Abstract
Purpose
Somatic mutations of the chromatin remodeling AT-rich interactive domain 1A (SWI-like) gene (ARID1A) have been identified in many human cancers, including breast cancer. The purpose of this study was to evaluate the nuclear expression of ARID1A in breast cancers by immunohistochemistry (IHC) and to correlate the findings to clinicopathologic variables including prognostic significance.
Methods
IHC was performed on tissue microarrays of 476 cases of breast cancer. Associations between ARID1A expression and clinicopathologic characteristics and molecular subtype were retrospectively analyzed.
Results
Low expression of ARID1A was found in 339 of 476 (71.2%) cases. Low expression of ARID1A significantly correlated with positive lymph node metastasis (p=0.027), advanced pathologic stage (p=0.001), low Ki-67 labeling index (p=0.003), and negative p53 expression (p=0.017). The ARID1A low expression group had significantly shorter disease-free and overall survival than the ARID1A high expression group (p<0.001 and p<0.001, respectively). Multivariate analysis demonstrated that low expression of ARID1A was a significant independent predictive factor for poor disease-free and overall survival in patients with breast cancer (disease-free survival: hazard ratio, 0.38, 95% confidence interval [CI], 0.20-0.73, p=0.004; overall survival: hazard ratio, 0.11, 95% CI, 0.03-0.46, p=0.003). In patients with luminal A type disease, patients with low ARID1A expression had significantly shorter disease-free and overall survival rates than patients with high ARID1A expression (p=0.022 and p=0.018, respectively).
Conclusion
Low expression of ARID1A is an independent prognostic factor for disease-free and overall survival in breast cancer patients and may be associated with luminal A type disease. Although the biologic function of ARID1A in breast cancer remains unknown, low expression of ARID1A can provide valuable prognostic information.
Keywords: ARID1A protein, Breast neoplasms, Immunohistochemistry, Prognosis
INTRODUCTION
Globally, breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death in women. In Korea, breast cancer is the second most common newly diagnosed malignancy in women (more than 15,000 new cases annually) [1]. Therefore, it is important to identify factors predictive of prognosis and therapeutic significance.
Recently, somatic mutations of the AT-rich interactive domain 1A (SWI-like) gene (ARID1A) located in chromosome 1p36 were identified in many human cancers, including breast cancer [2,3,4]. BAF250a, the protein encoded by ARID1A, is a key component of the multiprotein SWI/SNF chromatin remodeling complex, which is critical for differentiation, proliferation, DNA repair, and tumor suppression [4,5]. ARID1A has recently been the subject of intense investigation because it has been found to be lost or mutated in various types of cancer, including ovarian clear cell carcinoma [6], endometrial carcinoma [7,8], cervical cancer [9], clear cell renal cell carcinoma [10], small intestinal carcinoma [11], malignant rhabdoid tumors [12], gastric carcinoma [13], non-small cell lung cancer [14], and urothelial bladder tumors [15]. Loss of ARID1A protein expression correlates closely with ARID1A mutations and can be used as a surrogate marker of ARID1A mutation [16,17].
Previous studies have implicated that loss of ARID1A expression is associated with an unfavorable outcome of breast cancer [2,18,19]. The relationship between ARID1A protein expression and clinicopathological variables, including prognostic significance, in breast cancer has been investigated only in a limited way, and details remain largely unknown. The purpose of this study was to evaluate the nuclear expression of ARID1A in 476 cases of Korean breast cancer by immunohistochemistry (IHC) and to correlate the findings to molecular subtype and clinicopathologic variables, including prognostic significance.
METHODS
Patients
Formalin-fixed and paraffin-embedded tissues from 476 consecutively resected primary breast cancers from patients treated at Soonchunhyang University Cheonan Hospital from 2001 to 2013 were retrospectively examined. The inclusion criteria for these samples were as follows: patients underwent curative surgeries, resected specimens were pathologically examined, and complete medical records were available. All patients received standardized comprehensive treatment. Two pathologists (H.D.C. and H.J.L.) reviewed hematoxylin and eosinstained slides of all cases, according to the 2012 World Health Organization classification [20]. Data regarding patient age at initial diagnosis, tumor size, histological type, histological tumor grade [21], lymph node status, and surgery type were also collected. Pathologic TNM classification and staging were performed for the 476 cases using the current TNM international staging system (seventh edition of the American Joint Committee on Cancer criteria). This study was approved by the institutional review boards at the Soonchunhyang University Cheonan Hospital (SCHCA 2015-04-009-002).
Construction of the tissue microarrays
For uniform and simultaneous protein expression analysis of multiple tissue samples, tissue microarrays (TMAs) were prepared. Representative core tissue sections 2 mm in diameter were taken from paraffin blocks and arranged in new TMA blocks using a manual TMA device (Superbiochips Laboratories, Seoul, Korea). In cases with variable histological features, the most representative area was selected for TMA construction. Six cores were sampled and included in the TMA block. Using a standard microtome, 4 µm-thick sections were cut from TMA blocks and were used to perform IHC.
Immunohistochemistry
ARID1A expression was analyzed by IHC. Four micrometer-thick sections from the TMA blocks were deparaffinized in xylene and rehydrated through gradually decreasing concentrations of ethanol in distilled water. IHC staining of the TMA samples was performed using a Benchmark® automatic immunostaining device (Ventana Medical Systems, Tucson, USA) and an UltraView™ Universal DAB detection kit (Ventana Medical Systems) according to the manufacturer's recommendations. The primary anti-ARID1A mouse monoclonal antibody (PSG3, SC-32761; Santa Cruz, Dallas, USA) was used at a dilution of 1:150. For negative controls, sections were treated omitting the primary antibody. For positive controls, normal breast tissue section staining was positive. Cells positive for ARID1A protein were defined as those with distinct brown granules located in cell nuclei. Two independent observers (H.D.C. and H.J.L.) read the slides in a blinded manner. Only epithelial cells were evaluated, and the result for each core was recorded separately. At the time of review, neither of the investigators was aware of the clinicopathologic data of the breast cancers, since all of the slides had been coded. The average maximal staining intensity (no staining [0], weak [1+], moderate [2+], or strong [3+]) for each of the two cores per sample was recorded. The extent of staining was also initially assessed on a three-point scale: 0, ≤10% positive cells; 1, 11%-50% positive cells; and 2, ≥51% positive cells. Subsequently, the total score was calculated by multiplying each score. According to these assessment criteria, the immunostaining results were classified as follows: scores of 0-2 indicated low or no expression of ARID1A protein, and scores of 3-6 indicated high expression of ARID1A protein [19].
IHC staining for estrogen receptor (ER; 1:50; Dako Co., Carpinteria, USA), progesterone receptor (PR; 1:50; Dako Co.), human epidermal growth factor receptor 2 (HER2; 1:200; Novocastra Laboratories Ltd., Newcastle, UK), Ki-67 (1:800; Dako Co.), cytokeratin 5/6 (CK5/6; 1:50; Dako Co.), epidermal growth factor receptor (EGFR; 1:100; Dako Co.), and p53 (1:1,200; Dako Co.) was also performed on 4 µm-sections of the TMA blocks. The IHC staining for ER and PR was evaluated using the Allred method [22]. An Allred score of 3 or higher was considered positive. HER2 expression was analyzed according to the general guidelines set by the American Society of Clinical Oncology/College of American Pathologists. When the IHC yielded equivocal results, HER2 status was determined using fluorescent in situ hybridization. The expression of Ki-67 was counted in 1,000 tumor cells, and the percentage of positive cells was categorized as ≥14%. For CK5/6 and EGFR expression, the cells were considered positive when the cytoplasmic and/or membranous reaction was ≥10%. The expression of p53 was counted in 1,000 tumor cells, and the percentage of positive cells was categorized as >10%. The phenotypes were classified as follows: luminal A type: ER and/or PR positive, HER2 negative, and Ki-67 index <14%; luminal B HER2 negative type: ER and/or PR positive, HER2 negative, and Ki-67 index ≥14%; luminal B HER2 positive type: ER and/or PR positive, HER2 positive, and any Ki-67 index; HER2 type: ER and PR negative and HER2 positive; triple-negative breast cancer (TNBC) basal type: ER, PR, and HER2 negative and CK5/6 and/or EGFR positive; and TNBC nonbasal type: ER, PR, HER2, CK5/6, and EGFR negative.
Statistical analyses
The analyses were performed using the software package SPSS version 19.0 for Windows (IBM Corp., Armonk, USA). Associations between ARID1A expression and the clinicopathologic characteristics were analyzed using Pearson chi-square test, Fisher exact test, or an independent t-test, according to test conditions. Survival curves were plotted using the Kaplan-Meier method, and statistical significance was assessed using the log-rank test. Disease-free survival (DFS) was defined as the interval between primary surgery and the last follow-up visit without disease or evidence of recurrence or metastasis of breast cancers (locoregional relapse, distant metastasis). Overall survival (OS) was defined as the interval between primary surgery and the last follow-up visit or death from any cause. The Cox proportional hazards model was used for multivariate analysis. A p-value <0.05 was considered statistically significant.
RESULTS
Patient characteristics and ARID1A immunoreactivity
The clinicopathological characteristics of the patients with primary breast cancer (n=476) are listed in Table 1. Patient age ranged from 24 to 81 years (median, 50.0 years; mean, 52.3 years). There were 470 (98.7%) female and six (1.3%) male patients. Of the 476 included samples, 241 patients (50.6%) underwent breast-conserving surgery, and 235 patients (49.4%) underwent mastectomy. The histological types included invasive ductal carcinoma not otherwise specified (432 samples, 90.8%), invasive lobular carcinoma (20 samples, 4.2%), and others (24 samples, 5.0%). The histological grade was available for 476 samples; 59 (12.4%) were grade 1, 243 (51.1%) were grade 2, and 174 (36.6%) were grade 3. Tumor sizes varied from 0.3 to 12 cm (mean, 2.42 cm). Among 476 patients for whom primary tumor size data were available, 230 (48.3%), 219 (46.0%), and 23 (4.8%) tumors were categorized as pT1, pT2, and pT3, respectively. Of the 476 patients, 173 (36.3%) had lymph node positivity at the time of surgery. The 476 patients were classified using the TNM classification system as follows: stage I, 169 patients (35.5%); stage II, 220 patients (46.2%); and stage III, 87 patients (18.3%). The proportions of patients positive for ER and PR expression were 66.8% and 36.1%, respectively. Upon analysis of HER2 expression, 17.4% of all patients were positive. The percentage of cases with high Ki-67 expression was 43.9%. CK5/6 and EGFR expression was found in 9.5% and 20.6% of cases, respectively. For p53 expression, 17.4% of patients were positive.
Table 1. Distribution of ARID1A status in 476 patients with breast cancer.
| Variable | No. (%) | ARID1A | p-value | Variable | No. (%) | ARID1A | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| High (n = 137) No. (%) |
Low (n = 339) No. (%) |
High (n = 137) No. (%) |
Low (n = 339) No. (%) |
||||||
| Age (yr) | 0.130 | Ki-67 (%) | 0.003 | ||||||
| < 50 | 231 (48.5) | 74 (32.0) | 157 (68.0) | < 14 | 267 (56.1) | 62 (23.2) | 205 (76.8) | ||
| ≥ 50 | 245 (51.5) | 63 (25.7) | 182 (74.3) | ≥ 14 | 209 (43.9) | 75 (35.9) | 134 (64.1) | ||
| Sex | 1.000 | CK5/6 | 0.731 | ||||||
| Female | 470 (98.7) | 135 (28.7) | 335 (71.3) | Positive | 45 (9.5) | 14 (31.1) | 31 (68.9) | ||
| Male | 6 (1.3) | 2 (33.3) | 4 (66.7) | Negative | 431 (90.5) | 123 (28.5) | 308 (71.5) | ||
| Operation | 0.023 | EGFR | 0.381 | ||||||
| BCS | 241 (50.6) | 82 (34.0) | 159 (66.0) | Positive | 98 (20.6) | 32 (32.7) | 66 (67.3) | ||
| Mastectomy | 235 (49.4) | 55 (23.4) | 180 (76.6) | Negative | 378 (79.4) | 105 (27.8) | 273 (72.2) | ||
| Histologic type | 0.122 | p53 | 0.017 | ||||||
| Ductal | 432 (90.8) | 130 (30.1) | 302 (69.9) | Positive | 83 (17.4) | 33 (39.8) | 50 (60.2) | ||
| Lobular | 20 (4.2) | 4 (20.0) | 16 (80.0) | Negative | 393 (82.6) | 104 (26.5) | 289 (73.5) | ||
| Others | 24 (5.0) | 3 (12.5) | 21 (87.5) | Molecular subtype | 0.089 | ||||
| Histologic grade | 0.056 | Luminal A | 205 (43.1) | 55 (26.8) | 150 (73.2) | ||||
| 1 | 59 (12.4) | 10 (16.9) | 49 (83.1) | Luminal B, HER2 (-) | 76 (16.0) | 25 (32.9) | 51 (67.1) | ||
| 2 | 243 (51.1) | 69 (28.4) | 174 (71.6) | Luminal B, HER2 (+) | 37 (7.8) | 16 (43.2) | 21 (56.8) | ||
| 3 | 174 (36.6) | 58 (33.3) | 116 (66.7) | HER2 | 46 (9.7) | 11 (23.9) | 35 (76.1) | ||
| T staging | 0.381 | TNBC, basal | 81 (17.0) | 26 (32.1) | 55 (67.9) | ||||
| T1 | 230 (48.3) | 67 (29.1) | 163 (70.9) | TNBC, nonbasal | 31 (6.5) | 4 (12.9) | 27 (87.1) | ||
| T2 | 219 (46.0) | 66 (30.1) | 153 (69.9) | Neoadjuvant chemotherapy | 0.002 | ||||
| T3 | 23 (4.8) | 3 (13.0) | 20 (87.0) | Yes | 29 (6.1) | 1 (3.4) | 28 (96.6) | ||
| LN metastasis | 0.027 | No | 447 (93.9) | 136 (30.4) | 311 (69.6) | ||||
| Negative | 303 (63.7) | 98 (32.3) | 205 (67.7) | Chemotherapy | 0.375 | ||||
| Positive | 173 (36.3) | 39 (22.5) | 134 (77.5) | Yes | 336 (70.6) | 101 (30.1) | 235 (69.9) | ||
| Stage | 0.001 | No | 140 (29.4) | 36 (25.7) | 104 (74.3) | ||||
| I | 169 (35.5) | 53 (31.4) | 116 (68.6) | Radiotherapy | 0.539 | ||||
| II | 220 (46.2) | 74 (33.6) | 146 (66.4) | Yes | 199 (41.8) | 54 (27.1) | 145 (72.9) | ||
| III | 87 (18.3) | 10 (11.5) | 77 (88.5) | No | 277 (58.2) | 83 (30.0) | 194 (70.0) | ||
| ER | 0.390 | Progression | 88 (18.5) | 11 (12.5) | 77 (87.5) | < 0.001 | |||
| Positive | 318 (66.8) | 96 (30.2) | 222 (69.8) | Locoregional relapse | 9 (1.9) | 1 (11.1) | 8 (88.9) | 0.298 | |
| Negative | 158 (33.2) | 41 (25.9) | 117 (74.1) | Distant metastases | 79 (16.6) | 10 (12.7) | 69 (87.3) | 0.001 | |
| PR | 0.527 | Death | 51 (10.7) | 2 (3.9) | 49 (96.1) | < 0.001 | |||
| Positive | 172 (36.1) | 53 (30.8) | 119 (69.2) | ||||||
| Negative | 304 (63.9) | 84 (27.6) | 220 (72.4) | ||||||
| HER2 | 0.425 | ||||||||
| Positive | 83 (17.4) | 27 (32.5) | 56 (67.5) | ||||||
| Negative | 393 (82.6) | 110 (28.0) | 283 (72.0) | ||||||
ARID1A=AT-rich interactive domain 1A; BCS=breast conserving surgery; LN=lymph node; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; EGFR=epidermal growth factor receptor; TNBC=triple-negative breast cancer.
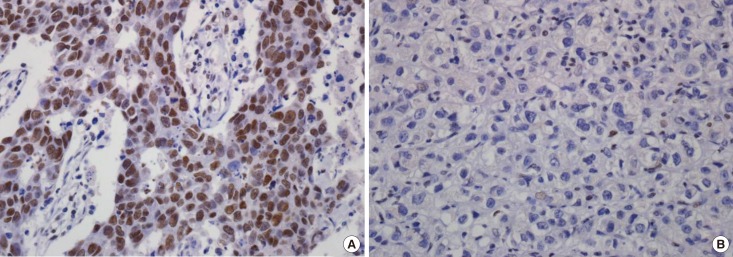
ARID1A protein expression in breast cancer appeared mainly in the nuclei of tumor cells (Figure 1). After evaluation of the 476 immunostained breast cancer specimens, 150 (31.5%) showed no positivity, 75 (15.8%) had score 1 positivity, 114 (23.9%) had score 2 positivity, 44 (9.2%) had score 3 positivity, 45 (9.5%) had score 4 positivity, and 48 (10.1%) had score 6 positivity. For the statistical analysis, the cases were subdivided into an ARID1A high expression group (scores 3, 4, and 6; n=137, 28.8%) and an ARID1A low expression group (scores 0, 1, and 2; n=339, 71.2%).
Figure 1. Immunohistochemical analyses of AT-rich interactive domain 1A (ARID1A) expression in breast cancer: (A) high and (B) low expression. ARID1A expressed in nuclei of the tumor cells (×400).
Correlations between ARID1A expression and clinicopathologic parameters
Low expression of ARID1A was significantly correlated with mastectomy (p=0.023), positive lymph node metastasis (p=0.027), advanced pathologic stage (p-stage, p=0.001), low Ki-67 labeling index (p=0.003), negative p53 expression (p=0.017), and neoadjuvant chemotherapy status (p=0.002) (Table 1). Weak correlations between low ARID1A expression level and low histologic grade (p=0.056) were also found without reaching formal statistical significance. Other clinicopathologic variables, including age, sex, histologic type, tumor size, ER positivity, PR positivity, HER2 positivity, CK5/6 positivity, EGFR positivity, molecular subtype, chemotherapy, and radiotherapy did not correlate with ARID1A expression.
Survival analysis
All patients were closely followed after surgery, with a median follow-up period of 39 months (range, 1-158 months). During follow-up, 88 patients (18.5%) relapsed, and 51 patients (10.7%) died. Patterns of relapse were reviewed, and we found that most patients had distant metastasis (n=79, 16.6%) rather than locoregional relapse (n=9, 1.9%) (Table 1). The OS rates for breast cancer patients with high and low ARID1A expression were 98.5% and 85.5%, respectively.
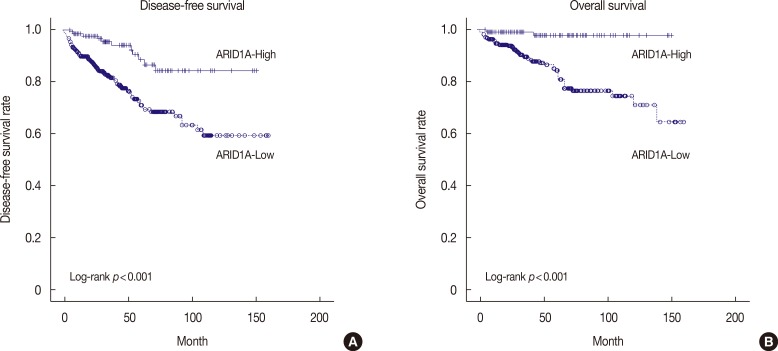
On univariate survival analysis, conventional prognostic parameters, including age, operation methods, tumor size, lymph node metastasis, and p-stage, reached significance for DFS and OS (p<0.05 for all) (Table 2). In addition, hormonal expression (ER or PR) and HER2 expression were factors affecting DFS or OS of breast cancer patients. Patients with low ARID1A expression had significantly shorter DFS and OS than patients with high ARID1A expression (p<0.001 and p<0.001, respectively) (Figure 2).
Table 2. Univariate and multivariate analysis results of disease-free survival and overall survival in 476 patients with breast cancer.
| Disease-free survival | Overall survival | |||
|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |
| p-value | p-value (HR, 95% CI) | p-value | p-value (HR, 95% CI) | |
| ARID1A expression (high vs. low) | < 0.001 | 0.004 (0.38, 0.20-0.73) | < 0.001 | 0.003 (0.11, 0.03-0.46) |
| Age ( < 50 yr vs. ≥ 50 yr) | 0.044 | 0.038 (1.58, 1.03-2.44) | 0.003 | < 0.001 (3.07, 1.64-5.77) |
| Operation (conserving surgery vs. mastectomy) | < 0.001 | 0.024 (0.52, 0.30-0.92) | < 0.001 | 0.286 (0.64, 0.28-1.46) |
| Histologic grade (1 vs. 2, 3) | 0.357 | 0.431 (0.67, 0.24-1.83) | 0.193 | 0.908 (1.09, 0.24-4.91) |
| Tumor size (T1 vs. T2 vs. T3) | < 0.001 | 0.270 (1.26, 0.84-1.88) | < 0.001 | 0.050 (1.66, 1.00-2.74) |
| LN metastasis (negative vs. positive) | < 0.001 | 0.008 (0.40, 0.20-0.79) | < 0.001 | 0.249 (0.56, 0.21-1.50) |
| Pathologic stage (I vs. II vs. III) | < 0.001 | 0.389 (1.28, 0.73-2.23) | < 0.001 | 0.234 (1.62, 0.73-3.60) |
| ER (positive vs. negative) | 0.089 | 0.208 (1.41, 0.83-2.40) | 0.015 | 0.037 (2.16, 1.05-4.48) |
| PR (positive vs. negative) | 0.275 | 0.539 (0.83, 0.47-1.49) | 0.502 | 0.092 (0.49, 0.21-1.13) |
| ER or PR (positive vs. negative) | 0.089 | 0.284 (1.27, 0.82-1.97) | 0.015 | 0.945 (0.97, 0.39-2.42) |
| HER2 (positive vs. negative) | 0.036 | 0.043 (0.60, 0.36-0.99) | 0.012 | 0.060 (0.54, 0.28-1.03) |
| Ki-67 ( < 14% vs. ≥ 14%) | 0.580 | 0.766 (0.92, 0.54-1.58) | 0.315 | 0.990 (1.00, 0.48-2.08) |
| p53 (positive vs. negative) | 0.847 | 0.945 (1.02, 0.54-1.94) | 0.271 | 0.296 (0.67, 0.31-1.43) |
HR=hazard ratio; CI=confidence interval; ARID1A=AT-rich interactive domain 1A; LN=lymph node; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
Figure 2. Kaplan-Meier survival curve for AT-rich interactive domain 1A (ARID1A). (A) Disease-free survival (p<0.001) and (B) overall survival (p<0.001) in breast cancer (n=476).
To evaluate whether ARID1A positivity in breast cancer was an independent predictor of DFS and OS, a multivariate analysis using the Cox proportional hazard model was performed with the following variables: age, operation methods, tumor size, lymph node metastasis, p-stage, hormonal expression (ER or PR), HER2 expression, and ARID1A expression. All variables with a p-value<0.05 in the univariate analysis were included in the multivariate Cox model. Age (p=0.038 and p<0.001 for DFS and OS, respectively), operation methods (p=0.024, only for DFS), lymph node metastasis (p=0.008, only for DFS), ER positivity (p=0.037, only for OS), and HER2 positivity (p=0.043, only for DFS) were significant prognostic factors for breast cancer patients (Table 2). Multivariate analysis identified low ARID1A expression as significant independent factor for poor DFS and OS in patients with breast cancer (DFS: hazard ratio, 0.38, 95% confidence interval [CI], 0.20-0.73, p=0.004; OS: hazard ratio, 0.11, 95% CI, 0.03-0.46, p=0.003, respectively).
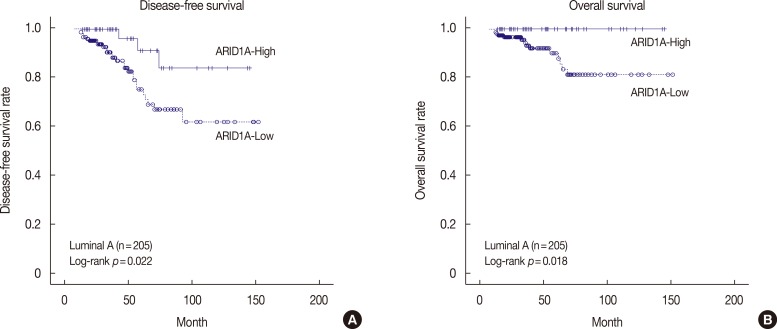
The DFS and OS of the ARID1A high and low expression groups, stratified according to molecular subtype, are shown in Figure 3 and Supplementary Figure 1 (available online). In patients with luminal A type disease, low ARID1A expression was associated with significantly shorter DFS and OS than high ARID1A expression (p=0.022 and p=0.018, respectively) (Figure 3). In patients with luminal B, HER2 negative type disease, both DFS and OS did not show any statistically significant differences according to ARID1A expression (p=0.874 and p=0.313, respectively) (Supplementary Figure 1A, B). In patients with luminal B, HER2 positive type disease, both DFS and OS did not show any statistically significant differences according to ARID1A expression (p=0.238 and p=0.067, respectively) (Supplementary Figure 1C, D). In patients with HER2 type disease, low ARID1A expression was associated with significantly shorter DFS than high ARID1A expression (p=0.016) (Supplementary Figure 1E). In contrast, OS was not significantly different between the ARID1A high and low expression groups (p=0.087) (Supplementary Figure 1F). In patients with TNBC basal type disease, both DFS and OS did not show any statistically significant differences according to ARID1A expression (p=0.144 and p= 0.114, respectively) (Supplementary Figure 1G, H). In patients with TNBC nonbasal type disease, both DFS and OS did not show any statistically significant differences according to ARID1A expression (p=0.258 and p=0.408, respectively) (Supplementary Figure 1I, J). In multivariate analysis using the Cox proportional hazard model, low ARID1A expression was not a significant independent prognostic factor for DFS and OS, according to molecular subtype.
Figure 3. Kaplan-Meier survival curve for AT-rich interactive domain 1A (ARID1A) in patients with luminal A type disease (n=205). (A) Disease-free survival (p=0.022) and (B) overall survival (p=0.018).
DISCUSSION
ARID1A functions as a tumor suppressor and may participate in both tumor initiation and progression in human cancers [23]. ARID1A is most frequently mutated in endometrium-derived tumors (about 50% of ovarian clear cell carcinomas and 30% of ovarian endometrioid carcinomas) [8,17]. Comprehensive molecular studies indicate that the ARID1A gene mutation rate is about 4% in breast cancers, but copy number loss occurs in 13%-35% of cases [2,24]. Low expression of ARID1A mRNA has been reported to be strongly associated with promoter hypermethylation of the ARID1A gene in invasive ductal carcinomas (86.4%) [25]. The authors identified an association of low ARID1A RNA or nuclear protein expression with more aggressive breast cancer phenotypes [2].
In our study, we used a large number of breast cancer samples (n=476) to detect the expression of ARID1A protein by IHC. The majority (339/476, 71.2%) of the breast cancer tissues exhibited low ARID1A expression. This percentage is consistent, but somewhat higher, than previous reports (Mamo et al. [2]: 64% [151/236]; Zhang et al. [18]: 56% [63/112]; and Zhao et al. [19]: 65.3% [324/496]). Differences in patient race and IHC methods may account for this discrepancy.
In this study, clinicopathological analysis revealed that low ARID1A expression in breast cancer was associated with mastectomy, lymph node metastasis, advanced p-stage, low Ki-67 labeling index, and negative p53 expression. Weak correlations between low ARID1A expression and low histologic grade were also found. With regard to lymph node metastasis and advanced p-stage, these results are similar to those reported in previous studies of breast cancer [2,18,19]. Our findings indicated that loss of ARID1A expression was related to low Ki-67 labeling index, negative p53 expression, and low histologic grade, which have been identified as good prognostic factors. Some studies reported the loss of ARID1A expression may be related to less invasive clinicopathologic features in colorectal cancer and gastric cancer [26]. However, one study reported that tumors with low ARID1A expression were associated with ER negativity, higher p53(+) percentage, higher Ki-67 labeling index, and TNBC subtype in breast cancer [18], with other authors reporting that low ARID1A expression was associated with ER negativity, high histologic grade, and higher p53(+) percentage [19]. A statistically significant inverse correlation between the mutational statuses of the ARID1A and TP53 genes in tumor samples of ovarian clear cell carcinoma and endometrial endometrioid carcinoma was reported [27]. The authors suggested that ARID1A and p53 collaborate to prevent tumorigenesis. In contrast, other researchers reported no significant relationship between loss of ARID1A expression and p53 overexpression in endometrial clear cell carcinoma [28]. These differences may be attributed to differences in the organ and carcinoma types, patient races or sample sizes, variations in antibodies, laboratory IHC methods, as well as other cofactors that affect tumor behavior. Therefore, additional studies on a larger cohort will be needed to confirm our findings.
In agreement with previous studies [2,18,19], patients with low ARID1A expression had worse DFS and OS than those with high ARID1A expression. The multivariate analysis also revealed that low ARID1A expression was a significant independent prognostic factor for shorter DFS and OS in patients with breast cancer. Thus, low ARID1A expression might be applied as a valuable prognostic marker for relapse and disease-related death in patients with breast cancer. In this study, we were also able to demonstrate an association between ARID1A expression and clinical outcomes according to the molecular subtype. In luminal A type disease, there was a significant trend toward shorter DFS and OS in the group with low ARID1A expression compared with the group with high ARID1A expression. With this result, we could speculate that luminal A type disease with loss of ARID1A expression might be correlated with poor clinical outcomes.
There are some limitations to the generalization of these results. First, the retrospective design and the small sample size drawn from a single institution resulted in selection bias. Second, the survival analysis was limited as the follow-up period was too short to determine 5-year survival rates. Third, the significant association between ARID1A expression and p53 and Ki-67 might be affected by several confounders. These need to be evaluated in subsequent studies. Despite these limitations, this is the first study to examine ARID1A expression in a large number of breast cancer patients in Korea.
Recent studies have focused on the possible mechanism of ARID1A mutation and protein expression loss in tumorigenesis. A relationship between ARID1A mutations and enhancer of zeste homologue 2 (EZH2) has been suggested. Inhibition of EZH2 methyltransferase acts in a synthetic lethal manner in ARID1A-mutated ovarian cancer cells, with ARID1A mutational status correlated with response to the EZH2 inhibitor, making it a potential target for targeted therapy [29].
In summary, we assessed clinicopathological correlations, molecular subtype, and prognostic significance of ARID1A expression by IHC in primary breast cancer. This is the first study to reveal the prognostic significance of ARID1A expression in a large number of breast cancer patients in Korea. Low expression of ARID1A is an independent predictive factor for poor DFS and OS in breast cancer patients and may be associated with luminal A type disease. The exact role of the ARID1A pathway in breast cancer is not clear. Additional functional studies using breast cancer cell lines and further validation with in vivo experiments are needed to elucidate the role of the ARID1A pathway in the tumorigenesis and progression of breast cancer.
Footnotes
This work was supported in part by the Soonchunhyang University Research Fund.
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
Supplementary Material
Kaplan-Meier survival curve for AT-rich interactive domain 1A (ARID1A) according to the luminal B, human epidermal growth factor receptor 2 (HER2), and triple-negative breast cancer (TNBC) subtype. (A) Disease-free survival (p=0.874) and (B) overall survival (p=0.313) in patients with in luminal B, HER2 negative type (n=76). (C) Disease-free survival (p=0.238) and (D) overall survival (p=0.067) in patients with in luminal B, HER2 positive type (n=37). (E) Disease-free survival (p=0.016) and (F) overall survival (p=0.087) in patients with in HER2 type (n=46). (G) Disease-free survival (p=0.144) and (H) overall survival (p=0.114) in patients with in TNBC basal type (n=81). (I) Disease-free survival (p=0.258) and (J) overall survival (p=0.408) in patients with in TNBC nonbasal type (n=31).
References
- 1.Yoon JH, Kim MJ, Kim EK, Moon HJ. Imaging surveillance of patients with breast cancer after primary treatment: current recommendations. Korean J Radiol. 2015;16:219–228. doi: 10.3348/kjr.2015.16.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mamo A, Cavallone L, Tuzmen S, Chabot C, Ferrario C, Hassan S, et al. An integrated genomic approach identifies ARID1A as a candidate tumor-suppressor gene in breast cancer. Oncogene. 2012;31:2090–2100. doi: 10.1038/onc.2011.386. [DOI] [PubMed] [Google Scholar]
- 3.Jones S, Li M, Parsons DW, Zhang X, Wesseling J, Kristel P, et al. Somatic mutations in the chromatin remodeling gene ARID1A occur in several tumor types. Hum Mutat. 2012;33:100–103. doi: 10.1002/humu.21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu JN, Roberts CW. ARID1A mutations in cancer: another epigenetic tumor suppressor? Cancer Discov. 2013;3:35–43. doi: 10.1158/2159-8290.CD-12-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson BG, Roberts CW. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer. 2011;11:481–492. doi: 10.1038/nrc3068. [DOI] [PubMed] [Google Scholar]
- 6.Itamochi H, Oumi N, Oishi T, Shoji T, Fujiwara H, Sugiyama T, et al. Loss of ARID1A expression is associated with poor prognosis in patients with stage I/II clear cell carcinoma of the ovary. Int J Clin Oncol. 2015;20:967–973. doi: 10.1007/s10147-015-0811-x. [DOI] [PubMed] [Google Scholar]
- 7.Bosse T, ter Haar NT, Seeber LM, v Diest PJ, Hes FJ, Vasen HF, et al. Loss of ARID1A expression and its relationship with PI3K-Akt pathway alterations, TP53 and microsatellite instability in endometrial cancer. Mod Pathol. 2013;26:1525–1535. doi: 10.1038/modpathol.2013.96. [DOI] [PubMed] [Google Scholar]
- 8.Mao TL, Shih IeM. The roles of ARID1A in gynecologic cancer. J Gynecol Oncol. 2013;24:376–381. doi: 10.3802/jgo.2013.24.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho H, Kim JS, Chung H, Perry C, Lee H, Kim JH. Loss of ARID1A/BAF250a expression is linked to tumor progression and adverse prognosis in cervical cancer. Hum Pathol. 2013;44:1365–1374. doi: 10.1016/j.humpath.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Park JH, Lee C, Suh JH, Chae JY, Kim HW, Moon KC. Decreased ARID1A expression correlates with poor prognosis of clear cell renal cell carcinoma. Hum Pathol. 2015;46:454–460. doi: 10.1016/j.humpath.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Kim MJ, Gu MJ, Chang HK, Yu E. Loss of ARID1A expression is associated with poor prognosis in small intestinal carcinoma. Histopathology. 2015;66:508–516. doi: 10.1111/his.12566. [DOI] [PubMed] [Google Scholar]
- 12.Rao Q, Xia QY, Wang ZY, Li L, Shen Q, Shi SS, et al. Frequent co-inactivation of the SWI/SNF subunits SMARCB1, SMARCA2 and PBRM1 in malignant rhabdoid tumours. Histopathology. 2015;67:121–129. doi: 10.1111/his.12632. [DOI] [PubMed] [Google Scholar]
- 13.Wiegand KC, Sy K, Kalloger SE, Li-Chang H, Woods R, Kumar A, et al. ARID1A/BAF250a as a prognostic marker for gastric carcinoma: a study of 2 cohorts. Hum Pathol. 2014;45:1258–1268. doi: 10.1016/j.humpath.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Xu X, Zhang M, Bai X, Li H, Kan L, et al. ARID1A is down-regulated in non-small cell lung cancer and regulates cell proliferation and apoptosis. Tumour Biol. 2014;35:5701–5707. doi: 10.1007/s13277-014-1755-x. [DOI] [PubMed] [Google Scholar]
- 15.Balbás-Martínez C, Rodríguez-Pinilla M, Casanova A, Domínguez O, Pisano DG, Gómez G, et al. ARID1A alterations are associated with FGFR3-wild type, poor-prognosis, urothelial bladder tumors. PLoS One. 2013;8:e62483. doi: 10.1371/journal.pone.0062483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda D, Mao TL, Fukayama M, Nakagawa S, Yano T, Taketani Y, et al. Clinicopathological significance of loss of ARID1A immunoreactivity in ovarian clear cell carcinoma. Int J Mol Sci. 2010;11:5120–5128. doi: 10.3390/ijms11125120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Zhang Y, Yang Y, Niu M, Sun S, Ji H, et al. Frequent low expression of chromatin remodeling gene ARID1A in breast cancer and its clinical significance. Cancer Epidemiol. 2012;36:288–293. doi: 10.1016/j.canep.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J, Liu C, Zhao Z. ARID1A: a potential prognostic factor for breast cancer. Tumour Biol. 2014;35:4813–4819. doi: 10.1007/s13277-014-1632-7. [DOI] [PubMed] [Google Scholar]
- 20.Lakhani SR EI, Schnitt SJ, Tan PH, van de Vijver MJ. WHO Classification of Tumours of the Breast. 4th ed. Lyon: International Agency for Research on Cancer; 2012. [Google Scholar]
- 21.Im S, Choi HJ, Yoo C, Jung JH, Jeon YW, Suh YJ, et al. Hedgehog related protein expression in breast cancer: gli-2 is associated with poor overall survival. Korean J Pathol. 2013;47:116–123. doi: 10.4132/KoreanJPathol.2013.47.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 23.Guan B, Gao M, Wu CH, Wang TL, Shih IeM. Functional analysis of in-frame indel ARID1A mutations reveals new regulatory mechanisms of its tumor suppressor functions. Neoplasia. 2012;14:986–993. doi: 10.1593/neo.121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornen S, Adelaide J, Bertucci F, Finetti P, Guille A, Birnbaum DJ, et al. Mutations and deletions of ARID1A in breast tumors. Oncogene. 2012;31:4255–4256. doi: 10.1038/onc.2011.598. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Sun Q, Shan M, Niu M, Liu T, Xia B, et al. Promoter hypermethylation of ARID1A gene is responsible for its low mRNA expression in many invasive breast cancers. PLoS One. 2013;8:e53931. doi: 10.1371/journal.pone.0053931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SY, Kim DW, Lee HS, Ihn MH, Oh HK, Park do J, et al. Loss of ATrich interactive domain 1A expression in gastrointestinal malignancies. Oncology. 2015;88:234–240. doi: 10.1159/000369140. [DOI] [PubMed] [Google Scholar]
- 27.Guan B, Wang TL, Shih IeM. ARID1A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Res. 2011;71:6718–6727. doi: 10.1158/0008-5472.CAN-11-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fadare O, Gwin K, Desouki MM, Crispens MA, Jones HW, 3rd, Khabele D, et al. The clinicopathologic significance of p53 and BAF-250a (ARID1A) expression in clear cell carcinoma of the endometrium. Mod Pathol. 2013;26:1101–1110. doi: 10.1038/modpathol.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bitler BG, Aird KM, Garipov A, Li H, Amatangelo M, Kossenkov AV, et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med. 2015;21:231–238. doi: 10.1038/nm.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan-Meier survival curve for AT-rich interactive domain 1A (ARID1A) according to the luminal B, human epidermal growth factor receptor 2 (HER2), and triple-negative breast cancer (TNBC) subtype. (A) Disease-free survival (p=0.874) and (B) overall survival (p=0.313) in patients with in luminal B, HER2 negative type (n=76). (C) Disease-free survival (p=0.238) and (D) overall survival (p=0.067) in patients with in luminal B, HER2 positive type (n=37). (E) Disease-free survival (p=0.016) and (F) overall survival (p=0.087) in patients with in HER2 type (n=46). (G) Disease-free survival (p=0.144) and (H) overall survival (p=0.114) in patients with in TNBC basal type (n=81). (I) Disease-free survival (p=0.258) and (J) overall survival (p=0.408) in patients with in TNBC nonbasal type (n=31).