This study tested whether injection of human cord blood mononuclear cells (CB-MNCs) combined with hyaluronan (HA) hydrogel improves cell therapy efficacy in a pig myocardial infarction model. The results indicate that combined CB-MNC and HA treatment improves heart performance and may be a promising treatment for ischemic heart diseases.
Keywords: Human cord blood cells, Hyaluronan, Myocardial infarction, Pig model
Abstract
Recent clinical trials using autologous bone marrow or peripheral blood cells to treat myocardial infarction (MI) show controversial results, although the treatment has a good safety profile. These discrepancies are likely caused by factors such as aging, systemic inflammation, and cell processing procedures, all of which might impair the regenerative capability of the cells used. Here, we tested whether injection of human cord blood mononuclear cells (CB-MNCs) combined with hyaluronan (HA) hydrogel improves cell therapy efficacy in a pig MI model. A total of 34 minipigs were divided into 5 groups: sham operation (Sham), surgically induced-MI plus injection with normal saline (MI+NS), HA only (MI+HA), CB-MNC only (MI+CB-MNC), or CB-MNC combined with HA (MI+CB-MNC/HA). Two months after the surgery, injection of MI+CB-MNC/HA showed the highest left ventricle ejection fraction (51.32% ± 0.81%) compared with MI+NS (42.87% ± 0.97%, p < .001), MI+HA (44.2% ± 0.63%, p < .001), and MI+CB-MNC (46.17% ± 0.39%, p < .001) groups. The hemodynamics data showed that MI+CB-MNC/HA improved the systolic function (+dp/dt) and diastolic function (−dp/dt) as opposed to the other experimental groups, of which the CB-MNC alone group only modestly improved the systolic function (+dp/dt). In addition, CB-MNC alone or combined with HA injection significantly decreased the scar area and promoted angiogenesis in the infarcted region. Together, these results indicate that combined CB-MNC and HA treatment improves heart performance and may be a promising treatment for ischemic heart diseases.
Significance
This study using healthy human cord blood mononuclear cells (CB-MNCs) to treat myocardial infarction provides preclinical evidence that combined injection of hyaluronan and human CB-MNCs after myocardial infarction significantly increases cell retention in the peri-infarct area, improves cardiac performance, and prevents cardiac remodeling. Moreover, using healthy cells to replace dysfunctional autologous cells may constitute a better strategy to achieve heart repair and regeneration.
Introduction
Stem cells are immature, self-renewing cells that have been proposed to improve cardiac function after myocardial infarction (MI). Among them, autologous bone marrow cells have been shown for use in repairing damaged cardiac tissue after MI in patients [1]. However, poor outcomes have also been reported in some clinical studies [2–4]. Age and diabetes may influence stem cell reparative functions [5–7]. For example, Heeschen et al. [8] reported that bone marrow mononuclear cells (MNC) impaired neovascularization capacity when the cells were isolated from chronic cardiomyopathy patients as opposed to cells isolated from a healthy source. In addition, MI-induced systemic inflammatory responses are also known to inversely affect therapeutic outcomes [9].
Recently, studies have suggested that human umbilical cord blood mononuclear cells (CB-MNC) may be a better alternative to bone marrow MNCs. Harvesting CB-MNCs is noninvasive and presents low risk to the mother and infant [10, 11]. Additionally, CB-MNCs contain large amounts of immature immune cells, suggesting that these cells will have a lower incidence of graft-versus-host disease compared with stem cells from various other sources [11, 12]. Human umbilical cord blood also contains a higher number of hematopoietic stem and progenitor cells compared with adult bone marrow [13, 14].
Apart from the choice of cells to be transplanted, the number cells retained in the myocardium can also affect overall cardiac function after cell therapy [15, 16]. Studies have demonstrated that the majority of the transplanted cells after intravenous transplantation were retained in other organs rather than in the heart [17]. Similarly, approximately 50%–90% cells were pumped out of the heart or dead within 24 hours after intramyocardial transplantation [18]. Only 1.3%–2.6% of the transplanted cells were detected in the infarcted myocardium 50–75 minutes after intracoronary delivery [19].
Hydrogel is well-established as an excellent material for use in regenerative medicine [20]. The chemical properties of hydrogel allow the material itself to be easily manipulated into a three-dimensional scaffold, which promotes cell adhesion and protein deposition, thus providing a suitable microenvironment for cellular growth [21–24]. Hyaluronan (HA) is a glycosaminoglycan that is abundant in the extracellular matrix [25]. Tang et al. [26] mentioned that the combination of umbilical vein endothelial cells and HA improved blood flow in a peripheral artery occlusive disease mouse model.
Because previous studies have demonstrated that HA-based hydrogel is suitable for implanting cells into the myocardium, it is of interest to investigate whether this material can be applied in CB-MNC transplantation [27]. In the present study, experiments were carried out to examine the hypothesis that HA administered in combination with CB-MNCs may increase cell retention and improve cardiac function after MI in a pig model.
Materials and Methods
Experimental Animals
All animal research procedures were approved by the National Cheng Kung University Institutional Animal Care and Use Committee. Sexually mature Lanyu minipigs (∼5 months old, mean body weight of 22.26 ± 0.78 kg) from National Taitung Animal Propagation Station were used. Anesthesia was administered to all animals before surgery and in vivo measurements. After an overnight fast, the pigs were injected with Zoletil (12.5 mg/kg; Virbac, Carros, France, https://www.virbac.com), Rompun (0.2 ml/kg; Bayer Healthcare, Leverkusen, Germany, http://www.bayer.com), and atropine (0.05 mg/kg; TaiYu, Taiwan, http://www.tai-yu.com.tw/home/index.php) before intubation. The pigs were attached to a respirator for intermittent positive pressure ventilation with a mixture of oxygen, air, and isoflurane (1.5%–2%; Baxter Healthcare, Guayama, PR, http://www.baxter.com). An indwelling needle was placed in an ear vein for continuous administration of saline and anesthetic drugs if necessary. Dextrose (glucose) and electrolyte solutions were administered during all surgical procedures. After surgery, analgesics (Keto; YSP, Taichung, Taiwan, http://www.ysp.com.tw) and antibiotics (Ampolin; YSP) were administered to relieve pain and prevent infection.
Isolation of Human Cord Blood MNCs and DiI Labeling
Human umbilical cord blood samples were kindly provided by StemCyte Taiwan, with the mother’s informed written consent of the donation for academic research purposes. MNCs from cord blood were isolated by Ficoll-Paque preparation according to the manufacturer’s instructions (density: 1.077g/ml; GE Healthcare BioScience, Uppsala, Sweden, http://www.gehealthcare.com). MNCs were cryopreserved with 10% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) in plasma and stored in a liquid nitrogen tank until use. Before use, CB-MNCs were thawed and centrifuged to remove DMSO. To trace these cells, CB-MNCs were labeled with DiI (cell tracker; Molecular Probes, Invitrogen, Eugene, OR, http://probes.invitrogen.com) according to the manufacturer’s protocol.
HA Hydrogel Preparation
A portion of 1% (wt/vol) HA powder (1,630 kDa; Sigma-Aldrich) was dissolved in phosphate-buffered saline at 4°C on the shaker overnight (16 hours). The resulting 1% HA hydrogel was stored in multiple microtubes before use.
Surgically Induced MI and Treatment Administration
A total of 34 animals were divided into 5 groups, and a sham operation was performed by opening the chest without coronary artery ligation. The treatment groups were defined as follows: MI+NS (normal saline), MI+CB-MNC (cell injection only), MI+HA (HA only), and MI+CB-MNC/HA (HA with cell injection). MI was induced by permanent midleft anterior descending coronary artery ligation. A 2-ml injection of normal saline (MI+NS), 1% hyaluronic acid (MI+HA) solution, or CB-MNC (total 1 × 108 cells) without HA (MI+CB-MNC) or with HA (MI+CB-MNC/HA) was administered over the infarct area at 24 hours after the surgery, through intramyocardial injection (40 sites, ∼50 μl per injection).
Immunosuppression Protocol
The minipigs that were cell-treated were placed on cyclosporine and methylprednisolone therapy to prevent rejection of the human cell transplants. Methylprednisolone (500 mg) IV was administered to each pig before cell transplantation (the day before MI surgery) and maintained for 3 days after surgery accompanied by cyclosporine (200 mg p.o.). Three days later, methylprednisolone (20 mg p.o.) and cyclosporine (200 mg p.o.) were administered once daily. Decreasing dosages were given to pigs over the following 8 weeks.
Echocardiography
Cardiac function was assessed by echocardiography before and immediately after MI and 1 and 2 months after surgery using Vivid 7 with a 3.5-MHz probe (GE Healthcare). The animals were placed in the lateral decubitus position. The anesthesia condition used for echocardiography was the same as that during surgery. Parasternal long-axis views were obtained with both M-mode and two-dimensional echo imaging. The left ventricular end-diastolic diameter (LVEDD) and end-systolic diameter (LVESD) were measured by the long axis view. The cursor was perpendicular to the ventricle at the papillary muscle insertion site. The left ventricle ejection fraction (LVEF) was calculated automatically by the echocardiography system as (LVEDV − LVESV)/LVEDV × 100%, where LVEDV is the left ventricular end diastolic volume calculated as 7.0 × LVEDD3/(2.4 + LVEDD), and LVESV is the left ventricle end-systolic volume calculated as 7.0 × LVESD3/(2.4 + LVESD).
Hemodynamics
After 2 months, hemodynamics was assessed by ventricular catheterization using 5.0 French gauge (1.67 mm diameter) pressure-volume sensing catheters (Millar Instruments, Houston, TX, http://millar.com). The blood was drawn from the right jugular vein to calibrate the electrical conductivity for volume conversion. The catheter was inserted into the right carotid artery and advanced to the left ventricle (LV). After stabilization, baseline LV pressure-volume loops were recorded. To change the preload, the inferior vena cava was transiently compressed through an incision in the upper abdomen. At the end of each catheterization, 10 ml of 25% saline was injected into the right atrium through the right jugular vein to determine the conductance. The volume and hemodynamic data were analyzed by commercial software (PVAN3.2; Millar Instruments).
Ratio of Scar Tissue and Collagen Content
After all in vivo measurements, the animals were sacrificed. The hearts were harvested, and the atria were removed. The heart was then cut into five sections from the apex to the midventricle and placed upright. Images were then collected, and the scar tissue (pale) was quantified by manual tracing and software calculation (ImageJ; NIH, Bethesda, MD, http://www.nih.gov). Collagen count was determined using Masson’s trichrome staining. Images were taken at 10 randomly selected areas from each section by digital microscopy at 100× magnification. The collagen content of each section was then quantified (Axio Vision; Carl Zeiss, Jena, Germany, http://www.zeiss.com) and averaged.
Immunohistochemistry and Fluorescence Microscopy
Fixed myocardial tissue sections were deparaffinized, rehydrated, and boiled in 10 mM sodium citrate (pH 6.0) for 10 minutes. This was followed by incubation with antibodies against isolectin (Molecular Probes, Invitrogen), SM22-α (Abcam, Cambridge, U.K., http://www.abcam.com), von Willebrand factor (Millipore, Billerica, MA, http://www.millipore.com), anti-human mitochondria (Millipore), and cardiac troponin I (Developmental Studies Hybridoma Bank) according to the manufacturer’s protocol. Samples were then incubated with conjugated secondary antibodies Alexa Fluor 488, 568, or 660 (Molecular Probes, Invitrogen). Finally, sections were incubated with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich), and all sections were observed under a fluorescence microscope.
Cell Retention Rate Analysis in the Injury Area
The DiI-positive cells in the injured area were observed. All tissue sections were deparaffinized and retrieved. Tissue samples were incubated with DAPI. The images were collected from 9 views (200× magnifications). Anti-human mitochondria antibodies were also used to trace transplanted cells. Blinded quantification was performed by manually counting DAPI and DiI or human mitochondria double positive cells in each section.
Capillary and Arteriole Density Quantification
The capillary density was stained by isolectin as an endothelial cell marker. The mature cardiomyocytes were stained by cardiac troponin I. The images were collected from 9 views (200× magnifications) at the border zone. The arteriole density was stained with SM22-α as a smooth muscle cell marker. The images were collected from 9 different views (100× magnifications) at the border zone. Blinded quantification was performed by manually counting each section.
Transplanted Cell Differentiation Into ECs and SMCs
CB-MNC differentiation into endothelial cells (ECs) was quantified by manually counting DiI-positive cells that demonstrated costaining with the vWF marker within each section. Similarly, CB-MNC differentiation into smooth muscle cells (SMCs) was quantified by manually counting DiI-positive cells that demonstrated costaining with the SMC marker smooth muscle actin (SMA) or SM22-α within each section.
Immune Cell Staining
The immune cells were stained with anti-CD3 antibodies and incubated at 4°C overnight. Anti-mouse polymer-horseradish peroxidase (Dako) antibodies were used as the secondary antibody. The ABC staining kit (Sigma-Aldrich) was used to label CD3-positive cells in the cell-injected area.
Statistical Analysis
The results are presented as means ± SEM. Statistical comparison was performed with Student’s t test or one-way or two-way analysis of variance. A probability value of less than .05 was considered statistically significant.
Results
Injection of CB-MNC/HA Improves Myocardial Function After MI
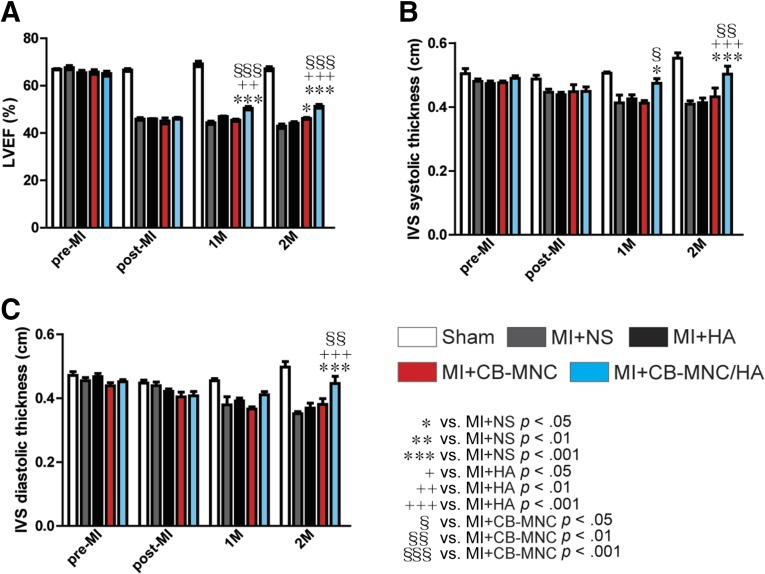
Immediately after the MI surgery, the MI-treated pigs displayed significant reduction in LVEF measurements (LVEF: 66.64% ± 0.58% in Sham, 45.93% ± 0.56% in MI+NS, 45.94% ± 0.13% in MI+HA, 45.10% ± 1.26% in MI+CB-MNC, and 46.25% ± 0.39% in MI+CB-MNC/HA; all p < .001 vs. Sham; Fig. 1A). After 2 months, the LVEF continued to decline in the MI+NS group (42.87% ± 0.97%; Fig. 1A), whereas the group treated with CB-MNC alone showed less decrease in LVEF (46.17% ± 0.39% vs. 42.87% ± 0.97, and MI+CB-MNC vs. MI+NS, p < .01; Fig. 1A). In contrast, a better result was seen with the group that was injected with CB-MNC with HA (CB-MNC/HA), because the LVEF measurement (50.49% ± 0.74%) displayed a better improvement compared with other treatment groups either 1 or 2 months after MI (Fig. 1A).
Figure 1.
Injection of CB-MNC/HA increases interventricular septum thickness after infarction. (A): The LVEF measured pre-MI, immediately after MI (post-MI), and 1 and 2 months after MI. (B, C): The systolic and diastolic interventricular septum thicknesses. The values are means ± SEM (n ≥ 6). ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001 versus MI+NS; +, p < .05; ++, p < .01; +++, p < .001 versus MI+HA; §, p < .05; §§, p < .01; §§§, p < .001 versus MI+CB-MNC. Abbreviations: CB-MNC, cord blood mononuclear cell; HA, hyaluronan; IVS, interventricular septum; LVEF, left ventricle ejection fraction; MI, myocardial infarction; NS, normal saline.
The interventricular septum (IVS) systolic and diastolic thickness measurements also indicated that CB-MNC alone did not show any significant improvement compared with MI+NS group (Fig. 1B, 1C). However, the group injected with CB-MNC/HA showed a significant increase in IVS systolic thickness (0.47 ± 0.01 cm) compared with both MI+NS (0.41 ± 0.02 cm, p < .05) and MI+CB-MNC (0.41 ± 0.01 cm) at 1 month after MI (Fig. 1B). A similar result was also seen at 2 months after MI, when the MI+CB-MNC/HA group showed an increased IVS systolic thickness (0.50 ± 0.02 cm) when compared with MI+NS (0.41 ± 0.01 cm, p < .001), MI+HA (0.41 ± 0.01 cm, p < .001), and MI+CB-MNC (0.43 ± 0.02 cm, p < .01).
Interestingly, significant improvement in IVS diastolic thickness was only seen with the CB-MNC/HA groups (0.40 ± 0.02 cm) at 2 months after MI, as opposed to MI+NS (0.35 ± 0.01 cm, p < .001), MI+HA (0.37 ± 0.01 cm, p < .001), and MI+CB-MNC (0.38 ± 0.02 cm, p < .01). These results thus suggest that the injection of CB-MNC alone into the myocardium prevented myocardial function loss, but the inclusion of HA (i.e., CB-MNC/HA) provided additional improvement in both the systolic and diastolic functions.
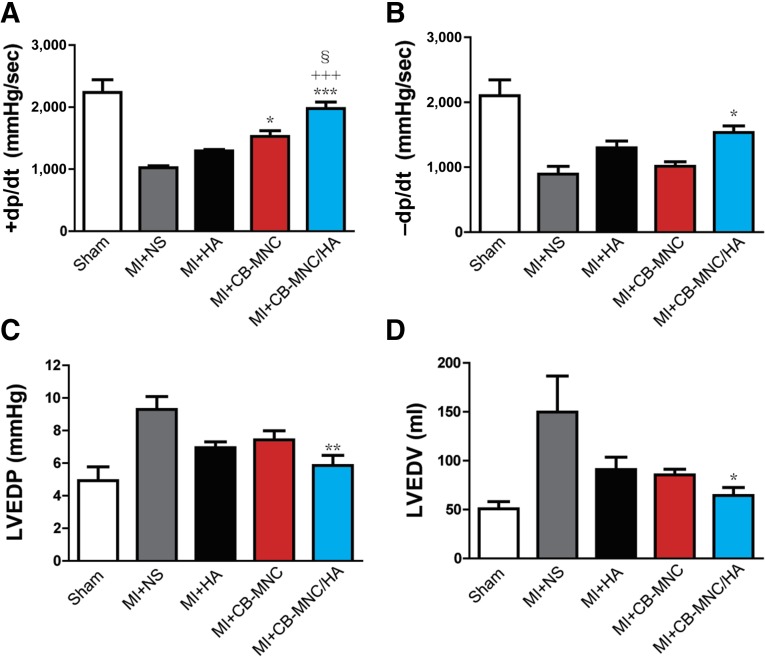
Injection of CB-MNC/HA Improves the Hemodynamics After MI
To investigate whether CB-MNC/HA improves heart performance compared with other treatment groups, catheterization was used to measure the cardiac hemodynamics 2 months after MI. As shown in Figure 2A and 2B, the MI+CB-MNC/HA group displayed the greatest +dp/dt and −dp/dt values in contrast to other treatment groups (p < .001), although the MI+CB-MNC group had a significantly better +dp/dt value compared with the MI+NS group (p < .05). Moreover, the LVEDP and LVEDV at 2 months after MI were significantly lower in the MI+ CB-MNC/HA group compared with the MI+NS group (Fig. 2C, 2D). Although, the HA-alone and CB-MNC-alone groups also had decreased LVEDP and LVEDV values, the results were not statistically significant. According to these data, the cell transplantation with or without HA improved the systolic function (+dp/dt), but the improvement in diastolic function was only seen in the MI+CB-MNC/HA group.
Figure 2.
Injection of CB-MNC/HA improves cardiac systolic and diastolic functions after infarction. (A–D): The +dp/dt (A), −dp/dt (B), LVEDP (C), and LVEDV (D) measured by ventricular catheterization at 2 months after infarction. The values are means ± SEM (n ≥ 6). ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001 versus MI+NS; +++, p < .001 versus MI+HA; §, p < .05. Abbreviations: CB-MNC, cord blood mononuclear cell; +dp/dt, systolic function; −dp/dt, diastolic function; HA, hyaluronan; LVEDP, left ventricular end diastolic pressure; LVEDV, left ventricular end diastolic volume; MI, myocardial infarction; NS, normal saline; sec, second.
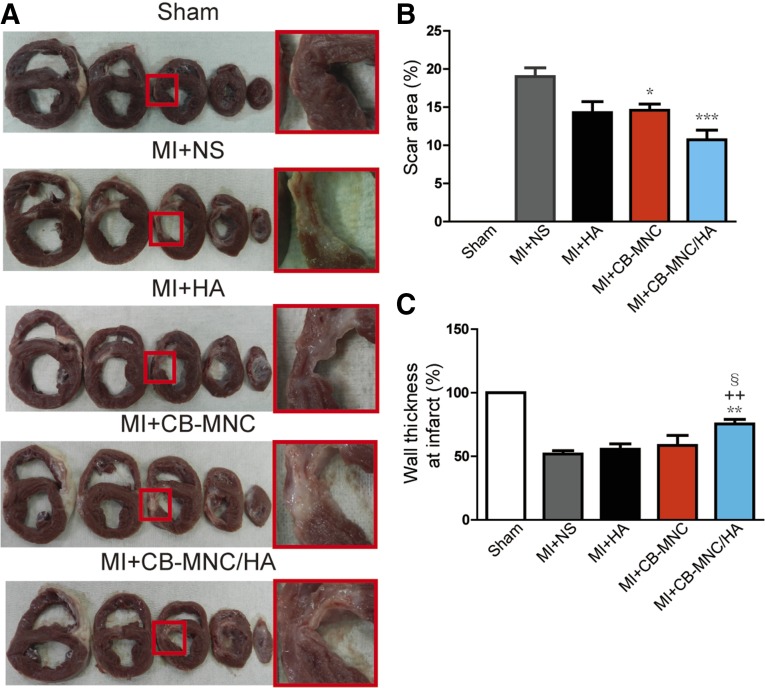
Injection of CB-MNC/HA Decreases Scar Size and Prevents the Loss of Wall Thickness in the Infarct Area
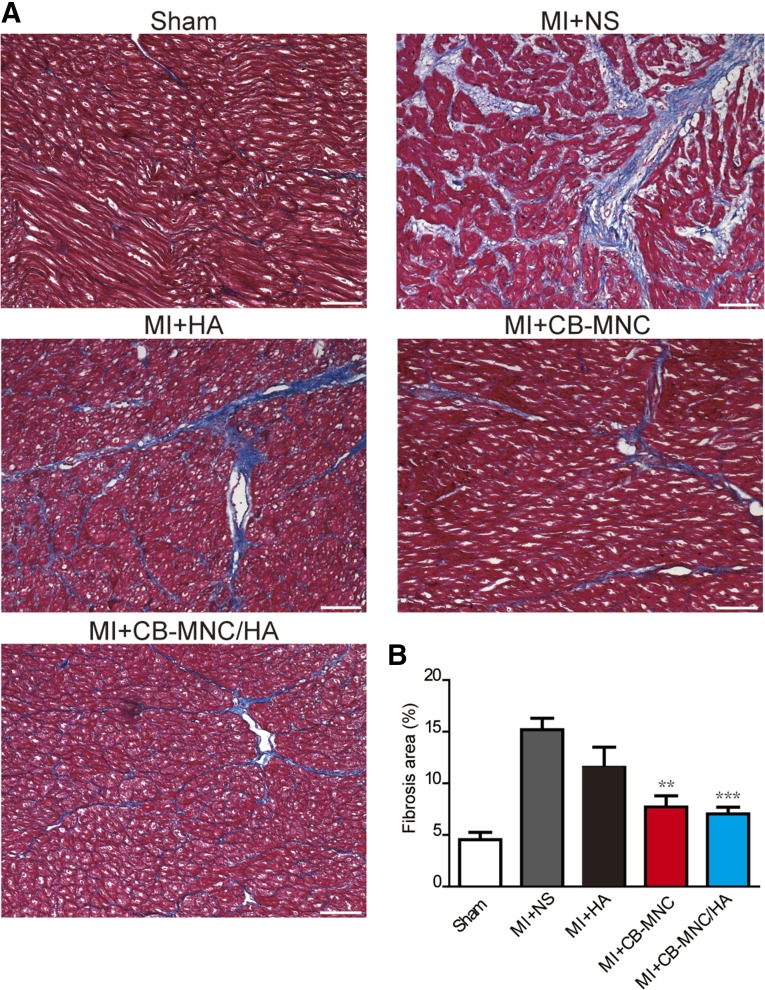
To further evaluate the therapeutic benefit of delivery of CB-MNC/HA, the morphology of the myocardium tissue was examined after the injection (Fig. 3A). The scar area was found to be significantly reduced at 2 months after MI in both the MI+CB-MNC and MI+CB-MNC/HA groups, with the latter group showing the greatest reduction (Fig. 3B). In addition, CB-MNC+HA injection prevented the loss of wall thickness compared with other treatment groups (51.83% ± 2.66% in MI+NS, 55.57% ± 4.22% in MI+HA, 58.60% ± 7.79% in MI+CB-MNC, and 76.29% ± 3.35 in MI+CB-MNC/HA; Fig. 3C). The injections of CB-MNC either with or without HA minimized the development of fibrosis during the 2-month period post-MI, because both treatments were shown to significantly attenuate collagen content in the remote area (7.7% ± 1.1% in MI+CB-MNC and 7.0% ± 0.7% in MI+CB-MNC/HA; Fig. 4A, 4B) compared with the MI+NS groups (15.2% ± 1.1%; Fig. 4A, 4B). These data indicated that the injection of CB-MNC/HA not only decreased scar size but also prevented heart dilatation.
Figure 3.
Injection of CB-MNC/HA decreases scar size and prevents wall dilatation. (A): Representative cross-section images showing the morphology of the myocardium. (B, C): Statistical analysis of scar area and wall thickness at infarct area. The values are means ± SEM (n ≥ 6). ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001 versus MI+NS; ++, p < .01; §, p < .05. Abbreviations: CB-MNC, cord blood mononuclear cell; HA, hyaluronan; MI, myocardial infarction; NS, normal saline.
Figure 4.
Injection of CB-MNC/HA attenuates collagen content at the remote area after infarction. (A): Representative images showing collagen content in the remote area. (B): Statistical analysis of collagen content at the remote area. The values are means ± SEM (n ≥ 6). ∗∗, p < .01; ∗∗∗, p < .001 versus MI+NS. Scale bars = 100 μm. Abbreviations: CB-MNC, cord blood mononuclear cell; HA, hyaluronan; MI, myocardial infarction; NS, normal saline.
Injection of CB-MNC/HA Increases Transplanted Cell Retention
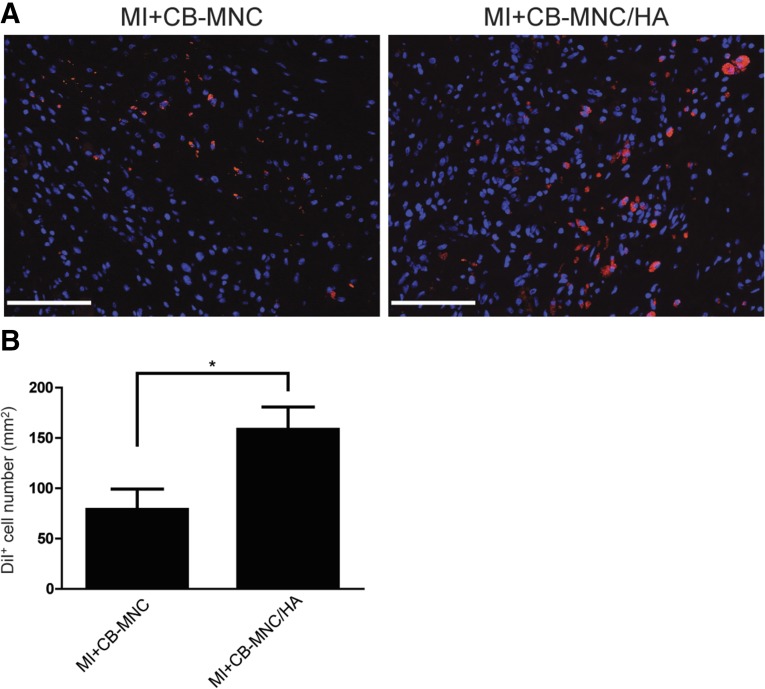
To examine the contribution of HA in cell retention, the number of Dil-labeled CB-MNCs remaining at the injected area at 2 months after MI was examined (Fig. 5A). The number of DiI-positive cells was found to be significantly higher in the CB-MNC/HA group compared with CB-MNC alone group (158.5 ± 22.3 vs. 79.2 ± 20.0 DiI+ cell number per mm2, p < .05; Fig. 5B). A strong signal for human mitochondria was also detected at 2 months after MI (supplemental online Fig. 1), further indicating that the transplanted cells could survive for at least 2 months after the injection.
Figure 5.
Injection of CB-MNC/HA improves cell retention 2 months after infarction. (A): Representative image of the DiI-positive cells detected at the injection area. (B): Statistical analysis of the DiI-positive cell retention rates. The values are means ± SEM (n ≥ 6). ∗, p < .05. Scale bars = 100 μm. Abbreviations: CB-MNC, cord blood mononuclear cell; DiI+, DiI-positive; HA, hyaluronan; MI, myocardial infarction.
Injection of CB-MNC/HA Increases Capillary and Arteriole Densities in the Peri-Infarct Area
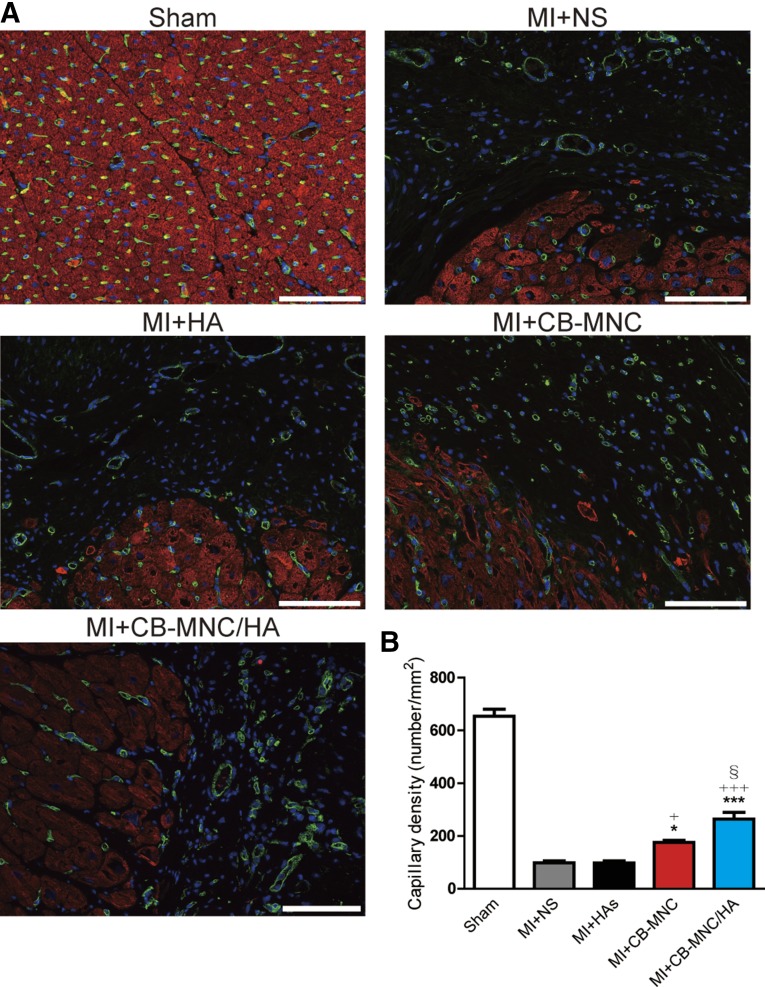
Whether HA with CB-MNC promotes angiogenesis 2 months after MI was explored. The capillary density was stained for isolectin in the peri-infarct area (Fig. 6A). Injection of the CB-MNC-only group increased the capillary density compared with the MI+NS and MI+HA groups (Fig. 6B). In contrast, injection of CB-MNC/HA had the highest increase in capillary density compared with other treatment groups (MI+NS, p < .001; MI+HA, p < .001; MI+CB-MNC, p < .05; Fig. 6B).
Figure 6.
Injection of CB-MNC/HA increases capillary density at the peri-infarct area. (A): Representative images of capillary, isolectin (green), cardiac troponin I (red), and DAPI (blue). (B): Statistical analysis of capillary density in the peri-infarct area. The values are means ± SEM. ∗, p < .05, ∗∗∗, p < .001 versus MI+NS; +, p < .05, +++, p < .001 versus MI+HA; §, p < .05. Scale bars = 100 μm. Abbreviations: CB-MNC, cord blood mononuclear cell; HA, hyaluronan; MI, myocardial infarction; NS, normal saline.
The formation of mature vascular structure was also investigated by staining smooth muscle cells in the peri-infarct area (supplemental online Fig. 2A). The small arteriolar density was modestly higher in the MI+CB-MNC group compared with the MI+NS group (supplemental online Fig. 2B). In the MI+CB-MNC/HA group, small arteriolar density was greater than MI+NS, MI+HA, and MI+CB-MNC (supplemental online Fig. 2B). Thus, the results suggest that CB-MNC combined with HA enhanced both angiogenesis and arteriogenesis and in turn improved the overall cardiac function.
Transplanted CB-MNCs Differentiate Into Endothelial Cells but Not Smooth Muscle Cells
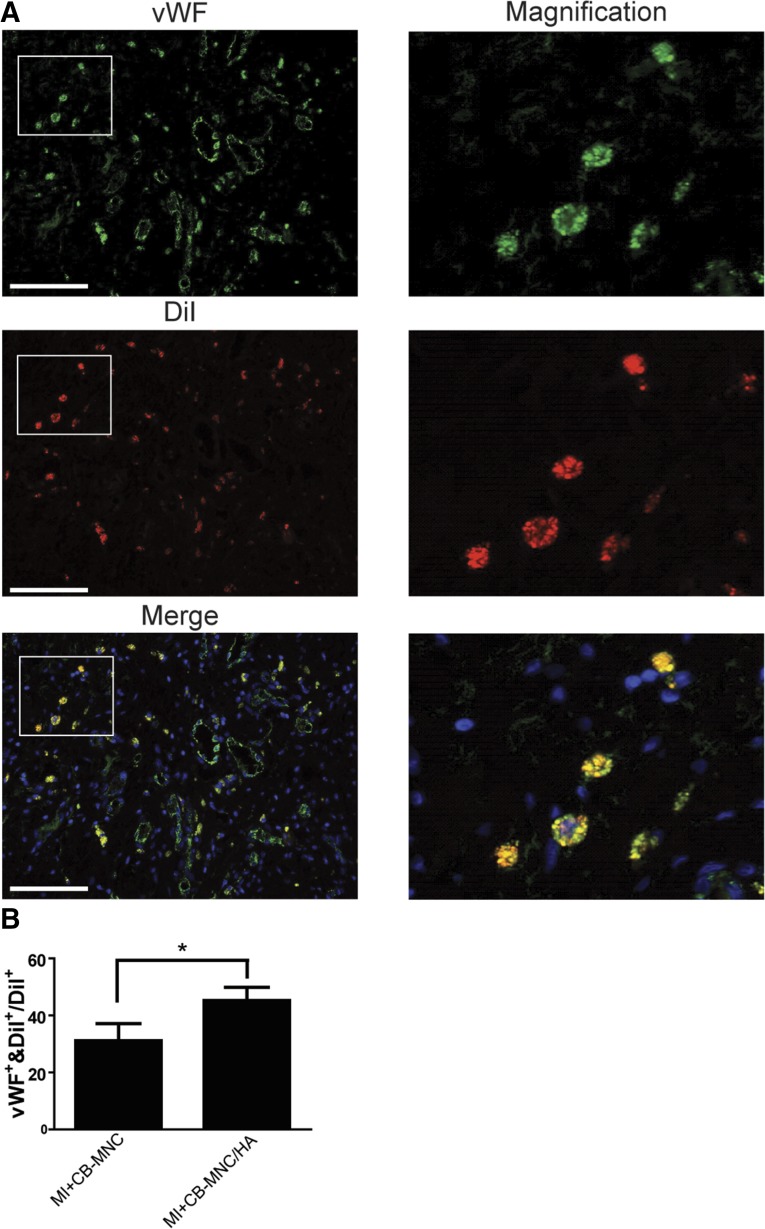
The presence of the differentiated human ECs derived from the transplanted CB-MNCs at the injection area was confirmed by the double staining for vWF and DiI (Fig. 7A). The differentiation ratio was measured by counting the vWF and DiI double positive cells divided by DiI-positive cells at the injury area. The CB-MNC/HA injection promoted more transplanted cells expressing EC marker than the MI+CB-MNC group (Fig. 7B). However, the smooth muscle cell marker SMA and DiI double positive cells were not markedly detected at the injection area (supplemental online Fig. 3), suggesting that the transplanted CB-MNCs have the capability to differentiate into ECs but not SMCs.
Figure 7.
HA promotes CB-MNC differentiation into endothelial cells. (A): Representative images of vWF and DiI double positive cells at the injury area. (B): Statistical analysis of differentiation rate measured at the peri-infarct area. The values are means ± SEM. ∗, p < .05. Scale bars = 100 μm. Abbreviations: CB-MNC, cord blood mononuclear cell; DiI+, DiI-positive; HA, hyaluronan; MI, myocardial infarction; vWF, von Willebrand factor.
Discussion
In the present study, human CB-MNCs were used alone or in combination with HA hydrogel to treat MI in a minipig model. Although CB-MNC-only injection was able to increase cardiac systolic function and reduce the scar size, the combined CB-MNC and HA treatment showed the best results in infarct size reduction and improved both systolic and diastolic function. The superior cardiac function improvement seen with the combination treatment might be because the HA hydrogel promotes higher CB-MNC retention and increases angiogenesis and arteriogenesis in the injured area. The current findings provide evidence that a CB-MNC-combined HA strategy may have potential for use in the clinic.
Cell therapy is a promising approach for treating acute MI. Several human clinical trials have shown that bone marrow cell transplantation has a beneficial effect in the MI heart [28–30]. Recently, some groups have reported different results with regard to the therapeutic efficacy of bone marrow cells [2, 4, 29]. Various factors may affect the therapeutic outcome of bone marrow cell therapy such as different processing and isolation methods and timing of cell harvest and administration. Even study size, population, and end-point selection may cause diverse outcomes [31]. Moreover, some chronic diseases, aging, and severe systemic immune response post-MI also act as negative regulators of endogenous stem cells [9, 32, 33]. Therefore, a young and healthy source of stem cells such as human umbilical cord blood cells, which can be isolated easily and stored before clinical use, may be a better alternative than dysfunctional bone marrow cells taken from patients [8, 34].
Previous studies have shown that umbilical cord blood cells have promising therapeutic effects in several kinds of diseases including ischemic heart disease and neonatal hypoxic-ischemic encephalopathy [35]. CB-MNCs contain many kinds of cell populations including hematopoietic stem cells, lymphocytes, monocytes, endothelial progenitor cells, and mesenchymal stem cells [35]. Each cell population has a different function and plays a different role in response to myocardium damage. CB-MNCs can contribute to cell protection and neovascularization in a paracrine manner by secreting antiapoptotic, anti-inflammatory, and neovascularization growth factors and cytokines such as basic fibroblast growth factor and vascular endothelial growth factor, which have been found to enhance endothelial cell proliferation and increase angiogenesis; hepatocyte growth factor, which regulates smooth muscle cell and pericyte growth; insulin-like growth factor-1, which has a cardioprotection effect through inducing Akt signaling; interleukin-10, which reduces immune response post-MI; and stromal derived factor-1, which acts as chemoattractant to recruit different cells to participate in cardiac regeneration [36–41].
Another issue that causes cell therapy failure is low cell retention rate after cell transplantation in the infarcted heart. Approximately 80% of cells are lost during the first 1–1.5 hours after cell injection into the myocardium [19]. There are many previously proposed hydrogel systems, such as using an injectable self-assembling peptide nanofiber with or without bone marrow MNCs to create a suitable microenvironment for increasing neovascularization and reducing scar size in the infarcted heart or using an injectable fibrin matrix and PEGylated-fibrinogen hydrogel combined with bone marrow MNCs or human embryonic stem cell-derived cardiomyocytes to promote neovascularization, increase wall thickness and cell graft area, and further improve cardiac performance [24, 42–44]. Another study that uses fibrin glue combined with skeletal myoblast also showed enhancement of arteriogenesis and retention of transplanted cells after intramyocardial injection [45]. These findings suggest that combination of hydrogel and stem cell may offer a potential solution for cardiac regeneration. Therefore, in this study, HA hydrogel combined with CB-MNC was used for the same approach after acute MI to investigate whether cell retention rate was improved. HA is a type of extracellular matrix with a rapid degradation rate (half-life: 15–25 hours) [46]. Although HA degraded rapidly, we still found that the cell retention rate was increased approximately twofold in the CB-MNC and HA combined group. Despite the short half-life of HA in vivo, it has been shown to benefit the injured heart by reducing scar formation and increasing cell survival and retention, neovascularization, and the efficacy of cell therapy in both rat and swine MI models [27, 46, 47]. In this study, we found that cell retention rate was increased approximately twofold in the CB-MNC and HA combined group. Based on previous studies, HA is able to upregulate COX2 signaling through CD44 and Toll-like receptor 4-HA binding receptors and prevent cardiac myoblast-H9C2 from ischemia-reperfusion-induced cell death [48–50]. Therefore, these findings suggest that HA may induce survival signaling to prevent cell apoptosis even when cells are under hypoxic stress and thus further benefit the injured heart. In addition, we observed that the capillary density and arteriole density in both the CB-MNC alone group and the CB-MNC/HA group were higher than other experimental groups. Additionally, in our previous study, we found that HA increased bone marrow MNC differentiation into vascular lineage cells and upregulated the gene expression of some proangiogenesis factors [27]. Moreover, human cord blood cells contain a large number of endothelial cell precursors [51] and those cells are able to differentiate into functional endothelial cells [52]. We found that some of the injected CB-MNCs that were labeled with DiI fluorescence dye coexpressed von Willebrand factor, a specific marker of mature endothelial cells. Furthermore, we observed that more injected CB-MNCs became endothelial cells in the CB-MNC/HA combined treatment group. Thus, transplanted human CB-MNCs with HA promoted angiogenesis and enhanced the differentiation of CB-MNCs into endothelial cells in the injured heart.
Although the combined CB-MNC and HA strategy improved cardiac performance post-MI, this study had some limitations. First, although immunosuppression drugs were administered throughout the whole study, an undetected, residual immune response may have been elicited toward the injected human cells. We observed some CD3+ T cells infiltrated in the injection area (supplemental online Fig. 4), a phenomenon that has also been observed in another study [53]. Second, it is not clear whether the therapeutic efficacy of this combined treatment would persist in chronic MI. Furthermore, the mechanism of action and the safety of this treatment also require further investigation before moving this protocol into clinical trials.
Conclusion
This study provides preclinical evidence that combined injection of HA and human CB-MNCs post-MI significantly increases cell retention in the peri-infarct area, improves cardiac performance, and prevents cardiac remodeling. Moreover, using healthy cells to replace dysfunctional autologous cells may constitute a better strategy to achieve heart repair and regeneration.
Supplementary Material
Acknowledgments
We are grateful to the Tai-Tung Animal Propagation Station and the National Cheng Kung University Animal Center for assistance with the pig experiments. We thank StemCyte, Taiwan, for providing human cord blood units donated for research purposes. This work was supported by the National Science Council (Grants NSC102-2314-B-006-067-MY3 and NSC102-2321-B-001-069-MY3), the National Health Research Institutes (Grant NHRI-EX103-10026SI), and the Academia Sinica Translational Medicine Program.
Author Contributions
M.-Y.C., T.-T.H., and C.-H.C.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; B.C.: data analysis and interpretation, manuscript writing; S.-M.H. and P.C.H.H.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
P.C.H.H. received compensated research support from Celgene Cellular Therapeutics and Meridigen Biotech Co. The other authors indicated no potential conflicts of interest.
References
- 1.Sanganalmath SK, Bolli R. Cell therapy for heart failure: A comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sürder D, Manka R, Lo Cicero V, et al. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: Effects on global left ventricular function. Circulation. 2013;127:1968–1979. doi: 10.1161/CIRCULATIONAHA.112.001035. [DOI] [PubMed] [Google Scholar]
- 3.Traverse JH, Henry TD, Ellis SG, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: The LateTIME randomized trial. JAMA. 2011;306:2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traverse JH, Henry TD, Pepine CJ, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: The TIME randomized trial. JAMA. 2012;308:2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheubel RJ, Zorn H, Silber RE, et al. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–2080. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 6.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 7.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 8.Heeschen C, Lehmann R, Honold J, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Takagawa J, Lam VC, et al. Donor myocardial infarction impairs the therapeutic potential of bone marrow cells by an interleukin-1-mediated inflammatory response. Sci Transl Med. 2011;3:100ra90. doi: 10.1126/scitranslmed.3002814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith AR, Wagner JE. Alternative haematopoietic stem cell sources for transplantation: Place of umbilical cord blood. Br J Haematol. 2009;147:246–261. doi: 10.1111/j.1365-2141.2009.07828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong XY, Zhang B, Asadollahi R, et al. Umbilical cord blood stem cells: What to expect. Ann N Y Acad Sci. 2010;1205:17–22. doi: 10.1111/j.1749-6632.2010.05659.x. [DOI] [PubMed] [Google Scholar]
- 12.Munoz J, Shah N, Rezvani K, et al. Concise review: Umbilical cord blood transplantation: Past, present, and future. Stem Cells Translational Medicine. 2014;3:1435–1443. doi: 10.5966/sctm.2014-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hows JM, Marsh JC, Bradley BA, et al. Human cord blood: A source of transplantable stem cells? Bone Marrow Transplant. 1992;9(suppl 1):105–108. [PubMed] [Google Scholar]
- 14.Lee MW, Jang IK, Yoo KH, et al. Stem and progenitor cells in human umbilical cord blood. Int J Hematol. 2010;92:45–51. doi: 10.1007/s12185-010-0619-4. [DOI] [PubMed] [Google Scholar]
- 15.Meluzín J, Mayer J, Groch L, et al. Autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction: The effect of the dose of transplanted cells on myocardial function. Am Heart J. 2006;152:975.e9–975.e15. doi: 10.1016/j.ahj.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Yuan C, Yu G, Yang T, et al. Enhanced therapeutic effects on acute myocardial infarction with multiple intravenous transplantation of human cord blood mononuclear cells. Int J Cardiol. 2013;168:2767–2773. doi: 10.1016/j.ijcard.2013.03.131. [DOI] [PubMed] [Google Scholar]
- 17.Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 18.Müller-Ehmsen J, Whittaker P, Kloner RA, et al. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann M, Wollert KC, Meyer GP, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 20.Slaughter BV, Khurshid SS, Fisher OZ, et al. Hydrogels in regenerative medicine. Adv Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 22.Pashuck ET, Stevens MM. Designing regenerative biomaterial therapies for the clinic. Sci Transl Med. 2012;4:160sr4. doi: 10.1126/scitranslmed.3002717. [DOI] [PubMed] [Google Scholar]
- 23.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 24.Davis ME, Motion JP, Narmoneva DA, et al. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111:442–450. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evanko SP, Tammi MI, Tammi RH, et al. Hyaluronan-dependent pericellular matrix. Adv Drug Deliv Rev. 2007;59:1351–1365. doi: 10.1016/j.addr.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang ZC, Liao WY, Tang AC, et al. The enhancement of endothelial cell therapy for angiogenesis in hindlimb ischemia using hyaluronan. Biomaterials. 2011;32:75–86. doi: 10.1016/j.biomaterials.2010.08.085. [DOI] [PubMed] [Google Scholar]
- 27.Chen CH, Wang SS, Wei EI, et al. Hyaluronan enhances bone marrow cell therapy for myocardial repair after infarction. Mol Ther. 2013;21:670–679. doi: 10.1038/mt.2012.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Assmus B, Honold J, Schächinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 29.Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: Double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 30.Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 31.Simari RD, Pepine CJ, Traverse JH, et al. Bone marrow mononuclear cell therapy for acute myocardial infarction: A perspective from the cardiovascular cell therapy research network. Circ Res. 2014;114:1564–1568. doi: 10.1161/CIRCRESAHA.114.303720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: A novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 33.Lepperdinger G. Inflammation and mesenchymal stem cell aging. Curr Opin Immunol. 2011;23:518–524. doi: 10.1016/j.coi.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Acosta SA, Franzese N, Staples M, et al. Human umbilical cord blood for transplantation therapy in myocardial infarction. J Stem Cell Res Ther. 2013;pii(suppl 4):S4-005. [PMC free article] [PubMed] [Google Scholar]
- 35.Pimentel-Coelho PM, Rosado-de-Castro PH, da Fonseca LM, et al. Umbilical cord blood mononuclear cell transplantation for neonatal hypoxic-ischemic encephalopathy. Pediatr Res. 2012;71:464–473. doi: 10.1038/pr.2011.59. [DOI] [PubMed] [Google Scholar]
- 36.Kanda S, Lerner EC, Tsuda S, et al. The nonreceptor protein-tyrosine kinase c-Fes is involved in fibroblast growth factor-2-induced chemotaxis of murine brain capillary endothelial cells. J Biol Chem. 2000;275:10105–10111. doi: 10.1074/jbc.275.14.10105. [DOI] [PubMed] [Google Scholar]
- 37.Fan CG, Zhang QJ, Tang FW, et al. Human umbilical cord blood cells express neurotrophic factors. Neurosci Lett. 2005;380:322–325. doi: 10.1016/j.neulet.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi H, DeBusk LM, Babichev YO, et al. Hepatocyte growth factor mediates angiopoietin-induced smooth muscle cell recruitment. Blood. 2006;108:1260–1266. doi: 10.1182/blood-2005-09-012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hausenloy DJ, Yellon DM. Cardioprotective growth factors. Cardiovasc Res. 2009;83:179–194. doi: 10.1093/cvr/cvp062. [DOI] [PubMed] [Google Scholar]
- 40.Liesz A, Suri-Payer E, Veltkamp C, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 41.Stellos K, Bigalke B, Langer H, et al. Expression of stromal-cell-derived factor-1 on circulating platelets is increased in patients with acute coronary syndrome and correlates with the number of CD34+ progenitor cells. Eur Heart J. 2009;30:584–593. doi: 10.1093/eurheartj/ehn566. [DOI] [PubMed] [Google Scholar]
- 42.Lin YD, Yeh ML, Yang YJ, et al. Intramyocardial peptide nanofiber injection improves postinfarction ventricular remodeling and efficacy of bone marrow cell therapy in pigs. Circulation. 2010;122(suppl):S132–S141. doi: 10.1161/CIRCULATIONAHA.110.939512. [DOI] [PubMed] [Google Scholar]
- 43.Ryu JH, Kim IK, Cho SW, et al. Implantation of bone marrow mononuclear cells using injectable fibrin matrix enhances neovascularization in infarcted myocardium. Biomaterials. 2005;26:319–326. doi: 10.1016/j.biomaterials.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 44.Habib M, Shapira-Schweitzer K, Caspi O, et al. A combined cell therapy and in-situ tissue-engineering approach for myocardial repair. Biomaterials. 2011;32:7514–7523. doi: 10.1016/j.biomaterials.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 45.Christman KL, Vardanian AJ, Fang Q, et al. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44:654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 46.Lai CY, Wu PJ, Roffler SR, et al. Clearance kinetics of biomaterials affects stem cell retention and therapeutic efficacy. Biomacromolecules. 2014;15:564–573. doi: 10.1021/bm401583b. [DOI] [PubMed] [Google Scholar]
- 47.Chen CH, Chang MY, Wang SS, et al. Injection of autologous bone marrow cells in hyaluronan hydrogel improves cardiac performance after infarction in pigs. Am J Physiol Heart Circ Physiol. 2014;306:H1078–H1086. doi: 10.1152/ajpheart.00801.2013. [DOI] [PubMed] [Google Scholar]
- 48.Riehl TE, Foster L, Stenson WF. Hyaluronic acid is radioprotective in the intestine through a TLR4 and COX-2-mediated mechanism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G309–G316. doi: 10.1152/ajpgi.00248.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Misra S, Toole BP, Ghatak S. Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J Biol Chem. 2006;281:34936–34941. doi: 10.1074/jbc.C600138200. [DOI] [PubMed] [Google Scholar]
- 50.Law CH, Li JM, Chou HC, et al. Hyaluronic acid-dependent protection in H9C2 cardiomyocytes: A cell model of heart ischemia-reperfusion injury and treatment. Toxicology. 2013;303:54–71. doi: 10.1016/j.tox.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Nieda M, Nicol A, Denning-Kendall P, et al. Endothelial cell precursors are normal components of human umbilical cord blood. Br J Haematol. 1997;98:775–777. doi: 10.1046/j.1365-2141.1997.2583074.x. [DOI] [PubMed] [Google Scholar]
- 52.Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gahremanpour A, Vela D, Zheng Y, et al. Xenotransplantation of human unrestricted somatic stem cells in a pig model of acute myocardial infarction. Xenotransplantation. 2013;20:110–122. doi: 10.1111/xen.12026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.