Abstract
Despite foundational work, we still do not know how therapist behaviors influence brain response and related treatment outcomes for alcohol-using adolescents. Therefore, we examined this question with 17 binge drinking youth (mean age = 16.62 years; 64.3% female; 42.9% Hispanic; 28.6% bi-/multi-racial). In this within-subjects design, all youth completed a baseline assessment, two therapy sessions, an fMRI scan, and were re-evaluated for behavior change at one-month post-treatment. During the fMRI session, youth were presented with two types of therapist responses from their treating therapist: higher-skill therapeutic statements prescribed in an empirically-supported addiction treatment (complex reflections) versus language standard within addiction treatment more broadly (closed questions). In terms of behavior change, at the one-month follow-up, youth showed significant reductions in their number of drinking days and binge drinking days post-treatment. Further, we found main effects for complex reflections and closed questions across the superior middle temporal gyrus and middle temporal gyrus (FWE-corrected, p<.05). Complex questions showed a relatively stronger response than closed questions within the bilateral anterior cingulate gyrus. Additionally, greater BOLD response in the parietal lobe during closed questions was significantly associated with less post-treatment drinking. Finally, lower BOLD responses during both complex reflections and closed questions in the precuneus were associated with greater post-treatment ratings of importance of changing drinking. This study represents a first step in understanding how certain therapist behaviors influence the developing adolescent brain and how that neural response may be associated with youth treatment outcomes in the context of addiction treatment.
Keywords: alcohol, motivational interviewing, fMRI, adolescent, therapist
I. Introduction
Not only do adolescents exhibit high levels of binge drinking (defined as ≥3 drinks/occasion for adolescent females; ≥4 drinks/occasion for adolescent males) [1], binge drinking is associated with a panoply of risk behaviors within this age group [2]. One of the greatest reasons for concern is that binge drinking has been directly linked to an increased incidence of accidents and injuries [1], which is the leading cause for mortality within this age group [3]. Importantly, despite engaging in this risk behavior, binge-drinking youth do not self-refer. Therefore, they are highly unlikely to seek, receive, or complete indicated addiction treatment [4]. Concretely, recent American surveys indicate that 89.6% of individuals with substance use disorders (SUDs) go untreated [5].
Subsequently, it is not only critical to improve access to brief behavioral treatments, it is also critical to improve their efficacy. In other words, one approach is to make behavioral treatments as powerful as possible, so that when youth receive them, they have the greatest possibility for behavior change, defined here as clinically-significant reductions in binge drinking and related harms [6]. One intervention that appears to be an ideal candidate for this context and age group is motivational interviewing (MI) [7]. While not initially developed for youth, this brief, empathic, and strength-based intervention has been well-received in terms of reaching and quickly facilitating therapeutic relationships, particularly with non-treatment seeking youth [8–10]. Qualitatively, adolescents report that the approach of MI resonates with them [11, 12].
Despite its promise, MI is not universally effective for youth. While one of the three strongest evidence-based addictions practices for this age group (http://www.nrepp.samhsa.gov), meta-analyses suggest that effect sizes for behavior change following MI are much less robust for adolescents (d = 0.17) [13] as compared with adults (d = 0.77) [14]. This may be because we do not fully understand how MI works, particularly with younger samples [15]. Thus, innovative approaches are needed to elucidate how MI operates.
One reason why MI might be less powerful with adolescents may stem from natural neurodevelopmental differences within this age group. Studies continue to highlight the unique structure and function of the brain during the adolescent years [16–18]. The field of developmental neuroscience is still unraveling the degree to which the adolescent brain is adaptive (i.e., enabling the drive to explore and gain new experiences) or overly disposed toward dangerous decision-making (i.e., an “immature” system programmed for risk) [19–21]. At this time, the prevailing theoretical frameworks [e.g., “dual process” [22]; “triadic” [23]; “imbalance” [24]], highlight a developmental mismatch, particularly between adolescents’ developing frontal control and reward systems. In this respect, less developed frontal control systems, including the medial frontal gyrus (MFG) and inferior frontal gyrus (IFG), may contribute to adolescents’ difficulty inhibiting impulsive behaviors, and bias youths’ selection of riskier decisions across myriad contexts. In terms of the reward system, critical nodes in the developing brain include the dopaminergic (DA)-pathways of the orbitofrontal cortex (OFC), nucleus accumbens (NAc), ventral striatum (VS), and medial prefrontal cortex (mPFC). These areas are important for adolescents’ evaluation of the magnitude and valence of rewards [e.g., 25, 26], along with processing of social information [e.g., 27, 28, 29]. During adolescence, these regions show relatively greater activation than during other periods of development. This may be a reflection of substantive neurodevelopmental changes during this period, including the redistribution of DA receptor density in the PFC, striatum, and NAc, causing a greater release of DA in response to rewarding events during this timeframe [e.g., 30]. This means that risk taking behaviors, such as drinking, which are inherently exciting, frightening, and fun, may indeed feel much more rewarding during adolescence [e.g., 31, 32]. While well-established in the developmental neurocognitive literature, these differences are only beginning to be examined within the treatment context [15, 33].
In terms of compelling candidates for how and why addiction therapy works (and how and why it does not) [34], most process research in the field of MI has relied upon examinations of audio-recorded MI treatment sessions. This work has revealed that certain statements made within the course of treatment, including those in favor of changing (reducing) substance use (change talk; CT; e.g., “I don’t like who I become when I’ve been drinking”) are strongly associated with successful behavior change [35, 36]. In contrast, this work has also shown that client statements in favor of continuing substance use (sustain talk; ST; e.g., “Drinking is fun”) are associated with continued substance use [34]. Interestingly, most of this research has focused on the client side of this relationship. Yet, studies point to the critical role of therapists in this clinical exchange [37–39]. For instance, following the seminal work of Patterson and Forgatch [40], Glynn and Moyers [41] found that when therapists utilized more skillful techniques prescribed in MI practice, including complex reflections (e.g., “You’ve seen what happens to people at parties when they have passed out”) young substance users provided significantly more CT, as compared with when therapists utilized approaches that are generally not recommended within MI practice and training [7], such as the use of closed questions (e.g., “Did you drink this weekend? How much?”; “Do your parents know?”). This is clinically relevant because many addiction treatment efforts, particularly with youth, rely heavily on therapists’ use of standard addictions approaches generally discouraged in MI, including use of closed questions.
In terms of the neural networks that might be relevant in this therapeutic exchange, adolescent studies have found greater brain response, measured by increases in blood oxygen level dependent (BOLD) activity during CT, in self-reflection and contemplation regions including the posterior cingulate gyrus and precuneus [15]. Importantly, these increases in BOLD activity have been inversely associated with post-treatment use behaviors. In other words, youth who showed greater BOLD response in these regions during CT showed greater reductions in cannabis use, dependence, and problems at the one-month follow-up [15]. Notably, other studies have also underscored the importance of using genuine therapeutic exchanges in research examining youth brain response. Specifically, another study found significantly greater BOLD response among emerging adult binge drinkers when they were re-presented with their own client language from a real MI session, as compared with youth who were re-presented with statements that “sounded like” client language but were not generated in the context of a true therapeutic session [42]. Further, studies continue to suggest that internal factors around importance, readiness, and motivation to change may be salient in this neural response-treatment outcome equation [43].
While behavioral studies of adults have supported the causal chain from therapist behaviors to treatment outcomes [44, 45], we still do not know how therapist behaviors influence treatment response for adolescent binge drinkers. Moreover, we do not know what neural mechanisms are relevant in this equation. Thus, in this study, we utilized a within-subjects design from a neurodevelopmental perspective to understand what happens to adolescents’ brains during two types of therapist statements. We then evaluated how that brain response related to post-treatment behavior change (number of drinking days; importance of changing drinking). Based on prior studies [15], we hypothesized that we would observe greater BOLD response in youths’ medial frontal gyrus (MFG), inferior frontal gyrus (IFG), and insula during therapist statements prescribed in MI (complex reflections) as opposed to the therapist statements that are more common in standard adolescent addiction treatment (closed questions). We also predicted that we would find an inverse association between youths’ BOLD response (MFG, IFG, insula) and post-treatment substance use behaviors.
II. Material and Methods
II.A Participants: Informed Consent and Description
Seventeen unique community-based youth participated in a translational study aimed at reducing adolescent health risk behaviors. Youth were recruited via local outreach methods (e.g., posted signs, word of mouth). All youth were required to be current binge drinkers, defined by current standards for this measurement with adolescents (during the past month ≥3 binge episodes, defined as ≥3 drinks/occasion for females and ≥4 drinks/occasion for males) [46]. To participate, youth were also required to be between 14–19 years of age, have a breath alcohol level of 0 (as measured by breathalyzer), and meet all requisite fMRI safety criteria [47]. Consistent with our prior work, all youth were screened for and excluded if they reported serious medical conditions (e.g., loss of consciousness >5 minutes in the last 6 months, history of brain disease or brain illness such as epilepsy), current psychosis, and/or serious psychopathology (as measured by current prescription medications for these disorders).
Youth aged 18–19 years provided their own informed consent. Youth <18 years of age provided informed assent. Consistent with other studies of high-risk youth [48], parent/guardian informed consent was obtained for youth via telephone. Parent/guardian consent conversations were audio-recorded and logged for proof of consent, and a copy of the consent document was mailed to back to parents. All youth received $60 for their participation. All study procedures were completed with documented approval from the university institutional review board and under the additional protection of a federal certificate of confidentiality. All study procedures reported herein are in line with Journal Article Reporting Standards (JARS) guidelines [49]. This sample was primarily female (64.3%), age 16.62 years (SD = 0.50), Hispanic (42.9%) and bi-/multi-racial (28.6%; see Table 1).
Table 1.
Demographic Characteristics of Participating Sample
| Variable | Mean | SD / % | Range | Significant Change from pre-post (p) |
|---|---|---|---|---|
| Gender | ||||
| Male (n) | 5 | 35.7% | ||
| Female (n) | 9 | 64.3% | ||
| Race/Ethnicity | ||||
| Hispanic | 42.9% | |||
| Bi/Multi-racial | 28.6% | |||
| Asian | 14.3% | |||
| Native American | 7.0% | |||
| Caucasian | 7.0% | |||
| Age (years) | 16.62 | 0.50 | 15–19 | |
| Age at first drink (years) | 13.86 | 1.23 | 11–15 | |
| Age at regular drinking (years) | 15.43 | 1.28 | 13–17 | |
| Baseline importance of changing | 2.62 | 3.22 | 0–10 | |
| Baseline past-month drinking days2 | 4.07 | 3.02 | 0–10 | |
| Baseline past-month binge drinking2 | 3.29 | 2.73 | 0–8 | |
| Baseline past-month cannabis days2 | 6.36 | 10.92 | 0–30 | |
| Baseline past-month tobacco days2 | 11.93 | 10.20 | 0–30 | |
| Baseline alcohol-related problems3 | 11.57 | 12.19 | 0–45 | |
| Follow-up importance of changing | 2.46 | 3.38 | 0–8 | .822 |
| Follow-up drinking days2 | 0.86 | 1.10 | 0–30 | <.001 |
| Follow-up binge drinking days2 | 0.43 | 0.85 | 0–30 | <.001 |
| Follow-up alcohol-related problems3 | 8.86 | 8.17 | 0–24 | .23 |
| Follow-up cannabis use days2 | 5.29 | 10.92 | 0–30 | .54 |
| Follow-up tobacco use days2 | 6.36 | 10.20 | 0–30 | .05 |
Readiness rulers (casaa.unm.edu);
Timeline FollowBack (measuring past month);
Rutgers Alcohol Problem Index (RAPI)
As most youth tend toward binge drinking on the days that they consume alcohol, consistent with our prior work with other samples of young drinkers [42], we focused target measurements on drinking days and binge drinking days, rather than drinks per drinking day. For this group of young people, in terms of current drinking, all youth reported drinking in the past month (M=4.07 drinking days; SD=3.02; Table 1), with most drinking days meeting criteria for binge drinking (M=3.29 binge drinking days; SD=2.73). Importantly, this sample also reported experiencing high levels of alcohol-related problems (M RAPI=11.57; SD=12.19). In addition, at the commencement of the study, most of the sample did not place a high value on the importance of changing their drinking (M=2.62; SD=3.22). As with many studies of adolescents who tend toward polysubstance use [50], this sample also reported some other substance use (past month substance use = 35.7% cannabis; 50% tobacco).
II.B Measures
All youth completed measures of demographics, substance use, and readiness to change, as well as fMRI measures. While recent adolescent studies support the reliability and validity of adolescent self-report in assessing substance use behavior [51, 52], in line with other recent adolescent substance use studies [53, 54], we employed a combination of objective biological measures in addition to self-report to provide an additional layer of information.
II.B.1 Demographic Measure
This measure queried age, gender, self-reported race/ethnicity, age at first drink, and age of first regular drinking.
II.B.2 Breath Alcohol
Breath alcohol was measured via breathalyzer (Alco-Sensor IV by Intoximeters; www.intox.com) at each appointment. In terms of objective alcohol use, none of the youth in this study had a breath alcohol level of >0.000 at any appointment during this study.
II.B.3 Timeline FollowBack. [TLFB; 55]
This an interviewer-administered measure that utilizes a calendar format to collect adolescents’ quantity and frequency of substance use during the past month. From this measure, we derived past month totals for overall number of alcohol use days and number of binge drinking days.
II.B.4 Rutgers Alcohol Problems Index [RAPI; 56]
This 23-item measure evaluated youths’ experience of alcohol-related problems. Items within this measure query whether and to what degree drinking has affected youth in numerous areas (e.g., difficulties cutting back and controlling drinking; problems with school performance).
II.B.5 Readiness Rulers (casaa.unm.edu)
This visual ruler analog evaluates youths’ perceived importance of changing their drinking, on a scale from 0, which denotes not at all important, to 10, which indicates extremely important.
II.C Procedures
Recent meta-analyses of MI with youth indicate that a single session of MI can generate observable behavior change (weighted M effect sizes range from d = 0.00–0.80), with the strongest effect sizes being observed within 6 months post addiction treatment (d = 0.32) [13]. Thus, for this study, we placed the fMRI session after the intervention that was likely to generate the most impact (Session 1). Having the fMRI between treatment sessions also facilitated the opportunity to discuss any concerns that came up during the fMRI experience during the second MI session. Related, in line with prior experimental designs that we have used in other fMRI-based studies of adolescent MI treatment response [15], in order to have the best chance at detecting behavior change, we scheduled the follow-up 1 month after the final treatment session (after Session 2).
Ultimately, for this study, all youth completed a psychosocial assessment, two MI sessions targeting reducing their drinking, an fMRI paradigm designed to assess the impact of therapist language on the adolescent brain, and a behavioral follow-up after one month. All youth completed the baseline behavioral assessment and the first MI session during their first appointment. One week later, all youth completed the fMRI scan, immediately followed by the second MI session. One month after the scan session, all youth completed their final behavioral follow-up.
II.C.1 Intervention Sessions
Similar to other studies of MI with adolescents [57], the intervention condition followed a manualized approach [58]. One master’s level therapist administered all interventions, which were closely overseen by the first author, an experienced MI therapist and trainer. Intervention integrity and fidelity were monitored and maintained by the first author, who reviewed random audio-recorded sessions with the study therapist during weekly supervision. Across both sessions, the therapist relied on MI-consistent approaches, including use of complex reflections, open-ended questions, affirmations, demonstration of accurate empathy, support of youths’ self-efficacy, and efforts to reduce youth resistance. With participant permission, all sessions were audio-recorded for the purposes of gathering the requisite statements for the neuroimaging paradigm and for maintaining intervention integrity and fidelity. Greater detail about intervention session content is readily available upon author request.
II.C.2 MRI Session
All participants were instructed to abstain from alcohol for 24 hours prior to the MRI scan, which was verified by self-report and breath alcohol (level of .000) prior to the scan session. No individuals were excluded due to non-compliance.
II.C.2.A. MRI Parameters
Once comfortable with the fMRI procedures, youth were placed into the scanner for a high-resolution structural scan. This scan provided image registration and normalization. We utilized the standard volume setting for all participants, which was monitored by our MR technicians. As verified in our post-scan questionnaire, no individuals reported any difficulties hearing the presented audio statements.
MR images were collected using a 3.0T Siemens Trio whole body scanner. A 12-channel receive head phased array coil combined with body coil transmission was employed to achieve greater sensitivity in cortical areas. T1-weighted anatomic images were collected with a 5-echo magnetization prepared rapid gradient echo (MPRAGE) sequence [TR/TE/TI = 2300/[1.65, 3.5, 5.36, 7.22, and 9.08 ms]/1.2 s, flip angle = 8°, FOV = 256×256 mm, slice thickness = 1 mm, field of view (FOV) = 256 × 256, Voxel size = 1×1×1 mm]. Functional MR images were collected using a single-shot gradient-echo echo planar sequence with ramp sampling correction using the intercommissural line (AC-PC) as a reference (TR: 2.0 s, TE: 27 ms, flip angle= 75°, matrix size: 64 × 64, 33 slices, voxel size: 3.75 × 3.27 × 4.55 mm, 4 runs, 120 images per run). Four dummy scans were acquired to let the MR signal reach steady state.
II.C.2.B. fMRI Therapist Statement Task
This task was designed to assess the effects of different types of therapist statements, those prescribed in the MI and broader clinical literature (complex reflections) [34] contrasted with standard therapist approaches often utilized in more widespread youth addiction practice (closed questions). This design was based upon prior translational studies with adolescents, which were focused on client language [15, 42]. Based on this foundational work, for this specific sample, we extracted 5 within-session client change talk (CT; e.g., “I’m worried what is going to happen to me when I black out”) and 5 sustain talk statements (ST; e.g., “I don’t want to stop drinking – it’s fun”). However, this evaluation targeted the evaluation of therapist behaviors on adolescent brain response.
For this new paradigm, the first author developed 2 unique complex reflections (“You’re worried about your drinking”; “You’ve seen what has happened to your friends after they’ve blacked out”) and 2 unique closed questions (“Did you drink this weekend?”; “Are you concerned about your safety?”). Importantly, an effort was made to match the semantic correspondence between the client and statement therapists for each trial; as a result, each set of statements “made sense” as a pair, with the therapist statement directly responding to each of the 5 within-session client statements (CT and ST). To be consistent with the youths’ clinical experience with the study therapist, all therapist statements were then audio-recorded by the study therapist in preparation for the fMRI paradigm. In other words, the authors paired five actual statements made by clients during therapy with two study therapist statements that were constructed and recorded specifically for the experiment across multiple trials.
II.C.3 Task Parameters
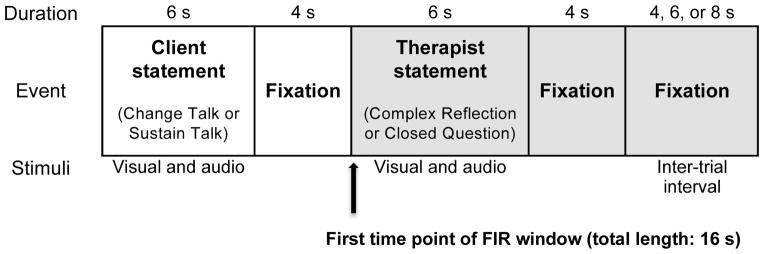
To assess the relationship between type of therapist language and youths’ BOLD response, youth were presented with 4 runs of the following client statement-therapist statement combinations, in an event-related design, over the course of four conditions: (1) client CT/therapist complex reflection, (2) client CT/therapist closed question, (3) client ST/therapist complex reflection, and (4) client ST/therapist closed question. The result was 220 trials encompassing all four of these conditions in a pseudo-random order. The conditions were not presented back-to-back; rather, the presentation included an inter-trial buffer during which no audio was presented. For each run, all trials started with a 6 second presentation of the client statement, followed by a 4-second fixation cross, a 6-second presentation of the therapist statement, and 4-second fixation cross. Trials were jittered using a variable inter-trial interval of 4, 6, or 8 seconds. All statements (client and therapist) were simultaneously presented auditorily (voices represented via headphones) and visually (words presented on screen). See Figure 1 for the schematic of a single trial. During the presentation of both the client statements and the therapist statements, participants were instructed to silently read their visually presented words and listen to the audio-recorded voices.
Figure 1.
Schematic of the fMRI Therapist Statement task. BOLD response to therapist statements was modeled as the 16-second time period from the onset of the audio-visual presentation of each therapist statement (indicated in gray).
Stimuli were delivered using Presentation (v. 16.3, Neurobehavioral Systems, Inc.). To ensure precise temporal integration of stimulus presentation and fMRI data acquisition, the timing of the stimulus presentation was controlled by trigger pulses from the magnet. Task stimuli were presented via rear projection to a mirror system that the participant viewed while in the magnet bore.
II.C.3.A. fMRI Data Analyses
II.C.3.A.1. Image processing
Functional imaging time series was processed using SPM8 (Wellcome Department of Imaging Neuroscience, London, UK). Pre-processing of these volumes started with slice timing correction to correct for temporal differences in acquisition time of the BOLD signal across slices within each volume. This was followed by motion correction using SPM’s realignment module [59]. Criteria for exclusion of data due to excessive motion were set at 2.5 mm (translation) and 2.5 degrees (rotation): 1 subject had high motion in all 3 runs and was therefore excluded from further analysis. All functional data were normalized [60] into the Montreal Neurological Institute (MNI) standard space template in SPM. The resultant time series was then smoothed using a 6 mm (full-width half-maximum, FWHM) Gaussian kernel. During the post-processing module, fMRI signal intensity was scaled by its grand mean, which was the averaged signal intensity over all intra-cerebral voxels, across all volumes acquired within a run. The time series data was high-pass filtered (filter cut-off frequency 1/128.0 Hz) to reduce low frequency noise in MRI signal. Serial correlations in the data were modeled using the autocorrelation correction with order 1 [AR(1)] [61]. Shape-free estimates of hemodynamic response functions were calculated using a finite impulse response approach.
II.C.3.A.2. Statistical analysis plan
We regressed both client and therapist statements for the four conditions separately using a finite impulse response (FIR) window of 8 time points (16 s) (see Figure 1). Parameter estimates were calculated for each time point in the finite impulse response window using a general linear model (GLM) with the motion parameters included as covariates of no interest. Estimates of the signed area under the curve per trial type were obtained. These beta maps were contrasted to generate images for (a) therapist complex reflections, (b) therapist closed questions and (c) complex reflections vs. closed questions. Group-level random-effect analyses were performed using one sample t-tests on contrasts of interest.
For Hypothesis 1, we compared BOLD response in response to therapists’ use of higher-skill statements prescribed according to MI practice (complex reflections) as contrasted with BOLD response in response to therapists’ use of statements according to standard addiction treatment (closed questions). To test Hypothesis 2, the association between youths’ BOLD response and post-treatment behavior change, we conducted correlations of the contrast images (therapist complex reflections, therapist closed questions and complex reflections vs. closed questions) against the following post-treatment outcome variables: follow-up total number of drinking days and follow-up importance to change. These analyses controlled for adolescents’ baseline frequency of drinking (past month drinking days).
We applied cluster size thresholding for all analyses [62] to control family wise error (FWE) rates and account for the effect of multiple voxel comparisons. For each contrast, AFNI’s (http://afni.nimh.nih.gov) 3dFWHMx function was used to estimate the residual variance smoothing kernel. By incorporating the smoothing kernel information, AFNI’s 3dClustSim function calculated a cluster-size threshold at p < 0.05 with a cluster-defining primary threshold of p < 0.005: values obtained for the cluster-size thresholds (k) have been indicated separately for each analysis in the results section. Cluster-size calculations were limited to a mask of cortical and subcortical gray matter. The anatomical localization for all regions of activation and visualization of results was performed using xjview toolbox (http://www.alivelearn.net/xjview).
III. Results
III.A Intervention Outcomes
We retained 82% (n=14) of youth at the one-month follow-up. Consistent with similar studies that have highlighted the challenges of retaining substance-using youth [48], missing youth were unreachable despite repeated staff efforts. In addition, one individual who participated in the final follow-up did not complete the final estimate of importance of changing their drinking, leaving a total n=13 for this comparison.
In terms of post-treatment behavior change, most youth evidenced clinically significant reductions from baseline to follow-up for past month drinking days and past month binge drinking days (p’s<.001; see Table 1) [6]. However, youth showed non-significant reductions in post-treatment alcohol-related problems and importance of changing drinking. In addition, although this intervention targeted alcohol (only), youth also showed non-significant reductions in their post-treatment cannabis and tobacco use (p’s≥05; see Table 1).
III.B. Main Effects of Therapist Language
III.B.1 Complex reflections
To evaluate the impact of therapists’ more skillful MI-consistent statements on the adolescent brain, we began by examining the main effects of complex reflections (vs. implicit baseline). When hearing their therapists’ complex reflections (e.g., “You’re worried about your drinking”), youth showed significant positive BOLD response across frontal (left inferior frontal gyrus; IFG), temporal (right superior temporal gyrus, left middle temporal gyrus), limbic (left amygdala), and occipital regions (left middle and inferior occipital gyri; d’s=2.47–5.96; k≥210 voxels; Table 2).
Table 2. Main Effects for complex reflections, closed questions, and their comparison.
Maximum loci of activation of BOLD response (FWE cluster corrected p<0.05). BA = Brodmann area; R= right; L = left.
| # voxels | Localization | x (mm) | y (mm) | z (mm) | t value | d value |
|---|---|---|---|---|---|---|
| Complex reflections | ||||||
| 6069 | L. Middle occipital gyrus | −32 | −90 | −6 | 10.32 | 5.96 |
| L. Inferior occipital gyrus | −20 | −90 | −10 | 9.06 | 5.23 | |
| 1855 | R. Superior temporal gyrus | 52 | −30 | 8 | 8.99 | 5.19 |
| R. Superior temporal gyrus; BA21 | 62 | −6 | −2 | 8.53 | 4.92 | |
| 3904 | L. Middle temporal gyrus | −54 | −18 | −4 | 8.07 | 4.66 |
| L. Middle temporal gyrus; BA22 | −58 | −28 | 2 | 7.4 | 4.27 | |
| 300 | L. Inferior frontal gyrus/ Pars triangularis | −44 | 28 | −2 | 4.38 | 2.53 |
| L. Inferior frontal gyrus/ Pars triangularis | −34 | 32 | 0 | 3.93 | 2.27 | |
| 230 | - | −22 | 4 | −12 | 4.32 | 2.49 |
| L. Amygdala | −24 | −8 | −14 | 4.28 | 2.47 | |
| Closed questions | ||||||
| 1850 | R. Superior temporal gyrus | 52 | −30 | 6 | 9.73 | 5.62 |
| R. Superior temporal gyrus | 62 | −6 | −4 | 9.13 | 5.27 | |
| 3412 | L. Middle temporal gyrus | −54 | −18 | −4 | 8.77 | 5.06 |
| L. Middle temporal gyrus | −62 | −16 | −4 | 8.75 | 5.05 | |
| 4503 | R. Inferior occipital gyrus | 28 | −94 | −2 | 8.16 | 4.71 |
| R. Calcarine gyrus | 20 | −100 | −6 | 7.55 | 4.36 | |
| Complex reflections > Closed questions | ||||||
| 231 | L. Anterior cingulate gyrus | −2 | 34 | 6 | 4.97 | 2.87 |
| R. Anterior cingulate gyrus | 4 | 36 | 0 | 4.34 | 2.51 | |
III.B.2 Closed questions
To evaluate the effect of therapists’ more standard addiction treatment language on the adolescent brain, we examined the main effects of closed questions (vs. implicit baseline). When hearing their therapists’ closed questions (e.g., “Do your parents know about your drinking?”), youth showed significant positive BOLD response in temporal (right superior temporal gyrus, left middle temporal gyrus) and occipital regions (right inferior occipital gyrus; right calcarine gyrus; d’s=4.36–5.62; k≥209 voxels; Table 2).
III.B.3 Comparison of complex reflections vs. closed questions
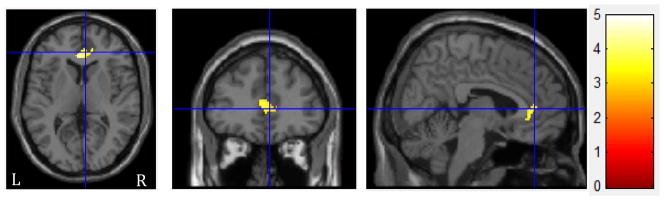
To determine whether adolescents show different neural responses to different types of therapeutic language, we then compared adolescent brain response to complex reflections versus closed questions. We found significantly greater BOLD response to complex reflections vs. closed questions in bilateral anterior cingulate (d’s=2.51–2.87; k≥231 voxels; Table 2, Figure 2). In contrast, there were no areas where youth showed greater BOLD response to closed questions as compared with complex reflections.
Figure 2.
Regions with increased BOLD response during complex reflections > closed questions have been overlaid onto sections of the canonical template. The crosshairs are positioned on right anterior cingulate (BA32). Activations are displayed at FWE cluster corrected p<0.05. The color bar indicates t range. Left (L) and right (R) sides are marked in the bottom of the figure.
III.B.4 Adolescents’ BOLD response to Therapist Language and Post-Treatment Behavior Change
We evaluated how adolescents’ brain response to different types of therapist statements related to post-treatment behavior change. All analyses herein were conducted while controlling for adolescents’ baseline number of drinking days.
III.B.5 Follow-up drinking days
There were no significant correlations between therapists’ complex reflections and adolescents’ post-treatment drinking days. For closed questions, we found a significant negative correlation between therapists’ closed questions and adolescents’ post-treatment drinking days in the parietal lobe with a peak in the angular gyrus (z’s=3.74–3.83; k≥206 voxels; Table 3). Specifically, adolescents’ greater brain response during closed questions was associated with better treatment response (fewer drinking days). There were no significant positive correlations between therapists’ closed questions and youth post-treatment drinking days. Further, in the comparison of youth brain response to complex reflections vs. closed questions, there were no regions in which BOLD response was significantly correlated with follow-up drinking days.
Table 3.
Relationship of BOLD activation during therapist language to youth treatment response, after controlling for baseline number of drinking days. Maximum loci of activation for correlation between follow-up behavior and beta maps (FWE cluster corrected p<0.05). BA = Brodmann area; R= right; L = left. Note: z values are included rather than d values, to better capture the magnitude of the correlation.
| # voxels | Localization | x (mm) | y (mm) | z (mm) | t value | z value |
|---|---|---|---|---|---|---|
| Follow-up drinking days: Negative correlation to closed questions | ||||||
| 208 | R. Angular gyrus | 50 | −46 | 30 | 5.6 | 3.93 |
| R. Angular gyrus | 46 | −50 | 36 | 5.33 | 3.81 | |
| R. Supramarginal gyrus | 60 | −40 | 28 | 5.16 | 3.74 | |
| Follow-up importance of changing drinking: Negative correlation to complex reflections | ||||||
| 345 | L. Precuneus | 2 | −74 | 44 | 5.32 | 3.74 |
| L. Precuneus | −12 | −60 | 36 | 5.29 | 3.73 | |
| L. Cuneus | −6 | −76 | 36 | 4.96 | 3.59 | |
| Follow-up importance of changing drinking: Negative correlation to closed questions | ||||||
| 1067 | R. Precuneus | 6 | −74 | 46 | 6.94 | 4.32 |
| L. Superior parietal lobule | −16 | −64 | 64 | 6.81 | 4.28 | |
| L. Precuneus | −2 | −68 | 56 | 5.96 | 3.99 | |
III.B.6 Follow-up importance of changing drinking
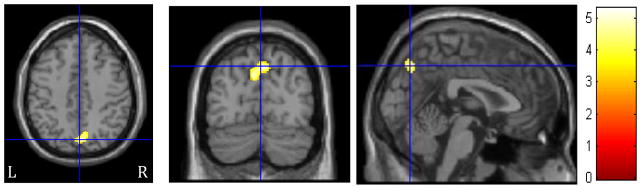
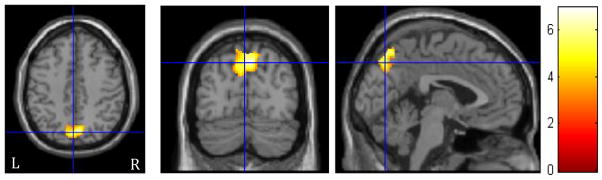
There were no significant positive correlations between therapists’ complex reflections and adolescents’ post-treatment importance of changing drinking. However, there was a significant negative relationship between therapists’ complex reflections and youths’ post-treatment evaluation of their importance of changing in the precuneus (z’s = 3.59–3.74; k≥197 voxels; Table 3, Figure 3). Specifically, less precuneus activation during complex reflections was associated with youths’ greater ratings of the importance of changing their drinking at the follow-up. Similarly, there were no significant positive correlations between therapists’ closed questions and adolescents’ post-treatment importance of changing drinking. Yet, we found a significant negative relationship between therapists’ closed questions and youths’ post-treatment importance of changing in the left superior temporal lobe and bilateral precuneus (z’s = 3.99–4.32; k≥197 voxels; Table 3, Figure 4). Meaning, less activation in these regions during closed questions was associated with youths’ higher ratings of the importance of changing their drinking at the follow-up. In the comparison of youth brain response to complex reflections vs. closed questions, there were no regions in which BOLD response was significantly correlated with follow-up importance of changing.
Figure 3.
Regions of significant negative correlation for adolescents between BOLD response during complex reflection (CR) and follow-up importance of changing drinking. The crosshairs are positioned on the left precuneus. Activations are displayed at FWE cluster corrected p<0.05. The color bar indicates t values. Left side (L) represents left hemisphere.
Figure 4.
Regions of significant negative correlation for adolescents between BOLD response during closed questions and follow-up importance of changing drinking. The crosshairs are positioned on the left precuneus (BA 7). Activations are displayed at FWE cluster corrected p<0.05. The color bar indicates t values. Left side (L) represents left hemisphere.
III.B.7 Post hoc examination of age
Given the importance of age on brain structure and function, we conducted a post hoc examination of the effects of normative age development on our target relationships of interest. In this analysis, we retained baseline number of drinking days as a covariate and added age to these analyses. When age was included as an additional covariate in the relationship between adolescents’ brain response to therapist language with follow-up drinking days, there were no longer significant correlations (positive or negative) between youth brain response to therapist language (complex reflections or closed questions) and follow-up drinking days.
We also included age as an additional covariate in the comparison between therapist language and follow-up importance of changing drinking. Even with age in the equation, we observed a very similar pattern to our initial comparison. As with the first set of comparisons, we did not see evidence of positive correlations between therapists’ complex reflections and youths’ post-treatment importance of changing drinking. However, we continued to observe a significant negative relationship between therapists’ complex reflections and youths’ post-treatment evaluation of their importance of changing their drinking in the precuneus (z’s =3.16–3.31; k≥198 voxels; Table 4). In other words, firmly in line with the initial results, less precuneus activation during complex reflections was associated with youths’ greater rating of the importance of changing their drinking at the follow-up. Similarly, as with the initial comparison, when age was entered as an additional covariate into the equation, we observed no significant positive correlations between therapists’ closed questions and adolescents’ post-treatment importance of changing their drinking. However, as with the first set of comparisons with age as a covariate, we still observed a significant negative relationship between therapists’ closed questions and youths’ post-treatment importance of changing in the bilateral precuneus and left superior parietal lobule (z’s =3.75–4.24; k≥192 voxels; Table 4). In other words, we continued to find evidence of a pattern of less activation in these key regions during closed questions associated with youths’ higher ratings of the importance of changing their drinking at the follow-up.
Table 4.
Relationship of BOLD activation during therapist language to youth treatment response, after controlling for both baseline number of drinking days and age. Maximum loci of activation for correlation between follow-up behavior and beta maps (FWE cluster corrected p<0.05). BA = Brodmann area; R= right; L = left. Note: z values are included rather than d values, to better capture the magnitude of the correlation.
| # voxels | Localization | x (mm) | y (mm) | z (mm) | t value | z value |
|---|---|---|---|---|---|---|
| Follow-up importance of changing drinking: Negative correlation to complex reflections | ||||||
| 242 | L. Precuneus | 2 | −74 | 44 | 4.83 | 3.31 |
| L. Precuneus | −12 | −62 | 36 | 4.81 | 3.30 | |
| L. Cuneus | −4 | −76 | 36 | 4.45 | 3.16 | |
| Follow-up importance of changing drinking: Negative correlation to closed questions | ||||||
| 953 | R. Precuneus | 4 | −74 | 50 | 8.00 | 4.24 |
| L. Precuneus | 0 | −78 | 46 | 7.14 | 4.04 | |
| L. Superior parietal lobule | −16 | −64 | 64 | 6.12 | 3.75 | |
IV. Discussion
An emerging body of work is beginning to explore the critical role of therapists in adolescent addiction treatment outcomes [37–39, 63]. Concomitantly, studies are also underscoring the importance of temporal sequence treatment process research, advocating for the collection of neuroimaging data prior to treatment outcomes to provide an empirical prospective link between brain response and treatment outcomes [64]. We utilized this precise approach to evaluate the connection between youth brain response to therapist statements, including the arguably more difficult prescribed approaches within MI (complex reflections) as contrasted with more fundamental therapist responses that are standard in many adolescent addictions treatment settings (closed questions). We then evaluated the relationships between youth BOLD response during these therapist statements and adolescents’ treatment outcomes across two salient indicators of behavior change: post-treatment drinking days and importance of changing drinking.
In line with other exciting studies in this area [13, 15], overall, among this ethnically-diverse sample of adolescents, these findings support that a single session of MI can generate detectable effects with substance-using adolescents. Here, youth showed clinically-significant behavior change in their quantity and frequency of drinking, as evidenced by a reduction by over half of their drinking days and binge drinking days at the one month follow-up [6]. However, in contrast to expectations, youth did not show clinically significant changes in alcohol-related problems or perceived importance of changing drinking post-treatment. As youth showed clinically relevant change in one set of alcohol behaviors, but not in the other, one interpretation is that the behavior changes observed here do not simply reflect an across-the-board regression to the mean [65], but rather indicate youths’ true, but notably partial, behavior change in the context of this intervention. Another explanation for the observed treatment outcomes revolves around the persistence of alcohol-related problems. While some have found significant reductions in youths’ substance-related problems at one-month post-treatment [15], due to the sustained nature of alcohol-related sanctions, many youth continue to contend with alcohol-related consequences months after successfully decreasing their drinking [66, 67].
In terms of our findings in the context of the importance of changing drinking measure, some contend that this metric is inherently difficult to use, as youths’ initial estimates may reflect their true low weighting of the importance of changing drinking, yet poor scores at treatment outcome can both reflect youths’ perception that they have “already” changed (for successful changers), as well as adolescents’ lack of interest in changing (for those who have not successfully changed). Better measures are needed for capturing more nuanced perceptions within the interim period as decisions are being made about whether to change [68].
One surprising outcome within this study was the powerful effect observed for closed questions. At this time, there is an increasing array of studies highlighting the “tough competition” of traditional addiction treatment [69]. To that end, when contrasted with what has been traditionally perceived as “ineffective” comparison groups (e.g., treatment as usual), MI does not consistently yield better outcomes [70, 71]. One clinical argument suggested by these data is that we do not fully understand the salience and potential cognitive power generated by more standard addictions treatment approaches (closed questions).
Concretely, the data in this study revealed that closed questions might have more clinical relevance than we initially proposed. Specifically, both closed questions and complex reflections led to equivalently solid and relevant responses in comparable regions as supported by strong effect sizes across right superior temporal gyrus and left middle temporal gyrus; regions implicated in the broader developmental addictions neuroscience literatures [e.g., 72, 73]. Additionally, complex reflections showed slightly stronger BOLD activation than closed questions in the bilateral anterior cingulate gyrus [ACG], a reward region that has been implicated as a region of risk in the adult neurobiology of addiction [74]. While, in this context, response in the ACG appears to be a positive finding, wherein stronger BOLD response to therapists’ complex reflections implies better response to treatment ingredients, it is also critical to consider that these findings potentially reflect developmental differences. For example, the adult literature suggests that greater activation of the AC is associated with alcohol craving and relapse [75, 76]. Interestingly, the evidence on the relationship between brain volume and function with alcohol use and alcohol related problems has been more mixed in the adolescent literature [77, 78]. Further, it appears that significant differences in AC functional brain response between drinkers and non-drinkers may disappear after a relatively circumscribed period of abstinence (e.g., 1 month) [79]. Ultimately, targeted research on this region is critical to understand its role in adolescent cognitive development, and the potential impact of early alcohol use on the healthy growth and development of this region [e.g., 80].
When evaluating treatment outcomes, we were surprised to find that brain response to complex reflections was unrelated to follow-up drinking days. Only closed questions significantly correlated with post-treatment behavior change, such that greater BOLD response to closed questions in the angular gyrus and supramarginal gyrus was associated with better reductions in post-treatment drinking days. As with developmental differences in ACG response, functional response across other fMRI tasks (e.g., cue exposure, verbal working memory), among adults and adolescents generally suggest that greater BOLD response of these parietal regions (e.g., angular gyrus; supramarginal gyrus) [81, 82] is indicative of risk response. However, the body of work in these areas, and in particular, their connection to treatment outcome, is still very much in its infancy. Concretely, this was also observed within this study, as these relationships appeared to subside when covarying for age. Thus, at this time, it is quite tricky to discern whether these findings may reflect the challenge of “overmodeling” the data, or potentially and compelling, the potential impact of age on these relationships. Future studies are needed in order to understand the influence of age on these relationships, whether greater response in these regions is a marker of risk or positive response, and how greater response in underevaluated parietal areas might relate to treatment outcomes for other adolescents.
Similarly, importance of changing drinking was significantly associated with brain response to both complex questions and closed questions, in an area highly relevant to self-reflection and introspection (precuneus) [83]. Notably, these relationships were robust to the inclusion of age as a covariate in these comparisons. Ultimately, across all comparisons, less activation in the precuneus was associated with a greater valence or estimate of the importance of changing. The involvement of the precuneus is particularly remarkable, as this region has not only been linked to youth risk behavior [84], it is also a hub of a key resting state network, the default mode network [85]. Thus, one reason for the inverse relationship between precuneus activation during closed questions and the follow-up importance of changing may be due to a quieting of this salient neural hub for successful changers and/or those continuing to consider change.
One crucial consideration in this equation is the role of neurodevelopment, particularly the implication of delayed frontal maturation. Specifically, while we did see response within some critical frontal regions (e.g., IFG) in response to complex reflections, we did not see the strength of the pattern expected in critical functional regions, nor the expected connection between frontal response and treatment outcomes. One reason for this might be that we are observing the difficulty that youth have in bringing their frontal lobes on-line; while this is often discussed in the context of self-control and impulsivity, it may also be relevant to adolescents’ ability to engage these frontal regions to participate in and respond to psychotherapy. Thus, one critical question is how best to engage and work with adolescents in the addiction treatment context while the prefrontal cortex [86] continues its final stretch of development (through age 25 for adolescent males) [87].
In terms of clinical implications, these data suggest two things. Most importantly, therapist behaviors were significantly correlated with youth brain response, and those brain responses were significantly connected to adolescents’ successful treatment outcomes. This is an exciting first step in understanding how, where, and which therapist behaviors prime the adolescent brain for behavior change. In other words, these data offer empirical support for observed relationships examined in the adult behavioral literature [44], reflecting that therapist behaviors do generate a significant neural response in adolescents, and, more meaningfully,, one that is directly related to positive behavior change.
These data also underscore the importance of continuing to evaluate the influence and outcome of un- or under-specified therapist interactions, such as the elusive “common factors” that might drive positive treatment outcomes. One explanation of these data is that the quality of the clinical relationship may be what was measured here. In line with the treatment arena [88–90], one cautious interpretation of this work is that these data suggest that strong and effective clinicians can change the adolescent brain and catalyze positive treatment outcomes, even when using approaches that can have been discouraged in the addictions treatment literature (e.g., closed questions) [69, 91]. While larger sample sizes are needed to offer firm support, this exploratory work suggests that the nature of therapeutic statements (complex reflection vs. closed question) may be less important than the therapist who makes them [92]. Future work must move towards quantifying and evaluating these unspecified factors, although this avenue is not completely intuitive. For example, there are many clinical and ethical issues inherent to randomizing youth to low-quality therapists and/or therapists who provide known poor therapeutic interactions. Future creative solutions are requisite.
While there are several strengths to the present study, our findings should be interpreted with an eye to the following limitations. First, and most importantly, while advances are being made in this direction, it is still not possible to conduct MI interventions within the context of fMRI; an in vivo, temporal evaluation of these relationships will offer a significant next step in understanding these data. Here we also speculate about the potential meaning of null findings; while we would be remiss not to report these outcomes, we also exert caution for the offered interpretations. This was a pilot study with a small sample size. Clearly, replication with a larger sample and a control condition is needed to empirically confirm findings. Similarly, because this was a small pilot study, we only had one therapist involved in the study; future studies would benefit from having multiple therapists to evaluate the comparative influence of intra-therapeutic factors. In addition, again due to the small sample size, we were unable to explore the associations of cannabis and tobacco use, including time since last use, on the observed findings; future work with a larger sample must explicitly examine the impact of age, along with these commonly co-occurring substances on our target relationships of interest. Finally, we acknowledge that it would be impractical to use neuroimaging in real-world treatment contexts; thus, we do not suggest that all youth should be imaged before receiving outpatient treatment. Rather, at this time, we suggest that this approach is useful in that it offers a creative and innovative approach to accelerate discovery.
V. Conclusions
In sum, these data offer a first step towards better understanding which youth brain systems should be targeted as influencing mechanisms of change within both the adolescent addictions neuroscience and treatment literatures. This is particularly important, given the still relatively sparse literature on how drinking affects the adolescent brain [93]. This study, which shows how the adolescent brain responds to therapist language, and how that activation may mediate treatment outcomes, offers a first step in this direction [94]. Critically, understanding these mechanisms among youth is essential not only for binge drinking interventions, but also for other problem behaviors, as the brain mechanisms of risk behavior appear to be consistent across substances of abuse [95].
Highlights.
This study aimed to evaluate how therapist language influences the adolescent brain.
We evaluated this question with 17 binge drinking youth.
All youth showed significant reductions in alcohol use post-treatment.
Therapist language was linked to brain response in parieto-temporal, reward, and self-reflection areas.
Brain response in these areas was associated with adolescent behavior change.
Acknowledgments
The authors would like to thank Hollis Karoly, MA for her assistance with this manuscript. These findings were presented in a symposium at the 2014 International Conference on Motivational Interviewing (ICMI).
Footnotes
Declaration of Interest: This research was supported by an Adolescent Alcohol Award from the University of New Mexico Center on Alcoholism, Substance Abuse, and Addictions and 1R01AA023658-01 (PI: Feldstein Ewing). The authors have no competing financial or other conflicts of interest relating to the data included in the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sarah W. Feldstein Ewing, Email: feldstei@ohsu.edu.
Jon M. Houck, Email: jhouck@unm.edu.
Uma Yezhuvath, Email: uma.yezhuvath@advancemri.com.
Ehsan Shokri Kojori, Email: eshoko@gmail.com.
Dustin Truitt, Email: dtruitt@mrn.org.
Francesca M. Filbey, Email: francesca.filbey@utdallas.edu.
References
- 1.CDC, Center for Disease Control and Prevention. Youth Risk Behavior Surveillance Survey. MMWR. 2012;61:SS-162. [Google Scholar]
- 2.Miller JW, et al. Binge drinking and associated health risk behaviors among high school students. Pediatrics. 2007;119:76–85. doi: 10.1542/peds.2006-1517. [DOI] [PubMed] [Google Scholar]
- 3.Heron M. N.C.f.H. Statistics, editor. Deaths: Leading causes for 2010. Hyattsville, MD: 2013. [Google Scholar]
- 4.Alegria M, et al. Disparities in treatment for substance use disorders and co-occurring disorders for ethnic/racial minority youth. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50(1):22–31. doi: 10.1016/j.jaac.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SAMSHA. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-41. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2011. [Google Scholar]
- 6.Miller WR, Manuel JK. How large must a treatment effect be before it matters to practitioners? An estimation method and demonstration. Drug and Alcohol Review. 2008;27:524–528. doi: 10.1080/09595230801956165. [DOI] [PubMed] [Google Scholar]
- 7.Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3. New York: Guilford Press; 2013. [Google Scholar]
- 8.Peterson PL, et al. Short-term effects of a brief motivational intervention to reduce alcohol and drug risk among homeless adolescents. Psychology of Addictive Behaviors. 2006;20(3):254–264. doi: 10.1037/0893-164X.20.3.254. [DOI] [PubMed] [Google Scholar]
- 9.McCambridge J, Slym RL, Strang J. Randomized controlled trial of motivational interviewing compared with drug information and advice for early intervention among young cannabis users. Addiction. 2008;103(11):1809–1818. doi: 10.1111/j.1360-0443.2008.02331.x. [DOI] [PubMed] [Google Scholar]
- 10.D’Amico EJ, et al. Brief motivational interviewing for teens at risk of substance use consequences: A randomized pilot study in a primary care clinic. Journal of Substance Abuse Treatment. 2008;35(1):53–61. doi: 10.1016/j.jsat.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Stern SA, et al. Project CHAT: A brief motivational substance abuse intervention for teens in primary care. Journal of Substance Abuse Treatment. 2007;32(2):153–165. doi: 10.1016/j.jsat.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 12.D’Amico EJ, Osilla KC, Hunter SB. Developing a group motivational interviewing intervention for first-time adolescent offenders at-risk for an alcohol or drug use disorder. Alcoholism Treatment Quarterly. 2010;28(4):417–436. doi: 10.1080/07347324.2010.511076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen CD, et al. Effectiveness of motivational interviewing interventions for adolescent substance use behavior change: A meta-analytic review. Journal of Consulting and Clinical Psychology. 2011;79:433–440. doi: 10.1037/a0023992. [DOI] [PubMed] [Google Scholar]
- 14.Hettema J, Steele J, Miller WR. Motivational Interviewing. Annual Review of Clinical Psychology. 2005;1(1):91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- 15.Feldstein Ewing SW, et al. Integrating brain and behavior: Evaluating adolescents’ response to a cannabis intervention. Psychology of Addictive Behaviors. 2013;27:510–525. doi: 10.1037/a0029767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 17.Blakemore SJ, Choudhury S. Development of the adolescent brain: Implications for executive function and social cognition. Journal of Child Psychology and Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 18.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 19.Mills KL, et al. The developmental mismatch in structural brain maturation during adolescence. Developmental Neuroscience. 2014;36:147–160. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- 20.Giedd JN. The digital revolution and adolescent brain evolution. J Adolesc Health. 2012;51:101–105. doi: 10.1016/j.jadohealth.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sercombe H. Risk, adaptation, and the functional teenage brain. Brain Cogn. 2014;89:61–69. doi: 10.1016/j.bandc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Somerville LH, Jones R, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ernst M. The triadic model perspective for the study of adolescent motivated behavior. Brain Cogn. 2014 doi: 10.1016/j.bandc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casey BJ, Getz S, Galvan A. The Adolescent Brain. Developmental Review. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galvan A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;12(4):6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galvan A, et al. Earlier Development of the Accumbens Relative to Orbitofrontal Cortex Might Underlie Risk-Taking Behavior in Adolescents. The Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyer AE, et al. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development. 2009;80(4):1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masten CL, et al. Neural correlates of social exclusion during adolescence: Understanding the distress of peer rejection. Soc Cogn Affect Neurosci. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dahl R. Understanding the Risky Business of Adolescence. Neuron. 2011:69. doi: 10.1016/j.neuron.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 32.Galvan A, et al. Risk-Taking and the Adolescent Brain: Who is at Risk? Developmental Science. 2007;10(2):F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- 33.Stanger C, et al. Neuroeconomics and adolescent substance abuse: individual differences in neural networks and delay discounting. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:745–755. doi: 10.1016/j.jaac.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller WR, Rose GS. Toward a theory of motivational interviewing. American Psychologist. 2009;64(6):527–537. doi: 10.1037/a0016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baer JS, et al. Adolescent change language within a brief motivational intervention and substance use outcomes. University of Washington; 2008. Manuscript under editorial review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaume J, et al. Does change talk during brief motivational interventions with young men predict change in alcohol use? Journal of Substance Abuse Treatment. 2013;44:177–185. doi: 10.1016/j.jsat.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 37.D’Amico EJ, et al. Group motivational interviewing for adolescents: Change talk and alcohol and marijuana outcomes. Journal of Consulting and Clinical Psychology. 2014 doi: 10.1037/a0038155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnett E, et al. From counselor skill to decreased marijuana use: Does change talk matter? Journal of Substance Abuse Treatment. 2014;46:498–505. doi: 10.1016/j.jsat.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carcone AI, et al. Provider communication behaviors that predict motivation to change in black adolescents with obesity. J Dev Behav Pediatr. 2013;34:599–608. doi: 10.1097/DBP.0b013e3182a67daf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patterson GR, Forgatch MS. Therapist behavior as a determinant for client noncompliance: A paradox for the behavior modifier. Journal of Consulting and Clinical Psychology. 1985;53(6):846–851. doi: 10.1037//0022-006x.53.6.846. [DOI] [PubMed] [Google Scholar]
- 41.Glynn LH, Moyers TB. Chasing change talk: The clinician’s role in evoking client language about change. Journal of Substance Abuse Treatment. 2010;39(1):65–70. doi: 10.1016/j.jsat.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 42.Feldstein Ewing SW, et al. Brain-based origins of change language: A beginning. Addictive Behaviors. 2014;39:1904–1910. doi: 10.1016/j.addbeh.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prisciandaro JJ, et al. Brain activation to cocaine cues and motivation/treatment status. Addiction Biology. 2012;19:240–249. doi: 10.1111/j.1369-1600.2012.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moyers TB, et al. From in-session behaviors to drinking outcomes: A causal chain for motivational interviewing. Journal of Consulting and Clinical Psychology. 2009;77(6):1113–1124. doi: 10.1037/a0017189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgenstern J, et al. Motivational interviewing: a pilot test of active ingredients and mechanisms of change. Psychology of Addictive Behaviors. 2012;26(859–869) doi: 10.1037/a0029674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feldstein SW. Binge drinking. In: Fisher GR, Roget N, editors. Encyclopedia of Substance Abuse Prevention, Treatment, and Recovery. Sage; 2009. [Google Scholar]
- 47.Filbey FM, et al. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33(6):1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montanaro E, Feldstein Ewing SW, Bryan AD. What works? An empirical perspective on how to retain youth in longitudinal HIV and substance risk reduction studies. Substance Abuse. 2014 doi: 10.1080/08897077.2014.970322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.APA. Reporting standards for research in Psychology. American Psychologist. 2008;63:839–851. doi: 10.1037/0003-066X.63.9.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karoly H, et al. Does incentive-elicited nucleus accumbens activation differ by substance of abuse? An examination with adolescents. Dev Cogn Neurosci. 2015 doi: 10.1016/j.dcn.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clark DB, Winters KC. Measuring risks and outcomes in substance use disorders prevention research. Journal of Consulting and Clinical Psychology. 2002;70(6):1207–1223. doi: 10.1037//0022-006x.70.6.1207. [DOI] [PubMed] [Google Scholar]
- 52.Marlatt GA, et al. Screening and brief intervention for high-risk college student drinkers: Results from a 2-year follow-up assessment. Journal of Consulting and Clinical Psychology. 1998;66(4):604–615. doi: 10.1037//0022-006x.66.4.604. [DOI] [PubMed] [Google Scholar]
- 53.Berk L, et al. Under pressure: Adolescent substance users show exaggerated neural processing of aversive interoceptive stimuli. Addiction. 2015 doi: 10.1111/add.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roese N, Jamieson D. Twenty years of bogus pipeline research: A critical review and meta-anlysis. Psychological Bulletin. 1993;114:363–375. [Google Scholar]
- 55.Sobell LC, Sobell MB. Time-line follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption. Humana Press; Totowa, N.J: 1992. pp. 73–98. [Google Scholar]
- 56.White HR, Labouvie EW. Towards the assessment of adolescent problem drinking. Journal of Studies on Alcohol. 1989;50:30–37. doi: 10.15288/jsa.1989.50.30. [DOI] [PubMed] [Google Scholar]
- 57.Walker DD, et al. Randomized controlled trial of motivational enhancement therapy with nontreatment-seeking adolescent cannabis users: A further test of the teen marijuana check-up. Psychology of Addictive Behaviors. 2011 doi: 10.1037/a0024076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feldstein Ewing SW, Bryan AD, Hutchison KE. M.R.N.U.o.N. Mexico, editor. The Adolescent Substance Use Check-Up: Intervention Manual. Albuquerque, NM: 2008. [Google Scholar]
- 59.Friston K, et al. Spatial Registration and normalization of images. Human Brain Mapping. 1995;2:165–189. [Google Scholar]
- 60.Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Human Brain Mapping. 1999;7(4):254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Purdon PL, Weisskoff RM. Effect of temporal autocorrelation due to physiological noise and stimulus paradigm on voxel-level false-positive rates in fMRI. Human Brain Mapping. 1998;6(4):239–49. doi: 10.1002/(SICI)1097-0193(1998)6:4<239::AID-HBM4>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forman SD, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 63.Feldstein SW, Forcehimes AA. Motivational interviewing with underage college drinkers: A preliminary look at the role of empathy and alliance. The American Journal of Drug and Alcohol Abuse. 2007;33(5):737–746. doi: 10.1080/00952990701522690. [DOI] [PubMed] [Google Scholar]
- 64.Schacht JP, Hutchison KE. Using clinical neuroscience to understand addictions treatment. In: Feldstein Ewing SW, Witkiewitz K, Filbey FM, editors. Neuroimaging and psychosocial addictions treament: An integrative guide for researchers and clinicians. London: Palgrave; in press. [Google Scholar]
- 65.McCambridge J, Kypri K, McElduff P. Regression to the mean and alcohol consumption: a cohort study exploring implications for the interpretation of change in control groups in brief intervention trials. Drug and Alcohol Dependence. 2014;135:156–159. doi: 10.1016/j.drugalcdep.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.LaChance HA, et al. What makes group MET work? A randomized controlled trial of college student drinkers in mandated alcohol diversion. Psychology of Addictive Behaviors. 2009;23(4):589–612. doi: 10.1037/a0016633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carey KB, et al. Individual-level interventions to reduce college student drinking: A meta-analytic review. Addictive Behaviors. 2007;32(11):2469–2494. doi: 10.1016/j.addbeh.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feldstein Ewing SW, Gaume J, Apodaca TR. Ambivalence: Prerequisite for success in motivational interviewing with adolescents? doi: 10.1111/add.13286. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller WR, Moyers TB. The forest and the trees: Relational and specific factors in addiction treatment. Addiction. 2014 doi: 10.1111/add.12693. [DOI] [PubMed] [Google Scholar]
- 70.Feldstein Ewing SW, et al. A model of motivational interviewing with Hispanic youth: Randomized clinical trial in addiction treatment. under review. [Google Scholar]
- 71.Bogenschutz MP, et al. Brief intervention for patients with problematic drug use presenting in emergency departments: A randomized clinical trial. JAMA Internal Medicine. 2014;174:1736–1745. doi: 10.1001/jamainternmed.2014.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Norman AL, et al. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug and Alcohol Dependence. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Squeglia LM, et al. Brain volume reductions in adolescent heavy drinkers. Developmental Cognitive Neuroscience. 2014;9:117–125. doi: 10.1016/j.dcn.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Volkow ND, Baler RD. Addiction science: Uncovering neurobiological complexity. Neuropharmacology. 2014;76:235–249. doi: 10.1016/j.neuropharm.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seo D, et al. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry. 2013;70:727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seo D, Sinha R. The neurobiology of alcohol craving and relapse. Handb Clin Neurol. 2014;125:355–368. doi: 10.1016/B978-0-444-62619-6.00021-5. [DOI] [PubMed] [Google Scholar]
- 77.Cheetham A, et al. Volumetric differences in the anterior cingulate cortex prospectively predict alcohol-related problems in adolescence. Psychopharmacology (Berl) 2014;231:1731–1742. doi: 10.1007/s00213-014-3483-8. [DOI] [PubMed] [Google Scholar]
- 78.Dager AD, et al. Functional magnetic resonance imaging (fMRI) response to alcohol pictures predicts subsequent transition to heavy drinking in college students. Addiction. 2014;109:585–595. doi: 10.1111/add.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brumback T, et al. Adolescent heavy drinkers’ amplified brain responses to alcohol cues decrease over one month of abstinence. Addictive Behaviors. 2015;46:45–52. doi: 10.1016/j.addbeh.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lisdahl KM, et al. Dare to Delay? The Impacts of Adolescent Alcohol and Marijuana Use Onset on Cognition, Brain Structure, and Function. Front Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seo D, et al. Sex differences in neural responses to stress and alcohol context cues. Human Brain Mapping. 2011;32:1998–2013. doi: 10.1002/hbm.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cservenka A, Herting MM, Nagel BJ. Atypical frontal lobe activity during verbal working memory in youth with a family history of alcoholism. Drug and Alcohol Dependence. 2012;123:98–104. doi: 10.1016/j.drugalcdep.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feldstein Ewing SW, et al. A preliminary examination of how serotonergic polymorphisms influence brain response following an adolescent cannabis intervention. Psychiatry Research: Neuroimaging. 2012;204:112–116. doi: 10.1016/j.pscychresns.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DeWitt SJ, Aslan S, Filbey FM. Adolescent risk-taking and resting state functional connectivity. Psychiatry Research. 2014;222:157–164. doi: 10.1016/j.pscychresns.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 85.Tomasi D, Volkow ND. Functional connectivity hubs in the human brain. Neuroimage. 2011;57:908–917. doi: 10.1016/j.neuroimage.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Leijenhorst L, et al. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb Cortex. 2010;20:61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- 87.Raznahan A, et al. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc Natl Acad Sci USA. 2014;111:1592–1597. doi: 10.1073/pnas.1316911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vanbuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. Journal of Behavioral Medicine. 2014;37:768–780. doi: 10.1007/s10865-013-9527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Roten Y, et al. Meta-analysis of the effects of MI training on clinicians’ behavior. Journal of Substance Abuse Treatment. 2013;45:155–162. doi: 10.1016/j.jsat.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 90.Gaume J, et al. Influence of Counselor Characteristics and Behaviors on the Efficacy of a Brief Motivational Intervention for Heavy Drinking in Young Men-A Randomized Controlled Trial. Alcoholism: Clinical and Experimental Research. 2014;38:2138–2147. doi: 10.1111/acer.12469. [DOI] [PubMed] [Google Scholar]
- 91.Moyers TB, Miller WR. Is low therapist empathy toxic? Psychology of Addictive Behaviors. 2013;27:878–884. doi: 10.1037/a0030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Imel ZE, et al. Distinctions wihtout a difference: Direct comparisons of psychotherapies for alcohol use disorders. Psychology of Addictive Behaviors. 2008;22(4):533–543. doi: 10.1037/a0013171. [DOI] [PubMed] [Google Scholar]
- 93.Feldstein Ewing SW, Blakemore S-J, Sakhardande A. The effect of alcohol consumption on the adolescent brain: A systematic review of MRI and fMRI studies of alcohol-using youth. Neuroimage: Clinical. 2014 doi: 10.1016/j.nicl.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Feldstein Ewing SW, Chung T. Neuroimaging mechanisms of change in psychotherapy for addictive behaviors: Emerging translational approaches that bridge biology and behavior. Psychology of Addictive Behaviors. 2013;27:329–335. doi: 10.1037/a0031491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nature Neuroscience. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]