Abstract
Many studies have examined the association between SLC6A3 3′-UTR VNTR polymorphism and smoking cessation; however, the results are inconclusive, primarily because of the small to moderate-size samples. The primary goal of this study was to determine whether this polymorphism has any effect on smoking cessation by a meta-analysis of all reported studies. We adopted a 9-repeat dominant model that considers 9-repeat and non 9-repeat as two genotypes and compared their frequencies in former vs. current smokers. Eleven studies with 5,480 participants were included. Considering the presence of study heterogeneity and differences in the availability of information from each study, three separate meta-analyses were performed with the Comprehensive Meta-Analysis statistical software (v. 2.0). The first meta-analysis provided evidence of association between the 9-repeat genotype and smoking cessation under the fixed-effects model (pooled odds ratio [OR] 1.13; 95% confidence interval [CI] 1.01, 1.27; P = 0.037) but not in the random-effects model (pooled OR 1.11; 95% CI 0.96, 1.29; P = 0.159). Given the marginal evidence of heterogeneity among studies (P = 0.10; I2 = 35.9%), which likely was caused by inclusion of an Asian-population treatment study with an opposite effect of the polymorphism on smoking cessation, we excluded these data, revealing a significant association between the 9-repeat genotype and smoking cessation under both the fixed- and random-effects models (pooled OR 1.15; 95% CI 1.02, 1.29; P = 0.02 for both models). By analyzing adjusted and unadjusted results, we performed the third meta-analysis, which showed consistently that the 9-repeat genotype was significantly associated with smoking cessation under both the fixed- and random-effects models (pooled OR 1.17; 95% CI 1.04, 1.31; P = 0.009 for both models). We conclude that the 3′-UTR VNTR polymorphism is significantly associated with smoking cessation, and smokers with one or more 9-repeat alleles have a 17% higher probability of smoking cessation than smokers carrying no such allele.
Keywords: Caucasians, Dopamine transporter, SLC6A3, Smoking Cessation, VNTR
Introduction
Although smoking, a chronic and relapsing addictive trait, has been related to a series of diseases, the prevalence of smoking is still high or increasing in some regions such as some Asian countries. Smoking leads to nearly 6 million deaths worldwide each year, with more than 5 million of those deaths resulting from direct tobacco use and more than 600,000 non-smokers dying secondary to second-hand smoke exposure.1 Thus, it is urgent that clinicians develop effective pharmacological methods of smoking cessation. There are many treatments available, such as bupropion, varenicline, and nicotine replacement therapy combined with behavioral counseling, but all have a relatively low success rate.
Many factors have been elucidated to explain the modest effectiveness of these medicines; e.g., genetic and environmental factors. Twin and family studies have shown that smoking behaviors are highly influenced by genetics.2–7 The heritability of smoking cessation is estimated to be approximately 50%.8, 9 To better understand the molecular mechanism underlying smoking cessation and to improve the strength of pharmacological treatments, researchers have focused on identifying genetic susceptibility factors for various smoking-related phenotypes, including cessation, as investigated in this study.
The dopaminergic reward pathway is widely implicated in the etiology of smoking and the development of nicotine dependence. Consequently, genes with a dopaminergic function have been considered promising candidates in smoking studies. Many studies have implicated a group of candidate genes in smoking behaviors.10–12 Nicotine, one of the important components of tobacco smoke, increases the synaptic concentrations of dopamine to a higher concentration than those triggered by natural rewards, which is considered a partial moderating factor of smoking persistence. Therefore, it is reasonable to infer that genes regulating extracellular dopamine concentrations are related to the risk of developing nicotine dependence and the ability to quit smoking. Smokers carrying the variants in those candidate genes associated with an increased synaptic concentration of dopamine are presumed to be more likely to quit smoking.13
The dopaminergic pathway consists of three sections: dopamine transporters (DAT), dopamine receptors, and enzyme targets, which collectively regulate the extracellular dopamine concentration. The DAT is a membrane-spanning protein that removes dopamine from the synapse,14–17 a primary way to stop the signal of dopamine neurotransmission. Consequently, numerous studies18–21 have concentrated on determining whether variants in SLC6A3 (also called DAT1), which encode the DAT protein, are associated with smoking behaviors, including cessation.
A 40-base-pair variable number tandem repeat (VNTR) in the 3′ un-translated region (UTR) of the SLC6A3 gene, consisting of 3–16 repeats, is frequently investigated in smoking cessation studies.19, 20, 22–25 In all reported studies in various ethnic populations, the common alleles of 3′-UTR VNTR polymorphism are the 10-repeat and 9-repeat. Other rare alleles are excluded in certain studies because of their low frequency. Although inconsistent results remain,26, 27 several functional studies28–31 have demonstrated that the 3′-UTR VNTR 9-repeat allele is associated with a risk of reduced DAT availability, which may weaken the dopaminergic function of DAT reuptake with increasing synaptic concentrations of dopamine. Therefore, it is plausible that the 3′-UTR VNTR 9-repeat allele plays a vital role in regulating the process of smoking cessation. Sabol et al.19 first reported a significant supportive association of SLC6A3 3′-UTR VNTR 9-repeat polymorphism with smoking cessation. However, this association was not replicated by follow-up studies.22, 23, 25, 32–34 Although negative or totally opposite results have been published, the general results indicate that smokers who carry one or more 9-repeat alleles are more likely to quit smoking than smokers who carry no such allele.
To date, two meta-analyses regarding on the association between SLC6A3 3′-UTR VNTR and smoking cessation have been reported.35, 36 Selecting three cross-sectional studies for meta-analysis, Munafo et al.35 found no significant association between this polymorphism and smoking cessation. Stapleton and coworkers 36 reported an independent meta-analysis including five studies with seven cohorts of subjects, performing two separate meta-analyses: one for primary data without adjustment for other variables and another for adjusted data where cohorts within studies were grouped into separate samples, and some odds ratios (ORs) were adjusted for age and sex. Their results from the unadjusted data suggested a trend toward smokers with 9-repeat genotypes having a greater likelihood of smoking cessation (OR 1.15; 95% confidence interval [CI] 0.97, 1.37; P = 0.08). From the adjusted data, there existed a statistically significant association of SLC6A3 3′-UTR 9-repeat genotypes with smoking cessation (OR 1.20; 95% CI 1.01, 1.37; P = 0.04). Since then, several more studies have been reported, including some with much larger samples; thus, it is important to conduct an updated meta-analysis regarding the effect of this polymorphism on smoking cessation.
Materials and Methods
Studies search strategy
Studies on the relation between SLC6A3 3′-UTR VNTR and smoking behaviors were selected from PubMed. The key words used were “dopamine transporter,” “DAT,” “DAT1,” “SLC6A3,” “smoking,” “nicotine,” “cigarette,” and “tobacco.” These key words underwent as many reciprocal combinations as possible to identify all the relevant studies. Electronic abstracts were reviewed according to the suggested standard inclusion and exclusion criteria by Moher et al.37 We also checked the references from the selected studies to find potentially additional studies that were not indexed by PubMed.
Study inclusion and data extraction
As reported by other researchers,35, 36 we used a comparison of former smokers with current smokers to define the phenotype of smoking cessation for those cross-sectional studies. By using a systematic reviewing process with appropriate selection criteria (see Supplementary Figure S1 for details), 13 papers on the association of SLC6A3 3′-UTR VNTR genotypes with smoking cessation were examined for possible inclusion. Because the data used in the David study38 consisted of the data from two other independent studies,39, 40 we excluded the study with the pooled sample and only included the two original studies for our current meta-analysis. Thus, a total of 12 smoking cessation-related studies were included in this meta-analysis, which consisted of four cross-sectional studies,19, 22, 23, 25, 41 one no-treatment longitudinal study,20 and six treatment longitudinal studies.32–34, 38, 41, 42 In the cross-sectional studies, surveyed participants were classified as either former smokers or current smokers. For the longitudinal studies, surveyed participants included only smokers who were followed over time and the proportion of those who have quitted at the time of follow-up was used to determine abstinence rate. By using a structured spreadsheet, we extracted the following data from the studies: authors and year of publication, type of study, sample origin, sample size, characteristics of participants (sex and ethnicity), and smoking status categorized according to the 3′-UTR VNTR genotype.
Classification of phenotypes and genotypes
In these cross-sectional studies, classification of smoking status was depended on self-report. Both Sabol et al. 19 and Vandenbergh et al. 23 defined current smokers as those who not only had smoked at least 100 cigarettes in their lifetimes but who also smoked either daily or occasionally at the time of the survey and former smokers as those who had smoked at least 100 cigarettes but not smoked immediately before the survey. In two other cross-sectional studies,22, 25 participants were classified as current or former smokers according to their answers to the following questions: “Are you currently smoking?” and “Have you ever smoked regularly?” Furthermore, the classification method of the non-treatment longitudinal study20 depended on the responses of participants who were smokers at baseline to the questions one year later, and biochemical verification as well.
In the treatment studies, participants included only smokers who were seeking to quit. Except for two studies reported by Ton et al.42 and Swan et al.,32 which classified subjects on the basis of a self-report, not only were the quitters in other treatment studies identified by self-report, their answers were confirmed by the expiratory CO or salivary cotinine concentration. Since smoking cessation is a dynamic process interrupted by relapses, the power to detect both treatment effects and association of the polymorphism with smoking cessation declines as the follow-up time increases after smoking cessation treatment. Between or within these studies, the point-prevalent smoking status was checked at various time frames. Thus, it was relatively difficult to determine which point-prevalent smoking result should be included. For consistency with previous meta-analyses,36 we used the data of these studies, 33, 34, 39–41 which were checked at the end of treatment (EOT) for meta-analysis, although O’Gara and colleagues indicated significant evidence of the effect of 3′-VNTR polymorphism on smoking cessation after 1 week post quit day. Furthermore, David et al.38 reported the abstinence at EOT, and Tashkin et al.41 presented the smoking status of participants at baseline. In the other two studies,32, 42 the investigators checked the prevalence of smoking at 12-month follow-up, as well as persistent smoking status. Although the cessation rate at the 12-month follow-up was lower than at the EOT, we used the point-prevalent smoking data from the two studies for this meta-analysis.
The 3′-UTR VNTR 9- and 10-repeat alleles were genotyped in all the studies (Supplementary Table 1). Although rare alleles of 3′-UTR VNTR were excluded because of the very low allele frequencies in some studies,20, 23, 33, 41 accumulating studies19, 22, 25, 32, 34, 38, 42 documented a corpus of data without ruling out the rare alleles. Furthermore, a dominant 9-repeat model, which assumes that carriers of the 9-repeat allele have a higher cessation rate than carriers of the no 9-repeat allele do, was adopted in all these studies. In this study, we applied the dominant 9-repeat model where the comparison is 9/* genotypes including 9/9, 9/10, and 9/X (X stands for variants other than 9 or 10 alleles) genotypes compared with 10/10 and other rare genotypes, which was different from the comparison of Stapleton et al.36 (9/9 and 9/10 genotypes vs. 10/10 genotypes).
Statistical analysis
Three meta-analyses were carried out to estimate the pooled odds ratio (OR) and 95% confidence interval (CI) of association of 3′-UTR VNTR 9/* genotypes with smoking cessation. The first two meta-analyses were based on the raw and unadjusted data extracted from the chosen studies. Given that Sabol et al. described the ORs of the variant in three cohorts stratified by different protocols,19 and that two of the studies reported two separate ORs adjusted for moderating factors including age and sex,20, 23 we analyzed the refined data, which included both adjusted and unadjusted data for the third meta-analysis.
Both random-effects and fixed-effects models were used. For the random-effects model, through using DerSimonian and Laird methods,43 the effect sizes of individual studies were calculated to generate a pooled OR and 95% CI. The effect sizes of the individual studies using the fixed-effects model were pooled using Mantel-Haenszel methods.43 Compared with the fixed-effects model, which assumes the effect sizes of different studies are identical and considers only within-study variation, the random-effects model is more conservative and generates a wider confidence interval by considering both between- and within-study variation. Therefore, the random-effects model is more applicable when heterogeneity exists; otherwise, use of the fixed-effects model is encouraged. The heterogeneity among studies is examined using a Q and I2 statistical test.44, 45 If the P value of the Q test is ≤ 0.05, there is significant evidence of heterogeneity among studies. The I2 test, which is related to Q value and degree of freedom, is used to judge the percentage of variation across studies which is caused by heterogeneity rather than chance. For example, when the value of I2 is 40%, meta-analysis results can inflate the percentage of variation across all included studies not explained by genotype to 40%, suggesting the presence of heterogeneity among studies.44 This shows moderate evidence of heterogeneity between studies if the I2 value is > 50%.45 The significance of the pooled OR is determined by a Z statistical test.
By plotting the log odds ratio of each individual study against the standard error of its log odds ratio, we applied the funnel plot, which assumes larger studies will be distributed near the average and smaller studies will be equally distributed on both sides of the average, to assess potential publication bias. When some studies deviate from the funnel-shaped distribution, the funnel plot is asymmetrical, suggesting the presence of publication bias. We also used Egger regression to assess the significance of publication bias.46 All of these analyses were performed with Comprehensive Meta-Analysis statistical software (v. 2.0).
Results
Basic information on the included studies
We included 12 studies published between 1999 and 2013 (Table 1). A total of 5,401 participants were extracted from the studies, of which the sample sizes ranged from 225 to 864. Except for the Han study,34 which was focused on subjects of Asian origin, all studies were primarily of Caucasian populations. There were different sex ratios in these studies. In particular, the samples of Ton et al. 42 and Han et al. 34 consisted of females only and males only, respectively. Furthermore, some studies 19, 22, 23, 25, 32, 42 classified the point-prevalent smoking status only by self-report, whereas other studies used both self-report and biochemical verification to define abstinence status. Although there was a wide range of cessation rates, from 20.2% to 61.0%, the three lowest cessation rates (i.e., 20.2%, 24.9%, and 33.1%) were recorded in three longitudinal studies20, 32, 42 in which the prevalence of smoking was recorded at a 12-month follow-up, whereas other studies checked smoking status at EOT or a time-point less than 12 months. A total of 2,337 (43.3%) of the 5,401 participants were identified with 9/* genotypes, which is in line with the frequencies in Caucasian-based studies, ranging from 31.3% to 51.1%, whereas it was only 10.2% in the Asian-based study.34
Table 1.
Characteristics of each study included in this meta-analysis (N = 5,401 participants)
| Study Name (Sample Origin) | Publication Year | Sample Size | % Caucasian | % Male | % Cessation (Measurement Method) | 9/* Genotype Frequency (%) |
|---|---|---|---|---|---|---|
| Sabol et al.19 (Israel) | 1999 | 514 | 87.5 | 47.5 | 44.9 (Self-report) | 46.5 |
| Jorm et al 22 (Australia) | 2000 | 409 | 100 | NR | 51.6 (Self-report) | 48.4 |
| Vandenbergh et al.23 (USA) | 2002 | 251 | 89 | NR | 61.0 (Self-report) | 45.8 |
| Lerman et al. (USA)39 | 2003 | 418 | 100 | 46.2 | 48.1 (Cotinine verified) | 47.8 |
| O’Gara et al.33 (UK) | 2007 | 563 | 86 | 41.6 | 54.2 (CO verified) | 42.6 |
| Swan et al.32 (USA) | 2007 | 323 | 100 | 37.5 | 33.1 (Self-report) | 51.1 |
| Ton et al.42 (USA) | 2007 | 554 | 93 | 0 (100% female) | 20.2 (Self-report) | 43.0 |
| David et al. (USA)40 | 2007 | 295 | 100 | 49.7 | 35.9 (Cotinine verified) | 43.7 |
| Han et al.34 (South Korea) | 2008 | 225 | 0 (100% Asians) | 100 | 40.0 (CO verified) | 10.2 |
| Styn et al.20 (USA) | 2009 | 864 | 100 | 48 | 24.9 (CO verified) | 44.3 |
| Tashkin et al. 41 (USA) | 2012 | 621 | >95 | 63.3 | 46.9 (CO and cotinine verified) | 47.2 |
| Gordiev et al.25 (Russia) | 2013 | 364 | 100 | 65.6a | 44.0 (Self-report) | 31.3 |
Notes: NR = not reported;
Percent male and female on the basis of all the samples in the study.
Findings from unadjusted data
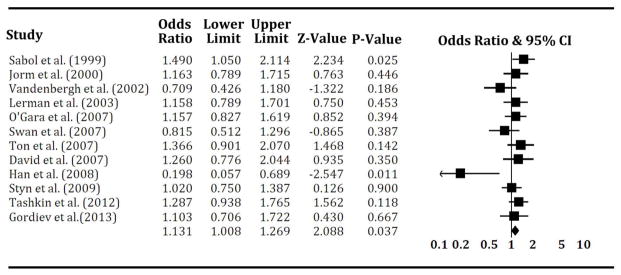
When analyzing all original data without adjustment for age, sex, and ethnicity, the pooled OR in the fixed-effects model was 1.13 (P = 0.037) with 95% CI ranging from 1.01 to 1.27 and in the random-effects model was 1.11 (P = 0.159) with 95% CI of 0.96 to 1.29. Results from the fixed-effects model indicate that one or more 9-repeat alleles confer a higher possibility of smoking abstinence (Table 2 and Fig. 1). However, in the first meta-analysis, the P value of the Q test is 0.10 and the value of the I2 test is 35.9%, providing marginal evidence of heterogeneity among studies. Under this circumstance, results from the random-effects model appear to be more acceptable, suggesting a trend in all unadjusted data to the genotypes of 3′-UTR VNTR 9-repeat correlating with the ability to achieve smoking cessation.
Table 2.
Results from the meta-analysis for smoking cessation stratified by dataset
| Dataset | Cohorts (N) | Estimate of heterogeneity | Fixed-effects model | Random-effects model | Bd | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | P (Q) | Pooled OR | 95% CI | P (Z) | Pooled OR | 95% CI | P(Z) | P(B) | ||
| Unadjusted data a | N=12 | 35.9 | 0.10 | 1.13 | 1.01, 1.27 | 0.037 | 1.11 | 0.96, 1.29 | 0.159 | 0.009 |
| Unadjusted data b | N=11 | 0.0 | 0.48 | 1.15 | 1.02, 1.29 | 0.020 | 1.15 | 1.02, 1.29 | 0.020 | 0.199 |
| Adjusted data c | N=13 | 0.0 | 0.58 | 1.17 | 1.04, 1.31 | 0.009 | 1.17 | 1.04, 1.31 | 0.009 | 0.197 |
Analysis of raw data extracted from all studies.
Analysis of raw data without data from the Han study.
Analysis of combined unadjusted data with adjusted data.
Publication bias.
Figure 1.
Forest plot of the first meta-analysis results on pooled effect of 3′-UTR 9-repeat genotypes on smoking cessation. The Z value and P value of each study are presented by rows. The central vertical solid line shows the null hypothesis where the OR is equal to 1. The OR and 95% CI of each study are represented by the square and horizontal bar, respectively. The diamond symbol indicates the estimated pooled OR, which was calculated under the fixed-effects model.
Of note, only one Asian-based study34 with an opposite result for the polymorphism was included in current meta-analysis. The frequency of 9/* genotypes in this study, by Han et al., was 10.2%, much lower than that in the Caucasian-based studies. Although the sample was relatively small (N = 225), the frequency of 9/* genotypes in this study was similar to the frequencies in other reports on the same ethnic population, where the range is from 9.2% to 16.0%.47–49 Thus, we speculate that inclusion of the study by Han et al. 34 might account for the heterogeneity detected among studies in the first meta-analysis.
In the second meta-analysis, we excluded the data from the Han study to calculate the unadjusted data for Caucasian samples only. The P value of the Q test was increased from 0.10 to 0.48, and the I2 value was reduced from 35.9% to 0.0% (Table 2), suggesting absence of heterogeneity among Caucasian populations. From the second meta-analysis, results indicate that the 3′ VNTR 9-repeat genotypes is significantly associated with the ability to achieve smoking cessation (P = 0.02 for both models). Please refer to Table 2 and Figure 2 for details. Because there was no evidence of heterogeneity among the Caucasian-based studies (P = 0.48, I2 = 0.0%), the fixed- and random-effects model are identical with the pooled OR being 1.15 and the 95% CI ranging from 1.02 to 1.29, suggesting that smokers carrying one or more 3′-UTR VNTR 9-repeat alleles have a 15% increase in the odds of smoking cessation compared with those carrying the non 9-repeat allele.
Figure 2.
Forest plot of the second meta-analysis results on pooled effect of 3′-UTR 9-repeat genotypes on smoking cessation. The Z value and P value of each study are presented by rows. The central vertical solid line shows the null hypothesis, where the OR is equal to 1. The OR and 95% CI of each study are represented by the square and horizontal bar, respectively. The diamond symbol marks the estimated pooled OR, which was calculated under the fixed-effects model.
Although there was no evidence of heterogeneity across 11 studies consisting of 4 cross-sectional surveys and 7 longitudinal studies, we still performed a separate analysis to determine whether different study designs conferred any influence on the final meta-analysis results. Such analyses show that the pooled OR was 1.16 with 95% CI of 0.94 to 1.42 in the 4 cross-sectional surveys (Supplementary Figure S2), and the pooled OR was 1.14 with 95% CI of 0.99 to 1.32 in the 7 longitudinal studies (Supplementary Figure S3). These results provide supporting evidence for the robustness of the finding resulted from our pooled analysis. Furthermore, this indicates that our meta-analysis results are not affected by the study design used in original reports.
Findings from adjusted data
In a study including three cohorts stratified by different protocols, Sabol et al. 19 documented the effect of 9/* genotypes on smoking cessation with a Cochran-Mantel-Haenszel analysis, demonstrating that the association of 9/* genotypes with smoking cessation was more significant than in the pooled data. By performing a logistic regression, Vandenbergh and coworkers 23 reported an adjusted OR for uncontrolled characteristics of participants, although there was no evidence of the association of 9/* genotypes with smoking cessation. Similarly, Styn et al. 20 increased the effect size of the variant for abstinence by using a logistic regression method to adjust the moderating factors, including sex, range of age, and time of enrollment. We carried out our third meta-analysis by analyzing both adjusted and unadjusted data to provide a more accurate estimated effect of the 9-repeat genotypes on smoking cessation. Consistently, results from the third meta-analysis showed strong evidence that 3′ VNTR 9/* genotypes are significantly associated with smoking cessation (P = 0.009 for both models). Both the fixed- and the random-effects models demonstrated that 9/* genotypes increase by 17% the likelihood of stopping smoking. For the third meta-analysis, the P value of the Q statistic is 0.58 and the percentage of variation across studies that is not explained by genetic variance is 0, indicating that there existed no heterogeneity among the studies. The pooled OR and other detailed information of the third meta-analysis are presented in Tables 2 and 3.
Table 3.
Results of meta-analysis for smoking cessation based on adjusted data
| Source | Sample size | Odds ratio | 95% CI | Weight | Weight (%) |
|---|---|---|---|---|---|
| Sabol et al. 1999 (cancer risk) | 104 | 3.28 | 1.10–9.78 | 3.22 | 1.15 |
| Sabol et al. 1999 (personality genetics) | 127 | 1.55 | 0.75–3.20 | 7.33 | 2.62 |
| Sabol et al. 1999 (sexual behavior) | 283 | 1.46 | 0.91–2.34 | 17.23 | 6.15 |
| Jorm et al. 2000 | 409 | 1.16 | 0.79–1.72 | 25.47 | 9.09 |
| Vandenbergh et al. 2002 (adjusted data) | 251 | 0.81 | 0.46–1.41 | 12.41 | 4.43 |
| Lerman et al. 2003 | 418 | 1.16 | 0.79–1.70 | 26.01 | 9.28 |
| O’Gara et al. 2007 | 563 | 1.16 | 0.83–1.62 | 34.07 | 12.16 |
| Swan et al. 2007 | 323 | 0.81 | 0.51–1.30 | 17.84 | 6.37 |
| Ton et al. 2007 | 554 | 1.37 | 0.90–2.07 | 22.2 | 7.92 |
| David et al. 2007 | 295 | 1.26 | 0.78–2.04 | 16.41 | 5.86 |
| Styn et al. 2009 (adjusted data) | 864 | 1.04 | 0.76–1.42 | 40.23 | 14.26 |
| Tashkin et al. 2012 | 621 | 1.29 | 0.94–1.77 | 38.42 | 13.71 |
| Gordiev et al. 2013 | 364 | 1.10 | 0.71–1.72 | 19.36 | 6.91 |
| Pooled analysis | 5176 | 1.17 | 1.04–1.31 | 280.1 | 100 |
Notes: Test for heterogeneity: X2 =10.46, d.f. =12 (P = 0.58), I2 = 0%. Test for overall effect under both fixed-effects and random-effects model: Z = 2.62 (P = 0.009).
Results from sensitivity and accumulative analyses
To determine whether the observed association between 3′ VNTR 9/* genotypes and smoking cessation was significantly biased by the features of a particular study, we performed sensitivity analysis for the last two meta-analyses by removing one study at a time. Based on relative large samples, these two plots showed that the pooled ORs of current meta-analyses were not greatly influenced by each individual study and fluctuated between 1.1 and 1.2 (Figure 3a, b). We also conducted an accumulative analysis for the second meta-analysis to ascertain whether the pooled effect of the 3′ VNTR 9/* genotypes on the higher possibility of smoking cessation differed by publication year. The accumulative test indicated that the pooled OR of the 3′ VNTR 9/* genotypes initially was reduced as publication year increased but became relatively stable after the year 2002 (Supplementary Figure S4).
Figure 3.
Plots of sensitivity analyses results for the last two meta-analyses. The Y axis stands for the pooled OR and the X axis for the individual study that was removed at each time from the included studies. The diamond symbols indicate the pooled OR, and the top and bottom horizontal bars mark the 95% CIs. (A) Plot of sensitivity test for the second meta-analysis. (B) Plot of sensitivity test for the third meta-analysis.
Identification of possible publication bias
The methods of Egger’s regression and funnel plot were used to evaluate potential publication bias for all the meta-analyses. For the first meta-analysis, the funnel plot is remarkably asymmetrical (Supplementary Figure S5a) and the P value of Egger’s regression is 0.009, suggesting a substantial publication bias. From the first funnel plot, we observed that only one Asian-based study34 deviated from the funnel. As described in the aforementioned sections, we excluded the Han study in the following two meta-analyses. We found that these Caucasian-based studies were symmetrically distributed in the second funnel plot (Supplementary Fig. S5b) and the P value of Egger’s regression is 0.199, indicating no publication bias in the second meta-analysis. For the third meta-analysis, we observed that no studies deviated from the funnel-shaped distribution in the plot, which is shown in Supplementary Figure S5c (Egger’s regression: t =1.37, d.f. = 11, P = 0.197).
Discussion
Although the meta-analysis reported by Munafo et al.35 revealed no significant effect of this polymorphism on smoking cessation, our current study indicates a significant association between 3′-UTR VNTR genotypes and smoking cessation, which is in accordance with the results of the Stapleton study.36 The non-significant association of this polymorphism with smoking cessation reported by Munafo et al. might be a result of the fact that their study analyzed only three studies, all of which had moderate sample sizes, perhaps creating a sample too small to reveal the nature of the link. On the basis of the previous36 and current study data, we conclude that 3′-UTR VNTR 9-repeat genotypes play an important role in smoking cessation in the Caucasian population, with the estimated effect of a 9-repeat allele being a 17% increase in the likelihood of smoking cessation.
Dopamine transporter, one of the important parts of the dopaminergic circuitry, modulates the extracellular dopamine concentration.14–17 The encoding SLC6A3 gene, located on chromosome 5p15, has been associated with addictive behaviors18–21, 50 and neuropsychiatric disorders such as attention deficit hyperactivity disorder (ADHD).51–54 The well-investigated polymorphism of VNTR is located in the 3′-end of the SLC6A3 UTR, influencing both mRNA transcription and stability. Although conflicting results have been described,26, 27 numerous functional studies28–31 indicate that a 3′-UTR VNTR 9-repeat allele is associated with the risk of decreased dopamine transporter binding potential or decreased amounts of SLC6A3 transcripts. This implies that under normal circumstances, there exists more abundant synaptic dopamine with longer persistence in the synapse of individuals who carry a 9-repeat allele than those who carry a non 9-repeat allele. Thus, individuals with a 9-repeat allele are less likely to become addicted and find quitting a drug easier. Considering that these conclusions were derived primarily from genetic epidemiologic studies, more functional studies are greatly needed. Nevertheless, it is worth noting that the observed association of the 3′-UTR VNTR polymorphism with the higher possibility of abstinence from smoking might result from other functional variants that are in tight linkage disequilibrium with 3′-UTR VNTR polymorphism. For example, several studies55–57 showed that the SLC6A3 promoter and 5′-UTR region were in modest linkage disequilibrium with the 3′-UTR VNTR region, and O’Gara and colleagues33 demonstrated that 3′-UTR VNTR was in linkage disequilibrium with rs27048 and intron 8 VNTR. Besides, Brookes et al.29 indicated that the presence of 3′-UTR VNTR and intron 8 VNTR were highly correlated with the increased amount of SLC6A3 transcript in post-mortem midbrain tissue.
When applying the 9-repeat dominant model to all samples, we detected heterogeneity among studies. Because only the Han study34 was focused on an Asian population, we inferred that this study contributed to the detected heterogeneity. We thus excluded this study from our remaining analyses. Further, prior Caucasian-based studies indicated a trend that smokers with one or more 9-repeat alleles were more likely to achieve smoking abstinence. However, Han et al.34 reported a significant trend in the opposite direction, where it was found that a SLC6A3 3′-UTR VNTR 9-repeat genotype was more common in the non-abstinence group than in the abstinence group (χ2 = 7.76; P = 0.01). This indicates that smoking cessation may be influenced by the ethnicity of the subjects. For example, although many Caucasian-based studies58–60 documented a significant association of Taq1A A1 allele with ever-smokers, two studies61, 62 based on subjects of Asian origin reported that the A2/A2 genotype was significantly associated with smoking risk. Considering the small sample size of the Han study,34 more Asian-based studies with significantly larger sample sizes are greatly needed to determine the effect of the polymorphism on smoking cessation in Asian populations.
Although we found a significant association between the 3′-UTR 9-genotypes and smoking cessation, this finding should be interpreted with caution because of the following potential limitations. Firstly, accruing evidence revealed that the cessation rate declined with time because of the substantial likelihood of smoking relapse.40 The treatment studies we used examined the prevalence of smoking at different time-points, which influenced the power of treatment effects and the association of polymorphism with smoking cessation. In contrast to the former smokers who had mostly quitted many years earlier in those cross-sectional studies, sustained quitters had followed only in short-term that might easily contribute to substantial smoking relapse in the longitudinal studies. Furthermore, different diagnostic criteria in defining quitters were used in these two types of studies, where the cross-sectional studies were based on relatively less stringent self-reports of smoking status and the longitudinal studies depended on both self-reports and biochemical verification. The factors might have contributed to the inconsistent results. Secondly, the different sex ratios and ages of subjects in these studies might impose limitations. An imaging study63 indicated that dopamine release following stimulant exposure tended to be greater in males than in females. In addition, several studies18, 64, 65 showed, as expected, that females had greater estrogen-induced dopamine activation in the striatum than did males. Thus, females with higher estrogen concentrations might be more easily prevented from addiction. Besides, Styn and colleagues,20 using a logistic regression to adjust the regulatory factors including sex and age, increased the effect of the variant on smoking cessation. Thirdly, because of the lack of sufficient studies on other polymorphisms, we could not examine gene-by-gene and gene-by-environmental interactions, which influence the pathogenesis of polygenic addictive behaviors, including smoking cessation. Lerman et al.39 observed a significant SLC6A3×DRD2 interactive effect on smoking cessation at the EOT, in that they found that individuals with A2A2 and 9-repeat genotypes were more likely to stop smoking than were those with A2A2 and non 9-repeat genotypes. Similarly, Swan et al.32 showed a significant interaction in that participants with A1/* (A1 or A2) and non 9-repeat genotypes were less likely to have stopped smoking at 12-month follow-up. In a fenfluramine test42 in a female cohort, Ton et al. documented a higher cessation rate among carriers of both the SLC6A3 3′-UTR VNTR 9-repeat allele and the DRD2 Taq1A A1 allele. Finally, most included studies failed to screen for underlying comorbid-related addictive and psychiatric disorders in control subjects, which may decrease the reliability of the results reported here.
In summary, results from our meta-analyses reveal a moderate effect of the 3′-UTR VNTR polymorphism on smoking cessation in Caucasians. Compared with smokers with non 9-repeat genotypes, smokers with 9-repeat genotypes have a 17% greater chance of quitting smoking. This indicates that the dopaminergic function of 3′-UTR VNTR 9-repeat genotypes is involved in regulating the process of smoking abstinence in the Caucasian population. Based on prior and current meta-analyses, more well-designed studies, in particular with large samples, are warranted to: 1) explore the molecular mechanism of 3′-UTR VNTR polymorphism for smoking cessation, 2) test the underlying effect of other variants, which are located in SLC6A3 and in LD with the 3′-UTR VNTR polymorphism, on smoking cessation, and 3) identify more novel variants potentially associated with smoking cessation. Once robust experimental evidence for the association of genetic variants with smoking cessation is established, it will be more effective to categorize smokers genetically into subgroups for smoking cessation intervention, which is a preliminary but crucial step for future personalized medicine.
Supplementary Material
Acknowledgments
We thank Dr. David L. Bronson for excellent editing of this manuscript. We also thank Dr. Sean P. David of Stanford University for providing the original genotyping data reported in his paper (David et al., 2007, Nicotine & Tobacco Research 9:821–833) to us. This study was supported in part by the Research Center for Air Pollution and Health of Zhejiang University, Ministry of Science and Technology of China (2012AA020405), National Natural Science Foundation of China grant 81273223, Young Scientists Fund of National Science Foundation of China (81301140), and NIH grant DA012844.
References
- 1.WHO. WHO Tobacco Fact sheet N°339. World Health Organization; 2014. http://www.who.int/mediacentre/factsheets/fs339/en/ [Google Scholar]
- 2.Carmelli D, Swan GE, Robinette D, Fabsitz R. Genetic influence on smoking--a study of male twins. N Engl J Med. 1992;327(12):829–833. doi: 10.1056/NEJM199209173271201. [DOI] [PubMed] [Google Scholar]
- 3.Heath AC, Martin NG. Genetic models for the natural history of smoking: evidence for a genetic influence on smoking persistence. Addict Behav. 1993;18(1):19–34. doi: 10.1016/0306-4603(93)90005-t. [DOI] [PubMed] [Google Scholar]
- 4.Heath AC, Cates R, Martin NG, Meyer J, Hewitt JK, Neale MC, et al. Genetic contribution to risk of smoking initiation: comparisons across birth cohorts and across cultures. J Subst Abuse. 1993;5(3):221–246. doi: 10.1016/0899-3289(93)90065-j. [DOI] [PubMed] [Google Scholar]
- 5.True WR, Heath AC, Scherrer JF, Waterman B, Goldberg J, Lin N, et al. Genetic and environmental contributions to smoking. Addiction. 1997;92(10):1277–1287. [PubMed] [Google Scholar]
- 6.Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98(1):23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res. 1999;1 (Suppl 2):S51–57. doi: 10.1080/14622299050011811. discussion S69–70. [DOI] [PubMed] [Google Scholar]
- 8.Xian H, Scherrer JF, Madden PA, Lyons MJ, Tsuang M, True WR, et al. The heritability of failed smoking cessation and nicotine withdrawal in twins who smoked and attempted to quit. Nicotine Tob Res. 2003;5(2):245–254. [PubMed] [Google Scholar]
- 9.Hardie TL, Moss HB, Lynch KG. Genetic correlations between smoking initiation and smoking behaviors in a twin sample. Addictive behaviors. 2006;31(11):2030–2037. doi: 10.1016/j.addbeh.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 10.David SP, Munafo MR. Genetic variation in the dopamine pathway and smoking cessation. Pharmacogenomics. 2008;9(9):1307–1321. doi: 10.2217/14622416.9.9.1307. [DOI] [PubMed] [Google Scholar]
- 11.Li MD. The genetics of nicotine dependence. Curr Psychiatry Rep. 2006;8(2):158–164. doi: 10.1007/s11920-006-0016-0. [DOI] [PubMed] [Google Scholar]
- 12.Ma Y, Yuan W, Jiang X, Cui WY, Li MD. Updated Findings of the Association and Functional Studies of DRD2/ANKK1 Variants with Addictions. Molecular neurobiology. 2014 doi: 10.1007/s12035-014-8826-2. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Li MD. Common and unique biological pathways associated with smoking initiation/progression, nicotine dependence, and smoking cessation. Neuropsychopharmacology. 2010;35(3):702–719. doi: 10.1038/npp.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, et al. Human dopamine transporter gene (DAT1) maps to chromosome 5p15. 3 and displays a VNTR. Genomics. 1992;14(4):1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- 15.Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16(5):905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 16.Caron MG. Images in neuroscience. Molecular biology, II. A dopamine transporter mouse knockout. The American journal of psychiatry. 1996;153(12):1515. doi: 10.1176/ajp.153.12.1515. [DOI] [PubMed] [Google Scholar]
- 17.Uhl GR. Dopamine transporter: basic science and human variation of a key molecule for dopaminergic function, locomotion, and parkinsonism. Movement disorders: official journal of the Movement Disorder Society. 2003;18 (Suppl 7):S71–80. doi: 10.1002/mds.10578. [DOI] [PubMed] [Google Scholar]
- 18.Lerman C, Caporaso NE, Audrain J, Main D, Bowman ED, Lockshin B, et al. Evidence suggesting the role of specific genetic factors in cigarette smoking. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 1999;18(1):14–20. doi: 10.1037//0278-6133.18.1.14. [DOI] [PubMed] [Google Scholar]
- 19.Sabol SZ, Nelson ML, Fisher C, Gunzerath L, Brody CL, Hu S, et al. A genetic association for cigarette smoking behavior. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 1999;18(1):7–13. doi: 10.1037//0278-6133.18.1.7. [DOI] [PubMed] [Google Scholar]
- 20.Styn MA, Nukui T, Romkes M, Perkins K, Land SR, Weissfeld JL. The impact of genetic variation in DRD2 and SLC6A3 on smoking cessation in a cohort of participants 1 year after enrollment in a lung cancer screening study. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2009;150B(2):254–261. doi: 10.1002/ajmg.b.30801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiemstra M, Engels RC, Barker ED, van Schayck OC, Otten R. Smoking-specific parenting and smoking onset in adolescence: the role of genes from the dopaminergic system (DRD2, DRD4, DAT1 genotypes) PloS one. 2013;8(4):e61673. doi: 10.1371/journal.pone.0061673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorm AF, Henderson AS, Jacomb PA, Christensen H, Korten AE, Rodgers B, et al. Association of smoking and personality with a polymorphism of the dopamine transporter gene: results from a community survey. Am J Med Genet. 2000;96(3):331–334. doi: 10.1002/1096-8628(20000612)96:3<331::aid-ajmg19>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Vandenbergh DJ, Bennett CJ, Grant MD, Strasser AA, O’Connor R, Stauffer RL, et al. Smoking status and the human dopamine transporter variable number of tandem repeats (VNTR) polymorphism: failure to replicate and finding that never-smokers may be different. Nicotine Tob Res. 2002;4(3):333–340. doi: 10.1080/14622200210142689. [DOI] [PubMed] [Google Scholar]
- 24.Sieminska A, Buczkowski K, Jassem E, Niedoszytko M, Tkacz E. Influences of polymorphic variants of DRD2 and SLC6A3 genes, and their combinations on smoking in Polish population. BMC medical genetics. 2009;10:92. doi: 10.1186/1471-2350-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordiev M, Engstrom PF, Khasanov R, Moroshek A, Sitdikov R, Dgavoronkov V, et al. Genetic analysis of polymorphisms in dopamine receptor and transporter genes for association with smoking among cancer patients. European addiction research. 2013;19(2):105–111. doi: 10.1159/000341711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, et al. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2005;46(5):745–751. [PubMed] [Google Scholar]
- 27.Martinez D, Gelernter J, Abi-Dargham A, van Dyck CH, Kegeles L, Innis RB, et al. The variable number of tandem repeats polymorphism of the dopamine transporter gene is not associated with significant change in dopamine transporter phenotype in humans. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2001;24(5):553–560. doi: 10.1016/S0893-133X(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 28.Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2000;22(2):133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- 29.Brookes KJ, Neale BM, Sugden K, Khan N, Asherson P, D’Souza UM. Relationship between VNTR polymorphisms of the human dopamine transporter gene and expression in post-mortem midbrain tissue. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2007;144B(8):1070–1078. doi: 10.1002/ajmg.b.30572. [DOI] [PubMed] [Google Scholar]
- 30.Mill J, Asherson P, Browes C, D’Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3′ UTR VNTR: Evidence from brain and lymphocytes using quantitative RT-PCR. American journal of medical genetics. 2002;114(8):975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- 31.Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S. The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. The pharmacogenomics journal. 2001;1(2):152–156. doi: 10.1038/sj.tpj.6500026. [DOI] [PubMed] [Google Scholar]
- 32.Swan GE, Jack LM, Valdes AM, Ring HZ, Ton CC, Curry SJ, et al. Joint effect of dopaminergic genes on likelihood of smoking following treatment with bupropion SR. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 2007;26(3):361–368. doi: 10.1037/0278-6133.26.3.361. [DOI] [PubMed] [Google Scholar]
- 33.O’Gara C, Stapleton J, Sutherland G, Guindalini C, Neale B, Breen G, et al. Dopamine transporter polymorphisms are associated with short-term response to smoking cessation treatment. Pharmacogenetics and genomics. 2007;17(1):61–67. doi: 10.1097/01.fpc.0000236328.18928.4c. [DOI] [PubMed] [Google Scholar]
- 34.Han DH, Joe KH, Na C, Lee YS. Effect of genetic polymorphisms on smoking cessation: a trial of bupropion in Korean male smokers. Psychiatric genetics. 2008;18(1):11–16. doi: 10.1097/YPG.0b013e3282df0939. [DOI] [PubMed] [Google Scholar]
- 35.Munafo M, Clark T, Johnstone E, Murphy M, Walton R. The genetic basis for smoking behavior: a systematic review and meta-analysis. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2004;6(4):583–597. doi: 10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- 36.Stapleton JA, Sutherland G, O’Gara C. Association between dopamine transporter genotypes and smoking cessation: a meta-analysis. Addict Biol. 2007;12(2):221–226. doi: 10.1111/j.1369-1600.2007.00058.x. [DOI] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 38.David SP, Strong DR, Leventhal AM, Lancaster MA, McGeary JE, Munafo MR, et al. Influence of a dopamine pathway additive genetic efficacy score on smoking cessation: results from two randomized clinical trials of bupropion. Addiction. 2013;108(12):2202–2211. doi: 10.1111/add.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lerman C, Shields PG, Wileyto EP, Audrain J, Hawk LH, Jr, Pinto A, et al. Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 2003;22(5):541–548. doi: 10.1037/0278-6133.22.5.541. [DOI] [PubMed] [Google Scholar]
- 40.David SP, Brown RA, Papandonatos GD, Kahler CW, Lloyd-Richardson EE, Munafo MR, et al. Pharmacogenetic clinical trial of sustained-release bupropion for smoking cessation. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2007;9(8):821–833. doi: 10.1080/14622200701382033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tashkin DP, Rabinoff M, Noble EP, Ritchie TL, Simmons MS, Connett J. Association of dopamine-related gene alleles, smoking behavior and decline in FEV1 in subjects with COPD: findings from the lung health study. Copd. 2012;9(6):620–628. doi: 10.3109/15412555.2012.712167. [DOI] [PubMed] [Google Scholar]
- 42.Ton TG, Rossing MA, Bowen DJ, Srinouanprachan S, Wicklund K, Farin FM. Genetic polymorphisms in dopamine-related genes and smoking cessation in women: a prospective cohort study. Behav Brain Funct. 2007;3:22. doi: 10.1186/1744-9081-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 44.Wang F, Simen A, Arias A, Lu QW, Zhang H. A large-scale meta-analysis of the association between the ANKK1/DRD2 Taq1A polymorphism and alcohol dependence. Human genetics. 2013;132(3):347–358. doi: 10.1007/s00439-012-1251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou QF, Li SB. Potential association of DRD2 and DAT1 genetic variation with heroin dependence. Neuroscience letters. 2009;464(2):127–130. doi: 10.1016/j.neulet.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Ueno S, Nakamura M, Mikami M, Kondoh K, Ishiguro H, Arinami T, et al. Identification of a novel polymorphism of the human dopamine transporter (DAT1) gene and the significant association with alcoholism. Molecular psychiatry. 1999;4(6):552–557. doi: 10.1038/sj.mp.4000562. [DOI] [PubMed] [Google Scholar]
- 49.Chen WJ, Chen CH, Huang J, Hsu YP, Seow SV, Chen CC, et al. Genetic polymorphisms of the promoter region of dopamine D2 receptor and dopamine transporter genes and alcoholism among four aboriginal groups and Han Chinese in Taiwan. Psychiatric genetics. 2001;11(4):187–195. doi: 10.1097/00041444-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Samochowiec J, Kucharska-Mazur J, Grzywacz A, Jablonski M, Rommelspacher H, Samochowiec A, et al. Family-based and case-control study of DRD2, DAT, 5HTT, COMT genes polymorphisms in alcohol dependence. Neuroscience letters. 2006;410(1):1–5. doi: 10.1016/j.neulet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Cook EH, Jr, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, et al. Association of attention-deficit disorder and the dopamine transporter gene. American journal of human genetics. 1995;56(4):993–998. [PMC free article] [PubMed] [Google Scholar]
- 52.Cornish KM, Manly T, Savage R, Swanson J, Morisano D, Butler N, et al. Association of the dopamine transporter (DAT1) 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Molecular psychiatry. 2005;10(7):686–698. doi: 10.1038/sj.mp.4001641. [DOI] [PubMed] [Google Scholar]
- 53.Rommelse NN, Altink ME, Arias-Vasquez A, Buschgens CJ, Fliers E, Faraone SV, et al. A review and analysis of the relationship between neuropsychological measures and DAT1 in ADHD. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2008;147B(8):1536–1546. doi: 10.1002/ajmg.b.30848. [DOI] [PubMed] [Google Scholar]
- 54.Gill M, Daly G, Heron S, Hawi Z, Fitzgerald M. Confirmation of association between attention deficit hyperactivity disorder and a dopamine transporter polymorphism. Molecular psychiatry. 1997;2(4):311–313. doi: 10.1038/sj.mp.4000290. [DOI] [PubMed] [Google Scholar]
- 55.Greenwood TA, Kelsoe JR. Promoter and intronic variants affect the transcriptional regulation of the human dopamine transporter gene. Genomics. 2003;82(5):511–520. doi: 10.1016/s0888-7543(03)00142-3. [DOI] [PubMed] [Google Scholar]
- 56.Kelada SN, Costa-Mallen P, Checkoway H, Carlson CS, Weller TS, Swanson PD, et al. Dopamine transporter (SLC6A3) 5′ region haplotypes significantly affect transcriptional activity in vitro but are not associated with Parkinson’s disease. Pharmacogenetics and genomics. 2005;15(9):659–668. doi: 10.1097/01.fpc.0000170917.04275.d6. [DOI] [PubMed] [Google Scholar]
- 57.Bergen AW, Conti DV, Van Den Berg D, Lee W, Liu J, Li D, et al. Dopamine genes and nicotine dependence in treatment-seeking and community smokers. Neuropsychopharmacology. 2009;34(10):2252–2264. doi: 10.1038/npp.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noble EP, St Jeor ST, Ritchie T, Syndulko K, St Jeor SC, Fitch RJ, et al. D2 dopamine receptor gene and cigarette smoking: a reward gene? Med Hypotheses. 1994;42(4):257–260. doi: 10.1016/0306-9877(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 59.Comings DE, Ferry L, Bradshaw-Robinson S, Burchette R, Chiu C, Muhleman D. The dopamine D2 receptor (DRD2) gene: a genetic risk factor in smoking. Pharmacogenetics. 1996;6(1):73–79. doi: 10.1097/00008571-199602000-00006. [DOI] [PubMed] [Google Scholar]
- 60.Li MD, Ma JZ, Beuten J. Progress in searching for susceptibility loci and genes for smoking-related behaviour. Clinical genetics. 2004;66(5):382–392. doi: 10.1111/j.1399-0004.2004.00302.x. [DOI] [PubMed] [Google Scholar]
- 61.Yoshida K, Hamajima N, Kozaki K, Saito H, Maeno K, Sugiura T, et al. Association between the dopamine D2 receptor A2/A2 genotype and smoking behavior in the Japanese. Cancer Epidemiol Biomarkers Prev. 2001;10(4):403–405. [PubMed] [Google Scholar]
- 62.Hamajima N, Ito H, Matsuo K, Saito T, Tajima K, Ando M, et al. Association between smoking habits and dopamine receptor D2 taqI A A2 allele in Japanese males: a confirmatory study. J Epidemiol. 2002;12(4):297–304. doi: 10.2188/jea.12.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, et al. Sex differences in striatal dopamine release in healthy adults. Biological psychiatry. 2006;59(10):966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 64.Dluzen DE, Anderson LI. Estrogen differentially modulates nicotine-evoked dopamine release from the striatum of male and female rats. Neuroscience letters. 1997;230(2):140–142. doi: 10.1016/s0304-3940(97)00487-4. [DOI] [PubMed] [Google Scholar]
- 65.Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, Brady KT. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: a review. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2006;8(5):627–638. doi: 10.1080/14622200600910793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.