Abstract
Objective
The study objective was to investigate epithelial changes in response to direct, repeated, acidified-pepsin exposures in an in vivo porcine model. We hypothesized that 12 acidified-pepsin applications to simulate reflux would elicit a vocal fold response characterized by inflammation, epithelial proliferation, and increased intercellular space; as well as changes in the gene expression of epithelial junctional proteins, ion transporter proteins, and pro-inflammatory cytokines.
Study Design
Prospective, in vivo study.
Methods
Pigs received acidified pepsin (pH=4) or saline (sham) applied directly to vocal folds. Larynges were collected following three exposures per week for four weeks. Vocal fold tissue morphology, collagen, and elastin were evaluated histologically. Gene expression of E-cadherin (Ecad), zona occludin-1 (ZO-1), cystic fibrosis transmembrane conductance regulator (CFTR), epithelial sodium channel (SCNN1α), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) were measured. Ultrastructural alterations, epithelial intercellular space diameter and microridge height were measured using transmission electron microscopy.
Results
There were no significant differences in histology, gene transcripts, epithelial ultrastructure, intercellular space, and microridge height after acidified-pepsin exposure.
Conclusions
Twelve simulated reflux challenges were insufficient to elicit epithelial changes which demonstrate the vigor of healthy vocal folds to direct, repeated acidified-pepsin exposures. These data increase our understanding of healthy vocal fold defenses and lay the groundwork for a prospective, uninjured, non-surgical, LPR model where pigs can be exposed directly to acidified-pepsin.
Introduction
Laryngopharyngeal reflux (LPR) is an extraesophageal manifestation of gastroesophageal reflux disease (GERD) in which gastric refluxate containing acid and pepsin directly contact laryngeal epithelium. LPR is a widely recognized disorder; however, there is still debate regarding pathophysiology, diagnosis, and treatment.1-4 Current literature related to LPR is lacking prospective controlled studies in which outcomes of reflux in healthy subjects is evaluated. Prospective animal studies are necessary to study pathophysiology of LPR, optimize our understanding of the disease, and improve treatment outcomes. The pig provides a unique opportunity to test hypotheses relating to laryngeal disease because porcine vocal folds are most similar to human vocal folds.5-7
The minimum acid exposure resulting in relevant laryngeal pathology is not well understood. There is a discrepancy in the human literature in which some investigators believe that any amount of laryngopharyngeal reflux is abnormal, while others have documented occasional pharyngeal reflux in healthy subjects.8,9 Animal studies have documented that a few times per week of gastric contents contacting the injured upper airway can result in significant pathology.10,11 In vitro research on healthy, porcine vocal folds have revealed impaired epithelial barrier function, and increased vocal fold ion transport, following a single acidic challenge (pH=3).12,13 In order to investigate these in vitro findings and their repercussions to the larynx in a more realistic manner, we tested if repeated reflux challenges result in a reproducible animal model to study LPR.
In this experiment, liquid refluxate is applied directly on the vocal folds of anesthetized animals via videoendoscopy. This method was chosen to simulate a clinical condition of healthy individuals being exposed to reflux with minimal manipulation. This study focuses on the vocal fold epithelia because the epithelia is the primary recipient of external challenges such as reflux. The vocal fold epithelia consists of stratified squamous cells connected by apical junctional complexes that together create a barrier to challenges. Epithelial cells also actively transport ions and water to regulate cell volume and vocal fold hydration.14 During an episode of LPR, gastric reflux makes contact with the vocal fold epithelia. The mechanism by which reflux alters epithelial function and the function of the underlying vocal fold tissue layers is not known. Investigating epithelial changes is important because alterations to connective tissue and muscle can cause voice disorders, and epithelial tissue may be one route by which these tissue planes are injured.
We hypothesized that 12 reflux exposures would result in quantifiable changes in vocal fold tissue morphology, collagen, and elastin; as well as alter gene expression of inflammatory cytokines, interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ). Inflammation and epithelial proliferation are two parameters that are diagnostic for reflux esophagitis.15,16 We also hypothesized that gene expression of epithelial barrier proteins E-cadherin (Ecad) and zona occludin-1 (ZO-1); and ion transporters, cystic fibrosis transmembrane conductance regulator (CFTR) and epithelial sodium channel (SCNN1α) would be altered. Finally, we predicted ultrastructural changes of the vocal fold epithelial cell, increases in epithelial intercellular space diameter and modifications of microridge height. Twelve exposures is double the number of reflux challenges that have been reported in previous animal studies.
Materials and Methods
Animal Procedure
The study was carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health as well as approval from Purdue Animal Care and Use Committee. Eight adult domestic pigs, 35kg - 60kg, were randomly assigned to a reflux (n = 4) and sham group (n = 4). All animals were sedated with a combination of Telazol and xylazine hydrochloride intramuscularly (IM). Sedation was maintained with inhaled isoflurane (1 to 5%) in 100% oxygen. After sedation, animals were placed in sternal recumbency and the larynx was visualized with a QIF 160 Olympus endoscope (Olympus USA Corp. Center Valley, PA). An endoscopic aspiration catheter (MILA International, Inc) was inserted to spray each challenge directly on the membranous portions of each vocal fold. The reflux group received 1.5 ml of 1.0 mg/ml acidified pepsin (pH 3-4) while the sham group received 1.5 ml of saline applied similarly. The endoscope was removed and the animal recovered. This procedure was repeated 3 times a week for 4 weeks (12 total challenges) in each animal. The animals were humanely sacrificed immediately following the final challenge with intravenous Beuthanasia-D Special (Schering Plough Animal Health Corp. Union, NJ). The larynx was immediately removed for sample processing. All animals received full autopsies to rule out any confounding diseases.
Histology and Histochemical Staining
A 6 mm punch biopsy of the true vocal fold of each animal was fixed in 10% neutral buffered formalin, processed and embedded in paraffin blocks. Tissue slices (5μm thick) were stained with hematoxylin and eosin (HE). Additional slides were stained with Masson's trichrome and Verhoeff-Van Gieson (VVG) stains for collagen and elastin, respectively. All slides were examined by a board-certified veterinary pathologist. In order to quantify any subtle changes due to reflux challenge, evidence of cell proliferation were investigated. Virtual slides were created using Aperio ScanScope (Aperio Technologies, Vista, CA). Scanned slides were analyzed using Aperio ImageScope software (v11.2.0.780) established algorithms. The positive pixel count (PPC) algorithm was utilized to detect epithelial nuclei (indicator of proliferation) and lamina proprial nuclei (indicator of cellular infiltrate). The PPC algorithm quantifies the amount of a specific stain present in a scanned slide by specifying a color, then the algorithm counts the number in a specified area. To compare the epithelial proliferation and lamina proprial cellular infiltrate between sham and reflux vocal folds, the number of nuclei based on pixel counts in the epithelium and lamina propria was quantified and standardized over the analysis area of each vocal fold by using the PPC algorithm. A similar method was used to quantify collagen and elastin in the Masson's trichrome and VVG stained slides, respectively.
Transmission electron microscopy
Vocal fold epithelium samples from 6 animals (3 sham and 3 reflux) were immediately fixed in 4% paraformaldehyde in 0.1 M sodium phosphate buffer and later with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer. Samples were post-fixed in buffered 1% osmium tetroxide containing 0.8% potassium ferricyanide, en bloc stained in aqueous 1% uranyl acetate, dehydrated with a graded series of ethanol, transferred into propylene oxide and embedded in Embed-812 resin. Ultrathin sections were cut on a Reichert-Jung Ultracut E ultramicrotome and stained with 2% uranyl acetate and lead citrate. Specimens were examined and photographed on a FEI Tecnai G2 20 electron microscope equipped with a LaB6 source and operating at 100kV. Ten representative fields from each vocal fold were captured and 10 randomly selected areas of intercellular space distance (ISD) or microridge height (MRH) within each image was analyzed via ImageJ (National Institutes of Health, Bethesda, MD). The mean value of ISD and MRH was computed for each animal by averaging the 100 spaces in the 10 photographs of each vocal fold.
Real-Time PCR Quantification
Vocal fold epithelium was separated from the underlying lamina propria and immediately frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted from frozen homogenized vocal fold epithelial tissue using Nucleospin® RNA isolation kit (Macherey-Nagel, Bethlehem, PA). cDNA was synthesized from total RNA using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Grand Island, NY), according to the manufacturer's instruction. The reactions were incubated in a thermal cycler for 10 minutes at 25°C, 120 minutes, at 37°C, 5 minutes at 85°C, and then held at 4°C.
TaqMan probes (Applied Biosystems) specific for porcine E-cadherin (Ecad), zona occludens-1 (ZO-1), cystic fibrosis transmembrane conductance regulator (CFTR), epithelial sodium channel (SCNN1α), tumor necrosis factor-1 (TNF-1), interleukin-1β, and interferon-γ (IFN-γ) were added to TaqMan Gene Expression Master Mix and to cDNA samples. Real-time PCR was performed using an Applied Biosystems 7500 Real Time PCR system. Reactions were performed as follows. Step 1: 50°C for 2 minutes; Step 2: 95°C for 10 minutes; Step 3 (40X): 95°C for 15 seconds followed by 60°C for 1 minute. The data obtained by real-time PCR was analyzed using the comparative threshold cycle (CT) method. In this method, the amount of the target gene, normalized to B-actin, and relative to a calibrator (sham vocal fold epithelial tissue), is given by 2ΔΔCT, where ΔΔCT = ΔCT (sample)–ΔCT (calibrator), and ΔCT is the CT of the target gene subtracted from the CT of B-actin. The average of four independent analyses for each gene and sample was calculated and was normalized to the endogenous gene B-actin.
Statistical Analysis
Statistical analysis included the Wilcoxon rank sum test or Mann-Whitney U test (Stata® 12.1, Statacorp, College Station, TX). An alpha level of 0.05 was selected for statistical significance.
Results
Histology
Vocal fold histology was similar across all animals regardless of challenge. Histological analyses revealed intact stratified squamous cells, 2-7 cell layers thick. The basilar layer of epithelium had 0-2 mitotic figures per high power field. The superficial lamina propria had few lymphocytes and rare plasma cells interspersed within a loose collagen matrix and small caliber vessels. The distribution of lymphocytes was consistent throughout all layers of the vocal fold lamina propria. We confirmed previous research showing that collagen was present throughout all layers of the vocal fold lamina propria, and was denser in the deeper layers of the lamina propria.17 Elastin fibers were denser in the superficial layer of the lamina propria, but were present throughout all layers of the vocal fold lamina propria.18 All histologic findings were similar across sham and reflux vocal folds.
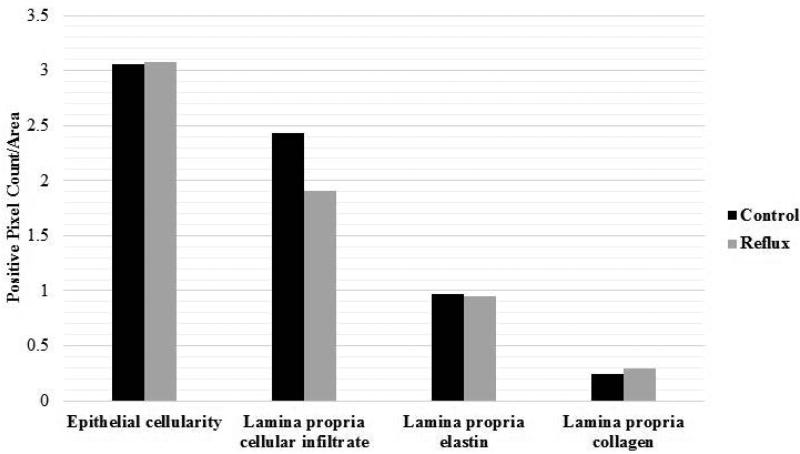
Quantitative analysis of digitally scanned slides of true vocal folds revealed similar epithelial cell proliferation (median sham = 3.26; reflux = 3.74), lamina proprial cellular infiltrate (median sham = 1.72; reflux = 1.18), amount of elastin (median sham = 1.05; reflux = 0.84), and collagen deposition (median sham = 0.25; reflux = 0.26) across reflux and sham groups (P > 0.05) (Figure 1 and 2).
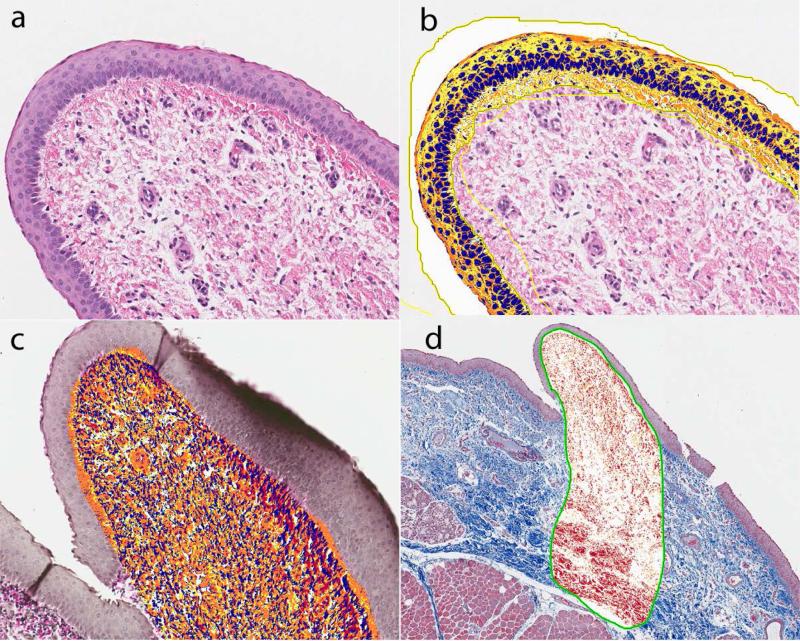
Figure 1.
Vocal fold epithelium and superficial lamina propria from a reflux animal stained with HE (a). Overlay of ImageScope software PPC algorithm to count epithelial nuclei (b), VVG elastin (c) and Masson's trichrome (d).
Figure 2.
Means and standard deviations for histologic findings in sham and reflux vocal folds.
Transmission Electron Microscopy
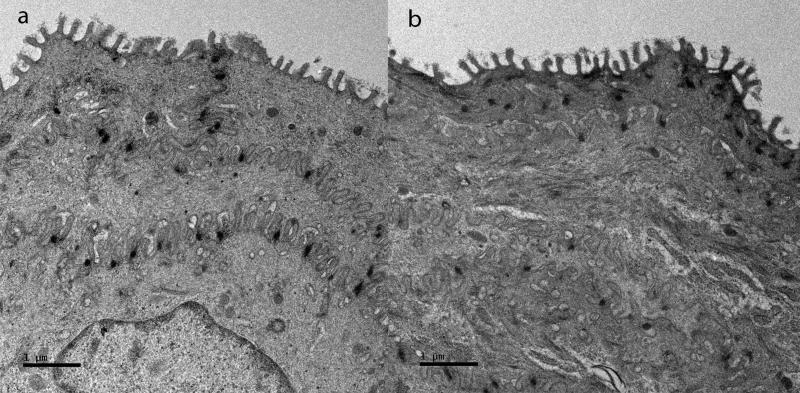
There were no ultrastructural alterations in the vocal fold epithelium. The intercellular space distance (ISD) and microridge height (MRH) of vocal fold epithelium was similar regardless of the challenge. Representative findings are shown (Figure 3.). The mean ISD was 0.25 μm (0.10-0.54) in shams; and 0.13 μm (0.07-0.22) in reflux vocal folds (P > 0.05). The mean MRH was 0.30 (0.28-0.34) in shams; and 0.28 (0.24-0.30) in reflux vocal folds (P > 0.05).
Figure 3.
Transmission electron photomicrograph of (a) sham and (b) reflux vocal fold epithelium. (Original magnification 2550X)
RT-qPCR
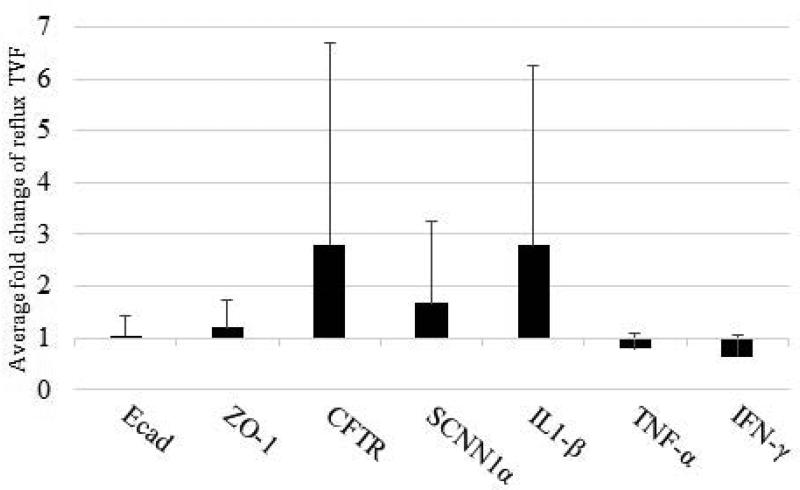
The fold difference of Ecad (1.05 ± 0.36), ZO-1(1.20 ± 0.54), CFTR (2.80 ± 3.90), SCNN1α (1.68 ± 1.56), IL-1β (2.79 ± 3.46), TNF-α (0.79 ± 0.29), and IFN-γ (0.65 ± 0.40) mRNA in the reflux vocal folds was not significantly different than the sham vocal folds (P > 0.05; Figure 4).
Figure 4.
Mean and standard deviations for gene transcripts in reflux compared to sham vocal folds. Data analyzed using delta CT method. E-cadherin (Ecad), zona-occludens-1 (ZO-1), cystic fibrosis transmembrane conductance regulator (CFTR), epithelial sodium channel (SCNN1α), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ).
Discussion
In this study, we sought to investigate whether 12 reflux challenges would induce vocal fold epithelial changes in a porcine model. Twelve exposures double the number of reflux challenges that have been reported in previous animal studies.10,11 A major strength of this study is that it attempts to mimic the clinical situation of human LPR more closely by challenging healthy, uninjured laryngeal epithelium. Our findings demonstrate that liquid acidified-pepsin applied directly to pig vocal folds did not significantly compromise epithelial structure or function as compared to a sham challenge. These data demonstrate that healthy vocal folds are robust and not vulnerable to 12 acidified-pepsin challenges. These data are also valuable because they demonstrate the usefulness of a pig model in future LPR studies; and provide a step forward in identifying the minimum threshold needed to elicit an epithelial response to reflux challenge in the healthy larynx.
The porcine larynx offers the greatest similarity to the human larynx than any other characterized animal model.5-7 These biological and physiological similarities are likely the same traits that are at play in human larynx and may translate to a reliable, reproducible model of LPR. Although our laryngeal reflux pig model focuses on the epithelia, the similarities to human vocal fold tissue in regards to connective tissue proteins, and intrinsic muscles make the porcine larynx appropriate for investigating biomechanical properties of the connective tissue and muscle in future studies. 17 The porcine model will also permit the investigation of the interaction of LPR with vocal fold injury from paralysis or trauma.
The data in this study result from the application of liquid refluxate directly on the vocal folds of anesthetized animals via videoendoscopy. Histological analysis did not reveal differences in vocal fold epithelia, lamina propria, and thyroarytenoid muscle between challenge groups. A mild scattering of lymphoid cells was present in the superficial lamina propria of both sham and reflux pig vocal folds similar to that reported by Barker et al. and were thus considered normal mucosal immunity within the pig larynx.7 In addition to tissue morphology, elastin and collagen amount, distribution, and morphology were similar between sham and reflux pig vocal folds.
Dilated intercellular spaces of esophageal epithelium is a hallmark ultrastructural lesion in GERD patients.19 Intercellular space was also significantly increased in laryngeal biopsies of GERD patients.20 Previous electron microscopic examination of ex vivo pig laryngeal tissue incubated in solution at a pH of 2.0 reported an increase in spaces between vocal fold epithelial cells.21 Microridges cover the vocal fold epithelial surface22 and are hypothesized to contribute to the adherence of mucus. Damage to the microridge structure could negatively impact the defense of the epithelia to reflux challenges. The ultrastructural morphology, intercellular space distance, and microridge height of the pig vocal fold epithelium were examined in this study and there were no differences between sham and reflux vocal folds.
The gene transcripts of epithelial barrier proteins (Ecad and ZO1), epithelial ion transporter proteins (CFTR and SCNN1α), and pro-inflammatory cytokines (IL-1β, TNF-α, and IFN-γ were compared in reflux and sham vocal folds. We chose Ecad, ZO-1, CFTR, and SCNN1α because they had been previously identified in the pig vocal fold epithelia and likely play a role in vocal fold epithelial defense to environmental challenges.23,24 Pro-inflammatory cytokines IL-1β and TNF-α are mediators of acute inflammation and their downstream effects on inflammatory cytokines and fibroblast proliferation may have important consequences in LPR. Furthermore, pepsin has been shown to induce up-regulation of IL-1 and TNF cytokine gene families in hypopharyngeal epithelial cells.25 IFN-γ plays an important role in innate immunity as well as adaptive immunity. IFN-γ has been shown to be up-regulated in human reflux esophagitis patients and Barrett's esophagus patients.26 Adaptive immunity, or immunological memory, is postulated to be involved in reflux esophagitis and thus IFN-γ may be modulated in LPR patients as well.27 Significant differences between reflux and sham vocal fold epithelia were not identified in any of the gene transcripts examined in this study.
Reasons for the lack of significant changes after 12 exposures to acidified-pepsin could include small sample size and the limited frequency of exposure to acidified-pepsin. Individual examination of data do not suggest that increasing sample size would significantly improve power and the sample size selected here is consistent with literature on the porcine animal model.6,28 The frequency and duration of the reflux challenge were based on previous research showing that as few as 3 experimental reflux episodes a week can result in injured laryngeal tissue if there is prior mucosal damage.10,11 However clinically, LPR is a chronic disease that can occur 2-5 times per day over many months in human patients.29 For this reason, an animal model has limitations for studying LPR because the duration and frequency needed to elicit a chronic disease are often not practical in a laboratory setting. More specifically to this study, the frequency and chronicity of LPR are challenging to replicate because multiple sedations are required and could bring harm to the research animals. We are currently investigating methodologies to expose unanesthetized animals to acidified-pepsin challenges more frequently.
The lack of significant change in epithelial structure and function is particularly striking because single, acute, acidic challenges applied to excised pig vocal fold epithelium, in vitro can alter barrier resistance and ion transport12,13 This current study was conducted in vivo, utilizing intact cardiovascular, immune, and neuromuscular systems that synergistically maintain homeostasis in a perturbed environment. These factors illustrate the importance of animal models to understand the pathophysiology of LPR and other laryngeal diseases. Previous studies have demonstrated reflux-induced damage to vocal folds that had been biopsied prior to initiation of the study.10,30 The role of acidified-pepsin on healthy vocal folds have not been examined. Our data suggest that healthy vocal folds are able to defend effectively against acidified-pepsin challenges.
Conclusion
Pigs were exposed to thrice weekly challenges with either reflux or saline applied to the vocal fold epithelia. Exposure to acidified pepsin did not significantly alter tissue morphology, ultrastructural morphology or epithelial intercellular space distance, gene transcripts of inflammatory cytokines, ion transporters, or epithelial barrier proteins. These data provide the groundwork for further investigations into developing animal models to understand the pathophysiology of LPR.
Acknowledgements
This project was funded by NIH RO1DC011759. The authors wish to thank the Purdue University Life Science Microscopy Laboratory and the Clinical Discovery Laboratory for their expertise in transmission electron microscopy and animal handling, respectively. We would also like to acknowledge Dr. Paul Snyder for his research expertise, and LeeAnn Grote for her personal attention to this project.
Footnotes
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Contributor Information
Abigail Durkes, Purdue University Department of Comparative Pathobiology, adcox@purdue.edu.
Preeti M. Sivasankar, Purdue University Department of Speech, Language, & Hearing Sciences
References
- 1.JA K. Laryngopharyngeal reflux is different from classic gastroesophageal reflux disease. - Abstract - Europe PubMed Central. Ear, Nose, & Throat Journal. 2002;81:7–9. [PubMed] [Google Scholar]
- 2.Morice AH. Is Reflux Cough Due to Gastroesophageal Reflux Disease or Laryngophary. Lung. 2008;186:S103–S106. doi: 10.1007/s00408-007-9038-6. [DOI] [PubMed] [Google Scholar]
- 3.Wang XY, Ye JY, Han DM. [Clinical value of 24-hour pH monitoring in patients with laryngopharyngeal reflux disease]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007;42:834–838. [PubMed] [Google Scholar]
- 4.Karkos PD, Wilson JA. Empiric treatment of laryngopharyngeal reflux with proton pump inhibitors: a systematic review. Laryngoscope. 2006;116:144–148. doi: 10.1097/01.mlg.0000191463.67692.36. [DOI] [PubMed] [Google Scholar]
- 5.Jiang JJ, Raviv JR, Hanson DG. Comparison of the phonation-related structures among pig, dog, white-tailed deer, and human larynges. Ann Otol Rhinol Laryngol. 2001;110:1120–1125. doi: 10.1177/000348940111001207. [DOI] [PubMed] [Google Scholar]
- 6.Gorti GK, Birchall MA, Haverson K, Macchiarini P, Bailey M. A preclinical model for laryngeal transplantation: anatomy and mucosal immunology of the porcine larynx. Transplantation. 1999;68:1638–1642. doi: 10.1097/00007890-199912150-00006. [DOI] [PubMed] [Google Scholar]
- 7.Barker E, Haverson K, Stokes CR, Birchall M, Bailey M. The larynx as an immunological organ: immunological architecture in the pig as a large animal model. Clin Exp Immunol. 2006;143:6–14. doi: 10.1111/j.1365-2249.2005.02950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koufman JA, Wiener GJ, Wu WC, Castell DO. Reflux laryngitis and its sequelae: the diagnostic role of ambulatory 24-hour pH monitoring. Journal of Voice. 1988;2:78–89. [Google Scholar]
- 9.Williams RB, Ali GN, Wallace KL, Wilson JS, De Carle DJ, Cook IJ. Esophagopharyngeal acid regurgitation: dual pH monitoring criteria for its detection and insights into mechanisms. Gastroenterology. 1999;117:1051–1061. doi: 10.1016/s0016-5085(99)70389-6. [DOI] [PubMed] [Google Scholar]
- 10.Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101:1–78. doi: 10.1002/lary.1991.101.s53.1. [DOI] [PubMed] [Google Scholar]
- 11.Little FB, Koufman JA, Kohut RI, Marshall RB. Effect of gastric acid on the pathogenesis of subglottic stenosis. Ann Otol Rhinol Laryngol. 1985;94:516–519. doi: 10.1177/000348948509400521. [DOI] [PubMed] [Google Scholar]
- 12.Erickson E, Sivasankar M. Simulated reflux decreases vocal fold epithelial barrier resistance. Laryngoscope. 2010;120:1569–1575. doi: 10.1002/lary.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson-Levendoski E, Sivasankar MP. Role for Ion Transport in Porcine Vocal Fold Epithelial Defense to Acid Challenge. Otolaryngol Head Neck Surg. 2011 doi: 10.1177/0194599811428273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sivasankar M, Fisher KV. Vocal folds detect ionic perturbations on the luminal surface: an in vitro investigation. J Voice. 2008;22:408–419. doi: 10.1016/j.jvoice.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Haggitt RC. Histopathology of reflux-induced esophageal and supraesophageal injuries. Am J Med. 2000;108(Suppl 4a):109s–111s. doi: 10.1016/s0002-9343(99)00346-0. [DOI] [PubMed] [Google Scholar]
- 16.Zentilin P, Savarino V, Mastracci L, et al. Reassessment of the diagnostic value of histology in patients with GERD, using multiple biopsy sites and an appropriate control group. Am J Gastroenterol. 2005;100:2299–2306. doi: 10.1111/j.1572-0241.2005.50209.x. [DOI] [PubMed] [Google Scholar]
- 17.Hahn MS, Kobler JB, Zeitels SM, Langer R. Quantitative and comparative studies of the vocal fold extracellular matrix II: collagen. Ann Otol Rhinol Laryngol. 2006;115:225–232. doi: 10.1177/000348940611500311. [DOI] [PubMed] [Google Scholar]
- 18.Hahn MS, Kobler JB, Starcher BC, Zeitels SM, Langer R. Quantitative and comparative studies of the vocal fold extracellular matrix. I: Elastic fibers and hyaluronic acid. Ann Otol Rhinol Laryngol. 2006;115:156–164. doi: 10.1177/000348940611500213. [DOI] [PubMed] [Google Scholar]
- 19.Tobey NA, Carson JL, Alkiek RA, Orlando RC. Dilated intercellular spaces: a morphological feature of acid reflux--damaged human esophageal epithelium. Gastroenterology. 1996;111:1200–1205. doi: 10.1053/gast.1996.v111.pm8898633. [DOI] [PubMed] [Google Scholar]
- 20.Franchi A, Brogelli B, Massi D, Santucci M, De Campora E, Gallo O. Dilation of intercellular spaces is associated with laryngo-pharyngeal reflux: an ultrastructural morphometric analysis of laryngeal epithelium. Eur Arch Otorhinolaryngol. 2007;264:907–911. doi: 10.1007/s00405-007-0295-z. [DOI] [PubMed] [Google Scholar]
- 21.Johnston N, Bulmer D, Gill GA, et al. Cell biology of laryngeal epithelial defenses in health and disease: further studies. Ann Otol Rhinol Laryngol. 2003;112:481–491. doi: 10.1177/000348940311200601. [DOI] [PubMed] [Google Scholar]
- 22.Gray SD. Cellular physiology of the vocal folds. Otolaryngol Clin North Am. 2000;33:679–698. doi: 10.1016/s0030-6665(05)70237-1. [DOI] [PubMed] [Google Scholar]
- 23.Fisher KV, Telser A, Phillips JE, Yeates DB. Regulation of vocal fold transepithelial water fluxes. J Appl Physiol. 2001;91:1401–1411. doi: 10.1152/jappl.2001.91.3.1401. [DOI] [PubMed] [Google Scholar]
- 24.Sivasankar M, Erickson E, Rosenblatt M, Branski RC. Hypertonic challenge to porcine vocal folds: effects on epithelial barrier function. Otolaryngol Head Neck Surg. 2010;142:79–84. doi: 10.1016/j.otohns.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samuels TL, Johnston N. Pepsin as a causal agent of inflammation during nonacidic reflux. Otolaryngol Head Neck Surg. 2009;141:559–563. doi: 10.1016/j.otohns.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 26.Zhong YQ, Lin Y, Xu Z. Expression of IFN-gamma and IL-4 in the esophageal mucosa of patients with reflux esophagitis and Barrett's esophagus and their relationship with endoscopic and histologic grading. Dig Dis Sci. 2011;56:2865–2870. doi: 10.1007/s10620-011-1696-9. [DOI] [PubMed] [Google Scholar]
- 27.Souza RF, Huo X, Mittal V, et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology. 2009;137:1776–1784. doi: 10.1053/j.gastro.2009.07.055. [DOI] [PubMed] [Google Scholar]
- 28.Woodson G. Developing a porcine model for study of vocal fold scar. J Voice. 2012;26:706–710. doi: 10.1016/j.jvoice.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Datta K, Datat R, Venkatesh MD, Dey D, Jaipurkar R. 24-hour dual-probe ambulatory pH-metry findings in cases of laryngopharyngeal reflux disease. Journal of Laryngology and Voice. 2011;1:18. [Google Scholar]
- 30.Adhami T, Goldblum JR, Richter JE, Vaezi MF. The role of gastric and duodenal agents in laryngeal injury: an experimental canine model. Am J Gastroenterol. 2004;99:2098–2106. doi: 10.1111/j.1572-0241.2004.40170.x. [DOI] [PubMed] [Google Scholar]