The present study assessed the effects of intraportal infusions of autologous bone marrow-derived mononuclear cells (MNCs) and/or CD133+ cells on liver function in patients with decompensated cirrhosis in a double-blind randomized controlled trial. No significant adverse events occurred in the present study. A transient improvement in the Model for End-Stage Liver Disease score was observed in subjects treated with CD133+ cells but not in the MNC or placebo group. Although the study was not powered to make definitive conclusions, the data justify further study of CD133+ therapy in cirrhotic patients.
Keywords: Cirrhosis, Hematopoietic stem cell, Regenerative medicine, Cell-based therapy
Abstract
The present study assessed the effects of intraportal infusions of autologous bone marrow-derived mononuclear cells (MNCs) and/or CD133+ cells on liver function in patients with decompensated cirrhosis. We randomly assigned 27 eligible patients to a placebo, MNCs, and/or CD133+ cells. Cell infusions were performed at baseline and month 3. We considered the absolute changes in the Model for End-Stage Liver Disease (MELD) scores at months 3 and 6 after infusion as the primary outcome. The participants and those who assessed the outcomes were unaware of the treatment intervention assignments. After 6 months, 9 patients were excluded because of liver transplantation (n = 3), hepatocellular carcinoma (n = 1), loss to follow-up (n = 3), and death (n = 2). The final analysis included 4 patients from the CD133+ group, 8 from the MNC group, and 6 from the placebo group. No improvement was seen in the MELD score at month 6 using either CD133+ cells or MNC infusions compared with placebo. However, at month 3 after infusion, a trend was seen toward a higher mean absolute change in the MELD score in patients who had received CD133+ cells compared with placebo (−2.00 ± 1.87 vs. −0.13 ± 1.46; p = .08). No significant adverse events occurred in the present study. A transient improvement in the MELD score was observed in subjects treated with CD133+ cells but not in the MNC or placebo group. Although the study was not powered to make definitive conclusions, the data justify further study of CD133+ therapy in cirrhotic patients.
Significance
Cell therapy is a new approach in liver disease. Several clinical experiments have been reported on the safety of bone marrow-derived stem cells to treat liver disorders. However, the effectiveness of these approaches in the long-term follow-ups of patients initiated controversial discussions among the scientific community. A double-blind randomized controlled trial was designed to address this concern scientifically. A transient improvement in the patients’ signs occurred; however, for a sustainable result, more work is needed. The results of multiple administrations of cells reported in the present study can be compared with the results from other single-injection studies.
Introduction
Cirrhosis is the final stage of chronic inflammation in the liver characterized by extensive fibrosis, architectural remodeling, regenerative nodules, and intrahepatic vascular shunts, which consequently results in the development of portal hypertension. Portal hypertension is the leading cause of cirrhosis complications and mortality [1]. Currently, orthotopic liver transplantation is the reference standard therapeutic intervention for advanced decompensated cirrhosis. However, the limiting factors of liver transplantation include the lack of organ availability, cost, and the complications of immunosuppression [2]. Cirrhosis is no longer perceived as a static stage. Rather, it is a dynamic and stepwise process that is potentially reversible before a specific, yet currently undetermined, stage. Clinical evidence of some degree of cirrhosis/fibrosis reversibility has been reported for hepatitis B and C, autoimmune hepatitis, alcoholic fatty liver disease, and hereditary hemochromatosis [3].
The introduction of stem cell therapy to liver disease has provided a promising approach in the future of regenerative medicine and could be the alternative to liver transplant that the health care system desperately seeks (review provided in [4, 5]). The transient therapeutic effects of bone marrow (BM)-derived mesenchymal stem cells (MSCs), CD34+ cells, CD133+ cells, and mononuclear cells (MNCs) on cirrhosis have been reported [6–18]. Earlier studies suggested that BM cells could transform into functional hepatocytes [19]. However, subsequent studies demonstrated that cell fusion and the paracrine effect of engrafted cells, rather than differentiation, were the principal mechanisms by which transplanted cells ameliorated cirrhosis [20, 21].
We previously reported the effect of BM-derived CD133+ cell and MNC transplantation in decompensated cirrhosis in a nonrandomized phase I trial of 6 patients (3 in each group) who were on the liver transplantation waiting list. The cell transplantation was well-tolerated, and the patients did not develop major cirrhosis-related complications during the 24-month follow-up period [22].
In the present report, we compared the effect of CD133+ versus MNCs on disease progression and clinical symptoms of decompensated cirrhosis in a randomized placebo-controlled trial. To the best of our knowledge, this is the first double-blind controlled trial of decompensated cirrhosis. Both selection and accidental bias were prevented by the randomization method, and the likelihood of chance as a source for the difference of the end outcome was eliminated. The use of comparable groups excluded the source of bias in treatment assignments. The trial was registered at the U.S. NIH Clinical Trials Database (ClinicalTrials.gov identifier NCT01120925; http://www.clinicaltrials.gov).
Materials and Methods
Patients
From March 2010 to June 2012, 27 patients diagnosed with decompensated cirrhosis at the Digestive Disease Research Institute, Tehran University of Medical Sciences, were recruited. At the time of enrollment, the patients were on the waiting list for liver transplantation. Cirrhosis was diagnosed by the clinical symptoms, biochemical indexes of liver failure, and liver histologic findings or compatible imaging findings (e.g., ultrasonography, computed tomography, or elastography). All cirrhotic patients had a decompensated disorder defined as either Child-Pugh class B or C.
The exclusion criteria included grade III or IV hepatic encephalopathy during the 6 months before study entry; refractory ascites; elevated serum transaminases (three times the normal values); active autoimmune hepatitis, manifested as serum γ-globulin more than twice the normal limit; serum creatinine of more than 1.5 mg/dl; positive HIV antibody; positive hepatitis C virus RNA quantitative polymerase chain reaction; hepatitis B virus DNA level of more than 200 IU/ml; primary sclerosing cholangitis; hepatocellular carcinoma (HCC); active infectious disease; grade 3 esophageal varices; a positive history of esophageal variceal bleeding 1 month before enrollment; portal and/or hepatic vein thrombosis diagnosed by Doppler ultrasonography; comorbid conditions that included cardiovascular, pulmonary, neurologic, and nephrologic problems; malignancy; substance abuse; alcohol consumption; and/or a hepatotoxic medication prescription at least 3 months before enrollment. The Ethics Committee of the Digestive Disease Research Institute, Tehran University of Medical Sciences, approved the present trial. The study was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Study Design
Randomization was performed to eliminate selection bias and equalize the treatment arms. The block randomization used in the present study guaranteed a balanced number of participants for each of the three groups during the course of the trial. Each block size was composed of characters (2 × number of treatments). The block size was short enough to prevent imbalance, yet long enough to prevent guessing allocation. The block size was not stated in the protocol; thus, the clinical care providers and those who assessed the outcomes were unaware of the block size and order. Five blocks, which included 6 patients per block, were randomly selected among all possible orders. In this randomized trial, the allocated equal numbers of patients in the experimental and placebo groups provided the most statistically efficient randomization ratio and maximized the statistical power for the considered sample size. The block randomization allocation sequence was generated at the Royan Cell Therapy Center. The corresponding general practitioner at the Digestive Diseases Research Institute enrolled the participants, and the cell Good Manufacturing Practice facility at Royan Institute assigned the participants to the groups according to the randomized block table. The participants, care providers for patients, and those who assessed the outcomes were unaware of the treatment intervention assignments.
The patients were randomly allocated into three groups, two intervention groups and one placebo group. The first and second groups received intraportal infusions of autologous BM-derived CD133+ cells and BM-derived MNCs, respectively. The placebo group received autologous cell-free serum. The cell infusions were performed at baseline and month 3. The patients were examined at Shariati Hospital (Tehran, Iran) 1 day before the intervention. BM aspiration and isolation of CD133+ cells and MNCs were performed at Royan Institute (Tehran, Iran). BM aspiration and cell separation were also performed for the placebo group, and the harvested cells were immediately frozen for future potential use. The patients were admitted to the hospital for transplantation and observed for 24 hours after infusion. An interventional radiologist conducted the infusion under ultrasound guidance. A total of 20 ml of the cell suspension was infused through a 20-gauge catheter (Terumo Medical Products, Tokyo, Japan, http://www.terumotmp.com) into the portal vein with the patient under light sedation and local anesthesia over 5–10 minutes. In the placebo group, 20 ml of autologous cell-free serum was infused through the portal vein. The trial protocol can be accessed on request from R.M.
Cell Preparation
Approximately 135–150 ml of BM was aspirated from the posterior iliac crest of patients under local anesthesia (lidocaine hydrochloride) and light intravenous sedation (midazolam; Darou Pakhsh, Iran; and sufentanil; Aburaihan Pharmaceutical Co., Tehran, Iran, http://www.aburaihan.co.ir) 1 day before cell transplantation. BM aspirate was collected in sodium citrate-containing bags (Beasat Industry, Iran). The samples were analyzed for any possible bacterial contamination using a BACTEC instrument (BD Bactec; BD Diagnostics, Franklin, NJ, http://www.bd.com). MNCs were counted using a NucleoCounter system (ChemoMetec AS, Allerod, Denmark, http://www.chemometec.com), and their viability was determined before processing. MNC isolation was performed under Current Good Manufacturing Process conditions in a clean room facility according to the available guidelines (FS209E and ISO14644). In brief, the cell suspensions were immediately centrifuged for 30 minutes at 400g over a Ficoll-Hypaque gradient (Lymphodex; Inno-Train, Kronberg im Taunus, Germany), and the MNCs were recovered at the interface. The cells were washed twice with phosphate-buffered saline (PBS)/EDTA. Subsequently, the harvested cell pellet was diluted in normal saline supplemented with 2.5% human serum albumin (HSA; Octapharma AG, Lachen, Switzerland, http://www.octapharma.com) to a final volume of 20 ml. Cell counts and viability were determined using a NucleoCounter system (ChemoMetec AS).
To enrich the CD133+ cells, aspirated BM was initially filtered through a 200-µm pore size filter and washed twice with PBS/EDTA, supplemented with 2.5% HSA solution. Next, the suspension was incubated with microbead-conjugated CD133 monoclonal antibody (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com). CD133+ cells were sorted using a CliniMACS cell separation system (Miltenyi Biotec) in the clean room according to the manufacturer’s instructions. The remaining red blood cells were removed by incubating the cells with 500 µl of ammonium chloride-based lysis reagent at room temperature for 10 minutes. Finally, the cells were washed twice with normal saline, counted, and assessed for viability using the trypan blue dye exclusion method. The cells were suspended in normal saline supplemented with 2% HSA to a final volume of 15–20 ml. All samples passed the standard criteria for sterility and pyrogenicity as assessed using the BD instrument (BD BACTEC 9120; BD Diagnostics) and LAL (limulus amebocyte lysate) test kit (Lonza, Walkersville, MD, http://www.lonza.com), respectively.
Flow Cytometry
Flow cytometry analysis of the expressed cell surface antigens in both groups was performed using a BD FACSCalibur flow cytometry system (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com), and the purity of isolated CD133+ cells was calculated using the International Society for Hematotherapy and Graft Engineering method. The characterization panel of the MNCs consisted of monoclonal antibodies for endothelial lineage markers (CD31 and vascular endothelial growth factor [VEGF] receptor), MSC markers (CD44, CD29, CD73, CD90, and CD105), and hematopoietic stem cell markers (CD3, CD11b, CD14, CD16, CD19, CD31, CD33, CD34, CD39, and CD45). The antibodies are listed in supplemental online Table 1.
For CD133 analysis, the cells were adjusted to a volume of 1–2 × 105 cells per milliliter and blocked with Fc receptor blocking reagent (Miltenyi Biotech) according to the manufacturer’s instructions. The cells were subsequently stained for 30 minutes at 4°C with fluorochrome-labeled monoclonal antibodies. The controls were appropriately diluted isotype-matched antibodies (supplemental online Table 1). Data from 10,000 events were analyzed using WinMDI, version 2.9. The samples were analyzed in duplicate.
Follow-Up
The patients were examined by a physician at baseline and months 1, 3, and 6 after infusion. At each follow-up visit, the patients were examined for signs and symptoms of ascites, edema, and encephalopathy. The following blood tests were requested at each visit: complete blood count, serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), serum alkaline phosphatase, serum total bilirubin, blood urea nitrogen, serum creatinine, serum α-fetoprotein, prothrombin time (PT), and international normalized ratio (INR). In addition, 10 ml of venous blood was obtained and stored at −70°C. Transabdominal ultrasonography with color Doppler was performed at baseline and month 6 after cell transplantation to assess the liver and portal vein. The radiologist was unaware of the patient’s intervention assignment. The patients were telephoned at month 12 after cell transplantation to assess their general condition and survival.
The primary outcome measure of the present study was the absolute changes in the Model for End-Stage Liver Disease (MELD) scores at months 3 and 6 after infusion. The secondary outcome measures included mortality and development of a poor outcome during the 12 months of follow-up. A poor outcome was defined as requiring liver transplantation, the development of HCC, or death during the follow-up period.
Statistical Analysis
Data are expressed as the mean ± SD, median and range, or frequency and percentage, as appropriate. The normality assumption for continuous variables was assessed using the Kolmogorov-Smirnov test. Group comparisons were performed using one-way analysis of variance with least significant difference correction. The different time points in each group were compared using the pairwise t test. Statistical analyses were performed with SPSS, version 19 (IBM Corp., Armonk, NY, http://www-01.ibm.com/software/analytics/spss/). A p value <.05 was considered statistically significant.
Results
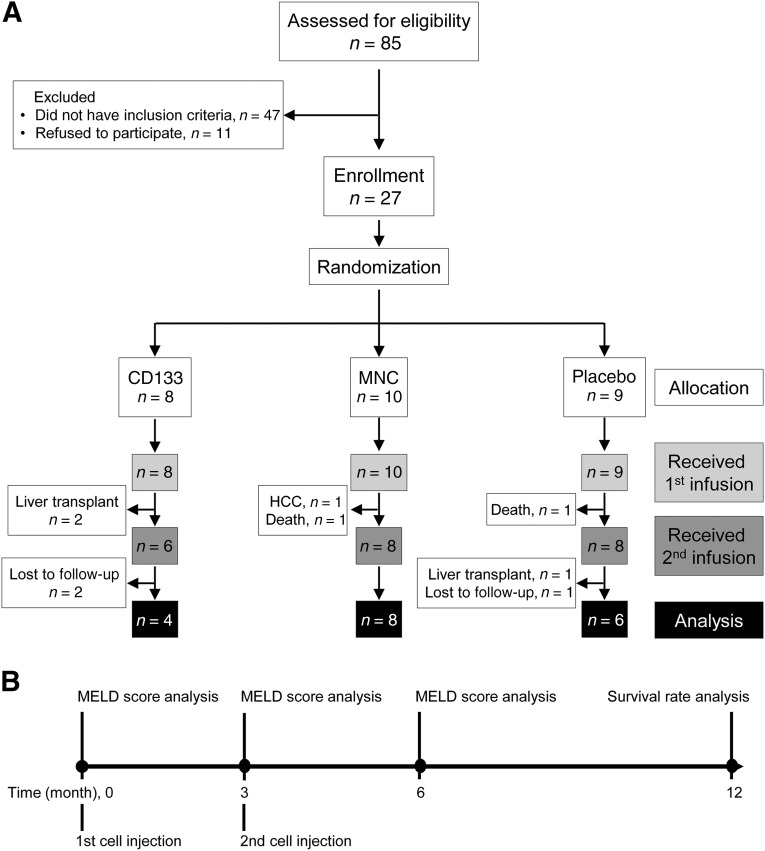
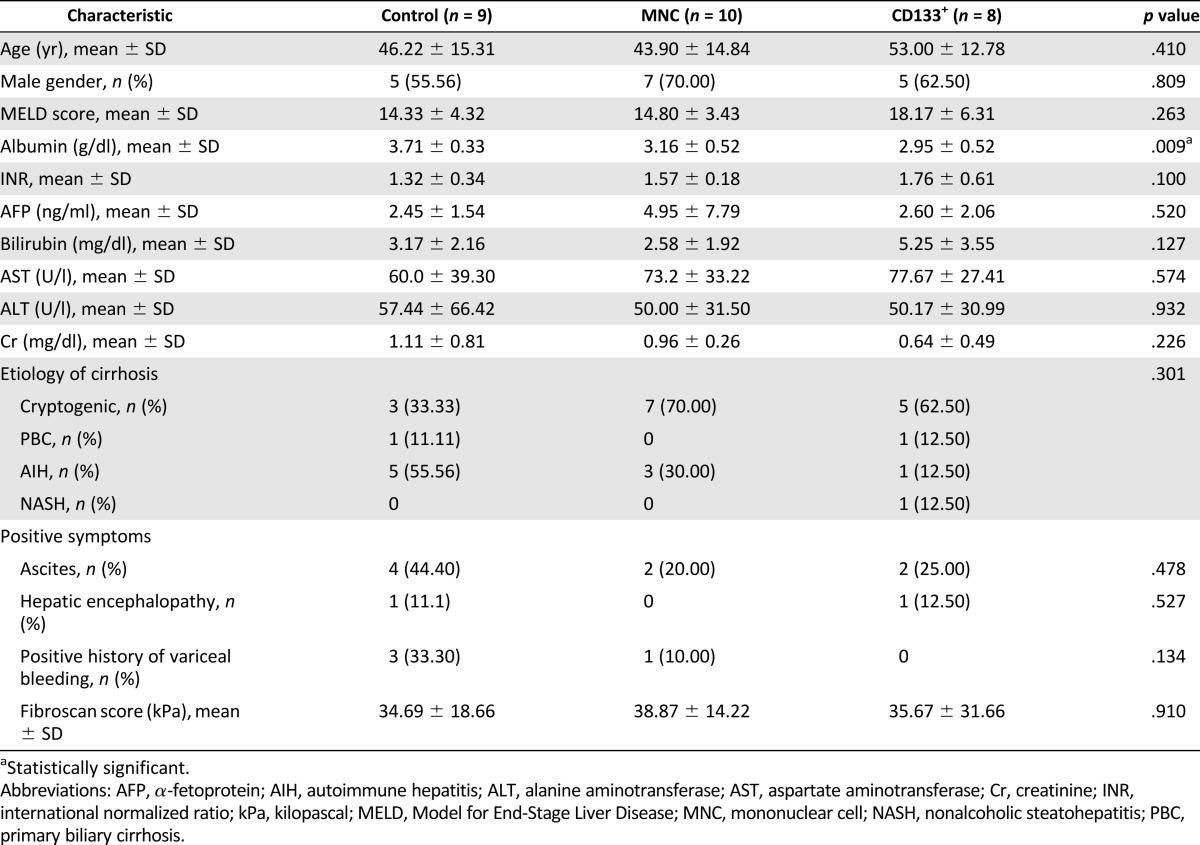
A total of 27 patients (17 men; 62.96%) were recruited. Overall, the median age at recruitment was 48 years (range, 20–76). The underlying causes of cirrhosis included cryptogenic cirrhosis (n = 15, 55.56%), autoimmune hepatitis (n = 9, 33.33%), primary biliary cirrhosis (n = 2, 7.41%), and nonalcoholic steatohepatitis (n = 1, 3.70%). The overall median baseline MELD score was 16 (range, 7–26), albumin 3.40 g/dl (range, 2.30–4.30), ALT 40 IU/L (range, 15–232), AST 62 IU/L (range, 29–160), total bilirubin 2.78 mg/dl (range, 0.99–9.85), and INR 1.55 (range, 1.00–2.70). We randomly assigned patients to three intervention groups (Fig. 1). The baseline MELD score, ALT, AST, total bilirubin, and INR levels did not significantly differ among the groups. However, the serum albumin levels were lower in both MNC and CD133+ groups compared with the placebo group (p = .009). In addition, the CD133+ group had a higher average MELD score compared with the other groups. The baseline characteristics of the patients are summarized in Table 1.
Figure 1.
Study flow diagram. (A): To eliminate selection bias and equalize the treatment arms, the 27 eligible patients were randomly divided into placebo, MNC, and CD133+ groups. At month 6, 9 patients were excluded during the study because of liver transplantation (n = 2), death (n = 2), HCC (n = 1), and “lost to follow-up” (n = 2). (B): Injection and analysis pipeline. Abbreviations: HCC, hepatocellular carcinoma; MELD, Model for End-Stage Liver Disease; MNC, mononuclear cell.
Table 1.
Baseline patient characteristics
Cell Preparation
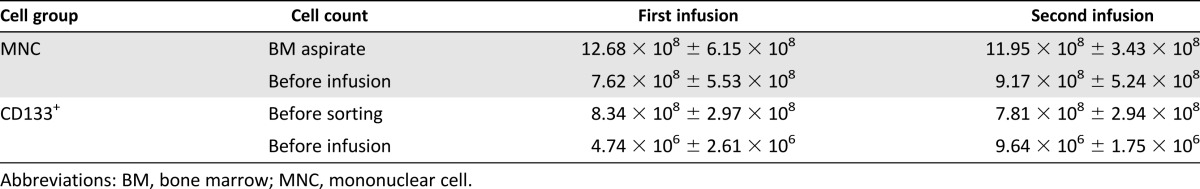
The mean volume of BM aspirates was 125 ± 21 ml for the first aspiration and 126 ± 13 ml for the second aspiration in the MNC group. For the CD133 group, the mean volume of BM aspirates was 125 ± 16 ml (first aspiration) and 120 ± 14 ml (second aspiration). The mean viabilities of the transplanted MNCs and CD133+ cells were more than 95% in both infusions. The cell counts of MNCs and CD133+ cells after preparation and at transplantation are summarized in Table 2. CD133+ cell purity was higher than 85% in the first and second infusions.
Table 2.
MNC and CD133+ cell count in bone marrow aspirate and before infusion
Liver Function Assessment After Therapy
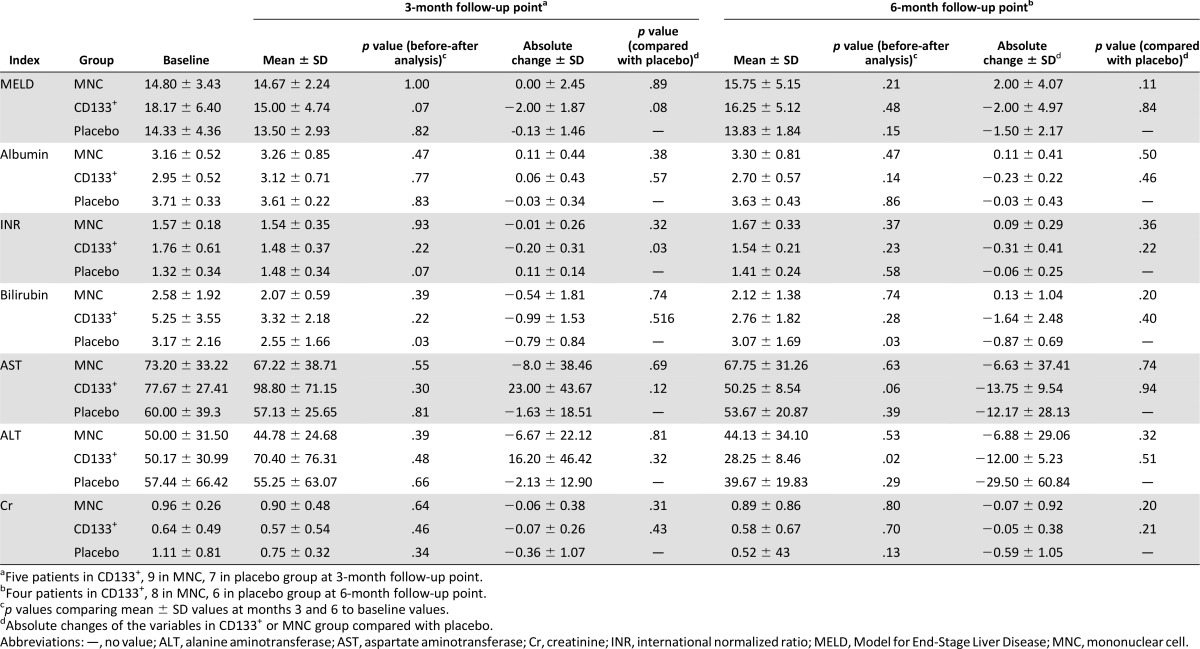
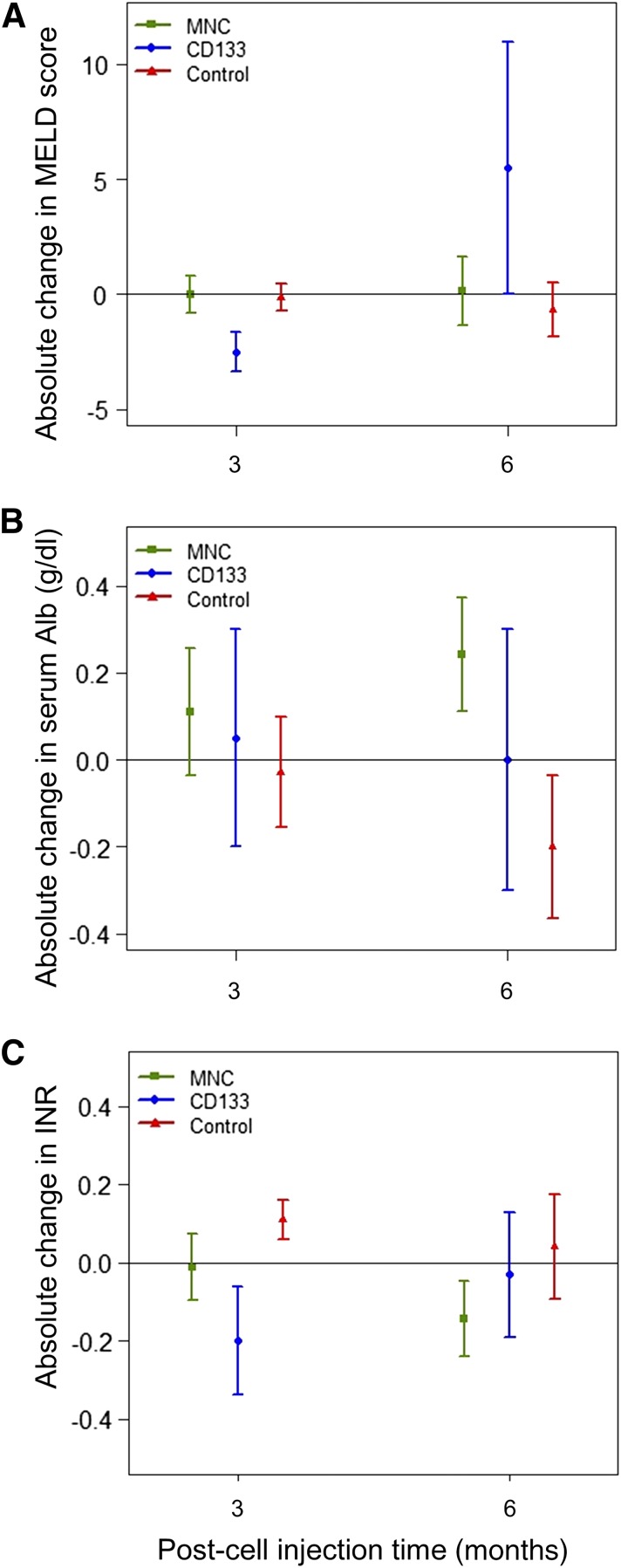
We compared the MELD scores at months 3 and 6 within each group. A decreasing trend was seen in the MELD score only in the CD133+ group at month 3 according to the before and after analysis (p = .07). At month 3 after infusion, we observed a trend toward a higher mean absolute change in the MELD score in patients who had received CD133+ cells (−2.00 ± 1.87) compared with those who received placebo (−0.13 ± 1.46; p = .08). The mean absolute change in the MELD score at month 3 was higher in the CD133+ group (−2.00 ± 1.87) than in the MNC group (0.00 ± 2.45; p = .09). At month 6 after infusion, the mean absolute change in the MELD score in the CD133+ group remained negative (−2.00 ± 4.97). In contrast, the MNC group had a positive mean absolute MELD score change (2.00 ± 0.4.07).
We observed a significant reduction in the absolute change of the INR in the CD133+ group (−0.20 ± 0.31) compared with the placebo group (0.11 ± 0.14, p = .03) at month 3. This effect was not observed at month 6 (Table 3). The effect of the intervention on the absolute changes in the MELD score, serum albumin, and INR over the course of the study is presented in Figure 2.
Table 3.
Changes in liver function test results after treatment
Figure 2.
Comparison of the outcomes of patients who received MNCs, CD133+ cells, or placebo over the course of the study. (A): Absolute change in MELD score in the three groups at two time points after cell transplantation. At month 3, the mean absolute change in the MELD score was higher in the CD133+ group than in the MNC group (Table 3). (B): Absolute change in serum albumin in the three groups at two time points after cell transplantation. (C): Absolute change in INR in the three groups at two time points after cell transplantation. A significant reduction in the absolute change in the INR in the CD133+ group was found compared with the placebo group at month 3. This effect was not observed at month 6 (Table 3). Data are presented as mean ± SD. Abbreviations: INR, international normalized ratio; MELD, Model for End-Stage Liver Disease; MNCs, mononuclear cells.
On an individual level, the MELD scores decreased in 3 of 9 patients from the MNC group, 4 of 5 from the CD133+ group, and 3 of 8 patients who had received placebo at month 3 compared with baseline. In addition, the MELD score decreased or remained unchanged in 2 of 8 patients from the MNC group, 3 of 4 from the CD133+ group, and 4 of 6 placebo group patients at both months 3 and 6 (supplemental online Fig. 1).
Patient Outcomes After Therapy
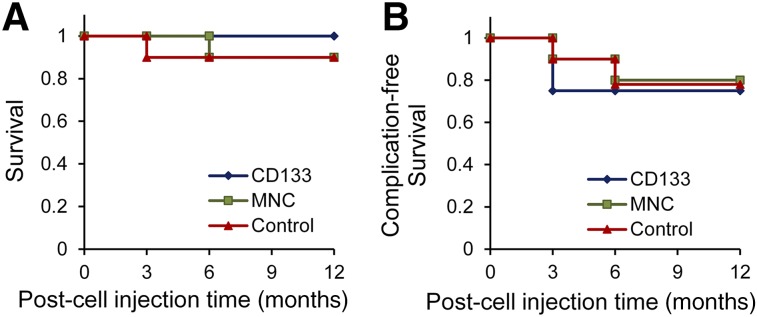
The patients were admitted to Shariati Hospital for transplantation and were observed for 24 hours after infusion. The patients' vital signs remained stable after the procedure. No procedural complications such as portal vein thrombosis, bleeding, infection, or renal failure occurred after the cellular or placebo infusions. Three patients underwent liver transplantation (two in the CD133+ group and one in the placebo group). These patients were excluded from the analysis. One patient in the MNC group developed HCC and 2 patients (1 from the MNC group and 1 from the placebo group) died during the course of the study and were excluded from the analysis. The placebo group patient died 1 month after receiving the first cell infusion, and the MNC patient died 6 months after receiving the first cell infusion. The baseline MELD scores of the 2 patients who died were 22 for the MNC patient and 20 for the placebo patient. Although no deaths occurred in the CD133+ group, the poor outcome rates did not significantly differ among the groups (Fig. 3). The mean baseline MELD scores for the patients who underwent liver transplantation, developed HCC, or died were 15.33 ± 8.51, 16.00, and 17.80 ± 5.85, respectively.
Figure 3.
Comparison of survival after MNC, CD133+, or placebo infusion in patients with decompensated cirrhosis. Survival (A) and complication-free survival (B) in three groups within 12 months of follow-up. One patient in the MNC group and one in the placebo group died and were excluded from the analysis (two deaths total). Hepatocellular carcinoma (HCC) and liver transplantation were considered complications. Two patients in the CD133+ group and one in the placebo group underwent liver transplantation. Furthermore, 1 patient in the MNC group developed HCC and was also excluded from the analysis. Abbreviation: MNC, mononuclear cell.
Discussion
Our study is the first randomized trial that has evaluated the efficacy of MNCs and CD133+ cells compared with placebo in improving liver function in patients with decompensated cirrhosis. MNCs, CD133+ cells, or placebo were infused through the portal vein of patients who were subsequently followed up for 6 months after infusion. At month 3 after infusion, we observed improved MELD score in the CD133+ group compared with the placebo group. However, the MELD score gradually increased after month 3 in the CD133+ group. It appears that CD133+ cells have a transient beneficial effect on decompensated cirrhosis. In accordance with our work, a recent trial protocol has been designed to compare the results of CD133+ infusion with granulocyte colony-stimulating factor in patients with cirrhosis of different etiologies [23]. The transient improvement in the MELD score in the CD133+ group was consistent with previous trials of hematopoietic stem cell transplantation in cirrhosis [6, 9, 11, 12, 14]. Furthermore, intraportal injection of autologous CD133+ cells expanded the remaining hepatic segments after hepatectomy and resulted in a 2.5-fold increase in mean proliferation rates of the segments compared with the placebo groups [18]. CD133+ cells have also been studied in other inflammatory conditions such as ischemic cardiomyopathy [24, 25]. Although the mechanism of action of CD133+ cells in tissue regeneration is not well understood, it seems they exert a partial effect via their proangiogenic properties [26, 27]. Pathologic angiogenesis is observed in chronic liver disease and results from the persistent inflammatory and hypoxic milieu. In this context, angiogenesis is the consequence of a vicious circle that alters the angioarchitecture of the liver and further drives fibrogenesis [28]. The therapeutic benefits of endothelial progenitor cell transplantation and VEGF have demonstrated fibrosis resolution, reduced portal hypertension, and improved survival in animal models [29, 30].
The improved MELD score at month 3 resulted from the reduced INR in the CD133+ group. The hemostatic abnormalities in patients with liver disease usually result from insufficient production of clotting factors and thrombocytopenia, commonly secondary to splenomegaly [31]. These abnormalities in cirrhotic patients result in increased PT and INR [32]. The improved INR in the CD133+ group might be indicative of the positive effect of the transplanted cells on hepatic regeneration capacity. The total bilirubin was also reduced in the CD133+ group, potentially indicating some immunomodulatory effects of the CD133+ cells as well. However, more studies are required to reveal the mechanism of interaction between CD133+ cells and liver parenchymal cells.
We did not find any therapeutic benefit based on the MELD score in the MNC group compared with the placebo group. This finding is in agreement with a report on the use of BM-MNCs in conjunction with standard medical treatment in decompensated alcoholic liver cirrhosis [17].
In order to exclude any confounding beneficial effects of other treatment modalities, we did not include cirrhotic patients with active autoimmune hepatitis or active chronic viral hepatitis B or C who required specific treatment for the underlying etiology of their cirrhosis. Nonetheless, the recruited patients had different etiologies. Therefore, the heterogeneity of our patients in terms of the causative factors of the cirrhosis could have resulted in an underestimation of the beneficial effects of CD133+ cell therapy in decompensated cirrhosis. However, we also observed significant changes in variables such as total bilirubin in the placebo group at months 3 and 6. This observation underscores the fluctuations commonly observed in patients with decompensated cirrhosis. Future trials with increased numbers of more homogenous patients at baseline are required to confirm our observation.
Conclusion
Although a transient improvement in liver function was observed in cirrhotic patients after repeated intraportal infusion of CD133+ cells but not MNCs, it was insufficient to effectively improve liver cirrhosis. The limited number of patients in the present study resulted in low statistical power. Moreover, our trial was limited by the different etiologies for cirrhosis and baseline patient heterogeneity. Further studies with higher numbers of patients are warranted to better clarify the effect of CD133+ infusion on cirrhosis.
Supplementary Material
Acknowledgments
This project was supported by the Small Business Development Centers at Royan Institute, the Iranian Council of Stem Cell Research and Technology, and the Digestive Disease Research Institute, Tehran University of Medical Sciences, Tehran, Iran.
Author Contributions
M.M. and M.V.: conception and design, provision of study material or patients, data analysis and interpretation, manuscript writing, final approval of manuscript; S. Moossavi: data analysis and interpretation, manuscript writing; S.N. and S. Mardpour: collection and/or assembly of data, data analysis and interpretation; S.A., M.A., V.A., N.J., S.-E.H., F.M., and M.B.: collection and/or assembly of data; M.S.: statistical analysis, final approval of the manuscript; N.A.: conception and design, provision of study material or patients, administrative support; R.M. and H.B.: conception and design, financial support, administrative support, provision of study material or patients, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Pinzani M, Rosselli M, Zuckermann M. Liver cirrhosis. Best Pract Res Clin Gastroenterol. 2011;25:281–290. doi: 10.1016/j.bpg.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Murray KF, Carithers RL., Jr AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407–1432. doi: 10.1002/hep.20704. [DOI] [PubMed] [Google Scholar]
- 3.Sohrabpour AA, Mohamadnejad M, Malekzadeh R. Review article: The reversibility of cirrhosis. Aliment Pharmacol Ther. 2012;36:824–832. doi: 10.1111/apt.12044. [DOI] [PubMed] [Google Scholar]
- 4.Irfan A, Ahmed I. Could stem cell therapy be the cure in liver cirrhosis? J Clin Exp Hepatol. 2015;5:142–146. doi: 10.1016/j.jceh.2014.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vosough M, Moslem M, Pournasr B, et al. Cell-based therapeutics for liver disorders. Br Med Bull. 2011;100:157–172. doi: 10.1093/bmb/ldr031. [DOI] [PubMed] [Google Scholar]
- 6.Gordon MY, Levicar N, Pai M, et al. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells. 2006;24:1822–1830. doi: 10.1634/stemcells.2005-0629. [DOI] [PubMed] [Google Scholar]
- 7.Terai S, Ishikawa T, Omori K, et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292–2298. doi: 10.1634/stemcells.2005-0542. [DOI] [PubMed] [Google Scholar]
- 8.Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, et al. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007;10:459–466. [PubMed] [Google Scholar]
- 9.Mohamadnejad M, Namiri M, Bagheri M, et al. Phase 1 human trial of autologous bone marrow-hematopoietic stem cell transplantation in patients with decompensated cirrhosis. World J Gastroenterol. 2007;13:3359–3363. doi: 10.3748/wjg.v13.i24.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan AA, Parveen N, Mahaboob VS, et al. Safety and efficacy of autologous bone marrow stem cell transplantation through hepatic artery for the treatment of chronic liver failure: A preliminary study. Transplant Proc. 2008;40:1140–1144. doi: 10.1016/j.transproceed.2008.03.111. [DOI] [PubMed] [Google Scholar]
- 11.Levicar N, Pai M, Habib NA, et al. Long-term clinical results of autologous infusion of mobilized adult bone marrow derived CD34+ cells in patients with chronic liver disease. Cell Prolif. 2008;41(suppl 1):115–125. doi: 10.1111/j.1365-2184.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pai M, Zacharoulis D, Milicevic MN, et al. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103:1952–1958. doi: 10.1111/j.1572-0241.2008.01993.x. [DOI] [PubMed] [Google Scholar]
- 13.Kharaziha P, Hellström PM, Noorinayer B, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: A phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21:1199–1205. doi: 10.1097/MEG.0b013e32832a1f6c. [DOI] [PubMed] [Google Scholar]
- 14.Salama H, Zekri AR, Zern M, et al. Autologous hematopoietic stem cell transplantation in 48 patients with end-stage chronic liver diseases. Cell Transplant. 2010;19:1475–1486. doi: 10.3727/096368910X514314. [DOI] [PubMed] [Google Scholar]
- 15.Peng L, Xie DY, Lin BL, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: Short-term and long-term outcomes. Hepatology. 2011;54:820–828. doi: 10.1002/hep.24434. [DOI] [PubMed] [Google Scholar]
- 16.Zhao W, Li JJ, Cao DY, et al. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World J Gastroenterol. 2012;18:1048–1058. doi: 10.3748/wjg.v18.i10.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spahr L, Chalandon Y, Terraz S, et al. Autologous bone marrow mononuclear cell transplantation in patients with decompensated alcoholic liver disease: A randomized controlled trial. PLoS One. 2013;8:e53719. doi: 10.1371/journal.pone.0053719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.am Esch JS, II, Knoefel WT, Klein M, et al. Portal application of autologous CD133+ bone marrow cells to the liver: A novel concept to support hepatic regeneration. Stem Cells. 2005;23:463–470. doi: 10.1634/stemcells.2004-0283. [DOI] [PubMed] [Google Scholar]
- 19.Jang YY, Collector MI, Baylin SB, et al. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004;6:532–539. doi: 10.1038/ncb1132. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Willenbring H, Akkari Y, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 21.Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- 22.Nikeghbalian S, Pournasr B, Aghdami N, et al. Autologous transplantation of bone marrow-derived mononuclear and CD133(+) cells in patients with decompensated cirrhosis. Arch Iran Med. 2011;14:12–17. [PubMed] [Google Scholar]
- 23.King A, Barton D, Beard HA, et al. REpeated AutoLogous Infusions of STem cells In Cirrhosis (REALISTIC): A multicentre, phase II, open-label, randomised controlled trial of repeated autologous infusions of granulocyte colony-stimulating factor (GCSF) mobilised CD133+ bone marrow stem cells in patients with cirrhosis. A study protocol for a randomised controlled trial. BMJ Open. 2015;5:e007700. doi: 10.1136/bmjopen-2015-007700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assmann A, Heke M, Kröpil P, et al. Laser-supported CD133+ cell therapy in patients with ischemic cardiomyopathy: Initial results from a prospective phase I multicenter trial. PLoS One. 2014;9:e101449. doi: 10.1371/journal.pone.0101449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasseri BA, Ebell W, Dandel M, et al. Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: The Cardio133 trial. Eur Heart J. 2014;35:1263–1274. doi: 10.1093/eurheartj/ehu007. [DOI] [PubMed] [Google Scholar]
- 26.Ratajczak J, Kucia M, Mierzejewska K, et al. Paracrine proangiopoietic effects of human umbilical cord blood-derived purified CD133+ cells—Implications for stem cell therapies in regenerative medicine. Stem Cells Dev. 2013;22:422–430. doi: 10.1089/scd.2012.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaipa G, Tilenni M, Straino S, et al. GMP-based CD133(+) cells isolation maintains progenitor angiogenic properties and enhances standardization in cardiovascular cell therapy. J Cell Mol Med. 2010;14(suppl 6B):1619–1634. doi: 10.1111/j.1582-4934.2009.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elpek GO. Angiogenesis and liver fibrosis. World J Hepatol. 2015;7:377–391. doi: 10.4254/wjh.v7.i3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taniguchi E, Kin M, Torimura T, et al. Endothelial progenitor cell transplantation improves the survival following liver injury in mice. Gastroenterology. 2006;130:521–531. doi: 10.1053/j.gastro.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Kwon J, Popov Y, et al. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology. 2014;146:1339–1350.e1. doi: 10.1053/j.gastro.2014.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caldwell SH, Hoffman M, Lisman T, et al. Coagulation disorders and hemostasis in liver disease: Pathophysiology and critical assessment of current management. Hepatology. 2006;44:1039–1046. doi: 10.1002/hep.21303. [DOI] [PubMed] [Google Scholar]
- 32.Dufour DR, Lott JA, Nolte FS, et al. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem. 2000;46:2027–2049. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.