The use of fresh, autologous, adipose-derived mesenchymal stem cells (ASCs) was studied for treatment of feline chronic gingivostomatitis. Cats received autologous ASCs, and immunomodulatory effects were assessed. The five cats that responded to treatment also exhibited systemic immunomodulation. Response to ASC therapy was seen only in cats with <15% CD8lo cells, suggesting relative absence of CD8lo cells may be a biomarker of treatment response.
Keywords: Adipose-derived stem cells, Fresh, Autologous, Cats, Gingivostomatitis, Oral mucosa, Immunomodulation
Abstract
Mesenchymal stem cells (MSCs) are a promising therapy for immune-mediated and inflammatory disorders, because of their potent immunomodulatory properties. In this study, we investigated the use of fresh, autologous, adipose-derived MSCs (ASCs) for feline chronic gingivostomatitis (FCGS), a chronic, debilitating, idiopathic, oral mucosal inflammatory disease. Nine cats with refractory FCGS were enrolled in this pilot study. Each cat received 2 intravenous injections of 20 million autologous ASCs, 1 month apart. Oral biopsies were taken before and at 6 months after the first ASC injection. Blood immune cell subsets, serum protein, and cytokine levels were measured at 0, 1, 3, and 6 months after treatment to assess immunomodulatory effects. Seven of the 9 cats completed the study. Five cats responded to treatment by either complete clinical remission (n = 3) or substantial clinical improvement (n = 2). Two cats were nonresponders. Cats that responded to treatment also exhibited systemic immunomodulation demonstrated by decreased numbers of circulating CD8+ T cells, a normalization of the CD4/CD8 ratio, decreased neutrophil counts, and interferon-γ and interleukin (IL)-1β concentration, and a temporary increase in serum IL-6 and tumor necrosis factor-α concentration. No clinical recurrence has occurred following complete clinical remission (follow-up of 6–24 months). In this study, cats with <15% cytotoxic CD8 T cells with low expression of CD8 (CD8lo) cells were 100% responsive to ASC therapy, whereas cats with >15% CD8lo cells were nonresponders. The relative absence of CD8lo cells may be a biomarker to predict response to ASC therapy, and may shed light on pathogenesis of FCGS and mechanisms by which ASCs decrease oral inflammation and affect T-cell phenotype.
Significance
This study is the first to demonstrate the safety and efficacy of fresh, autologous, adipose-derived stem cell systemic therapy for a naturally occurring, chronic inflammatory disease in cats. The findings demonstrate that this therapy resulted in complete clinical and histological resolution or reduction in clinical disease severity and immune modulation in most cats. This study also identified a potentially useful biomarker that could dictate patient enrollment and shed light on immune modulation mechanism. As a naturally occurring animal model, FCGS also provides a strategic platform for potentially translatable therapy for the treatment of human oral inflammatory disease.
Introduction
Immune-mediated, oral mucosal inflammatory diseases are prevalent in the human population and include oral lichen planus, stomatitis, pemphigus, and pemphigoid [1, 2]. These disorders cause painful mucosal lesions that markedly reduce quality of life and often require long-term immunosuppressive therapy with significant associated risks and side effects. The pathogenesis of these diseases is complex and heterogeneous, but consistently involves tissue infiltration primarily by activated effector T and B cells, with a skew toward a Th1 phenotype [3–5].
Naturally occurring diseases in client-owned animal species serve as useful animal models of human disease, as they reflect the complex genetic, environmental, and physiologic variation present in outbred populations. Feline chronic gingivostomatitis (FCGS) is a severe, idiopathic, oral inflammatory disease of cats that is estimated to affect 0.7%–10% of the general cat population [6–10]. Clinical signs are moderate to severe oral pain and discomfort, including inappetence, reduced grooming, weight loss, and hypersalivation [7, 8, 11]. FCGS can be debilitating, and severely affected cats are often euthanized. Approximately 70% of cats respond to the current standard of care for FCGS, which is full-mouth or near full-mouth tooth extraction. The remaining 30% of cats do not respond to tooth extraction and require lifelong therapy with antibiotics, corticosteroids, and pain medication (refractory FCGS) [7]. Spontaneous disease resolution has not been reported in FCGS-affected cats. The pathogenesis of FCGS is poorly understood but is thought to be due to the host immune system responding inappropriately to chronic oral antigenic stimulation secondary to underlying oral disease or clinical or subclinical viral infections [11–14].
Adult mesenchymal stromal/stem cells (MSCs) are adherent, fibroblast-like, multipotent stem cells [15, 16] that can be isolated from multiple tissue types, including adipose tissue. Adipose-derived MSCs (ASCs) have been isolated from humans and several domestic animal species, including cats [17–23]. Autologous ASCs are nonimmunogenic, safe in people and animals, and have been used clinically in horses and people for more than 8 years with no significant adverse reactions reported other than transient fever in people, occasional transfusion reactions in cats, and self-resolving inflammatory flares in horses [22, 24, 25]. MSCs’ regenerative ability is attributed in part to their ability to modulate both innate and adaptive immune responses [26–30]. MSCs inhibit T-cell proliferation, alter B-cell function, downregulate MHC II on antigen-presenting cells, and inhibit dendritic cell maturation and differentiation [26, 27, 29, 30]. MSCs are being used in phase I–III human clinical trials for inflammatory diseases including Crohn disease and graft versus host disease with variably promising results [26, 31–33]. Like the mentioned human inflammatory mucosal lesions, FCGS is characterized by CD8 T-cell inflammation and a dysregulated immune response [8, 9]. The ability of MSCs to inhibit T-cell proliferation and induce T-cell anergy suggested that FCGS may be a promising disease target for MSC therapy and a possible animal model that mimics chronic human oral lesions.
The purpose of this study was to evaluate the clinical, histopathologic, and immunologic effects resulting from systemic administration of fresh, autologous ASCs in a cohort of cats affected by FCGS that did not respond to previous conventional therapy. We hypothesized that ASC therapy would result in systemic immune modulation, reduction of the inflammatory lesions, and improvement of clinical signs. We found that ASCs administered systemically resulted in complete clinical remission or substantial clinical improvement in five of the seven cats. This improvement was correlated with systemic immune modulation and reduced inflammatory lesions. Cats that responded to ASC therapy had increased percentages of circulating total CD8 T cells and decreased percentages of cytotoxic CD8 T cells with low expression of CD8 (CD8lo) cells prior to therapy, suggesting that circulating CD8+ T cells and CD8 T-cell subsets may be promising biomarkers for patient selection, monitoring response to therapy, and elucidating how ASCs modulate oral inflammation and decrease T-cell activation.
Materials and Methods
Study Population
This study was conducted with approval of the Institutional Animal Care and Use Committee, and the Clinical Trials Review Board, University of California, Davis. All owners signed an informed consent. Nine client-owned cats with refractory FCGS, nonresponsive to full-mouth tooth extractions and immunosuppressive therapies, were recruited to the study. Inclusion criteria included cats affected by FCGS only, with no other primary comorbidities, that did not respond to full-mouth extraction performed at least 6 months before enrollment. If corticosteroid or other immunosuppressive therapy was prescribed, it had to be discontinued 2 weeks prior to and for the entire duration of the trial. Full-mouth dental radiographs were obtained to confirm the absence of retained root tips and to rule out any other underlying pathologies. All cats were screened and tested negative for feline immune deficiency virus and feline leukemia virus infection.
Study Design
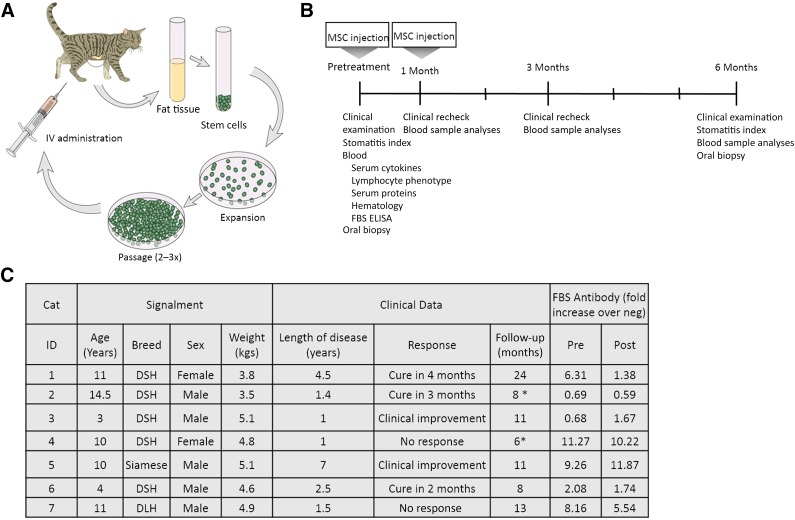
The study design is illustrated in Figure 1. Cats that met the inclusion criteria had subcutaneous abdominal fat collected under general anesthesia. Peripheral blood samples were obtained before treatment and at 1, 3, and 6 months after treatment for a complete blood cell count, serum biochemistry profile, serum protein analyses, lymphocyte phenotyping, and cytokine analyses. In addition, presence of anti-bovine serum albumin (anti-fetal bovine serum [FBS]) antibodies was evaluated before and at 6 months after ASC administration. Oral biopsies were collected before ASC administration (n = 9) and at 6 months after administration (n = 3). Clinical disease severity was evaluated using a Stomatitis Disease Activity Index (SDAI) scoring system [34]. The SDAI scoring was performed at the time of study enrollment and at the exit examination (supplemental online Fig.1) [34]. Briefly, each cat’s owner completed a brief questionnaire and scored the appetite, activity level, grooming behavior, and perceived oral comfort on a scale of 0–3. In addition, 2 veterinary dentists specialists (B.A., F.V.), experienced in FCGS evaluation, scored the severity of oral inflammatory lesions as 0 (no lesion), 1 (mild), 2 (moderate), or 3 (severe). The SDAI score for each cat was calculated at each time point (range: 0, no disease, to 20, severe disease). A final examination was performed at 6 months after the first ASC treatment.
Figure 1.
Images present the study design (A) and timeline (B) as well as signalment and clinical data (C). ∗, Animals are deceased due to unrelated causes. Abbreviations: DSH, domestic shorthair; ELISA, enzyme-linked immunosorbent assay; FBS, fetal bovine serum; neg, negative; post, after treatment; pre, before treatment.
During the study period, the cats received only opioid analgesic management (i.e., buprenorphine or oxymorphone) without any immunosuppressive, antibiotic, or nonsteroidal anti-inflammatory medication. To evaluate the true therapeutic efficacy and safety of autologous ASCs administered systemically, we elected to administer only ASCs and no additional immunosuppressive or antibiotic therapy during the entire 6-month period of the study. Our outcome measures (i.e., lymphocyte subsets, inflammatory parameters) could all potentially be altered by steroid therapy and would confound data analysis. In addition, as the mechanism(s) by which ASCs heal oral tissues and alter immune subsets is unknown, concurrent administration of immunosuppressive agents could alter ASC efficacy. In addition, blood from six cats that presented to the Dentistry and Oral Surgery service for mild dental disease was used to generate reference ranges for variables where robust reference intervals were not available (i.e., CD4 and CD8 numbers and serum IgA).
ASC Isolation and Expansion
ASC isolation and expansion were performed at the Regenerative Medicine Laboratory at the William R. Pritchard Veterinary Medical Teaching Hospital, according to previously established protocols [17]. Briefly, ASCs were cultured in low-glucose Dulbecco’s modified Eagle’s medium (DMEM; Corning Life Sciences, Manassas, VA, http://www.cellgro.com), 10% FBS (HyClone Inc., Logan, UT, http://promo.gelifesciences.com), and 1% penicillin/streptomycin (Thermo Fisher Scientific, Waltham, MA, http://www.thermofisher.com) in tissue culture flasks (Nunc, Roskilde, Denmark, http://www.thermofisher.com) and incubated at 37°C in 5% carbon dioxide. Cells were passaged once they reached approximately 70% confluence. Fresh, expanded, early-passage cells were used for treatment (second or third passage) and the remaining cells were cryopreserved. For the subsequent dose (at 4 weeks after the first dose), an aliquot of first-passage cells were thawed and cultured expanded for 72 hours to regain cell viability and function prior to infusion, effectively using second- or third-passage cells. Cells are provided in glass vials to avoid plastic adherence while awaiting administration.
ASC Phenotyping
Surface protein expression on fetal MSC lines was determined using flow cytometry, as described previously [17]. All antibodies were purchased from the Leukocyte Antigen Biology Laboratory, University of California, Davis (UCD), unless otherwise indicated. Antibodies included MHC II (42.3), CD18 (FE3.9F2), CD90 (CA1.4G8), CD44 (IM7; BioLegend, San Diego, CA, http://www.biolegend.com), and CD105 (SN6; eBioscience, San Diego, CA, http://www.ebioscience.com). For unconjugated antibodies, a mouse IgG-phycoerythrin antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, http://www.jacksonimmuno.com) was used for secondary labeling. Canine CD8a (CA9.JD3), rat immunoglobulin G-allophycocyanin (IgG-APC) (eBR2a; eBioscience), and mouse IgG-APC (MCA928; AbD Serotec, Kidlington, Oxford, UK, http://www.abdserotec.com) were used as isotype controls. Samples were run on a flow cytometer (Cytomics FC500; Beckman Coulter, Brea, CA, http://www.beckmancoulter.com). Flow cytometry data were analyzed using FlowJo flow cytometry software (Tree Star, Ashland, OR, http://company.flowjo.com)
Peripheral Blood Mononuclear Cell Proliferation Assay
Peripheral blood mononuclear cell (PBMC) isolation and mixed leukocyte reactions were carried out as described previously [58] with modifications described in this section. Histopaque 1119 (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) was mixed with Ficoll-Paque (GE Healthcare, Piscataway, NJ, http://www.gelifesciences.com) and diluted with tissue culture water for a final specific gravity of 1.066. This diluted Ficoll-Paque was layered over the Histopaque. Whole blood was diluted with modified Tyrode’s/HEPES buffer containing EDTA (12 mM NaHCO3, 138 mM NaCl, 2.9 mM KCl, 10 mM HEPES, and 1 M EDTA), and layered on top of the diluted Ficoll-Paque layer. The blood was centrifuged and PBMCs were collected and resuspended in activation medium (DMEM plus 10% FBS plus 1% penicillin/streptomycin), and stored on ice until plating. PBMCs were activated with 5 mg/ml concanavalin A (ConA; Sigma-Aldrich). To measure proliferation via 5-bromo-2′-deoxyuridine (BrdU), cells were collected and processed per manufacturer’s instructions (BrdU Flow Kit; BD Biosciences, Franklin Lakes, NJ, http://www.bdbiosciences.com), stained with a viability dye (Fixable Viability Dye eFluor780; eBioscience), and anti-BrdU conjugated to Alexa Fluor647 (clone MoBU-1; Thermo Fisher Scientific), and analyzed on a flow cytometer (Cytomics FC500). Flow cytometry data were analyzed using FlowJo flow cytometry software (Tree Star).
ASC Treatment
Each cat received an ASC transfusion 10–14 days after fat harvest. All cats were admitted 1 day prior to treatment. Intravenous (i.v.) fluid administration (lactated Ringer solution) was initiated at least 30 minutes before treatment. A single dose (2 mg/kg) of diphenhydramine was administered subcutaneously 20 minutes before treatment. Each cat received 2 i.v. transfusions of 20 × 106 (approximately 5 million ASCs per kg) fresh autologous ASCs. Each dose of 20 million ASCs was administered slowly, over a period of 20–30 minutes. Each dose was drawn from the glass vial into the syringes in 4 separate aliquots (approximately 5 million cells at a time) just before administration to prevent ASC adherence to syringe plastic. All cats were hospitalized for 48–72 hours after transfusion to monitor for adverse reactions. Opioid analgesics were administered during hospitalization every 8–12 hours to be consistent with the cat’s opioid analgesic management.
Histology and Immunohistochemistry
Oral biopsies were fixed en bloc in 10% neutral buffered formalin. Transverse sections were embedded in paraffin and 5-µm sections were cut, mounted, and stained with hematoxylin and eosin according to standard laboratory protocols. Immunohistochemistry was performed on 4-µm-thick, formalin-fixed, paraffin-embedded tissue sections, mounted on charged slides and air-dried overnight. Sections were deparaffinized in xylene and rehydrated through graded alcohols to phosphate-buffered saline. Endogenous peroxidases were quenched with 0.3% hydrogen peroxide for 30 minutes in methanol prior to rehydration. Antigen retrieval was performed in Dako Target Retrieval Solution (S1699; Agilent Technologies, Glostrup, Denmark, http://www.dako.com) for 30 minutes at 95°C and then cooled for 20 minutes. Sections were blocked for 20 minutes in 10% normal horse serum in Dulbecco’s phosphate-buffered saline (DPBS). Primary antibodies and dilutions were: rat anti-CD3 (clone 3-12, diluted 1:10; Leukocyte Antigen Biology Laboratory, UCD School of Veterinary Medicine, Davis, CA, http://www.vetmed.ucdavis.edu); and rabbit anti-CD20 (NeoMarker RB-9013-P1; 1:300; Thermo Fisher Scientific). Primary antibodies were incubated for 1 hour, rinsed, and detected with a streptavidin-horseradish peroxidase label (anti-rabbit link, or anti-rat link; 4+ Detection System GR608, or GR607 and HP604, respectively; Biocare Medical, Concord, CA, http://biocare.net). Each reagent was incubated for 10 minutes at room temperature, and two 3-minute rinses occurred between each reagent application. Detection was visualized with Vector NovaRED for peroxidase (SK-4800; Vector Laboratories, Burlingame, CA, http://vectorlabs.com), per the manufacturer’s instructions. Sections were counterstained in Mayer’s hematoxylin. Nonspecific background was evaluated with duplicate sections that received diluent in place of the primary antibody. Biopsies were interpreted by a board-certified veterinary pathologist (N.V.).
Hematology and Protein Analysis
Blood samples were collected into potassium EDTA vacutainers (BD Biosciences). White blood cells, including lymphocytes, and neutrophils, were quantified by an automated analyzer (Bayer ADVIA 120; Bayer Diagnostics, Tarrytown, NY, http://healthcare.bayer.com). Serum was isolated from whole blood collected without an anticoagulant. After clotting, blood was centrifuged (1,000g for 10 minutes) to isolate serum, and aliquots were stored at −80°C until analyzed. Serum biochemical profile was determined by an automated analyzer (Cobas c501; Roche Diagnostics International, Risch, Switzerland, http://www.roche-diagnostics.ch). Samples for serum IgA and IgG (radial immunodiffusion) and protein electrophoresis were shipped on dry ice overnight to the Cornell University Veterinary Diagnostic Laboratory (Ithaca, NY).
Cytokine Enzyme-Linked Immunosorbent Assays
Interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 were measured in serum before and at 1, 3, and 6 months after the first ASC therapy, using feline-specific enzyme-linked immunosorbent assay (ELISA) kits (Duosets; R&D Systems, Minneapolis, MN, http://www.rndsystems.com). ELISA kits were run following the manufacturer's instructions except that samples were diluted by at least one-quarter in reagent diluent to dilute out serum effects. All ELISA samples were read on a Synergy HT Multi-Mode microplate reader with Gen5 software (Biotek, Winooski, VT, http://www.biotek.com).
Anti-Bovine Serum Albumin ELISA
The anti-bovine serum albumin (BSA) ELISA was adapted from Gershwin et al. [35]. ELISA plates were coated with 1 μg of BSA (Thermo Fisher Scientific) in 100 μl of carbonate-bivarbonate buffer (Fraction V; Thermo Fisher Scientific) in a carbonate-bicarbonate buffer (63.5 mM carbonate, pH 9.5), overnight at 4°C. The wells were then blocked by adding 100 µl of 1% rabbit serum albumin (Sigma-Aldrich) in DPBS (Thermo Fisher Scientific) to each well, followed by incubation at 37°C for 1 hour. The wells were washed with DPBS plus 0.1% Tween 20 (“wash buffer”; EMD Millipore, San Diego, CA, www.emdmillipore.com) once for 10 minutes, followed by 6 brief washes. Feline serum samples were diluted 1:5,000 in wash buffer and plated in triplicate (100 µl per well) Known negative and highly positive feline samples were run as assay controls. Plates were incubated at 37°C for 1 hour, then washed as described above. Rabbit anti-feline IgG heavy and light chain (H&L)-HRP, 100 µl (Southern Biotech, Birmingham, AL, http://www.southernbiotech.com) diluted 1:10,000 was added to each well. The plates were incubated at 37°C for 1 hour, then washed as above. Freshly prepared 3,3′,5,5′-tetramethylbenzidine peroxidase substrate, 100 µl (KPL, Gaithersburg, MD. http://www.kpl.com) was added to each well and plates were incubated at room temperature in the dark until color developed. The colorimetric reaction was stopped with 100 µl of 2N H2SO4, and plates were read at 450–540 nm on a Synergy HT Multi-Mode microplate reader with Gen5 software (Biotek). The increase in color relative to the negative control sample was determined for each patient sample.
Flow Cytometry
We labeled 100 μl aliquots of whole blood (EDTA) with 25 μl mouse anti-feline CD4 (clone FE1.7B12), CD8-α (clone FE1.10E9), or CD21 (clone CA2.1D6) (Leukocyte Antigen Biology Laboratory). Red blood cells were lysed with an ammonium chloride lysing buffer (154 mM ammonium chloride, 10 mM potassium bicarbonate, 100 μM EDTA, pH 7.2). Cells were pelleted and washed twice in flow buffer (DPBS with 1% equine serum [HyClone]; 5 mM EDTA, pH 7.2; and 0.01% sodium azide). Antibodies were detected using a phycoerythrin-conjugated donkey anti-mouse IgG H&L F(ab′)2, diluted 1:50 in flow buffer (Jackson Immunotech). Samples were read on a Beckman Coulter FC500 Flow Cytometer with Cytomics software , and analyzed using FlowJo software (Tree Star). Approximately 20,000 events were collected within a lymphocyte scatter gate, and cell fluorescence was analyzed for cells within this gate.
Statistical Analyses
Data analysis was performed using GraphPad Prism version 6.05 software (GraphPad Software, San Diego, CA, http://www.graphpad.com). Statistical significance between two groups was determined by two-tailed paired t tests (stomatitis index, biomarkers), and between time points by two-way analysis of variance (cytokine and T-cell phenotype analyses). The number of cats in the nonresponding group (n = 2) prohibited accurate statistical analyses comparing responder cats to nonresponders for most parameters. As such, basic descriptive statistics were used in these situations. p values <.05 were considered statistically significant.
Results
Feline ASCs Have a Typical MSC Surface Phenotype and Inhibit Lymphocyte Proliferation In Vitro
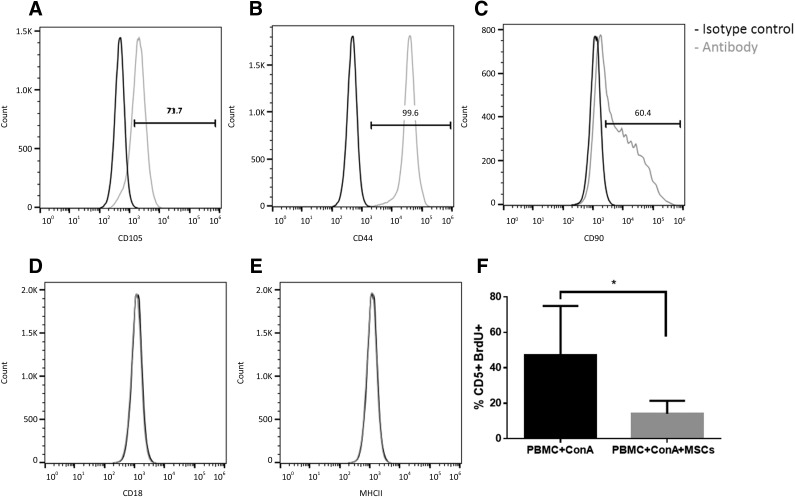
Feline ASCs were uniformly positive for CD105, CD44, and CD90, and negative for CD18 and MHCII (Fig. 2A–2E). ASCs significantly suppressed T-cell proliferation when stimulated with ConA in coculture with allogeneic PBMCs (n = 5; p = .03) (Fig. 2F), as measured by BrdU incorporation.
Figure 2.
Feline adipose-derived mesenchymal stem cells (ASCs) expressed surface markers consistent with an MSC phenotype. They were CD105+ (A), CD44+ (B), CD90+ (C), CD18− (D), and MHC II− (E). (F): They also suppressed proliferation of activated PBMCs in mixed leukocyte reactions experiments (n = 5; p = .03). Abbreviations: BrdU, 5-bromo-2′-deoxyuridine; ConA, concanavalin A; MSC, mesenchymal stem cell; PBMC, peripheral blood mononuclear cell.
ASC Treatment Induced Marked Clinical Improvement in Cats With FCGS
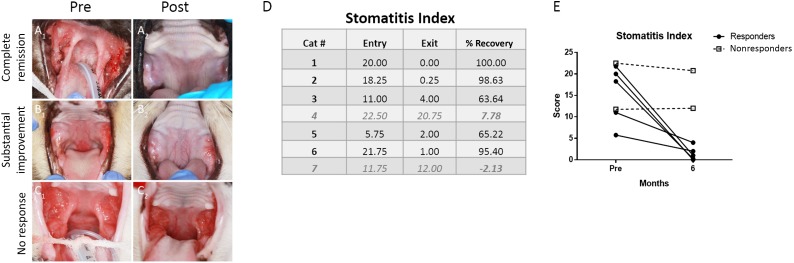
Nine cats were enrolled in the study; however, 2 cats discontinued the clinical trial within the first 2 months of the study because of the owner’s decision to administer corticosteroids to the cats. Seven cats completed the study and their signalment is presented in Figure 1. All cats had full-mouth tooth extractions and were considered nonresponsive to any therapeutic intervention. All had chronic, severe mucosal inflammation in the caudal oral cavity and various other locations within the oral cavity, with a disease duration of 1–7 years (mean: 2.7 years). During treatment, two cats experienced immediate transfusion reactions characterized by rapid respiration, urination, vomiting, and apathy. These reactions were infusion-rate dependent and spontaneously resolved within 10–15 minutes, after which the transfusion was completed at a slower rate (2 million cells per minute). No other short- or long-term adverse reactions were noted in any of the cats. Of the 7 cats, 5 responded to treatment and exhibited either complete clinical remission (n = 3) or substantial clinical improvement (n = 2) within 1–4 months of the first ASC administration (Fig. 3A1, 3A2, 3B1, 3B2). Two cats had either minimal or no clinical response (Fig. 3C1, 3C2).
Figure 3.
Clinical measure of disease severity. All cats had severe oral mucosal inflammation at the caudal oral cavity (A1, B1, C1). Clinical response among the responder cats was characterized by complete clinical remission (A2) in three cats and substantial clinical improvement in two cats (B2). No response was observed in two cats (C2). The stomatitis activity disease index (SDAI) was used to score disease severity of all cats that completed the study. (D): Table showing the SDAI scores at entry and at exit of the study with a calculation of percent recovery. Data for nonresponders are in gray and italicized type (cats 4 and 7). (E): Graph of SDAI scores at entry and exit indicating five responding cats (black circles) and two nonresponding cats (open boxes). Abbreviations: Post, after treatment; pre, before treatment.
Clinical assessment of disease severity, by means of the SDAI, confirmed our clinical observations (Fig. 3D, 3E). In general, the improvement of clinical signs corresponded with improvement of the oral mucosal lesions. The responder cats began eating more, gaining weight, resuming grooming behavior, and resuming sociability. The owners reported a return to pre-FCGS activity levels in four responder cats and near return to pre-FCGS activity in one responder cat. The two cats that did not respond had static SDAI and the owners reported the same activity levels as with historic immunosuppressive therapy. There was no significant difference between responder and nonresponder stomatitis index before treatment, indicating that clinical severity does not predict response (Fig. 3D, 3E).
Clinical and Histopathologic Correlation Between Responders and Nonresponder Cats
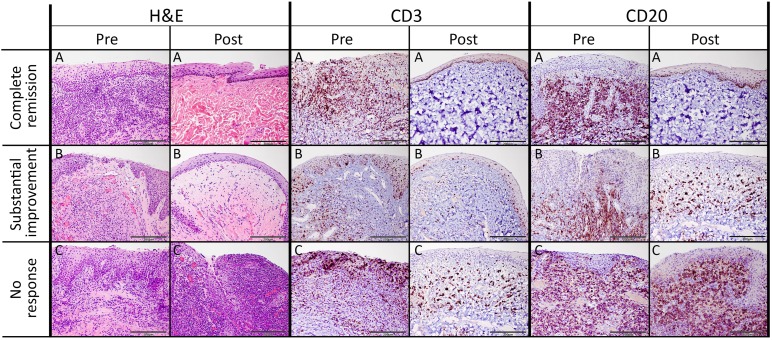
Oral mucosal biopsies were obtained from all cats prior to study enrollment. Post -SC treatment oral biopsy specimens were available from one cat that achieved complete clinical remission, one cat that exhibited substantial improvement, and one cat that did not respond to treatment. All biopsy specimens were analyzed histologically and post-treatment biopsy specimens were compared with the corresponding pretreatment biopsy. All cats that showed improvement in SDAI clinical scores also exhibited improved tissue inflammation on histopathologic examination (Fig. 4).
Figure 4.
Histologic and immunohistochemical evaluation of the feline chronic gingivostomatitis oral mucosa. Histomorphology of sections from all patients pretreatment was consistent with severe lymphoplasmacytic and neutrophilic stomatitis accompanied by epithelial hypertrophy and multifocal ulcerations ([A–C], H&E column). Pretreatment CD3+ T cells were present within the epithelium and submucosa, and CD20+ B cells were mainly present within the subepithelial stroma. Sections obtain from a cat with complete clinical remission ([A], Post columns) had histology consistent with normal mucosa. Histomorphology of sections from a substantial responder ([B], Post columns) was consistent with mild lymphoplasmacytic stomatitis accompanied by mild epithelial hyperplasia and superficial stromal edema. After treatment, moderate numbers of CD3+ T cells were observed within the epithelium and stroma in sections form partial responders, and moderate numbers of CD20+ T cells were located in the subepithelial stroma. Histomorphology of sections obtained from a nonresponder cat after treatment ([C], Post columns) was consistent with severe chronic lymphoplasmacytic and neutrophilic ulcerative stomatitis, which was histologically similar to treatment. Distribution of CD3+ and CD20+ cells was similar to that observed before treatment. Scale bar = 200 μm. Abbreviations: H&E, hematoxylin and eosin; post, after treatment; pre, before treatment.
In all pretreatment biopsy specimens, the epithelium and subepithelial stroma were heavily infiltrated by lymphocytes, plasma cells, and neutrophils, with occasional Mott cells, mast cells, and histiocytes. The surface epithelium was multifocally ulcerated and hyperplastic, with multiple rete pegs extending deep into the subjacent stroma. Immunohistochemistry revealed that CD3+ cells were present within the epithelium and subepithelial stroma, whereas CD20+ cells were restricted to the subepithelial stroma (Fig. 4).
Upon completion of the study, a complete return to normal tissue architecture was observed in a cat with complete clinical remission. Rare lymphocytes were observed within subepithelial stroma, with no evidence of epithelial hyperplasia, ulceration, or inflammation. The absence of CD3 and CD20 lymphocytes was confirmed by immunohistochemistry (Fig. 4A).
There was mild lymphoplasmacytic infiltration in the oral mucosal sections from a cat with substantial clinical improvement. There was no evidence of neutrophilic inflammation or ulceration. Occasional rete pegs were present and superficial stroma was moderately expanded by edema. Moderate numbers of CD3+ T cells were scattered randomly within the epithelium and the stroma, whereas moderate numbers of CD20+ B cells were primarily located in the subepithelial stroma (Fig. 4B).
Histologic sections of oral mucosa from a nonresponsive cat after treatment were consistent with severe, chronic, lymphoplasmacytic and neutrophilic ulcerative stomatitis, which was identical to the findings before treatment. There were moderate numbers of randomly scattered CD3+ T cells intraepithelially and within the subepithelial stroma. The subepithelial stroma was densely and diffusely infiltrated by CD20+ B cells (Fig. 4C).
ASC Administration Modulates Immune Cell Subsets: Cats With FCGS Have High Circulating CD8+ T Cells That Normalize With Therapy
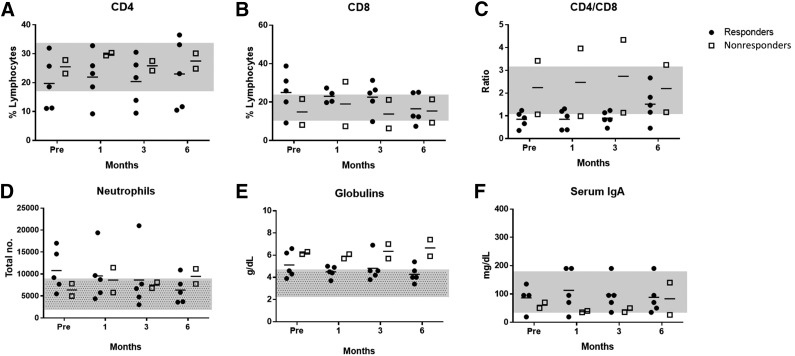
Cats with FCGS typically have systemic evidence of inflammation, including blood neutrophilia, polyclonal hypergammaglobulinemia, and increased expression of proinflammatory serum cytokines [9, 36]. The cats in this study recapitulated this phenotype. Neutrophil counts were generally elevated above the reference interval in responding cats (responders pre-injection: 10.7 × 103 ± 4.8 × 103; reference range: 2.0 × 103–9.0 × 103) and returned to normal or near-normal levels within 6 months after the first injection (6 months: 6.3 × 103 ± 3.0 × 103) (Fig. 5A), whereas nonresponding cats showed no change in neutrophil number and remained in or near the reference interval throughout the study (pre-injection: 6.4 × 103 ± 2.0 × 103; 6 months: 9.5 × 103 ± 2.4 × 103) (Fig. 5A). Nonresponding cats had higher total serum globulin levels and lower serum IgA levels than responding cats; however, neither group showed a change after ASC therapy (Fig. 5B, 5C).
Figure 5.
Circulating inflammatory cells and serum immunoglobulins. Flow cytometric analysis of whole blood for CD4 (A), CD8 (B), and neutrophils (D), including the calculated ratio of CD4 to CD8 T cells (C). Globulin (E) and IgA (F) concentrations were measured in serum. (D, E): Patterned gray bars represent standard reference intervals obtained from University of California, Davis, reference intervals. (A–C, F): Plain gray bars represent the range values of control cats (n = 6), which were used for parameters that did not have standard reference intervals. Abbreviations: Pre, before treatment.
Most cats had normal percentages of circulating CD4+ T cells (with 2 cats having decreased percentages) and this did not change over 6 months regardless of clinical response (Fig. 5D). In contrast, in 3 of 5 cats that responded to ASC therapy, baseline circulating CD8+ T-cell levels were elevated above the reference range (Fig. 5E), resulting in a low CD4/CD8 ratio in 4 of the 5 responder cats at time 0 (Fig. 5F). CD8+ T cells remained high 1 month after the first injection (responders pre-injection: 29% ± 18.57%, non-FCGS control group: 16% ± 4.18%). However, by 6 months post-ASC treatment, the percentage of CD8 T cells had nearly normalized in all responder cats, with a resultant normalization of the blood CD4/CD8 ratio by 6 months in 4 of the 5 responder cats (Fig. 5E, 5F). One of the nonresponding cats showed a consistently low percentage of CD8 T cells and high CD4/CD8 ratio throughout. Interestingly, this cat had a very high B-cell count in peripheral blood samples. The other nonresponder showed higher than normal CD8 percentage and low CD4/CD8 ratio, which did not change throughout the study (Figs. 5E, 5F).
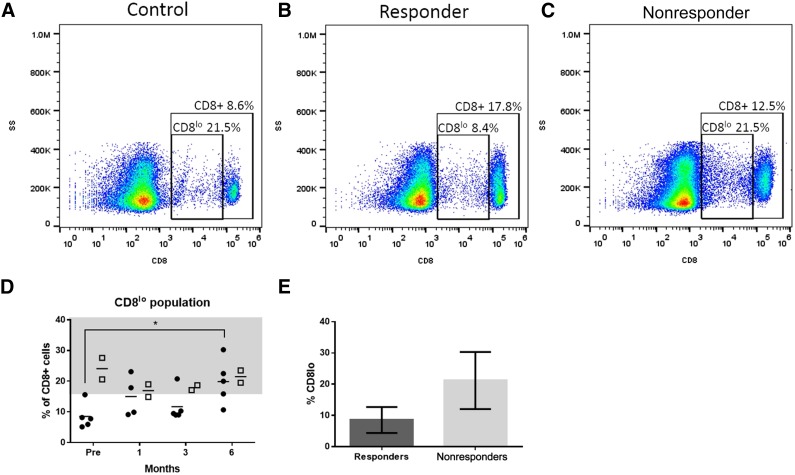
Increased Percentage of CD8lo Cells in the Blood of Cats With FCGS Predicts Response to MSC Infusion
Circulating CD8+ T cells were further examined to identify a population of CD8+hi and a population of CD8+lo cells (Fig. 6A, 6B). A majority (>80%) of these CD8lo cells were CD5+, confirming that they were CD8lo T cells (data not shown). Cats that responded to ASC therapy had a significantly lower percentage of CD8lo cells (as a percentage of total CD8+ T cells) than normal cats and cats that did not respond to therapy (Fig. 6A–6C). By 6 months after the first injection, the CD8lo population increased significantly, such that 3 of the 5 responder cats were within the control range and were no longer significantly different from the nonresponders (Fig. 6D). The level of <15% CD8lo cells in the blood of cats with FCGS was 100% predictive of response to ASC therapy in this small cohort.
Figure 6.
Low numbers of a subset of CD8+ cells (CD8lo) predict response to adipose-derived mesenchymal stem cell (ASC) therapy and normalize after treatment. CD8+ T cells can be divided into CD8hi and CD8lo populations. (A–C): Representative flow cytometry gating scheme for CD8+ and CD8lo populations for (A) control non-FCGS cat, (B) responder feline chronic gingivostomatitis (FCGS)-affected cat, and (C) nonresponder FCGS-affected cat, including the percent of total lymphocytes that were CD8+, and the percent of CD8+ cells that were CD8lo. (D): Summary of CD8lo percentages of CD8+ cells based on flow cytometry gates described in (A–C) for all cats in the study. Responder cats had a lower percentage of CD8lo cells before ASC treatment than at 6 months after (multiple comparisons 2-way analysis of variance, p < .05). (E): Comparison of the percent of CD8+ cells that were CD8lo in responder and nonresponder groups before ASC treatment for use as a biomarker to predict response to treatment. Responders had a lower percentage of CD8+ cells that were CD8lo than nonresponders (unpaired t test, p = .04).
Cats With FCGS That Responded to ASC Transfusion Had Increased Serum IL-6 Levels
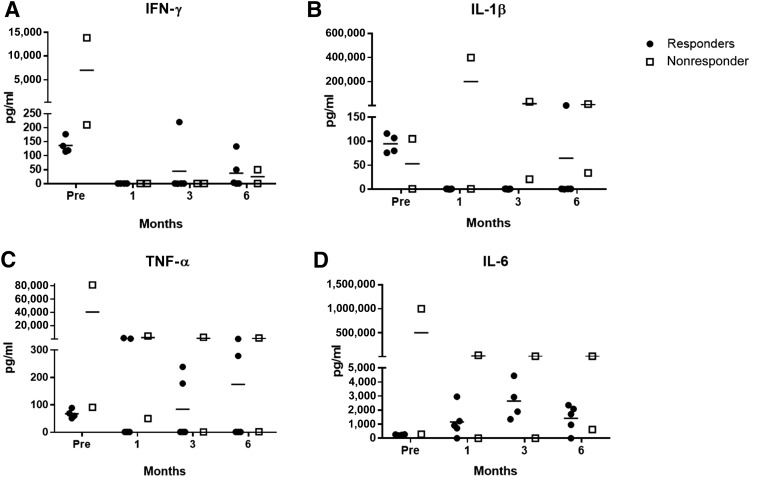
The levels of the proinflammatory serum cytokines TNF-α, IL-1β, and IFN-γ were generally elevated in all cats prior to ASC transfusion (Fig. 7). One of the nonresponding cats exhibited a marked proinflammatory cytokine profile, indicative of a severe systemic inflammatory state, throughout the study with only IFN-γ normalizing (Fig. 7). Although an outlier, we elected to include this cat, given the small number of patients in this pilot study. The other nonresponding cat showed very low levels of all cytokines (Fig. 7). Both IFN-γ and IL-1β levels decreased in most cats after the first ASC injection and stayed low or undetectable with variable, small increases in IFN-γ (n = 2) and IL-1β (n = 1) at later time points (Fig. 7A, 7B). TNF-α was more variable, with three responder cats showing decreased TNF-α levels, whereas two maintained high serum concentrations (Fig. 7C). Interestingly, the two cats that achieved complete clinical cure maintained high serum TNF-α levels throughout the study. Interestingly, serum IL-6 was unmeasurable at baseline in all but the one proinflammatory, nonresponding outlier cat. Response to therapy was associated with an increase in serum IL-6 level in all responder cats by 3 months that plateaued through the 6-month time point (Fig. 7D).
Figure 7.
Plots of serum cytokine data. Serum cytokines were variably detectable in all cats in the study. Enzyme-linked immunosorbent assay results for serum levels of IL-6 (A), IFN-γ (B), TNF-α (C), and IL-1β (D). Note that one nonresponder showed very high expression of most serum cytokines, indicating a severe systemic proinflammatory state. Abbreviations: IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
BSA Antibody Levels
Feline ASCs were cultured in FBS and, as such, we wanted to determine (a) whether cats developed antibodies to BSA, the primary protein in FBS; and (b) if the development of antibodies was associated with treatment failure. All cats had varying amounts of BSA antibody before ASC administration; however, titers did not change after cell administration (i.e., they stayed high or stayed low) and did not correlate with the response (or absence of a response) to ASC therapy.
Discussion
This is the first study to investigate the use of ASCs for treatment of severe oral mucosal inflammatory disease in a naturally occurring animal model. We found that systemically administered fresh, autologous ASCs can achieve cure or substantial reduction in inflammatory lesions associated with FCGS, resulting in regeneration of the oral mucosa and improved clinical signs. Although this safety and efficacy study did not explicitly include a no-treatment control group, spontaneous recovery or even significant clinical improvement has never been reported in cats with refractory FCGS. Cats that responded to therapy had a skewed peripheral blood CD4/CD8 ratio due to increased circulating CD8+ cells, with an increased percentage of CD8lo cells being highly predictive of response to MSC therapy. Full clinical response to therapy was delayed, generally took 2–4 months after the first ASC transfusion, and followed an increase in serum IL-6 concentration (and, for those cats with complete cure, a concurrent increase in serum TNF-α level) with a resultant normalization of the CD4/CD8 ratio, a reduction in blood neutrophil number, and a general reduction of the proinflammatory serum cytokines, IFN-γ and IL-1β. Transfusion reactions were rare and were transfusion-rate dependent. Cats had varying amounts of anti-BSA antibody before ASC administration that did not change after cell administration and did not correlate with the presence or absence of response to therapy.
In this study, we demonstrated that i.v. infusion of a relatively high dose (20 million cells per cat, equivalent to approximately 5 million cells per kg) of autologous ASCs is safe and well tolerated in cats with severe oral inflammatory disease. During cell administration, we observed rare transfusion reactions that were dependent on the rate of cell administration. A safe infusion rate was determined (2 million cells per minute) that eliminated these reactions. Cats that had transfusion reactions recovered rapidly and were no less likely to respond to ASC treatment than those cats that did not exhibit transfusion reactions. The reactions were independent of anti-BSA antibody titers. Our findings suggest that previous FBS exposure via vaccination does not predispose animals to react to autologous i.v. stem cell injections with cells grown in FBS-containing media. Transfusion reactions have been previously reported in a study where cats received low doses of ASCs [22]. However, there were differences in our studies that might explain the relatively minor transfusion reactions we noted that were easy to eliminate by slowing the transfusion. For example, cats in the other study received a substantially lower dose of cells; however, the cells were allogeneic, had been frozen in dimethyl sulfoxide (DMSO)/FBS, and were not culture expanded after cryopreservation before administration [22].
One objective of this study was to determine if a biomarker could be found that could both predict response to ASC therapy and help reduce the list of possible mechanism(s) by which ASCs work to heal oral inflammation that is secondary to chronic antigenic stimulation and characterized by T-cell activation in tissues. We found that circulating CD8+ T cells were elevated in most cats with FCGS prior to ASC treatment (hence, these cats had a skewed CD4/CD8 ratio) and, of these CD8+ cells, very few were CD8lo. In fact, a decreased CD8lo percentage within the CD8+ blood cells was 100% predictive of response to ASC therapy.
CD8lo cells have been characterized in humans, mice, and cats. These cells are associated with chronic viral infections such as Epstein-Barr virus [37] and HIV in humans, and feline immunodeficiency (FIV) in cats [38–40], as well as chronic antigen stimulation in a mouse skin-graft model. In cats, CD8lo cells express CD44, CD49d, and CD18, as well as high MHCII and low or absent CD62L, consistent with an activated phenotype [38–40]. They have strong anti-FIV suppressor activity [38] and can be used as a biomarker to differentiate cats with FIV infection from FIV-vaccinated cats [41]. Receptor expression on CD8 cells may dictate whether a CD8 cell has a suppressive or cytotoxic phenotype. Decreased receptor expression of CD8 limits the ability of cells to be classically activated to a cytotoxic phenotype and increases suppressive functions. These CD8lo cells likely represent a subset of activated CD8 effector/suppressor cells capable of downregulating the activation of naïve T cells and resulting from repeated and/or continuous exposure to self-antigen. The decreased percentage of CD8lo cells in cats with FCGS that responded to ASC therapy may imply that these cats have decreased suppressor function and that ASC administration supports the expansion of CD8lo cells helping to resolve the inflammatory oral response. In nonresponders, these cells are not lacking before treatment and, thus, ASCs do not induce a response via this mechanism.
ASC administration resulted in complete clinical remission or substantial clinical improvement in five of seven cats with FCGS that completed the study. This clinical improvement was associated with histologic clearing of both CD3+ T cells and CD20+ B cells from the lesions of responder cats. If we had screened for CD8 predominance in blood and a concurrent decrease in CD8lo cells, we could have predicted with 100% accuracy the response to therapy in this small cohort of cats. The elevation of CD8+ T cells found in circulation support the theory that FCGS is an inappropriate response to chronic, oral, antigenic stimulation from clinical/subclinical viral infections [11–14]. In the case of FCGS, it is likely that CD8+ T cells are contributing to the inflammatory lesions, and we observe correlation between the reduction of CD8+ T cells in circulation and the reduction in clinical signs and histologic markers of inflammation. A predominance of CD8+ T cells has been reported previously, suggestive of an underlying cytotoxic cell-mediated immune response [9]. We did not collect frozen tissue samples in this cohort of cats; therefore, we were unable to discriminate between CD4+ and CD8+ tissue cells in this study.
Our data suggest that ASC administration to cats with FCGS has immunomodulatory effects. Aside from the reduction in CD8+ T cells, responding cats show a reduction in the systemic inflammatory response following ASC treatment, including reduced levels of circulating neutrophils and serum IFN-γ and IL-1β. The cats that responded with complete clinical remission showed a sustained increase in serum TNF-α levels and all responder cats showed elevation in serum IL-6 concentration. IL-6 is a pleiotropic cytokine involved in pro- and anti-inflammatory processes [42]. In humans, IL-6 induces IL-21 production under Th1 priming conditions, which promotes Th17 differentiation [43–45]. However, IL-21 also promotes IL-10 secretion and inhibits IFN-γ production in the developing Th17 cells, preventing the generation of pathogenic Th1/Th17 effector cells. IL-21 also inhibits the differentiation of CD8+ T cells. This inhibition of excessive CD8+ T-cell differentiation, leading to functional T-cell exhaustion, may be important for the protective role of IL-21 in chronic viral infections [46–50].
IL-6 is a major regulator of the balance between regulatory T cells (Tregs) and effector Th17 cells [51]. Th17 cells are key players in the pathogenesis of autoimmune diseases, whereas Tregs maintain tolerance and restrain excessive effector T-cell responses [51]. IL-6, together with transforming growth factor (TGF)-β, induces Th17 cells, whereas IL-6 inhibits TGF-β-induced Treg differentiation [52–56]. However, this effect of IL-6 seems to be restricted to the development of naïve CD4 T cells, whereas transgenic mice in which serum IL-6 levels are constitutively elevated actually have more Foxp3+ natural Tregs than nontransgenic mice, and those Tregs normally suppress proliferation of naïve T cells in vitro [57]. Hence, in the context of chronic immune activation, this function of IL-6 is likely more relevant. ASCs constitutively express TGF-β, whereas IL-6 is expressed upon activation with phytohemagglutinin [58]. IL-6 is secreted by ASCs from all species tested to date, including horses, dogs, and cats [58, 59]. Ongoing work in our laboratory is focused on both in vitro and in vivo exploration of Th17 and Treg cells in cats.
Clinical trials using MSC products have often favored frozen aliquots of allogeneic cells for trials focused on chronic inflammatory lesions or acute ischemic lesions because of the relative ease of US Food and Drug Administration approval for “lots” of frozen cells and the advantage of an “off-the-shelf” product that can be administered without the lag time associated with cell expansion. Recently, however, it has been shown that 2–3 days of culture and stem cell expansion for patients receiving allogeneic stem cell transfusions may increase cell viability and cell activation readiness [60]. In addition, the use of DMSO as a cryoprotectant may account for the higher incidence of transfusion reactions seen with frozen cells and potentially interfere with therapeutic outcomes [60, 61]. Our group favors fresh cells to frozen cells for therapeutic administration and we believe much of our efficacy data in cats with FCGS and in other ongoing clinical trials are related to the infusion of freshly expanded cells.
There remains controversy and a paucity of data, in any given species, to support the use of autologous versus allogenic stem cells in terms of efficacy and outcome [60, 61]. There is some evidence that i.v. administration of human MSCs may induce an inflammatory cell response [62–64] and it has been shown in horses that completely unmatched allogeneic cell infusion can result in anti-MSC antibody production, although the significance of antibody development is not known [25]. Although the convenience and practicality of using allogeneic cell sources for treatment in the clinical setting is attractive, we elected to use autologous cells as our first line of therapy to maximize the potential for efficacy while minimizing interference from an immune response. In our hands, the autologous cells were safe and efficacious for this particular disease process and, given that the disease is chronic, the time needed for cell expansion does not hinder patient health. Additional investigation using fresh, allogeneic cells to treat this disease is ongoing. It appears that the use of allogeneic cells may be permissible in some animal species, even when administered systemically, whereas the use of allogeneic cells may be less feasible in other species. As with the issue of fresh versus frozen stem cells, there is a lack of long-term, randomized, placebo-controlled studies to make definitive statements. However, as FCGS is a common disease with limited therapeutic options, approaching these questions in a naturally occurring model of disease becomes practical.
Response to ASC therapy was often delayed by up to 4 months after the first injection, even in cats who fully responded. This delayed response may be due to ASCs acting through a systemic mechanism that prevents activation of new T cells or slowly provides regulatory signals to activated cells, which would then reveal clinical response only as activated pathogenic cells undergo apoptosis or reach an exhausted state. This delayed response is important for clinicians to note to avoid a premature conclusion that therapy was ineffective.
Conclusion
In summary, the i.v. injection of ASCs in cats diagnosed with FCGS that have not responded to conventional therapy is safe and >70% effective. Cell therapy was most effective in cats with decreased percentages of blood CD8lo cells. Successful ASC therapy resulted in (a) complete clinical remission or reduction in clinical disease severity, (b) histologic resolution of the oral lesions, (c) reduction of total circulating CD8+ T cells (and increased CD8lo cells), (d) resolution of neutrophilia and reduction of serum proinflammatory cytokine concentrations (IL-1β and IFN-γ), and (e) increases in serum IL-6 levels. Although the full mechanisms by which ASC treatment reduces inflammation in this model remain to be elucidated, this study demonstrates the clinical potential of ASC therapy for oral inflammatory lesions characterized by CD8+ cells and T-cell activation. We also identified a potentially useful biomarker that could both dictate patient enrollment (decreased percentage of CD8lo cells) as well as shed light on the mechanisms by which ASCs modulate healing in these inflammatory lesions. These results are encouraging both for the treatment of a refractory, severe lesion in cats, and as a potentially translatable therapy for the treatment of human oral inflammatory disease.
Supplementary Material
Acknowledgments
Financial support for this study was provided by NIH Grant 1R21DE024711-01, the WINN Feline Foundation for a Miller Trust grant, and the George and Phyllis Miller Feline Health Trust of the San Francisco Foundation, and it was administered by the Center of Companion Animal Health, UCD. The SDAI used in this study is a modified version of the SDAI originally developed by Dr. Jamie Anderson. Finally, we thank John Doval for assistance with the figures. This study was performed at the Department of Surgical and Radiological Sciences and the Department of Pathology, Microbiology and Immunology, School of Veterinary Medicine, University of California, Davis.
Author Contributions
B.A.: conception and design, financial support, provision of study material or patients, manuscript writing, data analysis and interpretation; E.M.-K.: manuscript writing, data analysis and interpretation; F.J.M.V. and M.R.B.: provision of study material or patients, collection and/or assembly of data, final approval of manuscript; A.K., N.F., W.J.M., and N.V.: data analysis and interpretation, final approval of manuscript; N.J.W.: collection and/or assembly of data, data analysis and interpretation; D.L.B.: conception and design, financial support, provision of study material or patients, manuscript writing, data analysis and interpretation.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Alpsoy E, Akman-Karakas A, Uzun S. Geographic variations in epidemiology of two autoimmune bullous diseases: Pemphigus and bullous pemphigoid. Arch Dermatol Res. 2015;307:291–298. doi: 10.1007/s00403-014-1531-1. [DOI] [PubMed] [Google Scholar]
- 2.Joly P, Litrowski N. Pemphigus group (vulgaris, vegetans, foliaceus, herpetiformis, brasiliensis) Clin Dermatol. 2011;29:432–436. doi: 10.1016/j.clindermatol.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Di Stasio D, Guida A, Salerno C, et al. Oral lichen planus: A narrative review. Front Biosci (Elite Ed) 2014;6:370–376. doi: 10.2741/E712. [DOI] [PubMed] [Google Scholar]
- 4.Omar AA, Hietanen J, Kero M, et al. Oral lichen planus and chronic junctional stomatitis: Differences in lymphocyte subpopulations. Acta Odontol Scand. 2009;67:366–369. doi: 10.1080/00016350903136605. [DOI] [PubMed] [Google Scholar]
- 5.Lavanya N, Jayanthi P, Rao UK, et al. Oral lichen planus: An update on pathogenesis and treatment. J Oral Maxillofac Pathol. 2011;15:127–132. doi: 10.4103/0973-029X.84474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girard N, Servet E, Biourge V, et al. Periodontal health status in a colony of 109 cats. J Vet Dent. 2009;26:147–155. doi: 10.1177/089875640902600301. [DOI] [PubMed] [Google Scholar]
- 7.Jennings MW, Lewis JR, Soltero-Rivera MM, et al. Effect of tooth extraction on stomatitis in cats: 95 cases (2000-2013) J Am Vet Med Assoc. 2015;246:654–660. doi: 10.2460/javma.246.6.654. [DOI] [PubMed] [Google Scholar]
- 8.Arzi B, Murphy B, Cox DP, et al. Presence and quantification of mast cells in the gingiva of cats with tooth resorption, periodontitis and chronic stomatitis. Arch Oral Biol. 2010;55:148–154. doi: 10.1016/j.archoralbio.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Harley R, Gruffydd-Jones TJ, Day MJ. Immunohistochemical characterization of oral mucosal lesions in cats with chronic gingivostomatitis. J Comp Pathol. 2011;144:239–250. doi: 10.1016/j.jcpa.2010.09.173. [DOI] [PubMed] [Google Scholar]
- 10.Healey KA, Dawson S, Burrow R, et al. Prevalence of feline chronic gingivo-stomatitis in first opinion veterinary practice. J Feline Med Surg. 2007;9:373–381. doi: 10.1016/j.jfms.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedersen NC. Inflammatory oral cavity diseases of the cat. Vet Clin North Am Small Anim Pract. 1992;22:1323–1345. doi: 10.1016/S0195-5616(92)50130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowers KL, Hawley JR, Brewer MM, et al. Association of Bartonella species, feline calicivirus, and feline herpesvirus 1 infection with gingivostomatitis in cats. J Feline Med Surg. 2010;12:314–321. doi: 10.1016/j.jfms.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennet PR, Camy GA, McGahie DM, et al. Comparative efficacy of a recombinant feline interferon omega in refractory cases of calicivirus-positive cats with caudal stomatitis: A randomised, multi-centre, controlled, double-blind study in 39 cats. J Feline Med Surg. 2011;13:577–587. doi: 10.1016/j.jfms.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lommer MJ, Verstraete FJ. Concurrent oral shedding of feline calicivirus and feline herpesvirus 1 in cats with chronic gingivostomatitis. Oral Microbiol Immunol. 2003;18:131–134. doi: 10.1034/j.1399-302x.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 15.Borjesson DL, Peroni JF. The regenerative medicine laboratory: Facilitating stem cell therapy for equine disease. Clin Lab Med. 2011;31:109–123. doi: 10.1016/j.cll.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 17.Arzi B, Kol A, Murphy B, et al. Feline foamy virus adversely affects feline mesenchymal stem cell culture and expansion: implications for animal model development. Stem Cells Dev. 2015;24:814–823. doi: 10.1089/scd.2014.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beggs KJ, Lyubimov A, Borneman JN, et al. Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant. 2006;15:711–721. doi: 10.3727/000000006783981503. [DOI] [PubMed] [Google Scholar]
- 19.Carrade DD, Borjesson DL. Immunomodulation by mesenchymal stem cells in veterinary species. Comp Med. 2013;63:207–217. [PMC free article] [PubMed] [Google Scholar]
- 20.Martinello T, Bronzini I, Maccatrozzo L, et al. Canine adipose-derived-mesenchymal stem cells do not lose stem features after a long-term cryopreservation. Res Vet Sci. 2011;91:18–24. doi: 10.1016/j.rvsc.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Quimby JM, Webb TL, Gibbons DS, et al. Evaluation of intrarenal mesenchymal stem cell injection for treatment of chronic kidney disease in cats: A pilot study. J Feline Med Surg. 2011;13:418–426. doi: 10.1016/j.jfms.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quimby JM, Webb TL, Habenicht LM, et al. afety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: Results of three sequential pilot studies. Stem Cell Res Ther. 2013;4:48. doi: 10.1186/scrt198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vieira NM, Brandalise V, Zucconi E, et al. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010;19:279–289. doi: 10.3727/096368909X481764. [DOI] [PubMed] [Google Scholar]
- 24.Kang MH, Park HM. Evaluation of adverse reactions in dogs following intravenous mesenchymal stem cell transplantation. Acta Vet Scand. 2014;56:16. doi: 10.1186/1751-0147-56-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kol A, Wood JA, Carrade Holt DD, et al. Multiple intravenous injections of allogeneic equine mesenchymal stem cells do not induce a systemic inflammatory response but do alter lymphocyte subsets in healthy horses. Stem Cell Res Ther. 2015;6:73. doi: 10.1186/s13287-015-0050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Ami E, Berrih-Aknin S, Miller A. Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases. Autoimmun Rev. 2011;10:410–415. doi: 10.1016/j.autrev.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 28.Le Blanc K, Pittenger M. Mesenchymal stem cells: Progress toward promise. Cytotherapy. 2005;7:36–45. doi: 10.1080/14653240510018118. [DOI] [PubMed] [Google Scholar]
- 29.Peroni JF, Borjesson DL. Anti-inflammatory and immunomodulatory activities of stem cells. Vet Clin North Am Equine Pract. 2011;27:351–362. doi: 10.1016/j.cveq.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Singer NG, Caplan AI. Mesenchymal stem cells: Mechanisms of inflammation. Annu Rev Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 31.Forbes GM, Sturm MJ, Leong RW, et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12:64–71. doi: 10.1016/j.cgh.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Prasad VK, Lucas KG, Kleiner GI, et al. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal™) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant. 2011;17:534–541. doi: 10.1016/j.bbmt.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Zhang LS, Liu QF, Huang K, et al. [Mesenchymal stem cells for treatment of steroid-resistant chronic graft-versus-host disease] Zhonghua Nei Ke Za Zhi. 2009;48:542–546. [PubMed] [Google Scholar]
- 34.Lommer MJ. Efficacy of cyclosporine for chronic, refractory stomatitis in cats: A randomized, placebo-controlled, double-blinded clinical study. J Vet Dent. 2013;30:8–17. doi: 10.1177/089875641303000101. [DOI] [PubMed] [Google Scholar]
- 35.Gershwin LJ, Netherwood KA, Norris MS, et al. Equine IgE responses to non-viral vaccine components. Vaccine. 2012;30:7615–7620. doi: 10.1016/j.vaccine.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 36.Harley R, Helps CR, Harbour DA, et al. Cytokine mRNA expression in lesions in cats with chronic gingivostomatitis. Clin Diagn Lab Immunol. 1999;6:471–478. doi: 10.1128/cdli.6.4.471-478.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trautmann A, Rückert B, Schmid-Grendelmeier P, et al. Human CD8 T cells of the peripheral blood contain a low CD8 expressing cytotoxic/effector subpopulation. Immunology. 2003;108:305–312. doi: 10.1046/j.1365-2567.2003.01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gebhard DH, Dow JL, Childers TA, et al. Progressive expansion of an L-selectin-negative CD8 cell with anti-feline immunodeficiency virus (FIV) suppressor function in the circulation of FIV-infected cats. J Infect Dis. 1999;180:1503–1513. doi: 10.1086/315089. [DOI] [PubMed] [Google Scholar]
- 39.Lehmann R, von Beust B, Niederer E, et al. Immunization-induced decrease of the CD4+:CD8+ ratio in cats experimentally infected with feline immunodeficiency virus. Vet Immunol Immunopathol. 1992;35:199–214. doi: 10.1016/0165-2427(92)90132-a. [DOI] [PubMed] [Google Scholar]
- 40.Willett BJ, Hosie MJ, Callanan JJ, et al. Infection with feline immunodeficiency virus is followed by the rapid expansion of a CD8+ lymphocyte subset. Immunology. 1993;78:1–6. [PMC free article] [PubMed] [Google Scholar]
- 41.Litster A, Lin JM, Nichols J, et al. Diagnostic utility of CD4%:CD8 low% T-lymphocyte ratio to differentiate feline immunodeficiency virus (FIV)-infected from FIV-vaccinated cats. Vet Microbiol. 2014;170:197–205. doi: 10.1016/j.vetmic.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Hoogduijn MJ, Roemeling-van Rhijn M, Engela AU, et al. Mesenchymal stem cells induce an inflammatory response after intravenous infusion. Stem Cells Dev. 2013;22:2825–2835. doi: 10.1089/scd.2013.0193. [DOI] [PubMed] [Google Scholar]
- 43.Caprioli F, Sarra M, Caruso R, et al. Autocrine regulation of IL-21 production in human T lymphocytes. J Immunol. 2008;180:1800–1807. doi: 10.4049/jimmunol.180.3.1800. [DOI] [PubMed] [Google Scholar]
- 44.Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 45.Zhou L, Ivanov II, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 46.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fröhlich A, Kisielow J, Schmitz I, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 48.Hinrichs CS, Spolski R, Paulos CM, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kastirr I, Maglie S, Paroni M, et al. IL-21 is a central memory T cell-associated cytokine that inhibits the generation of pathogenic Th1/17 effector cells. J Immunol. 2014;193:3322–3331. doi: 10.4049/jimmunol.1400775. [DOI] [PubMed] [Google Scholar]
- 50.McPhee CG, Bubier JA, Sproule TJ, et al. IL-21 is a double-edged sword in the systemic lupus erythematosus-like disease of BXSB.Yaa mice. J Immunol. 2013;191:4581–4588. doi: 10.4049/jimmunol.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kimura A, Kishimoto T. IL-6: Regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 52.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 53.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dominitzki S, Fantini MC, Neufert C, et al. Cutting edge: Trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4+CD25 T cells. J Immunol. 2007;179:2041–2045. doi: 10.4049/jimmunol.179.4.2041. [DOI] [PubMed] [Google Scholar]
- 55.Fantini MC, Becker C, Monteleone G, et al. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 56.Veldhoen M, Hocking RJ, Atkins CJ, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 57.Fujimoto M, Nakano M, Terabe F, et al. The influence of excessive IL-6 production in vivo on the development and function of Foxp3+ regulatory T cells. J Immunol. 2011;186:32–40. doi: 10.4049/jimmunol.0903314. [DOI] [PubMed] [Google Scholar]
- 58.Carrade DD, Lame MW, Kent MS, et al. Comparative analysis of the immunomodulatory properties of equine adult-derived mesenchymal stem cells. Cell Med. 2012;4:1–11. doi: 10.3727/215517912X647217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kol A, Foutouhi S, Walker NJ, et al. Gastrointestinal microbes interact with canine adipose-derived mesenchymal stem cells in vitro and enhance immunomodulatory functions. Stem Cells Dev. 2014;23:1831–1843. doi: 10.1089/scd.2014.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frey NV, Lazarus HM, Goldstein SC. Has allogeneic stem cell cryopreservation been given the ‘cold shoulder’? An analysis of the pros and cons of using frozen versus fresh stem cell products in allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;38:399–405. doi: 10.1038/sj.bmt.1705462. [DOI] [PubMed] [Google Scholar]
- 61.Parody R, Caballero D, Márquez-Malaver FJ, et al. To freeze or not to freeze peripheral blood stem cells prior to allogeneic transplantation from matched related donors. Eur J Haematol. 2013;91:448–455. doi: 10.1111/ejh.12140. [DOI] [PubMed] [Google Scholar]
- 62.Pezzanite LM, Fortier LA, Antczak DF, et al. Equine allogeneic bone marrow-derived mesenchymal stromal cells elicit antibody responses in vivo. Stem Cell Res Ther. 2015;6:54. doi: 10.1186/s13287-015-0053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moll G, Rasmusson-Duprez I, von Bahr L, et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells. 2012;30:1565–1574. doi: 10.1002/stem.1111. [DOI] [PubMed] [Google Scholar]
- 64.Isakova IA, Lanclos C, Bruhn J, et al. Allo-reactivity of mesenchymal stem cells in rhesus macaques is dose and haplotype dependent and limits durable cell engraftment in vivo. PLoS One. 2014;9:e87238. doi: 10.1371/journal.pone.0087238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.