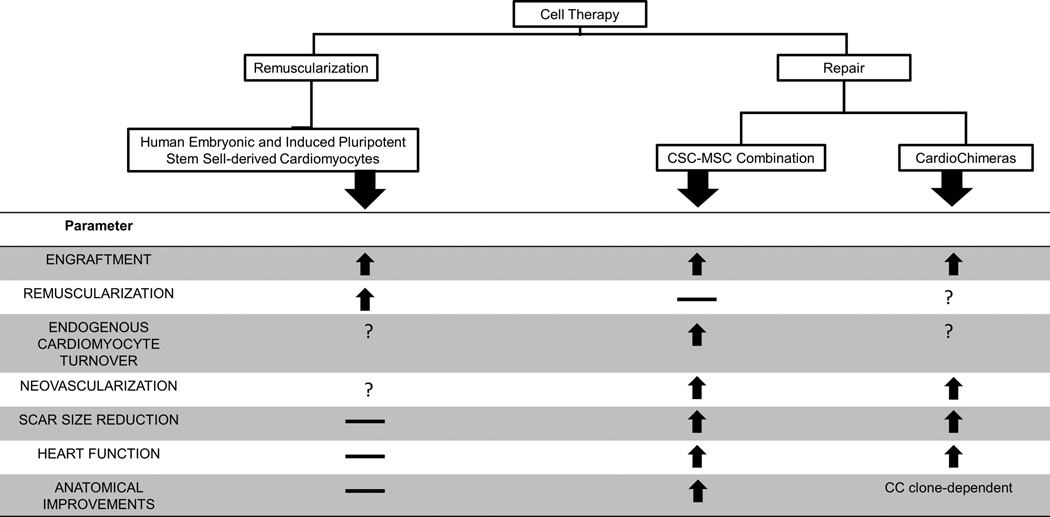
A growing body of work supports cell-based therapeutics as a promising strategy for treating cardiovascular disease1. Patients who receive stem cells, previously isolated from the bone marrow (e.g. MSCs)2–5 or the heart (cardiac stem cells; CSCs)6, 7 experience improved cardiac anatomy, increased functional capacity and quality of life. With regard to MSCs and CSCs, scar tissue is reduced and replaced by contractile myocardium2, 3, 6, 7, accompanied by increased tissue perfusion, due most likely to neovascularization and improved endothelial function, globally8 and locally4; 1–3, 5.
Early studies hypothesized that the therapeutic capacity of transplanted cells derives from their ability to differentiate into cardiomyocytes9. However, this concept has not yet been meaningfully achieved. In a recent study from Chong et al., non-human primates were administered 109 human embryonic stem cells-derived cardiomyocytes following myocardial infarction (MI). In this study, they achieved engraftment but no functional recovery9. Similar findings were recently reported by others10. Thus, engraftment and differentiation is not sufficient for cardiac repair.
In contrast, transplantation of cells with lower capacity to form new myocytes does significantly enhance repair1. This seemingly paradoxical effect coupled with important new mechanistic insights underlying cardiac biology raises the idea that the heart possesses regenerative capacity which may be a therapeutically targeted. Notably, after the landmark discovery by Anversa et al11, a series of studies12 showed that human myocyte turnover occurs during life, a phenomenon upregulated by injury13. Notably, Bergmann et al. estimates that under physiologic conditions, 35% of healthy myocytes by age 70 develop post-natally12.
Cell therapy promotes repair by enhancing endogenous cardiomyocyte turn over14, 15. For example, although few MSCs directly transdifferentiate into cardiomyocytes following transplantation14, 16, the majority of them establish cell-cell interactions with host myocardium and stimulate endogenous cardiomyocyte turnover and differentiation of CSCs14, 16, 17. Similarly, although CSCs transplantation was thought to promote repair via direct remuscularization18, it was later shown to also stimulate endogenous myocyte turnover and progenitor cell recruitment15.
Importantly, CSCs hold minimal endogenous regenerative activity14, 19, which may be significantly enhanced following cell-cell contact with MSCs14, 17. These cell-cell interactions closely resemble interactions within stem cell niches20, and prompted the idea of combining both cell types into a single cell therapeutic (Combo). Compared to monocellular therapeutic schemes, Combo demonstrated superior capacity to induce and sustain myocardial repair21, 22. Engraftment, heart function and scar size reduction were enhanced, whereas the rate of cardiomyocyte turnover and endothelial function were significantly increased21. From a mechanistic standpoint, the interactions of MSCs with CSCs are thought to involve production and exchange of growth factors, microRNAs, microvesicles and mitochondria with the host myocardium and/or each other1,
Quijada and colleagues23 have advanced the concept of cell combination therapy and suggest that Combo may promote repair through mechanisms involving fusion between MSCs and CSCs. The resulting hybrid cells (dubbed CardioChimeras or CCs) exhibit enhanced regenerative capacity, compared to each cell type alone.
Although it is unknown whether CCs exist naturally in humans, Quijada et al. employed ex-vivo bioengineering to test this idea in mice. Compared to the parent cells, CCs exhibited enhanced cardiovascular lineage commitment. When co-cultured with cardiomyocytes, CCs prevented maladaptive hypertrophy and apoptosis, at a level similar to Combo or each cell type alone, although only one of the CC clones enhanced cardiac gene transcription to a level comparable to Combo. When transplanted in mice with MI, CCs were equally effective to Combo, although the degree of recovery varied between different CC preparations23.
From a translational standpoint, CCs represent an interesting modality. It introduces cell fusion, previously associated with an increased risk for arrhythmias24, 25, as a potentially useful strategy for myocardial regeneration.
In parallel, it raises a number of concerns. For example, stochastic fusion of CSCs and MSCs will likely result in significant variability in generating therapeutically-competent CCs. To this end, further optimization of the methodology will be required before it becomes clinically relevant (i.e. prospective identification and selection of CSC and MSC founder clones). Similarly, it is unclear whether CCs retain the immunotolerant MSC phenotype that would make them an attractive alternative to Combo. Last, it will be important to understand how the elimination of MSC-CSC interactions, which are an essential stem cell niche property, and which we have found play an important role for regeneration14, may affect the therapeutic outcome in the clinical setting.
In summary, the findings by Quijada et al. further support the finding that combination cell therapy provides the most promising cell-based strategy for enhancing cardiac repair14, 21, 22. The concept of cell-cell fusion as an additional mechanism of action is exciting and its potential applicability into the clinical setting warrants further investigation.
Figure 1.
Acknowledgments
FUNDING SOURCES.
This study was funded by the National Institutes of Health grants (awarded to J.M.H.): R01 HL107110, R01 HL094849. JMH is also supported by the NIH grants R01 HL110737, R01 HL084275 and 5UM HL113460; and grants from the Starr foundation and the Soffer Family Foundation.
Footnotes
DISCLOSURES.
Drs. Hare and Hatzistergos disclose a relationship with Vestion that includes equity, board membership, and consulting. Vestion did not contribute funding to this study. Dr. Hare discloses equity in Kardia and a grant from Biocardia.
References
- 1.Karantalis V, Balkan W, Schulman IH, Hatzistergos KE, Hare JM. Cell-based therapy for prevention and reversal of myocardial remodeling. American journal of physiology Heart and circulatory physiology. 2012;303:H256–H270. doi: 10.1152/ajpheart.00221.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golpanian S, El-Khorazaty J, Mendizabal A, DiFede DL, Suncion VY, Karantalis V, Fishman JE, Ghersin E, Balkan W, Hare JM. Effect of aging on human mesenchymal stem cell therapy in ischemic cardiomyopathy patients. J Am Coll Cardiol. 2015;65:125–132. doi: 10.1016/j.jacc.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karantalis V, DiFede DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, Fishman J, Pattany P, McNiece I, Conte J, Schulman S, Wu K, Shah A, Breton E, Davis-Sproul J, Schwarz R, Feigenbaum G, Mushtaq M, Suncion VY, Lardo AC, Borrello I, Mendizabal A, Karas TZ, Byrnes J, Lowery M, Heldman AW, Hare JM. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res. 2014;114:1302–1310. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–S64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Premer C, Blum A, Bellio MA, Schulman IH, Hurwitz BE, Parker M, Dermarkarian CR, DiFede DL, Balkan W, Khan A, Hare JM. Allogeneic Mesenchymal Stem Cells Restore Endothelial Function in Heart Failure by Stimulating Endothelial Progenitor Cells. EBioMedicine. 2015;2:467–475. doi: 10.1016/j.ebiom.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riegler J, Tiburcy M, Ebert A, Tzatzalos E, Raaz U, Abilez OJ, Shen Q, Kooreman NG, Neofytou E, Chen V, Wang M, Meyer T, Tsao PS, Connolly AJ, Couture LA, Gold JD, Zimmermann WH, Wu JC. Human Engineered Heart Muscles Engraft and Survive Long-Term in a Rodent Myocardial Infarction Model. Circ Res. 2015 doi: 10.1161/CIRCRESAHA.115.306985. published on August 19 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. NEnglJMed. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 12.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andra M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisen J. Dynamics of Cell Generation and Turnover in the Human Heart. Cell. 2015;161:1566–1575. doi: 10.1016/j.cell.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE. Bone Marrow Mesenchymal Stem Cells Stimulate Cardiac Stem Cell Proliferation and Differentiation Novelty and Significance. Circulation research. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, Sun B, Aminzadeh M, Marban E. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med. 2013;5:191–209. doi: 10.1002/emmm.201201737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. ProcNatlAcadSciUSA. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki G, Iyer V, Lee TC, Canty JM., Jr Autologous mesenchymal stem cells mobilize cKit+ and CD133+ bone marrow progenitor cells and improve regional function in hibernating myocardium. Circ Res. 2011;109:1044–1054. doi: 10.1161/CIRCRESAHA.111.245969. [DOI] [PubMed] [Google Scholar]
- 18.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 19.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karantalis V, Suncion-Loescher VY, Bagno L, Golpanian S, Wolf S, Sanina C, Premer C, Kanelidis AJ, McCall F, Wang B, Balkan W, Rodriguez J, Rosado M, Morales AR, Hatzistergos KE, Natsumeda M, Margitich IS, Hernandez-Schulman I, Gomes SA, Mushtaq M, DiFede Velazquez DL, Fishman JE, Pattany PM, Zambrano JP, Heldman AW, Hare JM. Synergistic Effects of Combined Cell Therapy for Chronic Ischemic Cardiomyopathy. JACC. 2015 doi: 10.1016/j.jacc.2015.08.879. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW. Enhanced Effect of Combining Human Cardiac Stem Cells and Bone Marrow Mesenchymal Stem Cells to Reduce Infarct Size and to Restore Cardiac Function After Myocardial Infarction Clinical Perspective. Circulation. 2013;127:213–223. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quijada P, Salunga HT, Hariharan N, Cubillo J, El-Sayed F, Moshref M, Bala KM, Emathinger JM, De La Torre A, Ormachea L, Alvarez R, Gude NA, Sussman MA. Cardiac Stem Cell Hybrids Enhance Myocardial Repair. Circ Res. 2015;117 doi: 10.1161/CIRCRESAHA.115.306838. xxx-xxx [in this issue] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu JM, Hsueh YC, Ch'ang HJ, Luo CY, Wu LW, Nakauchi H, Hsieh PC. Circulating cells contribute to cardiomyocyte regeneration after injury. Circ Res. 2015;116:633–641. doi: 10.1161/CIRCRESAHA.116.304564. [DOI] [PubMed] [Google Scholar]
- 25.Shadrin IY, Yoon W, Li L, Shepherd N, Bursac N. Rapid fusion between mesenchymal stem cells and cardiomyocytes yields electrically active, non-contractile hybrid cells. Sci Rep. 2015;5:12043. doi: 10.1038/srep12043. [DOI] [PMC free article] [PubMed] [Google Scholar]