Abstract
Background
The influence of patients’ preoperative nutritional status on their clinical outcome has already been proven. Therefore, patients with malnutrition are in need of additional therapeutic efforts. However, for pancreatic surgery, evidence suggesting the adequacy of existing nutritional assessment scores to estimate malnutrition associated with postoperative outcome is limited.
Objective
The aim of the observational trial “Nutritional Risk in Major Abdominal Surgery (NURIMAS) Pancreas” is to prospectively assess and analyze different nutritional assessment scores for their prognostic value on postoperative complications in patients undergoing pancreatic surgery.
Methods
All patients scheduled to receive elective pancreatic surgery at the University Hospital of Heidelberg will be screened for eligibility. Preoperatively, 12 nutritional assessment scores will be collected and patients will be assigned either at risk or not at risk for malnutrition. The postoperative course will be followed prospectively and complications according to the Clavien-Dindo classification will be recorded. The prognostic value for complications will be evaluated for every score in a univariable and multivariable analysis corrected for known risk factors in pancreatic surgery.
Results
Final data analysis is expected to be available during Spring 2016.
Conclusions
The NURIMAS Pancreas trial is a monocentric, prospective, observational trial aiming to find the most predictive clinical nutritional assessment score for postoperative complications. Using the results of this protocol as a knowledge base, it is possible to conduct nutritional risk-guided intervention trials to prevent postoperative complications in the pancreatic surgical population.
Trial Registration
germanctr.de: DRKS00006340; https://drks-neu.uniklinik-freiburg.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00006340 (Archived by WebCite at http://www.webcitation.org/6bzXWSRYZ)
Keywords: Diagnosis Related Group system, malnutrition, nutritional assessment, nutritional score, pancreatic surgery
Introduction
Existing Evidence and Need for a Trial
Malnutrition is estimated as one of the leading causes for loss of health [1]. For hospitalized patients, the direct negative impact of malnutrition has broadly been examined [2-7]. Patients with tumorous diseases as well as patients being treated in intensive care units or in geriatric hospitals are mostly affected by negative impact of malnutrition [8-11].
To detect malnutrition, several scores have been developed. A recently published systematic review with meta-analysis investigated 32 scores with regard to their validity and predictive value for the population of hospitalized patients. The review indicated that only a small portion of scores had been fully validated and in particular, only limited scores are available for surgery. Development of new scores was considered redundant and they were not able to achieve higher sensitivity or specificity. Thus, trials investigating different scores in a specific patient population have been claimed necessary [12].
The population of surgical patients is specifically at high risk for being malnourished [13]. For some surgical indications, malnutrition has been proven as a risk of postoperative complications [14-16]. Regarding pancreatic surgery, limited data are available due to insufficient sample sizes or inhomogeneous populations. For example, the most recent pancreas-specific trial showed a correlation between the nutritional risk index and wound infections in patients after pancreaticoduodenectomy [17]. In addition, in this trial, the small sample size of 64 patients represents the major limitation.
Aim of the Trial
“Nutritional Risk in Major Abdominal Surgery (NURIMAS) Pancreas” (DRKS00006340) is a monocentric, prospective, observational trial with one study arm. The aim of this trial is to find the best suitable clinical nutritional assessment score to predict postoperative complications in patients undergoing pancreatic surgery.
Methods
Study Population
The study population will comprise adult patients undergoing pancreatic surgery at the Department of General, Visceral and Transplantation Surgery at the University Hospital of Heidelberg. All underlying diseases leading to a primary pancreatic resection will be included. Thus, the analysis will give information on a broad and representative population as seen in high-volume surgical centers (Textbox 1).
Eligibility criteria.
Inclusion criteria
Age ≥ 18 and ≤ 90 years
Elective pancreatic surgery
Written informed consent
Exclusion criteria
Any former pancreatic-surgical procedures
Language problems
Inability to understand the trial
Diagnostic Intervention (Nutritional Assessment Scores)
Based on the most recent systematic review about existing nutritional assessment scores by Van Bokhorst-de van der Schueren [12], 11 scores have been selected that are in use in surgical patient populations [18-27]. Recently, the European Society of Clinical Nutrition and Metabolism (ESPEN) published a new consensus definition of malnutrition, the ESPEN malnutrition criteria [28]. Table 1 presents a summary of the 12 nutritional assessment scores that will be evaluated.
Table 1.
Nutritional assessment scores.
| Name | Classification for nutritional riska |
| Nutritional Risk Index [18] | Normal/mild/moderate/severe |
| Nutritional Risk Screening Score and Revised Version [19,26] | Low/ moderate/high |
| Subjective Global Assessment [20] | No/ moderate/severe |
| Malnutrition Universal Screening Tool [21] | Low/ medium/high |
| Mini-Nutritional Assessment and Revised Version [22,27] | Normal/ at risk/malnourished |
| Short Nutritional Assessment Questionnaire [23] | Low/ moderate/severe |
| Imperial Nutritional Screening System I [24] | Not at risk/at risk |
| Imperial Nutritional Screening System II [24] | Green/amber/red |
| Nutritional Risk Classification [25] | Low/at risk |
| ESPEN Malnutrition Criteria [28] | Normal/malnourished |
aThe highest class for nutritional risk determined by the scores will be used as the study end point “at risk for malnutrition” for statistical evaluation.
Outcome Measures
The primary end point is postoperative morbidity and mortality. The most suitable score is defined as the score with the highest association of malnutrition and postoperative complication expressed as the highest lower bound of the 95% confidence interval of odds ratio.
Secondary end points are length of hospital stay, length of stay in intensive care unit, comprehensive complication index [29], place of discharge (discharge to home or discharge to rehabilitation or care facility), necessity of postoperative parenteral or enteral nutrition, and impact of malnutrition as diagnosis on hospital costs and Diagnosis Related Group (DRG) case cost.
Trial Site and Sample Size Calculation
The trial will be conducted at the Department of General, Visceral and Transplantation Surgery at the University Hospital of Heidelberg. Prevalence of malnutrition in pancreatic cancer is known to be 88% [30]. We calculated sample size with a lower prevalence of 70% for all pancreatic diseases. With a specificity and sensitivity of 95% and a confidence interval of 0.05, a total of 260 patients will be needed [31]. Patients will be consecutively recruited until the study population will consist of 260 patients with major pancreatic resections (pancreaticoduodenectomy, distal pancreatic resection, or total pancreatectomy). Based on the department’s data (about 500 eligible pancreatic resections), recruitment will be completed within 12 months after inclusion of the first patient.
Planned Study Conduct and Trial Visits
All patients visiting the Department of General, Visceral and Transplantation Surgery at the University Hospital of Heidelberg and scheduled to receive elective operations will be screened. Eligible patients will be consecutively informed about the study purpose and conduct. After giving a written informed consent, patients will be questioned and examined (Visit 1) according to the investigated nutritional assessment scores (Table 2). Further, other known risk factors for postoperative complications will be noted [32,33]. If the operation is delayed for any reason, patients will be re-evaluated as long as preoperative data from questionnaires are not older than 36 hours at the time of actual operation.
Table 2.
Flowchart of the NURIMAS trial-course of examinations.
| Visit | 1 | 2 | 3 | 4 |
| Preoperative | POD 3-7 | POD 10-14 | Discharge or POD 30 | |
| Eligibility | X |
|
|
|
| Informed consent | X |
|
|
|
| Baseline data | X |
|
|
|
| Nutritional scores | X |
|
|
|
| Laboratory analyses | X | x | x | x |
| Assessment of surgical procedure |
|
x |
|
|
| Assessment of complications |
|
x | x | x |
| Serious adverse events |
|
x | x | x |
| Secondary end points | x | x | x |
After the operation, the clinical course will be followed prospectively. Therefore, 3 planned visits will be performed. The first visit will be performed on postoperative days (PODs) 3-7, the second visit on PODs 10-14, and the last visit on the day of discharge or not later than POD 30. During these visits, complications according to Textbox 2 [34-41] will be assessed. Every postoperative complication will be rated according to the validated classification by Clavien-Dindo [42]. Further, on postoperative visits, the status of secondary end points will be evaluated.
Assessed postoperative complications.
• Postoperative pancreatic fistula [34]
• Bile leak [35]
• Postpancreatectomy hemorrhage [36]
• Delayed gastric emptying [37]
• Surgical site infection [38]
• Other infections and sepsis [39]
• Chylous ascites (triglycerides in drainage) [40]
• Serious adverse event [41]
Data Management and Monitoring
All required information according to this protocol will be recorded on a paper-based case report form. After the last visit, data will be entered in a password-protected and validated relational database (SQL Server 2008 Express). After the last patient’s last visit, database will be soft-locked. A monitoring will be performed on 100% of data necessary to evaluate the primary end point. Of the remaining data, 20% are randomly selected. Finally, the database will be closed and made available for statistical analysis.
Statistical Analysis
The included scores use different numbers of nutritional risk classes. To compare the scores, evaluation of the primary end point patients will be dichotomized by each nutritional assessment score as “at risk” or “not at risk” using the respective highest nutritional risk determined by each score (Table 1). Further, patients will be dichotomized whether they had at least one major complication (Clavien-Dindo III-V) or not. Hence, for every nutritional assessment score, a contingency table will be created (Figure 1). Positive predictive value, specificity, sensitivity, and c-index will be calculated. Association between every nutritional assessment score and major complication will be expressed as odds ratio with 95% confidence interval. Univariable significance of association will be tested with a chi-square test without Yate’s correction at a level of significance of 5%. A multivariable logistic regression model will be used for evaluation of primary end point at a level of significance of 5%. Covariates will be age (years) and operation time (minutes). Factors will be malignancy; gender; laparoscopy; intraoperative radiotherapy; resection of vessels (portal vein, superior mesenteric artery, or vein); inclusion in an interventional trial; American Society of Anaesthesiologists (ASA) physical status classification system; prior upper gastrointestinal surgery; pancreatic surgery associated risks (amylase in drainage >5000 IE/U on POD 1, biliary stent); and preoperative serum albumin level less than 35 g/L. Subgroup analysis will be performed separately for different types of pancreatic resections and different nutritional risk classes will be determined by each score (Table 1).
Figure 1.
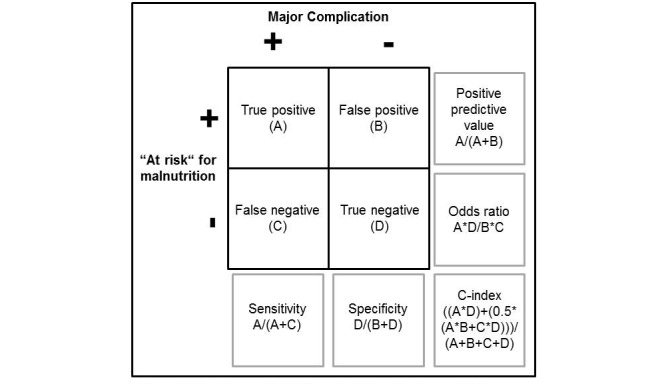
Contingency table for calculation of primary study endpoint for the prognostic value of every nutritional assessment score.
Secondary end points will be analyzed descriptively by tabulation of the measures of the empirical distributions. According to the level of the variables, means, SDs, medians, 1st and 3rd quartiles, minimum and maximum, or absolute and relative frequencies will be reported, respectively. Descriptive P values of the corresponding statistical tests and associated 95% confidence intervals will be given. Statistical analysis will be performed with program R [43].
Methods for Minimizing Bias
Minimizing Selection Bias
All patients will be consecutively screened and if found to be eligible, informed consent will be obtained in the single-arm study. Number of screened, included, and analyzed patients will be reported and differences will be explained.
Minimizing Performance and Detection Bias
Preoperative data capturing and outcome assessment will be performed by 2 different investigators. Statistical analysis will be performed after closure of database.
Minimizing Attrition Bias
Statistical measurements such as imputation will be taken to minimize risk of bias due to incomplete outcome data [44]. The trial will be reported according to the Standards for Reporting of Diagnostic Accuracy (STARD) statement [45]. The trial is registered with Deutsches Register Klinischer Studien (DRKS00006340). To avoid the risk of selective reporting, the trial protocol with full information about end points and profound explanation of planned statistical analysis is hereby published according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement where appropriate [46]. Report on cost issues and validation of the ESPEN criteria for malnutrition is planned separately.
Minimizing Other Bias
Any financial relationship or any conflict of interest that could inappropriately influence the work within this project will be stated explicitly. Confounding will be minimized by inclusion of covariates and factors in the statistical analysis of the primary end point as mentioned in the “Statistical Analysis” section.
Ethics and Informed Consent
The NURIMAS Pancreas trial is conducted in accordance with the Declaration of Helsinki in its actual version [47]. According to the professional code for physicians in Germany (§15 BOÄ), the trial protocol was reviewed and approved by the Ethics Committee of the medical faculty of the University of Heidelberg.
Before inclusion in the NURIMAS Pancreas trial, patients will be informed both orally and in writing about all relevant aspects of the trial (eg, the aims, methods, the anticipated benefits, potential risks of the study, and the discomfort it may entail). The patients’ free decision to participate will be documented by signature on the informed consent form. All patient-related information is subject to medical confidentiality and to the Federal Data Protection Act. Pseudonymized data transfer will be performed. Third parties will not have any insight into original data.
Results
Final data analysis is expected to be completed during Spring 2016.
Discussion
The NURIMAS trial is a monocentric, prospective, observational trial aiming to find the most suitable clinical nutritional assessment score to predict major postoperative complications associated with malnutrition. Thus, an important lack of knowledge in preoperative risk assessment in patients undergoing pancreatic surgery will be worked-off. Upon this knowledge, further trials can rely on a validated nutritional risk and evaluate the benefit of nutritional interventions potentially preventing postoperative complications.
Acknowledgments
This is an investigator-initiated study and PP is the primary investigator. For this study, no additional funding source is available. However, the resources and the facilities available at the University of Heidelberg are availed for conducting the trial. Only the primary investigator has access to the final dataset.
Abbreviations
- ASA
American Society of Anaesthesiologists
- DRG
Diagnosis Related Group
- ESPEN
European Society of Clinical Nutrition and Metabolism
- IMBI
Institute of Medical Biometry and Informatics
- POD
postoperative day
- SAE
serious adverse events
- SDGC
Study Center of the German Surgical Society
- SPIRIT
Standard Protocol Items: Recommendations for Interventional Trials
- STARD
Standards for Reporting of Diagnostic Accuracy
Footnotes
Conflicts of Interest: None declared.
References
- 1.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez M, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin AA, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro Karen Courville. Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De LD, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais Don C. Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes Francis Gerry R. Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo J, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer A, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan K M Venkat. Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn Felipe Rodriguez. Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg Nicolas J C. Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf Marieke J. van OJ, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams Sean R M. Witt E, Wolfe F, Woolf AD, Wulf S, Yeh P, Zaidi Anita K M. Zheng Z, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 Dec 15;380(9859):2197–2223. doi: 10.1016/S0140-6736(12)61689-4.S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 2.Bistrian BR, Blackburn GL, Vitale J, Cochran D, Naylor J. Prevalence of malnutrition in general medical patients. JAMA. 1976 Apr 12;235(15):1567–1570. [PubMed] [Google Scholar]
- 3.Bistrian BR, Blackburn GL, Hallowell E, Heddle R. Protein status of general surgical patients. JAMA. 1974 Nov 11;230(6):858–860. [PubMed] [Google Scholar]
- 4.Pirlich M, Luhrmann N, Schütz T. Mangelernährung bei Klinikpatienten: Diagnostik und klinische Bedeutung. Akt Ernähr-Med. 1999;24:260–266. [Google Scholar]
- 5.Gariballa SE, Parker SG, Taub N, Castleden CM. Influence of nutritional status on clinical outcome after acute stroke. Am J Clin Nutr. 1998 Aug;68(2):275–281. doi: 10.1093/ajcn/68.2.275. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=9701183 . [DOI] [PubMed] [Google Scholar]
- 6.Kyle UG, Pirlich M, Schuetz T, Luebke HJ, Lochs H, Pichard C. Prevalence of malnutrition in 1760 patients at hospital admission: A controlled population study of body composition. Clin Nutr. 2003 Oct;22(5):473–481. doi: 10.1016/s0261-5614(03)00049-9.S0261561403000499 [DOI] [PubMed] [Google Scholar]
- 7.Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998 Mar;34(4):503–509. doi: 10.1016/s0959-8049(97)10090-9.S0959804997100909 [DOI] [PubMed] [Google Scholar]
- 8.Nixon DW, Heymsfield SB, Cohen AE, Kutner MH, Ansley J, Lawson DH, Rudman D. Protein-calorie undernutrition in hospitalized cancer patients. Am J Med. 1980 May;68(5):683–690. doi: 10.1016/0002-9343(80)90254-5. [DOI] [PubMed] [Google Scholar]
- 9.Aviles A, Yañez J, López T, García EL, Guzmán R, Díaz-Maqueo JC. Malnutrition as an adverse prognostic factor in patients with diffuse large cell lymphoma. Arch Med Res. 1995;26(1):31–34. [PubMed] [Google Scholar]
- 10.Giner M, Laviano A, Meguid MM, Gleason JR. In 1995 a correlation between malnutrition and poor outcome in critically ill patients still exists. Nutrition. 1996 Jan;12(1):23–29. doi: 10.1016/0899-9007(95)00015-1.0899900795000151 [DOI] [PubMed] [Google Scholar]
- 11.Bruun LI, Bosaeus I, Bergstad I, Nygaard K. Prevalence of malnutrition in surgical patients: Evaluation of nutritional support and documentation. Clin Nutr. 1999 Jun;18(3):141–147. doi: 10.1054/clnu.1999.0006.clnu.1999.0006 [DOI] [PubMed] [Google Scholar]
- 12.van Bokhorst-de van der Schueren Marian AE, Guaitoli PR, Jansma EP, de Vet Henrica CW. Nutrition screening tools: Does one size fit all? A systematic review of screening tools for the hospital setting. Clin Nutr. 2014 Feb;33(1):39–58. doi: 10.1016/j.clnu.2013.04.008.S0261-5614(13)00108-8 [DOI] [PubMed] [Google Scholar]
- 13.Sungurtekin H, Sungurtekin U, Balci C, Zencir M, Erdem E. The influence of nutritional status on complications after major intraabdominal surgery. J Am Coll Nutr. 2004 Jun;23(3):227–232. doi: 10.1080/07315724.2004.10719365. [DOI] [PubMed] [Google Scholar]
- 14.Kuzu MA, Terzioğlu H, Genç V, Erkek AB, Ozban M, Sonyürek P, Elhan AH, Torun N. Preoperative nutritional risk assessment in predicting postoperative outcome in patients undergoing major surgery. World J Surg. 2006 Mar;30(3):378–390. doi: 10.1007/s00268-005-0163-1. [DOI] [PubMed] [Google Scholar]
- 15.Schiesser M, Müller S, Kirchhoff P, Breitenstein S, Schäfer M, Clavien P. Assessment of a novel screening score for nutritional risk in predicting complications in gastro-intestinal surgery. Clin Nutr. 2008 Aug;27(4):565–570. doi: 10.1016/j.clnu.2008.01.010.S0261-5614(08)00029-0 [DOI] [PubMed] [Google Scholar]
- 16.Schiesser M, Kirchhoff P, Müller MK, Schäfer M, Clavien P. The correlation of nutrition risk index, nutrition risk score, and bioimpedance analysis with postoperative complications in patients undergoing gastrointestinal surgery. Surgery. 2009 May;145(5):519–526. doi: 10.1016/j.surg.2009.02.001.S0039-6060(09)00078-6 [DOI] [PubMed] [Google Scholar]
- 17.Shinkawa H, Takemura S, Uenishi T, Sakae M, Ohata K, Urata Y, Kaneda K, Nozawa A, Kubo S. Nutritional risk index as an independent predictive factor for the development of surgical site infection after pancreaticoduodenectomy. Surg Today. 2013 Mar;43(3):276–283. doi: 10.1007/s00595-012-0350-2. [DOI] [PubMed] [Google Scholar]
- 18.The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group Perioperative total parenteral nutrition in surgical patients. N Engl J Med. 1991 Aug 22;325(8):525–532. doi: 10.1056/NEJM199108223250801. [DOI] [PubMed] [Google Scholar]
- 19.Reilly HM, Martineau JK, Moran A, Kennedy H. Nutritional screening: Evaluation and implementation of a simple Nutrition Risk Score. Clin Nutr. 1995 Oct;14(5):269–273. doi: 10.1016/s0261-5614(95)80063-8.S0261-5614(95)80063-8 [DOI] [PubMed] [Google Scholar]
- 20.Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN. What is subjective global assessment of nutritional status? 1987. Classical article. Nutr Hosp. 2008;23(4):400–407. [PubMed] [Google Scholar]
- 21.Elia M. Screening for malnutrition: A multidisciplinary responsibility. Development and use of the ‘Malnutrition Universal Screening Tool’ (‘MUST’) for adults. Redditch, Worcestershire: Malnutrition Advisory Group, a Standing Committee of BAPEN; 2003. [2015-10-11]. http://www.bapen.org.uk/pdfs/must/must_exec_sum.pdf . [Google Scholar]
- 22.Guigoz Y, Vellas B, Garry P. Mini Nutritional Assessment: A practical assessment tool for grading the nutritional state of elderly patients. Facts and Research in Gerontology (Nutrition) 1994;4(Suppl 2):15–59. [Google Scholar]
- 23.Kruizenga HM, Seidell JC, de Vet HC, Wierdsma NJ, van Bokhorst-de van der Schueren MA. Development and validation of a hospital screening tool for malnutrition: The Short Nutritional Assessment Questionnaire (SNAQ) Clin Nutr. 2005 Feb;24(1):75–82. doi: 10.1016/j.clnu.2004.07.015.S0261-5614(04)00124-4 [DOI] [PubMed] [Google Scholar]
- 24.Tammam JD, Gardner L, Hickson M. Validity, reliability and acceptability of the Imperial Nutritional Screening System (INSYST): A tool that does not require the body mass index. J Hum Nutr Diet. 2009 Dec;22(6):536–544. doi: 10.1111/j.1365-277X.2009.01004.x.JHN1004 [DOI] [PubMed] [Google Scholar]
- 25.Kovacevich DS, Boney AR, Braunschweig CL, Perez A, Stevens M. Nutrition risk classification: A reproducible and valid tool for nurses. Nutr Clin Pract. 1997 Feb;12(1):20–25. doi: 10.1177/011542659701200120. [DOI] [PubMed] [Google Scholar]
- 26.Kondrup J, Allison SP, Elia M, Vellas B, Plauth M, Educational Clinical Practice Committee‚ European Society of Parenteral Enteral Nutrition (ESPEN) ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003 Aug;22(4):415–421. doi: 10.1016/s0261-5614(03)00098-0.S0261561403000980 [DOI] [PubMed] [Google Scholar]
- 27.Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: Developing the Short-Form Mini-Nutritional Assessment (MNA-SF) J Gerontol A Biol Sci Med Sci. 2001 Jun;56(6):M366–M372. doi: 10.1093/gerona/56.6.m366. [DOI] [PubMed] [Google Scholar]
- 28.Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van GA, Klek S, Muscaritoli M, Nyulasi I, Ockenga J, Schneider SM, de van der Schueren M A E. Singer P. Diagnostic criteria for malnutrition: An ESPEN Consensus Statement. Clin Nutr. 2015 Jun;34(3):335–340. doi: 10.1016/j.clnu.2015.03.001.S0261-5614(15)00075-8 [DOI] [PubMed] [Google Scholar]
- 29.Slankamenac K, Nederlof N, Pessaux P, de JJ, Wijnhoven BP, Breitenstein S, Oberkofler CE, Graf R, Puhan MA, Clavien P. The comprehensive complication index: A novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg. 2014 Nov;260(5):757–762. doi: 10.1097/SLA.0000000000000948.00000658-201411000-00006 [DOI] [PubMed] [Google Scholar]
- 30.La TM, Ziparo V, Nigri G, Cavallini M, Balducci G, Ramacciato G. Malnutrition and pancreatic surgery: Prevalence and outcomes. J Surg Oncol. 2013 Jun;107(7):702–708. doi: 10.1002/jso.23304. [DOI] [PubMed] [Google Scholar]
- 31.Carley S, Dosman S, Jones SR, Harrison M. Simple nomograms to calculate sample size in diagnostic studies. Emerg Med J. 2005 Mar;22(3):180–181. doi: 10.1136/emj.2003.011148. http://emj.bmj.com/cgi/pmidlookup?view=long&pmid=15735264 .22/3/180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013 Jan;216(1):1–14. doi: 10.1016/j.jamcollsurg.2012.09.002.S1072-7515(12)01135-0 [DOI] [PubMed] [Google Scholar]
- 33.Kawai M, Kondo S, Yamaue H, Wada K, Sano K, Motoi F, Unno M, Satoi S, Kwon A, Hatori T, Yamamoto M, Matsumoto J, Murakami Y, Doi R, Ito M, Miyakawa S, Shinchi H, Natsugoe S, Nakagawara H, Ohta T, Takada T. Predictive risk factors for clinically relevant pancreatic fistula analyzed in 1,239 patients with pancreaticoduodenectomy: multicenter data collection as a project study of pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2011 Jul;18(4):601–608. doi: 10.1007/s00534-011-0373-x. [DOI] [PubMed] [Google Scholar]
- 34.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M, International Study Group on Pancreatic Fistula Definition Postoperative pancreatic fistula: an International Study Group (ISGPF) definition. Surgery. 2005 Jul;138(1):8–13. doi: 10.1016/j.surg.2005.05.001.S0039606005002291 [DOI] [PubMed] [Google Scholar]
- 35.Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, Christophi C, Banting S, Brooke-Smith M, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey J, Greig P, Rees M, Nimura Y, Figueras J, DeMatteo RP, Büchler MW, Weitz J. Bile leakage after hepatobiliary and pancreatic surgery: A definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011 May;149(5):680–688. doi: 10.1016/j.surg.2010.12.002.S0039-6060(10)00678-1 [DOI] [PubMed] [Google Scholar]
- 36.Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Büchler MW. Postpancreatectomy hemorrhage (PPH): An International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007 Jul;142(1):20–25. doi: 10.1016/j.surg.2007.02.001.S0039-6060(07)00105-5 [DOI] [PubMed] [Google Scholar]
- 37.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Büchler MW. Delayed gastric emptying (DGE) after pancreatic surgery: A suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007 Nov;142(5):761–768. doi: 10.1016/j.surg.2007.05.005.S0039-6060(07)00301-7 [DOI] [PubMed] [Google Scholar]
- 38.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: A modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992 Oct;13(10):606–608. [PubMed] [Google Scholar]
- 39.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent J, Moreno R, Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013 Feb;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Gaag NA, Verhaar AC, Haverkort EB, Busch OR, van Gulik TM, Gouma DJ. Chylous ascites after pancreaticoduodenectomy: Introduction of a grading system. J Am Coll Surg. 2008 Nov;207(5):751–757. doi: 10.1016/j.jamcollsurg.2008.07.007.S1072-7515(08)01004-1 [DOI] [PubMed] [Google Scholar]
- 41.ICH Guideline for GOOD CLINICAL PRACTICE E6(R1) 1996. [2015-07-22]. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf .
- 42.Dindo D, Demartines N, Clavien P. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004 Aug;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae.00000658-200408000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [2015-07-21]. http://www.r-project.org/ [Google Scholar]
- 44.Schafer JL. Multiple imputation: A primer. Stat Methods Med Res. 1999 Mar;8(1):3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 45.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet Henrica CW, Standards for Reporting of Diagnostic Accuracy Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative. Ann Intern Med. 2003 Jan 7;138(1):40–44. doi: 10.7326/0003-4819-138-1-200301070-00010.200301070-00010 [DOI] [PubMed] [Google Scholar]
- 46.Chan A, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, Hróbjartsson A, Mann H, Dickersin K, Berlin JA, Doré CJ, Parulekar WR, Summerskill William S M. Groves T, Schulz KF, Sox HC, Rockhold FW, Rennie D, Moher D. SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Ann Intern Med. 2013 Feb 5;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583.1556168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Medical Association World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013 Nov 27;310(20):2191–2194. doi: 10.1001/jama.2013.281053.1760318 [DOI] [PubMed] [Google Scholar]


