Abstract
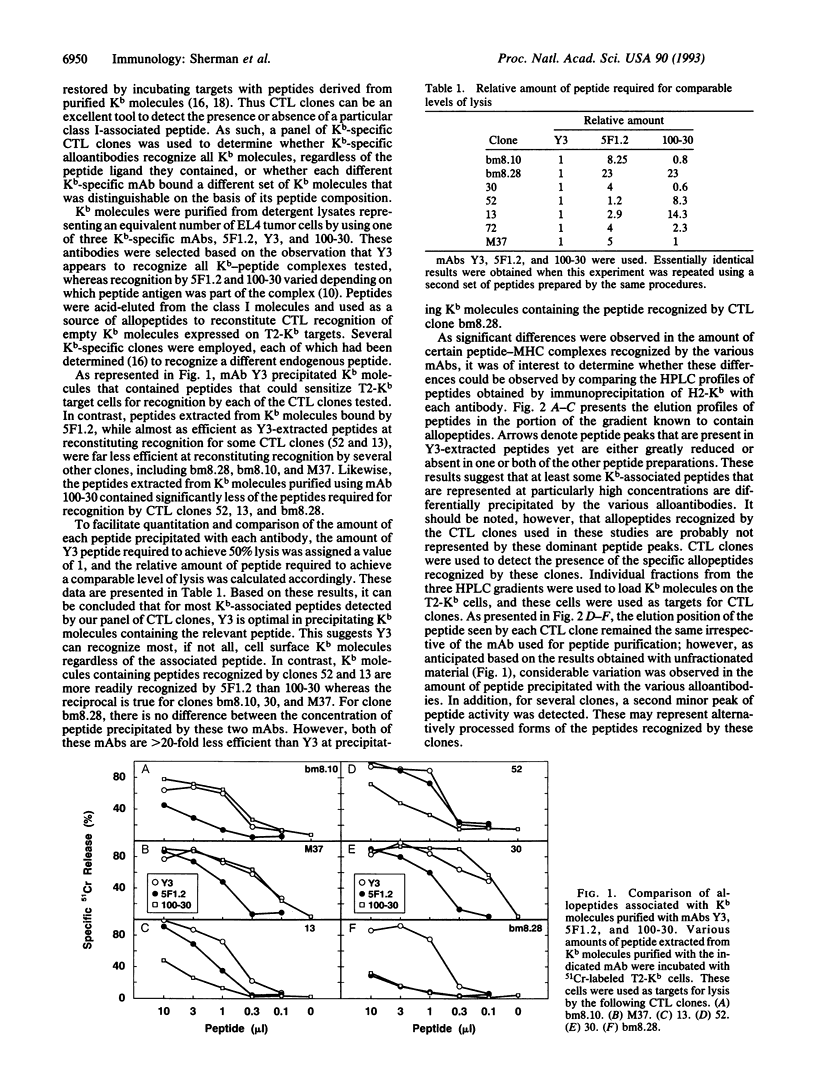
Molecules encoded by a single major histocompatibility complex class I gene can associate with any one of a large number of peptide ligands. T-cell receptors have the capacity to discriminate among these peptide-class I complexes and in many cases bind only a single peptide-class I complex with sufficient affinity to trigger effector function. In contrast, it is generally assumed that class I-specific alloantibodies are indifferent to peptide heterogeneity, being directed toward allele-specific determinants on the molecule. In this report, three monoclonal antibodies were used to precipitate Kb molecules from cell lysates. Surprisingly, in each case a different set of peptides was found to be associated with Kb as detected by peptide-dependent Kb-specific alloreactive cytolytic T lymphocytes or by biochemical resolution. These results demonstrate that the affinity of binding by alloantibodies can be affected by the endogenous peptide ligand.
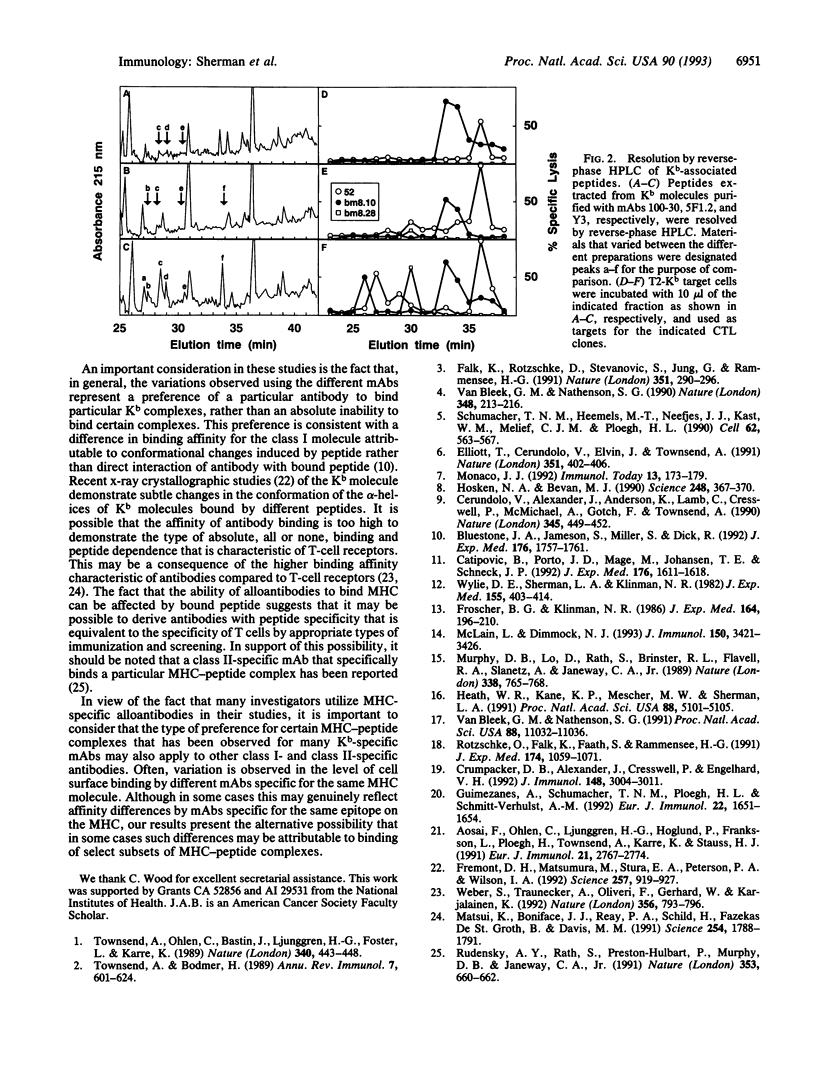
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aosai F., Ohlen C., Ljunggren H. G., Höglund P., Franksson L., Ploegh H., Townsend A., Kärre K., Stauss H. J. Different types of allospecific CTL clones identified by their ability to recognize peptide loading-defective target cells. Eur J Immunol. 1991 Nov;21(11):2767–2774. doi: 10.1002/eji.1830211118. [DOI] [PubMed] [Google Scholar]
- Bluestone J. A., Jameson S., Miller S., Dick R., 2nd Peptide-induced conformational changes in class I heavy chains alter major histocompatibility complex recognition. J Exp Med. 1992 Dec 1;176(6):1757–1761. doi: 10.1084/jem.176.6.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catipović B., Dal Porto J., Mage M., Johansen T. E., Schneck J. P. Major histocompatibility complex conformational epitopes are peptide specific. J Exp Med. 1992 Dec 1;176(6):1611–1618. doi: 10.1084/jem.176.6.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerundolo V., Alexander J., Anderson K., Lamb C., Cresswell P., McMichael A., Gotch F., Townsend A. Presentation of viral antigen controlled by a gene in the major histocompatibility complex. Nature. 1990 May 31;345(6274):449–452. doi: 10.1038/345449a0. [DOI] [PubMed] [Google Scholar]
- Crumpacker D. B., Alexander J., Cresswell P., Engelhard V. H. Role of endogenous peptides in murine allogenic cytotoxic T cell responses assessed using transfectants of the antigen-processing mutant 174xCEM.T2. J Immunol. 1992 May 15;148(10):3004–3011. [PubMed] [Google Scholar]
- Elliott T., Cerundolo V., Elvin J., Townsend A. Peptide-induced conformational change of the class I heavy chain. Nature. 1991 May 30;351(6325):402–406. doi: 10.1038/351402a0. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Fremont D. H., Matsumura M., Stura E. A., Peterson P. A., Wilson I. A. Crystal structures of two viral peptides in complex with murine MHC class I H-2Kb. Science. 1992 Aug 14;257(5072):919–927. doi: 10.1126/science.1323877. [DOI] [PubMed] [Google Scholar]
- Froscher B. G., Klinman N. R. Immunization with SV40-transformed cells yields mainly MHC-restricted monoclonal antibodies. J Exp Med. 1986 Jul 1;164(1):196–210. doi: 10.1084/jem.164.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimezanes A., Schumacher T. N., Ploegh H. L., Schmitt-Verhulst A. M. A viral peptide can mimic an endogenous peptide for allorecognition of a major histocompatibility complex class I product. Eur J Immunol. 1992 Jun;22(6):1651–1654. doi: 10.1002/eji.1830220647. [DOI] [PubMed] [Google Scholar]
- Heath W. R., Kane K. P., Mescher M. F., Sherman L. A. Alloreactive T cells discriminate among a diverse set of endogenous peptides. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5101–5105. doi: 10.1073/pnas.88.12.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosken N. A., Bevan M. J. Defective presentation of endogenous antigen by a cell line expressing class I molecules. Science. 1990 Apr 20;248(4953):367–370. doi: 10.1126/science.2326647. [DOI] [PubMed] [Google Scholar]
- Matsui K., Boniface J. J., Reay P. A., Schild H., Fazekas de St Groth B., Davis M. M. Low affinity interaction of peptide-MHC complexes with T cell receptors. Science. 1991 Dec 20;254(5039):1788–1791. doi: 10.1126/science.1763329. [DOI] [PubMed] [Google Scholar]
- McLain L., Dimmock N. J. A monoclonal antibody produced during infection which recognizes an epitope of influenza hemagglutinin only in the context of H-2k MHC class I antigen. J Immunol. 1993 Apr 15;150(8 Pt 1):3421–3426. [PubMed] [Google Scholar]
- Monaco J. J. A molecular model of MHC class-I-restricted antigen processing. Immunol Today. 1992 May;13(5):173–179. doi: 10.1016/0167-5699(92)90122-N. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Lo D., Rath S., Brinster R. L., Flavell R. A., Slanetz A., Janeway C. A., Jr A novel MHC class II epitope expressed in thymic medulla but not cortex. Nature. 1989 Apr 27;338(6218):765–768. doi: 10.1038/338765a0. [DOI] [PubMed] [Google Scholar]
- Rudensky AYu, Rath S., Preston-Hurlburt P., Murphy D. B., Janeway C. A., Jr On the complexity of self. Nature. 1991 Oct 17;353(6345):660–662. doi: 10.1038/353660a0. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Faath S., Rammensee H. G. On the nature of peptides involved in T cell alloreactivity. J Exp Med. 1991 Nov 1;174(5):1059–1071. doi: 10.1084/jem.174.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher T. N., Heemels M. T., Neefjes J. J., Kast W. M., Melief C. J., Ploegh H. L. Direct binding of peptide to empty MHC class I molecules on intact cells and in vitro. Cell. 1990 Aug 10;62(3):563–567. doi: 10.1016/0092-8674(90)90020-f. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Weber S., Traunecker A., Oliveri F., Gerhard W., Karjalainen K. Specific low-affinity recognition of major histocompatibility complex plus peptide by soluble T-cell receptor. Nature. 1992 Apr 30;356(6372):793–796. doi: 10.1038/356793a0. [DOI] [PubMed] [Google Scholar]
- Wylie D. E., Sherman L. A., Klinman N. R. Participation of the major histocompatibility complex in antibody recognition of viral antigens expressed on infected cells. J Exp Med. 1982 Feb 1;155(2):403–414. doi: 10.1084/jem.155.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bleek G. M., Nathenson S. G. The structure of the antigen-binding groove of major histocompatibility complex class I molecules determines specific selection of self-peptides. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11032–11036. doi: 10.1073/pnas.88.24.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]



