Abstract
Purpose
Deregulation of microRNA-370 (miR-370) has been reported in various cancers, in which it can act as either an oncogene or a tumor suppressor gene. However, the clinicopathologic significance of miR-370 expression in breast cancer has not been studied.
Methods
The expression of miR-370 was determined with quantitative real-time polymerase chain reaction in 60 formalin-fixed, paraffin-embedded primary breast cancer tissues. Additionally, the protein expression levels of previously known targets of miR-370, such as FOXM1, FOXO1, and FOXO3a, were detected using immunohistochemistry. Finally, we analyzed its correlation with target protein expression, clinicopathologic features, and clinical outcome.
Results
High levels of miR-370 expression correlated with lymph node metastasis (p=0.009), advanced stage (p=0.002), and frequent perineural invasion (p=0.042). Moreover, patients with high miR-370 expression had poor disease-free survival compared with the low-expression group. However, no correlation was observed between miR-370 and its target protein expression.
Conclusion
Our results indicate that upregulation of miR-370 in breast cancer is correlated with breast cancer progression and that it might be a potential biomarker for predicting clinical outcomes.
Keywords: Breast neoplasms, MicroRNA-370, Prognosis, Real-time polymerase chain reaction
INTRODUCTION
MicroRNAs (miRNAs) are endogenous, 19-22 nucleotide-long, noncoding, single-stranded RNAs that regulate gene expression at the posttranscriptional level by inhibiting translation or inducing mRNA degradation [1,2]. Dysregulation of microRNAs has been reported in various human cancers, including breast cancer. Previous miRNA profiling studies have proposed potential biomarkers for diagnosis, classification, prognosis, and response to specific treatment. One miRNA profiling study with 76 breast cancers and 10 normal breast tissues showed that an expression signature comprised of 15 miRNAs distinguished between normal and cancerous tissues with 100% accuracy [3]. Several previous studies have proposed miRNA signatures that can stratify molecular subgroups of breast cancer [4,5]. In addition, some miRNA signatures that are able to predict the tumor progression and prognosis of patients with breast cancer have been identified [6,7].
Recent controversial studies have found that micro- RNA-370 (miR-370) is deregulated in cancer cells and may play a key role in tumorigenesis and progression by acting as an oncogene or a tumor suppressor gene. For example, miR-370 was reported to be upregulated and to function as an oncogene in human prostate cancer, Wilms tumor, gastric cancer, and acute myeloid leukemia. However, it has also been reported to be downregulated and to function as a tumor suppressor gene in laryngeal squamous cell carcinoma, gastric carcinoma, acute myeloid leukemia, cholangiocarcinoma, hepatocellular carcinoma (HCC), ovarian cancer, and oral squamous cell carcinoma [8,9,10,11,12,13,14,15,16,17,18]. The clinical significance of miR-370 in breast cancer has not yet been reported.
The goal of this study was to assess the miR-370 expression level in formalin-fixed, paraffin-embedded (FFPE) primary breast cancer tissue and to investigate whether miR-370 expression is associated with conventional clinicopathologic parameters and clinical outcome. Additionally, we tried to correlate miR-370 expression with previously identified target protein expression levels.
METHODS
Patient and tissue specimens
For quantitative real-time polymerase chain reaction (qRT-PCR) analysis of miRNAs, archived FFPE specimens that had previously been used for routine pathological examination were collected from 60 patients who had undergone surgery for primary breast cancer from 2007 to 2012 at Hanyang University Medical Center. Hematoxylin and eosin (H&E)-stained slides, pathology reports, and other medical records were reviewed to confirm the diagnoses as well as to establish the clinicopathologic characteristics of the tumors, such as the patient's age, tumor size, histologic grade, American Joint Committee on Cancer/International Union Against Cancer (AJCC-UICC) stage, lymphovascular invasion, perineural invasion, lymph node metastasis, status of hormone receptors, human epidermal growth factor receptor 2 (HER2) status, and patient follow-up information. The American Society of Clinical Oncology and the College of American Pathologists guidelines were used for interpreting hormone receptor and HER2 testing results. The patients included 60 women, ranging in age from 32 to 78 years (mean, 50 years). None of the patients had received preoperative chemotherapy or radiotherapy. The median follow-up period was 38.1 months (range, 1-85 months). This study design was approved by the Institutional Review Board of Hanyang University Hospital (IRB number: 2014-12-002).
RNA extraction and reverse transcription
Representative blocks were selected, in which the tumor comprised more than 70% of the total area. Total RNA was isolated from five sections of 10 µm-thick FFPE tissue sections using a miRNeasy FFPE Kit (Qiagen, Germantown, USA) according to the manufacturer's instructions. RNA concentrations and purity were measured using a NanoDrop 2000 (NanoDrop Technologies, Waltham, USA). Total RNA was converted to complementary DNA using a Universal cDNA Synthesis Kit (Exiqon, Vedbaek, Denmark) according to the manufacturer's instructions.
Quantitative real-time polymerase chain reaction
The reverse transcription products were amplified and detected with qRT-PCR using an ExiLENT SYBR Green Master mix and microRNA Locked Nucleic Acid PCR primers specific for miR-370 (Exiqon). RNU6B expression was used as a reference. A CFX96 real-time detection system (Bio-Rad, Hercules, USA) was used to perform qRT-PCR. The PCR parameters were as follows: 95℃ for 15 minutes, followed by 40 cycles of 95℃ for 10 seconds, and 60℃ for 1 minute. At the end of the PCR cycles, a melting curve analysis was performed. Each sample was examined in triplicate and the expression of miRNAs was defined based on the threshold cycle (Ct); relative expression levels were normalized using the ddCt method with respect to RNU6B.
Construction of tissue microarray
H&E-stained slides were used to define the most morphologically representative, well-fixed, and nonnecrotic areas. Single tissue cores (2 mm in diameter) were sampled from each paraffin block and assembled into a recipient paraffin block using a tissue microarray (TMA) instrument (AccuMax Array; ISU ABXIS, Seoul, Korea).
Immunohistochemistry
The expression of FOXO1, FOXM1, and FOXO3a was evaluated with immunohistochemical staining of 4-µm-thick sections from TMA blocks. The sections were first deparaffinized in xylene and then rehydrated through graded ethanol washes. For antigen retrieval, we performed autoclave heating of the sections at 100℃ for 30 minutes in a sodium citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked with peroxidase blocking solution (S2023; DakoCytomation, Carpinteria, USA). TMA slides were incubated with primary antibodies at 4℃ overnight and incubated with labeled polymer (DAKO REAL EnVision/HRP, K5007; DakoCytomation) for 30 minutes at room temperature. The primary antibodies were rabbit antihuman FOXM1 (sc-502; Santa Cruz Biotechnology, Santa Cruz, USA), FOXO1 (#2880; Cell Signaling, Danvers, USA) and FOXO3a (sc-11351; Santa Cruz Biotechnology) polyclonal antibodies used at a 1:100 dilution. 3,3'-Diaminobenzidine was used as a chromogen for detection, and Mayer's hematoxylin counterstain was applied.
Interpretation of immunohistochemical staining
All slides were evaluated independently by two experienced pathologists in a blinded fashion without any knowledge of the patient data. The percentage of positive tumor cells and intensity of staining were assessed using a semiquantitative scoring system. The intensity score was assigned as follows: 0 (negative), 1 (weak), 2 (intermediate), and 3 (strong). The proportion score was as follows: 0 (0%-5%), 1 (6%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (76%-100%). Immunoreactivity for FOXM1, FOXO1, and FOXO3 was defined as cells showing a nuclear staining pattern with or without cytoplasmic staining in the tumor cells with minimal background staining. The expression of FOX proteins was determined by multiplying the intensity and proportion scores.
Statistical analysis
SPSS version 21.0 (IMB Corp., Armonk, USA) software for windows was used for the statistical analysis. A chi-square test was used to analyze the relationship between miR-370 and various clinicopathologic parameters. An independent Student t-test was used to analyze the association between miR-370 and the expression of its potential target proteins. Both disease free-survival (DFS) and overall survival (OS) were evaluated to determine the prognostic value of miR-370 expression using the Kaplan-Meier method. Multivariable survival analyses using the Cox proportional hazard model was performed to evaluate an independent prognostic factor. A p-value of 0.05 was used to determine statistical significance.
RESULTS
Correlation of miR-370 expression with clinicopathologic features
The median value of the relative expression of miR-370 compared to RNU6B was used as a cutoff value to divide all patients into two groups: the low-miR-370 expression group (relative expression <0.0063, n=30) and the high-miR-370 expression group (relative expression ≥0.0063, n=30). As shown in Table 1, the high miR-370 expression group was positively associated with lymph node metastasis (p=0.009), advanced AJCC stage (p=0.002), and frequent perineural invasion (p=0.042).
Table 1. Association of miR-370 expression and clinicopathologic characteristics in patients with breast cancer.
| Clinicopathologic feature | miR-370, No. (%) | p-value | |
|---|---|---|---|
| Low | High | ||
| Histological grade | 0.430 | ||
| Grade 1 & 2 | 16 (44.4) | 20 (55.6) | |
| Grade 3 | 14 (58.3) | 10 (41.7) | |
| Primary tumor size (cm) | 0.279 | ||
| <2 | 13 (61.9) | 8 (38.1) | |
| ≥2 | 17 (43.6) | 22 (56.4) | |
| LN metastasis | 0.009 | ||
| Not identified | 22 (66.7) | 11 (33.3) | |
| Present | 8 (29.6) | 19 (70.4) | |
| AJCC stage, 7th ed | 0.002 | ||
| I & II | 27 (64.3) | 15 (35.7) | |
| III & IV | 3 (16.7) | 15 (83.3) | |
| Lymphovascular invasion | 0.439 | ||
| Not identified | 17 (56.7) | 13 (43.3) | |
| Present | 13 (43.3) | 17 (56.7) | |
| Perineural invasion | 0.042 | ||
| Not identified | 28 (57.1) | 21 (42.9) | |
| Present | 2 (18.2) | 9 (81.8) | |
| Estrogen receptor | 0.792 | ||
| Negative | 13 (54.2) | 11 (45.8) | |
| Positive | 17 (47.2) | 19 (52.8) | |
| Progesterone receptor | 0.785 | ||
| Negative | 9 (45.0) | 11 (55.0) | |
| Positive | 21 (52.5) | 19 (47.5) | |
| HER2 amplification | 0.143 | ||
| Negative | 25 (56.8) | 19 (43.2) | |
| Positive | 5 (31.2) | 11 (68.8) | |
miR-370=microRNA-370; LN=lymph node; AJCC=American Joint Committee on Cancer; HER2=human epidermal growth factor receptor 2.
Correlation of miR-370 expression with prognosis in breast cancer
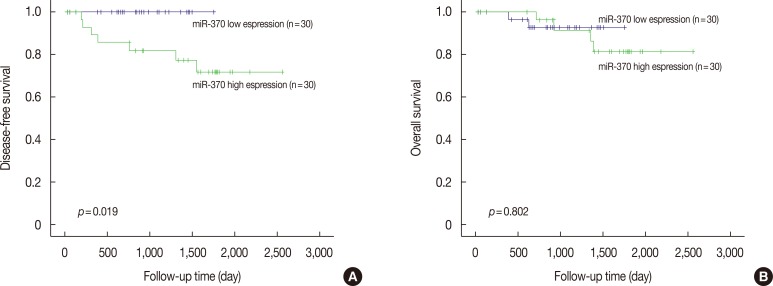
The Kaplan-Meier curves with a log-rank test demonstrated that patients who had high miR-370 expression appeared to be significantly associated with worse recurrence-free survival than those who had low expression (Figure 1). However, there was no significant association between miR-370 and overall survival. miR-370 expression failed to be an independent prognostic biomarker after adjusting for AJCC stage and histologic grade. On survival analyses stratified by molecular subtypes, luminal A (estrogen receptor [ER]/progesterone receptor [PR]-positive, HER2-negative, low Ki-67 index), luminal B (ER/PR-positive, HER2-positive or negative, High Ki-67 index), HER2-positive (ER/PR-negative, HER2-positive), and basal-like carcinoma (ER/PR-negative, HER2-negative), there was no significant correlation between the low and high miR-370 expression groups.
Figure 1. The survival curves comparing the recurrence-free survival (A) and overall survival (B) of breast cancer with high or low microRNA-370 (miR-370) expression (Kaplan-Meier curves with log-rank tests).
Association between miR-370 and target protein expression
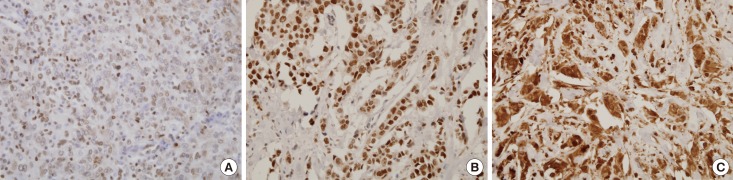
Previous in vitro studies have reported direct targets of miR-370 in several cancer types, including the Forkhead transcription factors FOXM1 and FOXO1 [10,13,14,15,19]. An online computational algorithm, targetscan (http://www.targetscan.org/), was employed to identify candidate target sites of miR-370, and it also revealed that FOXM1 and FOXO1 had potential target sequences in their 3'-UTR regions. In addition, FOXO3a expression has been known to inversely correlate with FOXM1 expression in ovarian and breast cancer [20,21]. To compare miR-370 expression with previously known targets, we performed immunohistochemical staining for FOX proteins in consecutive sections (Figure 2). However, mean FOX protein expression levels were not significantly different according to miR-370 status (Table 2).
Figure 2. Representative microphotographs of immunohistochemical staining of FOX proteins in breast cancer (×400). The tumor cells shows positive nuclear staining. (A) FOXO1 (intensity score 2), (B) FOXO3a (intensity score 3), and (C) FOXM1 (intensity score 3).
Table 2. Association between miR-370 expression and FOX protein expression in breast cancers.
| No. | Immunoreactive score (mean±SD) | p-value | ||
|---|---|---|---|---|
| FOXO1 | miR-370-Low | 29 | 0.50±1.353 | 0.092 |
| miR-370-High | 28 | 0.07±0.262 | ||
| FOXO3a | miR-370-Low | 29 | 6.55±3.158 | 0.528 |
| miR-370-High | 28 | 5.96±3.805 | ||
| FOXM1 | miR-370-Low | 29 | 2.33±1.842 | 0.411 |
| miR-370-High | 28 | 2.76±2.189 |
miR-370=microRNA-370; SD=standard deviation.
DISCUSSION
Although an increasing number of studies have demonstrated that the dysregulation of miR-370 occurs in various types of cancer, no prior studies of miR-370 in human breast cancer were found in the literature. In the present study, we found that breast cancer patients with higher miR-370 expression levels tended to have regional lymph node metastasis, perineural invasion, and an advanced tumor stage. Moreover, elevated miR-370 expression was associated with shorter recurrence-free survival. We tried to validate our results using a publicly available miRNA expression database, SurvMicro [22]. Six datasets were available for breast cancer, two of which revealed that the low miR-370 expression group was significantly associated with poor prognosis. Another four datasets revealed no significant differences. Taken together, the data from this study and six independent datasets suggest that the impact of miR-370 expression levels on clinical outcome is still controversial, and an additional study with a large cohort of patients is necessary.
Several studies have demonstrated that the expression of miR-370 acts as tumor suppressor gene in several types of cancers. miR-370 was reported to be downregulated and to function as a tumor suppressor gene by targeting FOXM1 in laryngeal squamous cell carcinoma, Helicobacter pylori-induced gastric carcinoma, and acute myeloid leukemia [13,14,15]. The paternal miR-370 allele was found to be silenced in cholangiocarcinoma, and miR-370 upregulation inhibits the growth of cholangiocarcinoma cells [16]. In an HCC cell line, miR-370 is involved in the signal transduction of the nuclear factor κB pathway and shows suppressive effects on the migration and invasion of HCC cells [17]. Moreover, miR-370 is downregulated in endometrioid ovarian cancer, and the restoration of miR-370 enhances chemosensitivity to cisplatin [23]. However, other studies have contradictory results, suggesting that miR-370 acts as an oncogenic miRNA. Upregulation of miR-370 in the Wilms tumor G401 cell line results in an increased proliferation rate, upregulation of cyclin-dependent kinase inhibitors, and downregulation of cyclin D1, which suggests a role for miR-370 in enhancing cell proliferation [9]. miR-370 was reported to be upregulated and to function as an oncogene by targeting FOXO1 in human prostate and gastric cancers [8,10]. In a study of the serum of gastric cancer patients, higher levels of miR-370 were observed in patients than in controls. Moreover, patients having more invasive or advanced tumors also exhibited a higher plasma level of miR-370 [11]. Taken together, the functional role of miR-370 in cancer is still controversial. Here, we evaluated the clinicopathologic relevance of miR-370 expression in clinical breast cancer specimens, and we revealed that upregulation of miR-370 was correlated with tumor progression and adverse clinical outcomes. However, no significant associations were found between miR-370 and the expression of its putative target proteins (FOXO1, FOXO3a, and FOXM1). Our results suggest that miR-370 functions as an oncogene in breast cancer; however, its target may be different from those that have been described previously. Further research is required to identify the mechanism of miR-370 dysregulation and its targets in breast cancer.
FFPE tissue samples are well-preserved, readily available, and cost-effective archival material in the clinical setting. Furthermore, they can be linked with complete clinical follow-up and various clinicopathologic data. Therefore, much interest has focused on whether FFPE tissue can be used in clinical validation studies. Although mRNA in FFPE blocks are easily degraded and chemically modified, miRNAs have been known to be well preserved because of their short nucleotide length. Previous studies have demonstrated that miRNAs are preserved in FFPE cancer tissues exhibiting near total mRNA degradation, and the expression levels in FFPE tissue are well correlated with those of corresponding freshly frozen samples [24,25,26,27]. These results reveal the suitability of FFPE tissues as promising resources for clinical assays of miRNA expression. However, the use of FFPE tissue for RNA-based experiments is still challenging, and the correlation of qRT-PCR in FFPE and matched fresh frozen tissues is necessary to determine its reliability. We are unable to provide the correlation data in the current study due to the absence of the corresponding fresh-frozen tumor tissue.
In conclusion, the current study identified correlations between miR-370 expression in breast cancer and clinicopathological characteristics. Our findings suggest that high levels of miR-370 are linked to tumor progression and worse disease-free survival. Although further studies are needed to clarify the precise mechanism of the oncogenic effect of miR-370 in breast cancer, understating the oncogenic role of miR-370 may provide the basic knowledge required for the development of potential prognostic biomarkers and specific miRNA inhibitors as targeted therapies.
Footnotes
This study was supported by a grant from the research fund of Hanyang University (HY-2012-MC) to Kiseok Jang.
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carleton M, Cleary MA, Linsley PS. MicroRNAs and cell cycle regulation. Cell Cycle. 2007;6:2127–2132. doi: 10.4161/cc.6.17.4641. [DOI] [PubMed] [Google Scholar]
- 3.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 4.Blenkiron C, Goldstein LD, Thorne NP, Spiteri I, Chin SF, Dunning MJ, et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8:R214. doi: 10.1186/gb-2007-8-10-r214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowery AJ, Miller N, Devaney A, McNeill RE, Davoren PA, Lemetre C, et al. MicroRNA signatures predict oestrogen receptor, progesterone receptor and HER2/neu receptor status in breast cancer. Breast Cancer Res. 2009;11:R27. doi: 10.1186/bcr2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foekens JA, Sieuwerts AM, Smid M, Look MP, de Weerd V, Boersma AW, et al. Four miRNAs associated with aggressiveness of lymph node-negative, estrogen receptor-positive human breast cancer. Proc Natl Acad Sci U S A. 2008;105:13021–13026. doi: 10.1073/pnas.0803304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothé F, Ignatiadis M, Chaboteaux C, Haibe-Kains B, Kheddoumi N, Majjaj S, et al. Global microRNA expression profiling identifies MiR-210 associated with tumor proliferation, invasion and poor clinical outcome in breast cancer. PLoS One. 2011;6:e20980. doi: 10.1371/journal.pone.0020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z, Sun H, Zeng W, He J, Mao X. Upregulation of MircoRNA-370 induces proliferation in human prostate cancer cells by downregulating the transcription factor FOXO1. PLoS One. 2012;7:e45825. doi: 10.1371/journal.pone.0045825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao X, Liu D, Yan X, Zhang Y, Yuan L, Zhang T, et al. Stat3 inhibits WTX expression through up-regulation of microRNA-370 in Wilms tumor. FEBS Lett. 2013;587:639–644. doi: 10.1016/j.febslet.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Fan C, Liu S, Zhao Y, Han Y, Yang L, Tao G, et al. Upregulation of miR-370 contributes to the progression of gastric carcinoma via suppression of FOXO1. Biomed Pharmacother. 2013;67:521–526. doi: 10.1016/j.biopha.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Lo SS, Hung PS, Chen JH, Tu HF, Fang WL, Chen CY, et al. Overexpression of miR-370 and downregulation of its novel target TGFβ-RII contribute to the progression of gastric carcinoma. Oncogene. 2012;31:226–237. doi: 10.1038/onc.2011.226. [DOI] [PubMed] [Google Scholar]
- 12.Garcća-Ortć L, Cristóbal I, Cirauqui C, Guruceaga E, Marcotegui N, Calasanz MJ, et al. Integration of SNP and mRNA arrays with microRNA profiling reveals that MiR-370 is upregulated and targets NF1 in acute myeloid leukemia. PLoS One. 2012;7:e47717. doi: 10.1371/journal.pone.0047717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yungang W, Xiaoyu L, Pang T, Wenming L, Pan X. miR-370 targeted FoxM1 functions as a tumor suppressor in laryngeal squamous cell carcinoma (LSCC) Biomed Pharmacother. 2014;68:149–154. doi: 10.1016/j.biopha.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y, Wang L, Zeng J, Shen L, Liang X, Yu H, et al. FoxM1 is overexpressed in Helicobacter pylori-induced gastric carcinogenesis and is negatively regulated by miR-370. Mol Cancer Res. 2013;11:834–844. doi: 10.1158/1541-7786.MCR-13-0007. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Zeng J, Zhou M, Li B, Zhang Y, Huang T, et al. The tumor suppressive role of miRNA-370 by targeting FoxM1 in acute myeloid leukemia. Mol Cancer. 2012;11:56. doi: 10.1186/1476-4598-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Zhao J, Zhang PY, Zhang Y, Sun SY, Yu SY, et al. MicroRNA-10b targets E-cadherin and modulates breast cancer metastasis. Med Sci Monit. 2012;18:BR299–BR308. doi: 10.12659/MSM.883262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu WP, Yi M, Li QQ, Zhou WP, Cong WM, Yang Y, et al. Perturbation of MicroRNA-370/Lin-28 homolog A/nuclear factor kappa B regulatory circuit contributes to the development of hepatocellular carcinoma. Hepatology. 2013;58:1977–1991. doi: 10.1002/hep.26541. [DOI] [PubMed] [Google Scholar]
- 18.Chang KW, Chu TH, Gong NR, Chiang WF, Yang CC, Liu CJ, et al. miR-370 modulates insulin receptor substrate-1 expression and inhibits the tumor phenotypes of oral carcinoma. Oral Dis. 2013;19:611–619. doi: 10.1111/odi.12046. [DOI] [PubMed] [Google Scholar]
- 19.Zhou M, Zeng J, Wang X, Guo Q, Huang T, Shen H, et al. MiR-370 sensitizes chronic myeloid leukemia K562 cells to homoharringtonine by targeting Forkhead box M1. J Transl Med. 2013;11:265. doi: 10.1186/1479-5876-11-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang L, Cao XC, Cao JG, Liu F, Quan MF, Sheng XF, et al. Casticin induces ovarian cancer cell apoptosis by repressing FoxM1 through the activation of FOXO3a. Oncol Lett. 2013;5:1605–1610. doi: 10.3892/ol.2013.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karadedou CT, Gomes AR, Chen J, Petkovic M, Ho KK, Zwolinska AK, et al. FOXO3a represses VEGF expression through FOXM1-dependent and -independent mechanisms in breast cancer. Oncogene. 2012;31:1845–1858. doi: 10.1038/onc.2011.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguirre-Gamboa R, Trevino V. SurvMicro: assessment of miRNA-based prognostic signatures for cancer clinical outcomes by multivariate survival analysis. Bioinformatics. 2014;30:1630–1632. doi: 10.1093/bioinformatics/btu087. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Zhang Z, Chen C, Liu Y, Si Q, Chuang TH, et al. MicroRNA-19a-3p inhibits breast cancer progression and metastasis by inducing macrophage polarization through downregulated expression of Fra-1 proto-oncogene. Oncogene. 2014;33:3014–3023. doi: 10.1038/onc.2013.258. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Chen J, Radcliffe T, Lebrun DP, Tron VA, Feilotter H. An array-based analysis of microRNA expression comparing matched frozen and formalin-fixed paraffin-embedded human tissue samples. J Mol Diagn. 2008;10:513–519. doi: 10.2353/jmoldx.2008.080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu A, Xu X. MicroRNA isolation from formalin-fixed, paraffin-embedded tissues. Methods Mol Biol. 2011;724:259–267. doi: 10.1007/978-1-61779-055-3_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Smyth P, Flavin R, Cahill S, Denning K, Aherne S, et al. Comparison of miRNA expression patterns using total RNA extracted from matched samples of formalin-fixed paraffin-embedded (FFPE) cells and snap frozen cells. BMC Biotechnol. 2007;7:36. doi: 10.1186/1472-6750-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall JS, Taylor J, Valentine HR, Irlam JJ, Eustace A, Hoskin PJ, et al. Enhanced stability of microRNA expression facilitates classification of FFPE tumour samples exhibiting near total mRNA degradation. Br J Cancer. 2012;107:684–694. doi: 10.1038/bjc.2012.294. [DOI] [PMC free article] [PubMed] [Google Scholar]