Abstract
Rationale
Although mammalian cardiac regeneration can occur in the neonatal period, the factors involved in this process remain to be established. As tissue and limb regeneration require concurrent reinnervation by the peripheral nervous system, we hypothesized that cardiac regeneration also requires reinnervation.
Objective
To test the hypothesis that reinnervation is required for innate neonatal cardiac regeneration.
Methods and Results
We crossed a Wnt1-Cre transgenic mouse with a double-tandem (td) Tomato reporter strain to identify neural crest-derived cell lineages including the peripheral autonomic nerves in the heart. This approach facilitated the precise visualization of subepicardial autonomic nerves in the ventricles using wholemount epifluorescence microscopy. Following resection of the left ventricular apex in 2-day-old neonatal mice, sympathetic nerve structures, which envelop the heart under normal conditions, exhibited robust re-growth into the regenerating myocardium. Chemical sympathectomy inhibited sympathetic regrowth and subsequent cardiac regeneration following apical resection, significantly (scar size as cross-sectional percentage of viable LV myocardium: n=9, 0.87±1.4% vs. n=6, 14.05±4.4%; p<0.01).
Conclusions
These findings demonstrate that the profound regenerative capacity of the neonatal mammalian heart requires sympathetic innervation. As such, these data offer significant insights into an underlying basis for inadequate adult regeneration following myocardial infarction, a situation where nerve growth is hindered by age-related influences and scar tissue.
Keywords: Cardiac regeneration, neonatal repair, cardiac innervation, sympathetic denervation, neonatal mouse cardiac myocyte, sympathic nervous system
INTRODUCTION
It has recently been established that unlike the limited cardiac regeneration demonstrated by the injured adult mammalian heart, the neonatal heart remains permissive for near-complete cardiac regeneration during a finite developmental period1, 2. In a similar fashion to other regeneration-competent species including the salamander3 and zebrafish4, 5, the dominant mechanism underlying cardiac regeneration in the neonatal mouse appears to be de-differentiation and proliferation of resident cardiac myocytes2. However, the underlying basis for regeneration and revascularization of the neonatal tissue are not fully understood. Other vertebrate6–9 and invertebrate10 models of tissue regeneration exhibit a complete dependence on reinnervation of the regenerating tissue by nerves of the peripheral nervous system (PNS). Despite the clinical importance of cardiac autonomic innervation, the neuroanatomy of the sympathetic nerve plexus innervating the ventricular myocardium remains incompletely characterized11.
To address these issues, we used a combination of genetic and pharmacologic tools to map the cardiac PNS and to test the role of peripheral nerve innervation in mammalian cardiac regeneration. Post-ganglionic, subepicardial sympathetic axons make up the bulk of nerve fibers in the ventricles11 and we demonstrate, for the first time, that these nerve fibers undergo robust re-growth and reinnervation during the regeneration of resected ventricular tissue. Furthermore, sympathectomy abrogates cardiac regeneration and promotes collagenous scar formation, demonstrating that innate mammalian cardiac regeneration in neonates is dependent on sympathetic innervation. Together these findings suggest that concurrent reinnervation of injured adult cardiac tissue is essential for functional and complete cardiac regeneration.
METHODS
A detailed description of the experimental procedure and statistical analysis is provided in the online data supplement. Briefly, Wnt1-Cre mice were crossed with tdTomato reporter mice (The Jackson Laboratory). Cre/+; tdTomato/+ animals were used in experiments where direct observation of nerves was required. BALB/cJ animals (The Jackson Laboratory) were used in apical resection experiments. Studies were performed on 2-day-old neonates and tissue was collected at either day 14- or day 21-post injury.
RESULTS
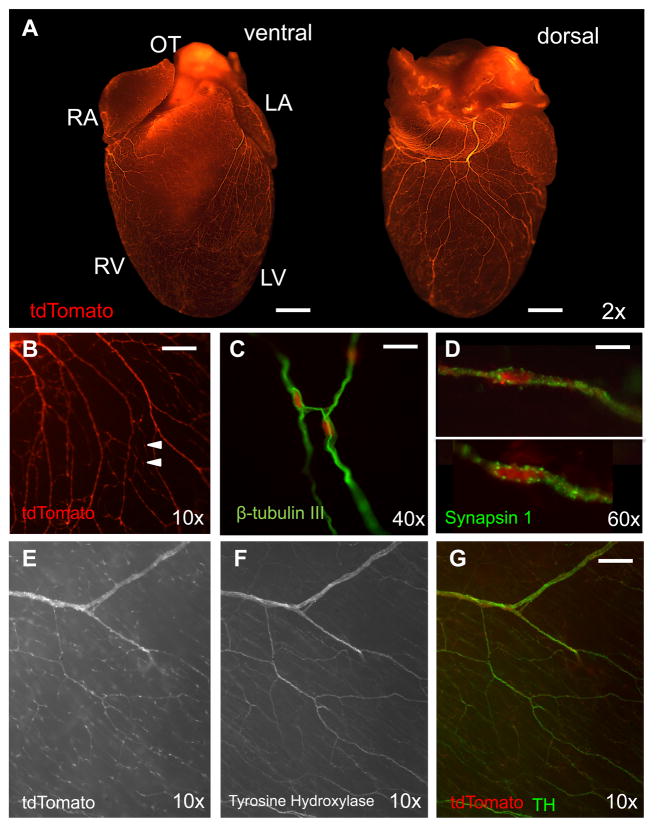
The proto-oncogene Wnt1 is only expressed during the development of the central nervous system and demarcates lineages derived from the neural crest12. The Wnt1-Cre transgenic mouse strain is a widely used and well-validated model for neural crest lineage tracing studies13, 14. Wnt1-Cre transgenic mice were mated with mice expressing the tdTomato reporter15 so that neural crest-derived cells, and their progeny, are permanently labeled with the red fluorescent tdTomato reporter. Using this system, together with whole-mount, broad focal plane, epifluorescent stereomicroscopy, we visualized the subepicardial neural network in unprecedented detail (Figure 1A, B).
Figure 1. Subepicardial distribution of post-ganglionic sympathetic nerve fibers in the mouse heart.
A, Crossing Wnt1-Cre transgenic mice with tdTomato reporter mice identifies nerve fibers throughout the entire subepicardium from the base of the heart to the apex of the ventricles (bar, 1 mm). B, The fibers are heavily varicosed (arrows) (bar, 200 μm). C, They stain positive for the neurofilament marker β-tubulin III (bar, 50 μm), the presynaptic marker synapsin 1 (D) (bar, 20 μm) and the sympathetic nerve fiber marker TH (E–G) (bar, 200 μm).
The structures observed in whole-mount were confirmed to be nerves by staining for β-tubulin III (Figure 1C) and the presynaptic marker synapsin1 (Figure 1D). In order to determine from which autonomic branch these nerves derive, we performed immunofluorescence histology against tyrosine hydroxylase (TH), and choline acetyltransferase (ChAT). Co-localization of TH+ fibers only with Wnt1-Cre+ fibers was observed at the base of the heart where nerve density is greatest (Figure 1E–G), which agrees with the published distribution of the sympathetic branch primarily to the subepicardium11, 13.
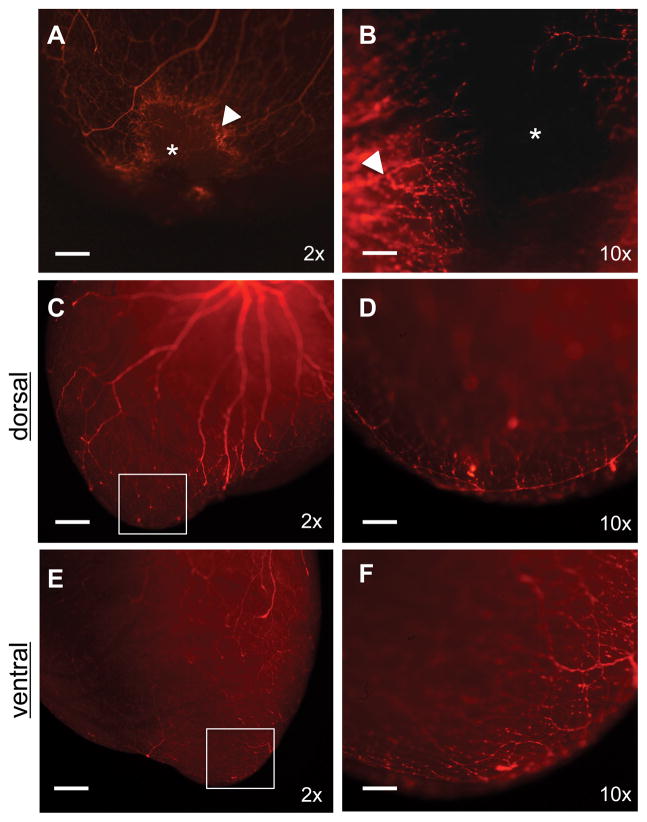
In contrast to the adult neonatal sympathetic neurons, sympathetic ganglia and dorsal root ganglia exhibit enhanced plasticity, both in vitro16 and in vivo17. Therefore, we assessed the ability of neonatal subepicardial sympathetic nerves to reinnervate ventricular myocardium following injury. We resected the apex of the left ventricle of 2-day-old Wnt1-Cre:tdTomato mice, as described18. Under normal conditions the neonate heart is capable of a robust regenerative response following apical resection within 21 days. At 14 days post-resection, we observed an area of heavy dendrite hyperinnervation at the injury border (Figure 2A), which is consistent with that described in studies of acute myocardial infarction19 and varicose fibers emerging from the border into the site of active regeneration (Figure 2B). By day 21 post-injury the entire apex had regenerated and had become reinnervated by organized, arborized and anastomosed fibers (Figure 2C–F).
Figure 2. Concurrent reinnervation and cardiac regeneration.
A, B, At day 14 post-resection the apex is in the process of regenerating (*) as nerves regrow into the injury (arrow marks site of hyperinnervation at injury border) (bars, 500 μm and 100 μm, respectively). C–F. After 21 days the apex has regenerated and fully reinnervated (bar, 500 μm). Boxes from C and E are expanded with greater magnification in D and F (bar, 100 μm). All images are of Wnt1-Cre;tdTomato hearts.
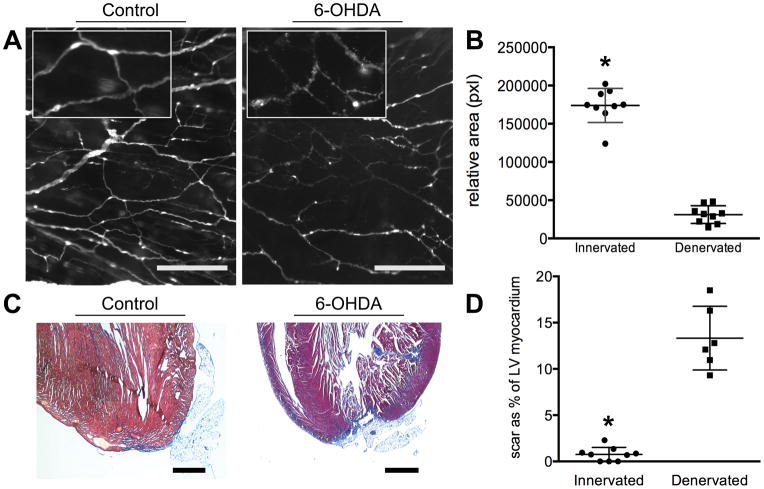
Denervation of tissue following injury in other animal models results in a block of the innate regenerative response with subsequent scar formation at the expense of functional tissue6, 8. Considering the vast abundance of sympathetic nerves associated with the ventricular myocardium and their ability to regrow in the model of neonatal cardiac regeneration, we sought to determine whether these nerves were a necessary component of the cardiac regenerative response. Beginning 48 hours after apical resection, chemical sympathectomy of adrenergic nerves20, 21 was induced by treatment of neonatal mice with 3 doses of 6-Hydroxydopamine hydrobromide (6-OHDA, 250 mg/kg, intraperitoneal injection) 48 hours apart. In response to 6-OHDA, the subepicardial sympathetic nerves demonstrated classical features of Wallerian degeneration22 throughout the surface of the heart (Figure 3A). Treatment resulted in robust denervation (n=9, 1.74 × 105±7447pxl; n=9, 3.11 × 104±3863pxl; p<0.01) of the subepicardial nerves in the heart, as quantified by densitometry (Figure 3B).
Figure 3. Cardiac innervation is required for myocardial regeneration.
A, Administering 6-OHDA to neonates induces Wallerian degeneration of sympathetic nerve fibers. Inset demonstrates degeneratio of nerve structure (epifluorescence, Wnt1-Cre;tdTomato. bar, 200 μm). B, Densitometry of Wnt1-Cre;tdTomato nerves from 21-day-old mouse hearts that are innervated compared to denervated (n=9, 1.74×105±7447pixels; n=9, 3.11×104±3863pixels; p<0.01). C, In the presence of sympathetic nerves the apex can regenerate with minimal fibrosis; visualized histologically with Masson’s Trichrome stain (blue). Small amounts of adipose are adherent to the apex on occasion. Following denervation with 6-OHDA innate regeneration is blocked and extensive scar and adipocyte deposition can be observed (bar, 1 mm). D, Scar area as a percentage of left ventricular (LV) myocardium in cross-section (n=9, 0.87±1.4% vs. n=6, 14.05±4.4%; p<0.01).
In the absence of 6-OHDA neonatal mouse hearts underwent robust reinnervation and regeneration of the ventricular apex following resection with little or no signs of injury (Figure 3C) (n=9, scar as a percentage of cross-sectional myocardial area = 0.87±1.4%,). In contrast, the hearts of sympathectomized mice consistently exhibited extensive scarring, lack of regeneration and failure to replace the ventricular myocardium following apical resection (Figure 3C) and denervation (n=6, 14.05±4.4%, p<0.01) (Figure 3D).
In summary, we have demonstrated that sympathetic denervation completely inhibits the ability of the neonatal heart to regenerate following injury. These findings correlate with those from other animal models of tissue regeneration, which describe a critical dependence on peripheral nerves in order to regenerate injured tissue6–10.
DISCUSSION
Despite the clinical importance of the cardiac autonomic system, there is still much to learn regarding the neuroanatomy of the mammalian heart. Here we combine a strong fluorescence lineage tracing reporter system with broad focal plane stereomicroscopy to visualize the vast cardiac sympathetic neural network in unprecedented detail. We demonstrate large nerve bundles entering the heart from the dorsal aspect, which then arborize and anastomose throughout all four chambers of the heart. The resulting dense network of nerve fibers makes extensive contact, via “en-passant” synapses, with subepicardial myocytes in the ventricles and with vessels of the cardiovasculature.
Direct visualization of these nerves facilitated investigation into the ability of the nerves to regenerate in vivo following injury and identified that cardiac regeneration is dependent on a nerve supply. Our data support the hypothesis that innervation is critical for innate cardiac regeneration exhibited by neonatal mice, as inhibition of sympathetic nerves completely blocks myocardial repair. Several other vertebrate and invertebrate models of tissue regeneration support this concept, including a recent report demonstrating that ablation of the parasympathetic branch of the autonomic system of neonatal mice by surgical vagotomy inhibits cardiac regeneration, despite an assumed presence of sympathetic nerves9. Together, these results suggest that contributions from both branches of autonomic nerves are needed to support full cardiac regeneration. Further studies are required to tease apart the relationship between the branches of the autonomic nervous system, the myocardium and the cardiovasculature and to address the structural changes in cardiac neuroanatomy that result from injury.
Substantial evidence is accumulating that implicates peripheral autonomic nerves in several aspects of tissue regeneration and homeostasis, including stem cell niche maintenance23, vasculogenesis and patterning24, and tissue hyperplasia6, 7. Cardiac nerves clearly function as more than just simple impulse conduits. A recent report demonstrated that uninjured rats treated with 6-OHDA to ablate the sympathetic while sparing the parasympathetic nerves, exhibit myocardial injury and fibrosis21. This injury was prevented when a neuroprotectant was co-administered, demonstrating that myocardial injury was a secondary response to sympathetic nerve loss.
Taken together, these data support the idea that peripheral cardiac autonomic nerves play a role not only in repair, but also in chronic disease. Peripheral neuropathies due to diabetes or heart transplant, are typically associated with idiopathic vasculopathy and heart failure25, 26. Further work is needed to investigate whether the loss of cardiac nerves in these patients results in a pathological loss of trophic support for the myocardium, vasculature or cardiac stem cell niches. Considering the physiological differences between fetal/embryonic and adult post-ganglionic nerves, these findings have important implications for improving adult cardiac regeneration, which has, as of yet, remained elusive.
Supplementary Material
Novelty and Significance.
What Is Known?
Whereas adult mammals, including humans, exhibit regenerative capacity inadequate to repair injury due to heart attack or other damage, the neonatal mouse is capable of regenerating injured myocardium during a finite developmental window.
The mammalian heart is extensively innervated by peripheral nerves.
Peripheral nerves are essential for tissue repair in newts and zebrafish.
What New Information Does This Article Contribute?
We used a Wnt1 transgenic reporter mouse, which allowed for high resolution visualization of cardiac sympathetic nerves.
Using this transgenic mouse we demonstrated concurrent nerve and myocardial tissue regrowth and repair at the site of cardiac regeneration in the neonatal mouse.
Cardiac regeneration in the neonatal mouse is critically dependent on sympathetic nerves, as denervation blocks cardiac tissue regeneration.
Our understanding of cardiac regeneration has advanced significantly in recent years. However, large-scale regeneration or full recovery remains elusive, suggesting that an important component of the regenerative process is being overlooked. Unlike the adult, the neonatal mouse heart retains an innate ability to regenerate following injury, providing a compelling model to study the mechanisms of mammalian cardiac regeneration. Several animal species exhibit a robust capacity to regenerate tissue and limbs and this process is critically dependent upon intact innervation. Here we demonstrate that neonatal cardiac regeneration is critically dependent on sympathetic nerves and identify a potentially novel strategic target for improving cardiac repair. This finding has important implications for adult regeneration following myocardial infarction where nerve growth is hindered by age related influences, disease processes and scar tissue.
Acknowledgments
SOURCES OF FUNDING
This research was supported by National Institutes of Health (NIH) grant R01 HL084275 to J.M.H. J.M.H. is also funded by NIH grants R01 HL110737, R01 HL107110 and 5UM 1HL113460 and grants from the Starr Foundation and the Soffer Family Foundation.
Nonstandard Abbreviations and Acronyms
- WNT1
Wingless-Type MMTV Integration Site Family, Member 1
- CRE
Cre recombinase
- LV
Left Ventricle
- PNS
Peripheral Nervous System
- TH
Tyrosine Hydroxylase
- ChAT
Choline Acetyltransferase
- 6-OHDA
6-hydroxydopamine
Footnotes
DISCLOSURES
Dr. Hare reported having a patent for cardiac cell-based therapy. He holds equity in Vestion and maintains a professional relationship with Vestion as a consultant and member of the Board of Directors and Scientific Advisory Board. Vestion Inc. did not play a role in the design and conduct of the study. The other authors report no conflicts.
References
- 1.Bryant DM, O’Meara CC, Ho NN, Gannon J, Cai L, Lee RT. A systematic analysis of neonatal mouse heart regeneration after apical resection. J Mol Cell Cardiol. 2015;79:315–318. doi: 10.1016/j.yjmcc.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh BN, Koyano-Nakagawa N, Garry JP, Weaver CV. Heart of newt: A recipe for regeneration. J Cardiovasc Transl Res. 2010;3:397–409. doi: 10.1007/s12265-010-9191-9. [DOI] [PubMed] [Google Scholar]
- 4.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 5.Lam NT, Currie PD, Lieschke GJ, Rosenthal NA, Kaye DM. Nerve growth factor stimulates cardiac regeneration via cardiomyocyte proliferation in experimental heart failure. PLoS One. 2012;7:e53210. doi: 10.1371/journal.pone.0053210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckley G, Wong J, Metcalfe AD, Ferguson MW. Denervation affects regenerative responses in mrl/mpj and repair in c57bl/6 ear wounds. J Anat. 2012;220:3–12. doi: 10.1111/j.1469-7580.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, Brockes JP. Nerve dependence in tissue, organ, and appendage regeneration. Trends Neurosci. 2012;35:691–699. doi: 10.1016/j.tins.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, Brockes JP. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science. 2007;318:772–777. doi: 10.1126/science.1147710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahmoud AI, O’Meara CC, Gemberling M, Zhao L, Bryant DM, Zheng R, Gannon JB, Cai L, Choi WY, Egnaczyk GF, Burns CE, Burns CG, MacRae CA, Poss KD, Lee RT. Nerves regulate cardiomyocyte proliferation and heart regeneration. Dev Cell. 2015 doi: 10.1016/j.devcel.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cebria F, Newmark PA. Morphogenesis defects are associated with abnormal nervous system regeneration following roboa rnai in planarians. Development. 2007;134:833–837. doi: 10.1242/dev.02794. [DOI] [PubMed] [Google Scholar]
- 11.Kimura K, Ieda M, Fukuda K. Development, maturation, and transdifferentiation of cardiac sympathetic nerves. Circ Res. 2012;110:325–336. doi: 10.1161/CIRCRESAHA.111.257253. [DOI] [PubMed] [Google Scholar]
- 12.Echelard Y, Vassileva G, McMahon AP. Cis-acting regulatory sequences governing wnt-1 expression in the developing mouse cns. Development. 1994;120:2213–2224. doi: 10.1242/dev.120.8.2213. [DOI] [PubMed] [Google Scholar]
- 13.Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- 14.Hatzistergos KETL, Saur D, Seidler B, Dymecki SM, Mai JJ, White IA, Balkan W, Kanashiro-Takeuchi RM, Hare JM. Title: Ckit+ cardiac progenitors of neural crest origin. PNAS. doi: 10.1073/pnas.1517201112. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaner NC, Patterson GH, Davidson MW. Advances in fluorescent protein technology. J Cell Sci. 2007;120:4247–4260. doi: 10.1242/jcs.005801. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda J. Nerve cells of adult and aged mice grown in a monolayer culture: Age-associated changes in morphological and physiological properties of dorsal root ganglion cells in vitro. Dev Neurosci. 1985;7:374–394. doi: 10.1159/000112304. [DOI] [PubMed] [Google Scholar]
- 17.Smith PG. Functional plasticity in the sympathetic nervous system of the neonatal rat. Exp Neurol. 1986;91:136–146. doi: 10.1016/0014-4886(86)90031-2. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoud AI, Porrello ER, Kimura W, Olson EN, Sadek HA. Surgical models for cardiac regeneration in neonatal mice. Nat Protoc. 2014;9:305–311. doi: 10.1038/nprot.2014.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114:1004–1021. doi: 10.1161/CIRCRESAHA.113.302549. [DOI] [PubMed] [Google Scholar]
- 20.Angeletti PU, Levi-Montalcini R. Sympathetic nerve cell destruction in newborn mammals by 6-hydroxydopamine. Proc Natl Acad Sci U S A. 1970;65:114–121. doi: 10.1073/pnas.65.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang YH, Jiang P, Yang JL, Ma DF, Lin HQ, Su WG, Wang Z, Li X. Cardiac dysregulation and myocardial injury in a 6-hydroxydopamine-induced rat model of sympathetic denervation. PLoS One. 2015;10:e0133971. doi: 10.1371/journal.pone.0133971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conforti L, Gilley J, Coleman MP. Wallerian degeneration: An emerging axon death pathway linking injury and disease. Nat Rev Neurosci. 2014;15:394–409. doi: 10.1038/nrn3680. [DOI] [PubMed] [Google Scholar]
- 23.Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li W, Kohara H, Uchida Y, James JM, Soneji K, Cronshaw DG, Zou YR, Nagasawa T, Mukouyama YS. Peripheral nerve-derived cxcl12 and vegf-a regulate the patterning of arterial vessel branching in developing limb skin. Dev Cell. 2013;24:359–371. doi: 10.1016/j.devcel.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr, Sowers JR. Diabetes and cardiovascular disease: A statement for healthcare professionals from the american heart association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 26.Schmauss D, Weis M. Cardiac allograft vasculopathy: Recent developments. Circulation. 2008;117:2131–2141. doi: 10.1161/CIRCULATIONAHA.107.711911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.