TO THE EDITOR: Multicentric Castleman disease (MCD) is usually a progressive systemic disease that has a worse prognosis than the unicentric form. The treatment of MCD is based on systemic therapies, such as chemotherapy and monoclonal antibodies. Imaging procedures play an important role in defining the response to treatment. However, no radiologic linchpins exist, due to the lack of evidence.
Castleman disease (CD) is a rare lymphoproliferative disorder first described by Benjamin Castleman, a pathologist at the Massachusetts General Hospital. CD may be unicentric or multicentric. Unicentric CD (UCD), the most common form, is localized and may often be successfully treated with local therapies. MCD is a systemic disease, occurs most commonly in the setting of human immunodeficiency virus (HIV) infection, is usually progressive, and has a worse prognosis. The management of MCD may be carried out by systemic therapies. In the past, the diagnostic imaging of CD has largely been supported by conventional structural imaging techniques, such as computed tomography (CT) and magnetic resonance imaging (MRI), as well as by traditional nuclear medicine examinations, such as 67Gallium scan. In the past two decades, 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET/CT) has become an established imaging tool in oncology. In addition, PET/CT has been generating increasing interest in the areas of infectious diseases [1] and fever of unknown origin [2], as well as with lymphoproliferative disorders such as CD [3,4] particularly in individuals with HIV infection [5]. Here, we present the case of a patient affected by MCD who underwent PET/CT for staging and restaging of the disease. In the diagnostic setting, PET/CT contributed to the identification of the most appropriate node to select for biopsy, whereas it provided objective information regarding the early response to therapy during the restaging.
CASE
In June 2012, a 50-year-old man presented to our hospital with a pruritic papular skin rash of the lower limbs, xerostomia associated with a 6 kg weight loss, asthenia, weakness of the lower limbs, and dyskinesia. The preliminary laboratory tests showed hypergammaglobulinemia, elevated liver enzymes, and values suggestive of an inflammatory state. Bilateral laterocervical and inguinal lymph nodes were observed on the medical evaluation. Additional tests revealed that the hypergammaglobulinemia was polyclonal with a prevalence of the immunoglobulin G (IgG) cluster (50 g/L) and increases in the light kappa and lambda chains. Moreover, C3 and C4 complement proteins were decreased, whereas beta-2 microglobulin was increased. The serology tests for cryoglobulin, antinuclear antibody (ANA), antimitochondrial antibody (AMA), anti-extractable nuclear antigen (ENA), antineutrophil cytoplasmic antibody (ANCA), anti-deoxyribonucleic acid (DNA), lupus anticoagulant (LAC), HIV, Hepatitis B virus (HBV), Salmonella, Brucella, Leishmania, Parvovirus, Strongyloides Stercoralis, Bartonella, Epstein-Barr virus (EBV), and cytomegalovirus (CMV) were negative. The CT scan showed slightly enlarged lymph nodes in the mediastinum, retroperitoneum, and iliac regions. Electromyography (EMG) reported mild sensorimotor neuropathy.
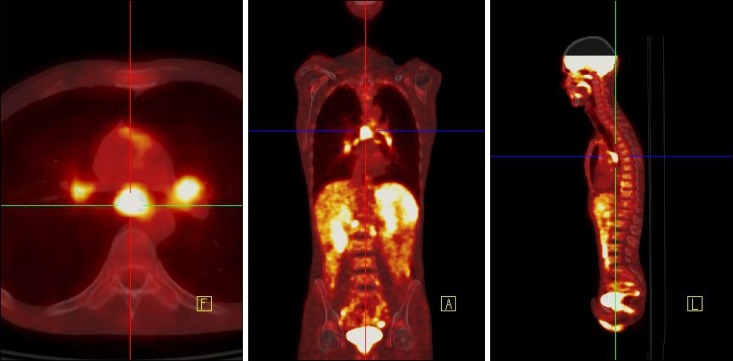
PET/CT scanning (Siemens Biograph PET/CT scanner) was performed from the base of the skull to the upper thighs nearly one hour after the intravenous administration of 370 MBq of 18F-FDG and after 6 hours fast. Emission data were acquired for a 3 min per bed position. PET demonstrated the presence of multiple foci of increased radiopharmaceutical uptake corresponding to adenopathies in the Barety, aortopulmonary, subcarinal, hilar, celiac, para-aortic, right common iliac, and bilateral inguinal spaces (Fig. 1). The PET findings well matched both the diagnoses of a low-grade lymphoma or an inflammatory granulomatous disease. The inguinal lymph node biopsy revealed presence of capsular fibrosis, histiocytosis, plasma cell infiltration, and interfollicular plasmacytosis with large CD30 positive cells. Immunohistochemistry showed the prevalence of mature IgG positive plasma cells with a significant presence of IgG4 positive cells. The pathological diagnosis was the plasma cell variant of MCD.
Fig. 1. Pre-therapy 18F-FDG PET/CT showing multiple areas of increased uptake corresponding to the enlarged nodes.
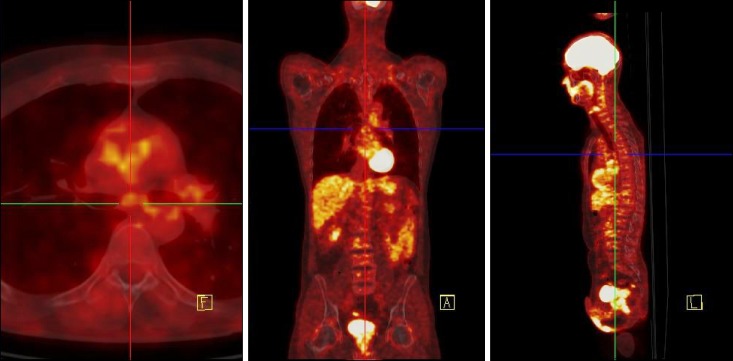
The patient underwent weekly administration of rituximab 375 mg/m2 for a total of 700 mg/cycle for four cycles. The treatment resulted in an initial gradual reduction in most of the symptoms. PET/CT performed four weeks after the start of the therapy showed disappearance of the radiopharmaceutical uptake in all the lymph nodes involved (Fig. 2). The patient's symptoms completely disappeared roughly six weeks after the start of the treatment.
Fig. 2. Post-therapy 18F-FDG PET/CT. The pathologic uptakes were almost completely disappeared.
DISCUSSION
MCD is a very rare disease, associated with various clinical manifestations and systemic symptoms, sometimes severe enough to be life-threatening. A definite treatment for MCD is still lacking, due to its relative rarity and heterogeneity. On the other hand, as is almost inevitable for rare diseases, the infrequency of CD has hampered wide-ranging clinical studies. Unresectable or widespread diffuse MCD may be treated with external beam radiation therapy, chemotherapy, and steroid therapy. However, strong prognostic features that may be used for the selection of the treatment remain to be identified. Keeping in mind that there are several effective, but no established, standard treatment options, we have focused our attention on the utility of the functional imaging carried out with PET/CT both in the diagnosis and in the evaluation of treatment. In our experience, PET/CT was helpful in finding the best lymph node to biopsy. Moreover, PET/CT provided us with an early reliable and objective parameter to support the use of rituximab as a therapeutic choice.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Meller J, Sahlmann CO, Scheel AK. 18F-FDG PET and PET/CT in fever of unknown origin. J Nucl Med. 2007;48:35–45. [PubMed] [Google Scholar]
- 2.Castaigne C, Tondeur M, de Wit S, Hildebrand M, Clumeck N, Dusart M. Clinical value of FDG-PET/CT for the diagnosis of human immunodeficiency virus-associated fever of unknown origin: a retrospective study. Nucl Med Commun. 2009;30:41–47. doi: 10.1097/mnm.0b013e328310b38d. [DOI] [PubMed] [Google Scholar]
- 3.Barua A, Vachlas K, Milton R, Thorpe JA. Castleman's disease - a diagnostic dilemma. J Cardiothorac Surg. 2014;9:170. doi: 10.1186/s13019-014-0170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Y, Li F, Chen L. Widespread hypermetabolic lesions due to multicentric form of Castleman disease as the cause of fever of unknown origin revealed by FDG PET/CT. Clin Nucl Med. 2013;38:835–837. doi: 10.1097/RLU.0b013e31829f8ebf. [DOI] [PubMed] [Google Scholar]
- 5.Rossotti R, Moioli C, Schiantarelli C, Orcese C, Puoti M. FDG-PET imaging in the diagnosis of HIV-associated multicentric Castleman disease: something is still missing. Top Antivir Med. 2012;20:116–118. [PMC free article] [PubMed] [Google Scholar]