Abstract
Lactobacillus plantarum is a Gram positive lactic acid bacterium commonly found in fermented food and in the gastro intestinal tract and is commonly used in the food industry as a potential starter probiotic. Recently, the consumption of food together with probiotics has tremendously increased. Among the lactic acid bacteria, L. plantarum attracted many researchers because of its wide applications in the medical field with antioxidant, anticancer, anti-inflammatory, antiproliferative, anti-obesity and antidiabetic properties. The present study aimed to investigate the in vitro importance of L. plantarum toward medical applications. Moreover, this report short listed various reports related to the applications of this promising strain. In conclusion, this study would attract the researchers in commercializing this strain toward the welfare of humans related to medical needs.
Keywords: Lactobacillus plantarum, Medical applications
1. Introduction
The human digestive system contains approximately four hundred different bacterial species and its abundance differs between individuals. Among them few probiotic Lactobacillus species namely, Lactobacillus acidophilus, Lactobacillus pentosus, Lactobacillus brevis, Lactobacillus lactis, Lactobacillus amylovorus, Lactobacillus casei, Lactobacillus bulgaricus, Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus rhamnosus specifically produce extracellular proteins, exopolysaccharides, bacteriocins and lipoteichoic acids which influence the health and physiology of the host by interacting with the epithelial cells and enhance the host immune system (Sanchez et al., 2010). Lactobacillus strains are recognized as safe for consumption because of their presence in food and their role in the gut defense mechanism. Of the Lactobacillus strains, L. plantarum is a Gram positive, short-rod, micro-aerophilic, acid-tolerant, non-spore forming, non-respiring, low G + C content, hetero-fermentative group of lactobacilli with a range of applications in the food industry as a starter culture and preservatives (Arasu et al., 2013). It is a non-spore forming bacterium which produces organic acids such as acetic acid, succinic acid and lactic acid as major metabolites. The antibacterial, antifungal and probiotic properties of LAB strains have been widely studied (Rejiniemon et al., 2015). L. plantarum grow under low buffering capacity in the stomach and other complex bile salt secretions in humans and other mammals. Besides applications in the food industry, L. plantarum has wide applications in the pharma industry by contributing significantly to human medicine without contributing to any side effects. Recently, L. plantarum has been applied in medical fields for the treatment of various chronic and cardiovascular diseases such as Alzheimer’s, Parkinson’s, diabetes, obesity, cancer, hypertension, urinogenital complications, liver disorders, etc. (Woo et al., 2014). The present study aimed to investigate the in vitro importance of L. plantarum related to the medical field.
2. Materials and methods
2.1. Isolation of Lactobacillus strains
Novel L. plantarum was isolated from the silage (Arasu et al., 2013). For the isolation process, the silage sample was serially diluted and spread on de Man–Rogosa (MRS) agar and incubated at 37 °C for three days. Morphological, biochemical and physiological characteristics of the strains were examined by following the reported literature. Phenotypic characteristics were studied using API 50CHB kits. The growth and fermentation patent of the strains under various sugars were determined by following the standard method.
2.2. Importance of L. plantarum
The in vitro application of the L. plantarum was reported by many researchers. Besides the applications of these strains in our lab, the importance of these strains reported by other researchers was summarized in this report.
3. Results and discussion
3.1. Identification and characterization of L. plantarum strains
The fermented food is commonly known for the presence of Lactobacillus strains. Besides protecting the nutritional quality, Lactobacillus strains were used for protecting the fermented food from various fungal pathogens. Many beneficial species of Lactobacillus strains were derived from the fermented food and other silage samples. The routine microbiological identification methods and 16S rRNA gene amplification followed by sequencing identified the strain as L. plantarum (Arasu et al., 2013). The physiological and biochemical identification study showed that the strain was catalase negative, Gram positive (Fig. 1). This novel strain was tolerant to a different range of salts especially NaCl and bile salts, pH of 4.0–8.0, temperatures of 28–45 °C, and with optimum cell growth at a temperature of 37 °C and pH 7.0 respectively. Similar to the literature the identified strain survived various biological barriers such as low pH, lytic enzymes, and bile salts in the upper GI tract (Vijayakumar et al., 2015). Carbohydrate assimilation test concluded that the strain was able to utilize a wide range of sugars especially monosaccharide’s and disaccharides respectively. Moreover, the production of extracellular enzymes such as amylase and protease was to its advantage. The above mentioned results were commonly observed in L. plantarum strains.
Figure 1.
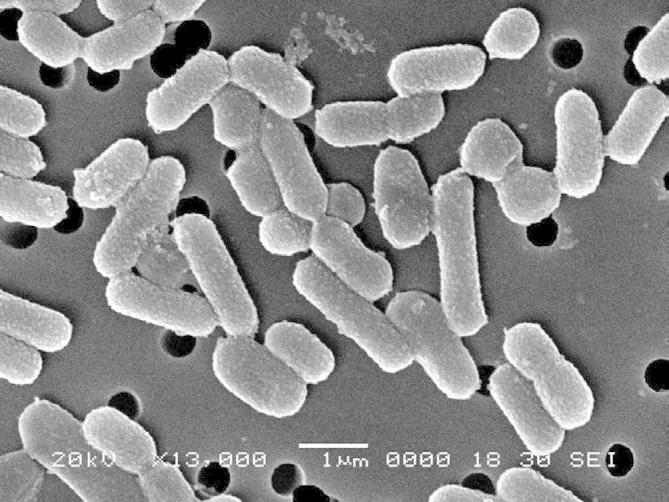
Micro-morphological image of Lactobacillus plantarum strain.
The bile salt tolerance level of the Lactobacillus strains was induced by the expression of proteins such as GshR4, Cfa2, Bsh1, OpuA, and AtpH (Hamon et al., 2011). Besides the tolerance level, the antagonistic properties of the novel Lactobacillus strains are important to prevent the spreading of the intestinal infections. In general, the probiotic Lactobacillus strains exhibited significant antimicrobial activity against various GI tract pathogens. The antimicrobial properties of the strains were mainly related to the secretion of the extracellular metabolites such as lactic acid, acetic acid, succinic acid, and bacteriocins.
3.2. Importance L. plantarum strains
Literature claimed that L. plantarum strains were widely studied for their applications in the medical field (Table 1). Especially, these strains were reported to posses the down regulation of the risk of cardiovascular diseases (Ahren et al., 2014), produce pro-inflammatory cytokines in the intestinal epithelial cells (Murofushi et al., 2015), produce varied concentrations of exopolysaccharide with anticancer property (Wang et al., 2014), reduce kidney stones (Sasikumar et al., 2014), enhance splenocytes in dendritic cells (Ku et al., 2014) and reduce the cholesterol level in the adipose tissue (Li et al., 2014. Recently, Ilavenil et al. (2015) claimed that the phenyl lactic acid recovered from L. plantarum promotes adipogenic activity in 3T3-L1. Interestingly, L. plantarum significantly induces mucosal, humoral and cellular immune responses (Shi et al., 2014) and protects against symptoms of irritable bowel syndrome (Stevenson et al., 2014). It inhibited the production of pro-inflammatory cytokines such as NF-κB and suppresses atherosclerotic plaque inflammation (Kim et al., 2013) and induces the enhanced production of cytokines in the human intestine (Salah et al., 2012). On the other hand, L. plantarum strains are mainly involved in the T-cell differentiation thereby improving immune responses toward antigens (Vissers et al., 2010) and preventing dermatitis by increment of type 1 helper T cell activation and regulatory T cell activation (Won et al., 2012).
Table 1.
Medical and pharmacological applications of Lactobacillus plantarum.
| Key finding | References |
|---|---|
| This study showed that L. plantarum together with blueberries significantly reduced hypertension and blood pressure. Therefore, this strain might be used for the down regulation of the risk of cardiovascular diseases | Ahren et al. (2014) |
| The exopolysaccharides obtained from L. plantarum significantly decreased the production of pro-inflammatory cytokines in the intestinal epithelial cells in a RP105/MD1-dependent manner | Murofushi et al. (2015) |
| This study reveals that the cell bound exopolysaccharide isolated from L. plantarum 78010 showed significant anticancer activity | Wang et al. (2014) |
| This study demonstrated that the L. plantarum expressing oxalate decarboxylase gene significantly degrades calcium oxalate in the kidney, thus protects the kidney from stones | Sasikumar et al. (2014) |
| The extra cellular products of L. plantarum revealed anticancer effects by increased trans-epithelial electrical resistance of H4 cells and decreased the secretion of pro-inflammatory cytokines IL-6 and IL-8 | Dimitrovski et al. (2014) |
| Oral supplementation of L. plantarum stimulated the expression of IL-12 and IFN-γ in splenocytes and activates MHC class II markers, CD80 and CD 86 in dendritic cells. This study confirmed that the probiotic strain has immune-modulatory effects | Ku et al. (2014) |
| This study showed that the L. plantarum ameliorates colitis by inhibiting the TLR-4-linked NF-κB and MAPK signaling pathways | Jang et al. (2014) |
| Administration of L. plantarum, regulates lipid metabolism in adipose tissues by lowering cholesterol level | Li et al. (2014) |
| Lactobacillus plantarum enhances the antiproliferative activity in the vascular smooth muscle cell through the suppression of cell cycle progression and expression of cell cycle-related proteins | Lee et al. (2014) |
| This study states that the oral administration of L. plantarum expressing the hemagglutinin (HA) gene of H9N2 AIV significantly induces the mucosal, humoral and cellular immune responses. Therefore, this vaccine could be used to prevent the spreading of H9N2 avian influenza virus and also transmission of AIV | Shi et al. (2014) |
| The reported data confirmed that the administration of L. plantarum significantly reduced levels of the total cholesterol, γ-glutamyl transpeptidase, low-density lipoprotein, glucose, homocysteine and interleukin-6 in postmenopausal women. These symptoms in postmenopausal women are an important risk factor for cardiovascular morbidity, especially stroke and coronary heart disease | Barreto et al. (2014) |
| The study demonstrates for the first time the protective role of L. plantarum on symptoms of irritable bowel syndrome | Stevenson et al. (2014) |
| This study confirmed that the oral administration of L. plantarum stimulates high levels of pro-inflammatory cytokine IL-12 and low levels of anti-inflammatory cytokine IL-10, whereas in hepatic and renal cells it induces the levels of alanine amino transferase, gamma glutamyl transferase, plasmatic triglycerides, total cholesterol, creatinine and urea concentrations | Salah et al. (2013) |
| Lipoteichoic acid obtained from L. plantarum inhibited the production of pro-inflammatory cytokines such as NF-κB and suppresses the atherosclerotic plaque inflammation | Kim et al. (2013) |
| This study confirmed that the administration of heat killed or live L. plantarum attenuates the symptom of Crohn’s disease and ulcerative colitis | Chiu et al. (2013) |
| L. plantarum recovered from Korean traditional pickle exhibited significant antioxidant activity. This study concluded that the administration of this strain has various oxidative effects | Arasu et al. (2014) |
| Lactobacillus plantarum derived from the intestine induces the enhanced production of cytokine | Salah et al. (2013) |
| Regular intake of the diet with Lactobacillus strains reduces the body weight and white cell size of the adipose tissue | Grover et al. (2012) |
| This study depicts that the supplementation of L. plantarum prevents dermatitis by increment of type 1 helper T cell activation and regulatory T cell activation | Won et al. (2012) |
| Lactobacillus plantarum exerts anti-cancer effects in 1,2-dimethyl hydrazine (DMH)-induced colorectal cancer | Kumar et al. (2012) |
| Oral administration of L. plantarum K68 prevents the spreading of ulcer and exhibited comparatively better anti-inflammatory and immune modulatory activities by inhibiting the synthesis of factor-α and prostaglandin E(2) in macrophage | Liu et al. (2011) |
| This study documented that the plantaricin A produced by L. plantarum stimulates in vitro proliferation and migration of human keratinocytes | Pinto et al. (2011) |
| This study concluded that the L. plantarum strains are mainly involved in the T-cell differentiation, thereby improving the immune responses toward antigens | Vissers et al. (2010) |
| Tight junction formation in the intestine is stimulated by in-vivo administration of L. plantarum | Anderson et al. (2010) |
| Bacterial infection causes a serious problem in curing wounds especially in the ulcer stage. In this report, external application of L. plantarum on the ulcer patient cures the wounds of the diabetic patient. The investigations concluded that levels of polymorphonuclear, apoptotic and necrotic cells were completely decreased | Pera et al. (2010) |
| Oral administrations of L. plantarum cure obstructive jaundice and protect the liver from different barriers | Zhang et al. (2010) |
| Lactobacillus plantarum coated with proteins and polysaccharides exhibited interesting hypocholesterolemic effects. This combination speeds up the degradation of hepatic cholesterol into bile acids | Jeun et al. (2010) |
| Lactobaciilus plantarum may improve human colon cancer. This is the first report about the pharmacological application of probiotic bacteria in the treatment of colon cancer and the mechanism of intestinal epithelial cells in immune responses | Paolillo et al. (2009) |
4. Conclusion
In conclusion, among the lactic acid bacteria, L. plantarum plays an important role in medical applications. The summarized recent report related to L. plantarum would be useful to the pharmaceutical industry for the preparation of medical formulations without side effects.
Acknowledgement
The Project was full financially supported by King Saud University, through Vice Deanship of Research Chairs.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahren I.L., Jie X., Önning G., Olsson C., Ahrné S., Molin G. Antihypertensive activity of blueberries fermented by Lactobacillus plantarum DSM 15313 and effects on the gut microbiota in healthy rats. Clin. Nutr. 2014:1–14. doi: 10.1016/j.clnu.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Anderson R.C., Cookson A.L., McNabb W.C., Park Z., McCann M.J., Kelly W.J., Roy N.C. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010;10:316. doi: 10.1186/1471-2180-10-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasu M.V., Jung M.W., Ilavenil S., Jane M., Kim D.H., Lee K.D., Park H.S., Hur T.Y., Choi G.J., Lim Y.C., Al-Dhabi N.A., Choi K.C. Isolation and characterization of antifungal compound from Lactobacillus plantarum KCC-10 from forage silage with potential beneficial properties. J. Appl. Microbiol. 2013;115:1172–1185. doi: 10.1111/jam.12319. [DOI] [PubMed] [Google Scholar]
- Arasu M.V., Kim D.H., Kim P.I., Jung M.W., Ilavenil S., Jane M., Lee K.D., Al-Dhabi N.A., Choi K.C. In vitro antifungal, probiotic and antioxidant properties of novel Lactobacillus plantarum K46 isolated from fermented sesame leaf. Ann. Microbiol. 2014;64(3):1333–1346. [Google Scholar]
- Barreto F.M., Simão A.N.C., Morimoto H.K., Lozovoy M.A.I.B., Dichi I., Miglioranza L.H.S. Beneficial effects of Lactobacillus plantarum on glycemia and homocysteine levels in postmenopausal women with metabolic syndrome. Nutrition. 2014;30(7–8):939–942. doi: 10.1016/j.nut.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Chiu Y.-H., Lu Y.-C., Ou C.-C., Lin S.-L., Tsai C.-C., Huang C.-T., Lin M.-Y. Lactobacillus plantarum MYL26 induces endotoxin tolerance phenotype in Caco-2 cells. BMC Microbiol. 2013;13:190. doi: 10.1186/1471-2180-13-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrovski D., Cencič A., Winkelhausen E., Langerholc T. Lactobacillus plantarum extracellular metabolites: in vitro assessment of probiotic effects on normal and cancerogenic human cells. Int. Microbiol. J. 2014;39(2):293–300. [Google Scholar]
- Grover S., Rashmi H.M., Srivastava A.K., Batish V.K. Probiotics for human health – new innovations and emerging trends. Gut Pathog. 2012;4:15. doi: 10.1186/1757-4749-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon E., Horvatovich P., Izquierdo E., Bringel F., Marchioni E., Aoudé-Werner D., Ennahar S. Comparative proteomic analysis of Lactobacillus plantarum for the identification of key proteins in bile tolerance. BMC Microbiol. 2011;29(11):63. doi: 10.1186/1471-2180-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilavenil S., Kim D.H., Arasu M.V., Srigopalram S., Sivanesan R., Choi K.C. Phenyllactic acid from Lactobacillus plantarum promotes adipogenic activity in 3T3-L1 adipocyte via up-regulation of PPAR-γ2. Molecules. 2015;20(8):15359–15373. doi: 10.3390/molecules200815359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S.-E., Han M.J., Kim S.-Y., Kim D.-H. Lactobacillus plantarum CLP-0611 ameliorates colitis in mice by polarizing M1 to M2-like macrophages. Int. Immunopharmacol. 2014;21(1):186–192. doi: 10.1016/j.intimp.2014.04.021. [DOI] [PubMed] [Google Scholar]
- Jeun J., Kim S., Cho S.Y., Jun H.J., Park H.J., Seo J.G., Chung M.J., Lee S.J. Hypocholesterolemic effects of Lactobacillus plantarum KCTC3928 by increased bile acid excretion in C57BL/6 mice. Nutrition. 2010;26(3):321–330. doi: 10.1016/j.nut.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Kim J.Y., Kim H., Jung B.J., Kim N.R., Park J.E., Chung D.K. Lipoteichoic acid isolated from Lactobacillus plantarum suppresses LPS-mediated atherosclerotic plaque inflammation. Mol. Cells. 2013;35(2):115–124. doi: 10.1007/s10059-013-2190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku H.-K., Lee H., Choi I.D., Ra J.-H., Kim T.-Y., Jeong J.-W., Kim S.-H., Sim J.-H., Ahn Y.-T. Immuno-stimulatory effect of Lactobacillus plantarum HY7712 via toll-like receptor 2 signaling pathway. Cytokine. 2014;70(1):52. [Google Scholar]
- Kumar R.S., Kanmani P., Yuvaraj N., Paari K.A., Pattukumar V., Thirunavukkarasu C., Arul V. Lactobacillus plantarum AS1 isolated from south Indian fermented food kallappam suppress 1,2-dimethyl hydrazine (DMH)-induced colorectal cancer in male wistar rats. Appl. Biochem. Biotechnol. 2012;166(3):620–631. doi: 10.1007/s12010-011-9453-2. [DOI] [PubMed] [Google Scholar]
- Lee J.-J., Kwon H., Lee J.-H., Kim D.-G., Jung S.-H., Ma J.Y. Fermented soshiho-tang with Lactobacillus plantarum enhances the antiproliferative activity in vascular smooth muscle cell. BMC Complement. Altern. Med. 2014;14:78. doi: 10.1186/1472-6882-14-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Nie S.-P., Ding Q., Zhu K.-X., Wang Z.-J., Xiong T., Gong J., Xie M.-Y. Cholesterol-lowering effect of Lactobacillus plantarum NCU116 in a hyperlipidaemic rat model. J. Funct. Food. 2014;8:340–347. [Google Scholar]
- Liu Y.W., Su Y.W., Ong W.K., Cheng T.H., Tsai Y.C. Oral administration of Lactobacillus plantarum K68 ameliorates DSS-induced ulcerative colitis in BALB/c mice via the anti-inflammatory and immunomodulatory activities. Int. Immunopharmacol. 2011;11(12):2159–2166. doi: 10.1016/j.intimp.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Murofushi Y., Villena J., Morie K., Kanmani P., Tohno M., Shimazu T., Aso H., Suda Y., Hashiguchi K., Saito T., Kitazawa H. The toll-like receptor family protein RP105/MD1 complex is involved in the immunoregulatory effect of exopolysaccharides from Lactobacillus plantarum N14O. Mol. Immunol. 2015;64(1):63–75. doi: 10.1016/j.molimm.2014.10.027. [DOI] [PubMed] [Google Scholar]
- Paolillo R., Carratelli C.R., Sorrentino S., Mazzola N., Rizzo A. Immunomodulatory effects of Lactobacillus plantarum on human colon cancer cells. Int. Immunopharmacol. 2009;9(11):1265–1271. doi: 10.1016/j.intimp.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Pera M.C., Rachid M.M., Gobbato N.M., Martinez M.A.H., Valdez J.C. Interleukin-8 production by polymorphonuclear leukocytes from patients with chronic infected leg ulcers treated with Lactobacillus plantarum. Clin. Microbiol. Infect. 2010;16(3):281–286. doi: 10.1111/j.1469-0691.2009.02793.x. [DOI] [PubMed] [Google Scholar]
- Pinto D., Marzani B., Minervini F., Calasso M., Giuliani G., Gobbetti M., Angelis M.D. Plantaricin A synthesized by Lactobacillus plantarum induces in vitro proliferation and migration of human keratinocytes and increases the expression of TGF-β1, FGF7, VEGF-A and IL-8 genes. Peptides. 2011;32(9):1815–1824. doi: 10.1016/j.peptides.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Rejiniemon T.S., Hussain R.R., Rajamani B. In-vitro functional properties of Lactobacillus plantarum iso-lated from fermented ragi malt. South Ind. J. Biol. Sci. 2015;1:15–23. [Google Scholar]
- Salah R.B., Trabelsi I., Mansour R.B., Lassoued S., Chouayekh H., Bejar S.A. New Lactobacillus plantarum strain, TN8, from the gastro intestinal tract of poultry induces high cytokine production. Anaerobe. 2012;18(4):436–444. doi: 10.1016/j.anaerobe.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Salah R.B., Trabelsi I., Hamden K., Chouayekh H., Bejar S. Lactobacillus plantarum TN8 exhibits protective effects on lipid, hepatic and renal profiles in obese rat. Anaerobe. 2013;23:55–61. doi: 10.1016/j.anaerobe.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Sanchez B., Urdaci M.C., Margolles A. Extracellular proteins secreted by probiotic bacteria as mediators of effects that promote mucosa–bacteria interactions. Microbiology. 2010;156:3232–3242. doi: 10.1099/mic.0.044057-0. [DOI] [PubMed] [Google Scholar]
- Sasikumar P., Gomathi S., Anbazhagan K., Abhishek A., Paul E., Vasudevan V., Sasikumar S., Selvam G.S. Recombinant Lactobacillus plantarum expressing and secreting heterologous oxalate decarboxylase prevents renal calcium oxalate stone deposition in experimental rats. J. Biomed. Sci. 2014;21:86. doi: 10.1186/s12929-014-0086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S.-H., Yang W.-T., Yang G.-L., Cong Y.-L., Huang H.-B., Wang Q., Cai R.-P., Ye L.-P., Hu J.-T., Zhou J.-Y., Wang C.-F., Li Y. Immunoprotection against influenza virus H9N2 by the oral administration of recombinant Lactobacillus plantarum NC8 expressing hemagglutinin in BALB/c mice. Virology. 2014;464–465:166–176. doi: 10.1016/j.virol.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Stevenson C., Blaauw R., Fredericks E., Visser J., Roux S. PP137-SUN: randomized clinical trial: effect of Lactobacillus plantarum 299V on symptoms of irritable bowel syndrome. Clin. Nutr. 2014;33(1):S71. doi: 10.1016/j.nut.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Vijayakumar M., Ilavenil S., Kim D.H., Arasu M.V., Priya K., Choi K.C. In-vitro assessment of the probiotic potential of Lactobacillus plantarum KCC-24 isolated from Italian rye-grass (Lolium multiflorum) forage. Anaerobe. 2015;32:90–97. doi: 10.1016/j.anaerobe.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Vissers Y.M., Snel J., Zuurendonk P.F., Smit B.A., Wichers H.J., Savelkoul H.F.J. Differential effects of Lactobacillus acidophilus and Lactobacillus plantarum strains on cytokine induction in human peripheral blood mononuclear cells. FEMS Immun. Med. Microbiol. 2010;59(1):60–70. doi: 10.1111/j.1574-695X.2010.00662.x. [DOI] [PubMed] [Google Scholar]
- Wang K., Li W., Rui X., Chen X., Jiang M., Dong M. Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014;63:133–139. doi: 10.1016/j.ijbiomac.2013.10.036. [DOI] [PubMed] [Google Scholar]
- Won T.J., Kim B., Lee Y., Bang J.S., Oh E.S., Yoo J.-S., Hyung K.E., Yoon J., Hwang S., Park E.S., Park S.-Y., Hwang K.W. Therapeutic potential of Lactobacillus plantarum CJLP133 for house-dust mite-induced dermatitis in NC/Nga mice. Cell. Immunol. 2012;277(1–2):49–57. doi: 10.1016/j.cellimm.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Woo J.-Y., Gu W., Kim K.-A., Jang S.-E., Han M.J., Kim D.-H. Lactobacillus pentosus var. plantarum C29 ameliorates memory impairment and inflammaging in a D-galactose-induced accelerated aging mouse model. Anaerobe. 2014;27:22–26. doi: 10.1016/j.anaerobe.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Zhang M., Wang X.Q., Zhou Y.K., Ma Y.L., Shen T.Y., Chen H.Q., Chu Z.X., Qin H.L. Effects of oral Lactobacillus plantarum on hepatocyte tight junction structure and function in rats with obstructive jaundice. Mol. Biol. Rep. 2010;37(6):2989–2999. doi: 10.1007/s11033-009-9866-y. [DOI] [PubMed] [Google Scholar]


