Abstract
Despite increased attention to traumatic brain injury (TBI), there remains no specific treatment and available interventions focus rather on the prevention of secondary injury. One of the reasons posited for the lack of a successful therapy is the amalgamation of various types of injuries under the same severity category in clinical trials. Informatics approaches have been suggested as a means to develop an improved classification system for TBI. As a result of federal interagency efforts, common data elements (CDEs) for TBI have now been developed. Further, the Federal Interagency Traumatic Brain Injury Research Informatics System (FITBIR) has been created and is now available for TBI researchers to both add and retrieve data. This chapter will discuss the goals, development, and evolution of the CDEs and FITBIR and discuss how these tools can be used to support TBI research. A specific exemplar using the CDEs and lessons learned from working with the CDEs and FITBIR are included to aid future researchers.
INTRODUCTION
Previously termed a silent epidemic (Centers for Disease Control, 2001), there has been increased attention to the problem of traumatic brain injury (TBI) in recent years primarily due to increased interest in sports-related concussion and combat-related TBI. This is evidenced by an increase in the number of PubMed citations on TBI (more than doubling from 1,844 in 2000 to 4,299 in 2013). Despite this increased emphasis in the biomedical and nursing literature, there remains no specific treatment for TBI and interventions continue to focus on the prevention of secondary injury. In response to this identified problem, the National Institute of Neurological Disorders and Stroke (NINDS), together with National Institute on Disability and Rehabilitation Research (NIDRR), the Defense and Veterans Brain Injury Center, and the Brain Injury Association of America, sponsored a workshop in October 2007 examining barriers to TBI clinical trial effectiveness, specifically the current classification system of TBI severity based solely on the Glasgow Coma Scale score. As a result of this workshop’s recommendations, an effort ensued to develop the common data elements (CDEs) for TBI and the Federal Interagency Traumatic Brain Injury Research Informatics System (FITBIR). This chapter discusses the goals, development, and evolution of the CDEs and FITBIR and how these tools can be used to support TBI research. A specific exemplar using the CDEs and lessons learned from working with the CDEs and FITBIR are included to aid future researchers.
Recommendations from the October 2007 Workshop for the Classification of TBI for Targeted Therapies specifically identified that (a) a set of CDEs should be developed and instituted in collaboration with the NINDS CDEs initiative and (b) a new databank should be launched in order to allow for data sharing and analysis (Saatman et al., 2008). The development of a new databank was desired as much of the current evidence base for the treatment of severe TBI came from the analysis of a similar resource, the U.S. Traumatic Coma Data Bank (TCDB). However, the TCDB data was gathered in the 1980s and focused solely on severe TBI, so it was viewed as outdated and too limited in scope. The development of the new database was recommended to characterize injury patterns across the life span and across injury severities and to improve injury classification, diagnosis, and treatment (Saatman et al., 2008).
DEVELOPMENT OF THE CDEs FOR TBI
The overarching purpose of the NINDS CDE Project (www.commondataelements.ninds.nih.gov) is to standardize data acquisition so that it is collected similarly across studies for the same constructs and to foster the movement of data into actionable information by enabling comparison across studies (National Insitute of Neurologic Disorders and Stroke, 2014). In response to the 2007 workshop recommendations, the Interagency Common Data Elements Project for TBI was launched in 2008 and the first set of recommendations for CDEs for TBI in adults was published (Version 1 [V1]) in 2010. CDEs V1 included various domains for data collection including demographics and clinical assessment, trial protocols, outcome, neuroimaging, and biomarkers (Haacke et al., 2010; Maas et al., 2010, 2011; Manley et al., 2010). The primary emphasis of V1 was the coding of data. Each data element identified in CDEs V1 had three levels of coding—basic, intermediate, and advanced—to allow for crosswalking of data from multiple studies when collapsed to the “basic” level if measured at different levels of specificity (Maas et al., 2011). Data elements were further classified as “core,” “supplemental,” or “emerging.” Data elements regarded as “core” were recommended to be collected for all clinical studies of TBI. This first version of the CDEs was subsequently followed by the publication of the pediatric CDEs for TBI (Adelson et al., 2012; Berger, Beers, Papa, & Bell, 2012; Miller, Odenkirchen, Duhaime, & Hicks, 2012).
Following the publication of the adult and pediatric CDEs for TBI V1, it was recognized that some modification and revision was necessary. There were several limitations with V1, including the realization that more than half of the 480 CDEs were classified as “core” and many of these were highly specific to population or setting and not broadly applicable (Hicks et al., 2013). Further, the first version lacked recommendations for mild TBI and was viewed as more focused on the acute phase following TBI. New workgroups, organized around the type of study (epidemiologic, acute hospitalized, rehabilitation for moderate-severe, and mild TBI/concussion), were then formed in 2012 to revisit the TBI CDEs. The specific procedures followed by the workgroups are detailed by Hicks and colleagues (2013). An emphasis of the workgroups was to clearly distinguish “core” elements from others in order to make data collection of the CDEs across multiple studies more feasible. This resulted in the reduction of core elements from 242 to 16. Those CDEs recommended data collection specific to the type of study questions (e.g., acute hospitalized or epidemiology). Data elements considered necessary to these study types were categorized as “basic” and all others as “supplemental” (replacing “emerging” category from V1). Significantly, draft CDEs were subject to external review prior to finalization and were endorsed by the American Association of Neurological Surgeons and the American Congress of Rehabilitation Medicine. The resulting Version 2 of the TBI CDEs is publically available at www.commondataelements.ninds.nih.gov/TBI.aspx#tab=Data_Standards. One excellent feature of the CDE website is that case report forms for the CDEs including questionnaires and instruments can be directly accessed from the website. Tests, tools, and questionnaires included in the CDEs are provided with a brief description and references supporting validity and reliability. For those instruments or tools that are copyrighted or trademarked, information is provided on how to obtain permission to use the resource.
EXEMPLAR OF CDE USE IN TBI RESEARCH
To date, two studies have published their experiences with implementing the V1 TBI CDEs in a prospective (Yue et al., 2013) and a retrospective (Stead et al., 2013) study, respectively. We recently consulted the TBI CDEs in the development of the Impact of Aging on the Immune Response to Injury (AIm:TBI) study protocol. The design is a prospective cohort study, which follows subjects to 6 months post injury or from enrollment for age- and gender-matched controls. The goal of the AIm:TBI study is to test a model of impairment and disability following mild TBI in adults in which aging modulates the immune response following TBI. The model is based on the Institute of Medicine’s Disability Framework (Pope & Tarlov, 1991) and examines measures in four domains, injury, impairment, functional limitation, and health-related quality of life, as well as transitional factors, which modify the individual response across all domains (see Figure 1.1).
FIGURE 1.1.
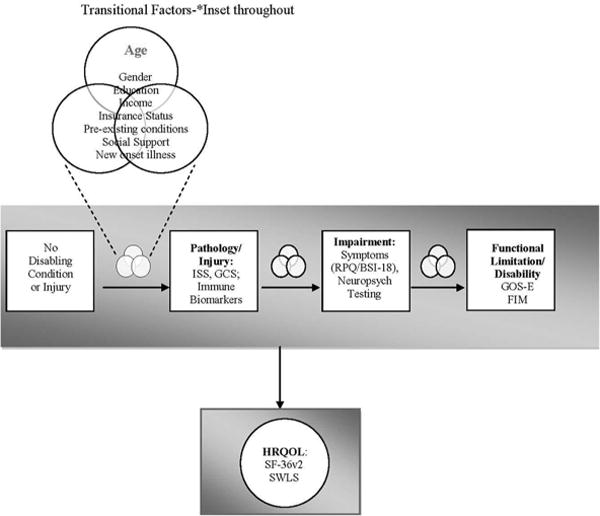
Conceptual model tested in the AIm:TBI study with associated common data elements.
Note. ISS = Injury Severity Score; GCS = Glasgow Coma Scale; RPQ = Rivermead Post-concussion questionnaire; BSI-18 = Brief Symptom Inventory-18; GOS-E = Glasgow Outcome Scale-Extended; FIM = Functional Independence Measure; SF-36 = MOS Short Form-36; SWLS = Satisfaction with Life Scale.
In study design, we referenced recommendations from the TBI CDEs to help tailor this study. This included reviewing core elements as well as relevant basic elements from the mild TBI, epidemiologic, and biospecimen and biomarker elements for their fit with our proposed model (Figure 1.1; Table 1.1). We also examined the CDEs for their specific applicability and validity with older adult populations.
TABLE 1.1.
Mapping of AIm:TBI Study Domains and Measures to Common Data Elements
| Domain | Measure | Common Data Elements | Core, Basic, or Supplementala |
|---|---|---|---|
| Transitional factors | Age | Birth date (age calculated from date of birth) | Core |
| Insurance status | Type of insurance | Supplemental | |
| Gender | Gender | Core | |
| Race/ethnicity | U.S. racial category; ethnicity | Core | |
| Education | Education level | Core | |
| Income | Employment status; income range | Core; supplemental | |
| Preexisting conditions | Medical history conditions coded by SNOMED | Core | |
| Social support | MOS Social Support Survey | Supplemental | |
| Pathology: injury | Injury | GCS: motor, eye, verbal, total score | Core |
| Loss of consciousness indicator | Basic | ||
| Posttraumatic amnesia indicator | Basic | ||
| Abbreviated Injury Scale region and scores | Basic | ||
| Injury mechanism E-code | Core | ||
| Findings from head imaging if any | Core | ||
| Impairment: symptoms | Symptom presence and burden | Rivermead Postconcussion Symptom Questionnaire (RPQ) | Basic |
| Brief Symptom Inventory-18 (BSI-18) | Basic | ||
| Neuropsychological impairment | Rey Auditory Verbal Learning Test (RAVLT) | Basic | |
| Trail Making Test (TMT) | Basic | ||
| Processing Speed Index from Wechsler Adult Intelligence Scale | Basic | ||
| Functional limitation/disability | Functional change/postinjury disability | Functional Independence Measure | Basic (motor and cognitive subscales only) all others supplemental |
| Glasgow Outcome Scale-Extended (GOS-E) | Core | ||
| Quality of life | Health-related quality of life | MOS Short Form-36 | Supplemental |
| Satisfaction with Life Scale (SWLS) | Basic |
Note. AIm:TBI = Impact of Aging on the Immune Response to Injury; E-code = External cause of injury code; GCS = Glasgow Coma Scale; MOS = Medical Outcomes Study; SNOMED = Systematized Nomenclature of Medicine.
The study questions of the AIm:TBI study fit both the epidemiologic and mild TBI/concussion research categories; data elements noted as basic may be for only one or both of the categories listed.
We were able to easily use CDEs in place of elements used in the pilot study to allow for the improved harmonization of the present study with that of other researchers. For example, for our construct of “Impairment” (Figure 1.1), we exchanged the head injury symptom checklist (HISC) used in the pilot study with the CDE Rivermead Postconcussion Symptom Questionnaire (RPQ). To enrich the assessment in some areas, we chose to add supplemental measures (e.g., social support; Short Form [SF]-36) to the study protocol.
We implemented the study protocol using the CDEs; data collection is ongoing. Subjects are able to complete visits within a 60- to 90-minute time-frame including all outcome measures and do not report the testing to be burdensome. The AIm:TBI study presently maintains >90% retention to 6 months, with more than 225 of 300 planned subjects recruited. Thus, researchers interested in using the CDEs should be able to balance the quality and quantity of data collected with subject burden.
The implementation of the CDEs into the study protocol required some additional staff training. For example, research staff were trained and validated by a licensed neuropsychologist to administer the recommended basic CDE neuropsychological tests. This training was able to be completed within the planned 3-month startup period. The neuropsychologist also performs ongoing review of a subset of the subject testing. Investigators planning to use neuropsychological testing should include these costs in planned study budgets.
In planning for the use of the Brief Symptom Inventory-18 (BSI-18), a mental health protocol was developed, should subjects endorse anxiety, depressive symptoms, or suicidal ideation. In the initial 2 years of the study, we have had approximately 20% of subjects enrolled endorse suicidal ideation within the prior week and have needed to implement the mental health protocol for further evaluation and referral. The prevalence of suicidal ideation in this sample of individuals in the acute/subacute period following mild TBI is similar to that reported in a prior study of chronic mild TBI with a mean length since injury of 5 years (Tsaousides, Cantor, & Gordon, 2011). Based on the prevalence, we strengthened our mental health protocol by having a psychiatrist specializing in the care of injured patients available for evaluation as needed. Research staff were also provided with the opportunity for additional specialized training to address suicidal ideation. Researchers planning to use the BSI-18 in similar samples should plan to have similar protocols and referral resources available.
FITBIR: CONSIDERATIONS FOR RESEARCHERS
FITBIR (https://fitbir.nih.gov/) was launched in mid-2012. It takes a “big data” approach, and its stated purpose is “to help accelerate TBI research by creating an infrastructure that integrates heterogeneous data sets allowing access to much more quality research data than an investigator would be able to collect independently” (FITBIR, 2014). FITBIR implements the data dictionary developed from the TBI CDEs. Investigators planning to submit applications for the funding of TBI research to the National Institutes of Health (NIH) or other federal sources like the Department of Defense should carefully read funding announcements to determine if they are required to submit to FITBIR and include this information in the data-sharing plan of the grant application.
An application approval process is in place in order for investigators to contribute data to or access data from FITBIR. In setting up their study databases, investigators need to carefully review the data dictionary in order to upload data meeting FITBIR quality standards. One of the challenges with the current data dictionary is that many FITBIR elements are coded alphanumerically rather than numerically. This can become a challenge if the use of a single data set for both FITBIR upload and data analysis is desired. The conversion of the study dataset from one format to the other may be required, necessitating additional human resources with informatics expertise for data management. It is critical that investigators have the necessary resources, both human and financial, to support data management associated with meeting FITBIR data-sharing requirements. FITBIR provides a cost model and project estimation tool for researchers to assist in estimating the necessary resources (FITBIR, 2014). Technical support is available to researchers through weekly FITBIR users’ conference calls and individual project consultations.
For investigators with approved projects, data are submitted quarterly to FITBIR. Investigators identify which data are considered experimental (i.e., testing study hypotheses). Core and basic CDEs used for experimental purposes are made available to other researchers contributing data to FITBIR 6 months following the end of the study funding period and 12 months for all other researchers (FITBIR, 2014). This is an important deadline for researchers to consider when planning for the dissemination of project findings.
In order to allow researchers to share data regarding individual subjects without disclosing personally identifiable information (PII), researchers create global unique identifiers (GUIDs) using a local tool. Pseudo-GUIDs are available for submitting data from retrospective studies when the data required to create one is not available. The generated GUID is a random number that does not contain any PII. The specific information required to create a GUID includes full name at birth; date of birth including month, day, and year; city and country of birth; and sex at birth. Therefore, investigators who will be submitting data to FITBIR from prospective studies need to include these items in their data collection plans. Additional tools available from FITBIR are the Protocol & Form Research Management System, which allows for the creation of case report forms linked to the FITBIR data dictionary, and the Medical Image Processing, Analysis, and Visualization, which is used for imaging data.
CONCLUSIONS
Integrating the CDEs for studies of TBI is feasible and does not add substantively to subject or researcher burden. In many cases, the recommended basic CDEs for each category may overlap, and it is up to the individual researcher to determine which is best for the planned study and sample. Newer computer-adapted tests such as the Patient Reported Outcomes Measurement Information System (PROMIS) measures and the NIH toolbox for neurological function hold some promise to address this gap, but require validation in TBI patients prior to widespread adoption. Depending on the CDEs selected for use, some additional staff training and validation may be needed prior to implementing the study protocol and may have implications for the study budget.
Building on the foundation of the TBI CDEs, FITBIR holds promise for accelerating TBI research by leveraging data from multiple studies. Investigators contributing data should consult the FITBIR/CDE data dictionary carefully in planning for database and case report form setup in order to minimize the post-processing of study data. It is also critical that researchers have adequate data management and informatics support on the study team. FITBIR should be an invaluable resource in the future for secondary data analysis as there are clear quality standards for the studies accepted to contribute data. Presently data available for analysis in FITBIR are limited as the project was recently launched. However, it should eventually allow for the examination of research questions that cannot presently be answered through a single study or through existing databases.
Acknowledgments
This work was supported, in part, by a grant from the NIH/National Institute of Neurologic Diseases and Stroke R01NS077913. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the granting agency.
References
- Adelson PD, Pineda J, Bell MJ, Abend NS, Berger RP, Giza CC, et al. Common data elements for pediatric traumatic brain injury: Recommendations from the working group on demographics and clinical assessment. Journal of Neurotrauma. 2012;29(4):639–653. doi: 10.1089/neu.2011.1952. http://dx.doi.org/10.1089/neu.2011.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger RP, Beers SR, Papa L, Bell M. Common data elements for pediatric traumatic brain injury: Recommendations from the biospecimens and biomarkers workgroup. Journal of Neurotrauma. 2012;29(4):672–677. doi: 10.1089/neu.2011.1861. http://dx.doi.org/10.1089/neu.2011.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Traumatic Brain Injury in the United States: A report to Congress. Atlanta, GA: CDC; 2001. [Google Scholar]
- Federal Interagency Traumatic Brain Injury Research Informatics System (FITBIR) FAQs. 2014 doi: 10.1891/0739-6686.33.1. Retrieved August 29, 2014 from https://fitbir.nih.gov/jsp/about/faqs.jsp. [DOI] [PMC free article] [PubMed]
- Haacke EM, Duhaime AC, Gean AD, Riedy G, Wintermark M, Mukherjee P, et al. Common data elements in radiologic imaging of traumatic brain injury. Journal of Magnetic Resonance Imaging. 2010;32(3):516–543. doi: 10.1002/jmri.22259. http://dx.doi.org/10.1002/jmri.22259. [DOI] [PubMed] [Google Scholar]
- Hicks R, Giacino J, Harrison-Felix C, Manley G, Valadka A, Wilde EA. Progress in developing common data elements for traumatic brain injury research: Version two—The end of the beginning. Journal of Neurotrauma. 2013;30(22):1852–1861. doi: 10.1089/neu.2013.2938. http://dx.doi.org/10.1089/neu.2013.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas AI, Harrison-Felix CL, Menon D, Adelson PD, Balkin T, Bullock R, et al. Standardizing data collection in traumatic brain injury. Journal of Neurotrauma. 2011;28(2):177–187. doi: 10.1089/neu.2010.1617. http://dx.doi.org/10.1089/neu.2010.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas AI, Harrison-Felix CL, Menon D, Adelson PD, Balkin T, Bullock R, et al. Common data elements for traumatic brain injury: Recommendations from the interagency working group on demographics and clinical assessment. Archives of Physical Medicine and Rehabilitation. 2010;91(11):1641–1649. doi: 10.1016/j.apmr.2010.07.232. [DOI] [PubMed] [Google Scholar]
- Manley GT, Diaz-Arrastia R, Brophy M, Engel D, Goodman C, Gwinn K, et al. Common data elements for traumatic brain injury: Recommendations from the biospecimens and biomarkers working group. Archives of Physical Medicine and Rehabilitation. 2010;91(11):1667–1672. doi: 10.1016/j.apmr.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Miller AC, Odenkirchen J, Duhaime AC, Hicks R. Common data elements for research on traumatic brain injury: Pediatric considerations. Journal of Neurotrauma. 2012;29(4):634–638. doi: 10.1089/neu.2011.1932. http://dx.doi.org/10.1089/neu.2011.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Neurologic Disorders and Stroke (NINDS) NINDS Common Data Elements. 2014 Retrieved August 29, 2014 from http://www.commondataelements.ninds.nih.gov/#page=Default.
- Pope AM, Tarlov AR, editors. Disability in America: A National Agenda for prevention. Washington, DC: National Academies Press; 1991. [Google Scholar]
- Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT. Classification of traumatic brain injury for targeted therapies. Journal of Neurotrauma. 2008;25(7):719–738. doi: 10.1089/neu.2008.0586. http://dx.doi.org/10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead LG, Bodhit AN, Patel PS, Daneshvar Y, Peters KR, Mazzuoccolo A, et al. TBI surveillance using the common data elements for traumatic brain injury: A population study. International Journal of Emergency Medicine. 2013;6(1):5. doi: 10.1186/1865-1380-6-5. http://dx.doi.org/10.1186/1865-1380-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaousides T, Cantor JB, Gordon WA. Suicidal ideation following traumatic brain injury: Prevalence rates and correlates in adults living in the community. Journal of Head Trauma Rehabilitation. 2011;26(4):265–275. doi: 10.1097/HTR.0b013e3182225271. http://dx.doi.org/10.1097/HTR.0b013e3182225271. [DOI] [PubMed] [Google Scholar]
- Yue JK, Vassar MJ, Lingsma HF, Cooper SR, Okonkwo DO, Valadka AB, et al. Transforming research and clinical knowledge in traumatic brain injury pilot: Multicenter implementation of the common data elements for traumatic brain injury. Journal of Neurotrauma. 2013;30(22):1831–1844. doi: 10.1089/neu.2013.2970. http://dx.doi.org/10.1089/neu.2013.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]


