Ferumoxytol was used as a labeling agent of human embryonic stem cell-derived cardiac progenitor cells (hESC-CPCs) to facilitate tracking by magnetic resonance imaging (MRI). The localization and dispersion of labeled cells could be effectively imaged and tracked at days 0 and 40 by MRI. Thus, under the described conditions, ferumoxytol can be used as a long-term, differentiation-neutral cell-labeling agent to track transplanted hESC-CPCs in vivo using MRI.
Keywords: Cardiac stem cell biology, Cell labeling, Stem cell therapy, Cardiovascular progenitors, Magnetic resonance imaging
Abstract
Given the limited regenerative capacity of the heart, cellular therapy with stem cell-derived cardiac cells could be a potential treatment for patients with heart disease. However, reliable imaging techniques to longitudinally assess engraftment of the transplanted cells are scant. To address this issue, we used ferumoxytol as a labeling agent of human embryonic stem cell-derived cardiac progenitor cells (hESC-CPCs) to facilitate tracking by magnetic resonance imaging (MRI) in a large animal model. Differentiating hESCs were exposed to ferumoxytol at different time points and varying concentrations. We determined that treatment with ferumoxytol at 300 μg/ml on day 0 of cardiac differentiation offered adequate cell viability and signal intensity for MRI detection without compromising further differentiation into definitive cardiac lineages. Labeled hESC-CPCs were transplanted by open surgical methods into the left ventricular free wall of uninjured pig hearts and imaged both ex vivo and in vivo. Comprehensive T2*-weighted images were obtained immediately after transplantation and 40 days later before termination. The localization and dispersion of labeled cells could be effectively imaged and tracked at days 0 and 40 by MRI. Thus, under the described conditions, ferumoxytol can be used as a long-term, differentiation-neutral cell-labeling agent to track transplanted hESC-CPCs in vivo using MRI.
Significance
The development of a safe and reproducible in vivo imaging technique to track the fate of transplanted human embryonic stem cell-derived cardiac progenitor cells (hESC-CPCs) is a necessary step to clinical translation. An iron oxide nanoparticle (ferumoxytol)-based approach was used for cell labeling and subsequent in vivo magnetic resonance imaging monitoring of hESC-CPCs transplanted into uninjured pig hearts. The present results demonstrate the use of ferumoxytol labeling and imaging techniques in tracking the location and dispersion of cell grafts, highlighting its utility in future cardiac stem cell therapy trials.
Introduction
Stem cell-based therapies have the potential to regenerate and repair various tissues, thereby providing hope of novel treatment options for many intractable diseases such as heart failure. The success of preclinical studies has partially relied on the use of appropriate large animals to establish reliable models for cellular transplantation [1–5]. Stem cell-based therapies in small animal models have yielded promising but variable results [6–8]. Several studies have supported the use of large animals, such as pigs, with a similar heart size and physiology to humans, to monitor the survival, engraftment, and efficacy of transplanted hESC-derived cardiac cells [2–4, 9–12]. However, the clinical application of cell-based therapies will require noninvasive imaging approaches that will allow in vivo monitoring of transplanted cells [7, 8, 13, 14].
Despite promising advances in cell labeling [6–8, 13–17], no single method to date has yielded a nontoxic, readily accessible, and accurate system for longitudinal monitoring of transplanted cells in the heart. Superparamagnetic iron oxide nanoparticles (SPIOs) are well characterized and widely used cell-labeling agents for magnetic resonance imaging (MRI) [15–17]. However, SPIO exposure has been associated with cytotoxicity, actin-cytoskeleton modulation, and carcinogenesis, which are undesirable side effects when labeling hESC-derived cardiac cells [18–22]. Fluorine-19 (19F) labeling might be a promising alternative for cell imaging, because it has little effect on stem cell viability, proliferation, and differentiation [23–25]. Furthermore, 19F MRI has no significant background signal from host tissue [26] and has been used in a cell therapy-based clinical trial [27]. However, many MRI facilities lack the specialized equipment required for 19F MR image acquisition, thereby reducing the accessibility of this technique [28].
The development of a simple, safe, and reproducible in vivo imaging technique to track the fate of transplanted hESC-CPCs would assist in the clinical translation of cell-based therapies. Recent studies have described the properties of the ultra-small superparamagnetic iron oxide nanoparticle, ferumoxytol (Feraheme; AMAG Pharmaceuticals, Inc., Waltham, MA, http://www.amagpharma.com) as a labeling agent [7, 14, 29–31]. Ferumoxytol interacts with cells via a synthetic carbohydrate coating and is currently used for the treatment of iron-deficiency anemia in the presence of chronic kidney disease [32]. Furthermore, ferumoxytol displays limited cytotoxicity and genotoxicity and might improve the viability of certain cell populations (i.e., microglia) [14, 33, 34].
The present proof-of-principal study aimed to define a ferumoxytol-based approach for cell labeling and subsequent in vivo MRI monitoring of hESC-CPCs transplanted into uninjured pig hearts. In the present study, we investigated the capacity of hESCs to uptake ferumoxytol during differentiation at varying labeling concentrations, while maintaining optimal survival and the ability to generate cardiovascular cell types. We hypothesized and subsequently demonstrated that ferumoxytol can be used as an effective, differentiation-neutral labeling agent for the in vivo MRI monitoring of hESC-CPCs in the pig heart.
Materials and Methods
Ferumoxytol Cell Labeling
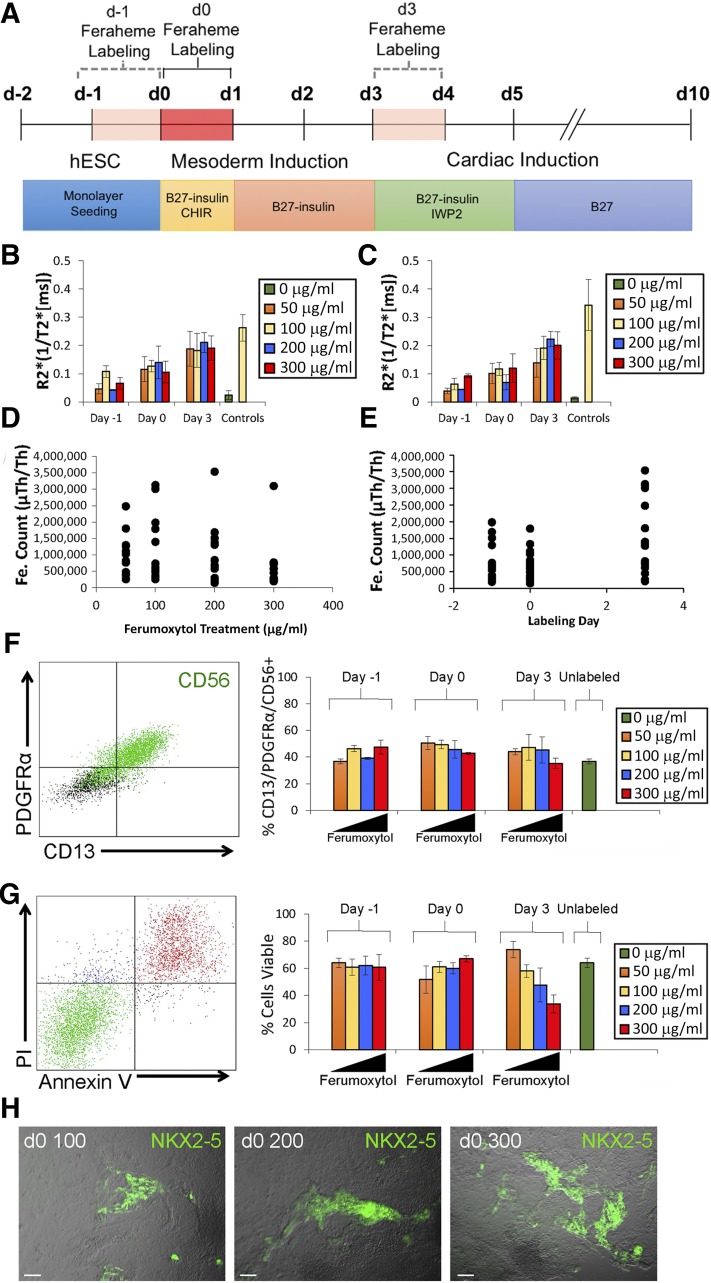
hESC/hESC-CPCs were treated with a 24-hour pulse of ferumoxytol at a concentration of 50 µg/ml, 100 µg/ml, 200 µg/ml, or 300 µg/ml. hESC/hESC-CPCs were exposed to ferumoxytol pulses at different stages of differentiation, including 1 day before differentiation start (d −1), day 0 of differentiation (d0), and day 3 after differentiation started (d3) (Fig. 1A). Next, the cells were washed three times with phosphate-buffered saline and media changed to a ferumoxytol-free medium.
Figure 1.
Analysis of signal intensity, viability, and differentiation of ferumoxytol-treated cells. (A): Timeline details of small molecules and media supplements (e.g., B27) used to guide differentiation and time points and duration of ferumoxytol labeling. Graphs of R2* values of ferumoxytol-labeled cells obtained by magnetic resonance (MR) imaging at day 4 (B) and day 10 (C) of differentiation. R2* values were calculated from T2*-weighted MR images of cells labeled for 24 hours with varying iron concentrations at days −1, 0, and 3 of hESC cardiac differentiation (n = 3, mean ± SEM). Views of unlabeled control (green) and positive control (yellow) representing 100 µg/ml pure ferumoxytol suspended in 50-µl agarose plugs are shown. Mass spectrometry data (in atom counts) comparing iron retention between cells treated with different iron concentrations (50, 100, 200, and 300 μg/ml) (D) and at different days of differentiation (day −1, day 0, and day 3) (E). (F): Flow cytometry analysis showing PDGFRα, CD56, and CD13 expression in corresponding ferumoxytol-labeling conditions (n = 3, mean ± SEM). (G): Flow cytometry analysis showing PI and Annexin V expression in corresponding ferumoxytol-labeling conditions (n = 3, mean ± SEM). Percentage of viable cells depicted graphically. (H): Field-of-view images showing NKX2-5 (green) expression in cells labeled at day 0 with 100 µg/ml, 200 µg/ml, and 300 µg/ml ferumoxytol. Scale bars = 100 μm. Abbreviations: CHIR, CHIR99021; d, day; hESC, human embryonic stem cell; PI, propidium iodide; μTh, Thurston measurement.
In Vitro MRI Cell Preparation
To determine the imaging potential and signal attenuation of ferumoxytol-labeled hESC-CPCs, the cells were harvested at days 4 and 10 of differentiation and resuspended in 50-μl agarose gel plugs for in vitro MRI.
Post-Sort Culture
Freshly sorted day 3 CD13+/ROR2+ cells were recultured on Matrigel-coated plates in Roswell Park Memorial Institute plus B27 for a recovery period of 24 hours before injection into the healthy pig heart (supplemental online Fig. 1).
Cell Injection and Animal Maintenance
Animal housing, maintenance, and experimentation were approved by, and performed in accordance with the guidelines set by, the Institutional Animal Care and Use Committee of the University of California and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. A total of 3 Yorkshire pigs weighing approximately 40 kg underwent thoracotomy and transplantation of ferumoxytol-labeled hESC-CPCs under direct visualization. Two injection sites were selected on the left ventricular free wall and marked with suture. Site 1 was injected with ferumoxytol-labeled CPCs. Site 2 was injected with unlabeled CPCs. A suspension of 4 × 107 cells (determined by hemocytometer) in approximately 300 μl of conditioned media was injected in each site using a 27-gauge needle. The pigs were imaged using T2-based MRI on the day of transplantation and again 40 days later. The pigs were immunosuppressed with cyclosporine (serum level of 100–120 ng/ml) and treated with ketoconazole (20 mg/kg) and trimethoprim sulfa (40 mg/kg) daily, which began 3 days before cell transplantation and was continued until euthanasia. After 40 days, the pigs were euthanized, and the hearts were harvested and sectioned for histological analysis. Detailed protocols are given in the supplemental online data and used published procedures.
Results
Variation in Signal Intensity Is Dependent on Ferumoxytol Exposure Day
The differentiation protocol efficiently generated precardiac mesoderm as shown by quantitative polymerase chain reaction and flow cytometry (supplemental online Fig. 2A–2C). Furthermore, under these conditions, differentiating cells gave rise to cardiomyocytes, smooth muscle cells, and endothelial cells in vitro (supplemental online Fig. 2C, 2D; supplemental online Video 1).
We analyzed the labeling efficiency of hESC/hESC-CPCs using 0, 50, 100, 200, and 300 μg/ml of ferumoxytol on day −1, d0, or d3 of differentiation (Fig. 1A). hESC-CPCs labeled on d3 revealed the highest levels of signal intensity across all concentrations, as determined by R2* values (ms−1) (Fig. 1B, 1C; supplemental online Table 1). Higher doses of ferumoxytol did not significantly increase the R2* values (ms−1; p > .05) in hESC-CPCs (Fig. 1B, 1C). Mass spectrometry data confirmed these findings, showing a positive correlation between higher intracellular iron and d3 ferumoxytol labeling, but not with increased ferumoxytol treatment concentrations (Fig. 1D, 1E; supplemental online Table 2). These results indicate that the signal intensity of the ferumoxytol-labeled cells is largely dependent on the day of exposure and that the ferumoxytol dose, at the concentrations tested, had little influence on cell labeling.
Ferumoxytol Affects Cell Viability and Differentiation
Under all labeling conditions, approximately 40% of cells adopted a PDGFRα+/CD13+/CD56+ precardiac mesoderm phenotype, comparable to that of the unlabeled control (p > .05; Fig. 1F). However, flow cytometric analysis with propidium iodide and annexin V revealed a significant increase in apoptotic cells (viability <50%) when higher concentrations of ferumoxytol (>200 μg/ml) were used on d3 (p < .05; Fig. 1G). Furthermore, cells labeled on d3 and d −1 failed to upregulate NKX2-5, a transcription factor important for cardiovascular lineage differentiation (supplemental online Fig. 3). However, labeling at d0 resulted in minimal apoptosis and was permissive for cardiac differentiation, as indicated by expression of NKX2-5eGFP, cardiac troponin T, α-actinin, CNN1, and CD31 (Fig. 1H; supplemental online Figs. 3, 4; supplemental online Video 2). These results demonstrate that ferumoxytol can adversely affect cell viability and differentiation in a temporal and dose-dependent manner. Accordingly, hESCs were labeled at d0 using 300 μg/ml of ferumoxytol, because this provided adequate signal intensity for MRI with no adverse effect on viability or cardiac differentiation.
Ferumoxytol-Labeled Cells Can Be Detected in Tissue Ex Vivo
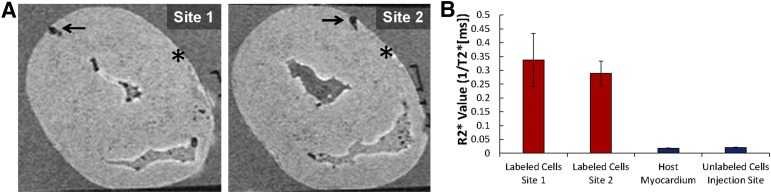
To determine whether ferumoxytol-labeled cells could be identified by ex vivo MRI, approximately 1.2 × 107 unsorted labeled or unlabeled hESC-CPCs were injected into two separate sites of explanted pig hearts (Fig. 2; supplemental online Fig. 5). T2*-weighted imaging revealed strong negative contrast in areas of labeled hESC-CPC injection (R2* value, 0.34 ± 0.096 ms−1 and 0.29 ± 0.04 ms−1; Fig. 2A, 2B; supplemental online Fig. 5; supplemental online Video 3). Unlabeled hESC-CPCs were indistinguishable from surrounding tissue (R2* value, 0.018 ± 0.002 ms−1 and 0.020 ± 0.001 ms−1) and could not be detected by MRI (Fig. 2A, 2B; supplemental online Fig. 5). Thus, ferumoxytol-labeled hESC-CPCs can be identified and imaged in porcine heart tissue using MRI.
Figure 2.
Ex vivo detection of ferumoxytol-labeled human embryonic stem cell-derived cardiac progenitor cells (hESC-CPCs) in pig hearts by magnetic resonance (MR) imaging. (A): Ex vivo MR images of porcine heart showing injection sites of ferumoxytol-labeled (arrow) and unlabeled (asterisk) hESC-CPCs; 1.2 × 107 hESC-CPCs were injected at each site. Labeling was done at day 0 of differentiation with 300 μg/ml of ferumoxytol. (B): R2* values (ms−1) of epicardium, endocardium, and labeled hESC-CPC injections (n = 3, mean ± SEM). R2* values were calculated from T2*-weighted MR images.
In Vivo Identification of Ferumoxytol-Labeled hESC-Derived Cardiac Cells by MRI
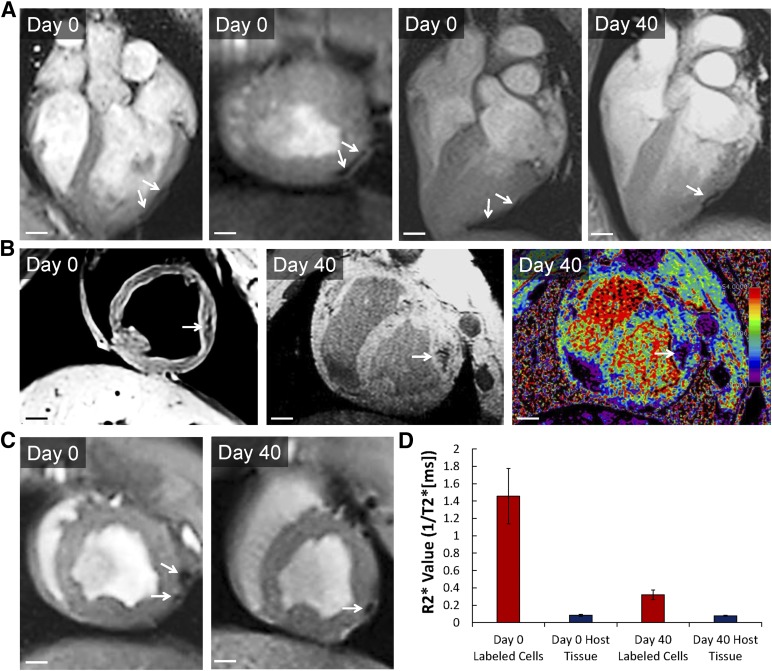
We transplanted approximately 4 × 107 ferumoxytol-labeled or unlabeled hESC-CPCs isolated by fluorescence-activated cell sorting based on coexpression of CD13 and ROR2 [1] (supplemental online Fig. 6) into two separate sites of the left ventricular free wall of adult pig hearts. MRI was performed at day 0 and day 40 after injection (n = 3) (Fig. 3; supplemental online Videos 4–6). Shortly after day 0 of cell transplantation, T2*-weighted imaging revealed a large area of strong negative contrast at the injection site (R2* value, 1.45 ± 0.31 ms−1), indicating the presence of cells within the myocardium of the left ventricle (Fig. 3; supplemental online Video 4). By contrast, unlabeled cells were indistinguishable from the surrounding heart tissue (R2* value, 0.083 ± 0.011 ms−1). Day 40 MRI detected a reduced area of negative contrast with decreased signal in the approximate anatomical location to that of day 0 imaging (R2* value, 0.32 ± 0.05 ms−1), suggesting a decrease in graft size and/or signal attenuation (Fig. 3; supplemental online Videos 5, 6).
Figure 3.
In vivo detection of ferumoxytol-labeled human embryonic stem cell-derived cardiac progenitor cells (hESC-CPCs) in pig hearts by magnetic resonance (MR) imaging. (A–C): Day 0 and day 40, in vivo T2*-weighted MR images from three porcine hearts showing locations of ferumoxytol-labeled (arrows) hESC-CPCs; 4 × 107 hESC-CPCs were injected. Scale bars = 1 cm. Labeling was done at day 0 of differentiation with 300 μg/ml of ferumoxytol. (D): R2* values (ms−1) measured from porcine host myocardium and labeled hESC-CPC injections at day 0 and day 40 (n = 3, mean ± SEM). R2* values were calculated from T2*-weighted MR images.
Ferumoxytol-Labeled hESC-CPCs Differentiate Toward Cardiac Linages In Vivo
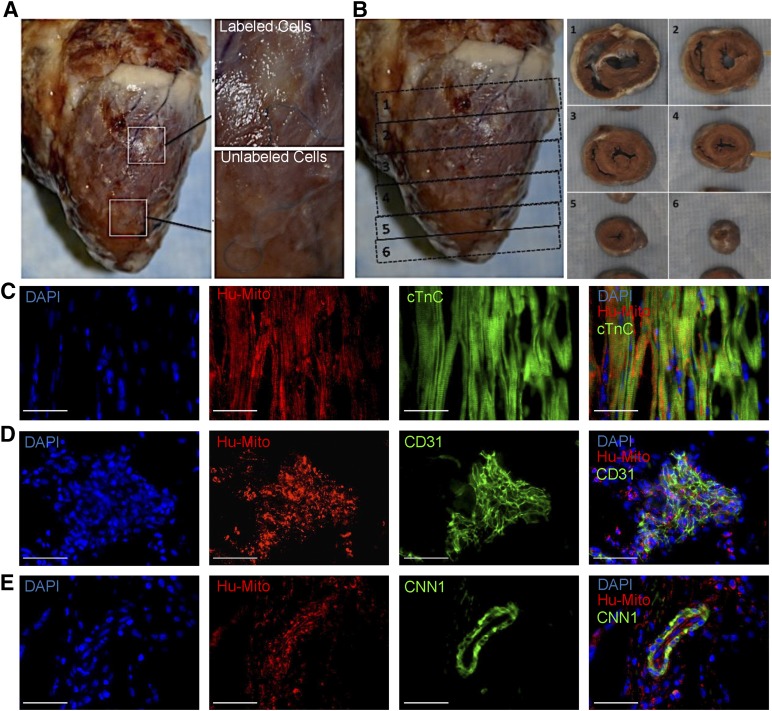
To further verify both cell retention and differentiation toward cardiac lineages, hearts were harvested on day 40, and areas showing negative contrast by MRI were analyzed for the presence of hESC-CPC-derived cells. Cell clusters (ranging from 5 to >500 cells) staining positive for human mitochondria and iron dextran were located at the outer myocardial layer near the epicardium, suggesting that hESC-derived cells retained ferumoxytol and remained near the site of injection (Fig. 4; supplemental online Figs. 7, 8). Consistent with previous reports, most transplanted cells (>90%) were not retained in the recipients’ heart [1–4, 8, 30, 34]. Nevertheless, the hESC-CPCs that remained gave rise to at least three definitive cardiac lineages, namely cardiomyocytes (cardiac troponin C [cTnC]), smooth muscle (CNN1), and endothelial cells (CD31) (Fig. 4C–4E). These results demonstrate that ferumoxytol-labeled hESC-CPCs can successfully differentiate toward definitive cardiac cell types after transplantation into live pig hearts.
Figure 4.
Ferumoxytol-labeled hESC-CPCs differentiation toward definitive cardiac cell types in vivo. (A): Porcine heart harvested at day 40 showing injection sites of ferumoxytol-labeled and unlabeled cells. (B): Transverse sections of day 40-harvested porcine heart; cell grafts are shown in sections 2 (ferumoxytol labeled) and 4 (unlabeled). (C–E): Stained frozen sections of ferumoxytol-labeled hESC-CPCs in injected hearts from sacrificed pigs (day 40). Immunohistochemical stains depict the expression of DAPI (blue), anti-human mitochondria (red), and cTnC (C), CD31 (D), and CNN1 (E) (green). Scale bars = 50 μm. Abbreviations: cTnC, cardiac troponin C; DAPI, 4′,6-diamidino-2-phenylindole; Hu-mito, human mitochondria.
Discussion
The present proof-of-principle report describes a transfection reagent-free, ferumoxytol-based labeling strategy for MRI monitoring of transplanted hESC-CPCs. We have demonstrated that labeling hESCs with 300 μg/ml of ferumoxytol on day 0 of differentiation offers optimal cell viability without compromising cardiac lineage commitment and adequate signal intensity with MRI. hESCs exposed to ferumoxytol at d −1 failed to differentiate into cardiomyocytes, which might have been because of the acute sensitivity of hESCs to a range of culture perturbations, as previously reported [38–41]. Furthermore, ferumoxytol labeling on d3 resulted in a significant decrease in cell viability, which might have contributed to the observed lack of cardiac differentiation. The sensitivity of day 3 cultures to ferumoxytol can be partially explained by the current hESC cardiac differentiation methods, resulting in some cell death at days 3–4, which might have been exacerbated by ferumoxytol labeling. It remains possible that ferumoxytol could also have a negative effect on differentiation, potentially accounting for the inability of d3-labeled hESC-CPCs to differentiate toward contractile cardiomyocytes. Our data suggest that the development of successful cell labeling strategies require a multifaceted approach, taking into consideration the differentiation method, time-point of labeling, labeling agent, and treatment concentrations. We reason that the ferumoxytol-labeling strategy used in the present study provides favorable conditions to cope with the most efficient and recently developed human pluripotent stem cell (hPSC) cardiac differentiation protocols.
We used an ex vivo imaging model to confirm that signal intensity was preserved in porcine cardiac tissue and that labeled cells could be adequately imaged with MRI. Sites of unlabeled hESC-CPCs injections were physically marked with sutures to correlate the injection site with the MR images, and, as anticipated, the unlabeled cells could not be visualized. However, the sites where ferumoxytol-labeled hESC-CPCs were injected demonstrated high R2* values, resulting from the high intracellular ferumoxytol concentration. Furthermore, in vivo studies in the pig demonstrated that MRI can monitor ferumoxytol-labeled cells for up to 40 days in the host myocardium. Immunohistochemical analysis of the explanted hearts in the area of the MRI signal revealed the presence of ferumoxytol-labeled hESC-derived cardiac cells. These results suggest that the MRI signal corresponds to the presence of labeled cells. Moreover, transplanted hESC-CPCs were able to differentiate toward cardiomyocytes (cTnC+), endothelium (CD31+), and smooth muscle (CNN1+) in vivo, suggesting that the differentiation process successfully continued after transplantation.
Previous rodent-based studies have demonstrated that iron-labeled ESCs, or their derivatives, can be imaged for up to 28 days when injected into mouse hindlimbs and hearts [6, 7, 31]. Furthermore, consistent with our findings, other groups have reported signal attenuation (ranging from 30% to 60%), cell migration, and cell loss at varying time points after transplantation [8, 14, 31, 35]. Recent studies, however, have shown that iron oxide labeling, combined with magnetic targeting, might improve graft retention in the rat heart [29, 30, 34, 36]. Thus, ferumoxytol cell labeling might have utility in both in vivo cardiac graft imaging and cell retention. The prospective value of the present study lies in its extended duration and the use of a large animal model. The pig is an excellent large animal model for preclinical studies for hESC-derived cardiac cell transplantation owing to its similar heart size and physiology to humans. These properties of the porcine model also make it ideal for MRI-based studies, for which image resolution is pivotal in designing future clinical applications.
We chose to deliver a single injection of hESC-CPCs into normal pig hearts to eliminate many of the variables resulting from the injury process. The hostile inflammatory environment, along with the active fibrosis and scar formation, could have interfered with the viability, maturation, and monitoring of the labeled cells. The present study was a proof-of-principle study to establish a reliable imaging technique for tracking transplanted hESC-CPCs that remain viable and differentiate into definite cardiac cell types. However, consistent with previous studies [3, 13, 37, 38], we observed that not all delivered cells were present after 40 days. Similar poor cellular retention was observed between ferumoxytol-labeled and unlabeled transplanted hESC-CPCs. Therefore, it is unlikely that ferumoxytol labeling exacerbates cellular loss after transplantation. Other possibilities, such as cell death at delivery, cell rejection despite immunosuppressive therapy, or that the cells were unable to engraft into their new environment and were cleared by the host immune system, warrant further investigation. Nonetheless, despite the low number of viable transplanted cells in the host myocardium, MRI was still able to accurately locate and image the labeled hESC-CPC grafts, thereby demonstrating the sensitivity of this cell monitoring technique.
Although more established methods for spatially identifying transplanted cells in vivo require animal sacrifice and histological analysis, we have demonstrated a practical and widely applicable tool for imaging at the cellular level. However, the low sample sizes (n = 3) used for this proof-of-principal study did not allow for statistically powered definitive conclusions to be drawn. Thus, future large animal in vivo studies with greater sample sizes are required. Furthermore, the present study did not compare the utility of this labeling protocol using different cell delivery methods, such as intracoronary, epicardial, and transendocardial. We observed a decline in signal intensity at 40 days compared with day 0. Although this was most likely a result of a significant reduction in the number of retained cells, we could not formally exclude that the reduction in signal intensity was caused by the prolonged time periods. Future studies are warranted to determine whether the ferumoxytol signal diminishes over time. The present study also did not determine the quantity or minimum volume necessary to produce a signal on MRI. Nevertheless, future hPSC cardiac treatments will likely incorporate multiple injection sites, with each site requiring far greater cell numbers than those used throughout the present study (∼4 × 107) to elicit a therapeutically relevant response in the setting of myocardial injury [3]. As such, the ferumoxytol-labeling strategy we have described could be appropriately used for translational studies and, possibly, clinical trials.
Conclusion
The results of the present proof-of-principle study have demonstrated that (a) a 24-hour pulse of ferumoxytol at the onset of differentiation is sufficient to label hESC-CPCs without compromising cell viability or differentiation; (b) MRI can accurately track the location of ferumoxytol-labeled hESC-CPCs transplanted into a pig’s heart for up to 40 days after delivery; and (c) transplanted ferumoxytol-labeled hESC-CPCs differentiate into three cardiovascular lineages, despite the low retention rate. Therefore, ferumoxytol can be used as a differentiation-neutral hESC-CPC-labeling agent that facilitates long-term MRI monitoring of transplanted cells in large animals.
Supplementary Material
Acknowledgments
We thank all the members of the Ardehali Laboratory for instructive discussions and suggestions. We also thank the University of California, Los Angeles (UCLA), Translational Research Imaging Center and Division of Laboratory Animal Medicine, especially Anthony Smithson, Sandra Duarte Vogel, and Janlee Jensen, for all their support and expertise in the handling of large animal models. Thanks are also due to the UCLA Flow Core, especially Jessica Scholes and Felicia Codrea. Furthermore, we especially thank Drs. Daniel Ennis, Kim Lien Nguyen, and Paul Finn for their valuable input and guidance. This work was supported in part by grants from the California Institute of Regenerative Medicine (CIRM) (Grant RC1-00354-1) to R.A. and the Eli & Edith Broad Center of Regenerative Medicine and Stem Cell Research Center at UCLA Research Award to R.A.
Author Contributions
R.J.P.S.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; S.K., F.H., J.E., and P.Z.: collection and/or assembly of data, data analysis and interpretation; S.A.: collection and/or assembly of data, manuscript writing; S.R.: conception and design, collection and/or assembly of data, data analysis and interpretation; P.H.: conception and design, provision of study material or patients, data analysis and interpretation; E.G.S. and A.G.E.: conception and design, data analysis and interpretation; M.K.: conception and design, collection and/or assembly of data; D.A.E.: data analysis and interpretation, manuscript writing, final approval of manuscript; R.A.: conception and design, financial support, manuscript writing, provision of study material or patients, data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Ardehali R, Ali SR, Inlay MA, et al. Prospective isolation of human embryonic stem cell-derived cardiovascular progenitors that integrate into human fetal heart tissue. Proc Natl Acad Sci USA. 2013;110:3405–3410. doi: 10.1073/pnas.1220832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blin G, Nury D, Stefanovic S, et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest. 2010;120:1125–1139. doi: 10.1172/JCI40120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chong JJ, Yang X, Don CW, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye L, Chang YH, Xiong Q, et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750–761. doi: 10.1016/j.stem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuleri KH, Feigenbaum GS, Centola M, et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebert SN, Taylor DG, Nguyen HL, et al. Noninvasive tracking of cardiac embryonic stem cells in vivo using magnetic resonance imaging techniques. Stem Cells. 2007;25:2936–2944. doi: 10.1634/stemcells.2007-0216. [DOI] [PubMed] [Google Scholar]
- 7.Li Z, Suzuki Y, Huang M, et al. Comparison of reporter gene and iron particle labeling for tracking fate of human embryonic stem cells and differentiated endothelial cells in living subjects. Stem Cells. 2008;26:864–873. doi: 10.1634/stemcells.2007-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tallheden T, Nannmark U, Lorentzon M, et al. In vivo MR imaging of magnetically labeled human embryonic stem cells. Life Sci. 2006;79:999–1006. doi: 10.1016/j.lfs.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Barad L, Schick R, Zeevi-Levin N, et al. Human embryonic stem cells vs human induced pluripotent stem cells for cardiac repair. Can J Cardiol. 2014;30:1279–1287. doi: 10.1016/j.cjca.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Rosen MR, Myerburg RJ, Francis DP, et al. Translating stem cell research to cardiac disease therapies: Pitfalls and prospects for improvement. J Am Coll Cardiol. 2014;64:922–937. doi: 10.1016/j.jacc.2014.06.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansen Of Lorkeers SJ, Eding JE, Vesterinen HM, et al. Similar effect of autologous and allogeneic cell therapy for ischemic heart disease: Systematic review and meta-analysis of large animal studies. Circ Res. 2015;116:80–86. doi: 10.1161/CIRCRESAHA.116.304872. [DOI] [PubMed] [Google Scholar]
- 12.van der Spoel TI, Jansen of Lorkeers SJ, Agostoni P, et al. Human relevance of pre-clinical studies in stem cell therapy: Systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc Res. 2011;91:649–658. doi: 10.1093/cvr/cvr113. [DOI] [PubMed] [Google Scholar]
- 13.Cheng K, Li TS, Malliaras K, et al. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circ Res. 2010;106:1570–1581. doi: 10.1161/CIRCRESAHA.109.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutova M, Frank JA, D’Apuzzo M, et al. Magnetic resonance imaging tracking of ferumoxytol-labeled human neural stem cells: Studies leading to clinical use. Stem Cells Translational Medicine. 2013;2:766–775. doi: 10.5966/sctm.2013-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17:484–499. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- 16.Egawa EY, Kitamura N, Nakai R, et al. A DNA hybridization system for labeling of neural stem cells with SPIO nanoparticles for MRI monitoring post-transplantation. Biomaterials. 2015;54:158–167. doi: 10.1016/j.biomaterials.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Scharf A, Holmes S, Thoresen M, et al. Superparamagnetic iron oxide nanoparticles as a means to track mesenchymal stem cells in a large animal model of tendon injury. Contrast Media Mol Imaging. 2015;10:388–397. doi: 10.1002/cmmi.1642. [DOI] [PubMed] [Google Scholar]
- 18.Berry CC, Wells S, Charles S, et al. Dextran and albumin derivatised iron oxide nanoparticles: Influence on fibroblasts in vitro. Biomaterials. 2003;24:4551–4557. doi: 10.1016/s0142-9612(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 19.Shubayev VI, Pisanic TR, 2nd, Jin S. Magnetic nanoparticles for theragnostics. Adv Drug Deliv Rev. 2009;61:467–477. doi: 10.1016/j.addr.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh N. Conference scene—Nanotoxicology: Health and environmental impacts. Nanomedicine (Lond) 2009;4:385–390. doi: 10.2217/nnm.09.20. [DOI] [PubMed] [Google Scholar]
- 21.Soenen SJ, Illyes E, Vercauteren D, et al. The role of nanoparticle concentration-dependent induction of cellular stress in the internalization of non-toxic cationic magnetoliposomes. Biomaterials. 2009;30:6803–6813. doi: 10.1016/j.biomaterials.2009.08.050. [DOI] [PubMed] [Google Scholar]
- 22.Soenen SJ, Nuytten N, De Meyer SF, et al. High intracellular iron oxide nanoparticle concentrations affect cellular cytoskeleton and focal adhesion kinase-mediated signaling. Small. 2010;6:832–842. doi: 10.1002/smll.200902084. [DOI] [PubMed] [Google Scholar]
- 23.Boehm-Sturm P, Mengler L, Wecker S, et al. In vivo tracking of human neural stem cells with 19F magnetic resonance imaging. PLoS One. 2011;6:e29040. doi: 10.1371/journal.pone.0029040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Partlow KC, Chen J, Brant JA, et al. 19F magnetic resonance imaging for stem/progenitor cell tracking with multiple unique perfluorocarbon nanobeacons. FASEB J. 2007;21:1647–1654. doi: 10.1096/fj.06-6505com. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz-Cabello J, Walczak P, Kedziorek DA, et al. In vivo “hot spot” MR imaging of neural stem cells using fluorinated nanoparticles. Magn Reson Med. 2008;60:1506–1511. doi: 10.1002/mrm.21783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahrens ET, Bulte JW. Tracking immune cells in vivo using magnetic resonance imaging. Nat Rev Immunol. 2013;13:755–763. doi: 10.1038/nri3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahrens ET, Helfer BM, O’Hanlon CF, et al. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn Reson Med. 2014;72:1696–1701. doi: 10.1002/mrm.25454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srivastava AK, Kadayakkara DK, Bar-Shir A, et al. Advances in using MRI probes and sensors for in vivo cell tracking as applied to regenerative medicine. Dis Model Mech. 2015;8:323–336. doi: 10.1242/dmm.018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khurana A, Chapelin F, Beck G, et al. Iron administration before stem cell harvest enables MR imaging tracking after transplantation. Radiology. 2013;269:186–197. doi: 10.1148/radiol.13130858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mani V, Adler E, Briley-Saebo KC, et al. Serial in vivo positive contrast MRI of iron oxide-labeled embryonic stem cell-derived cardiac precursor cells in a mouse model of myocardial infarction. Magn Reson Med. 2008;60:73–81. doi: 10.1002/mrm.21642. [DOI] [PubMed] [Google Scholar]
- 31.Castaneda RT, Khurana A, Khan R, et al. Labeling stem cells with ferumoxytol, an FDA-approved iron oxide nanoparticle. J Vis Exp. 2011:e3482. doi: 10.3791/3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu M, Cohen MH, Rieves D, et al. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am J Hematol. 2010;85:315–319. doi: 10.1002/ajh.21656. [DOI] [PubMed] [Google Scholar]
- 33.Neubert J, Wagner S, Kiwit J, et al. New findings about iron oxide nanoparticles and their different effects on murine primary brain cells. Int J Nanomedicine. 2015;10:2033–2049. doi: 10.2147/IJN.S74404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thu MS, Bryant LH, Coppola T, et al. Self-assembling nanocomplexes by combining ferumoxytol, heparin and protamine for cell tracking by magnetic resonance imaging. Nat Med. 2012;18:463–467. doi: 10.1038/nm.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen S, Alcantara D, Josephson L. A magnetofluorescent nanoparticle for ex-vivo cell labeling by covalently linking the drugs protamine and Feraheme. J Nanosci Nanotechnol. 2011;11:3058–3064. doi: 10.1166/jnn.2011.4164. [DOI] [PubMed] [Google Scholar]
- 36.Khurana A, Nejadnik H, Chapelin F, et al. Ferumoxytol: A new, clinically applicable label for stem-cell tracking in arthritic joints with MRI. Nanomedicine (Lond) 2013;8:1969–1983. doi: 10.2217/nnm.12.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terrovitis J, Stuber M, Youssef A, et al. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation. 2008;117:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.732073. [DOI] [PubMed] [Google Scholar]
- 38.Calderon D, Planat-Benard V, Bellamy V, et al. Immune response to human embryonic stem cell-derived cardiac progenitors and adipose-derived stromal cells. J Cell Mol Med. 2012;16:1544–1552. doi: 10.1111/j.1582-4934.2011.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.