Infantile hemangiomas (IHs) are the most common vascular tumor and arise from a hemangioma stem cell (HemSC). Propranolol has proved efficacious against IHs. A selective β2-adrenergic receptor (AR) antagonist mirrored propranolol’s effects on HemSCs. These results show that propranolol acts on HemSCs in IH to suppress proliferation and promote apoptosis in a dose-dependent fashion via β2AR perturbation.
Keywords: Hemangioma, Propranolol, β-Adrenergic receptor, Cell proliferation, Cell death, Mitogen-activated protein kinase, cAMP, Stem cell
Abstract
Infantile hemangiomas (IHs) are the most common vascular tumor and arise from a hemangioma stem cell (HemSC). Propranolol has proved efficacious for problematic IHs. Propranolol is a nonselective β-adrenergic receptor (βAR) antagonist that can lower cAMP levels and activate the mitogen-activated protein kinase (MAPK) pathway downstream of βARs. We found that HemSCs express β1AR and β2AR in proliferating IHs and determined the role of these βARs and the downstream pathways in mediating propranolol’s effects. In isolated HemSCs, propranolol suppressed cAMP levels and activated extracellular signal-regulated kinase (ERK)1/2 in a dose-dependent fashion. Propranolol, used at doses of <10−4 M, reduced cAMP levels and decreased HemSC proliferation and viability. Propranolol at ≥10−5 M reduced cAMP levels and activated ERK1/2, and this correlated with HemSC apoptosis and cytotoxicity at ≥10−4 M. Stimulation with a βAR agonist, isoprenaline, promoted HemSC proliferation and rescued the antiproliferative effects of propranolol, suggesting that propranolol inhibits βAR signaling in HemSCs. Treatment with a cAMP analog or a MAPK inhibitor partially rescued the HemSC cell viability suppressed by propranolol. A selective β2AR antagonist mirrored propranolol’s effects on HemSCs in a dose-dependent fashion, and a selective β1AR antagonist had no effect, supporting a role for β2AR signaling in IH pathobiology. In a mouse model of IH, propranolol reduced the vessel caliber and blood flow assessed by ultrasound Doppler and increased activation of ERK1/2 in IH cells. We have thus demonstrated that propranolol acts on HemSCs in IH to suppress proliferation and promote apoptosis in a dose-dependent fashion via β2AR perturbation, resulting in reduced cAMP and MAPK activation.
Significance
The present study investigated the action of propranolol in infantile hemangiomas (IHs). IHs are the most common vascular tumor in children and have been proposed to arise from a hemangioma stem cell (HemSC). Propranolol, a nonselective β-adrenergic receptor (βAR) antagonist, has proven efficacy; however, understanding of its mechanism of action on HemSCs is limited. The presented data demonstrate that propranolol, via βAR perturbation, dose dependently suppresses cAMP levels and activated extracellular signal-regulated kinase 1/2. Furthermore, propranolol acts via perturbation of β2AR, and not β1AR, although both receptors are expressed in HemSCs. These results provide important insight into propranolol’s action in IHs and can be used to guide the development of more targeted therapy.
Introduction
Infantile hemangiomas (IHs) are benign vascular tumors affecting approximately 4%–10% of infants [1, 2]. The natural history of IHs is well documented and includes rapid proliferation in the first months of life, a plateau phase up to approximately 1 year, followed by involution into a fibrofatty residuum by early childhood [3]. Although IHs are benign and eventually undergo spontaneous regression, rapid growth of the tumor during the proliferative phase can result in serious morbidity and even mortality. Complications can include bleeding and congestive heart failure, permanent visual impairment, and unstable airway obstruction, leading to respiratory distress [4–8].
Propranolol, a nonselective β-adrenergic receptor (βAR) antagonist, has been found to be an effective drug for treating IH [9–13]. Despite its efficacy in treating the morbidities of IH, adverse effects have been described, including symptomatic bradycardia, hypotension, hypoglycemia, and hypoglycemia-induced seizures [14, 15]. The mechanisms of action of propranolol in IHs have yet to be fully elucidated. It has been proposed that its effects are mediated via βAR inhibition on two cell types in IHs, hemangioma stem cells (HemSCs) and hemangioma endothelial cells (HemECs). Greater knowledge of the mechanism by which propranolol affects IHs could guide the development of therapies that minimize the potential adverse effects and maximize efficacy.
Studies of propranolol using IH-derived cells have suggested that it targets multiple cell types in IHs. Propranolol has antiproliferative and antiangiogenic effects on HemECs [16–18] and antiproliferative and increased contractility on IH-derived α-smooth muscle actin-positive mural cells [19]. Propranolol has been shown to induce the death of HemSCs, the cell type proposed to be the origin of IHs [20–23].
βARs are G-protein-coupled receptors that promote cellular proliferation and survival [17, 18, 24–27]. In cultured endothelial or tumor cells, propranolol has been shown to both reduce cAMP levels and simultaneously activate the mitogen-activated protein kinase (MAPK) pathway downstream of βAR inhibition [24–28]. Propranolol lowers cAMP levels by inhibiting the Gαs/adenylyl cyclase downstream pathway [28]. Propranolol activation of extracellular signal-regulated kinase (ERK)1/2 is thought to be independent of a G-protein pathway, possibly involving β-arrestins [29–33]. The roles of cAMP and ERK1/2 regulation subsequent to propranolol treatment in HemSCs have yet to be fully elucidated.
In the present study, we examined the mechanisms by which propranolol affects HemSC cellular behavior, with a focus on the downstream βAR signaling pathways involving cAMP and MAPK. We demonstrate that propranolol reduces cAMP levels and activates ERK1/2 in HemSCs in a dose-dependent manner. At lower concentrations (<10−4 M), propranolol suppression of cAMP levels correlated with reduced HemSC proliferation. At higher concentrations (≥10−5 M), propranolol reduced cAMP levels and increased ERK1/2 activity, which correlated with HemSC apoptosis and cytotoxicity at the >10−4 M concentration. Experiments using isoprenaline (βAR agonist), bucladesine (cAMP analog), and U0126 (MAPK inhibitor) partially rescued the HemSC viability suppressed by propranolol. Propranolol’s effects on HemSCs were primarily mediated through β2ARs, because ICI-118,551 (ICI), a β2-specific adrenergic receptor antagonist, mirrored that of propranolol. Finally, using an IH mouse model, we have demonstrated that propranolol rescues abnormal blood vessel development and activates ERK1/2 in vivo. Taken together, our results suggest that propranolol mediates its effects on HemSCs by inhibiting βAR signaling to suppress cAMP levels and induce MAPK signaling.
Materials and Methods
Tissue Collection and Cell Culture
The Columbia University institutional review board approved the collection and use of tissues (protocol no. IRB-AAA9976). Preparation of hemangioma specimens for immunohistochemistry consisted of overnight fixation in 4% paraformaldehyde, sucrose soaking, and freezing in Tissue-Tek O.C.T. compound (Sakura Finetek, Tokyo, Japan, http://www.sakura-finetek.com) 7-μM frozen sections were made. HemSC isolation was performed, and HemSCs were characterized as described previously [21]. In brief, tissues with regions of proliferating IH (supplemental online Table 1) were disassociated with collagenase, and HemSCs were selected by CD133+ magnetic bead isolation (Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com). HemSCs were cultured in Endothelial Cell Growth Medium-2 (EGM-2; Lonza, Walkersville, MD, http://www.lonza.com) with 20% fetal bovine serum (FBS; Life Technologies, Carlsbad, CA, http://www.lifetechnologies.com). Bone marrow-derived mesenchymal stem cells (MSCs) were purchased (Lonza) and grown in EGM-2 with 20% FBS. Three cell lines were tested for each assay (supplemental online Table 1).
Immunohistochemistry
Fixed frozen IH tissue sections and IH xenograft tissues were stained as previously described [34]. The antibodies included β1AR (1:400; Abcam, Cambridge, U.K., http://www.abcam.com) and β2AR (1:500; Abcam), CD133 (1:50; EMD Millipore, Billerica, MA, http://www.emdmillipore.com), CD31 (1:50; Dako, Glostrup, Denmark, http://www.dako.com), and phosphorylated ERK1/2 (pERK1/2) (P-p44/42; 1:100; Cell Signaling Technology, Beverly, MA, http://www.cellsignal.com) and were detected with either Alexa Fluor 488 or 594 secondary antibodies (Invitrogen, Carlsbad, CA, http://www.invitrogen.com). From each IH xenograft, the number of activated ERK1/2-positive cells was determined and divided by the total number of cells from three representative high-power fields (HPFs).
Reagents
Propranolol hydrochloride (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) was reconstituted at 100 mM in pH 3.0 water. Atenolol (Sigma-Aldrich) was reconstituted at 50 mM in dimethyl sulfoxide (DMSO). ICI-118,551 hydrochloride (Sigma-Aldrich) was reconstituted at 25 mM in sterile pH 7.0 water. Isoprenaline hydrochloride (Sigma-Aldrich) and dibutyryl cyclic adenosine monophosphate (bucladesine; R&D Systems, Minneapolis, MN, http://www.rndsystems.com), a cAMP analog, were both reconstituted in water to a concentration of 100 mM. U0126, a MEK1/MEK2 inhibitor, was reconstituted in DMSO to a concentration of 25 mM.
cAMP Assay
The cAMP levels in HemSCs were determined using the LANCE Ultra cAMP kit (PerkinElmer Life and Analytical Sciences, Waltham, MA, http://www.perkinelmer.com). The HemSCs were washed and resuspended in the provided stimulation buffer (Hanks’ balanced saline solution, bovine serum albumin, isobutylmethylxanthine, HEPES buffered saline solution) and seeded (1,000 per well) on a 96-well plate. The cells were then treated with drugs for 30 minutes. Tracer and ULight-anti-cAMP working solutions were added and incubated at room temperature for 1 hour. The time-resolved fluorescence resonance energy transfer signal was determined using the EnVision Multilabel Plate Reader (PerkinElmer Life and Analytical Sciences). cAMP levels were determined using a standard curve, and data were interpolated using a comprehensive curve fitting (nonlinear regression) and Prism (GraphPad Software, Inc., San Diego, CA, http://www.graphpad.com). Each condition was used in triplicate, and the experiments were performed at least two times. A representative experiment is presented in the figures.
To determine whether βARs are coupled to Gαs or Gαi in HemSCs, the cells were treated with isoprenaline, with or without 10 μM forskolin, over a 6-log dose range by serial dilutions with water for 30 minutes. Next, the cAMP levels were measured as described to determine whether βARs were coupled to Gαs or Gαi in HemSCs.
ERK1/2 Western Blotting
Cells were cultured on fibronectin-coated plates and treated with various concentrations of βAR antagonists and incubated for 30 minutes. The cells were lysed in TENT buffer (50 mM Tris [pH 8.0], 2 mM EDTA, 150 mM NaCl, 1% Triton-X-100) with 1% Halt Protease Inhibitor (Thermo Scientific, Wilmington, DE, http://www.thermoscientific.com), 1% phosphatase inhibitor (Thermo Scientific), and 0.5% sodium orthovanadate (FIVEphoton Biochemicals, San Diego, CA, http://www.fivephoton.com). Western blotting was performed for ERK1/2 (p44/42, 1:1,000; Cell Signaling Technology) and pERK1/2 (P-p44/42, 1:500; Cell Signaling Technology). The blots were stripped and then probed for α-tubulin (1:10,000; Sigma-Aldrich) to normalize protein loading. Experiments were performed at least three times, and a representative experiment is presented in the figures.
Proliferation Assay
HemSCs (5,000–10,000 per well) were seeded at subconfluency on a fibronectin-coated 24-well plate in EGM-2 with 20% FBS. Four hours later, the media were removed and the cells treated with various concentration of drug in serum-free media (SFM; Life Technologies) supplemented with 1% FBS. After 24–72 hours, the cell number was determined using the Cell Counting Kit-8 (Dojindo Molecular Technologies Inc., Gaithersburg, MD, http://www.dojindo.com) and using a standard curve. Each condition was used in triplicate, and the experiments performed at least three times. A representative experiment is presented in the figures.
Cytotoxicity/Digital Imaging Microscopy System Assay
HemSCs (4,000 cells per well) were seeded on a fibronectin-coated 96-well plate in EGM-2 with 20% FBS. The next day, when the HemSCs had reached confluence, the media were removed, and cells were treated with various concentrations of drug in SFM with 0.1% FBS. After 24 hours, the cells were incubated in 10 μg/ml fluorescein diacetate and 0.5% Eosin-Y for 20 minutes. Cell viability was determined by fluorescence using the Digital Imaging Microscopy System (DIMSCAN; BioImaging Solutions, Inc., San Diego, CA, http://www.bioimagimgsolutions.com) [35]. Each condition was used in triplicate or sextuplet, and experiments were performed at least three times. A representative experiment is presented in the figures.
Apoptosis Assays
Annexin V Assay
HemSCs were seeded in EGM-2 with 20% FBS media on 6-cm2 plates. After 4 hours, increasing concentrations of propranolol in SFM with 0.1% FBS were added to HemSCs. After 6 hours, Annexin V expression levels were measured using the Annexin V-FITC Apoptosis Kit (BioVision, Inc., Milpitas, CA, http://www.biovision.com). Antibody detection was performed using the FACSCalibur flow cytometer (BD Biosciences).
Caspase-3 Assay
HemSCs were seeded in EGM-2 with 20% FBS media and allowed to settle for 4 hours. HemSCs were treated at increasing concentrations of propranolol in SFM with 0.1% FBS for 24 hours. The protein lysates were collected, and caspase-3 activation was quantified using the Caspase-3 Human ELISA Kit (Life Technologies).
IH Mouse Model
All animal studies were performed with approval from Columbia University’s institutional animal care and use committee (approval no. AAAG5852). To study the effects of propranolol on HemSCs in vivo, a xenograft mouse model of IH was used as previously described [20]. In brief, 1.5 × 106 HemSCs (n = 2) suspended in 200 µL of Corning Matrigel Matrix (Corning, Corning, NY, http://www.corning.com) was implanted subcutaneously into the flanks of female 6–8-week-old NCrNude immunodeficient mice (n = 4; Taconic Biosciences, Hudson, NY, http://www.taconic.com). Propranolol, which was provided in drinking solution, was initiated the day of IH xenografting. Propranolol was diluted to 270 µM in 5% dextrose water (vehicle), and daily consumption was measured to calculate the treatment dosage, which averaged 40 mg/kg daily.
Blood flow within the IH Matrigel implant was analyzed using a VEVO 2100 Ultrasound Imaging System (VisualSonics, Toronto, ON, Canada, http://www.visualsonics.com) on a Doppler setting on days 14 and 21 of IH development. The mice were anesthetized with isoflurane and restrained in a supine position. The region of interest was fully scanned, with the transducer positioned at its largest longitudinal section over the implant to optimize the spatial resolution of the image, maximizing the detail. Next, two-dimensional images were captured in uniform steps of 0.05 mm. The images of blood flow were analyzed using software provided by VisualSonics.
The mice were sacrificed after 21 days. The Matrigel implants were collected and fixed overnight at 4°C in 10% formalin. The implants were dehydrated and embedded in paraffin for histological analysis. Vessel density and caliber were counted in 3–4 HPFs per implant (n = 4 for each group). Vessel density was determined as the number of vessels (whether longitudinally or axially oriented) per HPF. The vessel diameter was measured according to the orientation. For longitudinally oriented vessels, the width was measured at three points and averaged, and the cross-section (axial) vessels were measured once. Vessels were identified as tubular structures with erythrocytes within.
Statistical Analysis
To determine the significance between the control and experimental groups in the in vitro studies, a two-sample independent measures t test was used. For analyses of more than two groups, analysis of variance (ANOVA) with the Tukey-Kramer post hoc test (family error rate, α = 0.05) was performed. The caspase-3 assay significance was determined for a series of four two-sample independent measures t tests with Bonferroni’s correction (family error rate, α = 0.05, corresponding to an individual hypothesis test error rate of α = 0.0127). Cohen’s d, which reflects the magnitude of the treatment effect, was calculated and reported; by convention, d > 0.8 corresponds to a large effect size. For in vivo studies, the nonparametric Mann-Whitney U test was used to compare the vehicle and propranolol treatment between the two cell populations. For all tests, p < .05 was considered statistically significant. Statistical analysis was performed using Minitab, version 16 (Minitab Inc., State College, PA, http://www.minitab.com).
Results
In IH Tissues HemSCs Expressed β1- and β2-Adrenergic Receptors
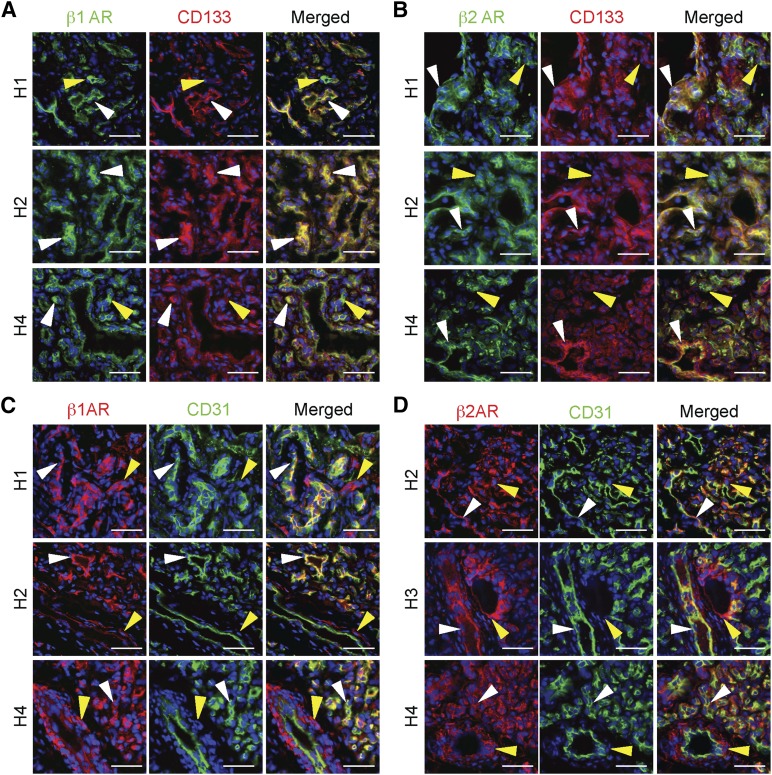
Because propranolol is a nonselective βAR antagonist with high affinity for the β1AR and β2AR [28], we determined their expression in HemSCs and HemECs in seven cutaneous proliferating hemangiomas. IH tissues are heterogeneous, and cells reside on a spectrum of CD133+ HemSCs localized adjacent to IH vessels and CD133+/CD31+ and CD31+ HemECs lining the IH vessels. β1AR was expressed in both CD133+ HemSCs and CD31+ HemECs (Fig. 1A, 1C). β2AR expression was strongest in CD133+ HemSCs, and only spotty expression was observed in HemECs (Fig. 1B, 1D). These results demonstrate that HemSCs in IHs express both β1AR and β2AR; thus, propranolol might be targeting the HemSCs, as well as the HemECs, in IH.
Figure 1.
Hemangioma stem cells (HemSCs) and hemangioma endothelial cells (HemECs) in proliferating infantile hemangiomas (IHs) expressed β1AR and β2AR. IH tissue sections were costained for β1AR or β2AR and either a stem cell marker (CD133) or an endothelial cell marker (CD31) (n = 7; representative images from three specimens shown). (A): β1AR and CD133 staining. White arrowheads mark β1AR-expressing CD133+ HemSCs. Yellow arrowheads mark cells that only express β1AR. (B): β2AR and CD133 staining. White arrowheads mark β2AR-expressing CD133+ HemSCs. Yellow arrowheads mark CD133− and β2AR+ cells. (C): β1AR and CD31 staining. White arrowheads mark β1AR-expressing CD31+ HemECs. Yellow arrowheads mark cells that only express β1AR localized to the perivascular region. (D): β2AR and CD31 staining. White arrowheads mark β2AR-expressing CD31+ HemECs. Yellow arrowheads mark CD31− and β2AR+ cells localized to perivascular regions. Scale bars = 50 μm. Abbreviations: β1AR, β1-adrenergic receptor; β2-adrenergic receptor.
Propranolol Dose Dependently Lowered cAMP Levels and Activated MAPK Signaling in HemSCs
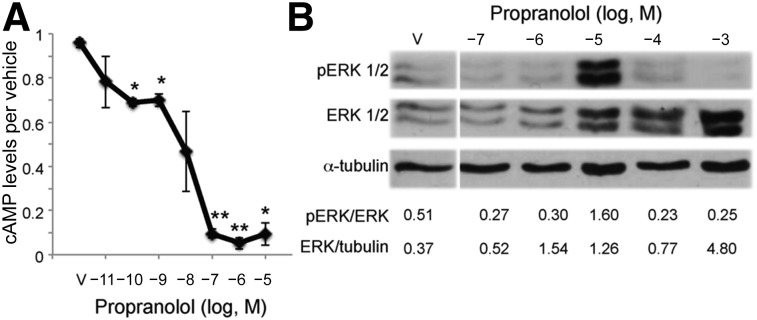
Propranolol has been shown in non-IH cell types to both reduce cAMP levels and increase MAPK signaling downstream of βARs [24–28]. To investigate the downstream βAR signaling pathways affected by propranolol, HemSCs were treated with increasing amounts of propranolol and the cAMP levels and MAPK and ERK1/2 activity determined [17, 18, 36]. Increasing doses of the βAR agonist, isoprenaline, resulted in a significant increase in cAMP levels at 20 nM (10−7.7 M) with an approximately threefold increase at 600 nM (10−6.2 M; supplemental online Fig. 1A). To confirm that isoprenaline regulated cAMP levels via Gαs activation, we assessed the ability of isoprenaline to increase cAMP in the presence of 10 μM forskolin, a compound known to activate adenylyl cyclase. Isoprenaline was still able to increase cAMP levels in this assay, suggesting βAR signals via Gαs in HemSCs (supplemental online Fig. 1B). In contrast, increasing doses of propranolol steadily decreased the cAMP levels over a 7-log dose range, with significance observed at 10−10 M and maximum suppression reached at 100 nM (10−7 M) propranolol (Fig. 2A).
Figure 2.
Propranolol dose dependently decreased cAMP levels and activated ERK1/2 in hemangioma stem cells (HemSCs). (A): HemSCs were treated with increasing doses of propranolol over a 7-log dose range (10−11 M to 10−5 M) and cAMP levels determined. Data presented as the fold-difference between propranolol-treated HemSCs relative to vehicle-treated HemSCs ± SEM; ∗, p < .005; ∗∗, p < .00002. (B): HemSCs were treated with increasing doses of propranolol over a 5-log dose range (10−7 M to 10−3 M), and ERK1/2 activation was determined at 30 minutes. Total and pERK1/2 expression was assessed by Western blot. Blots were serially stained for α-tubulin as a protein-loading control. Ratios of total ERK1/2 to α-tubulin and pERK1/2 to total ERK1/2 as determined by densitometry are presented below the blots. Abbreviations: ERK, extracellular signal-regulated kinase; pERK, phosphorylated ERK; V, vehicle.
Next, we determined the dose-dependent effects of propranolol on MAPK activity on HemSCs. HemSCs were treated with increasing amounts (7-log dose range) of propranolol, and ERK1/2 phosphorylation and total ERK expression were determined. Propranolol increased the total ERK1/2 levels in a dose-dependent manner (Fig. 2B). ERK1/2 activation was observed specifically at 10−5 M propranolol (Fig. 2B), consistent with a previous study in which propranolol activated ERK1/2 in 293T cells that ectopically expressed either β1AR or β2AR [28]. To assess whether the propranolol effects on ERK1/2 are specific to HemSCs, bone marrow-derived MSCs were treated with propranolol at 50 μM (10−4.3 M) and 100 μM (10−4 M). In MSCs, propranolol also increased ERK1/2 phosphorylation (supplemental online Fig. 2). Thus, high-dose propranolol increased ERK activation in multiple stem cell lines.
Propranolol Suppressed HemSC Proliferation and Induced HemSC Apoptosis and Cytotoxicity
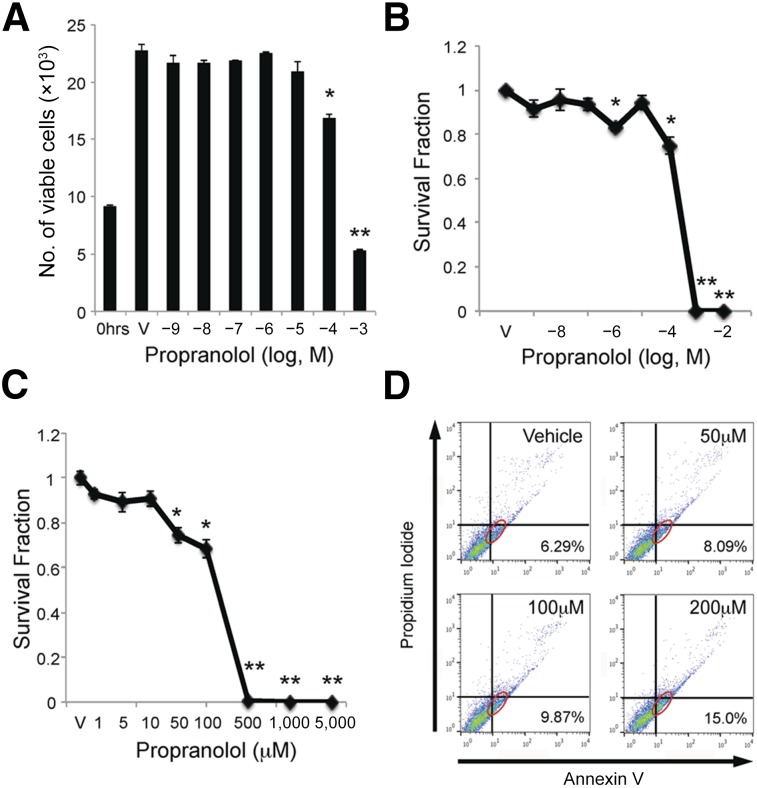
We next investigated the effects of propranolol on HemSC proliferation and death. HemSCs were treated with either vehicle or increasing amounts of propranolol, ranging from 10−9 M to 10−3 M, and the total number of viable cells was determined after 24 and 48 hours. Vehicle-treated HemSCs grew 2.5-fold after 24 hours (Fig. 3A). A significant decrease of viable HemSCs was observed at 10−4 M propranolol after 24 hours and 10−9 M propranolol after 48 hours (Fig. 3A; supplemental online Fig. 3). At doses greater than 10−4 M propranolol, the number of viable cells decreased to less than the number of HemSCs at time 0 (supplemental online Fig. 3A), suggesting propranolol induced HemSC death, as well as suppressing their proliferation.
Figure 3.
Propranolol inhibited proliferation and induced apoptosis of hemangioma stem cells (HemSCs). (A): HemSCs were treated with increasing doses of propranolol over a 10-log dose range and the number of viable HemSCs determined at time 0 before treatment or 24 hours after treatment. ∗, p < .002 and ∗∗, p < .001 compared with vehicle. (B): HemSCs were treated with increasing doses of propranolol over an 8-log dose range (10−9 M to 10−2 M), and viability was assessed by Digital Imaging Microscopy System (DIMSCAN) assay at 24 hours. ∗, p < .0005; ∗∗, p < .000001. (C): HemSCs were treated with increasing doses of propranolol from 1 μM to 5 mM, and viability was assessed by DIMSCAN assay at 24 hours. ∗, p < .001; ∗∗, p < .00001. (B, C): Data presented as survival fraction of propranolol-treated HemSCs relative to vehicle controls ± SEM. (D): HemSCs were treated with 50 μM, 200 μM, and 400 μM propranolol (corresponding to LD10, LD50, and LD90), and Annexin V assay was performed at 24 hours. Annexin V detects actively apoptotic cells (x-axis), and propidium iodide (y-axis) detects necrotic cells. Annexin V-positive and propidium iodide-negative apoptotic HemSCs are circled in red and their percentages shown in the lower right corner. Abbreviation: V, vehicle.
The effect of an 8-log dose range of propranolol on HemSC cytotoxicity was assessed using a DIMSCAN assay [35]. In this assay, drugs are introduced to confluent monolayers to assess cell survival independent of the drugs’ effects on proliferation, and cytotoxicity is defined as less than 10% of HemSCs in the survival fraction [35]. After 24 hours, propranolol concentrations less than 100 μM (10−4 M) did not significantly affect HemSC survival. At concentrations greater than 100 μM (10−4 M), propranolol resulted in a sharp and significant decrease in the survival fraction (Fig. 3B). To more precisely determine the cytotoxic concentration of propranolol on HemSCs, a narrower range from 1 μM to 5 mM propranolol was assessed (Fig. 3C). After 24 hours, a significant reduction in HemSC survival was observed at 50 μM, and cytotoxicity was achieved at somewhere between 100 μM (10−4 M) and 500 μM propranolol. HemSC survival was not observed (0% viability) at doses of ≥500 μM. The LD50 of propranolol for HemSCs was determined to be approximately 200 μM, averaged from HemSCs isolated from 3 different IH specimens (supplemental online Table 1).
We used Annexin V and caspase-3 assays to determine whether propranolol-induced HemSC cell death occurred via an apoptotic pathway. Cell surface expression of Annexin V, a marker of active apoptosis, and propidium iodide incorporation, as a marker of dead cells, was determined by FACS analysis of HemSCs treated with vehicle or 50 μM, 100 μM, or 200 μM propranolol for 6 hours. Increasing doses of propranolol were associated with increased Annexin V positivity (Fig. 3D). An increase in HemSC apoptosis was not observed when cells were exposed to propranolol for more than 48 hours, suggesting propranolol rapidly induced HemSC apoptosis (data not shown). To confirm the Annexin V results, caspase-3 activity, a marker of apoptosis, was determined in HemSCs treated with vehicle or 50 μM, 200 μM, or 400 μM propranolol (cytotoxicity at LD10, LD50, and LD90, respectively) in the presence or absence of serum for 24 hours. A significant increase in caspase-3 activity was observed at 100 μM propranolol that increased with the higher propranolol concentrations (supplemental online Fig. 4). Thus, cytotoxic doses of propranolol (>10−4 M) significantly increased Annexin V positivity and caspase-3 activation, suggesting propranolol rapidly induces HemSC apoptosis.
Propranolol’s Antiproliferative Effects in HemSCs Are Mediated via βARs
We previously showed that isolated HemSCs express β1AR and β2AR and that propranolol inhibited HemSC proliferation [21]. We screened β1AR and β2AR transcript levels in the HemSC populations (n = 8) and found that most HemSC populations expressed high levels of β2AR and low levels of β1AR, including those used for the present studies (supplemental online Table 1).
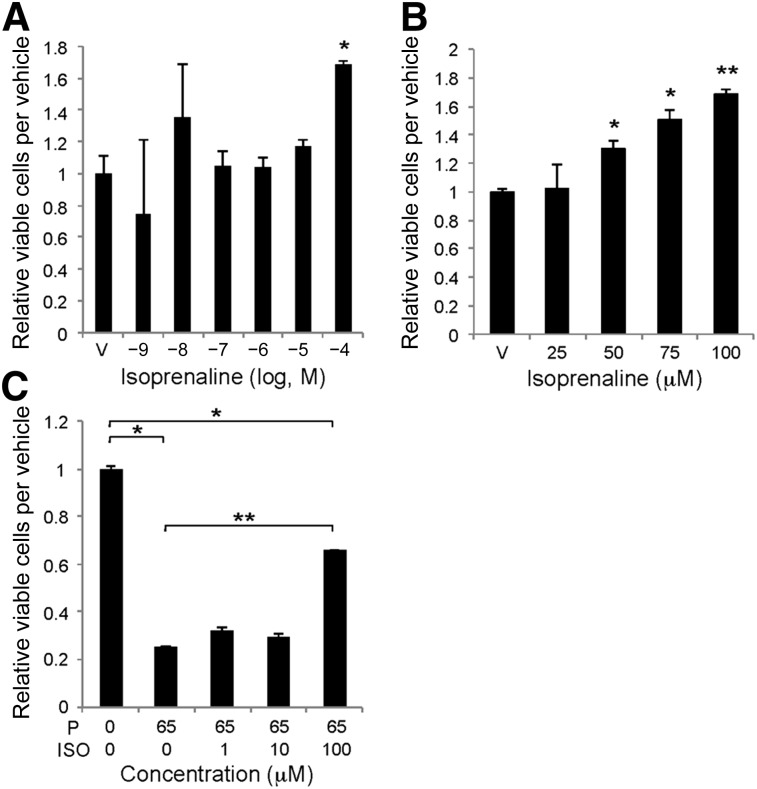
To investigate whether the propranolol effects on HemSCs are mediated via perturbation of βAR signaling, HemSCs were treated with increasing amounts of the βAR-specific agonist, isoprenaline, over a 6-log dose range, and proliferation was determined at 48 hours. A dose of 10−4 M isoprenaline resulted in an ∼1.7-fold increase in HemSC proliferation (Fig. 4A). In a narrower dose range from 25 to 100 μM, significant HemSC proliferation was induced at 50 μM (10−4.3 M) isoprenaline (Fig. 4B). We determined the ability of increasing amounts of isoprenaline to reverse the antiproliferative effects of propranolol after 24 hours. A dose of 100 μM (10−4 M) isoprenaline significantly increased HemSC proliferation—2.64-fold in the presence of 65 μM (10−4.2 M) propranolol relative to 65 μM propranolol alone (Fig. 4C). However, HemSC proliferation was still significantly decreased relative to the vehicle-treated cells, suggesting that the propranolol effects on HemSC proliferation were mediated by both βAR-dependent and βAR-independent mechanisms.
Figure 4.
Isoprenaline induced hemangioma stem cell (HemSC) proliferation and blocked propranolol’s antiproliferative effects on HemSCs. (A): HemSCs were treated with increasing doses of isoprenaline over a 6-log dose range (10−9 M to 10−4 M), and proliferation was determined at 48 hours. ∗, p < .05. (B): HemSCs were treated with a narrower dose range of isoprenaline from 25 μM to 100 μM (10−4.6 M to 10−4 M), and proliferation was determined at 48 hours. ∗, p < .05; ∗∗, p < .005. (C): HemSCs were pretreated with increasing doses of isoprenaline for 1 hour. Next, 65 μM propranolol (antiproliferative dose) was added and proliferation determined at 24 hours. ∗, p < .05; ∗∗, p < .0005. (A–C): Data presented as fold-difference to vehicle-treated HemSCs ± SEM. Abbreviations: ISO, isoprenaline; P, propranolol; V, vehicle.
A cAMP Analog or MAPK Inhibition Partially Rescued Propranolol Effects on HemSCs
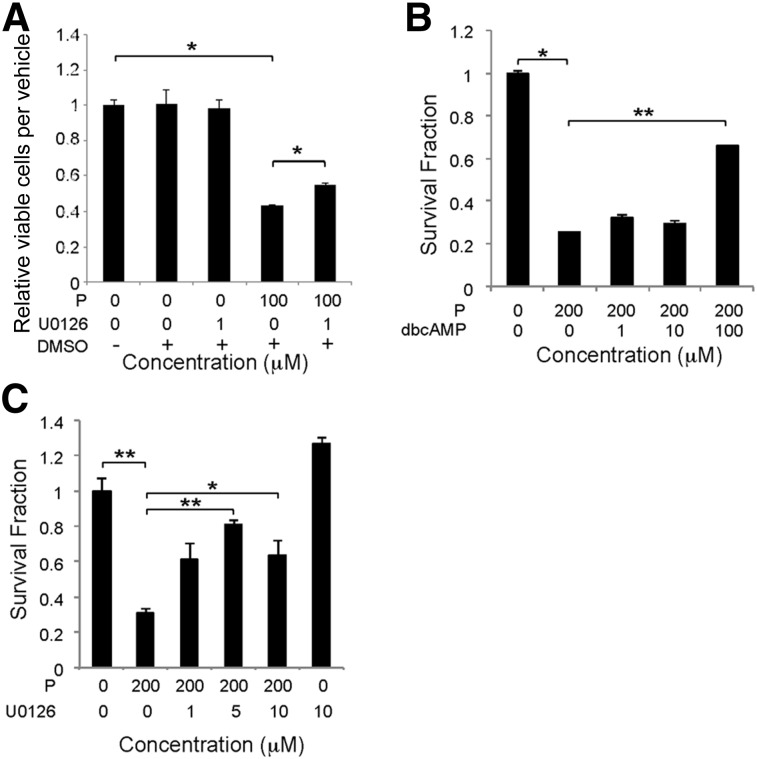
At 10−5 M propranolol, suppression of cAMP levels and ERK activation (Fig. 2) correlated with the antiproliferative and antisurvival effects of propranolol (Fig. 3). To determine whether these two signaling events were necessary for propranolol to exert its cellular effects, HemSC proliferation and survival was assessed in the presence of bucladesine, a cAMP analog, or U0126, a MEK1/2 inhibitor. U0126 partially rescued the decrease in cell viability caused by100 µM propranolol, but bucladesine did not (Fig. 5A; data not shown). To determine whether cAMP suppression was necessary for propranolol-induced cytotoxicity at doses greater than 10−4 M, increasing doses of bucladesine were added to HemSCs exposed to 200 μM propranolol (LD50), and HemSC survival was assessed at 24 hours. At 100 μM, bucladesine partially reversed propranolol-induced HemSC cytotoxicity, suggesting that cAMP suppression was necessary for HemSC death at higher concentrations (≥10−4 M; Fig. 5B). U0126 alone did not affect HemSC survival, but it did partially rescue propranolol-induced cell death (Fig. 5C). Together, these results demonstrate that propranolol via MAPK activation inhibited HemSC proliferation, and both cAMP suppression and MAPK activation mediated propranolol-induced HemSC cell death.
Figure 5.
Ectopic cAMP or mitogen-activated protein kinase inhibition partially rescued the propranolol-induced effects on hemangioma stem cells (HemSCs). (A): HemSCs, in either the presence or absence of 100 µM propranolol were treated with 1 µM U0126 or DMSO as a vehicle control, and the number of viable HemSCs was determined at 48 hours. ∗, p < .05. Data presented as fold-difference between treatment group and control ± SEM. (B): HemSCs, in either the presence or absence of 200 µM propranolol (proapoptotic dose), were treated with increasing doses of dbcAMP, and cell viability was determined by Digital Imaging Microscopy System (DIMSCAN) at 24 hours. ∗, p < .02; ∗∗, p < .0001. (C): HemSCs, either in the presence or absence of 200 µM propranolol (proapoptotic dose), were treated with increasing doses of U0126, and cell viability was determined by DIMSCAN at 24 hours. ∗, p < .05; ∗∗, p < .005. Data presented as survival fraction relative to control ± SEM (B, C). Abbreviations: dbcAMP, dibutyryl cAMP; DMSO, dimethyl sulfoxide; P, propranolol.
Propranolol Effects on HemSCs Were Mediated via Inhibition of β2AR
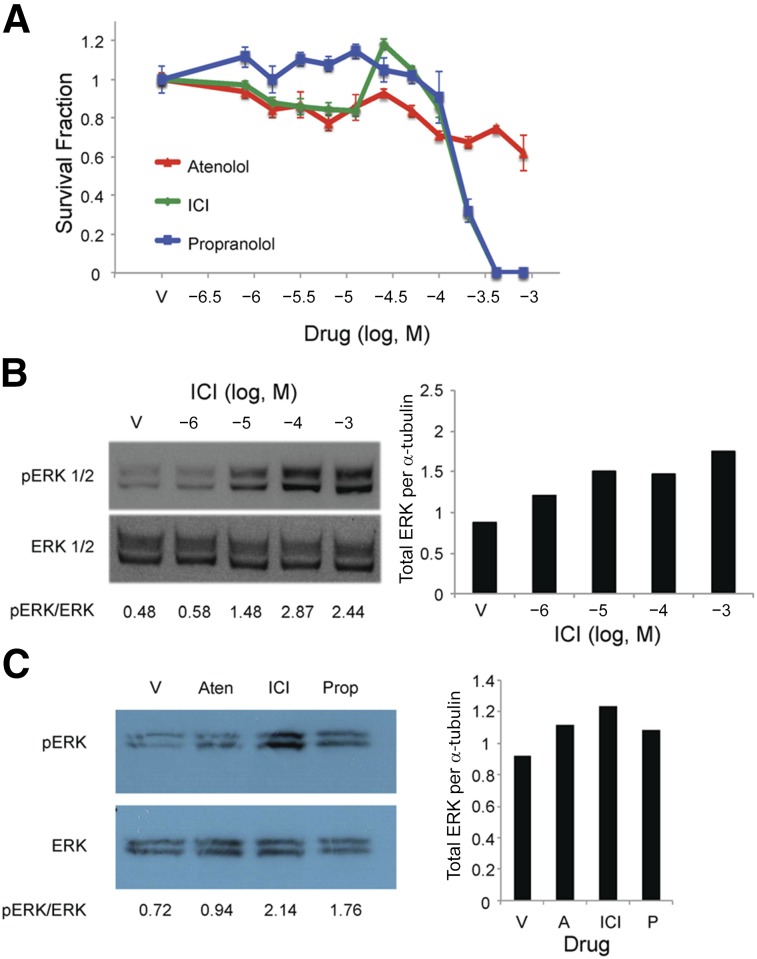
The HemSC populations studied expressed high levels β2AR and low levels of β1AR (supplemental online Table 1). We investigated whether the β2AR-specific antagonist, ICI-118,551 had similar effects on HemSCs, because propranolol is a pan-βAR antagonist, and compared its effects with that of the β1AR-specific antagonist, atenolol. HemSCs were exposed to a 4-log dose of propranolol (pan-βAR antagonist), atenolol (β1AR-specific antagonist), and ICI 118,551 (β2AR-specific antagonist). HemSC survival was determined after 24 hours. ICI mirrored the propranolol cytotoxic effects on HemSCs at 10−4 M to 10−3 M (Fig. 6A). Although atenolol modestly decreased cell viability (Fig. 6A), the effects of atenolol were similar to those observed for vehicle (DMSO) alone, indicating that the reduced HemSC survival resulted from DMSO and not the β1AR-specific antagonist (supplemental online Fig. 5). The effects of either propranolol or ICI when compared individually with atenolol on HemSC survival were statistically significant by ANOVA at 200 μM, 400 μM, and 800 μM (10−3.09 M, 10−3.39 M, 10−3.69 M, respectively). In contrast, no significant difference was seen in the fold-change in the cytotoxic effects between ICI and propranolol, suggesting propranolol targets β2AR in HemSCs. We observed a similar response in MSCs, in which ICI induced significant MSC cytotoxicity, similar to that of propranolol, which was not observed with atenolol (supplemental online Fig. 6A). MSCs also expressed higher levels of β2AR transcripts compared with β1AR (data not shown).
Figure 6.
β2-Adrenergic receptor inhibition mirrored propranolol’s effects on hemangioma stem cell (HemSC) viability and ERK1/2 activation. (A): HemSCs were treated with increasing doses of atenolol, ICI, or propranolol (10−6.5 M to 10−3 M), and HemSC viability was assessed by Digital Imaging Microscopy System (DIMSCAN) assay at 24 hours. Data presented as survival fraction of propranolol-treated HemSCs relative to vehicle controls ± SEM. At doses of 200 μM propranolol or greater, HemSC viability was significantly greater for cells treated with atenolol compared with those treated with either ICI or propranolol (p < .005 at 200 μM, p < .001 at 400 μM, and p < .005 at 800 μM). No significant difference was seen in cell viability between the cells treated with ICI and propranolol. (B): HemSCs were treated with increasing doses of ICI over a 4-log dose range (10−6 M to 10−3 M), and ERK1/2 activation was determined at 30 minutes by Western blot. (C): HemSCs were treated with 100 μM atenolol, ICI, or propranolol, and ERK1/2 activation was determined at 30 minutes by Western blot. (B, C): Blots were serially stained for α-tubulin as a protein-loading control. Ratios of pERK1/2 to total ERK1/2 as determined by densitometry are presented below the blots. Ratio of total ERK1/2 to α-tubulin presented in bar graphs to the right. Abbreviations: A, atenolol; Aten, atenolol; ERK, extracellular signal-regulated kinase; pERK, phosphorylated ERK; ICI, ICI-118,551; P, propranolol; Prop, propranolol; V, vehicle.
The effect of ICI on HemSC proliferation relative to propranolol and atenolol was tested. After 72 hours, 100 μM ICI reduced the number of viable HemSCs similar to that observed for propranolol (supplemental online Fig. 6B). The β1AR-specific antagonists, atenolol and metoprolol, did not affect HemSC cell viability in this assay (supplemental online Fig. 6B; data not shown).
We next assessed the effects of β1AR versus β2AR inhibition on ERK1/2 activation. ICI treatment of HemSCs increased total ERK expression and activated ERK1/2 at 10−5 M through 10−3 M (Fig. 6B, 6C). Although a slight increase in total ERK was observed, 100 μM atenolol did not activate ERK1/2 in HemSCs (Fig. 6C). Taken together, β2AR-specific antagonism of HemSCs mirrored the antiproliferative, antisurvival, and ERK1/2 activation effects of propranolol, suggesting propranolol targets β2AR in HemSCs to elicit its biological effects.
Propranolol Affects Vascular Development in a Xenograft Mouse Model of IH
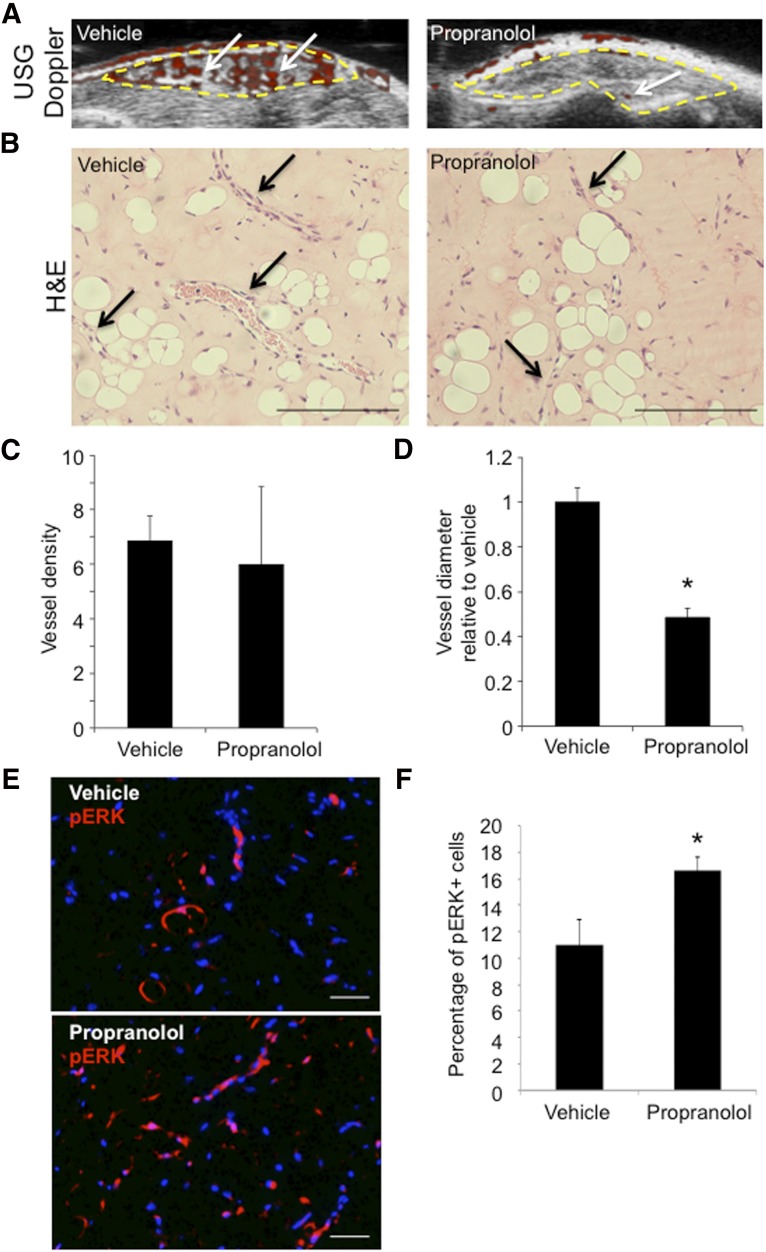
To assess how propranolol affects HemSCs and IH development in vivo, we adapted a previously described mouse model [20]. In the IH mouse model, HemSCs resuspended in Matrigel are implanted subcutaneously in immunocompromised mice, and IH vessel development progresses over 3 weeks. The mice were treated with propranolol or vehicle 40 mg/kg daily. Using the surface area conversion factor of 1/12 [37–39], the mice received a human equivalent dose of 3.3–4.8 mg/kg daily. IH Matrigel implants from propranolol-treated mice had reduced blood flow at 14 and 21 days after implantation, measured by Doppler ultrasound, compared with vehicle (data not shown; Fig. 7A). Histological analysis of the 21-day IH Matrigel implants (Fig. 7B) demonstrated that propranolol did not affect blood vessel density (Fig. 7C) but did significantly reduce the vessel diameter relative to the vehicle-treated implants (Fig. 7D). The reduced vessel caliber correlated with a loss of Doppler-detectable flow in the propranolol treatment group. Propranolol also significantly increased the number of cells that expressed phosphorylated ERK1/2 within the IH Matrigel implant (Fig. 7E), consistent with the results from our in vitro studies. Thus, propranolol improved vessel development in the IH mouse model that correlated with MAPK pathway activation.
Figure 7.
Propranolol reduced vessel caliber and increased ERK1/2 phosphorylation in an infantile hemangioma (IH) mouse model. Hemangioma stem cell (HemSC) Matrigel implants were xenografted into the flanks of immunocompromised mice, which were split into two treatment groups: vehicle and propranolol for 3 weeks (n = 2 cell populations; four Matrigel implants per treatment group). (A): Ultrasound-guided Doppler of implants at 21 days after implantation demonstrated reduced blood flow (red; white arrows) in implants from the propranolol treatment group compared with vehicle. (B): H&E of implant sections at 21 days after implantation. Black arrows highlight blood vessels. (C): Quantification of average blood vessel number per high-power field (HPF) in implants from vehicle and propranolol treatment. (D): Quantification of average blood vessel diameter in propranolol-treated group compared with vehicle for both H49 and H50. ∗, p < .0001 (C, D); n = 4–5 HPF for each line and treatment. (E): Vehicle- and propranolol-treated implants stained for pERK1/2. (F): Quantification of the number of pERK1/2-positive cells divided by the total number of cells. ∗, p < .05. Scale bars = 50 µm. Abbreviations: ERK, extracellular signal-regulated kinase; pERK, phosphorylated ERK; USG, ultrasonography.
Discussion
In IH tissues, HemSCs expressed both β1AR and β2AR. In isolated HemSCs, propranolol treatment dose dependently decreased cAMP levels and activated the MAPK pathway downstream of βARs. At low doses (IC50 of 65 μM), propranolol decreased cell viability by inhibiting HemSC proliferation and promoting HemSC apoptosis. In contrast, at high doses (LD50 of 200 μM [10−3.7 M]), propranolol was cytotoxic against HemSCs. The βAR agonist, isoprenaline, a cAMP analog, and a MAPK inhibitor partially rescued the propranolol-induced antiproliferative and antisurvival effects on HemSCs. The β2AR-specific antagonist, ICI-118,551, mirrored the propranolol effects on HemSC proliferation and survival of HemSCs. In an IH mouse model, propranolol reduced abnormal vessel dilation and increased p-ERK expression. Taken together, these data demonstrated that the propranolol effects on HemSCs are mediated in part via β2AR inhibition and suggest a role for β2AR signaling and its downstream cAMP and MAPK pathways in HemSC pathophysiology.
Both activation and inactivation of βAR has been shown to induce MAPK signaling. This could occur because multiple pathways downstream of βAR lead to activation of the downstream MAPK pathway. MAPK activation downstream of βAR stimulation can occur downstream of a GαS/AC/cAMP/PKA pathway [28] or through a Gβ/γ/Ras pathway [24–27]. Alternatively, propranolol, a βAR antagonist, has been shown to also lead to MAPK activation, possibly by disrupting β-arrestin function in decreasing the MAPK activity induced by G-protein-dependent pathways [29, 30, 32, 33, 40]. We found that propranolol activated ERK1/2 in isolated HemSCs and in an IH mouse model. The addition of an MEK inhibitor blocked the propranolol antiproliferative and antisurvival effects on HemSCs, suggesting that propranolol uses MAPK signal activation downstream of βAR inhibition to mediate its effects on HemSCs. Moreover, ICI mirrored the propranolol effects on ERK1/2 activation, suggesting that propranolol activates the MAPK pathway via inhibition of β2AR in HemSCs. This is consistent with the findings from previous studies, which have shown that propranolol activates MAPK through β2ARs [30, 33].
We found that high-dose propranolol rapidly induced HemSC apoptosis, with induction observed within 6 hours by Annexin V assay and 24 hours by caspase-3 assay. This is in contrast to previous studies, in which it was shown that propranolol induced HemSC death by a nonapoptotic pathway [21, 23, 41]. The difference between the present study and others mostly likely resulted from the timing of the apoptosis assessment. Kum and Khan [23] assessed the propranolol effects on apoptosis after 72 hours of propranolol treatment. In contrast, we reported that surface Annexin V expression was not increased at 24 hours of treatment [21, 41]. Annexin V can only be detected in cells actively undergoing apoptosis; thus, we believe we did not assess apoptosis at the appropriate time, because most cells were already dead, confirmed by propidium iodide incorporation. The results we have presented have demonstrated that >50 μM propranolol rapidly induced HemSC apoptosis within 6–24 hours of treatment. Propranolol cytotoxicity was observed at propranolol concentrations of 10−4 M.
The clinically used dosage of propranolol for IH treatment ranges from 1 to 3 mg/kg daily [11, 42, 43]. Although the serum concentration of propranolol in IH-treated infants has not been published, the plasma concentrations for adults taking 0.5–1.5 mg/kg per day ranged from 10−6.8 M to 10−6.3 M [44, 45]. In the present study, the clinical relative dose range of 10−6.5 M to 10−5.8 M propranolol both inhibited HemSC proliferation and induced HemSC apoptosis. However, this dose range is less than the concentration necessary to induce HemSC cytotoxicity. Thus, it is possible that the current propranolol dosing in IH patients reduces HemSC numbers but might allow for residual pools of IH stem cells, which might contribute to regrowth. IH rebound has been observed in as high as 25% of patients after discontinuation of propranolol therapy [46]. Thus, increasing the dose of propranolol given to patients with IH might induce HemSC cytotoxicity and prevent the rebound observed with propranolol discontinuation. In support of this idea, we found improved vessel morphology in the IH mouse model at a human equivalent dose of propranolol of 3.3–4.8 mg/kg per day.
Different cell populations in IHs express different levels of βAR subtypes, with high levels of β2AR expression seen in HemSCs and IH-derived pericytes [19]. In contrast, β1AR and β2AR are equally expressed in HemECs [17]. We have demonstrated that HemSCs in tissues express both β1AR and β2AR, but cultured HemSCs predominantly express β2AR. Propranolol has been suggested to target HemECs [17, 21, 47, 48] and hemangioma-derived pericytes [19], and the present study and others [21–23, 41] have demonstrated that propranolol also targets HemSCs. Thus, propranolol mediates its effects by targeting multiple cell types in IHs. Our in vitro data have demonstrated that a β2AR-specific, but not β1AR-specific, antagonist replicated the propranolol effects in HemSCs, suggesting that inhibition of β2AR activity alone is sufficient for the propranolol effects against HemSCs. Alternatively, the low β1AR expression in cultured HemSCs might be responsible for the absence of β1AR-specific antagonist responses. Further studies using βAR-specific antagonists in the IH mouse model might address this issue more effectively. Anecdotal clinical reports of using β1AR-specific antagonists in 3 cases of subglottic hemangiomas had mixed success [49]. However, a β1AR-specific antagonist might not effectively target HemSCs and IH-derived pericytes that also express β2AR.
Conclusion
Our study has provided important details for understanding the mechanism of propranolol action in the treatment of IH. Although propranolol affects HemSCs in part by disrupting βAR signaling, likely β2AR, a number of questions regarding the antiproliferative and cytotoxic effects of propranolol remain unanswered, including whether propranolol targets other receptor pathways to affect proliferation or cell viability. Given our findings, the possibility of targets other than βARs remains a possibility, especially at the high, cytotoxic dose. We are in the process of investigating these possibilities, and our findings could further enhance our understanding of the mechanism of action of propranolol in IH treatment and allow for the development of more effective treatment strategies.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant K08 1K08 HL102068-01 and Columbia University CTSA Grant UL1 TR000040 to J.K.W., NIH Grant 1R21 EB016515-01 to C.J.S., NIH Grant 1R01 HL112626 to J.K.K., and NIH Grant T35 5T35HL007616-34 to R.W.E. We thank Valeriya Borishenko for technical assistance.
Author Contributions
N.C.O.M. and R.W.E.: performance of experiments, generation and assembly of data, manuscript writing; A.K.E. and A.A.K.: performance of experiments, generation and assembly of data; Q.K.T., J.E.K., and M.W.: performance of experiments, generation of data; A.W.: statistical analysis; J.K.K.: financial support, data analysis and interpretation, final approval of manuscript; C.J.S. and J.K.W.: conception and design, financial support, manuscript writing, data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Mulliken JB, Fishman SJ, Burrows PE. Vascular anomalies. Curr Probl Surg. 2000;37:517–584. doi: 10.1016/s0011-3840(00)80013-1. [DOI] [PubMed] [Google Scholar]
- 2.Frieden IJ, Haggstrom AN, Drolet BA, et al. Infantile hemangiomas: Current knowledge, future directions. Proceedings of a research workshop on infantile hemangiomas, April 7–9, 2005, Bethesda, Maryland, USA. Pediatr Dermatol. 2005;22:383–406. doi: 10.1111/j.1525-1470.2005.00102.x. [DOI] [PubMed] [Google Scholar]
- 3.Kwon EK, Seefeldt M, Drolet BA. Infantile hemangiomas: An update. Am J Clin Dermatol. 2013;14:111–123. doi: 10.1007/s40257-013-0008-x. [DOI] [PubMed] [Google Scholar]
- 4.Chamlin SL, Haggstrom AN, Drolet BA, et al. Multicenter prospective study of ulcerated hemangiomas. J Pediatr. 2007;151:684–689. doi: 10.1016/j.jpeds.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 5.Haggstrom AN, Drolet BA, Baselga E, et al. Prospective study of infantile hemangiomas: Clinical characteristics predicting complications and treatment. Pediatrics. 2006;118:882–887. doi: 10.1542/peds.2006-0413. [DOI] [PubMed] [Google Scholar]
- 6.Boon LM, Burrows PE, Paltiel HJ, et al. Hepatic vascular anomalies in infancy: A twenty-seven-year experience. J Pediatr. 1996;129:346–354. doi: 10.1016/s0022-3476(96)70065-3. [DOI] [PubMed] [Google Scholar]
- 7.Arneja JS, Mulliken JB. Resection of amblyogenic periocular hemangiomas: Indications and outcomes. Plast Reconstr Surg. 2010;125:274–281. doi: 10.1097/PRS.0b013e3181c49708. [DOI] [PubMed] [Google Scholar]
- 8.Bitar MA, Moukarbel RV, Zalzal GH. Management of congenital subglottic hemangioma: Trends and success over the past 17 years. Otolaryngol Head Neck Surg. 2005;132:226–231. doi: 10.1016/j.otohns.2004.09.136. [DOI] [PubMed] [Google Scholar]
- 9.Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 10.Sans V, de la Roque ED, Berge J, et al. Propranolol for severe infantile hemangiomas: Follow-up report. Pediatrics. 2009;124:e423–e431. doi: 10.1542/peds.2008-3458. [DOI] [PubMed] [Google Scholar]
- 11.Lou Y, Peng WJ, Cao Y, et al. The effectiveness of propranolol in treating infantile haemangiomas: A meta-analysis including 35 studies. Br J Clin Pharmacol. 2014;78:44–57. doi: 10.1111/bcp.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128:e259–e266. doi: 10.1542/peds.2010-0029. [DOI] [PubMed] [Google Scholar]
- 13.Bauman NM, McCarter RJ, Guzzetta PC, et al. Propranolol vs prednisolone for symptomatic proliferating infantile hemangiomas: A randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2014;140:323–330. doi: 10.1001/jamaoto.2013.6723. [DOI] [PubMed] [Google Scholar]
- 14.Frieden IJ, Drolet BA. Propranolol for infantile hemangiomas: Promise, peril, pathogenesis. Pediatr Dermatol. 2009;26:642–644. doi: 10.1111/j.1525-1470.2009.00977.x. [DOI] [PubMed] [Google Scholar]
- 15.Lawley LP, Siegfried E, Todd JL. Propranolol treatment for hemangioma of infancy: Risks and recommendations. Pediatr Dermatol. 2009;26:610–614. doi: 10.1111/j.1525-1470.2009.00975.x. [DOI] [PubMed] [Google Scholar]
- 16.Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: Insights into the molecular mechanisms of action. Br J Dermatol. 2010;163:269–274. doi: 10.1111/j.1365-2133.2010.09848.x. [DOI] [PubMed] [Google Scholar]
- 17.Chim H, Armijo BS, Miller E, et al. Propranolol induces regression of hemangioma cells through HIF-1α-mediated inhibition of VEGF-A. Ann Surg. 2012;256:146–156. doi: 10.1097/SLA.0b013e318254ce7a. [DOI] [PubMed] [Google Scholar]
- 18.Ji Y, Chen S, Li K, et al. The role of β-adrenergic receptor signaling in the proliferation of hemangioma-derived endothelial cells. Cell Div. 2013;8:1. doi: 10.1186/1747-1028-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee D, Boscolo E, Durham JT, et al. Propranolol targets the contractility of infantile haemangioma-derived pericytes. Br J Dermatol. 2014;171:1129–1137. doi: 10.1111/bjd.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan ZA, Boscolo E, Picard A, et al. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J Clin Invest. 2008;118:2592–2599. doi: 10.1172/JCI33493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong A, Hardy KL, Kitajewski AM, et al. Propranolol accelerates adipogenesis in hemangioma stem cells and causes apoptosis of hemangioma endothelial cells. Plast Reconstr Surg. 2012;130:1012–1021. doi: 10.1097/PRS.0b013e318267d3db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Mai HM, Zheng J, et al. Propranolol inhibits angiogenesis via down-regulating the expression of vascular endothelial growth factor in hemangioma derived stem cell. Int J Clin Exp Pathol. 2014;7:48–55. [PMC free article] [PubMed] [Google Scholar]
- 23.Kum JJ, Khan ZA. Propranolol inhibits growth of hemangioma-initiating cells but does not induce apoptosis. Pediatr Res. 2014;75:381–388. doi: 10.1038/pr.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hentrich F, Göthert M, Greschuchna D. Involvement of cAMP in modulation of noradrenaline release in the human pulmonary artery. Naunyn Schmiedebergs Arch Pharmacol. 1985;330:245–247. doi: 10.1007/BF00572440. [DOI] [PubMed] [Google Scholar]
- 25.Bockaert J, Pin JP. Molecular tinkering of G protein-coupled receptors: An evolutionary success. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruni P, Burns DL, Hewlett EL, et al. Effects of pertussis toxin on cAMP and cGMP responses to carbamylcholine in N1E-115 neuroblastoma cells. Mol Pharmacol. 1985;28:229–234. [PubMed] [Google Scholar]
- 27.Li H, Fong C, Chen Y, et al. Beta-adrenergic signals regulate adipogenesis of mouse mesenchymal stem cells via cAMP/PKA pathway. Mol Cell Endocrinol. 2010;323:201–207. doi: 10.1016/j.mce.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 28.Galandrin S, Bouvier M. Distinct signaling profiles of beta1 and beta2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol Pharmacol. 2006;70:1575–1584. doi: 10.1124/mol.106.026716. [DOI] [PubMed] [Google Scholar]
- 29.Azzi M, Charest PG, Angers S, et al. Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci USA. 2003;100:11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker JG, Hall IP, Hill SJ. Agonist and inverse agonist actions of beta-blockers at the human beta 2-adrenoceptor provide evidence for agonist-directed signaling. Mol Pharmacol. 2003;64:1357–1369. doi: 10.1124/mol.64.6.1357. [DOI] [PubMed] [Google Scholar]
- 31.Kenakin T. Principles: Receptor theory in pharmacology. Trends Pharmacol Sci. 2004;25:186–192. doi: 10.1016/j.tips.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Wisler JW, DeWire SM, Whalen EJ, et al. A unique mechanism of beta-blocker action: Carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci USA. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nobles KN, Xiao K, Ahn S, et al. Distinct phosphorylation sites on the β(2)-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin. Sci Signal. 2011;4:ra51. doi: 10.1126/scisignal.2001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu JK, Adepoju O, De Silva D, et al. A switch in Notch gene expression parallels stem cell to endothelial transition in infantile hemangioma. Angiogenesis. 2010;13:15–23. doi: 10.1007/s10456-009-9161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keshelava N, Frgala T, Krejsa J, et al. DIMSCAN: A microcomputer fluorescence-based cytotoxicity assay for preclinical testing of combination chemotherapy. Methods Mol Med. 2005;110:139–153. doi: 10.1385/1-59259-869-2:139. [DOI] [PubMed] [Google Scholar]
- 36.Lamy S, Lachambre MP, Lord-Dufour S, et al. Propranolol suppresses angiogenesis in vitro: Inhibition of proliferation, migration, and differentiation of endothelial cells. Vascul Pharmacol. 2010;53:200–208. doi: 10.1016/j.vph.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Freireich EJ, Gehan EA, Rall DP, et al. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50:219–244. [PubMed] [Google Scholar]
- 38.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 39.U.S. National Institutes of Health. Equivalent Surface Area Dosage Conversion Factors. Available at https://ncifrederick.cancer.gov/lasp/acuc/frederick/Media/Documents/ACUC42.pdf. Accessed April 16, 2015.
- 40.Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 41.England RW, Hardy KL, Kitajewski AM, et al. Propranolol promotes accelerated and dysregulated adipogenesis in hemangioma stem cells. Ann Plast Surg. 2014;73(suppl 1):S119–S124. doi: 10.1097/SAP.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: Report of a consensus conference. Pediatrics. 2013;131:128–140. doi: 10.1542/peds.2012-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735–746. doi: 10.1056/NEJMoa1404710. [DOI] [PubMed] [Google Scholar]
- 44.Wong L, Nation RL, Chiou WL, et al. Plasma concentrations of propranolol and 4-hydroxypropranolol during chronic oral propranolol therapy. Br J Clin Pharmacol. 1979;8:163–167. doi: 10.1111/j.1365-2125.1979.tb05815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mansur AP, Avakian SD, Paula RS, et al. Pharmacokinetics and pharmacodynamics of propranolol in hypertensive patients after sublingual administration: Systemic availability. Braz J Med Biol Res. 1998;31:691–696. doi: 10.1590/s0100-879x1998000500014. [DOI] [PubMed] [Google Scholar]
- 46.Shah S, Frieden I, Baselga E et al. Rebound after discountinuation of propranolol in the therapy of infantile hemangiomas: A retrospective study. Paper presented at: 20th International Workshop on Vascular Anomalies; April 1–4, 2014; Melbourne, Victoria, Australia. [Google Scholar]
- 47.Stiles J, Amaya C, Pham R, et al. Propranolol treatment of infantile hemangioma endothelial cells: A molecular analysis. Exp Ther Med. 2012;4:594–604. doi: 10.3892/etm.2012.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji Y, Li K, Xiao X, et al. Effects of propranolol on the proliferation and apoptosis of hemangioma-derived endothelial cells. J Pediatr Surg. 2012;47:2216–2223. doi: 10.1016/j.jpedsurg.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Blanchet C, Nicollas R, Bigorre M, et al. Management of infantile subglottic hemangioma: Acebutolol or propranolol? Int J Pediatr Otorhinolaryngol. 2010;74:959–961. doi: 10.1016/j.ijporl.2010.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.