagic Fever Virus in Ticks, Southwestern Europe, 2010. Emerg Infect Dis. 2012;18: 179–180. 10.3201/eid1801.111040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smirnova SE, Nepesova NM, Tachmuradov G, Kir’Yanova AM, Chumakov MP. Data on studying Crimean hemorrhagic fever in Turkmen SSR. NAMRU-T804. Tr Inst Polio Virus Entsef Akad Med Nauk SSSR. 1971;19: 86–91. [Google Scholar]
- 33.Ceianu CS, Panculescu-Gatej RI, Coudrier D, Bouloy M. First Serologic Evidence for the Circulation of Crimean-Congo Hemorrhagic Fever Virus in Romania. Vector-Borne Zoonotic Dis. 2012;12: 718–721. 10.1089/vbz.2011.0768 [DOI] [PubMed] [Google Scholar]
- 34.Mustafa ML, Ayazi E, Mohareb E, Yingst S, Zayed A, Rossi C, et al. Crimean-Congo haemorrhagic fever, Afghanistan, 2009. Emerg Infect Dis. 2011;17: 1940–1941. 10.3201/eid1710.110061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tantawi H, Shony M, Al-Tikriti SK. Antibodies to Crimean-Congo haemorrhagic fever virus in domestic animals in Iraq: a seroepidemiological survey. Int J Zoon. 1981;8: 115–120. [PubMed] [Google Scholar]
- 36.Pak TP. Epidemiology of Crimean hemorrhagic fever in Tadzhik SSR. NAMRU-T1188. Inst Polio Virus Entsef, Akad Med Nauk SSSR Moskva. 1970. p. 26.
- 37.Swanepoel R, Shepherd AJ, Leman P, Shepherd SP, Mcgillivray M, Erasmus MJ, et al. Epidemiologic and clinical features of Crimean-Congo hemorrhagic fever in Southern Africa. Amercian J Top Med Hygeine. 1987;36: 120–132. [DOI] [PubMed] [Google Scholar]
- 38.Darwish M, Imam I, Omar F, Hoogstraal H. Results of a preliminary seroepidemiological survey for Crimean-Congo hemorrhagic fever virus in Egypt. Acta virol. 1978;22: 77 [PubMed] [Google Scholar]
- 39.Horton KC, Wasfy M, Samaha H, Abdel-Rahman B, Safwat S, Abdel Fadeel M, et al. Serosurvey for zoonotic viral and bacterial pathogens among slaughtered livestock in egypt. Vector Borne Zoonotic Dis. 2014;14: 633–9. 10.1089/vbz.2013.1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodrigues FM, Padbidri VS, Ghalsasi GR, Gupta NP, Mandke VB, Pinto BD, et al. Prevalence of Crimean haemorrhagic fever-Congo virus in Jammu & Kashmir State. Indian J Med Res. 1986; 134–138. [PubMed] [Google Scholar]
- 41.Yadav PD, Gurav YK, Mistry M, Shete AM, Sarkale P, Deoshatwar AR, et al. Emergence of Crimean-Congo hemorrhagic fever in Amreli District of Gujarat State, India, June to July 2013. Int J Infect Dis. 2014;18: 97–100. 10.1016/j.ijid.2013.09.019 [DOI] [PubMed] [Google Scholar]
- 42.Mourya DT, Yadav PD, Shete AM, Gurav YK, Raut CG, Jadi RS, et al. Detection, isolation and confirmation of Crimean-Congo hemorrhagic fever virus in human, ticks and animals in Ahmadabad, India, 2010–2011. PLoS Negl Trop Dis. 2012;6: e1653 10.1371/journal.pntd.0001653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darwish MA, Hoogstraal H, Roberts TJ, Ghazi R, Amer T. A sero-epidemiological survey for Bunyaviridae and certain other arboviruses in Pakistan. Trans R Soc Trop Med Hyg. 1983;77: 446–450. 10.1016/0035-9203(83)90108-6 [DOI] [PubMed] [Google Scholar]
- 44.Sun S, Dai X, Aishan M, Wang X, Meng W, Feng C, et al. Epidemiology and phylogenetic analysis of Crimean-Congo hemorrhagic fever viruses in Xinjiang, China. J Clin Microbiol. 2009;47: 2536–2543. 10.1128/JCM.00265-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chumakov M, Smirnova S. Detection of antibodies to CHF in wild and domestic animal blood sera from Iran and Africa. NAMRU T1072. Tezisy 17 Nauch Sees Inst Posvyashch Aktual Probl Virus Profil Virus Zabolev. 1972; 367–368. [Google Scholar]
- 46.Williams RJ, Al-Busaidy S, Mehta FR, Maupin GO, Wagoner KD, Al-Awaidy S, et al. Crimean-congo haemorrhagic fever: a seroepidemiological and tick survey in the Sultanate of Oman. Trop Med Int Health. 2000;5: 99–106. 10.1046/j.1365-3156.2000.00524.x [DOI] [PubMed] [Google Scholar]
- 47.Khan AS, Maupin GO, Rollin PE, Noor AM, Shurie HH, Shalabi AG, et al. An outbreak of Crimean-Congo hemorrhagic fever in the United Arab Emirates, 1994–1995. Am J Trop Med Hyg. 1997;57: 519–525. [DOI] [PubMed] [Google Scholar]
- 48.Berezin V V., Chumakov MP, Stolbov DN, Butenko AM. On the problem of natural hosts of Crimean hemorrhagic fever virus in Astrakhan region. (In Russian). (In English: NAMRU3-T912). Tr Inst Polio Virusn Entsefalitov Akad Med Nauk SSSR. 1971;19: 210–216. [Google Scholar]
- 49.Chumakov M. Contribution to 30 years of investigation of Crimean hemorrhagic fever. NAMRU-T950. Tr Inst Polio Virus Entsef Akad Med Naul USSR. 1974;22: 5–18. [Google Scholar]
- 50.Papa A, Velo E, Papadimitriou E, Cahani G, Kota M, Bino S. Ecology of the Crimean-Congo hemorrhagic fever endemic area in Albania. Vector Borne Zoonotic Dis. 2009;9: 713–716. 10.1089/vbz.2008.0141 [DOI] [PubMed] [Google Scholar]
- 51.Lugaj A, Bërxhol K. Serological Survey of CCHFV in Cattle in 10 Regions of Albania. IMPACT Int J Res Applied, Nat Soc Sci (IMPACT IJRANSS). 2014;2: 55–60. http://www.impactjournals.us/journals.php?id=14&jtype=2&page=8 [Google Scholar]
- 52.Lugaj A, Koni M, Mertens M, Groschup MH, Berxholi K. Serological survey of Crimean-Congo hemorrhagic fever virus in cattle in Berat and Kolonje, Albania. Albanian j agric sci. 2014; 325–328. [Google Scholar]
- 53.Lugaj A, Bërxholi K. Serological survey of Crimean-Congo hemorrhagic fever virus CCHFV in cattle in Kukes Rreshen, and Gjirokastra regions of Albania. 2nd International conference on Research and Education (ICRAE), ISSN: 2308-0825. Shkodra, Albania; 2014.
- 54.Matevosyan KS, Semashko I, Rubin S, Chumakov M. Antibodies to CHF virus in human and cattle blood sera from Armenia SSR (NAMRU-T939). Tr Inst Polio Virus Entsef Akad Med Nauk SSSR. 1974;22: 173–175. [Google Scholar]
- 55.Chumakov MP, Ismailova S, Rubin S, Smirnova S, Zgurskaya G, Khankishiev AS, et al. Detection of crimean hemorrhagic fever foci in Azerbaijan SSR from results of serological investigations of domestic animals (NAMRU-T941). Tr Inst Polio Virus Entsef Akad Med Nauk SSSR. 1970;18: 120–122. [Google Scholar]
- 56.Vasilenko S, Katsarov G, Mikhailov A, Teckharova M, Levi V, Levi S, et al. Crimean hemorrhagic fever (CHF) in Bulgaria. Tr Inst Polio Virus Entsef. 1971;19: 100–111. [Google Scholar]
- 57.Hassanein K, El-Azazy O, Yousef H. Detection of Crimean-Congo haemorrhagic fever virus antibodies in humans and imported livestock in Saudi Arabia. Trans R Soc Trop Med Hyg. 1997;91: 536–537. [DOI] [PubMed] [Google Scholar]
- 58.Horvath L. Incidence of antibodies to Crimean hemorrhagic fever virus in animals. Acta Microbiol Acad Sci Hung. 1975;22: 61–66. [PubMed] [Google Scholar]
- 59.Horvath LB. Precipitating antibodies to Crimean haemorrhagic fever virus in human sera collected in Hungary. Acta Microbiol Acad Sci Hung. 1976;23: 331–335. [PubMed] [Google Scholar]
- 60.Mourya DT, Yadav PD, Shete A, Majumdar TD, Kanani A, Kapadia D, et al. Serosurvey of Crimean-Congo hemorrhagic Fever virus in domestic animals, Gujarat, India, 2013. Vector Borne Zoonotic Dis. 2014;14: 690–2. 10.1089/vbz.2014.1586 [DOI] [PubMed] [Google Scholar]
- 61.Lotfollahzadeh S, Nikbakht Boroujeni GR, Mokhber Dezfouli MR, Bokaei S. A serosurvey of Crimean-Congo haemorrhagic fever virus in dairy cattle in Iran. Zoonoses Public Health. 2011;58: 54–9. 10.1111/j.1863-2378.2009.01269.x [DOI] [PubMed] [Google Scholar]
- 62.Telmadarraiy Z, Ghiasi SM, Moradi M, Vatandoost H, Eshraghian MR, Faghihi F, et al. A survey of Crimean-Congo haemorrhagic fever in livestock and ticks in Ardabil Province, Iran during 2004–2005. Scand J Infect Dis. 2010;42: 137–41. 10.3109/00365540903362501 [DOI] [PubMed] [Google Scholar]
- 63.Chinikar S, Ghiasi SM, Naddaf S, Piazak N, Moradi M, Razavi MR, et al. Serological Evaluation of Crimean-Congo Hemorrhagic Fever in Humans with High-Risk Professions Living in Enzootic Regions of Isfahan Province of Iran and Genetic Analysis of Circulating Strains. Vector-Borne Zoonotic Dis. 2012;12: 733–738. 10.1089/vbz.2011.0634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mostafavi E, Haghdoost A, Khakifirouz S, Chinikar S. Spatial Analysis of Crimean Congo Hemorrhagic Fever in Iran. Am J Trop Med Hyg. 2013; 10.4269/ajtmh.12-0509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al-Yabis AS, Al-Thamery AAK, Hasony HJ. Seroepidemiology of Crimean-Congo haemorrhagic fever in rural community of Basrah. Med J Basrah Univ. 2005;23: 30–35. [Google Scholar]
- 66.Semashko I, Dobritsa P, Bashkirtsev V, Chumakov M. Results from investigating blood sera from healthy persons, animals, and birds collected in southern Kazakhstan for antibodies to CHF-Congo virus. NAMRU-T1128. Mater 9 Simp Ekol Virus. 1975; 43–44. [Google Scholar]
- 67.Fajs L, Humolli I, Saksida A, Knap N, Jelovšek M, Korva M, et al. Prevalence of Crimean-Congo Hemorrhagic Fever Virus in Healthy Population, Livestock and Ticks in Kosovo. PLoS ONE. 2014;9: e110982 10.1371/journal.pone.0110982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Umoh J, Ezeokoli C, Ogwu D. Prevalence of antibodies to Crimean-haemorrhagic fever-Congo virus in cattle in northern Nigeria. Int J Zoonoses. 1983;10: 151–4. [PubMed] [Google Scholar]
- 69.Berezin V, Chumakov M, Rubin S, Stolbov D, Butenko A, Bashkirtsev V. Contribution to the ecology of Crimean hemorrhagic fever virus in the lower Volga River (NAMRU-T836). Arboviruses. 1969;2: 120–122. [Google Scholar]
- 70.Badalov M, Butenko A, Karinskaya G, Leshchinskaya E, Rubin S, Tkachenko E, et al. Results of serological investigation of the rural population and domestic animals in Rostov Oblast in connection with the problem of prevention (NAMRU-T834). Mater 16 Nauch Sess Inst Polio Virus Entsef. 1969;2: 117–118. [Google Scholar]
- 71.Karinskaya G, Chumakov M, Butenko A, Badalow M, Rubin S. Investigation of Antibodies to Crimean Hemorrhagic Fever Virus in Animal Blood Samples from Rostov Oblast. A Transl Crime Hemorrhagic fever Misc Publ Entomol Soc Am. 1974; 147–149. [Google Scholar]
- 72.Kuchin V, Yanovich T, Butenko A, Kirsanova K. Serological Examination for Antibodies to CHF Virus in Domestic Animals of Rostov Oblast. A Transl Crime Hemorrhagic fever Misc Publ Entomol Soc Am. 1974; 149–150. [Google Scholar]
- 73.Chunikhin SP, Chumakov MP, Butenko A, Smirnova SE, Taufflieb R, Camicas JL, et al. Results investigating human and domestic and wild animal blood sera in the Senegalese Republic (Western Africa) for antibodies to Crimean hemorrhagic fever virus. (In Russian). (In English: NAMRU-3, T810). Mater 16 Nauch Sess Inst Polio Virus Entsef. Moscow, October 21–23; 1969;2: 158–160. [Google Scholar]
- 74.Swanepoel R, Shepherd a J, Leman P a, Shepherd SP. Investigations following initial recognition of Crimean-Congo haemorrhagic fever in South Africa and the diagnosis of 2 further cases. S Afr Med J. 1985;68: 638–641. [PubMed] [Google Scholar]
- 75.Adam I a, Mahmoud MA, Aradaib IE. A seroepidemiological survey of Crimean Congo hemorrhagic fever among Cattle in North Kordufan State, Sudan. Virol J. Virology Journal; 2013;10: 178 10.1186/1743-422X-10-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smirnova SE, Daniyarov OA, Zgurskaya GN, Kasymov KT, Pavlovich AN, Pak TP, et al. Serological investigation of humans and animals in Tadzhik SSR for antibodies to Crimean hemorrhagic fever virus (from the 1968 data). NAMRU-T964. Tr Inst Polio Virus Entsef Akad Med Nauk SSSR. 1971;19: 66–71. [Google Scholar]
- 77.Kirya B, Semenov B, Tretiyakov A, Gromashevsky V, Madzhomba E. Preliminary report on investigating animal sera from East Africa for antibodies to Congo virus by the agar gel diffusion and precipitation method. NAMRU-T1073. Tezisy 17 Nauch Sees Inst Posvyashch Aktual Probl Virus Profil Virus Zabolev. 1972; 368–9. [Google Scholar]
- 78.Nabeth P, Cheikh DO, Lo B, Faye O, Vall IOM, Niang M, et al. Crimean-Congo Hemorrhagic Fever, Mauritania. Emerg Infect Dis. 2004;10: 2143–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Humolli I, Dedushaj I, Zupanac TA, Muçaj S. Epidemiological, serological and herd immunity of Crimean-Congo haemorrhagic fever in Kosovo. Med Arh. 2010;64: 91–93. [PubMed] [Google Scholar]
- 80.Mostafavi E, Chinikar S, Moradi M, Bayat N, Meshkat M, Fard MK, et al. A case report of Crimean Congo hemorrhagic Fever in ostriches in Iran. Open Virol J. 2013;7: 81–3. 10.2174/1874357901307010081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Papa A, Chaligiannis I, Kontana N, Sourba T, Tsioka K, Tsatsaris A, et al. A novel AP92-like Crimean-Congo hemorrhagic fever virus strain, Greece. Ticks Tick Borne Dis. 2014;5: 590–3. 10.1016/j.ttbdis.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 82.Mostafavi E, Chinikar S, Esmaeili S, Amiri FB, Tabrizi AMA, KhakiFirouz S. Seroepidemiological Survey of Crimean-Congo Hemorrhagic Fever Among Sheep in Mazandaran Province, Northern Iran. Vector-Borne Zoonotic Dis. 2012;12: 739–742. 10.1089/vbz.2011.0958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilson ML, Leguenno B, Guillaud M. Distribution of Crimean-Congo hemorrhagic viral antibody in Senegal: environmental and vectorial correlates. Am J Trop Med Hyg. 1990;43: 557–566. [DOI] [PubMed] [Google Scholar]
- 84.Papa A, Sidira P, Kallia S, Ntouska M, Zotos N, Doumbali E, et al. Factors associated with IgG positivity to Crimean-Congo hemorrhagic fever virus in the area with the highest seroprevalence in Greece. Ticks Tick Borne Dis. Elsevier GmbH.; 2013;4: 417–420. 10.1016/j.ttbdis.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 85.Yu-Chen Y, Ling-Xiong K, Ling L, Yu-Qin Z, Feng L, Bao-Jian C, et al. Characteristics of Crimean-Congo hemorrhagic fever virus (Xinjiang strain) in China. Am J Trop Med Hyg. 1985;34: 1179–1182. [PubMed] [Google Scholar]
- 86.Chumakov M, Vafakulov BK, Zavodova T, Karmysheva VY, Maksumov S, Mart’Yanova LI, et al. Cases of Crimean hemorrhagic fever transmission in Uzbekistan through contacts with the blood of a sick cow and a patient and also by tickbites (NAMRU-T1111). Tr Inst Polio Virus Entsef Akad Med Nauk SSSR. 1974;22: 29–34. [Google Scholar]
- 87.Chinikar S, Ghiasi SM, Moradi M, Goya MM, Shirzadi MR, Zeinali M, et al. Geographical distribution and surveillance of Crimean-Congo hemorrhagic fever in Iran. Vector Borne Zoonotic Dis. 2010;10: 705–8. 10.1089/vbz.2009.0247 [DOI] [PubMed] [Google Scholar]
- 88.Kadanali A, Erol S, Ozkurt Z, Ozden K. Epidemiological risk factors for Crimean-Congo hemorrhagic fever patients. Turk J Med Sci. 2009;39: 829–832. 10.3906/sag-0904-49 [DOI] [Google Scholar]
- 89.Sargianou M, Panos G, Tsatsaris A, Gogos C, Papa A. Crimean-Congo hemorrhagic fever: seroprevalence and risk factors among humans in Achaia, western Greece. Int J Infect Dis. International Society for Infectious Diseases; 2013;17: e1160–e1165. 10.1016/j.ijid.2013.07.015 [DOI] [PubMed] [Google Scholar]
- 90.Christova I, Gladnishka T, Taseva E, Kalvatchev N, Tsergouli K, Papa A. Seroprevalence of Crimean-Congo Hemorrhagic Fever Virus, Bulgaria. Emerg Infect Dis. 2013;19: 177–179. 10.3201/eid1901.120299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lwande OW, Irura Z, Tigoi C, Chepkorir E, Orindi B, Musila L, et al. Seroprevalence of Crimean Congo Hemorrhagic Fever Virus in Ijara District, Kenya. Vector-Borne Zoonotic Dis. 2012;12: 727–732. 10.1089/vbz.2011.0914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharifi-Mood B, Metanat M, Alavi-Naini R. Prevalence of crimean-congo hemorrhagic Fever among high risk human groups. Int J high risk Behav Addict. 2014;3: e11520 10.5812/ijhrba.11520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Camicas JL, Wilson ML, Cornet J, Digoutte J, Calvo M, Adam F, et al. Ecology of ticks as potential vectors of Crimean-Congo hemorrhagic fever virus in Senegal: epidemiological implications. Arch Virol. 1990;1: 303–322. [Google Scholar]
- 94.Fakoorziba MR, Golmohammadi P, Moradzadeh R, Moemenbellah-Fard MD, Azizi K, Davari B, et al. Reverse Transcription PCR-Based Detection of Crimean-Congo Hemorrhagic Fever Virus Isolated from Ticks of Domestic Ruminants in Kurdistan Province of Iran. Vector-Borne Zoonotic Dis. 2012;12: 794–799. 10.1089/vbz.2011.0743 [DOI] [PubMed] [Google Scholar]
- 95.Zeller HG, Cornet JP, Diop a, Camicas JL. Crimean-Congo hemorrhagic fever in ticks (Acari: Ixodidae) and ruminants: field observations of an epizootic in Bandia, Senegal (1989–1992). J Med Entomol. 1997;34: 511–516. http://www.ncbi.nlm.nih.gov/pubmed/9379454 [DOI] [PubMed] [Google Scholar]
- 96.Watts DM, Ksiasek T, Unthicum K, Hoogstraal H. Crimean-Congo hemorrhagic fever The arboviruses epidemiology and ecology, vol 2 CRC; Mona T. P. Boca Raton, FL; 1988. pp. 177–260. [Google Scholar]
- 97.Gunes T, Poyraz O, Vatansever Z. Crimean-Congo Hemorrhagic Fever Virus in Ticks Collected from Humans, Livestock, and Picnic Sites in the Hyperendemic Region of Turkey. Vector-Borne Zoonotic Dis. 2011;11: 1411–1416. 10.1089/vbz.2011.0651 [DOI] [PubMed] [Google Scholar]
- 98.Papadopoulos O, Koptopoulos G. Isolation of Crimean-Congo hemorrhagic fever (CCHF) virus from Rhipicephalus bursa ticks in Greece. Acta Microbiol Hell. 1978;23: 20–28. [Google Scholar]
- 99.Keshtkar-Jahromi M, Sajadi MM, Ansari H, Mardani M, Holakouie-Naieni K. Crimean-Congo hemorrhagic fever in Iran. Antiviral Res. 2013;100: 20–8. 10.1016/j.antiviral.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Berezin V V., Povalishina TP, Ermakova RM, Stolbov DN. On the role of birds in feeding immature stages of Hyalomma plumbeum plumbeum ticks-vectors of hemorrhagic fever of the Crimean type in foci of the Volga Delta. (In Russian). (In English, NAMRU3- Tl98). Tr Inst Polio Virusn Entsefalitov Akad Med Nauk SSSR. 1965;7: 296–303. [Google Scholar]
- 101.Blagoveshchenskaya N, Donets M, Zaruna L, Kondratenko VF, Kuchin V. Study of susceptibility to Crimean hemorrhagic fever (CHF) virus in European and long-eared hedgehogs (In Russian). (In English: NAMRU-T985). Tezisy Konf Vop Med Virus. 1975; 269–270. [Google Scholar]
- 102.Zarubinsky VY, Klisenko GA, Kuchin V V., Timchenko V V., Shanoyan NK. Application of the indirect hemagglutination inhibition test for serological investigation of Crimean hemorrhagic fever focus in Rostov Oblast. (In Russian). (In English, NAMRU3-T1145). Sb Tr Inst Virus Im D I Ivanov Akad Med Nauk SSSR. 1975;2: 73–77. [Google Scholar]
- 103.Smirnova SE, Zgurskaya GN, Nepesova NM, Pak TP, Chumakov MP, Chunikhin SP. Examination of animal blood samples in Central Asia for antibodies to Crimean hemorrhagic fever virus (CHF). (In Russian). (In English, NAMRU3-T820). Mater 16 Nauch Sess Inst Polio Virus Entsef. Moscow, October; 1969;2: 146–47. [Google Scholar]
- 104.Tkachenko E, Khanun K, Berezin V. Serological investigation of human and animal sera in agar gel diffusion and precipitation (AGDP) test for the presence of antibodies of Crimean hemorrhagic fever and Grand Arbaud viruses. (In Russian). (In English, NAMRU3-T620). Mater 16 Nauch Sess Inst Polio Virus Entsef. Moscow, October; 1969;2: 265. [Google Scholar]
- 105.Chunikhin SP, Chumakov MP, Smirnova SE, Pak TP, Pavlovich AN, Kuima AU. Division into biocenotic groups of mammals and ixodid ticks in Crimean hemorrhagic foci of southern Central Asia. (In Russian). (In English, NAMRU3-T821). Mater 16 Nauch Sess Inst Polio Virus Entsef. Moscow, October; 1969. pp. 156–57. [Google Scholar]
- 106.Arata AA. The importance of small mammals in public health. Int Biol Program. 1975;5: 349–59. [Google Scholar]
- 107.Dacheux L, Cervantes-Gonzalez M, Guigon G, Thiberge JM, Vandenbogaert M, Maufrais C, et al. A preliminary study of viral metagenomics of french bat species in contact with humans: Identification of new mammalian viruses. PLoS ONE. 2014;9 10.1371/journal.pone.0087194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ishii A, Ueno K, Orba Y, Sasaki M, Moonga L, Hang BM, et al. A nairovirus isolated from African bats causes haemorrhagic gastroenteritis and severe hepatic disease in mice. Nat commincations. 2014; 10.1038/ncomms6651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Široký P, Bělohlávek T, Papoušek I, Jandzik D, Mikulíček P, Kubelová M, et al. Hidden threat of tortoise ticks: high prevalence of Crimean-Congo haemorrhagic fever virus in ticks Hyalomma aegyptium in the Middle East. Parasit Vectors. 2014;7: 101 10.1186/1756-3305-7-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Capek M, Literak I, Kocianova E, Sychra O, Najer T, Trnka A, et al. Ticks of the Hyalomma marginatum complex transported by migratory birds into Central Europe. Ticks Tick Borne Dis. Elsevier GmbH.; 2014;5: 489–493. 10.1016/j.ttbdis.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 111.Jameson LJ, Morgan PJ, Medlock JM, Watola G, Vaux AGC. Importation of Hyalomma marginatum, vector of Crimean-Congo haemorrhagic fever virus, into the United Kingdom by migratory birds. Ticks Tick Borne Dis. Elsevier GmbH.; 2012;3: 95–99. 10.1016/j.ttbdis.2011.12.002 [DOI] [PubMed] [Google Scholar]
- 112.Gale P, Stephenson B, Brouwer a., Martinez M, de la Torre a., Bosch J, et al. Impact of climate change on risk of incursion of Crimean-Congo haemorrhagic fever virus in livestock in Europe through migratory birds. J Appl Microbiol. 2012;112: 246–257. 10.1111/j.1365-2672.2011.05203.x [DOI] [PubMed] [Google Scholar]
- 113.Berezin V, Stolbov D, Povalishchina T, Zimina Y. On the role of rooks in the epidemiology of Crimean hemorrhagic fever in Astrakhan Oblast (NAMRU-T376). Tr Inst Pollo Virus Entsef Akad Mad Nauk SSSR. 1965;7: 304–311. [Google Scholar]
- 114.Zeller HG, Cornet JP, Camicas JL. Crimean-Congo haemorrhagic fever virus infection in birds: field investigations in Senegal. Res Virol. 1994;145: 105–9. http://www.ncbi.nlm.nih.gov/pubmed/8059064 [DOI] [PubMed] [Google Scholar]
- 115.Zeller HG, Cornet JP, Camicas JL. Experimental transmission of Crimean-Congo hemorrhagic fever virus by West African wild ground-feeding birds to Hyalomma Marginatum Rufipes ticks. Am J Trop Med. 1994;50: 676–681. [DOI] [PubMed] [Google Scholar]
- 116.Swanepoel R, Leman PA, Burt FJ, Jardine J, Verwoerd DJ, Capua I, et al. Experimental infection of ostriches with Crimean-Congo haemorrhagic fever virus. Epidemiol Infect. 1998;121: 427–32. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2809542&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Causey OR, Kemp GE, Madbouly MH, David-West TS. Congo virus from domestic livestock, African hedgehog, and arthropods in Nigeria. Am J Trop Med Hyg. 1970;19: 846–850. [DOI] [PubMed] [Google Scholar]
- 118.Rabinovich V, Blagoveshchenskaya N, Butenko A, Zarubina L, Kondratenko VF, Milyutin V. Virological and Serological Examination of Wild Animals and Birds in Rostov Oblast CHF focus (NAMRU-T650). Third Regional Workshop at Rostov-on-Don in May 1970. 1970. pp. 138–139.
- 119.Smirnova SE. A Comparative Study of the Crimean Hemorrhagic Fever-Congo Group of Viruses. Arch Virol. 1979;62: 137–143. [DOI] [PubMed] [Google Scholar]
- 120.Shepherd AJ, Swanepoel R, Cornel A, Mathee O. Experimental studies on the replication and transmission of Crimean-Congo hemorrhagic fever virus in some African tick species. Am J Trop Med Hyg. 1989;40: 326–331. http://www.ncbi.nlm.nih.gov/pubmed/2494900 [DOI] [PubMed] [Google Scholar]
- 121.Fagbami AH, Tomori O, Fabiyi A, Isoun T. Experimental Congo virus (Ib-AN 7620) infection in primates. Virologie. 1975;26: 33–37. http://www.ncbi.nlm.nih.gov/pubmed/814708 [PubMed] [Google Scholar]
- 122.Obukhova V, Klisenko G, Lapina T. Indirect hemagglutination inhibition test for serological diagnosis of CHF (NAMRU-T1378.pdf. Sborn Tr Inst Virus Im D I Ivanov Akad Med Nauk SSSR. 1978;3: 110–113. [Google Scholar]
- 123.Swanepoel R, Struthers JK, Erasmus MJ, Shepherd SP, McGillivray GM, Erasmus BJ, et al. Comparison of techniques for demonstrating antibodies to Rift Valley fever virus. J Hyg (Lond). 1986;97: 317–329. 10.1017/S0022172400065414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Donets M, Rezapkin G, Ivanov A, Tkachenko E. Immunosorbent Assays for Diagnosis of Crimean-Congo Hemorrhagic Fever (CCHF). Am J Trop Med Hyg. 1982;31: 156–162. [DOI] [PubMed] [Google Scholar]
- 125.Qing T, Saijo M, Lei H, Niikura M, Maeda A, Ikegami T, et al. Detection of immunoglobulin G to Crimean-Congo hemorrhagic fever virus in sheep sera by recombinant nucleoprotein-based enzyme- linked immunosorbent and immunofluorescence assays. J Virol Methods. 2003;108: 111–116. http://www.ncbi.nlm.nih.gov/pubmed/12565161 [DOI] [PubMed] [Google Scholar]
- 126.Kagunyu AW, Wanjohi J. Camel rearing replacing cattle production among the Borana community in Isiolo County of Northern Kenya, as climate variability bites. Pastor Res Policy Pract. 2014;4: 13 10.1186/s13570-014-0013-6 [DOI] [Google Scholar]
- 127.Rodriguez LL, Maupin G, Ksiazek TG, Rollin PE, Khan AS, Schwarz TE, et al. Molecular investigation of a multisource outbreak of Crimean-Congo hemorrhagic fever in the United Arab Emirates. Am J Trop Med Hyg. 1997;57: 512–518. [DOI] [PubMed] [Google Scholar]
- 128.Jaureguiberry S, Tattevin P, Tarantola A, Legay F, Tall A, Nabeth P, et al. Imported Crimean-Congo hemorrhagic fever. J Clin Microbiol. 2005;43: 4905–4907. 10.1128/JCM.43.9.4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lumley S, Atkinson B, Dowall SD, Pitman JK, Staplehurst S, Busuttil J, et al. Non-fatal case of crimean-congo haemorrhagic fever imported into the United Kingdom (ex Bulgaria), June 2014. Eurosurveillance. 2014;19: 3–5. 10.2807/1560-7917.ES2014.19.30.20864 [DOI] [PubMed] [Google Scholar]