Abstract
Purpose
A systematic literature review was done to determine the relationship between elevated CRP and prognosis in people with solid tumors. C-reactive protein (CRP) is a serum acute phase reactant and a well-established inflammatory marker. We also examined the role of CRP to predict treatment response and tumor recurrence.
Methods
MeSH (Medical Subject Heading) terms were used to search multiple electronic databases (PubMed, EMBASE, Web of Science, SCOPUS, EBM-Cochrane). Two independent reviewers selected research papers. We also included a quality Assessment (QA) score. Reports with QA scores <50% were excluded. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) methodology was utilized for this review (S1 PRISMA Checklist).
Results
271 articles were identified for final review. There were 45% prospective studies and 52% retrospective. 264 had intermediate QA score (≥50% but <80%); Seven were adequate (80% -100%); A high CRP was predictive of prognosis in 90% (245/271) of studies—80% of the 245 studies by multivariate analysis, 20% by univariate analysis. Many (52%) of the articles were about gastrointestinal malignancies (GI) or kidney malignancies. A high CRP was prognostic in 90% (127 of 141) of the reports in those groups of tumors. CRP was also prognostic in most reports in other solid tumors primary sites.
Conclusions
A high CRP was associated with higher mortality in 90% of reports in people with solid tumors primary sites. This was particularly notable in GI malignancies and kidney malignancies. In other solid tumors (lung, pancreas, hepatocellular cancer, and bladder) an elevated CRP also predicted prognosis. In addition there is also evidence to support the use of CRP to help decide treatment response and identify tumor recurrence. Better designed large scale studies should be conducted to examine these issues more comprehensively.
Introduction
Approximately 1,638,910 new cancer diagnoses and about 577,190 deaths occurred in the US in 2012, mostly from solid tumors [1]. Prognostication in cancer can be either subjective or objective. In the former, dependent on clinician skill and experience, it is often inaccurate and usually overly optimistic [2]. Prognostication is an important clinical skill for oncologists. Despite advances in medical technology and biology, it is still an inexact science [2], even with extensive and expensive investigations [3]. Objective determination of prognosis can be based on a combination of tumor, patient, and environmental factors. The use of biological tumor markers to help prognostication (alone or combined with other parameters) has appeal. An ideal potential tumor marker should have a long half-life, be measured accurately and precisely by a simple and inexpensive blood test. It is also important that it be sensitive to change so that it can be followed over time through serial measurements. A few biologic markers meet these criteria [4]. C-reactive protein (CRP) is one.
Rationale
CRP is an acute phase reactant, which reflects tissue injury [5]. The half-life is 19 hours in both health and disease. CRP secretion by hepatocytes appears controlled by interleukin 6 (IL-6). Interleukin-1 (IL-1) and tumor necrosis factor (TNF) also stimulate CRP synthesis [6]. CRP is a stable downstream marker of inflammation, unlike the pro-inflammatory cytokines, which have short half-lives (minutes) [7, 8]. In chronic inflammatory diseases, serial CRP levels have been correlated with disease severity, and response to therapy [9]. Many large prospective studies now support the role of CRP in prediction of coronary artery disease [10, 11], though controversies exist [12].
Chronic inflammation has been linked to cancer at tumor initiation, but may also be associated with invasive potential and disease progression [13, 14]. A relationship has been proposed between systemic inflammation and various cancer symptoms [15]. A strong positive correlation between high CRP and high IL-6 levels was shown in advanced pancreatic cancer [16]. Elevated CRP levels have been linked to shorter survival in several common cancers [17].
Objectives
In this paper, we describe the results of a systematic review of the relationship between elevated serum CRP and life expectancy in people with solid tumors. We also examined its role in the prediction of treatment response and risk of tumor recurrence.
Methods
Eligibility criteria
Only articles in English were included. Original reports of any studies of solid malignancies in adults were scrutinized. All study designs were included. The following articles were excluded: all non-English literature, basic research, animal research, all pediatric and hematological malignancies, and studies where prognostic parameters were not assessed, or serum CRP levels not measured. Editorial letters and comments were also excluded. Review papers were consulted, but for discussion purposes only.
Information sources
Electronic databases included: PubMed (1966 to December 2012); EMBASE (1988 to 2012); Web of Science (1980 to 2012); SCOPUS (1965 to 2012); and the EBM-Cochrane Central Register of Controlled trials and EBM-Cochrane Database of Systematic Reviews (Up to 2012). The search was repeated at the end of data analysis.
Search
PubMed search of CRP or related MeSH terms (c—reactive protein/c-reactive) with (AND) neoplasm/neoplasms/cancer in all fields with (AND) prognosis/mortality/survival OR survival rate/treatment outcome/treatment/outcome was done including other databases. Search words/terms were as follows:
Study selection
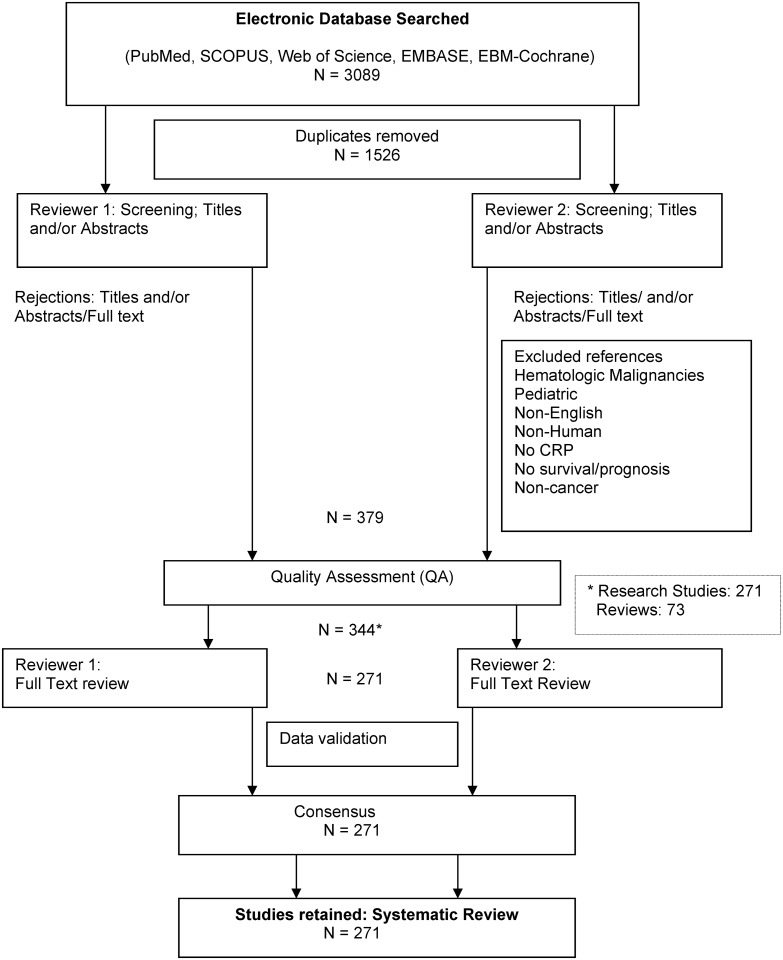
A qualified medical librarian (see Acknowledgements) reviewed the search strategy. The first literature screen (Fig 1) was based on article title. If that was irrelevant, the abstract was also reviewed (by NBB, SS and DW) before an exclusion decision. Abstracts (and when necessary the full text) of the remaining articles were then assessed. The reviewers (NBB, SS, ST and DW) met periodically to discuss reasons of exclusion or inclusion of selected papers. Retained articles were then subjected to quality assessment (S1 Appendix).
Fig 1. Studies Selection.
Quality assessment
A quality assessment (QA) system was developed. This was based on existing guidelines [18, 19] for observational cohort prognostic studies. The tool combined five criteria:
Study design
Patient selection
Prognostic variables
Follow-up
Data analysis
A score of 0–2 was assigned to each—if a study met the conditions in full (score of 2), partially (score of 1), or not at all (score of 0) (S1 Appendix). The total was expressed as a percentage of the maximum possible score. A score of 80–100% was ranked as an adequate study; ≥50 but <80% as intermediate; articles that scored <50% were considered inadequate and excluded from the review (S2 Appendix).
Data collection
Data were extracted using a custom designed extraction sheet (till 2010) and later utilizing the Research Electronic Data Capture (REDCap) [20] forms with same sheet. REDCap is a secure web application to create and manage data. The following information was collected: author name, publication date, study title, study design, quality assessment score and grade, number of patients and controls (when available), cancer type, extent of disease, main outcome, main results related to prognosis or treatment outcomes, CRP cut-off points, assay method, mean CRP value, survival definition, median survival duration, CRP sensitivity and specificity as a predictor of prognosis, treatment outcomes or recurrence, other parameters assessed for prognosis, strongest predictors of prognosis, statistical analysis used, and possible co-morbid contributors to increased CRP levels other than cancer (like infection, chemo- or radiotherapy, surgery). The descriptor term “cutoff” refers to the value an investigator/author used to determine an “elevated” CRP even if that level was within the biochemical reference range.
Summary measures
There was substantial variation in study design and cancer primary sites. A meta-analysis was therefore inappropriate. For each study article we estimated the minimum sample size necessary to detect a difference at P ≤ 0.05. We used the general rule of n = 10 per variable. The estimated minimum sample size was compared to the actual size of the study. Studies with insufficient sample sizes were considered underpowered. Predictors by multivariate analysis were stratified by relative risk (RR) and statistical significance (p-value):
When RR<2 or >0.5
When RR>2 or <0.5
When RR>5 or <0.2
When RR>10 or <0.1
When p<0.05
When p<0.01
When p<0.001
We followed the PRISMA statement (S1 PRISMA Checklist) to design and report our systematic review [21].
Results
Study selection
The search identified three thousand and eighty nine (3089) citations: fourteen hundred sixty-six (1466) in PubMed, eight hundred two (802) in Web of Science, three hundred twenty (320) in SCOPUS, three hundred eleven (311) in EMBASE and one hundred ninety (190) in the Cochrane database. After removal of duplicates, fifteen hundred twenty six (1526) remained. Irrelevant studies were then removed. These included those where survival or prognosis was not an outcome, studies where CRP was not studied as a prognostic marker, animal/cell-line based studies, letters and editorials, and those that did not fit our inclusion criteria. Seven hundred thirty one (731) papers were left. Next, three hundred (300) studies in hematologic malignancies, ten (10) non-English articles and forty two (42) pediatric reports were removed. Subsequent to the quality assessment (QA) of the three hundred seventy nine (379) studies retained, thirty five (ten prospective, twenty five retrospective) were inadequate by QA score and excluded. Then two hundred seventy one (271) research studies and seventy-three (73) review papers remained (Fig 1). Survival and outcome measures differed between studies. As a result, no direct study comparisons were possible.
Two hundred seventy one original articles constituted the final analysis (Fig 1). Only seven of these scored ≥ 80% in the QA (all were prospective and longitudinal, and with a control group in three). Two hundred sixty four (264) had an intermediate QA score. One hundred twenty nine (129) of the 271 did not describe their patient selection procedures. Examples included whether patients were screened for infections, the timing of CRP measurement in relation to factors that could raise CRP level (like chemo- or radiation therapy), and invasive procedures. The sensitivity and specificity of the predictive prognostic value of CRP were reported in only four studies [22–25], two in melanoma, one each in cancer of the esophagus and lung. A power analysis was described in two [26, 27]; CRP was an independent prognostic marker of survival in one but not the other. The reference level of CRP for evaluation of responses varied both for RCC and GI studies.
Study characteristics by study design
Forty five percent (n = 122) of the studies were prospective and 52% (N = 142) of the 271 studies were retrospective; the remaining 3% (N = 7) combined retrospective and prospective design. In the prospective studies, median sample size was 121 (range 15–9605) versus 146 (range 32–9608) in the retrospective. High CRP predicted prognosis in 82% (100/122) of the prospective studies. In 13% (16/122) of prospective studies, this was by univariate analysis only. In 18% (22/122), CRP was not prognostic of survival (Table 1). Only 16% (20 of 122) of the prospective studies had a control group (CG) (Table 1). Overall CRP predicted prognosis in 90% (245/271) of studies; 80% by multivariate analysis (MVA) and 20% by univariate analysis (UVA) (Table 2).
Table 1. Characteristics by Study Design.
| Study Type | Number of Studies | Sample Size | Study Outcomes # (%) | |||
|---|---|---|---|---|---|---|
| (%)* | Median | Range | 1 | 2 | 3 | |
| Prospective | 122 (45) | 121 | (15–9605) | 100 (82) | 22 (18) | 16 (13) |
| No control group | 102 (84) | 117 | (15–9605) | |||
| Control group | 20 (16) | 156 | (54–687) | |||
| Retrospective | 142 (52) | 146 | (32–9608) | 128 (90) | 14 (10) | 29 (20) |
| Combined + | 7 (3) | 98 | (58–325) | 7 (100) | 0 | 0 |
1: Number of Studies Where CRP was a Prognostic Predictor.
2: Number of Studies Where CRP was Not a Prognostic Predictor.
3: Number of Studies Where CRP was a Prognostic Predictor by Univariate Analysis Only.
* Percent (rounded to the closest whole number) compared to total number of studies.
# Percent compared to study type.
+ Both prospective and retrospective data.
Table 2. Study Characteristics by Tumor Type.
| Cancer Type | Number of Studies | Study Outcomes | ||
|---|---|---|---|---|
| 1 (%) | 2 (%) | 3 (%) | ||
| Digestive Tumors* | 90 | 81 (90) | 9 (10) | 16 (18) |
| Renal cell carcinoma | 51 | 46 (90) | 5 (10) | 12 (24) |
| Pancreas | 24 | 23 (96) | 1 (4) | 7 (29) |
| Lung | 24 | 22 (92) | 2 (8) | 2 (8) |
| Hepatocellular carcinoma (HCC) | 10 | 10 (100) | 0 (0) | 1 (10) |
| Melanoma | 5 | 5 (100) | 0 (0) | 0 (0) |
| Breast | 7 | 4 (57) | 3 (43) | 0 (0) |
| Prostate | 9 | 7 (78) | 2 (22) | 0 (0) |
| Bladder | 12 | 12 (100) | 0 (0) | 2 (17) |
| Heterogeneous | 15 | 14 (93) | 1 (7) | 2 (13) |
| Others | 24 | 21 (88) | 3 (13) | 6 (25) |
1: Number of Studies Where CRP was a Prognostic Predictor.
2: Number of Studies Where CRP was Not a Prognostic Predictor.
3: Number of Studies Where CRP was a Prognostic Predictor on Univariate Analysis Only.
* Digestive tumors include esophageal, gastroesophageal and intestinal tumors.
Study characteristics by tumor type
1. Renal cell carcinoma
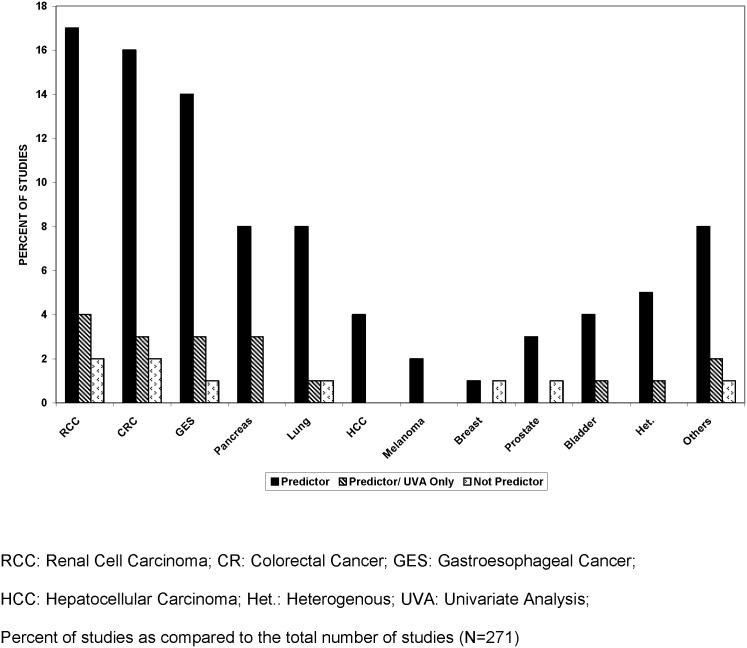
Fifty one (19%) studies looked at renal cell carcinoma. Of these, CRP was prognostic in 90% (46 of 51). In 12 of 51 (24%), CRP predicted prognosis on univariate analysis only [28–37]. CRP was not predictive of prognosis in five studies [38] (Table 2, Fig 2).
Fig 2. CRP Prediction of Prognosis by Tumor Primary Site.
1.1 Prognosis: Forty-six of the 51 studies in renal cell carcinoma (90%) had prognosis as a primary outcome. In thirty three of the 46, CRP was a strong predictor of survival by multivariate analysis (Table 3). In the other 12 of the 46, CRP predicted prognosis by univariate analysis only [28–37]. One of these was underpowered [39]; none of the other eleven studied the prognostic value of CRP as a primary outcome measure (Table 3).
Table 3. CRP as a Predictor of Prognosis, Treatment Outcome or Tumor Recurrence in Renal Cell Carcinoma.
| Publication Year (Reference) | Main Outcome | CRP cut-offs* (mg/L) # | Study Design | Quality Score (%) | Sample Size | Disease Extent | Strongest Predictors by MVA ϕ | |
|---|---|---|---|---|---|---|---|---|
| PROGNOSIS | ||||||||
| 1998 [144] | 5 year Survival Post Curative Resection | Negative vs. positive | Retrospective | 55 | 111 | All stages | CRP | b, x |
| T stage | a, x | |||||||
| 1999 [145] | Pre-treatment Serum Markers and Clinical Parameters | ≥8 | Prospective | 65 | 99 | Metastatic | SICAM-1 | —, y |
| CRP | —, x | |||||||
| ESR | —, x | |||||||
| 2006 [146] | APP in Potentially Curative Resection | >10 | Prospective + Retrospective | 60 | 43 Prospective 57 Retrospective | All stages | CRP | c, y |
| Grade | b, y | |||||||
| Sex | b, x | |||||||
| 2006 [147] | Estimation by Fractional Polynomials | Not reported | Retrospective | 65 | 425 | Metastatic | Age | x |
| LN, liver, bone metastasis | —, y,x,y | |||||||
| CRP | —, x | |||||||
| Neutrophils | —, y | |||||||
| 2007 [148] | Preoperative Serum CRP | >5 | Prospective | 65 | 101 | Localized | DSS: pT staging | b, y |
| CRP | b, x | |||||||
| RFS: CRP | b, z | |||||||
| pT Staging | b, y | |||||||
| 2007 [149] | GPS and Cancer-specific Survival | >10 | Prospective | 70 | 119 | Metastatic | Biochemical: Calcium | b, y |
| CRP | b, y | |||||||
| Albumin | b, y | |||||||
| WCC | a, y | |||||||
| Scoring systems: GPS | b, z | |||||||
| MSKCC | a, y | |||||||
| 2007 [150] | Stages Treated with Nephrectomy–Survival UISS v. Model with CRP | Continuous Categorical: ≤4.0, 4.1–23.0, >23.0 | Prospective | 65 | 313 | All Stages | CRP (Cat.) | —, y |
| Metastasis | b, z | |||||||
| ECOG PS | b, y | |||||||
| 2007 [151] | Survival, Treatment Response: IL-2 Based Therapy | 8mg/L | Retrospective With Control | 60 | 55 + 144 Controls | Metastatic | CRP | b, y |
| IL-12 | a, x | |||||||
| 2008 [50] | Survival: Primary Operable Tumor Recurrence | >10mg/L | Prospective | 75 | 83 | All Stages | CRP | d, y |
| T-stage | d, x | |||||||
| Necrosis | d, y | |||||||
| 2008 [33] | Prognosis: RCC Extending IVC | 6mg/L | Retrospective | 55 | 46 | All Stages | CRP | b, — |
| LN Metastasis | b, y | |||||||
| 2008 [51] | CRP, Tumoral IL-6, COX-2 Expression & Survival | 10mg/L | Retrospective | 60 | 60 | Resectable | CRP | b, x |
| TNM | c, x | |||||||
| 2008 [152] | Systemic Symptoms on Survival | 3mg/L | Retrospective | 55 | 252 | All Stages | CRP | b, y |
| Systemic Symptoms | c, z | |||||||
| 2008 [123] | Survival in Cytoreductive Nephrectomy | 5mg/L | Prospective | 65 | 40 | Metastatic | CRP kinetics | b, z |
| Poor ECOG | —, y | |||||||
| Number of Mastectomy | —, y | |||||||
| Bone Metastasis | —, x | |||||||
| 2009 [122] | CRP Kinetics & Survival | Normalized & Non-Normalized | Retrospective | 60 | 108 | Metastatic | Normal CRP | a, x |
| Non-Normal CRP | b, z | |||||||
| ECOG PS | a, z | |||||||
| LDH | a, z | |||||||
| 2009 [153] | Survival Prediction Model with CRP | 5mg/L | Prospective with Control | 75 | 249 Control-290 | Locally Advanced | CRP | a, x |
| Distant Metastasis | b, z | |||||||
| 2009 [32] | Worst Grade Component Survival, Recurrence | ≥10 mg/L | Retrospective | 50 | 314 | All Stages | CRP | a,— |
| Distant Metastasis | c, z | |||||||
| 2010 [53] | Preoperative CRP Survival, Metastasis | Continuous | Prospective | 85 | 130 | All Stages | Pre-operative CRP | a, z |
| Pre-operative Platelets | a, z | |||||||
| 2010 [49] | Preoperative and Postoperative CRP to Predict Outcome | Continuous | Prospective | 70 | 110 | Localized | Post-operative CRP | a, z |
| T-stage | d, — | |||||||
| 2010 [48] | Pre-operative Prognostic Significance of CRP | 15mg/L | Retrospective | 60 | 286 | All Stages | Log (CRP) | a, y |
| M-Stage | b, z | |||||||
| Necrosis | a, y | |||||||
| MVI (invasion) | b, y | |||||||
| RBC | b, y | |||||||
| WBC | b, z | |||||||
| 2011 [37] | Lifestyle Factors on CRP and Overall Survival | 2mg/L | Prospective | 70 | 257 | Localized | Pre-operative CRP | a, — |
| 2011 [54] | CRP and Thrombocytosis on Survival | 8mg/L | Retrospective | 55 | 177 | Resectable | CRP | b, x |
| Tumor size | b, y | |||||||
| 2011 [154] | CRP on Survival, Predictive Survival Model | 3mg/L | Retrospective | 50 | 94 | Metastasis (Bone) | CRP | b, x |
| Sarcoma Differentiate | b, z | |||||||
| Bone Involvement | b, y | |||||||
| Extraosseus Metastasis | b, x | |||||||
| ALP | c, x | |||||||
| 2011 [55] | CRP, CRP Kinetics: Survival and Recurrence | 10mg/L | Retrospective | 55 | 263 | Resectable | Non-normal CRP | —, z |
| Anemia | —,— | |||||||
| Thrombocytosis | —,- | |||||||
| 2011 [155] | Prognosis of Metastatic RCC; Validity of MSKCC | 3mg/L | Retrospective | 50 | 473 | Metastasis | CRP | b, z |
| Diagnosis→Metastasis (Time) | b, z | |||||||
| Hemoglobin | a, y | |||||||
| Calcium | a, y | |||||||
| LDH | a, x | |||||||
| Liver metastasis | b, z | |||||||
| Bone metastasis | a, y | |||||||
| Node metastasis | a, y | |||||||
| 2011 [56] | Factors Associated WithSurvival, Recurrence | 4 mg/L | Retrospective | 50 | 32 | Metastasis | CRP | b, x |
| 2011 [156] | Post-operative CRP, pre-operative albumin and survival | 2 mg/L | Retrospective | 50 | 40 | Resectable | Postoperative CRP | a, x |
| Preoperative Albumin | c, x | |||||||
| 2012 [157] | mGPS and Prognosis | 10 mg/L (mGPS) | Prospective | 70 | 169 | All Stages | mGPS | b, z |
| Necrosis | a, x | |||||||
| 2012 [158] | Systemic inflammation, Tumor inflammatory cells, Tumor Necrosis & Survival | 10mg/ L (mGPS) | Prospective | 60 | 79 | Resectable | mGPS | c, z |
| 2012 [159] | Survival & Treatment Response with Sunitinib | 3mg/L | Retrospective | 50 | 41 | All Stages | Elevated CRP | —/— |
| Normal CRP | d, x | |||||||
| 2012 [160] | Molecular-targeted agents, Survival & Treatment Response | 8mg/L | Retrospective | 55 | 52 | Metastasis | CRP | a, y |
| Neutrophilia | a, x | |||||||
| 2012 [161] | Hyponatremia on Survival with Molecular Targeted Therapy | 10mg/L | Retrospective | 50 | 87 | Metastasis | Severe Hyponatremia | b, x |
| Mild Hyponatremia | c, z | |||||||
| CRP | a, y | |||||||
| Neutrophilia | b, x | |||||||
| 2012 [162] | WBC, CRP and Survival, Optimal Threshold of CRP | 25mg/L | Retrospective | 55 | 327 | Resectable | CRP | b, x |
| T stage | b, x | |||||||
| N stage | b, y | |||||||
| M stage | c, z | |||||||
| Nuclear grade | b, z | |||||||
| Karnofsky | b, y | |||||||
| 2012 [163] | Prognostic significance of Osteopontin A, Carbonic Anhydrase IX, CRP; alone and combined | Continuous | Retrospective | 55 | 216 | All Stages | CRP | a, z |
| CA-9 | b, y | |||||||
| N stage | b, z | |||||||
| M stage | b, z | |||||||
| 2012 [164] | Pre-operative CRP | Continuous Categorical: <4mg/L, 4-10mg/L, >10mg/L | Retrospective | 55 | 1161 | All Stages | Metastasis | b, z |
| G4 Differentiation | b, y | |||||||
| CRP (Continuous) | a, z | |||||||
| CRP (Categorical) | a, z | |||||||
| CRP (Categorical) | b, z | |||||||
| TREATMENT RESPONSE AND TUMOR RECURRENCE | ||||||||
| 1992 [40] | CRP and IL-2 Response | >10 | Prospective | 60 | 15 | Metastatic | CRP | — |
| 1992 [41] | Serum IL-6, pre-IL-2 | >50 | Prospective | 50 | 138+ 70 controls | Metastatic | CRP | — |
| IL-6 | — | |||||||
| 1999 [42] | Cytoreductive Surgery Subgroups | ≥1ng/ml | Retrospective | 50 | 58 | Metastatic | CRP | — |
| 2003 [43] | Prognostic System Post-IL-2 + INF-α | ≥11 | Retrospective | 55 | 425 | Metastatic | WBC | a, z |
| CRP | a, y | |||||||
| LDH | a, x | |||||||
| Number of Metastasis | a, x | |||||||
| Time to Metastasis | a, y | |||||||
| 2004 [44] | APP, Performance Status and Survival post-IFN-α | ≤10 vs. >10 | Prospective+ Retrospective | 55 | 26 Retrospective 32 Prospective | Advanced | CRP | b, x |
| 2005 [45] | Pre-treatment (IL-2) Biohumoral and Clinical Factors | ≥8 | Retrospective | 60 | 110 | Metastatic | CRP | b, x |
| DFI | b, x | |||||||
| 2005 [46] | Prognostic Factors Post-Allogeneic Stem Cell Transplant | Normal or not | Prospective | 65 | 70 | Advanced | CRP | b, z |
| LDH | b, x | |||||||
| KPS | a, x | |||||||
| 2006 [47] | Response and Survival Post IFN-α then IL-2. | ≥8 | Retrospective | 55 | 99 | Metastatic | Nuclear grade | b, y |
| Mastectomy | b, x | |||||||
| LDH | b, x | |||||||
| CRP | b, x | |||||||
| 2006 [52] | CRP, Thrombocytosis and Recurrence | >10 | Retrospective | 55 | 178 | All stages | Metastasis | d, z |
| CRP | c, z | |||||||
| Tumor grade | b, x | |||||||
| Tumor size | a, y | |||||||
| 2008 [50] | Primary Operable Tumor Recurrence | >10mg/L | Prospective | 75 | 83 | All Stages | CRP | b, x |
| UISS | b, z | |||||||
| SSIGN | b, x | |||||||
| 2008 [51] | CRP, Tumoral IL-6, COX-2 Expression & Recurrence Free Survival | 10mg/L | Retrospective | 60 | 60 | Resectable | CRP | b, x |
| TNM | b, x | |||||||
| 2009 [32] | Worst Grade Component And Recurrence | ≥10 mg/L | Retrospective | 50 | 314 | All Stages | CRP | c, x |
| 2010 [53] | Preoperative CRP and Metastasis | Continuous | Prospective | 85 | 130 | All Stages | Pre-operative CRP | —, z |
| SSIGN | d,— | |||||||
| 2010 [48] | Pre-operative CRP and Disease Free Survival | 15mg/L | Retrospective | 60 | 286 | All Stages | Log (CRP) | b, z |
| Stage | a, y | |||||||
| MVI (invasion) | b, z | |||||||
| 2010 [49] | Postoperative CRP to Predict Recurrence | Continuous | Prospective | 70 | 110 | Localized | Post-operative CRP | a, z |
| T-stage | d, z | |||||||
| 2011 [54] | CRP and Recurrence | 8mg/L | Retrospective | 55 | 177 | Resectable | CRP | b, x |
| Tumor size | b, y | |||||||
| 2011 [55] | Post-nephrectomy CRP, CRP Kinetics & Recurrence | 10mg/L | Retrospective | 55 | 263 | Resectable | High Pre-operative CRP | -/z |
| Non-normal CRP | —, z | |||||||
| MVI | —/— | |||||||
| Tumor Necrosis | —/- | |||||||
| 2011 [56] | Risk Factors for Metastasis | 4mg/L | Retrospective | 50 | 32 | Metastasis | CRP | b, y |
| Symptoms | c, z | |||||||
| Size | b, x | |||||||
| Histologic Grade | a, y | |||||||
| Sarcoma Component | d, z | |||||||
| MVI | a, x | |||||||
| 2011 [156] | Post-operative CRP, Pre-operative Albumin and Recurrence | 2mg/L | Retrospective | 50 | 40 | Resectable | Post-operative CRP | d, x |
* All CRP levels reported in results correspond to serum levels unless otherwise specified.
# Since CRP values are reported in different units, for uniformity purposes we converted all values to mg/L unless otherwise specified.
ϕ Strongest predictors by MVA were stratified by relative risk (RR) and statistical significances (p) as follows:
a RR<2 or >0.5
b RR>2 or <0.5
c RR>5 or <0.2
d RR>10 or <0.1
x p<0.05
y p<0.01
z p<0.001
—values not reported or no MVA
Abbreviations: ALP: Alkaline Phosphatase; APP: Acute Phase Protein(s); DFI: Disease Free Interval; DSS: Disease Specific Survival; Score; LDH: Lactate Dehydrogenase; LN: lymph Node(s); MP: Medroxyprogesterone; MSKCC: Memorial Sloan-Kettering Cancer Center; MVI: Micro vascular Invasion; MVA: Multivariate Analysis; RFS: Recurrence Free Survival; RR: Relative Risk; SSIGN: Stage Size Grade Necrosis; UISS: University of California Los Angeles Integrated Staging System; WCC: White Cell Count.
1.2. Treatment response: Thirteen of the 51 studies in renal cell carcinoma had treatment response and prognosis as a primary outcome [38, 40–51]. In 12 of the thirteen, CRP independently predicted both treatment response and prognosis. Six studies [40, 44, 48–51] investigated CRP and treatment response as a primary outcome. High CRP predicted treatment response in all except one [38]. This study was also underpowered, and the primary objective was not treatment response (Table 3). Treatment responses (ill-defined) were evaluated after resection of localized tumors and after cytokine based therapies (IL-2 infusions, IF-α) in metastatic RCC. Low CRP level was associated with better treatment responses overall in 11 of thirteen studies.
1.3. Tumor recurrence: Six of the 51 renal cell carcinoma studies [32, 52–56] investigated recurrence and survival as primary outcomes. In three studies, elevated CRP independently predicted both tumor recurrence and prognosis [54, 56] (Table 3). One of these [55] examined CRP kinetics (change in CRP over time) and identified non-normalization of postoperative CRP as a predictor of recurrence.
2. Gastrointestinal malignancies
Of 90 studies, 48 were in colorectal and 42 in esophageal, gastric or gastroesophageal cancers. In colorectal cancer, high CRP strongly predicted survival in 36 (75%) studies. High CRP was an independent prognostic indicator in most reports, 31 of 36 (65%). Only two [57, 58] were negative. In another ten studies [26, 59–66] CRP predicted prognosis by univariate analysis only; one of these was underpowered (Table 2, Fig 2). Elevated CRP independently predicted prognosis in thirty five of the 42 (71%) studies in gastroesophageal cancer.
2.1. Prognosis: Most studies (81 of 90) in gastrointestinal malignancies had survival as a study outcome. Eighty percent (65 of 81) investigated CRP and prognosis as the primary outcome. High CRP was an independent predictor of survival in 56% (45 of 81) and a strong predictor in 25% (20 of 81). It was a predictor on univariate analysis only in 20% (16 0f 81). In two studies [26, 67], CRP predicted prognosis (but not independently of disease stage). Once this was considered, in those two, CRP was not a statistically significant prognostic predictor (Table 4) [165–225].
Table 4. CRP as a Predictor of Prognosis, Treatment Outcome or Tumor Recurrence in Digestive Tumors.
| Publication Year (Reference) | Main Outcome | CRP cut-offs* (mg/L) # | Study Design | Quality Score (%) | Sample Size | Disease Extent | Strongest Predictors by MVA ϕ | |
|---|---|---|---|---|---|---|---|---|
| PROGNOSIS | ||||||||
| Colorectal | ||||||||
| 1998 [165] | Preoperative CRP and Clinicopathologic Factors | >8 | Prospective | 65 | 120 | All stages | CRP | — |
| 2000 [166] | PAI-1 and CRP Post-resection | >9.8 | Prospective | 70 | 594 | All stages | CRP | a, z |
| 2003 [167] | Pre-/postoperative CRP in Curative Resection | >10 | Prospective | 65 | 174 | Dukes’ A, B, C | CSS CRP | c, y |
| Dukes | c, x | |||||||
| Age | a, x | |||||||
| 2003 [168] | Deprivation, CRP in Curative Resection | >10 | Prospective | 65 | 174 | Dukes’ B, C | CSS: Age | b, z |
| Dukes’ | b, x | |||||||
| CRP | b, x | |||||||
| 2004 [169] | Perioperative APP; IL-1,6 network | ≥10 | Prospective | 60 | 75 | All stages | CRP | — |
| 2004 [170] | CRP in Potentially Curative Resection | >10 | Prospective | 65 | 147 | Duke's B, C | Dukes | c, z |
| CRP | b, z | |||||||
| Age | a, y | |||||||
| 2004 [171] | PH vs. Laparotomy Effects on Markers in Liver Metastasis | >2 | Prospective | 70 | 24 PH + 9 laparotomy | Liver metastasis | DFS: CRP | —, y |
| HGF | —, x | |||||||
| 2005 [172] | IL-6, TNFα, CRP in Local Resection | ≥7 | Prospective | 70 | 74 + 25 controls | All stages | Unclear: CRP | — |
| IL-6 | — | |||||||
| 2005 [173] | T-lymphocyte Infiltration + Preoperative CRP | >10 | Prospective | 60 | 147 | Dukes’ B, C | CSS: CRP | b, z |
| Stage | b, z | |||||||
| Age | b, y | |||||||
| 2006 [174] | Nutritional and Inflammatory Status in Palliative Treatment | >10 | Prospective | 60 | 51 | Advanced | PS | b, x |
| GPS | b, x | |||||||
| Treatment type | a, y | |||||||
| 2006 [175] | CRP in patients receiving adjuvant 5-FU Post- curative Resection | >10 | Prospective | 60 | 222 | Duke’s A, B, C | No adjuvant chemotherapy CRP | b, x |
| Age | a, x | |||||||
| Adjuvant chemotherapy CRP | c, x | |||||||
| 2007 [131] | GPS: Post Resection | >10 | Prospective | 75 | 316 | All stages | mGPS | a, y |
| Age | a, x | |||||||
| 2007 [176] | Ki-67 Expression, CRP and Survival | 10mg/L | Retrospective | 60 | 147 | Curative | CRP | b, z |
| Dukes | b, y | |||||||
| Age | a, y | |||||||
| 2007 [177] | mGPS and Prognosis | 10mg/L | Prospective | 70 | 233 | All Stages | mGPS | b, z |
| Platelet | b, x | |||||||
| Monocyte | b, y | |||||||
| Neutrophil | b, y | |||||||
| WBC | a, z | |||||||
| TNM | a, x | |||||||
| Age | a, y | |||||||
| 2007 [178] | Pre-operative Score for Prognosis With Liver Metastasis | 10mg/L | Prospective with Control | 75 | 560 | Resectable | IRT | a, z |
| Metastasis Number | b, x | |||||||
| 2007 [179] | GPS and Post operative Mortality Prediction | 10mg/L | Retrospective | 65 | 315 | All Stages | GPS | a, x |
| 2008 [180] | Preoperative and Perioperative CRP Levels and Prognosis | 5mg/L | Prospective | 80 | 212 | All Stages | CRP | c, x |
| Differerentiation | b, x | |||||||
| Stages | c, x | |||||||
| 2008 [181] | Preoperative CRP in CEA Independent Stage I or II CRC | 5mg/L | Retrospective | 60 | 300 | All Stages | CRP | a, x |
| 2008 [182] | Preoperative CRP and Prognosis | 5mg/L | Retrospective | 65 | 116 | All Stages | CRP | d, z |
| Stage | b, y | |||||||
| Poor Differentiation | b, x | |||||||
| 2008 [183] | Pre-treatment Levels of IL-6, CRP | 9.7mg/L | Retrospective with control | 65 | 76, C: 35 | All Stages | Tumor Residue | —/ y |
| CRP | —/ y | |||||||
| CA 19–9 | —/ x | |||||||
| 2008 [184] | Systemic Inflammatory Response (SIR); GPS; Gene Polymorphism | 10mg/L (GPS) | Prospective | 55 | 56 | Advanced | GPS: 1 | d, x |
| Albumin | c, y | |||||||
| Primary Site | c, x | |||||||
| 2009 [185] | Emergency (ER) Presentation, Preoperative mGPS and Survival | 10mg/L | Prospective | 70 | 188 | Curative | mGPS | b, x |
| Presentation, ER/Elective | b, x | |||||||
| 2009 [186] | Systemic Inflammatory Response (SIR) with Liver Metastasis | 10mg/L | Retrospective | 65 | 93 | Metastasis | CRP | b, x |
| Number of Tumors | b, x | |||||||
| Hepatectomy | b, x | |||||||
| Lung metastasis | b, x | |||||||
| 2009 [187] | Local (Klintrup and Jass score) v. Systemic Inflammatory Response (mGPS) and Prognosis | 10mg/L (mGPS) | Retrospective | 60 | 287 | Curative | mGPS | b, z |
| Dukes | b, x | |||||||
| Age | a, x | |||||||
| Klintrup | b, x | |||||||
| 2009 [188] | mGPS and Survival | 10mg/L (mGPS) | Retrospective | 60 | 112 | Unresectable | mGPS | c, y |
| 2010 [189] | Survival Predictors in Stage IV metastasis | <50, 50–150, >150 | Retrospective | 55 | 541 | Advanced | CRP | a, x |
| Chemotherapy | a, z | |||||||
| PS | b, z | |||||||
| Hb | a, z | |||||||
| Weight Loss | b, z | |||||||
| Anorexia | b, z | |||||||
| Fatigue | b, z | |||||||
| Blood Transfusion | b, z | |||||||
| 2010 [190] | Pre-resection GPS and Survival | 10mg/L (GPS) | Prospective | 65 | 63 | Metastasis | GPS | b, x |
| Liver metastasis | b, x | |||||||
| 2010 [191] | Obesity, Insulin Resistance, Inflammation, Angiogenesis and Survival | 4.1 | Prospective | 60 | 344 | All Stages | CRP | a |
| VEGF-A | a, x | |||||||
| Ang-2 | a, x | |||||||
| 2010 [192] | Systemic inflammatory Response Before Curative Resection and Survival | 10mg/L (mGPS) | Retrospective | 55 | 320 | All Stages | mGPS | a, z |
| Age | a, z | |||||||
| Smoking | a, x | |||||||
| Dukes | a, z | |||||||
| POSSUM | a, x | |||||||
| 2011 [193] | mGPS and Prognosis, Effect of Adjuvant Chemotherapy | 5mg/L (mGPS) | Retrospective | 55 | 219 | Specific Stages, Stage II and III | mGPS | c, y |
| Pathology | c, y | |||||||
| 2011 [194] | Hsp70, Acute Phase Proteins (CRP, C1 Inhibitor, C3, C9) and Prognosis | 4.7mg/L | Retrospective | 65 | 175 | All Stages | CRP | b, x |
| sHsp70 | a, x | |||||||
| 2011 [195] | Pre-operative Comorbidity, Systemic Inflammation and Survival | 10mg/L (mGPS) | Retrospective | 55 | 302 | All Stages | mGPS | a, z |
| Age | a, z | |||||||
| TNM | a, z | |||||||
| Peterson | a, y | |||||||
| ACE-27 | a, y | |||||||
| 2011 [63] | CRP & Prognosis: Peritoneal Carcinomatosis + CRC | 35mg/L, Other Cutoffs | Retrospective | 50 | 50 | Advanced | CRP | —/z |
| 2012 [196] | Preoperative Thrombocytosis and Survival After Surgery | Continuous | Retrospective | 55 | 453 | All Stages | CRP | a, x |
| CEA | a, x | |||||||
| Tumor Number | b, x | |||||||
| Platelet | a, x | |||||||
| 2012 [197] | GPS in Synchronous and Metachronous Liver Metastasis | 10mg/L (GPS) | Retrospective | 50 | 40 | All Stages | GPS 2 | c, y |
| CA19-9 | d, z | |||||||
| CEA | c, y | |||||||
| 2012 [198] | GPS and survival: Undergoing Curative Surgery | 10mg/L (GPS) | Retrospective | 55 | 366 | Specific Stages, TNM Stage II & III | GPS | b, z |
| LN Mets | a, z | |||||||
| Lymphatic Invasion | b, x | |||||||
| Invasion depth | b, y | |||||||
| Esophagus | ||||||||
| 2003 [199] | Clinical outcomes & Predictors Before Therapy | ≥ 5 | Retrospective | 60 | 356 | All stages | TNM | a, z |
| Weight Change | a, x | |||||||
| CRP | a, x | |||||||
| 2003 [200] | Clinicopathological & the Prognostic Value of Pre-operative CRP | 10mg/L | Retrospective | 60 | 150 | All Stages | CRP (low vs. high) | a, x |
| LN status | b, y | |||||||
| 2005 [201] | Pretreatment CRP in Chemo/radiation | ≥6 | Prospective | 65 | 67 + 20 controls | All stages | CRP | —, y |
| 2006 [202] | Preoperative CRP in Adeno- and Squamous Cell Carcinoma Post-Resection | ≥50 | Prospective | 60 | 291 | All stages | pT stage | —, z |
| CRP | a, x | |||||||
| R classification | —, x | |||||||
| Transthoracic approach | a, x | |||||||
| 2006 [203] | Clinico-pathological Status & Preop. CRP | >5 and >10 | Prospective | 70 | 120 | All stages | CRP>10 | b, z |
| LN metastases | b, z | |||||||
| 2008 [204] | GPS and Survival Prior nCRT | 10mg/L (GPS) | Retrospective | 70 | 48 | Specific Stages, Stage II and III | GPS | a, y |
| 2009 [205] | Biomarkers and Survival | <5mg/L, ≥ 5mg/L | Prospective | 65 | 123 | All Stages | CRP | d, z |
| Treatment | b, y | |||||||
| Albumin | b, z | |||||||
| 2010 [27] | nCRT Followed by Surgery | 8mg/L, 10mg/L | Prospective with Control | 70 | 90, C: 105 | Resectable | CRP | c, z |
| UICC | b, y | |||||||
| Radicality | c, z | |||||||
| 2010 [206] | GPS and Survival in Oesophageal Carcinoma (SCC) | 10mg/L | Prospective | 75 | 65 | Locally Advanced | GPS | a, y |
| LN Number | a, x | |||||||
| Curability | a, x | |||||||
| 2011 [207] | Locally Advanced Disease Undergoing Induction CRT | 3mg/L | Retrospective | 55 | 34 | Advanced | High CRP (After Chemotherapy) | -/ x |
| 2011 [208] | GPS in Homogenous Esophageal Cancer | 10mg/L (GPS) | Retrospective | 65 | 495 | esectable | SCC: GPS1 | a, z |
| GPS2 | b, y | |||||||
| Adeno: GPS1 | a, y | |||||||
| GPS2 | b, z | |||||||
| 2011 [209] | Inflammatory Markers Surgical Resection & Prognosis | 10mg/L mGPS | Retrospective | 55 | 112 | Resectable | + LN Ratio | b, z |
| mGPS | b, z | |||||||
| 2012 [210] | Local/Systemic Inflammatory Response & Survival | 10mg/L mGPS | Prospective | 60 | 121 | All Stages | mGPS | d, z |
| + LN ratio | b, z | |||||||
| CD68 (K-M Score) | a, x | |||||||
| 2012 [129] | CRP and Albumin & Risk stratification | 5 mg/L (Fuzzy Score) | Retrospective | 55 | 271 | All Stages | Fuzzy | a, y |
| BMI | a, z | |||||||
| Treatment | a, z | |||||||
| TNM Stage | b, z | |||||||
| 2012 [24] | Serum CRP and Histological Subtype | 5.75 mg/L | Prospective with control | 70 | 53 C:90 | All Stages | EC, CRP | -/ y |
| ESCC, CRP | -/ x | |||||||
| Gastro-esophageal | ||||||||
| 2006 [211] | IL-1β, IL-6, IL-8, TNF-α mRNA, Protein: Tumoral & Systemic Levels | >10 | Prospective | 70 | 56 + 22 controls | All stages | CRP | b, x |
| IL-1β infiltrate | — | |||||||
| 2006 [203] | GPS in Inoperable Cancer | >10 | Prospective | 60 | 258 | All stages | Active treatment, GPS | a, z |
| Stage TNM | a, z | |||||||
| Treatment | a, y | |||||||
| Supportive treatment Stage | a, x | |||||||
| 1982 [212] | Postoperative Survival and Pretreatment CEA, Albumin, CRP, ACT, AGP | >10 | Prospective | 55 | 104 | All stages | ACT | — |
| CRP | — | |||||||
| AGP | — | |||||||
| 2007 [108] | Factors Predictive of Death. Risk Prediction Model | 5mg/L | Prospective | 70 | 220 | All Stages | CRP | a, x |
| WL Rate | a, x | |||||||
| Karnofsky | b, y | |||||||
| Stage IV | c, z | |||||||
| 2008 [127] | GPS & ECOG-PS: Survival & Treatment Response | 10mg/L | Prospective | 60 | 65 | All Stages | GPS | a, z |
| 2010 [213] | Pre-treatment Clinical Prognostic Factors and Survival | 10mg/L (GPS) | Retrospective | 60 | 217 | All Stages | mGPS | b, z |
| TNM Stage | a, z | |||||||
| Position | a, z | |||||||
| Age | a, z | |||||||
| 2011 [214] | Tumor proliferation, Systemic Inflammatory Response and Survival | 10mg/L mGPS | Prospective | 60 | 100 | All Stages | mGPS | b, z |
| LN ratio | a, x | |||||||
| Tumor Differentiation | b, z | |||||||
| Klintrup | b, x | |||||||
| Ki-67 | a, x | |||||||
| Gastric | ||||||||
| 1983 [215] | Preoperative CEA, CRP, GGT, PHI, Pseudouridine, ACT, AAG | >20 | Prospective | 70 | 200 + 73 C | All stages | Gastric CRP | — |
| Colorectal Dukes | — | |||||||
| 2010 [102] | Preoperative CRP and Survival | 3mg/L | Prospective with controls | 80 | 170, C: 405 | Resectable | CRP | —/ y |
| 2010 [216] | Hypoalbuminemia, High CRP and Survival | ≤10, >10 | Retrospective | 60 | 217 | All Stages | Continuous CRP | b, z |
| Categorical CRP | b, z | |||||||
| Act. Pall. | b, z | |||||||
| TNM IV | b, z | |||||||
| 2011 [217] | Preoperative CRP | 5mg/L | Retrospective | 60 | 204 | Curative | Preoperative CRP | b, x |
| Tumor Stage | b, x | |||||||
| LN Invasion | b, x | |||||||
| 2011 [218] | GPS and Prognosis | 10mg/L TGPS, 5mg/L MGPS | Retrospective | 65 | 232 | Resectable | TGPS | b, x |
| Stage | d, z | |||||||
| MGPS | b, x | |||||||
| 2011 [219] | Peritoneal Dissemination and Prognosis | 20mg/L | Retrospective | 55 | 79 | Metastasis | CRP | b, y |
| Albumin | a, x | |||||||
| Ascites | a, x | |||||||
| ECOG PS | a, z | |||||||
| 2011 [97] | Clinical Status, Laboratory factors and Survival | 10mg/L GPS | Retrospective | 55 | 402 | Metastasis | GPS 1 | a, z |
| GPS 2 | a, z | |||||||
| ECOG PS | a, x | |||||||
| 2012 [220] | CRP & Potential Prognostic Factors | 10mg/L | Retrospective | 50 | 61 | Metastasis | CRP | b, y |
| Gender | b, y | |||||||
| 2012 [221] | mGPS and Prognosis | 10mg/L mGPS | Retrospective | 55 | 1710 | All Stages | mGPS | a, y |
| Tumor Stage | b, z | |||||||
| Age | a, y | |||||||
| 2012 [222] | GPS and Survival | 10mg/L GPS | Retrospective | 50 | 83 | Advanced | GPS | a, y |
| Age | b, y | |||||||
| 2012 [223] | NLR and mGPS in Advanced Stage | 10mg/LmGPS | Retrospective | 55 | 104 | Unresectable | mGPS1 | a, z |
| mGPS2 | a, y | |||||||
| NLR | a, x | |||||||
| LN Mets | a, y | |||||||
| 2012 [228] | GPS before curative surgery and survival | 10mg/L GPS | Retrospective | 55 | 366 | Specific stages: TNM Stage II & III | GPS | b, z |
| LN Metastasis | a, z | |||||||
| LN Invasion | b, x | |||||||
| Invasion Depth | b, y | |||||||
| 2012 [128] | Markers of Systemic Inflammatory Response and Prognosis | 10mg/L mGPS | Prospective | 60 | 120 | All Stages | mGPS | b, z |
| LN Ratio | b, z | |||||||
| Gastric + Colorectal | ||||||||
| 2000 [224] | Metastasis, KPS Anthropometry, Appetite, Blood Markers, and CRP | >10 | Prospective | 70 | 91 | Locally Advanced or Metastatic | CRP | —, z |
| KPS | —, y | |||||||
| Mets | —, x | |||||||
| Other Gastrointestinal | ||||||||
| 2004 [225] | Albumin, CRP | >10 | Retrospective | 60 | 165 | Advanced | GPS | —, z |
| Tumor type | —, y | |||||||
| Age | —, x | |||||||
| Tumor Recurrence | ||||||||
| Colorectal | ||||||||
| 1995 [226] | APR (CRP) | >5 | Prospective | 70 | 36 | Duke’s B/C | CRP | — |
| 2001 [227] | CEA, CA19-9 and CRP | >0.5 ng/ml | Prospective | 60 | 82 | Dukes’ A, B,C | CRP | — |
| CA 19–9 | — | |||||||
| 2007 [178] | Pre-operative Inflammatory Response Scoring System & Recurrence | 10mg/L | Prospective with Control | 75 | 560 | Resectable | IRT | a, z |
| Number of metastasis | b, x | |||||||
| Esophagus | ||||||||
| 2003 [228] | Outcomes Post-Recurrence | ≥10 | Prospective + Retrospective | 55 | 258 | All stages | S-p53-Abs | d, z |
| CRP | c, y | |||||||
| 2011 [208] | GPS and Recurrence in Homogenous Esophageal Cancer | 10mg/L GPS | Retrospective | 65 | 495 | Resectable | SCC: GPS1 | b, y |
| GPS2 | b, z | |||||||
| Adeno: GPS1 | a, x | |||||||
| GPS2 | b, z | |||||||
| Gastric | ||||||||
| 2011 [97] | Laboratory Factors and Progression | 10mg/L GPS | Retrospective | 55 | 402 | Metastasis | CRP | a, z |
| ECOG PS | a, x | |||||||
| Bone Metastasis | a, y | |||||||
| 2012 [220] | CRP and Gastric Cancer Progression | 10mg/L | Retrospective | 50 | 61 | Metastasis | CRP | —/ z |
| 2012 [229] | Inflammation Based Prognostic Score and Recurrence | 5mg/L | Retrospective | 60 | 197 | Locally Advanced | Inflammatory Score | a, x |
| TNM | d, z | |||||||
| Serous Invasion | a, x | |||||||
| 2012 [222] | GPS and Recurrence | 10mg/L GPS | Retrospective | 50 | 83 | Advanced | GPS | a, y |
| Gastric + Colorectal | ||||||||
| 2000 [224] | CRP, Metastasis, KPS and Blood Markers | >10 | Prospective | 70 | 91 | Locally Advanced or Metastatic | CRP | —, z |
| KPS | —, y | |||||||
| Mets | —, x | |||||||
| Treatment Response and/or Staging | ||||||||
| Colorectal | ||||||||
| 1995 [230] | Pre-treatment APP (4); Response to Immuno- chemotherapy | >10 | Prospective | 55 | 24 | Metastatic | CRP | — |
| Albumin | — | |||||||
| α1-AT | — | |||||||
| 2006 [175] | Adjuvant 5-FU Post Resection + Survival | >10 | Prospective | 60 | 222 | Dukes A, B, C | CRP | c, x |
| 2011 [193] | mGPS and Response in Potentially Curative Resection | 5 mg/L (mGPS) | Retrospective | 55 | 219 | Specific Stage: Stage II | mGPS | b, y |
| Pathology | b, x | |||||||
| Esophagus | ||||||||
| 2005 [201] | Pretreatment CRP in CRT + Survival | ≥6 | Prospective | 65 | 67 + 20 controls | All stages | CRP | — |
| 2011 [207] | Locally Advanced Disease Under Induction CRT | 3mg/L | Retrospective | 55 | 34 | Advanced | CRP (Post CRT) | -/y |
| Gastro-esophageal | ||||||||
| 2008 [127] | GPS, ECOG-PS & Clinical Response | 10mg/L | Prospective | 60 | 65 | All Stages | GPS | —/x |
Notes: (86, 89) are survival studies where treatment response was also an outcome.
* All CRP levels reported in results correspond to serum levels unless otherwise specified.
# Since CRP values are reported in different units, for uniformity purposes we converted all values to mg/L unless otherwise specified.
ϕ Strongest predictors by MVA were stratified by relative risk (RR) and statistical significances (p) as follows:
a RR<2 or >0.5
b RR>2 or <0.5
c RR>5 or <0.2
d RR>10 or <0.1
x p<0.05
y p<0.01
z p<0.001
—Values not reported or no MVA
Abbreviations: AAG: α1 acid glycoprotein; α1-AT: α1 Antitryspsin; ACE-27: Adult Comorbidity Evaluation-27: ACT: α1 Antichymotryspsin; ALP: Alkaline Phosphatase; APP: Acute Phase Protein(s); CEA: Carcinoembryonic Antigen; CRT: Chemoradiotherapy; FU: Fluorouracil: GGT: Gamma Glutamyl Transferase; GPS: Glagcow Prognostic Score; HGF: Hepatocyte Growth Factor; HsP: Heat Shock Protein; IAP: Immunosuppressive Acid Protein; IL: Interleukin; LN: Lymph Node; MVA: Multivariate Analysis; PAI: Plasminogen Activator Inhibitor-1; PH: Partial Hepatectomy; PHI: Phosphohexose Isomerase; PS: Performance Status; RR: Relative Risk; SCC: Squamous Cell Carcinoma; TNF: Tumor Necrosis Factor; ↑: Increase; ↓: Decrease.
2.2. Treatment response or tumor stage: CRP predicted treatment response in six studies [127, 175, 193, 201, 207, 230] (Table 4). It did not predict stage in one study [57] but this was underpowered (Table 4). Treatment responses were evaluated after curative resection followed by adjuvant 5-Flurouracil (5-FU) in localized GI tumors. Responses after neo-adjuvant chemotherapy, chemo-radiotherapy, and IL-2 infusions (with either 5FU or surgery) were observed in advanced tumors. In 4 of the five studies, high CRP level was associated with poorer responses.
2.3. Tumor recurrence: Ten of the 90 GI studies investigated recurrence as a primary outcome. In six of the ten, high CRP independently predicted recurrence. One study did not [59]; it included both retrospective and prospective cohorts. Furthermore, CRP prediction of recurrence was not the main outcome [226–230] (Table 4). High CRP was a strong predictor of recurrence in the rest of the other studies.
3. Other Solid Tumors
24 studies (each) investigated CRP and prognosis in pancreatic and lung cancer. CRP predicted prognosis in 23 of 24 (96%) studies in pancreatic cancer [68–71], 22 of 24 (92%) in lung cancer [25, 72–74], all 10 in hepatocellular carcinoma (HCC) [75–77], all 5 in melanoma [23, 78], 4 of 7 (57%) in breast cancer [79, 80], 12 of 12 (100%) in bladder cancer [81–83], 7 of 9 (78%) in prostate cancer [84–86] and 21 of 24 (88%) others (cervical cancer, ovarian cancer, bone and soft tissue etc.) [87–91]. 14 of 15 (93%) studies of heterogeneous cancers found high CRP to be a predictor of prognosis [92–94] (Table 2, Fig 2).
CRP and prognosis by univariate analysis
CRP as a prognostic indicator was investigated as the primary outcome in most of these studies. Eighteen percent of all studies (48 of 271) found CRP prognostic only by univariate analysis. The forty eight consisted of 12 in renal cell carcinoma; 10 in colorectal cancer; 6 in gastroesophageal; 7 in pancreas; 2 each in lung and bladder; 2 in heterogeneous groups; 1 in hepatocellular cancer and 6 in others (ovarian, primary bone and soft tissue cancers, oral squamous cell carcinoma, hepatocellular carcinoma and malignant histiocytoma) [231–246] (S3 Appendix). The median sample size was one hundred fifty five (range 38–9608). Thirty included various disease stages, and another 18 advanced, or metastatic/recurrent disease [59, 63, 66, 95–101]. One had an adequate quality score [102], forty three intermediate. 4 were underpowered [39, 59, 95, 103].
Negative studies
Overall, CRP was not prognostic in 26 of 271 studies (17 prospective, 9 retrospective) (S4 Appendix). These included 9 in digestive tumors; 5 in renal cell carcinoma; 3 in breast; 2 each in lung and prostate; 1 in pancreas; 1 in heterogeneous and 3 in other cancers patients. Median sample size was one hundred thirty eight (range 31–329). 15 of the 26 included various disease stages [57, 58, 65, 80, 104–110]. The others were resectable/unresectable or advanced/locally advanced and/or metastatic disease [38, 111–113]. Although all had intermediate quality scores, three were also underpowered [38, 57, 112]. In most negative studies, CRP as a prognostic indicator was not the primary outcome measure [247–255] (S4 Appendix).
Additional parameters used for prognosis
CRP was used alone in 6% (15 of 271). Many studies considered more than one parameter for prognostic purpose. Demographic characteristics (age, gender, sex) were included as prognostic parameters in 66% of studies (170/256). Common clinicopathologic parameters included with CRP were: stage (TNM, Dukes, others) 23% (59/256); metastasis (lymph node, liver, others) 17%; performance status (ECOG, KPS, others) 16%; tumor characteristics (histology, site, diameter, size) 16%; WBC 13%. Biochemical parameters used with CRP (specifically in renal cell carcinoma) were: albumin (alone or as hypoalbuminemia), LDH, and interleukins (IL-6, IL-8, IL-2). In digestive tumors common biochemical parameters used were: albumin (alone or hypoalbuminemia), carcinoembryonic antigen (CEA), cancer antigen 19–9 (CA19-9) and interleukins (IL-6, IL-8, IL-2).
Discussion
Summary of evidence
Efforts to improve prognostication in cancer had limited success [114]. The number of cancer prognostic biomarkers validated as clinically useful is small, despite extensive research [115, 116]. Many studies have been underpowered. These studies are also difficult to interpret and compare because of heterogeneous study designs. This has prevented meta-analyses of prognostic biologic markers [4, 117]. We encountered this same difficulty during this systematic review.
Although thirty four percent of the studies (92 of 271) used an elevated CRP cut-off point of >10mg/L, the rest varied. The cut-off value was not reported at all in twenty one studies, and simply as present/absent, or positive/negative in others. Reported cut-off values extended over a wide range: 0.5ng/ml, 1ng/ml, >94nmol/L, >2mg/L, >5mg/L, >8mg/L, > 11 or 12 mg/L, > 35 or 50 mg/L. This made meaningful study comparisons difficult. We tried to standardize if not, cut-off values, then at least the units used. All the studies (except one of the high sensitivity CRP) used CRP. Most (>90%) of CRP levels were reported either in milligram per liter or milligram per deciliter (mg/L or mg/dL).
High sensitivity CRP (hs-CRP), tumoral CRP and CRP kinetics have also been utilized for disease progression and prognosis. Increased hs-CRP has been associated with late recurrence in renal cell carcinoma [119] and with increased mortality in breast cancer [79] and in men with lung cancer [118]. Tumoral CRP (increased locally within the tumor) may be superior to serum CRP for prognosis and recurrence [120]. Determined by CRP gene expression, tumoral CRP values are more personalized and rather a target for individualized therapy [121]. CRP kinetics may predict survival [122], recurrence [55] and clinical course [123] in cancer. Human CRP gene is located on the chromosome 1q21-23, spans 1.9 kb and has two exons. CRP gene polymorphism has been associated with increased cancer risk and worse prognosis, mainly in colorectal cancer [124, 125].
Various prognostic scoring systems and instruments have been developed utilizing CRP along with other clinical parameters. Prognostic Inflammatory Nutritional Index, PINI (CRP, Alpha-1 Acid Glycoprotein, albumin and prealbumin) [126]; Glasgow Prognostic Scale or Modified Glasgow Prognostic Scale (CRP, albumin); [50, 127, 128] Fuzzy Logic Based Prognostic Score (CRP and albumin) [129]; Biomarker Based Score (CRP, albumin, Gamma- Glutamyl Transferase (GGT) and HDL) [130]. GPS/mGPS and Fuzzy score only differ by CRP cutoffs. We included studies which utilized the Glasgow Prognostic Score or modified Glasgow Prognostic Score, as identified by the search criteria. We have not included studies which utilized Fuzzy score except for discussion purpose. The dominant biochemical component in both GPS and mGPS is CRP [131, 132]. One study defined mGPS as an Inflammation Based Index (IBI) and utilized it as a validated prognostic index for HCC [76].
CRP is a non-specific marker of inflammation. It can be elevated for many reasons: infection, invasive procedures, or medications [133, 134]. Inadequate screening for known non-cancer CRP-modifying factors may have significantly influenced values. In addition, it is accepted that sensitivity, specificity, positive and negative predictive values should be used to validate and compare any test against a gold standard [135]. Only two studies reported this data.
Inflammatory cells are tumor promoters. They produce an attractive environment for tumor growth, induce DNA damage, promote angiogenesis, and favor neoplastic spread and metastasis [92], and so may affect prognosis [17]. Several explanations exist for the proposed relationship between inflammation and the natural history of cancer. First, tumor growth itself can cause inflammation of surrounding tissue and increase plasma CRP [136]. Second, tumor cells produce various cytokines and chemokines that attract leukocytes. Some cancer cells express CRP and secrete interleukin-6 and interleukin-8, which stimulate liver CRP production [14, 136]. Studies have also shown that IL-6 blocks p-53 induced apoptosis. CRP-positivity develops a favorable microenvironment for the tumor cells through acute inflammatory cytokine network system maintenance [73]. Finally, CRP may be part of the host tumor immune response [136]. Evidence also suggests a causal role for chronic inflammation in several malignancies [14, 136, 137].
Cytokines and their surrogate markers (like CRP and IL-6 receptor) can be elevated both locally and systemically in different solid tumors. In renal cell carcinoma, the imbalance between pro-inflammatory cytokines and their anti-inflammatory counterpart is the therapeutic rationale behind immunotherapy [51, 138]. Colorectal cancer seems linked to chronic inflammation (both local and systemic) from genesis to progression [139]. Similar observations have been made in pancreatic [16] and lung cancers [140]. Those tumors are also highly associated with the cancer anorexia-cachexia syndrome, which itself may in part be due to inflammation [141].
The role of CRP as a prognostic marker for cardiovascular risk is widely known. Although studies have included large sample sizes, some skepticism still exists [12, 133]. It is noteworthy that a recently published study of 270,000 hospital patients, showed that high CRP levels not only predicted all-cause mortality (compared to the low/or normal CRP group), but also higher cancer mortality [142]. This study was retrospective and may have suffered from selection bias; those who had CRP measured were sicker and so had a higher risk of death.
In our review, most studies (over half) which met inclusion and quality criteria were in gastrointestinal and renal cell carcinoma. We were surprised not to see more investigations in lung and pancreatic cancer, since they are often considered clinically to have an inflammatory component. This was perhaps influenced by publication bias and selective reporting, i.e. positive studies published while some negative studies may not even be submitted for publication [143].
In our review, CRP appeared to be a valuable prognostic predictor particularly in digestive tumors and renal cell carcinoma. It may also help predict tumor recurrence and treatment response in those diseases. CRP was compared to other clinical and biochemical factors in these tumors. In renal cell carcinoma, grade, TNM staging, albumin and lactate dehydrogenase (LDH), were among the strongest prognostic predictors by multivariate analysis. Age, Dukes’ stage, albumin, carcinoembryonic antigen (CEA) and the Glasgow Prognostic Score were amongst those in gastrointestinal tumors.
Does CRP add any extra information to these other predictors? CRP can be easily and reliably measured. However, it is a non-specific marker. Levels can rise for numerous reasons independent of the cancer; this also reduces the value of single versus serial CRP measurements. Longitudinal studies of CRP values were largely absent. Since CRP cut-off points differed among studies, and the sensitivity and specificity comparisons with different prognostic variables were unreported, it was impossible to conclude with certainty whether CRP was a better predictor than others. In the negative studies, the role of CRP as a prognostic predictor was not the primary outcome and most were underpowered to detect a difference.
Limitations
This review had several limitations. Survival and treatment outcomes in the literature were defined and reported inconsistently. Identification of studies depended on CRP being indexed, so we may have been more likely to identify positive studies. Quality assessment was conducted with no cross-validation. The QA system had been piloted on 10 studies picked randomly before the review. This showed it could distinguish between studies in the three QA categories (see Appendices). No meta-analysis or direct study comparisons were done because of the methodological issues described. For similar reasons side by side study comparisons were not possible.
Conclusions
Increased CRP level predicted prognosis in most (90%) of the studies in solid tumors which met inclusion and quality criteria identified in this systematic review. More than half of all studies (52%) were in gastrointestinal malignancies or renal cell carcinoma. High CRP predicted prognosis in most reports (90%) in these two tumor groups. In addition CRP predicted prognosis in most reports in other solid tumors, so it may also be a clinically useful predictor in lung, pancreas, hepatocellular, and bladder cancers. CRP appeared to be a valuable (and probably under-recognized) prognostic predictor in these tumors. It may also have a role in determining treatment response, and tumor recurrence. The balance of evidence supports wider (and perhaps routine) use of CRP by oncologists for staging, assessment of tumor response and prognostication in at least these two tumor types. These conclusions and recommendations must be tempered by the intermediate quality of most studies.
Despite some methodological issues, CRP appears valuable to help predict prognosis and other important clinical outcomes in many solid tumors. Better quality prospective longitudinal studies on the role of CRP as a prognostic indicator are needed to confirm these observations.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLS)
(XLS)
(DOC)
Acknowledgments
We would like to thank our librarians, Jodith Janes, MSLS, AHIP and Woody Lorelei, MLIS for their help and advice to construct the search for this systematic review. Also, we are grateful to Dr. Paul Glare for his advice and opinion regarding this review.
Data Availability
All data are fully available without restriction. Data files uploaded as supporting information files (zipped).
Funding Statement
No external funding was utilized to conduct this systematic review.
References
- 1. Cancer Facts and Figures 2012. American Cancer Society. 2012.
- 2. Christakis NA, Lamont EB. Extent and determinants of error in physicians' prognoses in terminally ill patients: prospective cohort study. West J Med. 2000. May;172(5):310–3. . Pubmed Central PMCID: 1070876. Epub 2008/08/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Glare PA, Sinclair CT. Palliative medicine review: prognostication. J Palliat Med. 2008. Jan-Feb;11(1):84–103. Epub 2008/03/29. eng. 10.1089/jpm.2008.9992 [DOI] [PubMed] [Google Scholar]
- 4. McShane LM, Altman DG, Sauerbrei W. Identification of clinically useful cancer prognostic factors: what are we missing? J Natl Cancer Inst. 2005. July 20;97(14):1023–5. . Epub 2005/07/21. eng. [DOI] [PubMed] [Google Scholar]
- 5. Morley JJ, Kushner I. Serum C-reactive protein levels in disease. Ann N Y Acad Sci. 1982;389:406–18. . Epub 1982/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 6. Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004. November 19;279(47):48487–90. . Epub 2004/09/01. eng. [DOI] [PubMed] [Google Scholar]
- 7. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003. June;111(12):1805–12. . Pubmed Central PMCID: 161431. Epub 2003/06/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whiteside T, editor. Cytokines in Human Health. Totowa, New Jersey: Humana Press Inc.; 2007. [Google Scholar]
- 9. Emery P, Gabay C, Kraan M, Gomez-Reino J. Evidence-based review of biologic markers as indicators of disease progression and remission in rheumatoid arthritis. Rheumatol Int. 2007. July;27(9):793–806. . Epub 2007/05/17. eng. [DOI] [PubMed] [Google Scholar]
- 10. Shishehbor MH, Bhatt DL, Topol EJ. Using C-reactive protein to assess cardiovascular disease risk. Cleve Clin J Med. 2003. July;70(7):634–40. . Epub 2003/07/29. eng. [DOI] [PubMed] [Google Scholar]
- 11. Koenig W, Lowel H, Baumert J, Meisinger C. C-reactive protein modulates risk prediction based on the Framingham Score: implications for future risk assessment: results from a large cohort study in southern Germany. Circulation. 2004. March 23;109(11):1349–53. . Epub 2004/03/17. eng. [DOI] [PubMed] [Google Scholar]
- 12. Kushner I, Sehgal AR. Is high-sensitivity C-reactive protein an effective screening test for cardiovascular risk? Arch Intern Med. 2002. April 22;162(8):867–9. . Epub 2002/04/23. eng. [DOI] [PubMed] [Google Scholar]
- 13. Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006. November 30;72(11):1605–21. . Epub 2006/08/08. eng. [DOI] [PubMed] [Google Scholar]
- 14. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002. December 19–26;420(6917):860–7. . Pubmed Central PMCID: 2803035. Epub 2002/12/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003. June 1;97(11):2919–25. . Epub 2003/05/27. eng. [DOI] [PubMed] [Google Scholar]
- 16. Barber MD F K, Ross JA. Relationship of serum levels of interleukin-6, soluble interleukin-6 receptor and tumor necrosis factor receptors to the acute-phase protein response in advanced pancreatic cancer. Clin Sci (Lond). 1999;96:83–7. [PubMed] [Google Scholar]
- 17. Mahmoud FA, Rivera NI. The role of C-reactive protein as a prognostic indicator in advanced cancer. Curr Oncol Rep. 2002. May;4(3):250–5. . Epub 2002/04/09. eng. [DOI] [PubMed] [Google Scholar]
- 18. Khan K, ter Riet G, Popay J, Nixon J, Kleijen J. Conducting a review, stage II: PHASE 5: study quality assessment. Undertaking systematic reviews of research on effectiveness: CRD’s guidance for those carrying out or commissioning reviews (CRD Report 4, 2nd ed) Centre for Reviews and Dissemination. 2001:5–20. [Google Scholar]
- 19. Lau F, Cloutier-Fisher D, Kuziemsky C, Black F, Downing M, Borycki E, et al. A systematic review of prognostic tools for estimating survival time in palliative care. J Palliat Care. 2007. Summer;23(2):93–112. . Epub 2007/09/15. eng. [PubMed] [Google Scholar]
- 20. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009. April;42(2):377–81. Pubmed Central PMCID: 2700030. Epub 2008/10/22. eng. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International journal of surgery (London, England). 2010;8(5):336–41. . Epub 2010/02/23. eng. [DOI] [PubMed] [Google Scholar]
- 22. Deichmann M, Benner A, Waldmann V, Bock M, Jackel A, Naher H. Interleukin-6 and its surrogate C-reactive protein are useful serum markers for monitoring metastasized malignant melanoma. J Exp Clin Cancer Res. 2000. September;19(3):301–7. Epub 2001/01/06. eng. [PubMed] [Google Scholar]
- 23. Deichmann M, Kahle B, Moser K, Wacker J, Wust K. Diagnosing melanoma patients entering American Joint Committee on Cancer stage IV, C-reactive protein in serum is superior to lactate dehydrogenase. Br J Cancer. 2004. August 16;91(4):699–702. . Pubmed Central PMCID: 2364774. Epub 2004/07/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lukaszewicz-Zajac M, Mroczko B, Kozlowski M, Niklinski J, Laudanski J, Siewko M, et al. Comparative evaluation of serum C-reactive protein (CRP) levels in the different histological subtypes of esophageal cancer (squamous cell carcinoma and adenocarcinoma of esophagus). J Clin Lab Anal. 2012. February;26(2):73–81. Epub 2012/04/03. eng. 10.1002/jcla.21486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leung EY, Scott HR, McMillan DC. Clinical utility of the pretreatment glasgow prognostic score in patients with advanced inoperable non-small cell lung cancer. J Thorac Oncol. 2012. April;7(4):655–62. Epub 2012/03/20. eng. 10.1097/JTO.0b013e318244ffe1 [DOI] [PubMed] [Google Scholar]
- 26. Wigmore SJ, McMahon AJ, Sturgeon CM, Fearon KC. Acute-phase protein response, survival and tumour recurrence in patients with colorectal cancer. Br J Surg. 2001. February;88(2):255–60. . Epub 2001/02/13. eng. [DOI] [PubMed] [Google Scholar]
- 27. Zingg U, Forberger J, Rajcic B, Langton C, Jamieson GG. Association of C-reactive protein levels and long-term survival after neoadjuvant therapy and esophagectomy for esophageal cancer. J Gastrointest Surg. 2010. March;14(3):462–9. Epub 2009/11/26. eng. 10.1007/s11605-009-1113-2 [DOI] [PubMed] [Google Scholar]
- 28. Ljungberg B, Grankvist K, Rasmuson T. Serum acute phase reactants and prognosis in renal cell carcinoma. Cancer. 1995. October 15;76(8):1435–9. . Epub 1995/10/15. eng. [DOI] [PubMed] [Google Scholar]
- 29. Masuda H, Kurita Y, Suzuki A, Kanbayashi T, Suzuki K, Fujita K. Prognostic factors for renal cell carcinoma: a multivariate analysis of 320 cases. Int J Urol. 1997. May;4(3):247–53. . Epub 1997/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 30. Inoue T, Hashimura T, Iwamura H, Takahashi T, Segawa T, Kakehi Y, et al. Multivariate analysis of prognostic determinants after surgery for renal cell carcinoma at Himeji National Hospital. Hinyokika Kiyo. 2000. April;46(4):229–34. . Epub 2000/06/09. eng. [PubMed] [Google Scholar]
- 31. Miyata Y, Koga S, Nishikido M, Noguchi M, Kanda S, Hayashi T, et al. Predictive values of acute phase reactants, basic fetoprotein, and immunosuppressive acidic protein for staging and survival in renal cell carcinoma. Urology. 2001. August;58(2):161–4. . Epub 2001/08/08. eng. [DOI] [PubMed] [Google Scholar]
- 32. Ito K, Yoshii H, Asakuma J, Sato A, Horiguchi A, Sumitomo M, et al. Clinical impact of the presence of the worst nucleolar grade in renal cell carcinoma specimens. Jpn J Clin Oncol. 2009. September;39(9):588–94. Epub 2009/06/27. eng. 10.1093/jjco/hyp068 [DOI] [PubMed] [Google Scholar]
- 33. Tanaka M, Fujimoto K, Okajima E, Tanaka N, Yoshida K, Hirao Y. Prognostic factors of renal cell carcinoma with extension into inferior vena cava. Int J Urol. 2008. May;15(5):394–8. Epub 2008/05/03. eng. 10.1111/j.1442-2042.2008.02017.x [DOI] [PubMed] [Google Scholar]
- 34. Wood SL, Rogers M, Cairns DA, Paul A, Thompson D, Vasudev NS, et al. Association of serum amyloid A protein and peptide fragments with prognosis in renal cancer. Br J Cancer. 2010. June 29;103(1):101–11. Pubmed Central PMCID: 2905280. Epub 2010/06/10. eng. 10.1038/sj.bjc.6605720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Falkensammer CE, Thurnher M, Leonhartsberger N, Ramoner R. C-reactive protein is a strong predictor for anaemia in renal cell carcinoma: role of IL-6 in overall survival. BJU Int. 2011. June;107(12):1893–8. Epub 2010/11/13. eng. 10.1111/j.1464-410X.2010.09817.x [DOI] [PubMed] [Google Scholar]
- 36. Johnson TV, Ali S, Abbasi A, Kucuk O, Harris WB, Ogan K, et al. Intratumor C-reactive protein as a biomarker of prognosis in localized renal cell carcinoma. J Urol. 2011. October;186(4):1213–7. Epub 2011/08/19. eng. 10.1016/j.juro.2011.06.014 [DOI] [PubMed] [Google Scholar]
- 37. Michigan A, Johnson TV, Master VA. Preoperative C-reactive protein level adjusted for comorbidities and lifestyle factors predicts overall mortality in localized renal cell carcinoma. Mol Diagn Ther. 2011. August 1;15(4):229–34. . Epub 2011/07/16. eng. [DOI] [PubMed] [Google Scholar]
- 38. Naglieri E, Lopez M, Lelli G, Morelli F, Amodio A, Di Tonno P, et al. Interleukin-2, interferon-alpha and medroxyprogesterone acetate in metastatic renal cell carcinoma. Anticancer Res. 2002. Sep-Oct;22(5):3045–51. . Epub 2003/01/18. eng. [PubMed] [Google Scholar]
- 39. Inoue A, Kunitoh H, Sekine I, Sumi M, Tokuuye K, Saijo N. Radiation pneumonitis in lung cancer patients: a retrospective study of risk factors and the long-term prognosis. Int J Radiat Oncol Biol Phys. 2001. March 1;49(3):649–55. . Epub 2001/02/15. eng. [DOI] [PubMed] [Google Scholar]
- 40. Broom J, Heys SD, Whiting PH, Park KG, Strachan A, Rothnie I, et al. Interleukin 2 therapy in cancer: identification of responders. Br J Cancer. 1992. December;66(6):1185–7. . Pubmed Central PMCID: 1978053. Epub 1992/12/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blay JY, Negrier S, Combaret V, Attali S, Goillot E, Merrouche Y, et al. Serum level of interleukin 6 as a prognosis factor in metastatic renal cell carcinoma. Cancer Res. 1992. June 15;52(12):3317–22. . Epub 1992/06/15. eng. [PubMed] [Google Scholar]
- 42. Fujikawa K, Matsui Y, Oka H, Fukuzawa S, Takeuchi H. Serum C-reactive protein level and the impact of cytoreductive surgery in patients with metastatic renal cell carcinoma. J Urol. 1999. December;162(6):1934–7. . Epub 1999/11/24. eng. [DOI] [PubMed] [Google Scholar]
- 43. Atzpodien J, Royston P, Wandert T, Reitz M. Metastatic renal carcinoma comprehensive prognostic system. Br J Cancer. 2003. February 10;88(3):348–53. . Pubmed Central PMCID: 2747541. Epub 2003/02/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bromwich E, McMillan DC, Lamb GW, Vasey PA, Aitchison M. The systemic inflammatory response, performance status and survival in patients undergoing alpha-interferon treatment for advanced renal cancer. Br J Cancer. 2004. October 4;91(7):1236–8. . Pubmed Central PMCID: 2409897. Epub 2004/09/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Casamassima A, Picciariello M, Quaranta M, Berardino R, Ranieri C, Paradiso A, et al. C-reactive protein: a biomarker of survival in patients with metastatic renal cell carcinoma treated with subcutaneous interleukin-2 based immunotherapy. J Urol. 2005. January;173(1):52–5. . Epub 2004/12/14. eng. [DOI] [PubMed] [Google Scholar]
- 46. Peccatori J, Barkholt L, Demirer T, Sormani MP, Bruzzi P, Ciceri F, et al. Prognostic factors for survival in patients with advanced renal cell carcinoma undergoing nonmyeloablative allogeneic stem cell transplantation. Cancer. 2005. November 15;104(10):2099–103. . Epub 2005/10/13. eng. [DOI] [PubMed] [Google Scholar]
- 47. Vogl UM, Zehetgruber H, Dominkus M, Hejna M, Zielinski CC, Haitel A, et al. Prognostic factors in metastatic renal cell carcinoma: metastasectomy as independent prognostic variable. Br J Cancer. 2006. September 18;95(6):691–8. . Pubmed Central PMCID: 2360513. Epub 2006/08/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jagdev SP, Gregory W, Vasudev NS, Harnden P, Sim S, Thompson D, et al. Improving the accuracy of pre-operative survival prediction in renal cell carcinoma with C-reactive protein. Br J Cancer. 2010. November 23;103(11):1649–56. Pubmed Central PMCID: 2994232. Epub 2010/11/11. eng. 10.1038/sj.bjc.6605973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Johnson TV, Abbasi A, Owen-Smith A, Young AN, Kucuk O, Harris WB, et al. Postoperative better than preoperative C-reactive protein at predicting outcome after potentially curative nephrectomy for renal cell carcinoma. Urology. 2010. September;76(3):766 e1–5. . Epub 2010/04/17. eng. [DOI] [PubMed] [Google Scholar]
- 50. Ramsey S, Lamb GW, Aitchison M, McMillan DC. Prospective study of the relationship between the systemic inflammatory response, prognostic scoring systems and relapse-free and cancer-specific survival in patients undergoing potentially curative resection for renal cancer. BJU Int. 2008. April;101(8):959–63. Epub 2008/01/15. eng. 10.1111/j.1464-410X.2007.07363.x [DOI] [PubMed] [Google Scholar]
- 51. Lamb GW, McArdle PA, Ramsey S, McNichol AM, Edwards J, Aitchison M, et al. The relationship between the local and systemic inflammatory responses and survival in patients undergoing resection for localized renal cancer. BJU Int. 2008. September;102(6):756–61. Epub 2008/04/04. eng. 10.1111/j.1464-410X.2008.07666.x [DOI] [PubMed] [Google Scholar]
- 52. Ito K, Asano T, Yoshii H, Satoh A, Sumitomo M, Hayakawa M. Impact of thrombocytosis and C-reactive protein elevation on the prognosis for patients with renal cell carcinoma. Int J Urol. 2006. November;13(11):1365–70. . Epub 2006/11/07. eng. [DOI] [PubMed] [Google Scholar]
- 53. Johnson TV, Abbasi A, Owen-Smith A, Young A, Ogan K, Pattaras J, et al. Absolute preoperative C-reactive protein predicts metastasis and mortality in the first year following potentially curative nephrectomy for clear cell renal cell carcinoma. J Urol. 2010. February;183(2):480–5. Epub 2009/12/17. eng. 10.1016/j.juro.2009.10.014 [DOI] [PubMed] [Google Scholar]
- 54. Cho DS, Kim SJ, Lee SH, Ahn HS, Kim YS, Kim SI. Prognostic significance of preoperative C-reactive protein elevation and thrombocytosis in patients with non-metastatic renal cell carcinoma. Korean J Urol. 2011. February;52(2):104–9. Pubmed Central PMCID: 3045714. Epub 2011/03/08. eng. 10.4111/kju.2011.52.2.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ito K, Yoshii H, Sato A, Kuroda K, Asakuma J, Horiguchi A, et al. Impact of postoperative C-reactive protein level on recurrence and prognosis in patients with N0M0 clear cell renal cell carcinoma. J Urol. 2011. August;186(2):430–5. Epub 2011/06/18. eng. 10.1016/j.juro.2011.03.113 [DOI] [PubMed] [Google Scholar]
- 56. Takayama T, Sugiyama T, Kai F, Suzuki T, Nagata M, Imanishi T, et al. Characteristics of aggressive variants in T1a renal cell carcinoma. J Cancer Res Clin Oncol. 2011. November;137(11):1653–9. Epub 2011/08/30. eng. 10.1007/s00432-011-1040-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yuceyar S, Erturk S, Dirican A, Cengiz A, Saner H. The role of acute-phase reactant proteins, carcinoembryonic antigen and CA 19–9 as a marker in the preoperative staging of colorectal cancer: a prospective clinical study. Int Surg. 1996. Apr-Jun;81(2):136–9. . Epub 1996/04/01. eng. [PubMed] [Google Scholar]
- 58. Kwon KA, Kim SH, Oh SY, Lee S, Han JY, Kim KH, et al. Clinical significance of preoperative serum vascular endothelial growth factor, interleukin-6, and C-reactive protein level in colorectal cancer. BMC Cancer. 2010;10:203 Pubmed Central PMCID: 2886042. Epub 2010/05/15. eng. 10.1186/1471-2407-10-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hannisdal E, Tveit KM, Theodorsen L, Host H. Host markers and prognosis in recurrent rectal carcinomas treated with radiotherapy. Acta Oncol. 1994;33(4):415–21. . Epub 1994/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 60. Chung YC, Chang YF. Significance of inflammatory cytokines in the progression of colorectal cancer. Hepatogastroenterology. 2003. Nov-Dec;50(54):1910–3. . Epub 2003/12/31. eng. [PubMed] [Google Scholar]
- 61. Crozier JE, McKee RF, McArdle CS, Angerson WJ, Anderson JH, Horgan PG, et al. Preoperative but not postoperative systemic inflammatory response correlates with survival in colorectal cancer. Br J Surg. 2007. August;94(8):1028–32. . Epub 2007/04/18. eng. [DOI] [PubMed] [Google Scholar]
- 62. Crozier JE, McMillan DC, McArdle CS, Angerson WJ, Anderson JH, Horgan PG, et al. Tumor size is associated with the systemic inflammatory response but not survival in patients with primary operable colorectal cancer. J Gastroenterol Hepatol. 2007. December;22(12):2288–91. . Epub 2007/11/23. eng. [DOI] [PubMed] [Google Scholar]
- 63. van de Poll MC, Klaver YL, Lemmens VE, Leenders BJ, Nienhuijs SW, de Hingh IH. C-reactive protein concentration is associated with prognosis in patients suffering from peritoneal carcinomatosis of colorectal origin. Int J Colorectal Dis. 2011. August;26(8):1067–73. Epub 2011/04/09. eng. 10.1007/s00384-011-1187-7 [DOI] [PubMed] [Google Scholar]
- 64. Bystrom P, Berglund A, Nygren P, Wernroth L, Johansson B, Larsson A, et al. Evaluation of predictive markers for patients with advanced colorectal cancer. Acta Oncol. 2012. September;51(7):849–59. Epub 2012/09/15. eng. 10.3109/0284186X.2012.705020 [DOI] [PubMed] [Google Scholar]
- 65. Miyata H, Yamasaki M, Kurokawa Y, Takiguchi S, Nakajima K, Fujiwara Y, et al. Prognostic value of an inflammation-based score in patients undergoing pre-operative chemotherapy followed by surgery for esophageal cancer. Exp Ther Med. 2011. September;2(5):879–85. . Pubmed Central PMCID: 3440840. Epub 2012/09/15. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Shibuya N, Kubota K. Clinical significance of tumor pathology for postoperative survival of patients undergoing surgery for stage IV colorectal cancer. Anticancer Res. 2012. August;32(8):3291–7. . Epub 2012/07/31. eng. [PubMed] [Google Scholar]
- 67. Chung YC, Chang YF. Serum C-reactive protein correlates with survival in colorectal cancer patients but is not an independent prognostic indicator. Eur J Gastroenterol Hepatol. 2003. April;15(4):369–73. . Epub 2003/03/26. eng. [DOI] [PubMed] [Google Scholar]
- 68. Sawaki A, Kanemitsu Y, Mizuno N, Takahashi K, Nakamura T, Ioka T, et al. Practical prognostic index for patients with metastatic pancreatic cancer treated with gemcitabine. J Gastroenterol Hepatol. 2008. August;23(8 Pt 1):1292–7. Epub 2008/08/15. eng. 10.1111/j.1440-1746.2006.04734.x [DOI] [PubMed] [Google Scholar]
- 69. Tanaka T, Ikeda M, Okusaka T, Ueno H, Morizane C, Hagihara A, et al. Prognostic factors in japanese patients with advanced pancreatic cancer treated with single-agent gemcitabine as first-line therapy. Jpn J Clin Oncol. 2008. November;38(11):755–61. Epub 2008/10/11. eng. 10.1093/jjco/hyn098 [DOI] [PubMed] [Google Scholar]
- 70. Haas M, Laubender RP, Stieber P, Holdenrieder S, Bruns CJ, Wilkowski R, et al. Prognostic relevance of CA 19–9, CEA, CRP, and LDH kinetics in patients treated with palliative second-line therapy for advanced pancreatic cancer. Tumour Biol. 2010. August;31(4):351–7. Epub 2010/05/19. eng. 10.1007/s13277-010-0044-6 [DOI] [PubMed] [Google Scholar]
- 71. La Torre M, Nigri G, Cavallini M, Mercantini P, Ziparo V, Ramacciato G. The glasgow prognostic score as a predictor of survival in patients with potentially resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2012. September;19(9):2917–23. Epub 2012/04/11. eng. 10.1245/s10434-012-2348-9 [DOI] [PubMed] [Google Scholar]
- 72. Masago K, Fujita S, Togashi Y, Kim YH, Hatachi Y, Fukuhara A, et al. Clinical significance of pretreatment C-reactive protein in patients with advanced nonsquamous, non-small cell lung cancer who received gefitinib. Oncology. 2010;79(5–6):355–62. Epub 2011/03/25. eng. 10.1159/000323486 [DOI] [PubMed] [Google Scholar]
- 73. Hara M, Yonei A, Ayabe T, Tomita M, Nakamura K, Onitsuka T. Postoperative serum C-reactive protein levels in non-small cell lung cancer patients. Ann Thorac Cardiovasc Surg. 2010. April;16(2):85–90. . Epub 2010/10/12. eng. [PubMed] [Google Scholar]
- 74. Gioulbasanis I, Pallis A, Vlachostergios PJ, Xyrafas A, Giannousi Z, Perdikouri IE, et al. The Glasgow Prognostic Score (GPS) predicts toxicity and efficacy in platinum-based treated patients with metastatic lung cancer. Lung Cancer. 2012. August;77(2):383–8. Epub 2012/05/04. eng. 10.1016/j.lungcan.2012.04.008 [DOI] [PubMed] [Google Scholar]
- 75. Ishizuka M, Kubota K, Kita J, Shimoda M, Kato M, Sawada T. Impact of an inflammation-based prognostic system on patients undergoing surgery for hepatocellular carcinoma: a retrospective study of 398 Japanese patients. Am J Surg. 2012. January;203(1):101–6. Epub 2011/03/25. eng. 10.1016/j.amjsurg.2010.09.030 [DOI] [PubMed] [Google Scholar]
- 76. Pinato DJ, Stebbing J, Ishizuka M, Khan SA, Wasan HS, North BV, et al. A novel and validated prognostic index in hepatocellular carcinoma: the inflammation based index (IBI). J Hepatol. 2012. November;57(5):1013–20. Epub 2012/06/27. eng. 10.1016/j.jhep.2012.06.022 [DOI] [PubMed] [Google Scholar]
- 77. Kinoshita A, Onoda H, Takano K, Imai N, Saeki C, Fushiya N, et al. Pretreatment serum C-reactive protein level predicts poor prognosis in patients with hepatocellular carcinoma. Med Oncol. 2012. December;29(4):2800–8. Epub 2012/03/31. eng. 10.1007/s12032-012-0220-1 [DOI] [PubMed] [Google Scholar]
- 78. Tartour E, Blay JY, Dorval T, Escudier B, Mosseri V, Douillard JY, et al. Predictors of clinical response to interleukin-2—based immunotherapy in melanoma patients: a French multiinstitutional study. J Clin Oncol. 1996. May;14(5):1697–703. . Epub 1996/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 79. Allin KH, Nordestgaard BG, Flyger H, Bojesen SE. Elevated pre-treatment levels of plasma C-reactive protein are associated with poor prognosis after breast cancer: a cohort study. Breast Cancer Res. 2011;13(3):R55 Pubmed Central PMCID: 3218944. Epub 2011/06/07. eng. 10.1186/bcr2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ravishankaran P, Karunanithi R. Clinical significance of preoperative serum interleukin-6 and C-reactive protein level in breast cancer patients. World J Surg Oncol. 2011;9:18 Pubmed Central PMCID: 3045973. Epub 2011/02/08. eng. 10.1186/1477-7819-9-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Morizane S. Serum C-reactive protein level is significant prognostic indicator in patients with advanced urothelial cancer treated with Gemcitabine-Cisplatin or Carboplatin-preliminary results. Central European Journal of Urology. 2012;65(2):62–6. 10.5173/ceju.2012.02.art1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Obata J, Kikuchi E, Tanaka N, Matsumoto K, Hayakawa N, Ide H, et al. C-reactive protein: A biomarker of survival in patients with localized upper tract urothelial carcinoma treated with radical nephroureterectomy. Urol Oncol. 2012. November 8 . Epub 2012/11/13. Eng. [DOI] [PubMed] [Google Scholar]
- 83. Yoshida S, Saito K, Koga F, Yokoyama M, Kageyama Y, Masuda H, et al. C-reactive protein level predicts prognosis in patients with muscle-invasive bladder cancer treated with chemoradiotherapy. BJU Int. 2008. April;101(8):978–81. Epub 2008/01/15. eng. 10.1111/j.1464-410X.2007.07408.x [DOI] [PubMed] [Google Scholar]
- 84. Beer TM, Lalani AS, Lee S, Mori M, Eilers KM, Curd JG, et al. C-reactive protein as a prognostic marker for men with androgen-independent prostate cancer: results from the ASCENT trial. Cancer. 2008. June;112(11):2377–83. Epub 2008/04/23. eng. 10.1002/cncr.23461 [DOI] [PubMed] [Google Scholar]
- 85. Ito M, Saito K, Yasuda Y, Sukegawa G, Kubo Y, Numao N, et al. Prognostic impact of C-reactive protein for determining overall survival of patients with castration-resistant prostate cancer treated with docetaxel. Urology. 2011. November;78(5):1131–5. Epub 2011/11/08. eng. 10.1016/j.urology.2011.07.1416 [DOI] [PubMed] [Google Scholar]
- 86. Shafique K, Proctor MJ, McMillan DC, Qureshi K, Leung H, Morrison DS. Systemic inflammation and survival of patients with prostate cancer: evidence from the Glasgow Inflammation Outcome Study. Prostate Cancer Prostatic Dis. 2012. June;15(2):195–201. Epub 2012/02/22. eng. 10.1038/pcan.2011.60 [DOI] [PubMed] [Google Scholar]
- 87. Polterauer S, Grimm C, Tempfer C, Sliutz G, Speiser P, Reinthaller A, et al. C-reactive protein is a prognostic parameter in patients with cervical cancer. Gynecol Oncol. 2007. October;107(1):114–7. . Epub 2007/07/10. eng. [DOI] [PubMed] [Google Scholar]
- 88. Hefler LA, Concin N, Hofstetter G, Marth C, Mustea A, Sehouli J, et al. Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clin Cancer Res. 2008. February 1;14(3):710–4. Epub 2008/02/05. eng. 10.1158/1078-0432.CCR-07-1044 [DOI] [PubMed] [Google Scholar]
- 89. Nakamura T, Matsumine A, Matsubara T, Asanuma K, Uchida A, Sudo A. Clinical significance of pretreatment serum C-reactive protein level in soft tissue sarcoma. Cancer. 2012. February 15;118(4):1055–61. Epub 2011/07/16. eng. 10.1002/cncr.26353 [DOI] [PubMed] [Google Scholar]
- 90. Kodama J, Miyagi Y, Seki N, Tokumo K, Yoshinouchi M, Kobashi Y, et al. Serum C-reactive protein as a prognostic factor in patients with epithelial ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 1999. January;82(1):107–10. . Epub 1999/04/07. eng. [DOI] [PubMed] [Google Scholar]
- 91. Yudoh K, Matsui H, Kamanori M, Ohmori K, Yasuda T, Tsuji H, et al. Prognostic value of the doubling time of serum C-reactive protein and alkaline phosphatase levels in primary bone and soft tissue tumors. Jpn J Cancer Res. 1996. December;87(12):1288–95. . Epub 1996/12/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. 2009. May 1;27(13):2217–24. Epub 2009/03/18. eng. 10.1200/JCO.2008.19.8440 [DOI] [PubMed] [Google Scholar]
- 93. Kanz R, Vukovich T, Vormittag R, Dunkler D, Ay C, Thaler J, et al. Thrombosis risk and survival in cancer patients with elevated C-reactive protein. J Thromb Haemost. 2011. January;9(1):57–63. Epub 2010/09/30. eng. 10.1111/j.1538-7836.2010.04069.x [DOI] [PubMed] [Google Scholar]
- 94. Del Fabbro E, Hui D, Nooruddin ZI, Dalal S, Dev R, Freer G, et al. Associations among hypogonadism, C-reactive protein, symptom burden, and survival in male cancer patients with cachexia: a preliminary report. J Pain Symptom Manage. 2010. June;39(6):1016–24. Epub 2010/05/12. eng. 10.1016/j.jpainsymman.2009.09.021 [DOI] [PubMed] [Google Scholar]
- 95. Ridwelski K, Meyer F, Ebert M, Malfertheiner P, Lippert H. Prognostic parameters determining survival in pancreatic carcinoma and, in particular, after palliative treatment. Dig Dis. 2001;19(1):85–92. . Epub 2001/06/01. eng. [DOI] [PubMed] [Google Scholar]
- 96. Gioulbasanis I, Georgoulias P, Vlachostergios PJ, Baracos V, Ghosh S, Giannousi Z, et al. Mini Nutritional Assessment (MNA) and biochemical markers of cachexia in metastatic lung cancer patients: interrelations and associations with prognosis. Lung Cancer. 2011. December;74(3):516–20. Epub 2011/06/03. eng. 10.1016/j.lungcan.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 97. Hwang JE, Kim HN, Kim DE, Choi HJ, Jung SH, Shim HJ, et al. Prognostic significance of a systemic inflammatory response in patients receiving first-line palliative chemotherapy for recurred or metastatic gastric cancer. BMC Cancer. 2011;11:489 Pubmed Central PMCID: 3226799. Epub 2011/11/23. eng. 10.1186/1471-2407-11-489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kohles N, Nagel D, Jungst D, Durner J, Stieber P, Holdenrieder S. Prognostic relevance of oncological serum biomarkers in liver cancer patients undergoing transarterial chemoembolization therapy. Tumour Biol. 2012. February;33(1):33–40. Epub 2011/09/21. eng. 10.1007/s13277-011-0237-7 [DOI] [PubMed] [Google Scholar]
- 99. Hwang EC, Hwang IS, Yu HS, Kim SO, Jung SI, Hwang JE, et al. Utility of inflammation-based prognostic scoring in patients given systemic chemotherapy first-line for advanced inoperable bladder cancer. Jpn J Clin Oncol. 2012. October;42(10):955–60. . Epub 2012/07/31. eng. [DOI] [PubMed] [Google Scholar]
- 100. Chua TC, Chong CH, Liauw W, Zhao J, Morris DL. Inflammatory markers in blood and serum tumor markers predict survival in patients with epithelial appendiceal neoplasms undergoing surgical cytoreduction and intraperitoneal chemotherapy. Ann Surg. 2012. August;256(2):342–9. Epub 2012/07/04. eng. 10.1097/SLA.0b013e3182602ad2 [DOI] [PubMed] [Google Scholar]
- 101. Chua W, Clarke SJ, Charles KA. Systemic inflammation and prediction of chemotherapy outcomes in patients receiving docetaxel for advanced cancer. Support Care Cancer. 2012. August;20(8):1869–74. Epub 2011/10/12. eng. 10.1007/s00520-011-1289-3 [DOI] [PubMed] [Google Scholar]
- 102. Chang CC, Sun CF, Pai HJ, Wang WK, Hsieh CC, Kuo LM, et al. Preoperative serum C-reactive protein and gastric cancer; clinical-pathological correlation and prognostic significance. Chang Gung Med J. 2010. May-Jun;33(3):301–12. . Epub 2010/06/30. eng. [PubMed] [Google Scholar]
- 103. Nakanishi H, Araki N, Kudawara I, Kuratsu S, Matsumine A, Mano M, et al. Clinical implications of serum C-reactive protein levels in malignant fibrous histiocytoma. Int J Cancer. 2002. May 10;99(2):167–70. . Epub 2002/04/30. eng. [DOI] [PubMed] [Google Scholar]
- 104. Mortensen RF, Rudczynski AB. Prognostic significance of serum CRP levels and lymphoid cell infiltrates in human breast cancer. Oncology. 1982;39(3):129–33. . Epub 1982/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 105. Lewenhaupt A, Ekman P, Eneroth P, Nilsson B. Tumour markers as prognostic aids in prostatic carcinoma. Br J Urol. 1990. August;66(2):182–7. . Epub 1990/08/01. eng. [DOI] [PubMed] [Google Scholar]
- 106. Ekman P, Lewenhaupt A. Serum tumour markers in human prostatic carcinoma. The value of a marker panel for prognostic information. Acta Oncol. 1991;30(2):173–5. . Epub 1991/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 107. Muller T, Marshall RJ, Cooper EH, Watson DA, Walker DA, Mearns AJ. The role of serum tumour markers to aid the selection of lung cancer patients for surgery and the assessment of prognosis. Eur J Cancer Clin Oncol. 1985. December;21(12):1461–6. . Epub 1985/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 108. Deans DA, Wigmore SJ, de Beaux AC, Paterson-Brown S, Garden OJ, Fearon KC. Clinical prognostic scoring system to aid decision-making in gastro-oesophageal cancer. Br J Surg. 2007. December;94(12):1501–8. . Epub 2007/08/19. eng. [DOI] [PubMed] [Google Scholar]
- 109. Kim DK, Oh SY, Kwon HC, Lee S, Kwon KA, Kim BG, et al. Clinical significances of preoperative serum interleukin-6 and C-reactive protein level in operable gastric cancer. BMC Cancer. 2009;9:155 Pubmed Central PMCID: 2694817. Epub 2009/05/22. eng. 10.1186/1471-2407-9-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lukaszewicz-Zajac M, Mroczko B, Gryko M, Kedra B, Szmitkowski M. Comparison between clinical significance of serum proinflammatory proteins (IL-6 and CRP) and classic tumor markers (CEA and CA 19–9) in gastric cancer. Clin Exp Med. 2011. June;11(2):89–96. Pubmed Central PMCID: 3087107. Epub 2010/10/13. eng. 10.1007/s10238-010-0114-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Heys SD, Ogston KN, Simpson WG, Walker LG, Hutcheon AW, Sarkar TK, et al. Acute phase proteins in patients with large and locally advanced breast cancer treated with neo-adjuvant chemotherapy: response and survival. Int J Oncol. 1998. September;13(3):589–94. . Epub 1998/07/31. eng. [DOI] [PubMed] [Google Scholar]
- 112. Tas F, Duranyildiz D, Argon A, Oguz H, Camlica H, Yasasever V, et al. Serum levels of leptin and proinflammatory cytokines in advanced-stage non-small cell lung cancer. Med Oncol. 2005;22(4):353–8. . Epub 2005/11/02. eng. [DOI] [PubMed] [Google Scholar]
- 113. Kelly L, White S, Stone PC. The B12/CRP index as a simple prognostic indicator in patients with advanced cancer: a confirmatory study. Ann Oncol. 2007. August;18(8):1395–9. . Epub 2007/05/22. eng. [DOI] [PubMed] [Google Scholar]
- 114. Glare P. Clinical predictors of survival in advanced cancer. J Support Oncol. 2005. Sep-Oct;3(5):331–9. . Epub 2005/10/13. eng. [PubMed] [Google Scholar]
- 115. Hayes DF, Bast RC, Desch CE, Fritsche H Jr., Kemeny NE, Jessup JM, et al. Tumor marker utility grading system: a framework to evaluate clinical utility of tumor markers. J Natl Cancer Inst. 1996. October 16;88(20):1456–66. . Epub 1996/10/16. eng. [DOI] [PubMed] [Google Scholar]
- 116. Schilsky RL, Taube SE. Tumor markers as clinical cancer tests—are we there yet? Semin Oncol. 2002. June;29(3):211–2. . Epub 2002/06/14. eng. [DOI] [PubMed] [Google Scholar]
- 117. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat Clin Pract Urol. 2005. August;2(8):416–22. . Epub 2006/02/17. eng. [PubMed] [Google Scholar]
- 118. Ko YJ, Kwon YM, Kim KH, Choi HC, Chun SH, Yoon HJ, et al. High-sensitivity C-reactive protein levels and cancer mortality. Cancer Epidemiol Biomarkers Prev. 2012. November;21(11):2076–86. Epub 2012/11/09. eng. 10.1158/1055-9965.EPI-12-0611 [DOI] [PubMed] [Google Scholar]
- 119. Elsberger B, Lankston L, McMillan DC, Underwood MA, Edwards J. Presence of tumoural C-reactive protein correlates with progressive prostate cancer. Prostate Cancer Prostatic Dis. 2011. June;14(2):122–8. Epub 2011/03/02. eng. 10.1038/pcan.2011.5 [DOI] [PubMed] [Google Scholar]
- 120. Nakatsu T, Motoyama S, Maruyama K, Usami S, Sato Y, Miura M, et al. Tumoral CRP expression in thoracic esophageal squamous cell cancers is associated with poor outcomes. Surg Today. 2012. July;42(7):652–8. Epub 2012/02/22. eng. 10.1007/s00595-012-0147-3 [DOI] [PubMed] [Google Scholar]
- 121. Saito K, Tatokoro M, Fujii Y, Iimura Y, Koga F, Kawakami S, et al. Impact of C-reactive protein kinetics on survival of patients with metastatic renal cell carcinoma. Eur Urol. 2009. May;55(5):1145–53. Epub 2008/10/22. eng. 10.1016/j.eururo.2008.10.012 [DOI] [PubMed] [Google Scholar]
- 122. Tatokoro M, Saito K, Iimura Y, Fujii Y, Kawakami S, Kihara K. Prognostic impact of postoperative C-reactive protein level in patients with metastatic renal cell carcinoma undergoing cytoreductive nephrectomy. J Urol. 2008. August;180(2):515–9. Epub 2008/06/14. eng. 10.1016/j.juro.2008.04.025 [DOI] [PubMed] [Google Scholar]
- 123. Yang SH, Huang CJ, Chang SC, Lin JK. Association of C-reactive protein gene polymorphisms and colorectal cancer. Ann Surg Oncol. 2011. July;18(7):1907–15. Epub 2011/02/05. eng. 10.1245/s10434-011-1575-9 [DOI] [PubMed] [Google Scholar]
- 124. Slattery ML, Curtin K, Poole EM, Duggan DJ, Samowitz WS, Peters U, et al. Genetic variation in C-reactive protein in relation to colon and rectal cancer risk and survival. Int J Cancer. 2011. June 1;128(11):2726–34. Pubmed Central PMCID: 3229275. Epub 2010/10/16. eng. 10.1002/ijc.25721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Walsh D, Mahmoud F, Barna B. Assessment of nutritional status and prognosis in advanced cancer: interleukin-6, C-reactive protein, and the prognostic and inflammatory nutritional index. Support Care Cancer. 2003. January;11(1):60–2. . Epub 2003/01/16. eng. [DOI] [PubMed] [Google Scholar]
- 126. Crumley AB, Stuart RC, McKernan M, McDonald AC, McMillan DC. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG-ps) in patients receiving palliative chemotherapy for gastroesophageal cancer. J Gastroenterol Hepatol. 2008. August;23(8 Pt 2):e325–9. . Epub 2007/07/25. eng. [DOI] [PubMed] [Google Scholar]
- 127. Dutta S, Crumley AB, Fullarton GM, Horgan PG, McMillan DC. Comparison of the prognostic value of tumour and patient related factors in patients undergoing potentially curative resection of gastric cancer. Am J Surg. 2012. September;204(3):294–9. Epub 2012/03/27. eng. 10.1016/j.amjsurg.2011.10.015 [DOI] [PubMed] [Google Scholar]
- 128. Wang CY, Lee TF, Fang CH, Chou JH. Fuzzy Logic-Based Prognostic Score for Outcome Prediction in Esophageal Cancer. IEEE Trans Inf Technol Biomed. 2012. August 2. . Epub 2012/08/10. Eng. [DOI] [PubMed] [Google Scholar]
- 129. Van Hemelrijck M, Eichholzer M, Faeh D, Rohrmann S. Ability of a biomarker-based score to predict death from circulatory disease and cancer in NHANES III. BMC Public Health. 2012;12:895 Pubmed Central PMCID: 3549794. Epub 2012/10/25. eng. 10.1186/1471-2458-12-895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. McMillan DC, Crozier JE, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007. August;22(8):881–6. . Epub 2007/01/25. eng. [DOI] [PubMed] [Google Scholar]
- 131. Proctor MJ, Morrison DS, Talwar D, Balmer SM, O'Reilly DS, Foulis AK, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer. 2011. February 15;104(4):726–34. Pubmed Central PMCID: 3049591. Epub 2011/01/27. eng. 10.1038/sj.bjc.6606087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am J Med. 2006. February;119(2):166 e17–28. . Epub 2006/01/31. eng. [DOI] [PubMed] [Google Scholar]
- 133. Koc M, Taysi S, Sezen O, Bakan N. Levels of some acute-phase proteins in the serum of patients with cancer during radiotherapy. Biol Pharm Bull. 2003. October;26(10):1494–7. . Epub 2003/10/02. eng. [DOI] [PubMed] [Google Scholar]
- 134. Bland M, editor. An Introduction to Medical Statistics. 3 rd ed NY: Oxford University Press; 2002. [Google Scholar]
- 135. Heikkila K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J Epidemiol Community Health. 2007. September;61(9):824–33. . Pubmed Central PMCID: 2703800. Epub 2007/08/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001. February 17;357(9255):539–45. . Epub 2001/03/07. eng. [DOI] [PubMed] [Google Scholar]
- 137. Ernstoff MS, Crocenzi TS, Seigne JD, Crosby NA, Cole BF, Fisher JL, et al. Developing a rational tumor vaccine therapy for renal cell carcinoma: immune yin and yang. Clin Cancer Res. 2007. January 15;13(2 Pt 2):733s–40s. . Epub 2007/01/27. eng. [DOI] [PubMed] [Google Scholar]
- 138. Fantini MC, Pallone F. Cytokines: from gut inflammation to colorectal cancer. Curr Drug Targets. 2008. May;9(5):375–80. . Epub 2008/05/14. eng. [DOI] [PubMed] [Google Scholar]
- 139. Jones JM, McGonigle NC, McAnespie M, Cran GW, Graham AN. Plasma fibrinogen and serum C-reactive protein are associated with non-small cell lung cancer. Lung Cancer. 2006. July;53(1):97–101. . Epub 2006/05/16. eng. [DOI] [PubMed] [Google Scholar]
- 140. Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006. June;83(6):1345–50. . Epub 2006/06/10. eng. [DOI] [PubMed] [Google Scholar]
- 141. Marsik C, Kazemi-Shirazi L, Schickbauer T, Winkler S, Joukhadar C, Wagner OF, et al. C-reactive protein and all-cause mortality in a large hospital-based cohort. Clin Chem. 2008. February;54(2):343–9. . Epub 2007/12/25. eng. [DOI] [PubMed] [Google Scholar]
- 142. Riley RD, Sauerbrei W, Altman DG. Prognostic markers in cancer: the evolution of evidence from single studies to meta-analysis, and beyond. Br J Cancer. 2009. April 21;100(8):1219–29. Pubmed Central PMCID: 2676559. Epub 2009/04/16. eng. 10.1038/sj.bjc.6604999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Masuda H, Kurita Y, Fukuta K, Mugiya S, Suzuki K, Fujita K. Significant prognostic factors for 5-year survival after curative resection of renal cell carcinoma. Int J Urol. 1998. September;5(5):418–22. . Epub 1998/10/22. eng. [DOI] [PubMed] [Google Scholar]
- 144. Hoffmann R, Franzke A, Buer J, Sel S, Oevermann K, Duensing A, et al. Prognostic impact of in vivo soluble cell adhesion molecules in metastatic renal cell carcinoma. Br J Cancer. 1999. April;79(11–12):1742–5. . Pubmed Central PMCID: 2362808. Epub 1999/04/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lamb GW, McMillan DC, Ramsey S, Aitchison M. The relationship between the preoperative systemic inflammatory response and cancer-specific survival in patients undergoing potentially curative resection for renal clear cell cancer. Br J Cancer. 2006. March 27;94(6):781–4. . Pubmed Central PMCID: 3216422. Epub 2006/03/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Royston P, Reitz M, Atzpodien J. An approach to estimating prognosis using fractional polynomials in metastatic renal carcinoma. Br J Cancer. 2006. June 19;94(12):1785–8. . Pubmed Central PMCID: 2361333. Epub 2006/06/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Komai Y, Saito K, Sakai K, Morimoto S. Increased preoperative serum C-reactive protein level predicts a poor prognosis in patients with localized renal cell carcinoma. BJU Int. 2007. January;99(1):77–80. . Epub 2006/09/08. eng. [DOI] [PubMed] [Google Scholar]
- 148. Ramsey S, Lamb GW, Aitchison M, Graham J, McMillan DC. Evaluation of an inflammation-based prognostic score in patients with metastatic renal cancer. Cancer. 2007. January 15;109(2):205–12. . Epub 2006/12/07. eng. [DOI] [PubMed] [Google Scholar]
- 149. Karakiewicz PI, Hutterer GC, Trinh QD, Jeldres C, Perrotte P, Gallina A, et al. C-reactive protein is an informative predictor of renal cell carcinoma-specific mortality: a European study of 313 patients. Cancer. 2007. September 15;110(6):1241–7. . Epub 2007/07/20. eng. [DOI] [PubMed] [Google Scholar]
- 150. Guida M, Casamassima A, Monticelli G, Quaranta M, Colucci G. Basal cytokines profile in metastatic renal cell carcinoma patients treated with subcutaneous IL-2-based therapy compared with that of healthy donors. J Transl Med. 2007;5:51 . Pubmed Central PMCID: 2169206. Epub 2007/10/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kawata N, Nagane Y, Yamaguchi K, Ichinose T, Hirakata H, Takahashi S. How do symptoms have an impact on the prognosis of renal cell carcinoma? Int J Urol. 2008. April;15(4):299–303. Epub 2008/04/03. eng. 10.1111/j.1442-2042.2008.01990.x [DOI] [PubMed] [Google Scholar]
- 152. Iimura Y, Saito K, Fujii Y, Kumagai J, Kawakami S, Komai Y, et al. Development and external validation of a new outcome prediction model for patients with clear cell renal cell carcinoma treated with nephrectomy based on preoperative serum C-reactive protein and TNM classification: the TNM-C score. J Urol. 2009. March;181(3):1004–12; discussion 12. Epub 2009/01/20. eng. 10.1016/j.juro.2008.10.156 [DOI] [PubMed] [Google Scholar]
- 153. Kume H, Kakutani S, Yamada Y, Shinohara M, Tominaga T, Suzuki M, et al. Prognostic factors for renal cell carcinoma with bone metastasis: who are the long-term survivors? J Urol. 2011. May;185(5):1611–4. Epub 2011/03/23. eng. 10.1016/j.juro.2010.12.037 [DOI] [PubMed] [Google Scholar]
- 154. Shinohara N, Abe T, Mochizuki T, Kashiwagi A, Kanagawa K, Maruyama S, et al. Is Memorial Sloan-Kettering Cancer Center risk classification appropriate for Japanese patients with metastatic renal cell carcinoma in the cytokine era? Urol Oncol. 2011. September 26 . Epub 2011/10/01. Eng. [DOI] [PubMed] [Google Scholar]
- 155. Soga N. The impact of preoperative serum albumin level and postoperative C-reactive protein nadir on the survival of patients with non-metastatic renal cell carcinoma with vessel thrombus after nephrectomy. Curr Urol. 2011;5:190–5. [Google Scholar]
- 156. Lamb GW, Aitchison M, Ramsey S, Housley SL, McMillan DC. Clinical utility of the Glasgow Prognostic Score in patients undergoing curative nephrectomy for renal clear cell cancer: basis of new prognostic scoring systems. Br J Cancer. 2012. January 17;106(2):279–83. Pubmed Central PMCID: 3261680. Epub 2011/12/15. eng. 10.1038/bjc.2011.556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Qayyum T, McArdle PA, Lamb GW, Going JJ, Orange C, Seywright M, et al. Prospective study of the role of inflammation in renal cancer. Urol Int. 2012;88(3):277–81. Epub 2012/03/02. eng. 10.1159/000334971 [DOI] [PubMed] [Google Scholar]
- 158. Fujita T, Iwamura M, Ishii D, Tabata K, Matsumoto K, Yoshida K, et al. C-reactive protein as a prognostic marker for advanced renal cell carcinoma treated with sunitinib. Int J Urol. 2012. October;19(10):908–13. Epub 2012/06/08. eng. 10.1111/j.1442-2042.2012.03071.x [DOI] [PubMed] [Google Scholar]
- 159. Yasuda Y, Saito K, Yuasa T, Kitsukawa S, Urakami S, Yamamoto S, et al. Prognostic impact of pretreatment C-reactive protein for patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. Int J Clin Oncol. 2012. August 11 . Epub 2012/08/14. Eng. [DOI] [PubMed] [Google Scholar]
- 160. Kawashima A, Tsujimura A, Takayama H, Arai Y, Nin M, Tanigawa G, et al. Impact of hyponatremia on survival of patients with metastatic renal cell carcinoma treated with molecular targeted therapy. Int J Urol. 2012. December;19(12):1050–7. Epub 2012/08/07. eng. 10.1111/j.1442-2042.2012.03115.x [DOI] [PubMed] [Google Scholar]
- 161. Bedke J, Chun FK, Merseburger A, Scharpf M, Kasprzyk K, Schilling D, et al. Inflammatory prognostic markers in clear cell renal cell carcinoma—preoperative C-reactive protein does not improve predictive accuracy. BJU Int. 2012. December;110(11 Pt B):E771–7. Epub 2012/11/09. eng. 10.1111/j.1464-410X.2012.11642.x [DOI] [PubMed] [Google Scholar]
- 162. Sim SH, Messenger MP, Gregory WM, Wind TC, Vasudev NS, Cartledge J, et al. Prognostic utility of pre-operative circulating osteopontin, carbonic anhydrase IX and CRP in renal cell carcinoma. Br J Cancer. 2012. September 25;107(7):1131–7. Pubmed Central PMCID: 3461155. Epub 2012/08/25. eng. 10.1038/bjc.2012.360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Steffens S, Kohler A, Rudolph R, Eggers H, Seidel C, Janssen M, et al. Validation of CRP as prognostic marker for renal cell carcinoma in a large series of patients. BMC Cancer. 2012;12:399 Pubmed Central PMCID: 3502607. Epub 2012/09/11. eng. 10.1186/1471-2407-12-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Nozoe T, Matsumata T, Kitamura M, Sugimachi K. Significance of preoperative elevation of serum C-reactive protein as an indicator for prognosis in colorectal cancer. Am J Surg. 1998. October;176(4):335–8. . Epub 1998/11/17. eng. [DOI] [PubMed] [Google Scholar]
- 165. Nielsen HJ, Christensen IJ, Sorensen S, Moesgaard F, Brunner N. Preoperative plasma plasminogen activator inhibitor type-1 and serum C-reactive protein levels in patients with colorectal cancer. The RANX05 Colorectal Cancer Study Group. Ann Surg Oncol. 2000. September;7(8):617–23. . Epub 2000/09/27. eng. [DOI] [PubMed] [Google Scholar]
- 166. McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg. 2003. February;90(2):215–9. . Epub 2003/01/30. eng. [DOI] [PubMed] [Google Scholar]
- 167. McMillan DC, Canna K, McArdle CS. The effect of deprivation and the systemic inflammatory response on outcome following curative resection for colorectal cancer. Br J Cancer. 2003. August 18;89(4):612–4. . Pubmed Central PMCID: 2376934. Epub 2003/08/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Miki C, Konishi N, Ojima E, Hatada T, Inoue Y, Kusunoki M. C-reactive protein as a prognostic variable that reflects uncontrolled up-regulation of the IL-1-IL-6 network system in colorectal carcinoma. Dig Dis Sci. 2004. June;49(6):970–6. . Epub 2004/08/18. eng. [DOI] [PubMed] [Google Scholar]
- 169. Canna K, McMillan DC, McKee RF, McNicol AM, Horgan PG, McArdle CS. Evaluation of a cumulative prognostic score based on the systemic inflammatory response in patients undergoing potentially curative surgery for colorectal cancer. Br J Cancer. 2004. May 4;90(9):1707–9. Pubmed Central PMCID: . Epub 2004/05/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. de Jong KP, Hoedemakers RM, Fidler V, Bijzet J, Limburg PC, Peeters PM, et al. Portal and systemic serum growth factor and acute-phase response after laparotomy or partial hepatectomy in patients with colorectal liver metastases: a prognostic role for C-reactive protein and hepatocyte growth factor. Scand J Gastroenterol. 2004. November;39(11):1141–8. . Epub 2004/11/17. eng. [DOI] [PubMed] [Google Scholar]
- 171. Nikiteas NI, Tzanakis N, Gazouli M, Rallis G, Daniilidis K, Theodoropoulos G, et al. Serum IL-6, TNFalpha and CRP levels in Greek colorectal cancer patients: prognostic implications. World J Gastroenterol. 2005. March 21;11(11):1639–43. . Epub 2005/03/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Canna K, McArdle PA, McMillan DC, McNicol AM, Smith GW, McKee RF, et al. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2005. February 28;92(4):651–4. . Pubmed Central PMCID: 2361875. Epub 2005/02/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Read JA, Choy ST, Beale PJ, Clarke SJ. Evaluation of nutritional and inflammatory status of advanced colorectal cancer patients and its correlation with survival. Nutr Cancer. 2006;55(1):78–85. . Epub 2006/09/13. eng. [DOI] [PubMed] [Google Scholar]
- 174. Crozier JE, McKee RF, McArdle CS, Angerson WJ, Anderson JH, Horgan PG, et al. The presence of a systemic inflammatory response predicts poorer survival in patients receiving adjuvant 5-FU chemotherapy following potentially curative resection for colorectal cancer. Br J Cancer. 2006. June 19;94(12):1833–6. . Pubmed Central PMCID: 2361334. Epub 2006/05/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Canna K, Hilmy M, McMillan DC, Smith GW, McKee RF, McArdle CS, et al. The relationship between tumour proliferative activity, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Colorectal Dis. 2008. September;10(7):663–7. . Epub 2007/11/17. eng. [DOI] [PubMed] [Google Scholar]
- 176. Leitch EF, Chakrabarti M, Crozier JE, McKee RF, Anderson JH, Horgan PG, et al. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. Br J Cancer. 2007. November 5;97(9):1266–70. . Pubmed Central PMCID: 2360467. Epub 2007/10/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Malik HZ, Prasad KR, Halazun KJ, Aldoori A, Al-Mukhtar A, Gomez D, et al. Preoperative prognostic score for predicting survival after hepatic resection for colorectal liver metastases. Ann Surg. 2007. November;246(5):806–14. . Epub 2007/10/31. eng. [DOI] [PubMed] [Google Scholar]
- 178. Ishizuka M, Nagata H, Takagi K, Horie T, Kubota K. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann Surg. 2007. December;246(6):1047–51. . Epub 2007/11/29. eng. [DOI] [PubMed] [Google Scholar]
- 179. Shiu YC, Lin JK, Huang CJ, Jiang JK, Wang LW, Huang HC, et al. Is C-reactive protein a prognostic factor of colorectal cancer? Dis Colon Rectum. 2008. April;51(4):443–9. Epub 2008/01/04. eng. 10.1007/s10350-007-9133-z [DOI] [PubMed] [Google Scholar]
- 180. Koike Y, Miki C, Okugawa Y, Yokoe T, Toiyama Y, Tanaka K, et al. Preoperative C-reactive protein as a prognostic and therapeutic marker for colorectal cancer. J Surg Oncol. 2008. December 1;98(7):540–4. Epub 2008/10/22. eng. 10.1002/jso.21154 [DOI] [PubMed] [Google Scholar]
- 181. Nozoe T, Mori E, Takahashi I, Ezaki T. Preoperative elevation of serum C-reactive protein as an independent prognostic indicator of colorectal carcinoma. Surg Today. 2008;38(7):597–602. Epub 2008/07/10. eng. 10.1007/s00595-007-3680-8 [DOI] [PubMed] [Google Scholar]
- 182. Groblewska M, Mroczko B, Wereszczynska-Siemiatkowska U, Kedra B, Lukaszewicz M, Baniukiewicz A, et al. Serum interleukin 6 (IL-6) and C-reactive protein (CRP) levels in colorectal adenoma and cancer patients. Clin Chem Lab Med. 2008;46(10):1423–8. Epub 2008/10/11. eng. 10.1515/CCLM.2008.278 [DOI] [PubMed] [Google Scholar]
- 183. Sharma R, Zucknick M, London R, Kacevska M, Liddle C, Clarke SJ. Systemic inflammatory response predicts prognosis in patients with advanced-stage colorectal cancer. Clin Colorectal Cancer. 2008. September;7(5):331–7. Epub 2008/09/17. eng. 10.3816/CCC.2008.n.044 [DOI] [PubMed] [Google Scholar]
- 184. Crozier JE, Leitch EF, McKee RF, Anderson JH, Horgan PG, McMillan DC. Relationship between emergency presentation, systemic inflammatory response, and cancer-specific survival in patients undergoing potentially curative surgery for colon cancer. Am J Surg. 2009. April;197(4):544–9. Epub 2008/07/11. eng. 10.1016/j.amjsurg.2007.12.052 [DOI] [PubMed] [Google Scholar]
- 185. Ishizuka M, Kita J, Shimoda M, Rokkaku K, Kato M, Sawada T, et al. Systemic inflammatory response predicts postoperative outcome in patients with liver metastases from colorectal cancer. J Surg Oncol. 2009. July 1;100(1):38–42. Epub 2009/04/29. eng. 10.1002/jso.21294 [DOI] [PubMed] [Google Scholar]
- 186. Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC. The relationship between the local and systemic inflammatory responses and survival in patients undergoing curative surgery for colon and rectal cancers. J Gastrointest Surg. 2009. November;13(11):2011–8; discussion 8–9. Epub 2009/09/22. eng. 10.1007/s11605-009-1034-0 [DOI] [PubMed] [Google Scholar]
- 187. Ishizuka M, Nagata H, Takagi K, Kubota K. Influence of inflammation-based prognostic score on mortality of patients undergoing chemotherapy for far advanced or recurrent unresectable colorectal cancer. Ann Surg. 2009. August;250(2):268–72. Epub 2009/07/30. eng. 10.1097/SLA.0b013e3181b16e24 [DOI] [PubMed] [Google Scholar]
- 188. Zacharakis M, Xynos ID, Lazaris A, Smaro T, Kosmas C, Dokou A, et al. Predictors of survival in stage IV metastatic colorectal cancer. Anticancer Res. 2010. February;30(2):653–60. . Epub 2010/03/25. eng. [PubMed] [Google Scholar]
- 189. Kobayashi T, Teruya M, Kishiki T, Endo D, Takenaka Y, Miki K, et al. Elevated C-reactive protein and hypoalbuminemia measured before resection of colorectal liver metastases predict postoperative survival. Dig Surg. 2010;27(4):285–90. Epub 2010/08/07. eng. 10.1159/000280021 [DOI] [PubMed] [Google Scholar]
- 190. Volkova E, Willis JA, Wells JE, Robinson BA, Dachs GU, Currie MJ. Association of angiopoietin-2, C-reactive protein and markers of obesity and insulin resistance with survival outcome in colorectal cancer. Br J Cancer. 2011. January 4;104(1):51–9. Pubmed Central PMCID: 3039823. Epub 2010/11/18. eng. 10.1038/sj.bjc.6606005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Richards CH, Leitch EF, Horgan PG, Anderson JH, McKee RF, McMillan DC. The relationship between patient physiology, the systemic inflammatory response and survival in patients undergoing curative resection of colorectal cancer. Br J Cancer. 2010. October 26;103(9):1356–61. Pubmed Central PMCID: 2990607. Epub 2010/09/30. eng. 10.1038/sj.bjc.6605919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Toiyama Y, Miki C, Inoue Y, Tanaka K, Mohri Y, Kusunoki M. Evaluation of an inflammation-based prognostic score for the identification of patients requiring postoperative adjuvant chemotherapy for stage II colorectal cancer. Exp Ther Med. 2011. January;2(1):95–101. . Pubmed Central PMCID: 3440627. Epub 2011/01/01. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Kocsis J, Meszaros T, Madaras B, Toth EK, Kamondi S, Gal P, et al. High levels of acute phase proteins and soluble 70 kDa heat shock proteins are independent and additive risk factors for mortality in colorectal cancer. Cell Stress Chaperones. 2011. January;16(1):49–55. Pubmed Central PMCID: 3024085. Epub 2010/08/24. eng. 10.1007/s12192-010-0220-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Roxburgh CS, Platt JJ, Leitch EF, Kinsella J, Horgan PG, McMillan DC. Relationship between preoperative comorbidity, systemic inflammatory response, and survival in patients undergoing curative resection for colorectal cancer. Ann Surg Oncol. 2011. April;18(4):997–1005. Epub 2010/11/03. eng. 10.1245/s10434-010-1410-8 [DOI] [PubMed] [Google Scholar]
- 195. Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Preoperative thrombocytosis is associated with survival after surgery for colorectal cancer. J Surg Oncol. 2012. December;106(7):887–91. Epub 2012/05/25. eng. 10.1002/jso.23163 [DOI] [PubMed] [Google Scholar]
- 196. Furukawa K, Shiba H, Haruki K, Fujiwara Y, Iida T, Mitsuyama Y, et al. The Glasgow prognostic score is valuable for colorectal cancer with both synchronous and metachronous unresectable liver metastases. Oncol Lett. 2012. August;4(2):324–8. . Pubmed Central PMCID: 3402729. Epub 2012/07/31. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Sugimoto K, Komiyama H, Kojima Y, Goto M, Tomiki Y, Sakamoto K. Glasgow prognostic score as a prognostic factor in patients undergoing curative surgery for colorectal cancer. Dig Surg. 2012;29(6):503–9. Epub 2013/02/09. eng. 10.1159/000346002 [DOI] [PubMed] [Google Scholar]
- 198. Ikeda M, Natsugoe S, Ueno S, Baba M, Aikou T. Significant host- and tumor-related factors for predicting prognosis in patients with esophageal carcinoma. Ann Surg. 2003. August;238(2):197–202. . Pubmed Central PMCID: 1422696. Epub 2003/08/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Shimada H, Nabeya Y, Okazumi S, Matsubara H, Shiratori T, Aoki T, et al. Elevation of preoperative serum C-reactive protein level is related to poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol. 2003. August;83(4):248–52. . Epub 2003/07/29. eng. [DOI] [PubMed] [Google Scholar]
- 200. Guillem P, Triboulet JP. Elevated serum levels of C-reactive protein are indicative of a poor prognosis in patients with esophageal cancer. Dis Esophagus. 2005;18(3):146–50. . Epub 2005/07/28. eng. [DOI] [PubMed] [Google Scholar]
- 201. Gockel I, Dirksen K, Messow CM, Junginger T. Significance of preoperative C-reactive protein as a parameter of the perioperative course and long-term prognosis in squamous cell carcinoma and adenocarcinoma of the oesophagus. World J Gastroenterol. 2006. June 21;12(23):3746–50. . Epub 2006/06/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Crumley AB, McMillan DC, McKernan M, Going JJ, Shearer CJ, Stuart RC. An elevated C-reactive protein concentration, prior to surgery, predicts poor cancer-specific survival in patients undergoing resection for gastro-oesophageal cancer. Br J Cancer. 2006. June 5;94(11):1568–71. . Pubmed Central PMCID: 2361311. Epub 2006/05/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Kobayashi T, Teruya M, Kishiki T, Endo D, Takenaka Y, Tanaka H, et al. Inflammation-based prognostic score, prior to neoadjuvant chemoradiotherapy, predicts postoperative outcome in patients with esophageal squamous cell carcinoma. Surgery. 2008. November;144(5):729–35. Epub 2008/12/17. eng. 10.1016/j.surg.2008.08.015 [DOI] [PubMed] [Google Scholar]
- 204. Wang CY, Hsieh MJ, Chiu YC, Li SH, Huang HW, Fang FM, et al. Higher serum C-reactive protein concentration and hypoalbuminemia are poor prognostic indicators in patients with esophageal cancer undergoing radiotherapy. Radiother Oncol. 2009. August;92(2):270–5. Epub 2009/02/07. eng. 10.1016/j.radonc.2009.01.002 [DOI] [PubMed] [Google Scholar]
- 205. Kobayashi T, Teruya M, Kishiki T, Kaneko S, Endo D, Takenaka Y, et al. Inflammation-based prognostic score and number of lymph node metastases are independent prognostic factors in esophageal squamous cell carcinoma. Dig Surg. 2010. August;27(3):232–7. Epub 2010/06/24. eng. 10.1159/000276910 [DOI] [PubMed] [Google Scholar]
- 206. Fujiwara H, Suchi K, Okamura S, Okamura H, Umehara S, Todo M, et al. Elevated serum CRP levels after induction chemoradiotherapy reflect poor treatment response in association with IL-6 in serum and local tumor site in patients with advanced esophageal cancer. J Surg Oncol. 2011. January 1;103(1):62–8. Epub 2010/10/30. eng. 10.1002/jso.21751 [DOI] [PubMed] [Google Scholar]
- 207. Vashist YK, Loos J, Dedow J, Tachezy M, Uzunoglu G, Kutup A, et al. Glasgow Prognostic Score is a predictor of perioperative and long-term outcome in patients with only surgically treated esophageal cancer. Ann Surg Oncol. 2011. April;18(4):1130–8. Epub 2010/10/29. eng. 10.1245/s10434-010-1383-7 [DOI] [PubMed] [Google Scholar]
- 208. Dutta S, Crumley AB, Fullarton GM, Horgan PG, McMillan DC. Comparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative resection of oesophageal cancer. World J Surg. 2011. August;35(8):1861–6. Epub 2011/05/04. eng. 10.1007/s00268-011-1130-7 [DOI] [PubMed] [Google Scholar]
- 209. Dutta S, Going JJ, Crumley AB, Mohammed Z, Orange C, Edwards J, et al. The relationship between tumour necrosis, tumour proliferation, local and systemic inflammation, microvessel density and survival in patients undergoing potentially curative resection of oesophageal adenocarcinoma. Br J Cancer. 2012. February 14;106(4):702–10. Pubmed Central PMCID: 3322960. Epub 2012/01/14. eng. 10.1038/bjc.2011.610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Deans DA, Wigmore SJ, Gilmour H, Paterson-Brown S, Ross JA, Fearon KC. Elevated tumour interleukin-1beta is associated with systemic inflammation: A marker of reduced survival in gastro-oesophageal cancer. Br J Cancer. 2006. December 4;95(11):1568–75. . Pubmed Central PMCID: 2360731. Epub 2006/11/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Rashid SA, O'Quigley J, Axon AT, Cooper EH. Plasma protein profiles and prognosis in gastric cancer. Br J Cancer. 1982. March;45(3):390–4. . Pubmed Central PMCID: 2010918. Epub 1982/03/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Crumley AB, Stuart RC, McKernan M, Going JJ, Shearer CJ, McMillan DC. Comparison of pre-treatment clinical prognostic factors in patients with gastro-oesophageal cancer and proposal of a new staging system. J Gastrointest Surg. 2010. May;14(5):781–7. Epub 2010/02/12. eng. 10.1007/s11605-010-1162-6 [DOI] [PubMed] [Google Scholar]
- 213. Crumley ABC. Interrelationship between tumor proliferation activity, leucocyte and macrophage infiltration, systemic inflammatory response and survival in patients selected for potentially curative resection. Ann Surg Oncol. 2011;18:2604–12. 10.1245/s10434-011-1658-7 [DOI] [PubMed] [Google Scholar]
- 214. de Mello J, Struthers L, Turner R, Cooper EH, Giles GR. Multivariate analyses as aids to diagnosis and assessment of prognosis in gastrointestinal cancer. Br J Cancer. 1983. September;48(3):341–8. . Pubmed Central PMCID: 2011475. Epub 1983/09/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Crumley AB, Stuart RC, McKernan M, McMillan DC. Is hypoalbuminemia an independent prognostic factor in patients with gastric cancer? World J Surg. 2010. October;34(10):2393–8. Epub 2010/07/06. eng. 10.1007/s00268-010-0641-y [DOI] [PubMed] [Google Scholar]
- 216. Nozoe T, Iguchi T, Adachi E, Matsukuma A, Ezaki T. Preoperative elevation of serum C-reactive protein as an independent prognostic indicator for gastric cancer. Surg Today. 2011. April;41(4):510–3. Epub 2011/03/25. eng. 10.1007/s00595-009-4297-x [DOI] [PubMed] [Google Scholar]
- 217. Nozoe T, Iguchi T, Egashira A, Adachi E, Matsukuma A, Ezaki T. Significance of modified Glasgow prognostic score as a useful indicator for prognosis of patients with gastric carcinoma. Am J Surg. 2011. February;201(2):186–91. Epub 2010/09/14. eng. 10.1016/j.amjsurg.2010.01.030 [DOI] [PubMed] [Google Scholar]
- 218. Iwasa S, Nakajima TE, Nakamura K, Takashima A, Kato K, Hamaguchi T, et al. Systemic chemotherapy for peritoneal disseminated gastric cancer with inadequate oral intake: a retrospective study. Int J Clin Oncol. 2011. February;16(1):57–62. Epub 2010/10/16. eng. 10.1007/s10147-010-0135-9 [DOI] [PubMed] [Google Scholar]
- 219. Shimura T, Kitagawa M, Yamada T, Ebi M, Mizoshita T, Tanida S, et al. C-reactive protein is a potential prognostic factor for metastatic gastric cancer. Anticancer Res. 2012. February;32(2):491–6. . Epub 2012/01/31. eng. [PubMed] [Google Scholar]
- 220. Jiang X, Hiki N, Nunobe S, Kumagai K, Kubota T, Aikou S, et al. Prognostic importance of the inflammation-based Glasgow prognostic score in patients with gastric cancer. Br J Cancer. 2012. July 10;107(2):275–9. Pubmed Central PMCID: 3394986. Epub 2012/06/21. eng. 10.1038/bjc.2012.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Kunisaki C, Takahashi M, Ono HA, Oshima T, Takagawa R, Kimura J, et al. Inflammation-based prognostic score predicts survival in patients with advanced gastric cancer receiving biweekly docetaxel and s-1 combination chemotherapy. Oncology. 2012;83(4):183–91. Epub 2012/08/15. eng. 10.1159/000341346 [DOI] [PubMed] [Google Scholar]
- 222. Jeong JH, Lim SM, Yun JY, Rhee GW, Lim JY, Cho JY, et al. Comparison of two inflammation-based prognostic scores in patients with unresectable advanced gastric cancer. Oncology. 2012;83(5):292–9. Epub 2012/09/12. eng. 10.1159/000342376 [DOI] [PubMed] [Google Scholar]
- 223. O'Gorman P, McMillan DC, McArdle CS. Prognostic factors in advanced gastrointestinal cancer patients with weight loss. Nutr Cancer. 2000;37(1):36–40. . Epub 2000/08/31. eng. [DOI] [PubMed] [Google Scholar]
- 224. Elahi MM, McMillan DC, McArdle CS, Angerson WJ, Sattar N. Score based on hypoalbuminemia and elevated C-reactive protein predicts survival in patients with advanced gastrointestinal cancer. Nutr Cancer. 2004;48(2):171–3. . Epub 2004/07/03. eng. [DOI] [PubMed] [Google Scholar]
- 225. McMillan DC, Wotherspoon HA, Fearon KC, Sturgeon C, Cooke TG, McArdle CS. A prospective study of tumor recurrence and the acute-phase response after apparently curative colorectal cancer surgery. Am J Surg. 1995. October;170(4):319–22. . Epub 1995/10/01. eng. [DOI] [PubMed] [Google Scholar]
- 226. Kara EK, M A; Erhan Y et al. The value of serum C-reactive protein, carcinoembryonic antigen and cancer-associated antigen 19.9 as prognostic factors for recurrence in colorectal cancer. Journal of B U On. 2001;6(1):91–4. [Google Scholar]
- 227. Shimada H, Kitabayashi H, Nabeya Y, Okazumi S, Matsubara H, Funami Y, et al. Treatment response and prognosis of patients after recurrence of esophageal cancer. Surgery. 2003. January;133(1):24–31. . Epub 2003/02/04. eng. [DOI] [PubMed] [Google Scholar]
- 228. Mohri Y, Tanaka K, Ohi M, Toiyama Y, Yasuda H, Inoue Y, et al. Inflammation-based prognostic score as a predictor of postoperative gastric cancer recurrence. Anticancer Res. 2012. October;32(10):4581–4. . Epub 2012/10/13. eng. [PubMed] [Google Scholar]
- 229. Simpson WG, Heys SD, Whiting PH, Eremin O, Broom J. Acute phase proteins and recombinant IL-2 therapy: prediction of response and survival in patients with colorectal cancer. Clin Exp Immunol. 1995. February;99(2):143–7. . Pubmed Central PMCID: 1534287. Epub 1995/02/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Miyake H, Sakai I, Muramaki M, Kurahashi T, Takenaka A, Fujisawa M. Prediction of response to combined immunotherapy with interferon-alpha and low-dose interleukin-2 in metastatic renal cell carcinoma: expression patterns of potential molecular markers in radical nephrectomy specimens. Int J Urol. 2009. May;16(5):465–71. . Epub 2009/05/27. eng. [DOI] [PubMed] [Google Scholar]
- 231. Ohno Y, Nakashima J, Ohori M, Hatano T, Tachibana M. Pretreatment neutrophil-to-lymphocyte ratio as an independent predictor of recurrence in patients with nonmetastatic renal cell carcinoma. J Urol. 2010. September;184(3):873–8. Epub 2010/07/21. eng. 10.1016/j.juro.2010.05.028 [DOI] [PubMed] [Google Scholar]
- 232. Melichar B, Krcmova L, Kalabova H, Holeckova P, Kasparova M, Plisek J, et al. Serum retinol, alpha-tocopherol and systemic inflammatory response in metastatic colorectal carcinoma patients treated with combination chemotherapy and cetuximab. Journal of nutritional science and vitaminology. 2010;56(4):222–6. . Epub 2010/10/07. eng. [DOI] [PubMed] [Google Scholar]
- 233. Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Inflammation-based prognostic system predicts postoperative survival of colorectal cancer patients with a normal preoperative serum level of carcinoembryonic antigen. Ann Surg Oncol. 2012. October;19(11):3422–31. Epub 2012/05/12. eng. 10.1245/s10434-012-2384-5 [DOI] [PubMed] [Google Scholar]
- 234. Wilop S, Crysandt M, Bendel M, Mahnken AH, Osieka R, Jost E. Correlation of C-reactive protein with survival and radiographic response to first-line platinum-based chemotherapy in advanced non-small cell lung cancer. Onkologie. 2008. December;31(12):665–70. Epub 2008/12/09. eng. 10.1159/000165054 [DOI] [PubMed] [Google Scholar]
- 235. Gondo T, Nakashima J, Ohno Y, Choichiro O, Horiguchi Y, Namiki K, et al. Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology. 2012. May;79(5):1085–91. Epub 2012/03/27. eng. 10.1016/j.urology.2011.11.070 [DOI] [PubMed] [Google Scholar]
- 236. Muller MW, Friess H, Koninger J, Martin D, Wente MN, Hinz U, et al. Factors influencing survival after bypass procedures in patients with advanced pancreatic adenocarcinomas. Am J Surg. 2008. February;195(2):221–8. . Epub 2007/12/25. eng. [DOI] [PubMed] [Google Scholar]
- 237. Skipworth RJ, Moses AG, Sangster K, Sturgeon CM, Voss AC, Fallon MT, et al. Interaction of gonadal status with systemic inflammation and opioid use in determining nutritional status and prognosis in advanced pancreatic cancer. Support Care Cancer. 2011. March;19(3):391–401. Epub 2010/03/12. eng. 10.1007/s00520-010-0832-y [DOI] [PubMed] [Google Scholar]
- 238. Furukawa K, Uwagawa T, Iwase R, Haruki K, Fujiwara Y, Gocho T, et al. Prognostic factors of unresectable pancreatic cancer treated with nafamostat mesilate combined with gemcitabine chemotherapy. Anticancer Res. 2012. November;32(11):5121–6. . Epub 2012/11/17. eng. [PubMed] [Google Scholar]
- 239. Frigeri M, De Dosso S, Castillo-Fernandez O, Feuerlein K, Neuenschwander H, Saletti P. Chemotherapy in patients with advanced pancreatic cancer: too close to death? Support Care Cancer. 2013. January;21(1):157–63. Epub 2012/06/01. eng. 10.1007/s00520-012-1505-9 [DOI] [PubMed] [Google Scholar]
- 240. Haas M, Laubender RP, Klose C, Schulz C, Mansmann U, Boeck S, et al. External validation of 2 prognostic indices for patients with advanced pancreatic cancer treated with first-line therapy. Pancreas. 2012. July;41(5):738–44. . Epub 2012/01/31. eng. [DOI] [PubMed] [Google Scholar]
- 241. Wang DS, Luo HY, Qiu MZ, Wang ZQ, Zhang DS, Wang FH, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012. December;29(5):3092–100. Epub 2012/04/06. eng. 10.1007/s12032-012-0226-8 [DOI] [PubMed] [Google Scholar]
- 242. Hefler-Frischmuth K, Seebacher V, Polterauer S, Tempfer C, Reinthaller A, Hefler L. The inflammation-based modified Glasgow Prognostic Score in patients with vulvar cancer. Eur J Obstet Gynecol Reprod Biol. 2010. March;149(1):102–5. Epub 2010/01/19. eng. 10.1016/j.ejogrb.2009.12.027 [DOI] [PubMed] [Google Scholar]
- 243. Grimm M, Lazariotou M. Clinical relevance of a new pre-treatment laboratory prognostic index in patients with oral squamous cell carcinoma. Med Oncol. 2012. September;29(3):1435–47. Epub 2011/08/20. eng. 10.1007/s12032-011-0045-3 [DOI] [PubMed] [Google Scholar]
- 244. Cho KS, Choi YD, Kim SJ, Kim CI, Chung BH, Seong do H, et al. A comprehensive prognostic stratification for patients with metastatic renal clear cell carcinoma. Yonsei medical journal. 2008. June 30;49(3):451–8. Pubmed Central PMCID: PMC2615339. Epub 2008/06/27. eng. 10.3349/ymj.2008.49.3.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Komura K, Inamoto T, Black PC, Koyama K, Katsuoka Y, Watsuji T, et al. Prognostic significance of body mass index in Asian patients with localized renal cell carcinoma. Nutr Cancer. 2011;63(6):908–15. Epub 2011/08/02. eng. 10.1080/01635581.2011.594207 [DOI] [PubMed] [Google Scholar]
- 246. Hotta K, Sho M, Fujimoto K, Shimada K, Yamato I, Anai S, et al. Prognostic significance of CD45RO+ memory T cells in renal cell carcinoma. Br J Cancer. 2011. October 11;105(8):1191–6. Pubmed Central PMCID: PMC3208496. Epub 2011/09/22. eng. 10.1038/bjc.2011.368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Park YH, Baik KD, Lee YJ, Ku JH, Kim HH, Kwak C. Late recurrence of renal cell carcinoma> 5 years after surgery: clinicopathological characteristics and prognosis. BJU international. 2012;110(11b):E553–E8. [DOI] [PubMed] [Google Scholar]
- 248. Ikeguchi M, Hatada T, Yamamoto M, Miyake T, Matsunaga T, Fukumoto Y, et al. Serum interleukin-6 and -10 levels in patients with gastric cancer. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2009;12(2):95–100. . Epub 2009/06/30. eng. [DOI] [PubMed] [Google Scholar]
- 249. Takagawa R, Kunisaki C, Makino H, Kosaka T, Ono HA, Akiyama H, et al. Efficacy of chemoradiotherapy with low-dose cisplatin and continuous infusion of 5-fluorouracil for unresectable squamous cell carcinoma of the esophagus. Dis Esophagus. 2009;22(6):482–9. Epub 2009/02/05. eng. 10.1111/j.1442-2050.2008.00935.x [DOI] [PubMed] [Google Scholar]
- 250. Lee WS, Baek JH, You DH, Nam MJ. Prognostic value of circulating cytokines for stage III colon cancer. The Journal of surgical research. 2013. June 1;182(1):49–54. Epub 2012/09/27. eng. 10.1016/j.jss.2012.08.051 [DOI] [PubMed] [Google Scholar]
- 251. Kwon HC, Kim SH, Oh SY, Lee S, Lee JH, Jang JS, et al. Clinicopathologic significance of expression of nuclear factor-kappaB RelA and its target gene products in gastric cancer patients. World J Gastroenterol. 2012. September 14;18(34):4744–50. . Pubmed Central PMCID: PMC3442213. Epub 2012/09/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Garcea G, Ladwa N, Neal CP, Metcalfe MS, Dennison AR, Berry DP. Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J Surg. 2011. April;35(4):868–72. Epub 2011/02/12. eng. 10.1007/s00268-011-0984-z [DOI] [PubMed] [Google Scholar]
- 253. Suh SY, Choi YS, Shim JY, Kim YS, Yeom CH, Kim D, et al. Construction of a new, objective prognostic score for terminally ill cancer patients: a multicenter study. Support Care Cancer. 2010. February;18(2):151–7. Epub 2009/04/22. eng. 10.1007/s00520-009-0639-x [DOI] [PubMed] [Google Scholar]
- 254. Six L, Polterauer S, Grimm C, Seebacher V, Tempfer C, Heinze G, et al. C-reactive protein serum levels are closely associated with lymph node status, but not with prognosis in patients with vulvar cancer. Eur J Obstet Gynecol Reprod Biol. 2008. April;137(2):217–21. . Epub 2007/04/27. eng. [DOI] [PubMed] [Google Scholar]
- 255. Kruse AL, Luebbers HT, Gratz KW. C-reactive protein levels: a prognostic marker for patients with head and neck cancer? Head & neck oncology. 2010;2:21 . Pubmed Central PMCID: PMC2921363. Epub 2010/08/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLS)
(XLS)
(DOC)
Data Availability Statement
All data are fully available without restriction. Data files uploaded as supporting information files (zipped).