Abstract
Aims
Crystal-storing histiocytosis (CSH) is a rare lesion composed of histiocytes with abnormal intra-lysosomal accumulation of immunoglobulin (Ig) as crystals, reported in patients with plasmacytic/ lymphoplasmacytic neoplasms. We report the clinicopathologic features of 13 patients with CSH and describe the proteomic composition of the crystals in 3 cases analyzed by mass spectrometry (MS).
Methods and results
There were 7 men and 6 women with a median age of 60 years (range, 33-79). CSH was generalized in 1 (8%) and localized in 12 (92%) patients involving various sites. CSH was associated with a low-grade B-cell lymphoma with plasmacytoid differentiation or a plasma cell neoplasm in all cases. In 10 (77%) cases, CSH represented more than 50% of the neoplastic infiltrate. By immunohistochemical studies, histiocytes were positive for monotypic kappa in 5 (50%), lambda in 4 (40%) cases; in 1 (10%) case, results were equivocal. MS analysis of the histiocyte contents in all 3 tested cases showed predominance of variable-region fragments of Ig light and/or heavy chains.
Conclusions
CSH is frequently associated with an underlying lymphoplasmacytic neoplasm. MS findings suggest that Ig alterations and/ or possibly defects in the ability of histiocytes to process Ig play a role in pathogenesis.
Keywords: crystal-storing histiocytosis, mass spectrometry, proteomic analysis, immunoglobulin, lymphoplasmacytic neoplasm
Introduction
Crystal-storing histiocytosis (CSH) is a rare lesion that results from the intra-lysosomal accumulation of immunoglobulins (Ig) as crystals within histiocytes. It can involve a variety of sites, such as bone marrow, lymph nodes, liver, spleen, gastrointestinal tract and kidney.1, 2 There is a localized form of CSH, which is slightly more frequent, that is confined to a single site. A generalized form that involves multiple organ sites also can occur. Crystal-storing histiocytosis is frequently associated with various types of B-cell lymphomas, plasma cell neoplasms, or rarely inflammatory disorders. 1, 3-7 Often, extensive involvement by CSH can obscure the underlying neoplasm making the recognition of the neoplasm challenging.
In the literature, CSH has been reported to show a prominent association with lymphoplasmacytic neoplasms expressing Ig kappa light chain without any association with a specific type of Ig heavy chain.8-11 These findings suggest that Ig may have a role in the pathogenesis of CSH. However, extensive studies to analyze the protein content of these crystals have not been performed.
In this study, we present the clinicopathologic characteristics of 13 patients with localized or generalized CSH. We also report the protein contents of the Ig crystals in 3 cases analyzed by mass spectrometry.
Materials and Methods
Study Group
We retrieved all CSH cases from the pathology files of our institution between 1999 and 2015. In addition, we obtained one case from a collaborating institution. Clinical and laboratory data were obtained by review of the medical record. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of each participating center. The overall collaboration was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center in Houston, Texas, USA.
Case Review and Immunohistochemical Methods
We reviewed hematoxylin-eosin stained slides prepared from formalin-fixed, paraffin-embedded tissue sections. In cases with bone marrow involvement, Wright-Giemsa–stained aspirate smears, touch imprints, and hematoxylin-eosin stained slides of aspirate clot and biopsy specimens were reviewed.
Immunohistochemical analysis was performed using 4-mm-thick formalin-fixed, paraffin-embedded tissue sections using heat-induced antigen retrieval, an avidin biotin-peroxidase complex method and an automated immunostainer (Ventana Biotech, Tucson, AZ, USA). A variable panel of antibodies was used as appropriate for establishing the diagnosis as described elsewhere.12 The antibodies used were reactive with the following antigens: CD3 (1:100) (Lab Vision; Thermo Fisher Scientifics, Fremont, CA); CD20 (1:200), CD68 (KP-1; 1:900;), CD138 (M1-15; 1:600), IgG (1:40000), IgM (1:3000), IgA (1:5000), kappa (1:20,000) and lambda (1:20,000) (Dako North America, Inc., Carpenteria, CA). Detection was performed with biotinylated secondary antibody, horseradish peroxidase and 3,3’-diaminobenzidine as a chromogen. Slides were counterstained with haematoxylin. Appropriate negative and positive control slides were run in parallel.
IgH sequencing
DNA was extracted from fixed, paraffin-embedded tissue sections using with the QIAamp DNeasy Tissue Kit (Qiagen, Valencia, CA, USA). PCR amplification of Ig heavy chain variable region (IGVH) genes was performed using consensus primers for variable and conserved regions: 5’-TGG(A/G)TCCG(A/C/G)CAG(G/C)C(T/C)CC(A/C/G/T)GG-3’ (FRII), 5’-TCGGATCCACGGC(T/C)(C/G)TGTATTACTGT-3’ (FRIII) and 5’-AACTGCAGAGGAGACGGTGACC-3’ (conserved region of JH segment). The details of the PCR conditions are described elsewhere.13 Amplification of ß-actin gene was performed to confirm that the DNA quality was adequate. Following gel electrophoresis, the PCR products from the rearranged bands underwent sequence analysis using the fluorescence dye terminator (Sanger) method performed by SeqWright DNA Technology Services (Houston, TX, USA).
Laser Capture Microdissection and Mass Spectrometry
Three cases of CSH with sufficient tissue were assessed by proteomic analysis. Areas to be analyzed were selected under bright-field microscopy from fixed, paraffin-embedded tissue sections using laser capture microdissection (Leica DM6000B Microdissection System, Wetzler, Germany). The tissue was collected into 0.5 ml microcentrifuge tube caps containing 10mM Tris/1mM EDTA/0.002% Zwittergent 3–16 (Calbiochem, San Diego, CA, USA) as described elsewhere.14 Following heating at 98°C (x 90 minutes) and sonication in a waterbath (x 60 mts), samples underwent overnight digestion with 1.5 ml of 1 mg/ml trypsin (Promega, Madison, WI, USA) at 37°C.
Following trypsin digestion and reduction with dithiothreitol, the generated products underwent separation by nanoflow liquid chromatography–electrospray tandem mass spectrometry using a ThermoFinnigan LTQ Orbitrap Hybrid Mass Spectrometer (Thermo Electron, Bremen, Germany) linked to an Eksigent nanoLC-2D HPLC system (Eksigent, Dublin, CA, USA) and recommended protocols described elsewhere.14 The raw data were analyzed using a three search algorithms (Sequest, Mascot and X!Tandem) to assign peptide and protein probability scores. The results were depicted using Scaffold (Proteome Software, Portland, OR) as a list of proteins based on the peptide spectra. The details of the settings have been described elsewhere.14
Results
Clinical Features
The study group included 7 men and 6 women with a median age of 60 years (range, 33-79). Patients presented with symptoms related to various underlying conditions as detailed in Table 1. In all patients, CSH was associated with an underlying neoplasm: extranodal marginal zone B-cell lymphoma (n=6), lymphoplasmacytic lymphoma (n=3), plasma cell myeloma (n=3), and splenic marginal zone B-cell lymphoma (n=1).
Table 1.
Clinico-pathologic characteristics of 13 cases of crystal storing histiocytosis
| Case#/ age/sex |
Symptoms prompting work-up |
Prior neoplasms |
Diagnosis | Serum paraprotein |
Sites of CSH |
BM (neoplasm /CSH) |
Histiocytes (heavy/light chain) |
Plasma cells (heavy/ light chain) |
Outcome, follow-up duration |
|---|---|---|---|---|---|---|---|---|---|
| 1/51/M | Persistent progressive abdominal pain; fatigue | None | CSH, PCM | IgG & free lambda; 0.9 g/dL | BM, stomach | Pos/ Pos | IgG/weak blush of kappa & lambda (equivocal, likely non-specific) | IgG/lambda | Unknown, 26 months |
| 2/75/M | Rib pain | None | CSH, PCM | IgG & free kappa; 1.3 & 0.1 g/dL | BM (sternum only) | Neg /Neg* | IgG/ kappa | IgG/kappa | Alive, 2 months |
| 3/46/F | Fatigue, anemia | None | CSH, PCM | IgA kappa; 1.1 g/dl | BM | Pos /Pos | NA/NA | NA/NA | Died in 33 months |
| 4/74/M | NA | Non-Hodgkin lymphoma | CSH, LPL | IgM gammopathy | Lymph node | Pos /Neg | IgM/NA | IgM/ NA | Unknown |
| 5/63/M | Persistent fatigue, lymphadenopathy | None | CSH, LPL; amyloid in abdominal soft tissue | IgM lambda; 0.6 gm/dL | Lymph node (right axillary) | Pos /Neg | NA/lambda | NA/lambda | Died, 16 months |
| 6/79/M | Fatigue, cyanosis | None | Focal CSH, LPL | IgM kappa; 2.1 g/dl | BM | Pos /Pos | NA/kappa | NA/ kappa | Died, 1 month |
| 7/43/M | Stomach cramps, rectal bleeding; stomach nodules on EGD | None | CSH, ENMZL | Absent | Stomach, esophagus | Neg/ Neg | IgA/kappa | IgA/ kappa | Alive, 36 months |
| 8/63/F | Abnormal mammogram | None | CSH, ENMZL | Absent | Breast | Neg /Neg | IgM /lambda | IgM/ lambda | Alive, 12 months |
| 9/50/F | Skin lesion | None | CSH, ENMZL | NA | Skin, upper back | NA | IgM/lambda | IgM/ lambda | Alive, 99 months |
| 10/33/F | Inner cheek mass | NA | CSH, ENMZL | NA | Inner cheek mass | NA | NA/kappa | NA/kappa | Unknown |
| 11/73/F | NA | NA | CSH, ENMZL | NA | Duodenum | NA | NA/kappa | too few plasma cells for assessment | Unknown |
| 12/58/M | NA | NA | Focal CSH, ENMZL | NA | Colon*** | NA | NA/NA | NA/NA | Died, 3 months |
| 13/68/F | Lymphocytosis | None | Focal CSH, SMZL | Absent | Spleen | Pos /Neg | NA/lambda | NA/lambda | Alive, 109 months |
No general BM involvement, only in sternum
BM, bone marrow; CSH, crystal storing histiocytosis; EGD, esophagogastroduodenoscopy; ENMZL, Extranodal marginal zone B-cell lymphoma; LPL, lymphoplasmacytic lymphoma; Neg, negative; NA, not available; Pos, positive; PCM, plasma cell myeloma; SMZL, splenic marginal zone B-cell lymphoma.
CSH involves colon only; lymphoma involves in stomach, duodenum and colon
Crystal-storing histiocytosis involved the bone marrow (n=4), gastrointestinal tract (n=3), lymph nodes (n=2), peritoneum (n=1), spleen (n=1), breast (n=1), skin (n=1) and soft tissue of the cheek (n=1). In 8 of 9 (89%) patients with adequate staging data, CSH was localized and confined to one organ system. In 1 (11%) patient who had plasma cell myeloma, CSH was generalized, extensively involving bone marrow and peritoneal cavity. Nine patients were tested for serum paraprotein: 3 patients with plasma cell neoplasm and 3 patients with LPL showed a serum paraprotein (range, 0.6 to 2.1 g/dL). Two patients with extranodal marginal zone B-cell lymphoma (MZL) and 1 patient with splenic MZL tested did not have a serum paraprotein.
Survival data were available for 10 patients, of which 5 patients were alive at last follow up. The median survival time was 32 months (range, 1.2 – 44 months). The clinical, laboratory and pathologic details of these cases are summarized in Table 1.
Histologic and Immunohistochemical Findings
Histologic findings of representative cases are demonstrated in figures 1, 2 and 3. In 10 cases, the histiocytes with crystals represented at least 50% of the total infiltrate in the biopsy specimen. In all cases, the histiocytes were cytologically benign with abundant cytoplasm and bland oval nuclei. These histiocytes contained numerous eosinophilic refractile crystals that distended their cytoplasm. Variable proportions of plasma cells and/or lymphocytes were associated with the crystal-laden histiocytes.
Figure 1.
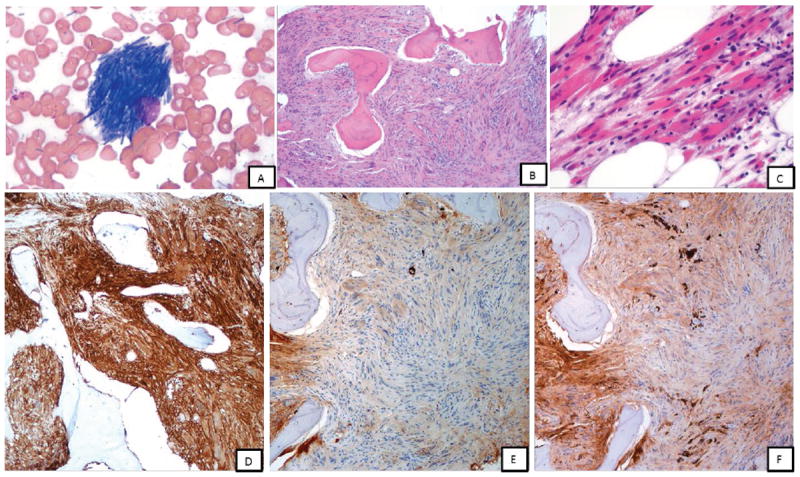
Case 1 (A-F): A, bone marrow touch imprint showing histiocytes with numerous crystalline inclusions within the cytoplasm, Giemsa-Wright stain, x1000; B-C, low and high power magnifications of bone marrow biopsy; marrow space demonstrates extensive spindle cell proliferation composed of polygonal-shaped histiocytes with crystalline cytoplasmic inclusions, H & E stain, x100 and x400; D, By immunohistochemistry, these histiocytes were positive for CD163 (x100, shown); CD68 (KP-1), SMA (focal) and IgG and negative for desmin, myogenin, EBER, melanoma cocktail, S-100, IgM, IgA (not shown); E and F, Staining for kappa and lambda immunoglobulin light chains highlight sparse plasma cells that show bright monotypic lambda staining; the spindle cells show weak blush of lambda and kappa (equivocal)
Figure 2.
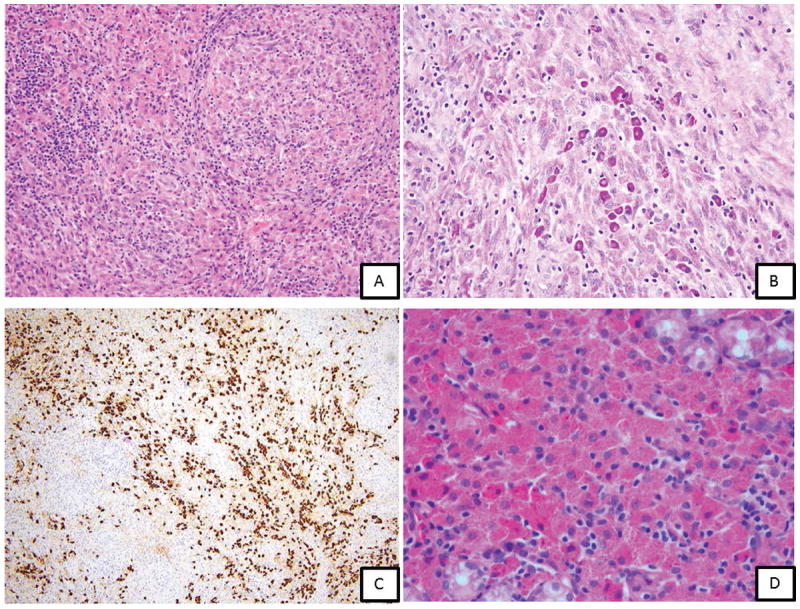
Case 4 (A-C): A 74 year old man with a history of perinephric non-Hodgkin lymphoma with lymphadenopathy and IgM gammopathy. A, Lymph node biopsy showing complete replacement of architecture by a combination of abnormal histiocytic proliferation (90%) as sheets of large bland histiocytes with pink granular crystalline cytoplasm and a lymphoplasmacytic proliferation (10%) as scattered islands with numerous Russell bodies and rare Dutcher bodies. B, PAS stain highlighting plasma cells and immunoglobulin-laden histiocytes; C, IgM positive in histiocytes and plasma cells; Case 7 (D): A 43 year old man with waxing and waning abdominal cramping. Endoscopy showed multiple, submucosal, firm, umbillicated nodules in the proximal stomach. D, Oxyntic type gastric mucosa showing crystal-storing histiocytosis associated with lymphoplasmacytic infiltrate (H & E stain, x400); Immunohistochemistry showed monotypic IgA kappa staining in CSH and lymphoplasmacytic infiltrate. PCR studies showed clonal immunoglobulin heavy chain gene rearrangement.
Figure 3.
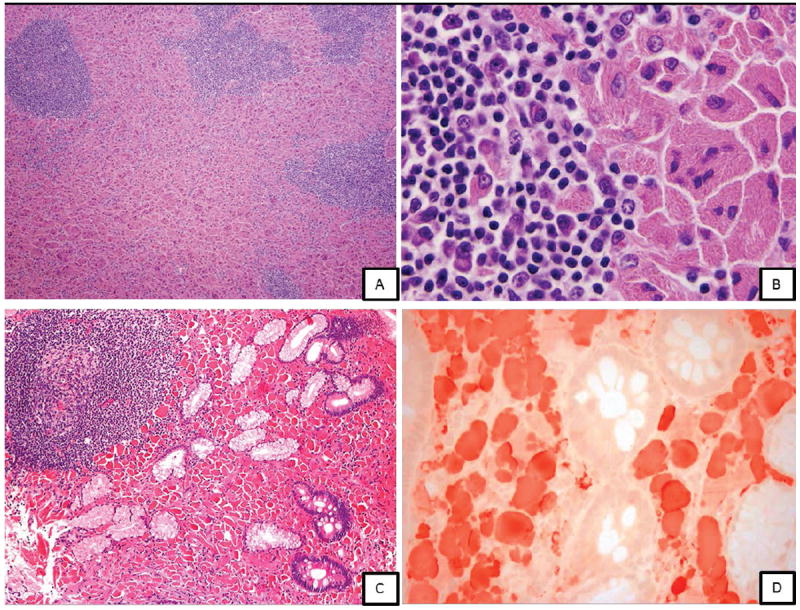
Case 5 (A, B): A, lymph node biopsy showing CSH associated with lymphoplasmacytic lymphoma (H&E, x100, H&E, x1000); Case 11 (C, D): C, Duodenal biopsy in a 73 year old woman showing CSH; D, Crystal-laden histiocytes positive for kappa
Immunohistochemical analysis in all cases showed that the histiocytes containing crystals were positive for CD68 and negative for CD138. Immunoglobulin light and heavy chain analysis was performed on 10 and 6 cases, respectively. The histiocytes were positive for monotypic kappa in 5 (50%) cases and lambda in 4 (40%) cases. In 1 case (case 1) the light chain results were equivocal. The types of heavy chains identified were IgM (n=3), IgG (n=2) and IgA (n=1). In 7 cases in which the light chain of the neoplastic cells could be assessed and compared with the histiocytes, the light was identical in both components. Notably, the intensity of staining in CSH component was weaker than the plasma cells.
Mass Spectrometry Results
Mass spectrometric proteomic analysis was successfully performed on 3 biopsy specimens (cases 1, 4 and 7) following laser capture microdissection of CSH component. Case 1 was an IgG lambda-positive plasma cell myeloma; case 4 was an IgM kappa positive LPL; and case 7 was an IgA kappa positive extranodal MZL. The highest content of the crystals in the histiocytes of these cases included Ig variable region fragments. Case 1 demonstrated fragments of Ig heavy chain variable region (IgH V-2); fragments of Ig light chain (presumably lambda) and Ig constant regions were not detected. Case 4 demonstrated fragments of both kappa chain variable (V-3) and also constant regions. In addition, there were fragments of IgM and IgG constant regions. Case 3 demonstrated only fragments of kappa variable region (V-1 and V-3); no Ig heavy chain was identified. The findings are shown in detail in figures 4 and 5.
Figure 4.
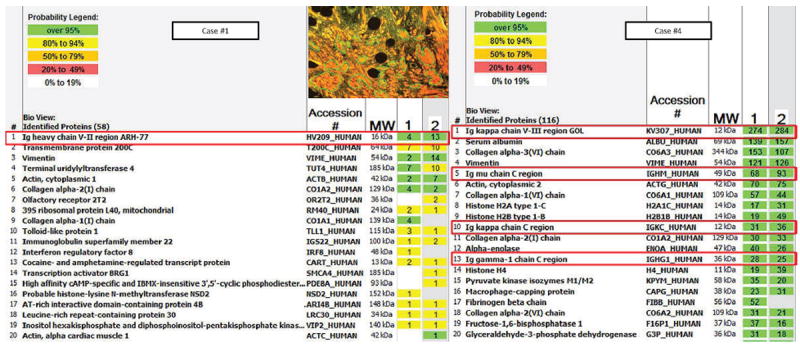
Scaffold analysis on cases 1 and 4 listing the proteins identified by mass spectrometry within the crystals based on detected peptide spectra. Columns 1 and 2 represent two different areas of microdissection. The numbers listed within each box is the number of unique peptide spectra detected of a given protein. Inset represents areas selected for analysis by laser capture microdissection.
Figure 5.
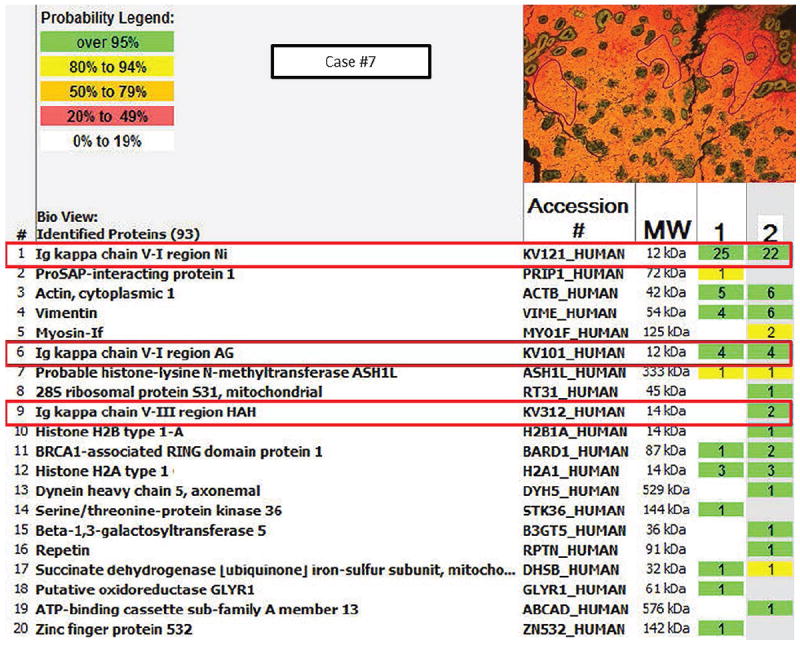
Scaffold analysis of case 7 showing the proteome components. Inset represents areas selected for analysis by laser capture microdissection.
IGH sequencing
We attempted to sequence IGH in the three cases analyzed by mass spectrometry. Monoclonal IGH rearrangement was identified in case #2 that showed IGHV1-2*04. Comparison of the sequence with the germline reference showed no mutations.
Discussion
Crystal-storing histiocytosis is a rare finding characterized by numerous histiocytes with a distinctive accumulation of intracytoplasmic Ig crystals. In this study, we present the clinical and pathologic findings of 13 cases of CSH, one of the largest case series in the literature.
Identification of CSH requires careful evaluation of histiocytic contents in an optimally stained H&E stained slide under high magnification and confirmation by immunohistochemical staining. A high degree of awareness and a thorough knowledge of the differential diagnosis are essential for performing appropriate stains. Conditions mimicking CSH include mycobacterial and fungal infections, mycobacterial spindle cell pseudotumor, malakoplakia, hemophagocytic lymphohistiocytic syndrome, storage diseases such as Gaucher’s, histiocytic lesions such as xanthogranuloma, Langerhans histiocytosis, fibrous histiocytoma, Rosai Dorfman disease and other tumors including rhabdomyoma, granular cell tumor and oncocytic neoplasms.
In this study group, localized CSH was the predominant presentation, in over 90% of patients. These lesions most often involved gastrointestinal tract and lymph nodes. These results are in contrast to the data published in literature based, on a review of 80 cases that showed relatively equal proportions of localized (58%) and generalized (42%) CSH.1 In that review, localized CSH most often involved the head and neck region and lungs.1 We do not have an explanation for the differences between our study group and the cases reported by others. Possible reasons for the discrepancy include a small number of study patients and the absence of complete staging data for 4 patients in the study group. As our institution is a referral center, referral bias is another possibility. We also suspect that cases of generalized CSH are most likely to be reported in the literature, because of their more striking clinical presentation and greater likelihood of having a complete workup.
In this study group, CSH was associated with an underlying lymphoma or plasmacytic neoplasm.1 In all patients but case 4, CSH was detected at the time of initial presentation without any preceding history of a neoplasm and therefore the initial diagnosis was challenging. In 5 patients (cases 1, 5, 7, 10 and 11), CSH formed a significant component of the lesion, and partially to more extensively obscured the underlying neoplasm, adding to the challenge. To illustrate this point, in case 1 the patient presented with progressive abdominal pain. Imaging studies showed thickening of the gastric wall and distal esophagus. Repeated open excisional biopsy specimens obtained from the stomach and lesser omentum showed a spindle cell proliferation involving fibroadipose tissue with a mixed inflammatory infiltrate and CSH was not appreciated. Subsequently, an L1 compression fracture was observed during a workup for a traumatic fall. At this time BM examination showed an extensive spindle cell proliferation associated with scattered monotypic plasma cells (shown in figure 1) and a touch imprint showed bundles of crystalline inclusions within histiocytes. Extensive immunohistochemical work-up led to the diagnosis of CSH and retrospective appreciation of CSH in the earlier biopsy specimens.
Based on these findings in this study, detection of CSH is very often a manifestation of underlying neoplasm, either lymphoma or plasma cell myeloma, and clinical workup is needed to exclude the possibility. In all cases showing CSH, appropriate immunohistochemical studies and PCR clonality assessment is recommended. Since on occasion immunohistochemical analysis of the histiocytes in CSH can be weak or non-specific, a thorough history and physical examination, imaging studies, CBC, bone marrow examination, serum and urine protein studies are particularly important. However, there are rare cases of CSH reported in literature in patients without an underlying malignancy, occurring mostly in the setting of a hyper-activated immune system, as is the case in patients with rheumatoid arthritis or Crohn disease. 1, 3, 4, 6, 15
In order to determine the protein content of the crystals within histiocytes, we used laser capture microdissection followed by tandem mass spectrometric analysis. To date, very little mass spectrometric data are available for CSH. In all three cases in this study the histiocytes contained Ig light and/or heavy chain fragments likely derived from the lymphoma or neoplastic plasma cells. Specifically, variable regions of Ig (V-1, V-3 of kappa, V-2 of IgH) were present in greatest abundance. The variable region of Ig is prone to acquiring mutations during gene rearrangement. Certain sequence alterations may impart changes in the Ig configuration that can potentially lead to protein crystallization and resistance or altered lysosomal degradation within the histiocytes, thereby leading to crystal accumulation. Alternatively, patients may have inherited or acquired histiocyte processing defects that result in Ig crystal formation.
The type of the heavy and/or light chain detected by mass spectrophotometry was concordant with the immunohistochemistry (IHC) findings. In case 3, no fragments of Ig heavy chain were detected although CSH was positive for IgA by IHC, suggesting the presence of heavy chain fragments at a much lower concentration than kappa light chain and other proteins. The presence of only Ig fragments within CSH may be the reason for weaker intensity of kappa and lambda IHC staining in histiocytes than neoplastic plasma cells. Weaker staining due to fixation artifacts and masking of antigens within the crystalline structure of the protein also cannot be excluded.
Preferential accumulation of Ig variable region fragments was also observed in two cases of CSH associated with kappa positive plasma cell neoplasms reported by others (summarized in table 2).16, 17 In one case of generalized CSH with an IgA kappa positive plasma cell neoplasm, Lebeau et al. showed excessive accumulation of kappa variable region fragments. Upon sequencing the kappa light chain, unusual amino acid substitutions were observed that conferred abnormal crystallization properties. Hence, the authors suggested a role of kappa light chain alterations in CSH pathogenesis. 16 Interestingly, analysis of the CSH contents in our case of lambda light chain associated plasma cell myeloma did not show any evidence of light chain fragments within the histiocytes. Only variable region fragments of heavy chains were identified without a constant domain. In this case, the complete absence of lambda light chain and sole representation of the variable region of IgH suggests, for the first time, the possible role of the Ig heavy chain moiety in the development of CSH, thus expanding the proposed hypothesis by Lebeau et al.16 Alterations in Ig heavy chains, such as heavy chain hinge region deletions can lead to abnormal disulphide bridge formation. Furthermore, any type of sequence change can prevent proper interaction of Ig heavy and light chains.8 Unfortunately, repeated attempts to sequence the IgH on this case were unsuccessful.
Table 2.
Results of protein composition of CSH lesions analyzed by mass spectrometry
| # | Neoplasm | Serum paraprotein | Site (lesion selected by LCM for MS analysis) | MS proteome components
|
IgH sequencing/ somatic hypermutation | |
|---|---|---|---|---|---|---|
| Heavy chain, region | Light chain, region | |||||
|
| ||||||
| 1 | Plasma cell myeloma | IgG lambda | Bone marrow (CSH, no neoplasm) | IgH, V-II* | Absent | NA |
|
| ||||||
| 2 | Lymphoplasmacytic lymphoma | IgM kappa | Lymph node (CSH, no neoplasm) | IgM, C | Kappa, V-III* | Rearranged, productive, IGHV1-2*04/ unmutated |
| IgG, C | Kappa, C | |||||
|
| ||||||
| 3 | Extranodal marginal zone | IgA kappa | Stomach (CSH-rich) | Absent | Kappa, V-I* | NA |
| B-cell lymphoma | Kappa, V-III | |||||
|
| ||||||
| 4Ref16 | Plasma cell neoplasm (MGUS) | IgA kappa | Liver | IgA, IgG | Kappa, V-I* | |
|
| ||||||
| 5Ref17 | Plasma cell neoplasm | IgA kappa | Brain | IgA, C | Kappa V-I* | NA |
| Kappa, C | ||||||
Most abundant component
C, constant region; CSH, crystal storing histiocytosis; IgH, immunoglobulin heavy chain; MS, mass spectrometry
In summary, in this study of 13 cases CSH was always associated with a lymphoma or plasma cell neoplasm. The histiocytes are often numerous and large with distended eosinophilic cytoplasm and can therefore obscure the underlying neoplasm, presenting a diagnostic challenge. Sometimes, detection of CSH can be the first clue that the patient has a lymphoma or a plasmacytic neoplasm and very rarely CSH can be a sole pathologic finding. The detection of Ig heavy or light chain variable region fragments by proteomic methods in this study suggests that alterations in Igs and/or possible defects in the ability of histiocytes to process Igs play a role in pathogenic accumulation of Ig within histiocytes.
Acknowledgments
We thank Lynn Baron for the IgH sequencing studies. Funding for this research was provided in part by the National Cancer Institute/ National Institutes of Health (R01CA138688, 1RC1CA146299, P50CA136411, and P50CA142509).
Footnotes
Author contributions
R.K-S. and K.H.Y. designed and conducted the study and performed the experimental analysis; R.K-S, Z.Y.X-M., R.N.M., A.D, D.Z., R.L, D.W., D.P.M., J.L.J, J.D.K, C.E.B.R., R.Z.O., L.J.M., and K.H.Y. contributed vital new reagents, resources, and analytical tools; A.D also performed mass spectrometric proteomic analysis; R.K-S, L.J.M., and K.H.Y. collected clinical and follow-up data under approval by the institutional review boards and the material transfer agreement, analyzed the data and wrote the manuscript; and all authors contributed vital strategies, participated in discussions, and provided scientific input.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Dogan S, Barnes L, Cruz-Vetrano WP. Crystal-storing histiocytosis: report of a case, review of the literature (80 cases) and a proposed classification. Head Neck Pathol. 2012;6:111–120. doi: 10.1007/s12105-011-0326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossi G, De Rosa N, Cavazza A, et al. Localized pleuropulmonary crystal-storing histiocytosis: 5 cases of a rare histiocytic disorder with variable clinicoradiologic features. Am J Surg Pathol. 2013;37:906–912. doi: 10.1097/PAS.0b013e31827b1618. [DOI] [PubMed] [Google Scholar]
- 3.Bosman C, Camassei FD, Boldrini R, et al. Solitary crystal-storing histiocytosis of the tongue in a patient with rheumatoid arthritis and polyclonal hypergammaglobulinemia. Arch Pathol Lab Med. 1998;122:920–924. [PubMed] [Google Scholar]
- 4.Ionescu DN, Pierson DM, Qing G, et al. Pulmonary crystal-storing histiocytoma. Arch Pathol Lab Med. 2005;129:1159–1163. doi: 10.5858/2005-129-1159-PCH. [DOI] [PubMed] [Google Scholar]
- 5.Joo M, Kwak JE, Chang SH, et al. Localized gastric crystal-storing histiocytosis. Histopathology. 2007;51:116–119. doi: 10.1111/j.1365-2559.2007.02710.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee WS, Kim SR, Moon H, et al. Pulmonary crystal-storing histiocytoma in a patient without a lymphoproliferative disorder. Am J Med Sci. 2009;338:421–424. doi: 10.1097/MAJ.0b013e3181ad3feb. [DOI] [PubMed] [Google Scholar]
- 7.Kaminsky IA, Wang AM, Olsen J, et al. Central nervous system crystal-storing histiocytosis: neuroimaging, neuropathology, and literature review. AJNR Am J Neuroradiol. 2011;32:E26–28. doi: 10.3174/ajnr.A1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green ED, Morrison LK, Love PE, et al. A structurally aberrant immunoglobulin paraprotein in a patient with multiple myeloma and corneal crystal deposits. Am J Med. 1990;88:304–311. doi: 10.1016/0002-9343(90)90159-b. [DOI] [PubMed] [Google Scholar]
- 9.Llobet M, Castro P, Barcelo C, et al. Massive crystal-storing histiocytosis associated with low-grade malignant B-cell lymphoma of MALT-type of the parotid gland. Diagn Cytopathol. 1997;17:148–152. doi: 10.1002/(sici)1097-0339(199708)17:2<148::aid-dc12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 10.Harada M, Shimada M, Fukayama M, et al. Crystal-storing histiocytosis associated with lymphoplasmacytic lymphoma mimicking Weber-Christian disease: immunohistochemical, ultrastructural, and gene-rearrangement studies. Hum Pathol. 1996;27:84–87. doi: 10.1016/s0046-8177(96)90143-4. [DOI] [PubMed] [Google Scholar]
- 11.Jones D, Bhatia VK, Krausz T, et al. Crystal-storing histiocytosis: a disorder occurring in plasmacytic tumors expressing immunoglobulin kappa light chain. Hum Pathol. 1999;30:1441–1448. doi: 10.1016/s0046-8177(99)90166-1. [DOI] [PubMed] [Google Scholar]
- 12.Kanagal-Shamanna R, Medeiros LJ, Lu G, et al. High-grade B cell lymphoma, unclassifiable, with blastoid features: an unusual morphological subgroup associated frequently with BCL2 and/or MYC gene rearrangements and a poor prognosis. Histopathology. 2012;61:945–954. doi: 10.1111/j.1365-2559.2012.04301.x. [DOI] [PubMed] [Google Scholar]
- 13.Yin CC, Medeiros LJ, Cromwell CC, et al. Sequence analysis proves clonal identity in five patients with typical and blastoid mantle cell lymphoma. Mod Pathol. 2007;20:1–7. doi: 10.1038/modpathol.3800716. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez FJ, Gamez JD, Vrana JA, et al. Immunoglobulin derived depositions in the nervous system: novel mass spectrometry application for protein characterization in formalin-fixed tissues. Lab Invest. 2008;88:1024–1037. doi: 10.1038/labinvest.2008.72. [DOI] [PubMed] [Google Scholar]
- 15.da Cruz Perez DE, Silva-Sousa YT, de Andrade BA, et al. Crystal-storing histiocytosis: a rare lesion in periapical pathology. Ann Diagn Pathol. 2012;16:527–531. doi: 10.1016/j.anndiagpath.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Lebeau A, Zeindl-Eberhart E, Muller EC, et al. Generalized crystal-storing histiocytosis associated with monoclonal gammopathy: molecular analysis of a disorder with rapid clinical course and review of the literature. Blood. 2002;100:1817–1827. [PubMed] [Google Scholar]
- 17.Orr BA, Gallia GL, Dogan A, et al. IgA/kappa-restricted crystal storing histiocytosis involving the central nervous system characterized by proteomic analysis. Clin Neuropathol. 2014;33:23–28. doi: 10.5414/NP300645. [DOI] [PMC free article] [PubMed] [Google Scholar]


