Regenerative therapies have the potential to cure chronic degenerative diseases but are limited by a shortage of donor organs and tissues and the need for immune suppression. This article proposes a 21st Century Grand Challenge that would address this significant medical need by coordinating the multidisciplinary expertise needed to manufacture functional and engraftable cells, tissues, or organs that could be made available to any patient without significant risk of rejection.
Summary
The aging population in the U.S. and other developed countries has led to a large increase in the number of patients suffering from degenerative diseases. Transplantation surgery has been a successful therapeutic option for certain patients; however, the availability of suitable donor organs and tissues significantly limits the number of patients who can benefit from this approach. Regenerative medicine has witnessed numerous recent and spectacular advances, making the repair or replacement of dysfunctional organs and tissues an achievable goal. Public-private partnerships and government policies and incentives would further catalyze the development of universally available donor tissues, resulting in broad medical and economic benefits. This article describes a Regenerative Medicine Grand Challenge that the Alliance for Regenerative Medicine recently shared with the White House’s Office of Science and Technology Policy in response to a White House call to action in scientific disciplines suggesting that the development of “universal donor tissues” should be designated as a Regenerative Medicine Grand Challenge. Such a designation would raise national awareness of the potential of regenerative medicine to address the unmet needs of many diseases and would stimulate the scientific partnerships and investments in technology needed to expedite this goal. Here we outline key policy changes and technological challenges that must be addressed to achieve the promise of a major breakthrough in the treatment of degenerative disease. A nationalized effort and commitment to develop universal donor tissues could realize this goal within 10 years and along the way result in significant innovation in manufacturing technologies.
Significance
Regenerative therapies, in which dysfunctional or degenerating cells, tissues, or organs are repaired or replaced, have the potential to cure chronic degenerative diseases. Such treatments are limited by a shortage of donor organs and tissues and the need for immune suppression to prevent rejection. This article proposes a 21st Century Grand Challenge that would address this significant medical need by coordinating a national effort to convene the multidisciplinary expertise needed to manufacture functional and engraftable cells, tissues, or organs that could be made available to any patient without significant risk of rejection—so-called universal donor tissues.
Introduction
Much of the world is facing a health care crisis because of an increasing prevalence of patients suffering from chronic degenerative diseases, combined with escalating health care costs. Regenerative therapies, in which dysfunctional or degenerating cells, tissues, or organs are repaired or replaced, have the potential to treat or cure such diseases, as evidenced by the success of organ and bone marrow transplantations in saving millions of lives. However, such treatments are limited by the acute shortage of donor organs and tissues, the need for potentially harmful immune suppression to prevent rejection, and the development of de novo antibodies in patients that complicates tissue matching between donors and recipients.
These limitations could be solved by a renewable source of regenerative cells, tissues and organs that could be provided to patients regardless of tissue type. This is an achievable goal given significant recent advances in the areas of stem cell biology, nanotechnology, bioengineering, materials science, genome editing, and transplant immunology. Worldwide, a number of stem cell-based therapies and tissue-based products have entered or completed early clinical testing [1]. However, bringing these types of products to fruition faces a number of commercialization challenges. Japan, another country whose aging population places a similar strain on its health care resources, passed the Regenerative Medicine Law in 2013, streamlining approval of cell-based technologies while safeguarding patient protections. This initiative is likely to bring considerable clinical and economic benefit to Japan. The U.S. should follow suit and take affirmative steps to support regenerative medicine.
Efforts are ongoing to promote U.S. policies that will further enable the development of the regenerative medicine field and the tremendous potential economic and health benefits that it promises. The Alliance for Regenerative Medicine (ARM), a U.S.-based organization comprising more than 250 companies, research institutions, patient advocacy groups, clinical centers, investors, and others globally, advocates a coordinated national strategy for regenerative medicine, including cell and gene therapy and other advanced therapies to bring new treatments to patients in an expedited and safe manner. ARM leaders have worked with policy makers in the U.S. and abroad on key initiatives such as greater use of manufacturing and development standards, facilitating expedited approval of products, and developing a reimbursement system that rewards innovation, especially for curative therapies and other initiatives to accelerate the development of safe and effective regenerative medicine technologies. As part of its efforts to advocate for the advancement of regenerative medicine, ARM recently shared a Regenerative Medicine Grand Challenge idea with the White House’s Office of Science and Technology Policy in response to a White House call to action in scientific disciplines [2] suggesting that the development of “universal donor tissue” be designated as a Regenerative Medicine Grand Challenge. Herein we summarize that proposal, including the challenges to be addressed and our suggestions for how the U.S. government can spearhead a nationally coordinated effort to address them.
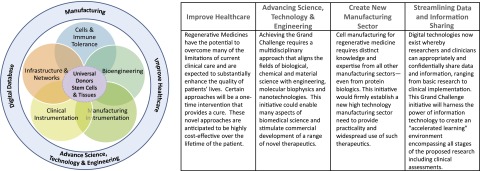
The key challenge ahead will be to convene and manage the multidisciplinary expertise needed to manufacture high quality, functional, and engraftable cells, tissues, or organs that could be made available to any patient, without significant risk of rejection—so-called universal donor tissue. (For clarity, in this article the phrase universal donor tissues is intended to describe cell banks covering the spectrum of human leukocyte antigen [HLA] genotypes. It is envisioned that these banked cells could provide HLA-matched cells, tissues, or organs for a majority in need and would be used in combination with nontoxic methods of achieving immune tolerance when needed.) Herein, and summarized on Figure 1 and in Table 1, we outline key scientific questions and technical and biological hurdles that need to be addressed to achieve the goal of universal donor tissue. Further, we suggest that a concerted, nationwide, and multidisciplinary effort to address these challenges could lead to clinical use of universal donor tissue within a decade. Such a concerted effort can best be orchestrated by federal leadership, for example by designating this effort as a Grand Challenge, as well as by taking other actions described in Table 2 and below.
Figure 1.
Rapid progress toward universal donor tissues and organs will require highly interdisciplinary consortia with expertise from diverse fields.
Table 1.
Key questions to achieve universal replacement tissues and organs

Table 2.
Actions needed by the federal government

Regenerative Cells for Tissue and Organ Repair and Organ Bioengineering
The paradigm-changing discoveries of reprogramming adult cells into human pluripotent stem cells and directly reprogrammed cell types has enabled the production of specific stem, progenitor, or mature cell types needed for engineering universal tissues and organs [3, 4]. Current preclinical and clinical transplantation studies using isolated cell populations will inform our understanding of the optimal cell type(s) and stage of maturation required for robust and sustained therapeutic function of each different tissue and organ. This knowledge, in turn, will help guide the selection of cell types needed to engineer specific functional and engraftable tissues and organs.
In addition to selection of cell types, engineering of tissues and organs will require methods to organize and pattern these cells into a three-dimensional structure with the appropriate cell interactions needed for function and full vascularization needed for delivery of oxygen and nutrients. During human development, organs and tissues are assembled over relatively long time periods (e.g., weeks to months), whereas to be clinically and commercially practical, engineered transplants must be ready for patients when they need them. Therefore, such transplantable tissue must be rapidly constructed, while accurately emulating the function and structure of these complex organs and tissues. Rapid generation of functional tissues for transplantation will require expertise in diverse disciplines, including cell and developmental biology, immunology, tissue engineering, cell manufacturing, robotics, and imaging [5, 6]. Coordination of strategies to meet these challenges will more rapidly lead to breakthroughs in both regenerative medicine and our basic knowledge of human biology.
A number of distinct approaches are now being pioneered to facilitate the manufacture of whole organs. Some of these approaches incorporate preformed tissue scaffolds on which cells are placed, whereas others require self-organization of deposited cells. Subtractive or top-down approaches start by decellularizing cadaveric animal or human organs to yield minimally immunogenic organ scaffolds that can be repopulated with either allogeneic HLA-matched or autologous stem/progenitor cells and then matured into an organ [7]. Self-organization of complex mixes of tissue stem cells, stroma, and other specialized cell types into ordered structures similar to adult organs suggests that formation of organ architecture is driven by organ-intrinsic mechanisms [8]. Nonetheless, innervation and extrinsic perfusion of self-assembled organs needs to be considered and will require additional patterning of blood vessels comprising endothelial and smooth muscle cells. These cells are derived from distinct cell lineages and highlight the complexity of constructing a fully functional organ.
Alternatively, as an example of a bottom-up approach, cell layers or sheets can be individually grown and, if needed, stacked into more complex structures including sheet and tubular organs and tissues such as cornea, esophagus, vagina, and skin [5]. Another bottom-up approach is to use robotic systems to repeatedly print or place individual cells or groups of cells in prespecified three-dimensional positions within a binding matrix to form tissue and organ structures [9]. It is likely that complex scaffolds will incorporate combinations of synthetic nanotechnology with natural extracellular matrix scaffolds to enhance cellular function, modulate immune response, or alter scaffold architecture/bioactivity at both micro- and nanoscales, so as to recapitulate both the structure and signaling properties of the matrix [10].
The ex vivo production processes highlighted above may be complemented by in vivo production of functional human organs in animals. It remains to be determined whether immunologically compatible human organs can be generated in other species [11]. A more straightforward application of in vivo organ generation would be the direct transplant of human organ rudiments into patients to facilitate final tissue patterning and maturation.
Each of the above approaches to organ generation will face challenges, but together they offer a set of complementary paths toward engineered tissues of various complexities—from skin and cartilage to pancreas, liver, kidney, and heart. Understanding how cells interact with one another and with scaffolds to yield functional organs will both require and engender advances in systems biology knowledge. Likewise, major progress in biomedical materials science will emerge and benefit multiple disciplines.
New Generation Technologies and Instrumentation for Cell, Tissue, and Organ Manufacturing
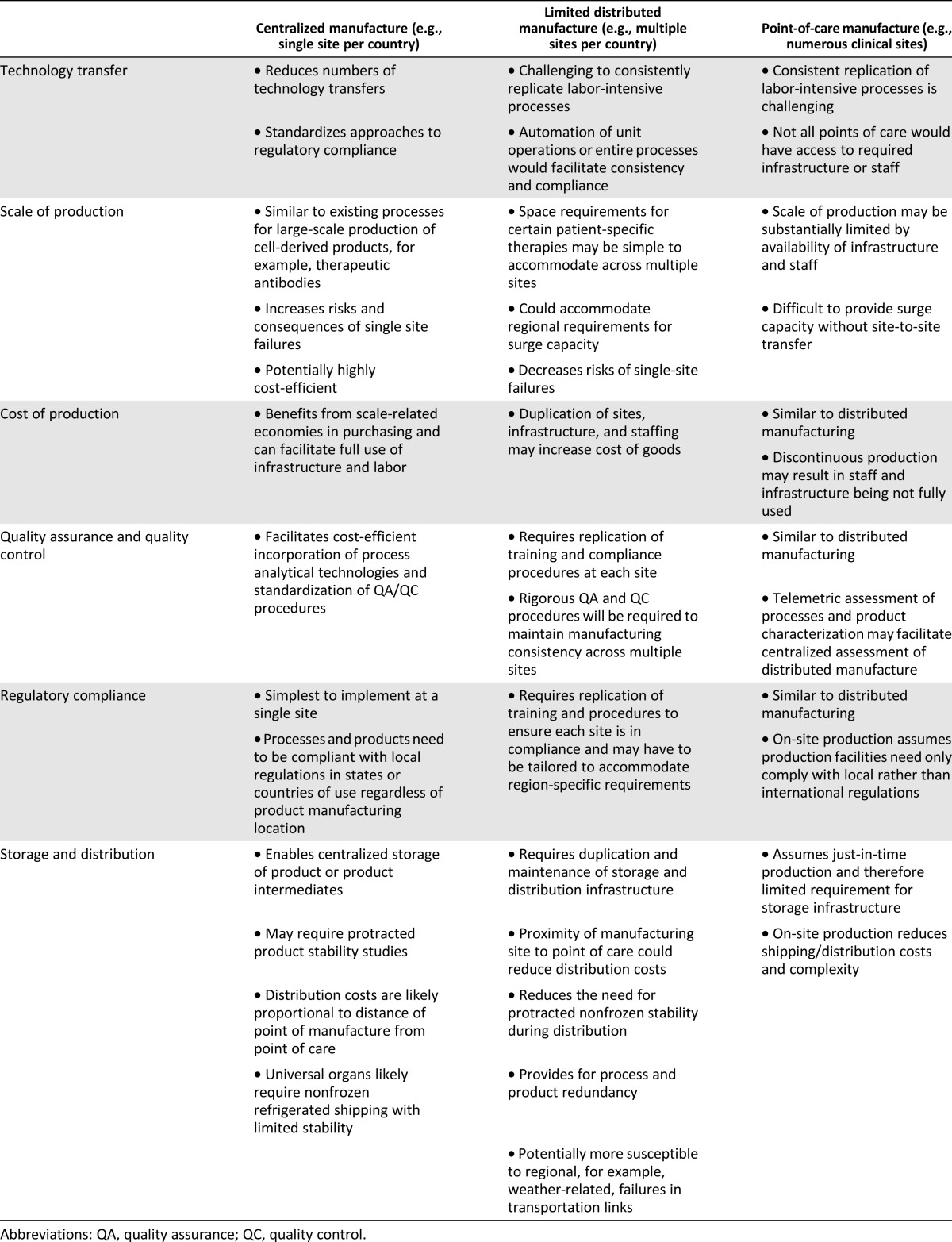
A major consideration for the regenerative medicine field is the need for large-scale, cost-effective production of cells and tissues in a good manufacturing practices (GMP)-compatible manner. Even relatively small and homogeneous organs such as the heart are comprised of billions of cells and multiple cell types. Whether the most efficacious therapy is a single cell type delivered alone or on a scaffold or a more complex preformed tissue or organ, any regenerative medicine product will require consistently manufacturing large quantities of cells. Although it is highly likely that distributed facilities and multidisciplinary staff, for example, industry/academic/clinical collaborations, will be required for initial clinical development, it is envisaged that ultimate commercial production of universal organs would be conducted in more centralized facilities. Centralized commercial production (one, or a few manufacturing centers, per country or continent) is likely to decrease cost of goods and reduce the complexities associated with securing skilled workforces and maintaining regulatory compliance that would be needed to ensure safe and consistent manufacture of universal organs. Although centralized production of such organs is likely to be the most feasible commercial approach, key merits and demerits of distinct production schemes are highlighted in Table 3. Regardless of which manufacturing scenario is used, efficient and reproducible production of biologically active cells will benefit from codevelopment of the methods, integrated systems, and instrumentation to produce the distinct cell types, tissues, or organs at the needed scale and will require appropriate media and reagents, instrumentation, automation, and robotics. Together, these elements will help to isolate, purify, expand, or grow the “product” with the foremost goal of ensuring endpoint bioactivity and safety [12].
Table 3.
Considerations for centralized and decentralized manufacturing
Routine ex vivo production of human organs will rely on further development of large-scale, closed-system bioreactors that are likely to incorporate nanoscale biosensing technology for monitoring metabolic function, growth and quality of cells/tissues. It must be considered that each cell bank, tissue, or organ will require several weeks to manufacture and that individual bioreactor systems likely will need to be at least 5–10 times the size of the manufactured organ. Therefore, further advances in automated cell handling systems and clean room technologies will be required to design and construct automated, high volume, parallel processing, and GMP-compatible instrumentation to fully enable the establishment and maintenance of cell banks and production for universal donor tissues and organs. In addition, standards relating to key issues such as cell potency assay development and validation and cell characterization will be important (ARM and several other public and private organizations are working to establish a standards-coordinating body to harmonize standards and standards development). It will also be critical to develop low-cost manufacture of GMP-grade essential growth media, enzymes, and growth factors to minimize production costs. Having the government work with private entities to standardize production of low-cost, quality reagents for this manufacturing sector could ultimately result in lower costs of therapies. In turn, development of the materials, processes, and instruments required to produce universal donor organs calls for a new manufacturing sector. Conquering these manufacturing hurdles alone may provide therapies using transplants of cells, with or without a matrix, for a variety of disorders.
Chemical and biomolecular engineering are providing new materials and platforms for efficient and scalable manufacture of various cell types. Thermoresponsive hydrogels and nanobridges are examples of new materials used in three-dimensional stem cell culture systems that are capable of producing higher-fold expansions and yields in a more manageable volume than traditional two-dimensional culture systems [13]. Similar to the White House’s Materials Genome Initiative and BRAIN Initiative, a new initiative in regenerative medicine will spur research and create new jobs in technology development, leading to lower manufacturing costs. Ultimately, and most importantly, this initiative will accelerate the delivery of new and more effective therapies to patients.
Long-Term Survival of Organs in the Absence of Nonspecific Immunosuppression
Autologous induced pluripotent stem cells (iPSCs) would theoretically be the ideal immune-compatible cell source for organ generation; however, the cost, time, and logistics associated with manufacturing GMP-compatible autologous iPSC-derived products may be prohibitive. Direct reprogramming methods may offer a more efficient way to achieve the rapid production of autologous specialized cell types [14], but it is likely that for cost-effective and large-scale organ production, allogeneic cells and/or scaffolds will be used. Therefore, the ultimate success of using bioengineered cells and organs for transplantation will require a concerted effort to overcome the key challenges associated with allogeneic cell or tissue transplantation: the immunological rejection of these products and assurance of long-term survival and safety. It is imperative to achieve immune tolerance for sustained function of transplanted organs and to eliminate, or at least minimize, the need for prolonged immunosuppression to prevent organ rejection. To date, long-term organ transplantation tolerance in humans has required transplantation of donor hematopoietic stem cells (HSCs) leading to stable “mixed chimerism” (coexistence of donor and host blood and immune cells) and immune tolerance of the transplanted organ [15]. This method is complicated by its requirement for dual transplantation of the immune system and the organ of interest and is associated with morbidity caused by myeloabalative conditioning needed for the HSC transplant.
Another approach to decrease the risk of immune rejection is to transplant organs that are generated using HLA-matched iPSC donors. These donors would be sourced from GMP-grade banks containing iPSCs selected for homozygosity of the most common HLA types in a given population [16]. Such iPSC “haplobanks” hold particular promise for providing HLA matches in populations that have low levels of HLA diversity. For more diverse populations containing rare HLA genotypes that are difficult to match, it will still be important to pursue other effective nontoxic methods to achieve tolerance of donor cells and tissues.
New strategies to improve donor cell engraftment and tolerance induction with minimal toxicity have been defined in animal models and are transitioning into clinical testing [17]. Methods being examined to prevent immune rejection of transplanted organs also include a variety of novel immunotherapies that could dampen immunogenicity of transplanted organs by manipulating the T-cell response. Further development of humanized animal model systems (small and large as appropriate) for testing transplantation tolerance and tissue and organ safety, survival, and function will also be required as a critical component of future universal donor organs and will undoubtedly also inform the development of more conventional therapeutic strategies for a myriad of autoimmune disorders.
Disease Understanding and Noninvasive Monitoring of Tissue and Organ Function
It will be critical to develop innovative noninvasive imaging tools, cell-labeling technologies, and methods for monitoring the survival, health, and function of transplanted cells, tissues, and organs deep within patients. Despite rapid progress in in vivo imaging, biomedical monitoring of transplanted cells, tissues, and organs remains an open clinical challenge. Fortunately, advances in nanoscience, molecular biology, and biomedical imaging are leading to new imaging technologies to assess implants with enhanced reliability, sensitivity, and diagnostic power. For example, biologists, physicists, and engineers are developing nanoscale probes that can highlight cell, tissue, and organ transplant location and provide noninvasive functional assessment [18–20]. A focused multidisciplinary collaborative endeavor could develop and implement a reliable, noninvasive monitoring system for widespread use at hospitals and clinics.
Value of Achieving the Goal
A new era in regenerative medicine and advanced therapy is approaching and offers the promise of treatments for chronic, debilitating conditions that will become more prevalent as our population ages. The field of regenerative medicine has now matured to the point that developing banks of genetically matched cells to create universal donor tissues is an achievable goal. This new paradigm in medicine is broad-based with seemingly diverse but critically interrelated stakeholders at every level of science, engineering, and medicine. What is now needed is a clear commitment by the federal government and stakeholders to making the development of universal donor tissues a national priority. The key roles that the government would need to play are highlighted in Table 2. This commitment will require sustained funding from public and private sources, as well as coordination across multiple federal agencies to define priorities, accelerate innovation, establish centers of excellence, and facilitate progress on a national level. Recognizing the complexity of specific regenerative medicine goals that require multidisciplinary expertise, new initiatives have coalesced around specific regenerative medicine goals such as the New Organ Liver Prize [21] to create a bioengineered liver and to facilitate translational studies. In addition, the Food and Drug Administration holds liaison meetings to convene groups of stakeholders to bridge and advance regulatory policies in specific areas (i.e., cardiovascular, endocrine, and others). Similar to the White House’s Materials Genome Initiative and BRAIN Initiative, a federal priority in regenerative medicine would deliver a valuable national resource, in this case by enabling a 10-year goal of developing universal donor tissues. Such an initiative would also catalyze innovation to realize new tools and technologies that will clearly position the U.S. as the global leader in regenerative medicine and bring new treatments and, most importantly, cures to patients.
Acknowledgments
S.E.A. is currently affiliated with Fate Therapeutics (San Diego, CA). N.D.D. is currently affiliated with Accendo Science Editing (San Francisco, CA) and NDA Partners (San Francisco, CA).
Author Contributions
A.T., S.E.A., L.C.K., N.D.D., D.V.S., J.A.W., K.J.W., and M.J.W.: conception and design, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
A.T. is a compensated employee of and has intellectual property rights with StemCells, Inc. S.E.A. is a compensated employee and has compensated intellectual property rights with Celgene Cellular Therapeutics and compensated stock options with Celgene Corporation and Fate Therapeutics. D.V.S. is an inventor on multiple patents and patent applications, including ones in the stem cell area and in the gene therapy area, and is a consultant and board member for several companies. The other authors indicated no potential conflicts of interest.
References
- 1.Ratcliffe E, Glen KE, Naing MW, et al. Current status and perspectives on stem cell-based therapies undergoing clinical trials for regenerative medicine: case studies. Br Med Bull. 2013;108:73–94. doi: 10.1093/bmb/ldt034. [DOI] [PubMed] [Google Scholar]
- 2. 21st Century Grand Challenges. Available at https://www.whitehouse.gov/administration/eop/ostp/grand-challenges. Accessed September 14, 2015.
- 3.Takahashi K, Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development. 2013;140:2457–2461. doi: 10.1242/dev.092551. [DOI] [PubMed] [Google Scholar]
- 4.Pappas DJ, Gourraud PA, Le Gall C, et al. Proceedings: Human leukocyte antigen haplo-homozygous induced pluripotent stem cell haplobank modeled after the California population: Evaluating matching in a multiethnic and admixed population. Stem Cells Translational Medicine. 2015;4:413–418. doi: 10.5966/sctm.2015-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams ML, Bhatia SK. Engineering the extracellular matrix for clinical applications: Endoderm, mesoderm, and ectoderm. Biotechnol J. 2014;9:337–347. doi: 10.1002/biot.201300120. [DOI] [PubMed] [Google Scholar]
- 6.Hunsberger J, Harrysson O, Shirwaiker R, et al. Manufacturing road map for tissue engineering and regenerative medicine technologies. Stem Cells Translational Medicine. 2015;4:130–135. doi: 10.5966/sctm.2014-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soto-Gutierrez A, Wertheim JA, Ott HC, et al. Perspectives on whole-organ assembly: Moving toward transplantation on demand. J Clin Invest. 2012;122:3817–3823. doi: 10.1172/JCI61974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sterneckert JL, Reinhardt P, Schöler HR. Investigating human disease using stem cell models. Nat Rev Genet. 2014;15:625–639. doi: 10.1038/nrg3764. [DOI] [PubMed] [Google Scholar]
- 9.Kolesky DB, Truby RL, Gladman AS, et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 2014;26:3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 10.Wong IY, Bhatia SN, Toner M. Nanotechnology: Emerging tools for biology and medicine. Genes Dev. 2013;27:2397–2408. doi: 10.1101/gad.226837.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rashid T, Kobayashi T, Nakauchi H. Revisiting the flight of Icarus: Making human organs from PSCs with large animal chimeras. Cell Stem Cell. 2014;15:406–409. doi: 10.1016/j.stem.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham JJ, Ulbright TM, Pera MF, et al. Lessons from human teratomas to guide development of safe stem cell therapies. Nat Biotechnol. 2012;30:849–857. doi: 10.1038/nbt.2329. [DOI] [PubMed] [Google Scholar]
- 13.Lei Y, Jeong D, Xiao J, et al. Developing defined and scalable 3D culture systems for culturing human pluripotent stem cells at high densities. Cell Mol Bioeng. 2014;7:172–183. doi: 10.1007/s12195-014-0333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Du Y, Deng H. Direct lineage reprogramming: Strategies, mechanisms, and applications. Cell Stem Cell. 2015;16:119–134. doi: 10.1016/j.stem.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Sachs DH, Kawai T, Sykes M. Induction of tolerance through mixed chimerism. Cold Spring Harb Perspect Med. 2014;4:a015529. doi: 10.1101/cshperspect.a015529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner M, Leslie S, Martin NG, et al. Toward the development of a global induced pluripotent stem cell library. Cell Stem Cell. 2013;13:382–384. doi: 10.1016/j.stem.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Czechowicz A, Weissman IL. Purified hematopoietic stem cell transplantation: The next generation of blood and immune replacement. Immunol Allergy Clin North Am. 2010;30:159–171. doi: 10.1016/j.iac.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen PK, Riegler J, Wu JC. Stem cell imaging: From bench to bedside. Cell Stem Cell. 2014;14:431–444. doi: 10.1016/j.stem.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srivastava AK, Kadayakkara DK, Bar-Shir A, et al. Advances in using MRI probes and sensors for in vivo cell tracking as applied to regenerative medicine. Dis Model Mech. 2015;8:323–336. doi: 10.1242/dmm.018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wegner KD, Hildebrandt N. Quantum dots: Bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem Soc Rev. 2015;44:4792–4834. doi: 10.1039/c4cs00532e. [DOI] [PubMed] [Google Scholar]
- 21. New Organ Liver Prize. Available at http://www.neworgan.org/prize.php. Accessed August 31, 2015.