T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive hematologic malignancy that affects both children and adults. Optimization of chemotherapy regimens over the last five decades has led to steady improvements in outcome for pediatric patients, who have a long-term survival rate of 80%. For adults, however, the five-year survival rate is only 35–40% and both pediatric and adult patients who suffer relapse have uniformly poor outcomes.1 Further, improvements in outcome will thus require introduction of new approaches and more specific, targeted therapies.
Testing of new therapeutic agents, has been restricted in part by the lack of a simple and efficient method for studying patient cells ex vivo. Most preclinical studies to date have relied on a relatively small number of permanent cell lines. Many of these lines were established from malignant effusions in patients with advanced-stage disease and have been maintained in culture for long periods, raising concerns about how well they reflect the biology of typical disease in patients. Studies involving primary T-ALLs, xenografted in immunodeficient mice are generally perceived to be better models of human disease, but are costly and time-consuming.
A relatively cheap and flexible alternative is study of primary cells in short-term ex vivo culture systems that are permissive for growth. Several groups have developed methods for in vitro culture of patient T-ALL samples, including culture in suspension, on stromal feeders and under reduced oxygen conditions.2–4 On the basis of the importance of NOTCH1 signaling in T-ALL,5 the Pflumio group has engineered MS5 bone marrow stromal feeders to express the Notch ligand DL1 and demonstrated that these MS5-DL1 feeders support growth of primary human T-ALL cells in vitro.2 We have successfully employed the MS5-DL1 feeder system in our own labs,6,7 but these cultures are limited by relatively modest cell growth and dependence on inclusion of both fetal bovine serum and human AB serum2 which are subject to significant lot-to-lot variability.
We previously reported a chemically defined, serum-free medium (WIT) that supports robust in vitro growth of normal and transformed human mammary epithelial cells in primary culture for extended periods.8 This medium provides all the essential nutrients for maintaining basic cellular metabolism without undefined supplements such as serum, pituitary extract, conditioned medium or drugs. As a result, normal human breast epithelial cells have proliferated in WIT medium over 70 population doublings, or nearly 1021-fold expansion of cell numbers.
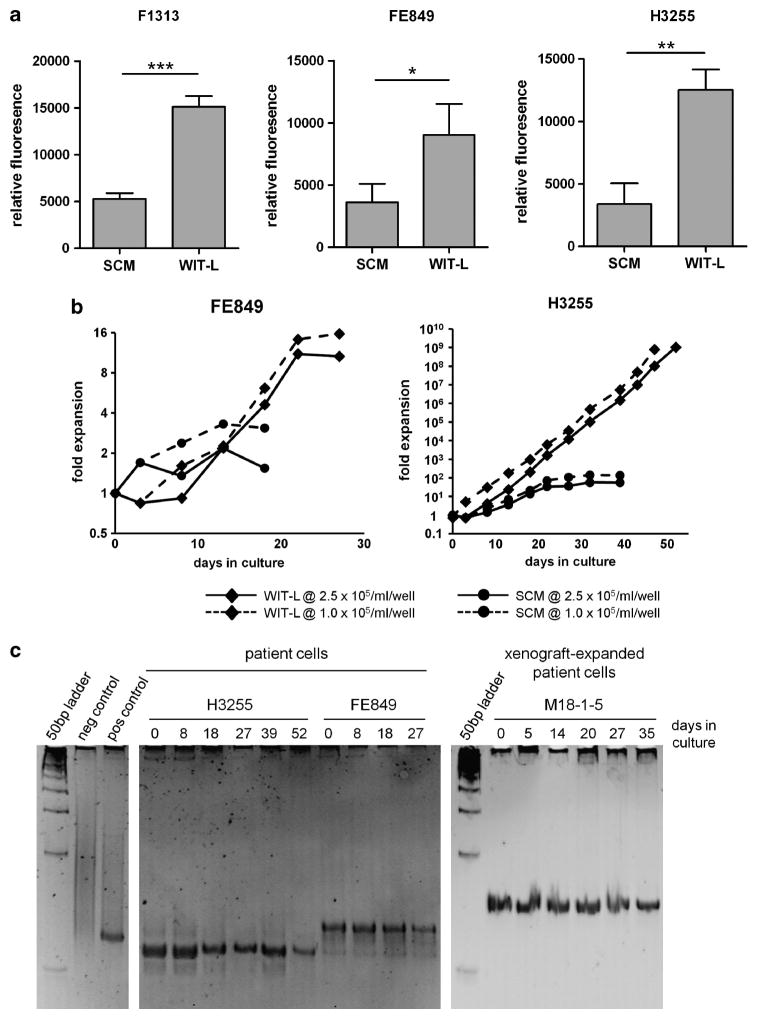
We devised a modification of WIT medium by supplementing it with recombinant Stem Cell Factor (SCF) (50 ng/ml), interleukin (IL)-2 (10 ng/ml), IL-7 (10 ng/ml) and insulin-like growth factor (IGF)-1 (10 ng/ml), referred to as WIT-L, and evaluated its ability to expand primary human T-lymphoblasts on MS5-DL1 feeders compared directly with fetal bovine and human serum-containing medium (SCM), which included supplemental SCF (50 ng/ml), Flt3L (20 ng/ml), IL-7 (10 ng/ml) and insulin (116 ng/ml) (Supplementary Table 1). T-ALL samples obtained directly from patient biopsy material (Supplementary Table 2) were seeded under identical conditions in SCM or WIT-L on MS5-DL1 stromal cells. In all cases, T-ALL cells showed superior growth in WIT-L than in SCM, and confirmed that MS5-DL1 feeders are required to support growth in vitro (Figures 1a and b and Supplementary Figure 1). To provide sufficient cells for further experiments, patient T-lymphoblasts were also first expanded as xenografts in NOD/Scid/Il2rg−/− (NSG) mice6 and then tested for growth in SCM vs WIT-L. Again, cells cultured in WIT-L grew significantly better than those cultured in SCM (Supplementary Figure 2). Xenograft-expanded cells were also cultured in WIT-L medium and manually counted at passage (every 4–6 days) for up to 6 weeks. WIT-L cultures typically exhibited logarithmic growth and attained maximal expansions of 40- to 100-fold when cultured at high-density, and 4000- to 10 000-fold when cultured at low-density (Supplementary Figure 3). Importantly, T-cell receptor γ heteroduplex analysis confirmed expansion of the original clone throughout the culture period (Figure 1c) and flow cytometric analysis confirmed cultures to be composed entirely of immature T-lineage human cells (data not shown). Of note, high-density cultures all began to regress after ~30 days, suggesting long-term renewing cells are not supported under these conditions. Low-density cultures did not show obvious regression; however, most were typically not carried beyond 30–50 days and thus are not particularly informative on this point. Further, it is clear that while some patient samples expand very well in culture (for example, H3255), others do not grow at all in either WIT-L or SCM media (n = 3, data not shown). Whether failure to grow in vitro is purely technical (for example, pertaining to the quality of the sample) or reflects an inherent difference in growth requirements for a subset of T-ALLs remains to be determined. Also, although our data show that 9 of 9 samples cultured under both sets of conditions grew better in WIT-L than SCM, we did not observe any that grew well in WIT-L, yet completely failed to expand in SCM.
Figure 1.
Serum-free WIT-L medium supports robust growth of primary T-ALL blasts in vitro. In vitro growth of primary T-ALL cells. (a) Resazurin reduction assay. T-lymphoblasts obtained directly from patient biopsy material were seeded in 24-well dishes at 2.5 × 105 viable cells per well on confluent monolayers of irradiated (50 Gy) MS5-DL1 feeders in 1 ml of either SCM or WIT-L medium. Four to six days later, cell growth was measured by resazurin reduction assay (Cell Titer Blue, Promega, Madison, WI, USA) with subtraction of values obtained from feeder-only control wells. Error bars indicate s.d. of assays performed in triplicate. Data depicted are representative of 2–4 experimental replicates. *P<0.05; **P<0.01; ***P<0.001 (Student’s t-test). (b) Growth curves. Patient T-lymphoblasts were seeded in 24-well dishes at 1.0 ×105 or 2.5 ×105 cells per well on irradiated MS5-DL1 monolayers in 1 ml of either SCM or WIT-L medium. Cultures were passaged every 4–6 days by reseeding at the initial density onto fresh feeders. Carry-over of feeders between passages was minimized by filtering manually disaggregated cell suspensions through 48 μm nylon mesh. Viable cell yields were counted at each passage (Vi-Cell, Coulter, Brea, CA, USA). (c) TCRγ clonality assay by heteroduplex analysis. Genomic DNA from patient T-ALL samples cultured in WIT-L medium on MS5-DL1 feeders was PCR amplified using consensus TCRγ primers. Resulting PCR products were heated at 94 °C for 5 min, transferred to ice for 1 h, then run on a 12% non-denaturing polyacrylamide gel and visualized by ethidium bromide staining. Positive control is a biopsy-proven T-cell non-Hodgkin lymphoma; negative control is a reactive lymph node.
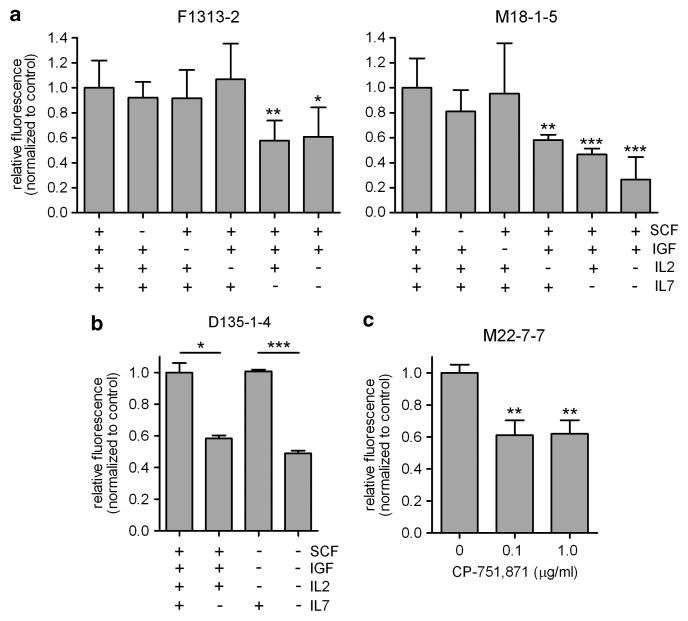
To define growth factor/cytokine requirements in the WIT-L/MS5-DL1 culture system, individual supplements were ‘left out’ of WIT-L one at a time. Consistent with other reports,9 supplemental IL-7 proved to be the most important factor (Figures 2a and b). We also noted that IL-2 improved the growth of one tumor (M18-1-5), whereas IGF-1 was dispensable in all cases tested. As we recently reported that signaling through IGF1R is important for T-ALL cell growth and leukemia-initiating activity,7 we explored the issue of IGF-dependence further. Interestingly, CP-751,871, an IGF1R blocking antibody in clinical trials (Figitumumab, Pfizer),10 significantly impaired cell growth (Figure 2c), suggesting that signaling through IGF1R does indeed transduce important growth signals in this model. This apparent paradox is likely explained by the inclusion of high concentrations of insulin (20 μg/ml or 3.44 μM) in the basal WIT-L medium; the Kd of insulin for IGF1R is 200–400 nM,11,12 making it likely that insulin is stimulating IGF1R in lymphoblasts under these conditions. Of note, CP-751,871 is specific for human IGF1R and thus, indirect effects on the mouse MS5-DL1 feeders are unlikely.
Figure 2.
Supplemental IL-7 and signaling through IGF1R are required for growth in vitro. In vitro growth of xenograft-expanded primary T-ALL cells as measured by resazurin reduction assay. (a), (b) Cells were cultured on MS5-DL1 feeders as in Figure 1, but with WIT-L media lacking the indicated growth factors/cytokines and assayed 4–6 days later. (c) Cells were cultured on MS5-DL1 feeders in WIT-L media as in Figure 1, but with addition of the indicated doses of IGF1R blocking antibody, CP-751,871, and assayed 3 days later. Error bars indicate s.d. of assays performed in triplicate. Statistical significance was calculated in comparison with relevant controls (complete WIT-L media in a; as indicated in b; no CP-751,871 in c). *P<0.05; **P<0.01; ***P<0.001 (Student’s t-test).
MS5 cells have been reported to secrete a variety of growth factors, including GM-CSF, IL-6, SCF, hepatocyte growth factor and an IL-3-like activity, as well as extracellular matrix proteins, including fibronectin, laminin and type I collagen.13 We attempted to replace the MS5-DL1 feeders with immobilized, recombinant DL1 ligand,14 but cells cultured in WIT-L medium or in MS5-DL1-conditioned WIT-L medium failed to grow under these conditions (data not shown), demonstrating that physical interaction with MS5 feeders and/or a secreted matrix protein provides critical signals for T-ALL cell growth.
In summary, the MS5-DL1/WIT-L culture system, supports robust growth of primary T-lymphoblasts in vitro for at least 3–4 weeks, achieving net clonal expansion of up to 100- to 10 000-fold, and thus can facilitate functional and pharmacologic studies that previously could only be performed using established T-ALL cell lines. The Pflumio group has reported up to 30% lentiviral transduction using their optimized protocol15 and we have achieved up to 20% transduction in our own hands, suggesting that gene transfer/knock-down studies are possible using this system. This system has been used successfully in the laboratories of two members of our groups (Aster and Weng), suggesting that this completely defined system will prove to be easily adopted by other groups in the field. We anticipate that further optimization of the medium formulation will yield additional gains in performance from primary cultures and eventually allow routine establishment of permanent cell lines. Among the possible applications are personalized therapy initiatives, in which patient leukemia cells are cultured in vitro immediately after biopsy and prospectively screened against panels of therapeutic agents.
Supplementary Material
Acknowledgments
We would like to thank Francoise Pflumio (CEA Fontenayaux-Roses, France) for providing the MS5-DL1 cell line and helpful advice and discussion. We would also like to thank P Ballerini (Hôpital Armand Trousseau, Paris, France), Thierry Leblanc (Hôpital Saint-Louis, Paris, France), K Schultz (BC Children’s Hospital, Vancouver, Canada) and L Matherly (Karmanos Cancer Institute, Detroit, Michigan) for providing patient samples. This work was supported by grants from the Canadian Institutes of Health Research/Terry Fox Foundation (APW), Leukemia and Lymphoma Society of Canada (APW), Leukemia and Lymphoma Society (JCA), the William Lawrence and Blanche Hughes Foundation (JCA), Harvard Catalyst Pilot Grant (TAI and JCA) and the National Institutes of Health/National Cancer Institute (R01CA146445 to TAI).
Footnotes
CONFLICT OF INTEREST
TAI receives royalties for WIT-P, -I, and -T media and was a consultant to Stemgent (2008–10). The remaining authors declare no competing financial interests.
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Contributor Information
TA. Ince, Email: TInce@med.miami.edu.
AP. Weng, Email: aweng@bccrc.ca.
References
- 1.Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008;111:1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong F, Brunet de la Grange P, Gerby B, Rouyez MC, Calvo J, Fontenay M, et al. NOTCH is a key regulator of human T-cell acute leukemia initiating cell activity. Blood. 2009;113:1730–1740. doi: 10.1182/blood-2008-02-138172. [DOI] [PubMed] [Google Scholar]
- 3.Chiu PP, Jiang H, Dick JE. Leukemia-initiating cells in human T-lymphoblastic leukemia exhibit glucocorticoid resistance. Blood. 2010;116:5268–5279. doi: 10.1182/blood-2010-06-292300. [DOI] [PubMed] [Google Scholar]
- 4.Cox CV, Martin HM, Kearns PR, Virgo P, Evely RS, Blair A. Characterization of a progenitor cell population in childhood T-cell acute lymphoblastic leukemia. Blood. 2007;109:674–682. doi: 10.1182/blood-2006-06-030445. [DOI] [PubMed] [Google Scholar]
- 5.Aster JC, Pear WS, Blacklow SC. Notch Signaling in Leukemia. Annu Rev Pathol. 2008;3:587–613. doi: 10.1146/annurev.pathmechdis.3.121806.154300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medyouf H, Gao X, Armstrong F, Gusscott S, Liu Q, Larson Gedman A, et al. Acute T-cell leukemias remain dependent on Notch signaling despite PTEN and INK4A/ARF loss. Blood. 2010;115:1175–1184. doi: 10.1182/blood-2009-04-214718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medyouf H, Gusscott S, Wang H, Tseng JC, Wai C, Nemirovsky O, et al. High-level IGF1R expression is required for leukemia-initiating cell activity in T-ALL and is supported by Notch signaling. J Exp Med. 2011;208:1809–1822. doi: 10.1084/jem.20110121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ince TA, Richardson AL, Bell GW, Saitoh M, Godar S, Karnoub AE, et al. Transformation of different human breast epithelial cell types leads to distinct tumor phenotypes. Cancer Cell. 2007;12:160–170. doi: 10.1016/j.ccr.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Barata J, Cardoso A, Boussiotis V. Interleukin-7 in T-cell acute lymphoblastic leukemia: An extrinsic factor supporting leukemogenesis? Leuk Lymphoma. 2005;46:483–495. doi: 10.1080/10428190400027852. [DOI] [PubMed] [Google Scholar]
- 10.Lacy MQ, Alsina M, Fonseca R, Paccagnella ML, Melvin CL, Yin D, et al. Phase I, pharmacokinetic and pharmacodynamic study of the anti-insulinlike growth factor Type 1 receptor monoclonal antibody Cp-751,871 in patients with multiple myeloma. J Clin Oncol. 2008;26:3196–3203. doi: 10.1200/JCO.2007.15.9319. JCO.2007.2015.9319. [DOI] [PubMed] [Google Scholar]
- 11.Kurtzhals P, Schäffer L, Sørensen A, Kristensen C, Jonassen I, Schmid C, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes. 2000;49:999–1005. doi: 10.2337/diabetes.49.6.999. [DOI] [PubMed] [Google Scholar]
- 12.Soos MA, Field CE, Siddle K. Purified hybrid insulin/insulin-like growth factor-I receptors bind insulin-like growth factor-I, but not insulin, with high affinity. Biochem J. 1993;290(Pt 2):419–426. doi: 10.1042/bj2900419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki J, Fujita J, Taniguchi S, Sugimoto K, Mori KJ. Characterization of murine hemopoietic-supportive (MS-1 and MS-5) and non-supportive (MS-K) cell lines. Leukemia. 1992;6:452–458. [PubMed] [Google Scholar]
- 14.Dallas MH, Varnum-Finney B, Martin PJ, Bernstein ID. Enhanced T-cell reconstitution by hematopoietic progenitors expanded ex vivo using the Notch ligand Delta1. Blood. 2007;109:3579–3587. doi: 10.1182/blood-2006-08-039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerby B, Armstrong F, de la Grange PB, Medyouf H, Calvo J, Verhoeyen E, et al. Optimized gene transfer into human primary leukemic T cell with NOD-SCID/leukemia-initiating cell activity. Leukemia. 2010;24:646–649. doi: 10.1038/leu.2009.235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.