Abstract
As a novel oral drug delivery system, proliposome was applied to improve the solubility of active components of Ginkgo biloba extract (GbE). There are currently few reports focusing on the pharmacokinetic characteristics of proliposome of GbE (GbP). A rapid and sensitive ultra performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) method for the simultaneous quantification of active components of GbP and a commercial tablet product (Ginaton) in rat plasma was developed and successfully validated. The method was applied to the comparative pharmacokinetic evaluation of GbP and Ginaton in rat plasma. The results indicated that GbP has a significant effect on absorption, elimination and bioavailability of flavonoids and terpenoid lactones in comparison with Ginaton. The obtained results would be helpful for evaluating the absorption mechanism in the gastrointestinal tract in pharmacokinetic level and guiding the development of the novel oral drug delivery system.
Keywords: Ginkgo biloba extract, Pharmacokinetics, Proliposome, Ultra performance liquid chromatography–tandem mass spectrometry
1. Introduction
Ginkgo, a living fossil tree, is the last surviving member of the family Ginkgoaceae that has been growing on earth for about 200 million years (Yoshitake et al., 2010). Ginkgo biloba extract (GbE) has been used medicinally for many centuries in China to treat lung ailments, such as asthma and bronchitis, and as a remedy for cardiovascular diseases (DeFeudis and Drieu, 2000, Surhio et al., 2014). EGb 761 (Tebonin®), the standardized G. biloba extract of G. biloba leaves, was launched by Dr. Willmar Schwabe (GmbH & Co) in 1976.
Recently, a number of preclinical and clinical research studies indicated that GbE has many positive effects, such as scavenging radicals, anti-oxidation, antitumor, and protective effects in the central nervous system, and therapeutic effects for cerebral and peripheral vascular diseases (Li et al., 2012, Liu et al., 2009, Lu et al., 2016, Koltermann et al., 2007). These benefits of GbE are presumed to result from the synergistic effect of two distinct groups of compounds: the flavonoids (quercetin, kaempferol, and isorhamnetin) and terpenoid lactones (bilobalide, ginkgolides A, B and C) (Chen et al., 2010, Chen et al., 2003). However, the oral bioavailabilities of the flavonoids are relatively low due to their poor solubility (Khaskheli et al., 2015). Therefore, an oral proliposome formulation loaded with GbE (GbP) was developed to enhance the absorption of ginkgo flavonoids. Some previous studies have reported the determination of flavonoids or/and terpenoid lactones of GbE in biological samples using LC–ELSD (Kiyani et al., 2014), GC–MS (Ivic et al., 2003), and LC–MS/MS (Wu et al., 2007, Ou et al., 2009). Among these analytical methods, LC–MS/MS has been supposed as one of the most effective techniques for the analysis of GbE components in biological samples. However, there have been few reports focusing on the pharmacokinetic characteristics of novel drug delivery systems of GbE, like GbE phospholipid complexes (Zhou et al., 2004), and there are no reports describing the PK profile of proliposome formulation of GbE.
In this study, a highly rapid, simple and sensitive ultra performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) method was developed for the simultaneous quantification of quercetin, kaempferol, isorhamnetin, bilobalide, ginkgolides A, ginkgolides B and ginkgolides C in rat plasma, and was applied to demonstrate the pharmacokinetic influences of an oral proliposome formulation loaded with GbE, as compared with a commercial tablet product (Ginaton) (Naureen et al., 2014). The results would be helpful for evaluating the absorption mechanism in the gastrointestinal tract at the pharmacokinetic level, and for guiding the development of novel oral drug delivery systems.
2. Materials and methods
2.1. Chemicals, reagents and animals
The commercial product Ginaton (Dr. Willmar Schwabe GmbH & Co. KG, Germany), a tablet with 40 mg of G. biloba extract, was used at its standardized composition of 9.6 mg of flavonoids and 2.4 mg of terpene lactone by weight. The powder of GbP, containing ∼24% flavonoids and ∼6% terpene lactones (mg/mg), was prepared in our laboratory.
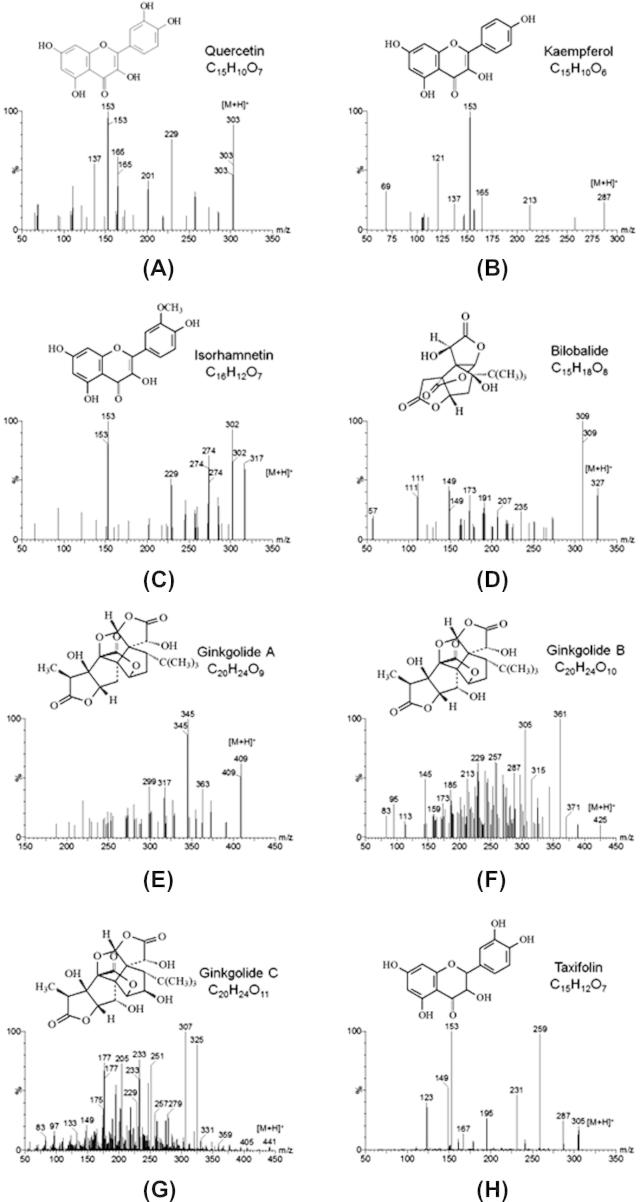
Strands of quercetin (QCT; MW = 302.043; Fig. 1A), kaempferol (KMF; MW = 286.048; Fig. 1B), isorhamnetin (ISR; MW = 316.058; Fig. 1C), bilobalide (BLL; MW = 326.101; Fig. 1D), ginkgolide A (GLA; MW = 408.142; Fig. 1E), ginkgolide B (GLB; MW = 424.137; Fig. 1F), ginkgolide C (GLC; MW = 440.132; Fig. 1G), taxifolin (MW = 304.058; IS, Fig. 1H) were purchased from the National Institute for Food and Drug Control of China. Ethyl acetate (EtOAc, 99.9%), acetonitrile (HPLC-grade), and formic acid (HCOOH, 99.9%) were obtained from Sigma–Aldrich Co. (MO, USA). Hydrochloric acid (HCl, 36–38%) and other chemical reagents of analytical grade or better were obtained from Sinopharm Chemical Reagent Co. (Shanghai, China).
Figure 1.
The product ion scan spectra and chemical structures of (A) quercetin, (B) kaempferol, (C) isorhamnetin, (D) bilobalide, (E) ginkgolide A, (F) ginkgolide B, (G) ginkgolide C and (H) taxifolin (IS).
Blank rat plasma (drug-free and anti-coagulated with heparin sodium) used for the calibration curve was prepared in our laboratory. Validation of the assay was also performed in our laboratory.
2.2. Chromatographic and mass spectrometry conditions
For quantitative analysis, the analysis of plasma samples was performed on a Waters Xevo TQ–S triple quadruple mass spectrometry (Waters, Milford, MA, USA) connected with a Waters Acquity UPLC system.
The Waters Xevo TQ–S triple quadrupole mass spectrometry was operated in positive electrospray ionization (ESI+) mode. Optimized instrumental parameters for mass spectral acquisition were as follows: capillary voltage at 3.0 kV; source voltage at 60 V; source temperature at 150 °C; desolvation temperature at 500 °C; cone gas flow at 150 L/h; and desolvation gas flow at 1000 L/h. Multiple-reaction monitoring (MRM) was employed for data acquisition. The optimized conditions of precursor to product ion pairs performed in multiple-reaction monitoring (MRM) mode are described in Table 1. The Mass Lynx software version 4.1 was used to control all parameters of UPLC and MS (Rangel-Ordóñez et al., 2010).
Table 1.
The information for MRM parameters used for the analytes in GbP and Ginaton (using taxifolin as IS).
| Compound | Precursor ion | Cone voltage (V) | Product ion | Collision energy (eV) |
|---|---|---|---|---|
| Quercetin | 303.096 | 20 | 152.982⁎ | 32 |
| 228.977 | 26 | |||
| Kaempferol | 287.096 | 20 | 152.980⁎ | 30 |
| 120.967 | 30 | |||
| Isorhamnetin | 317.096 | 20 | 152.903⁎ | 30 |
| 302.048 | 24 | |||
| Bilobalide | 327.160 | 40 | 111.063⁎ | 16 |
| 148.993 | 22 | |||
| Ginkgolide A | 409.160 | 50 | 345.103⁎ | 18 |
| 363.115 | 14 | |||
| Ginkgolide B | 425.160 | 42 | 305.112⁎ | 24 |
| 361.105 | 18 | |||
| Ginkgolide C | 441.160 | 48 | 325.040⁎ | 22 |
| 177.022 | 40 | |||
| Taxifolin (IS) | 305.096 | 20 | 152.977⁎ | 16 |
| 148.943 | 22 |
Quantitative transitions.
Chromatographic separations of prepared samples were obtained using a Waters Acquity BEH C18 column (2.1 mm × 50 mm, 1.7 μm) maintained at 40 °C. A 0.2-mm filter (Waters, Milford, MA, USA) was applied before the analytical column. The mobile phase was delivered at 0.4 ml/min, including acetonitrile (containing 0.01% formic acid) (A) and water (containing 0.01% formic acid) (B). The gradient program can be described as follows, 0–0.6 min, 5–30% A; 0.6–3 min, 30–80% A; 3–4 min, 80–20% A; 4–4.1 min, 20–5% A; 4.1–6 min, 5–5% A. The temperature of the auto sampler was set at 20 °C and the injection volume was 2 μl.
2.3. Preparation of standards and quality control samples
Standard stock solutions of investigated samples were prepared in acetonitrile. The appropriate amounts of quercetin, kaempferol, isorhamnetin, bilobalide, ginkgolide A, ginkgolide B, ginkgolide C and taxifolin were separately weighed and dissolved as the stock solutions. The stock solutions were then mixed and diluted with acetonitrile to prepare a final mixed standard working solution containing 200 ng/ml of quercetin, 75 ng/ml of kaempferol, 150 ng/ml of isorhamnetin, 750 ng/ml of bilobalide, 150 ng/ml of ginkgolide A, 250 ng/ml of ginkgolide B, and 250 ng/ml of ginkgolide C. The IS (taxifolin) working solution was diluted with acetonitrile to give a concentration of 5 ng/ml.
Calibration standard solutions were prepared by spiking the appropriate amount of the final mixed standard working solution into 50 μl drug-free rat plasma, resulting in a nominal concentration range of 0.266–66.66 ng/ml for quercetin, 0.1–25 ng/ml for kaempferol, 0.2–50 ng/ml for isorhamnetin, 0.266–66.66 ng/ml for bilobalide, 0.2–50 ng/ml for ginkgolide A, 0.333–83.33 ng/ml for ginkgolide B, and 0.333–83.33 ng/ml for ginkgolide C.
For validation of the method, low, medium and high concentration levels of the standard solution containing quercetin (0.666, 6.666 and 53.33 ng/ml), kaempferol (0.25, 2.5 and 20 ng/ml), isorhamnetin (0.5, 5 and 40 ng/ml), bilobalide (0.666, 6.666 and 53.33 ng/ml), ginkgolide A (0.5, 5 and 40 ng/ml), ginkgolide B (0.833, 8.333 and 66.66 ng/ml) and ginkgolide C (0.833, 8.333 and 66.66 ng/ml) were used for preparing the quality control (QC) plasma samples. All of the solutions were stored at 4 °C (Saleem et al., 2008).
2.4. Preparation of plasma samples
To a 50 μl of rat plasma, 50 μl of 4 M hydrochloric acid (containing 10 mM ascorbic acid) and 50 μl of IS (5 ng/ml taxifolin) were added in turn. The mixture was vortexed for 10 s. The resulting mixture was then acid hydrolyzed at 80 °C for 30 min in a water bath. After cooling immediately to room temperature, 600 μl of ethyl acetate was added and vortexed for 1 min. The mixture was next centrifuged at 10,836g for 10 min. The supernatant was dried at 37 °C under a mild stream of nitrogen (Qureshi et al., 2015). The residue was reconstituted in 100 μl of acetonitrile, and the samples were centrifuged at 10,836g for 10 min. 80 μl of the resulting supernatant was used for further LC–MS/MS analysis.
2.5. Method validation
The method used for simultaneous quantitative determination of seven active components of GbP and Ginaton in rat plasma was validated for specificity, sensitivity, linearity, accuracy, intra-and inter-day precision, matrix effect, extraction recovery and stability, as described below.
2.5.1. Specificity and selectivity
The specificity of the method was demonstrated by comparing the chromatograms of blank plasma samples from six rats to those of corresponding standard samples spiked with analytes and IS (5 ng/ml).
2.5.2. Sensitivity and linearity
The calibration curve consisted of seven concentration levels, and the sample with each concentration level was prepared as described above. The linearity was confirmed by plotting the peak area ratio of the transition pair of analytes to that of IS against the nominal concentrations, through least squares linear regression analysis, described in the form of y = ax + b(1/x2 weighed).
Sensitivity of the method was determined by the lower limit of quantification (LLOQ), which was defined as the lowest concentration point of the standard curve, at which the concentration can be reliably and reproducibly measured with a corresponding signal-to-noise ratio of 10. The LLOQ can be accurately quantified within a 20% bias of the nominal concentration and with a precision error not exceeding 20%.
2.5.3. Accuracy and precision
The accuracy, intra- and inter-day precision of the method were determined by measuring six replicates of QC samples at low, medium, and high concentration levels on three validation days, respectively. The accuracy was expressed as the relative error (RE), and the intra- and inter-day precision was determined as the relative standard deviation (RSD) (Safi et al., 2015a).
2.5.4. Matrix effect and extraction recovery
The matrix effects of analytes were determined by analyzing three replicates of QC samples at low, medium, and high concentration levels, by comparing the peak areas of analytes dissolved in the post-extraction plasma blank with those of pure standard solution containing equivalent amounts of analytes. The matrix effect of IS was also evaluated with the same method.
The extraction recoveries of analytes were evaluated by comparing the peak areas of the analytes extracted from plasma samples with those of post-extraction spiked plasma blank. The extraction recovery of IS was also evaluated in the same way at a concentration of 5 ng/ml.
2.5.5. Dilution integrity
The dilution integrity experiment was performed to validate the dilution step that might be required for some of the actual rat samples with concentrations of analytes greater than the upper limit of quantification (ULOQ) (Zhou et al., 2011). Six replicate dilution QC samples containing quercetin (800 ng/ml), kaempferol (300 ng/ml), isorhamnetin (600 ng/ml), bilobalide (800 ng/ml), ginkgolide A (600 ng/ml), ginkgolide B (1000 ng/ml), and ginkgolide C (1000 ng/ml) were diluted with spiking blank rat plasma by 15-fold high quality control (HQC) concentrations, and their concentrations were calculated by applying the dilution factor of 15 against the calibration curve. Accuracy and precision error should be within ±20%.
2.5.6. Stability
The stability of analytes in rat plasma was assessed by analyzing QC samples at three concentration levels under various conditions, including three freeze–thaw cycles stability (−20 °C to room temperature as a cycle), short-term temperature stability (room-temperature 20 °C for 4 h), long-term stability (−20 °C for 2 weeks), and post-preparation stability (the extracted QC samples maintained in the auto sampler at room temperature for 12 h). All the stability studies were conducted by analyzing replicates (n = 3) of QC samples at three concentration levels. The results were compared with those of freshly prepared QC samples, and the percentage of concentration deviation was calculated.
2.6. Pharmacokinetic study
Male Wistar rats (285 ± 5 g) were obtained from the Laboratory Animal Center of Jilin University (Changchun, China), and acclimated to the circumstance at a temperature of 25 °C, 12 h light/dark cycle with free access to water and food for 7 days before the experiment. The experimental protocol was approved by the Laboratory Animal Center of Jilin University (license No. SCXK–(JI) 2011-0003). Before drug administration, the rats were fasted for over 12 h and had free access to water (Safi et al., 2015b).
For the pharmacokinetic study, twelve rats were randomly divided into two groups. Rats in group 1 were given a single oral dose of 25 mg/kg Ginaton, while rats in group 2 were administered a single oral dose of 25 mg/kg GbP. Blood samples of approximately 0.5 ml were collected into heparinized centrifuge tubes from the fossa orbitalis vein before dosing, and at 0.083, 0.167, 0.333, 0.5, 1, 2, 4, 6, 8, 10, 12 and 24 h post-dosing, and were immediately centrifuged at 10,836g for 10 min. The plasma supernatants were transferred into clean 1.5 ml Eppendorf tubes and stored at −20 °C prior to analysis.
2.7. Statistical analysis
The pharmacokinetic parameters for Ginaton and GbP were calculated using the Drug and Statistics 3.0 program (DAS, life science college of Jilin University, Changchun, China). Data were expressed as the mean ± standard deviations (S.D.). The identification of statistical significance between different groups was carried out with Student’s t tests. A P value of <0.05 was considered statistically significant.
3. Results and discussion
3.1. Method development
3.1.1. Sample preparation
Sample preparation is one of the most important steps for simultaneous quantification of the two kinds of analytes investigated (flavonoids and terpene trilactones) in rat plasma. Three techniques of protein precipitation, liquid–liquid extraction and solid phase extraction have been frequent approaches. With comprehensive inspection of cost, simplicity and efficiency, the liquid–liquid extraction technique (used ethyl acetate as extraction solution) was the best choice. In this study, when the calibration curve was established by the routine method to prepare plasma sample, cross-interference between the ginkgo flavonoids and terpenoids was found both in positive mode, as described in Zhao et al., (2008). Compared to the standard curve directly established by mixed standard solutions of analytes, the linear relationship of the calibration curve deteriorated and the slopes of the regression curves also prominently changed in both negative and positive mode. Therefore, three key operations in the preparation of plasma samples should be noted, which were (1) adding a certain amount of ascorbic acid to the plasma samples during sample preparation; (2) cooling samples immediately to room temperature before ethyl acetate extraction; (3) reconstituting the dried residues in absolute acetonitrile before LC–MS/MS.
3.1.2. Selection of IS
The confirmation of a suitable internal standard substance is a critical step in biological sample analysis. An ideal IS for LC–MS/MS should be a stable isotope-labeled analyte, but it is difficult to obtain such a compound, especially for the simultaneous determination of seven analytes. Several possible internal standards were tested in the experimental conditions, including baicalin, domperidone and taxifolin. Baicalin, as a kind of flavonoids, was first considered as an IS. However, it could be hydrolyzed to baicalin through the required acid hydrolysis process. Domperidone was relatively stable with the sample preparation process, but its extraction efficiency was not stable using ethyl acetate as an extraction solvent in contrast with seven analytes. Taxifolin, with a chemical structure similar to ginkgo flavonoids, was selected as the appropriate IS, because its chromatographic behavior, extraction efficiency and ionization properties were close to those of the analytes (Noor et al., 2015).
3.1.3. Mass spectrometry
UPLC–MS/MS has been emerging as a powerful analytical technique for the determination of compounds in biological samples with improved sensitivity, selectivity and specificity. In this study, a standard solution (500 ng/ml) of analytes and IS was directly infused along with the mobile phase into the source of the mass spectrometer (Huang et al., 2004). The key mass spectrometry parameters, such as capillary voltage, source voltage, source temperature, desolvation temperature, cone gas flow, desolvation gas flow, cone voltage and collision energy were carefully optimized in both positive and negative electrospray ionization modes (Batool et al., 2015). The positive electrospray ionization mode (ESI+) was found to be more appropriate for the determination of analytes and IS than the negative electrospray ionization (ESI−) mode, because of the weak mass spectrometric response of bilobalide and ginkgolide A in ESI− mode (Butt et al., 2015). Moreover, additives such as formic acid helped to promote better ionization efficiency of the analytes, which facilitated the formation of [M+H]+. The full-scan mass spectra of quercetin, kaempferol, isorhamnetin, bilobalide, ginkgolide A, ginkgolide B, ginkgolide C and taxifolin showed predominant protonated precursor [M+H]+ ions at m/z 303, 287, 317, 327, 409, 425, 441 and 305, respectively. As shown by the product ion spectra, the main product ions of quercetin, kaempferol, isorhamnetin, bilobalide, ginkgolide A, ginkgolide B, ginkgolide C and taxifolin were m/z 153, 229, and 137 (Fig. 1A), m/z 153, 121 and 165 (Fig. 1B), m/z 153, 302 and 274 (Fig. 1C), m/z 309, 111 and 149 (Fig. 1D), m/z 345, 363 and 327 (Fig. 1E), m/z 361, 305 and 257 (Fig. 1F), m/z 325, 177 and 307 (Fig. 1G), and m/z 153, 149 and 259 (Fig. 1H). For all compounds, the highest stable signal product ions were selected for quantification for each component in MRM mode (Biber, 2003). The MRM transition m/z 425 → 361 of ginkgolide B was abandoned due to the strong interference of baseline signal, particularly at lower concentrations even though it was the most abundant (Drieu and DeFeudis, 2000) The product ion of bilobalide at m/z 309 ([M+H−H2O]+) was also not used since it was not stable during the process of ionization. The final optimization results are shown in Table 1.
3.1.4. Chromatography
Chromatographic conditions were further optimized to produce the best sensitivity, efficiency and chromatographic peak shape. Compared with HPLC, UPLC provided increased sensitivity, better peak shape and higher analysis speed. Acetonitrile had higher mass spectrometric response and lower background noise than methanol, and was chosen as the organic phase. It was observed that pH of the mobile phase had a significant impact on peak shape and sensitivity of analytes and IS. The sensitivity significantly increased with the use of 0.01% formic acid (pH = 3.33) when compared with the mobile phase containing no electrolyte or 0.1% formic acid (pH = y72.75). However, the lower concentration of formic acid (<0.01%) had a negative impact on separation selectivity (Ashraf et al., 2015). Hence, the optimal concentration of formic acid in the mobile phase was 0.01%. To eliminate the matrix effect and to obtain a high sensitivity and sample high-throughput, gradient elution program optimized was finally adopted in the study. Furthermore, to maintain the pH of the mobile phase in whole gradient elution program, acetonitrile and water were both added to 0.01% formic acid. The total run time was only 6 min for seven analytes (Chen et al., 2011).
3.2. Method validation
3.2.1. Specificity
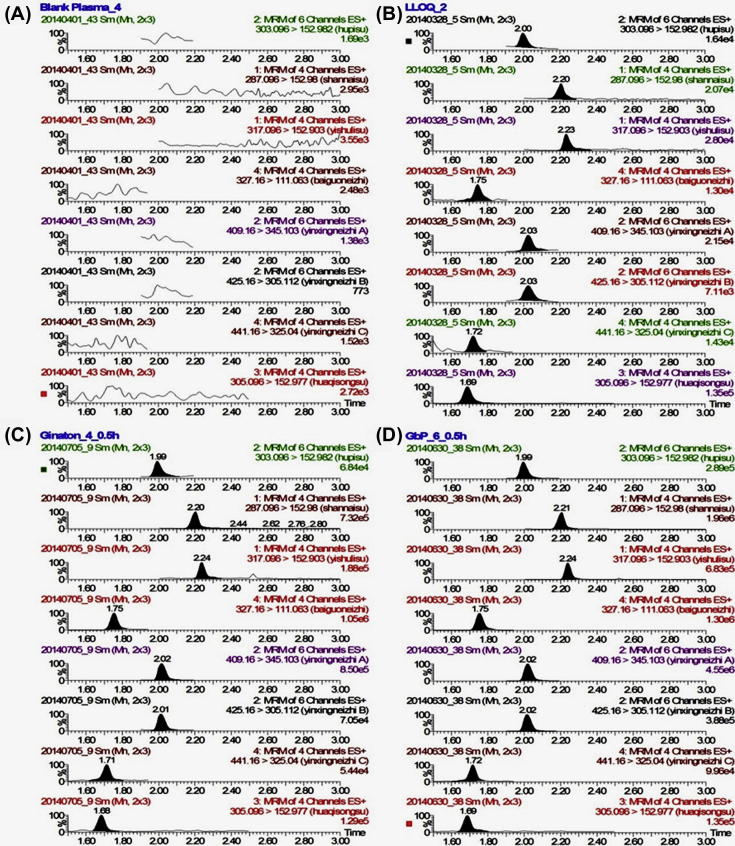
The specificity of the proposed method was demonstrated by comparing the MRM chromatograms of quercetin, kaempferol, isorhamnetin, bilobalide, ginkgolide A, ginkgolide B, ginkgolide C and IS obtained from a blank rat plasma sample and a spiked plasma sample. As shown in Fig. 2, no significant interference from endogenous substances was observed in the chromatograms of drug-free rat plasma at the retention times of the analytes and IS. The retention times of quercetin, kaempferol, isorhamnetin, bilobalide, ginkgolide A, ginkgolide B, ginkgolide C and IS were 2.00, 2.20, 2.23, 1.75, 2.03, 2.03, 1.72 and 1.69 min, respectively.
Figure 2.
Chromatograms of (A) drug-free rat plasma, (B) spiked plasma at the limit of quantitation, (C) 0.5 h plasma sample after a single oral administration of Ginaton, (D) 0.5 h plasma sample after a single oral administration of GbP.
3.2.1.1. Calibration curve and lower limit of quantification (LLOQ)
The calibration curves for the seven analytes in the biological samples were found to be linear in the concentration range tested. The regression coefficients (r) were higher than 0.995, showing a good linearity over the concentration range.
The LLOQ of quercetin, kaempferol, isorhamnetin, bilobalide, ginkgolide A, ginkgolide B, ginkgolide C was 0.266, 0.1, 0.2, 0.266, 0.2, 0.333 and 0.333, respectively, and the precision and accuracy of the seven analytes at the LLOQ were less than 20%, indicating that this method was sufficiently sensitive for the quantitative evaluation of the seven compounds.
3.2.2. Accuracy and precision
The results of the intra-day and inter-day precision and accuracy for seven analytes in three QC levels are summarized in Table 2. The intra-day and inter-day precisions (RSD) ranged from 0.76% to 8.99% and the accuracy (RE) was within −12.62–10.98%. The results of this method demonstrated that the intra-day and inter-day precision and accuracy were within an acceptable range.
Table 2.
Intra-day and inter-day precisions and accuracies for the determination of the seven analytes (n = 3 days, 6 replicates per day).
| Compounds and added concentration (ng/ml) | Intra-day (n = 6) |
Inter-day (n = 6) |
||||
|---|---|---|---|---|---|---|
| Found concentration (ng/ml) | Precision RSD (%) | Accuracy RE (%) | Found concentration (ng/ml) | Precision RSD (%) | Accuracy RE (%) | |
| Quercetin | ||||||
| 0.666 | 0.65 ± 0.03 | 4.99 | −1.90 | 0.66 ± 0.03 | 3.11 | −0.73 |
| 6.666 | 5.83 ± 0.13 | 2.35 | −12.54 | 5.83 ± 0.14 | 2.77 | −12.62 |
| 53.33 | 46.80 ± 1.20 | 2.08 | −12.24 | 46.68 ± 0.98 | 2.27 | −12.48 |
| Kaempferol | ||||||
| 0.25 | 0.25 ± 0.02 | 6.75 | 1.33 | 0.26 ± 0.02 | 3.72 | 2.22 |
| 2.5 | 2.38 ± 0.05 | 3.46 | −4.67 | 2.33 ± 0.09 | 4.87 | −6.78 |
| 20 | 19.19 ± 0.65 | 2.53 | −4.07 | 19.75 ± 0.61 | 6.39 | −6.27 |
| Isorhamnetin | ||||||
| 0.5 | 0.50 ± 0.04 | 5.94 | 0.67 | 0.51 ± 0.03 | 8.99 | 2.44 |
| 5 | 4.67 ± 0.08 | 2.55 | −6.60 | 4.65 ± 0.11 | 0.76 | −6.93 |
| 40 | 36.87 ± 0.89 | 2.14 | −7.83 | 37.05 ± 0.97 | 4.92 | −7.39 |
| Bilobalide | ||||||
| 0.666 | 0.60 ± 0.03 | 4.38 | −10.66 | 0.59 ± 0.03 | 4.29 | −10.99 |
| 6.666 | 6.54 ± 0.18 | 2.86 | −1.94 | 6.45 ± 0.21 | 5.26 | −3.28 |
| 53.33 | 53.44 ± 1.47 | 2.63 | 0.21 | 51.78 ± 1.76 | 6.98 | −2.91 |
| Ginkgolide A | ||||||
| 0.5 | 0.49 ± 0.04 | 6.89 | −3.00 | 0.47 ± 0.03 | 8.22 | −5.67 |
| 5 | 5.17 ± 0.12 | 3.41 | 3.4 | 5.27 ± 0.18 | 3.92 | 5.34 |
| 40 | 43.48 ± 1.24 | 2.42 | 4.85 | 42.36 ± 1.26 | 5.65 | 5.90 |
| Ginkgolide B | ||||||
| 0.833 | 0.91 ± 0.05 | 3.45 | 9.04 | 0.92 ± 0.03 | 3.67 | 10.98 |
| 8.333 | 8.89 ± 0.26 | 3.34 | 6.62 | 8.83 ± 0.29 | 3.33 | 5.96 |
| 66.66 | 69.62 ± 2.34 | 2.57 | 4.44 | 69.01 ± 1.75 | 2.29 | 3.52 |
| Ginkgolide C | ||||||
| 0.833 | 0.81 ± 0.08 | 7.51 | −3.36 | 0.80 ± 0.06 | 1.82 | −3.56 |
| 8.333 | 8.36 ± 0.17 | 2.07 | 0.26 | 8.22 ± 0.23 | 5.71 | −1.42 |
| 66.66 | 65.99 ± 1.97 | 2.64 | −1.01 | 65.73 ± 1.64 | 0.82 | −1.39 |
3.2.3. Matrix effect and extraction recovery
Matrix effects and extraction recovery of the seven analytes and IS are shown in Table 3. The observed matrix effects of the investigated analytes at three different concentration levels in rat plasma were found to be in the range of 87.0–106.5% with an RSD of less than 7.7%, demonstrating that the matrix effect on the ionization of the analytes was negligible. The extraction recovery ranged from 86.0% to 104.6% with an RSD of less than 7.4%, indicating that the liquid–liquid extraction procedure was efficient.
Table 3.
Matrix effects and extraction recoveries of the seven analytes and IS in rat plasma (n = 3).
| Compounds | Matrix effect (%) |
Extraction recovery (%) |
||||
|---|---|---|---|---|---|---|
| LQC | MQC | HQC | LQC | MQC | HQC | |
| Quercetin | 90.2 (7.7) | 91.9 (4.9) | 106.5 (1.2) | 100.8 (7.2) | 94.1 (1.6) | 96.1 (0.9) |
| Kaempferol | 91.2 (2.5) | 91.7 (2.7) | 100.2 (0.7) | 101.3 (3.7) | 85.0 (4.1) | 88.5 (1.3) |
| Isorhamnetin | 96.2 (7.5) | 98.6 (1.8) | 98.6 (1.6) | 88.1 (4.3) | 88.5 (4.0) | 86.0 (1.9) |
| Bilobalide | 87.0 (3.0) | 99.0 (3.3) | 94.8 (0.4) | 93.6 (6.2) | 93.8 (5.3) | 98.0 (1.0) |
| Ginkgolide A | 95.9 (6.4) | 95.2 (1.9) | 98.9 (0.3) | 92.9 (5.5) | 90.5 (5.1) | 93.4 (0.4) |
| Ginkgolide B | 94.1 (1.9) | 101.5 (0.8) | 97.6 (0.8) | 104.5 (3.6) | 85.2 (4.2) | 93.4 (0.6) |
| Ginkgolide C | 98.2 (2.1) | 103.8 (2.0) | 93.5 (1.8) | 87.7 (7.4) | 90.8 (3.8) | 96.2 (2.0) |
| Taxifolin | 95.7 (3.3) | 97.5 (1.0) | 99.9 (4.4) | 104.6 (4.0) | 94.6 (5.2) | 94.2 (4.2) |
Note: percentage RSDs are in parentheses.
3.2.4. Dilution integrity
To test the dilution integrity, high concentrated samples at six replicates of each were diluted 15-fold by blank rat plasma, clean-up and analyzed. As shown in Table 4, the dilution integrity accuracy (RE) of the seven analytes after 15-fold dilution ranged from −14.75% to 13.53% with a precision (RSD) of less than 9.87%.
Table 4.
The dilution integrity experiment of the seven analytes (n = 6).
| Compounds | Diluted concentration (ng/ml) | Found concentration (ng/ml) | Precision RSD (%) | Accuracy RE (%) |
|---|---|---|---|---|
| Quercetin | 53.33 | 50.19 ± 2.34 | 4.66 | −5.90 |
| Kaempferol | 20 | 17.80 ± 0.64 | 3.62 | −10.98 |
| Isorhamnetin | 40 | 45.41 ± 1.26 | 2.77 | 13.53 |
| Bilobalide | 53.33 | 45.46 ± 0.54 | 1.19 | −14.75 |
| Ginkgolide A | 40 | 38.39 ± 0.62 | 1.60 | −4.02 |
| Ginkgolide B | 66.66 | 67.18 ± 1.61 | 2.40 | 0.78 |
| Ginkgolide C | 66.66 | 68.68 ± 6.77 | 9.87 | 3.02 |
3.2.5. Stability
The stability of the seven analytes in rat plasma was evaluated under different storage conditions. The results are summarized in Table 5. The analytes were found to be stable through three freeze–thaw cycles (RE ranged from −8.36% to 9.63%, RSD were within 0.29–9.92%), at a temperature of −20 °C for 2 weeks (RE ranged from −10.36% to 6.52%, RSD were within 1.53–8.29%), at room temperature of 20 °C for 4 h (RE ranged from −9.64% to 6.20%, RSD were within 1.11–6.94%) and for 12 h in the autosampler after sample preparation (RE ranged from −10.94% to 10.67%, RSD were within 0.40–9.49%.
Table 5.
Stability of the seven analytes in rat plasma (n = 3) under different storage conditions.
| Compounds and added concentration (ng/ml) | Freeze-thraw stability |
Long-term stability |
Short-term temperature stability |
Post-preparation stability |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Found concentration (ng/ml) | Precision RSD (%) | Accuracy RE (%) | Found concentration (ng/ml) | Precision RSD (%) | Accuracy RE (%) | Found concentration (ng/ml) | Precision RSD (%) | Accuracy RE (%) | Found concentration (ng/ml) | Precision RSD (%) | Accuracy RE (%) | |
| Quercetin | ||||||||||||
| 0.666 | 0.65 ± 0.05 | 7.70 | −1.90 | 0.64 ± 0.05 | 8.29 | −4.01 | 0.66 ± 0.04 | 5.29 | −0.40 | 0.68 ± 0.04 | 5.14 | 2.60 |
| 6.666 | 6.25 ± 0.10 | 1.60 | −6.19 | 6.28 ± 0.32 | 5.09 | −5.83 | 6.02 ± 0.07 | 1.11 | −9.64 | 5.94 ± 0.15 | 2.58 | −10.94 |
| 53.33 | 52.70 ± 1.59 | 3.02 | −1.19 | 51.59 ± 2.03 | 3.94 | −3.26 | 51.08 ± 2.00 | 3.91 | −4.22 | 51.38 ± 0.88 | 1.72 | −3.66 |
| Kaempferol | ||||||||||||
| 0.25 | 0.24 ± 0.01 | 5.53 | −2.07 | 0.24 ± 0.01 | 4.17 | −4.67 | 0.25 ± 0.02 | 6.51 | 0.53 | 0.25 ± 0.02 | 9.49 | −1.2 |
| 2.5 | 2.63 ± 0.08 | 3.08 | 5.12 | 2.66 ± 0.08 | 3.07 | 6.32 | 2.48 ± 0.10 | 4.13 | −0.88 | 2.48 ± 0.08 | 3.30 | −0.88 |
| 20 | 21.62 ± 0.82 | 3.81 | 8.09 | 19.04 ± 0.41 | 2.13 | −4.59 | 20.39 ± 0.50 | 2.47 | 1.93 | 20.57 ± 0.37 | 1.80 | 2.87 |
| Isorhamnetin | ||||||||||||
| 0.5 | 0.52 ± 0.01 | 1.12 | 3.33 | 0.47 ± 0.02 | 3.23 | −5.33 | 0.50 ± 0.02 | 4.19 | −0.67 | 0.51 ± 0.04 | 8.55 | 2.00 |
| 5 | 5.32 ± 0.02 | 0.29 | 6.33 | 5.07 ± 0.14 | 2.81 | 1.4 | 5.16 ± 0.28 | 5.44 | 3.13 | 5.00 ± 0.03 | 0.61 | 0.07 |
| 40 | 43.85 ± 1.23 | 2.81 | 9.63 | 39.51 ± 1.10 | 2.76 | −1.23 | 42.28 ± 1.08 | 2.54 | 6.20 | 43.15 ± 0.61 | 1.42 | 7.88 |
| Bilobalide | ||||||||||||
| 0.666 | 0.67 ± 0.03 | 3.86 | 0.64 | 0.67 ± 0.02 | 3.50 | −0.13 | 0.63 ± 0.03 | 4.52 | −5.88 | 0.61 ± 0.03 | 5.29 | −8 |
| 6.666 | 6.56 ± 0.19 | 2.84 | −1.55 | 7.10 ± 0.21 | 2.95 | 6.52 | 6.17 ± 0.22 | 3.62 | −7.4 | 6.08 ± 0.14 | 2.37 | −8.75 |
| 53.33 | 54.64 ± 1.07 | 1.97 | 2.45 | 54.46 ± 2.88 | 5.29 | 2.11 | 51.47 ± 0.97 | 1.88 | −3.49 | 53.02 ± 0.92 | 1.74 | −0.59 |
| Ginkgolide A | ||||||||||||
| 0.5 | 0.46 ± 0.04 | 9.48 | −8 | 0.49 ± 0.01 | 1.53 | −2.07 | 0.46 ± 0.03 | 6.94 | −7.33 | 0.55 ± 0.02 | 2.76 | 10.67 |
| 5 | 4.91 ± 0.07 | 1.50 | −1.9 | 5.07 ± 0.16 | 3.11 | 1.34 | 5.01 ± 0.07 | 1.40 | 0.14 | 4.92 ± 0.14 | 2.76 | −1.54 |
| 40 | 42.30 ± 0.17 | 0.39 | 5.76 | 41.84 ± 1.77 | 4.23 | 4.61 | 41.64 ± 0.85 | 2.04 | 4.11 | 42.02 ± 0.70 | 1.67 | 5.06 |
| Ginkgolide B | ||||||||||||
| 0.833 | 0.85 ± 0.05 | 6.19 | 1.82 | 0.85 ± 0.04 | 4.35 | 1.68 | 0.87 ± 0.02 | 2.45 | 4.76 | 0.82 ± 0.01 | 1.52 | −1.44 |
| 8.333 | 8.26 ± 0.18 | 2.13 | −0.93 | 8.46 ± 0.29 | 3.49 | 1.51 | 8.50 ± 0.14 | 1.60 | 2.05 | 8.01 ± 0.21 | 2.67 | −3.84 |
| 66.66 | 65.09 ± 1.29 | 1.98 | −2.36 | 63.37 ± 2.82 | 4.46 | −4.93 | 67.04 ± 1.76 | 2.62 | 0.58 | 65.74 ± 0.31 | 0.47 | −1.38 |
| Ginkgolide C | ||||||||||||
| 0.833 | 0.76 ± 0.08 | 9.92 | −8.36 | 0.75 ± 0.03 | 3.87 | −10.36 | 0.80 ± 0.01 | 1.25 | −3.96 | 0.88 ± 0.05 | 5.85 | 5.24 |
| 8.333 | 8.56 ± 0.32 | 3.74 | 2.68 | 8.54 ± 0.51 | 5.92 | 2.44 | 8.41 ± 0.29 | 3.40 | 0.92 | 7.95 ± 0.23 | 2.95 | −4.60 |
| 66.66 | 71.95 ± 0.74 | 1.03 | 7.93 | 65.80 ± 2.61 | 3.97 | −1.29 | 70.16 ± 1.88 | 2.67 | 5.25 | 67.21 ± 0.27 | 0.40 | 0.83 |
3.3. Pharmacokinetics study
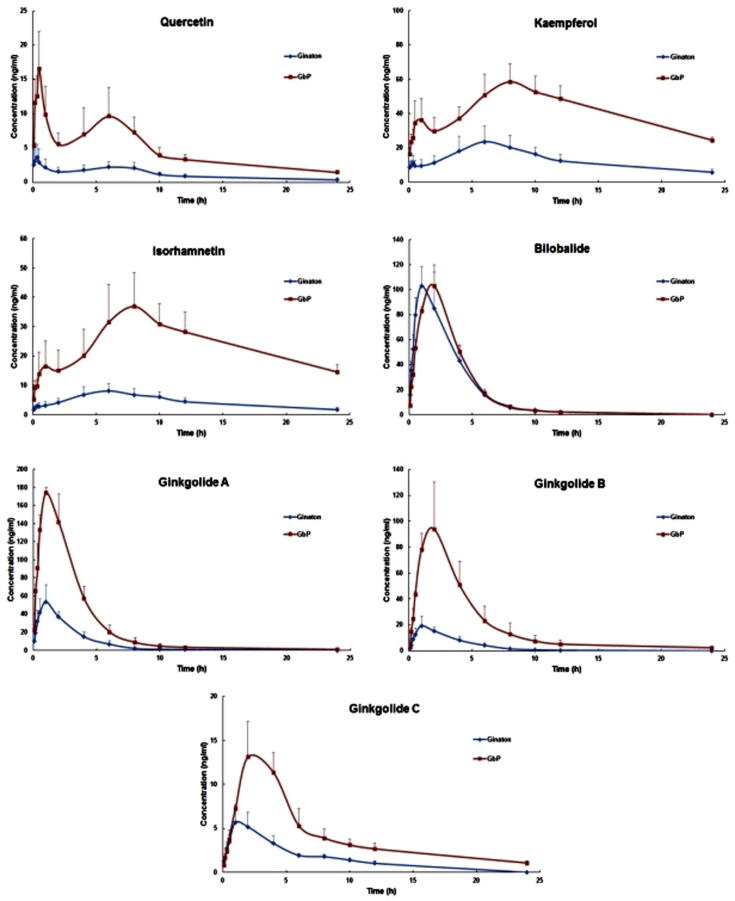
The developed and validated method was applied to the pharmacokinetic evaluation of the seven analytes in rat plasma after oral administration of Ginaton and GbP (Fig. 3). The method was proven to be sensitive enough for the quantification of these analytes in rat plasma (Kleijnen and Knipschild, 1992). The pharmacokinetic parameters, including the time of the maximum concentrations (Tmax), the maximum plasma concentration (Cmax), the elimination half-time (T1/2), the area under concentration–time curve (AUC0−t), and the plasma clearance (CL) were calculated through a non-compartment model, as displayed in Table 6.
Figure 3.
Mean concentration–time curves of quercetin, kaempferol, isorhamnetin, bilobalide, ginkgolide A, ginkgolide B and ginkgolide C in rat plasma after a single oral administration of Ginaton and GbP (n = 6).
Table 6.
Pharmacokinetics parameters of seven analytes after an oral administration (n = 6).
| Compounds | AUC0−t (ng/ml/h) | Cmax (ng/ml) | Tmax (h) | T1/2 (h) | CL (ml/h) |
|---|---|---|---|---|---|
| Quercetin | |||||
| Ginaton | 28.47 ± 10.44 | 3.81 ± 2.35 | 0.31 ± 0.07 | 8.81 ± 0.29 | 12720.04 ± 4198.09 |
| GbP | 112.41 ± 34.32$ | 16.78 ± 5.08$ | 0.47 ± 0.07# | 10.52 ± 2.26 | 3012.87 ± 740.40$ |
| Kaempferol | |||||
| Ginaton | 310.07 ± 92.96 | 23.65 ± 8.95 | 6.33 ± 0.82 | 10.04 ± 1.28 | 621.73 ± 192.20 |
| GbP | 976.87 ± 138.15$ | 60.13 ± 9.56$ | 8.00 ± 1.26⁎ | 12.68 ± 1.51# | 161.40 ± 13.86$ |
| Isorhamnetin | |||||
| Ginaton | 106.72 ± 33.44 | 8.41 ± 2.60 | 5.67 ± 0.82 | 7.71 ± 0.68 | 1235.96 ± 378.93 |
| GbP | 566.59 ± 156.33$ | 37.51 ± 11.75$ | 7.67 ± 0.82# | 12.96 ± 1.90$ | 176.70 ± 35.62$ |
| Bilobalide | |||||
| Ginaton | 384.58 ± 93.57 | 102.99 ± 15.51 | 1.17 ± 0.41 | 2.35 ± 0.72 | 612.04 ± 156.76 |
| GbP | 406.83 ± 43.23 | 103.97 ± 15.26 | 1.83 ± 0.41⁎ | 2.72 ± 1.30 | 560.09 ± 61.94 |
| Ginkgolide A | |||||
| Ginaton | 166.34 ± 20.69 | 53.42 ± 18.83 | 1.00 ± 0.00 | 1.61 ± 0.14 | 1117.64 ± 138.65 |
| GbP | 618.80 ± 110.07$ | 173.97 ± 5.68$ | 1.00 ± 0.00 | 5.74 ± 0.78$ | 304.71 ± 54.59$ |
| Ginkgolide B | |||||
| Ginaton | 74.42 ± 8.58 | 19.80 ± 6.98 | 1.50 ± 0.55 | 2.38 ± 0.61 | 1509.77 ± 169.71 |
| GbP | 461.24 ± 173.57$ | 96.98 ± 32.94$ | 1.67 ± 0.52 | 8.85 ± 0.62$ | 262.57 ± 102.28$ |
| Ginkgolide C | |||||
| Ginaton | 31.70 ± 6.76 | 5.68 ± 1.84 | 1.00 ± 0.00 | 5.81 ± 1.08 | 2569.97 ± 506.86 |
| GbP | 99.35 ± 19.56$ | 13.32 ± 3.76# | 2.67 ± 1.03# | 9.08 ± 0.82$ | 909.29 ± 185.36$ |
Difference from corresponding Ginaton group, P < 0.05.
Difference from corresponding Ginaton group, P < 0.01.
Difference from corresponding Ginaton group, P < 0.001.
As showed in Fig. 3 and Table 6, the pharmacokinetic parameters of quercetin, kaempferol, isorhamnetin, ginkgolide A, ginkgolide B and ginkgolide C showed significant differences in AUC0−t and Cmax. The values of AUC0−t and Cmax of quercetin, kaempferol, isorhamnetin, ginkgolide A, ginkgolide B and ginkgolide C in the GbP group significantly increased (P < 0.001) while the AUC0−t and Cmax of bilobalide showed no obvious difference when compared with the Ginaton group. It could be inferred that novel drug delivery systems of proliposome could lead to better absorption and higher bioavailability of tested ginkgo flavonoids and terpenoid lactones, except for bilobalide. These results were consistent with the effects of proliposome described in the literature (Ashraf et al., 2013).
The Tmax of quercetin, kaempferol, isorhamnetin, bilobalide, ginkgolide B and ginkgolide C in GbP group showed a tendency to increase when compared to those in the Ginaton group, of which the values of quercetin, kaempferol, isorhamnetin, bilobalide and ginkgolide C showed remarkable differences (P < 0.05). So it could be inferred that the proliposome formulation could also postpone the arrival of peak values of ginkgo flavonoids in rat plasma.
Compared with those in the Ginaton group, T1/2 of seven analytes (but not quercetin and bilobalide in the GbP group) was also significantly different (P < 0.05). The T1/2 values for kaempferol, isorhamnetin, ginkgolide A, ginkgolide B and ginkgolide C were remarkably prolonged. As for CL of seven analytes, the values of CL for quercetin, kaempferol, isorhamnetin, ginkgolide A, ginkgolide B and ginkgolide C in GbP group were remarkably increased (P < 0.001), while that of bilobalide showed no significant difference when compared with the Ginaton group. Therefore, it could be speculated that the proliposome formulation could increase the elimination time of ginkgo flavonoids and terpenoid lactones, except for bilobalide.
4. Conclusions
In this paper, a highly rapid, simple and sensitive UPLC–MS/MS method was developed for the simultaneous quantification of quercetin, kaempferol, isorhamnetin, bilobalide, ginkgolides A, B and C in plasma of rats with the lower limit of quantification of 0.266, 0.1, 0.2, 0.266, 0.2, 0.333 and 0.333, respectively. This method required a 6 min chromatographic run time and used 50 μl plasma samples. Moreover, this method was first subjected to comparative pharmacokinetic evaluation between a commercial tablet product of Ginaton and an oral proliposome formulation carrying GbE (GbP) in rat plasma, with the results showing that the proliposome formulation had a significant effect on absorption, elimination and bioavailability of G. biloba flavonoids and terpenoid lactones in comparison with the ordinary tablet. The current results would be helpful for speculating the absorption mechanism in the gastrointestinal tract at the pharmacokinetic level, and for guiding the development of novel oral drug delivery systems.
Acknowledgments
This research is supported by UMRG (RG257-13AFR) IPPP (PG038-2013B) and FRGS (FP038-2013B).
Footnotes
Peer review under responsibility of King Saud University.
References
- Ashraf M.A., Ullah S., Ahmad I., Qureshi A.K., Balkhair K.S., Rehman M.A. Green biocides, a promising technology: current and future applications. J. Sci. Food Agric. 2013;94(3):388–403. doi: 10.1002/jsfa.6371. [DOI] [PubMed] [Google Scholar]
- Ashraf M.A., Khan A., Sarfraz M., Ahmad M. Effectiveness of silica based Sol-gel microencapsulation method for odorants and flavours leading to sustainable environment. Front. Chem. 2015;3:42. doi: 10.3389/fchem.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batool S., Khalid A., Chowdury A.J.K., Sarfraz M., Balkhair K.S., Ashraf M.A. Impacts of azo dye on ammonium oxidation process and ammonia oxidizing soil bacteria. RSC Adv. 2015;5:34812–34820. [Google Scholar]
- Biber A. Pharmacokinetics of Ginkgo biloba extracts. Pharmacopsychiatry. 2003;36(Suppl. 1):S32–S37. doi: 10.1055/s-2003-40446. [DOI] [PubMed] [Google Scholar]
- Butt M.A., Ahmad M., Fatima A., Sultana S., Zafar M., Yaseen G., Ashraf M.A., Shinwari Z.K., Kayani S. Ethnomedicinal uses of plants for the treatment of snake and scorpion bite in Northern Pakistan. J. Ethnopharmacol. 2015;2015:1–14. doi: 10.1016/j.jep.2015.03.045. [DOI] [PubMed] [Google Scholar]
- Chen J.S., Huang P.H., Wang C.H., Lin F.Y., Tsai H.Y., Wu T.C. Nrf-2 mediated heme oxygenase-1 expression, an antioxidant-independent mechanism, contributes to anti-atherogenesis and vascular protective effects of Ginkgo biloba extract. Atherosclerosis. 2011;214:301–309. doi: 10.1016/j.atherosclerosis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Chen J.W., Chen Y.H., Lin F.Y., Chen Y.L., Lin S.J. Ginkgo biloba extract inhibits tumor necrosis factor-induced reactive oxygen species generation, transcription factor activation, and cell adhesion molecule expression in human aortic endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2003;23:1559–1566. doi: 10.1161/01.ATV.0000089012.73180.63. [DOI] [PubMed] [Google Scholar]
- Chen Z.-P., Sun J., Chen H.-X., Xiao Y.-Y., Liu D., Chen J. Comparative pharmacokinetics and bioavailability studies of quercetin, kaempferol and isorhamnetin after oral administration of Ginkgo biloba extracts, Ginkgo biloba extract phospholipid complexes and Ginkgo biloba extract solid dispersions in rats. Fitoterapia. 2010;81:1045–1052. doi: 10.1016/j.fitote.2010.06.028. [DOI] [PubMed] [Google Scholar]
- DeFeudis F.V., Drieu K. In vitro studies of the pharmacological and biochemical activities of Ginkgo biloba extract (EGb761) and its constituents. In: van Beek T.A., editor. Ginkgo Biloba. Harwood Academic Publishers; Amsterdam: 2000. pp. 279–301. [Google Scholar]
- Drieu K., DeFeudis F.V. In vivo studies of the pharmacological and biochemical activities of Ginkgo biloba extract (EGb761) and its constituents. In: van Beek T.A., editor. Ginkgo Biloba. Harwood Academic Publishers; Amsterdam: 2000. pp. 303–329. [Google Scholar]
- Huang S.Y., Jeng C., Kao S.C., Yu J.J.H., Liu D.Z. Improved haemorrheological properties by Ginkgo biloba extract (Egb761) in type 2 diabetes mellitus complicated with retinopathy. Clin. Nutr. 2004;23:615–621. doi: 10.1016/j.clnu.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Ivic L., Sands T.T., Fishkin N., Nakanishi K., Kriegstein A.R., StrØmgaard K. Terpene trilactones from Ginkgo biloba are antagonists of cortical glycine and GABAA receptors. J. Biol. Chem. 2003;278:49279–49285. doi: 10.1074/jbc.M304034200. [DOI] [PubMed] [Google Scholar]
- Kleijnen J., Knipschild P. Ginkgo biloba. Lancet. 1992;340:1136–1139. doi: 10.1016/0140-6736(92)93158-j. [DOI] [PubMed] [Google Scholar]
- Koltermann A., Hartkorn A., Koch E., Furst R., Vollmar A.M., Zahler S. Ginkgo biloba extract EGb761 increases endothelial nitric oxide production in vitro and in vivo. Cell. Mol. Life Sci. 2007;64:1715–1722. doi: 10.1007/s00018-007-7085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaskheli A.A., Talpur F.N., Ashraf M.A., Cebeci A., Jawaid S., Afridi H.I. Monitoring the Rhizopus oryzae lipase catalyzed hydrolysis of castor oil by ATR-FTIR spectroscopy. J. Mol. Catal. B Enzym. 2015;113:56–61. [Google Scholar]
- Kiyani S., Ahmad M., Zafar M.A., Sultana S., Khan M.P.Z., Ashraf M.A., Hussain J., Yaseen G. Ethnobotanical uses of medicinal plants for respiratory disorders among the inhabitants of Gallies-Abbottabad, Northern Pakistan. J. Ethnopharmacol. 2014;156:47–60. doi: 10.1016/j.jep.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Li L., Zhao Y.-S., Du F.-F., Yang J.-L., Xu F., Niu W. Intestinal absorption and presystemic elimination of various chemical constituents present in GBE50 extract, a standardized extract of Ginkgo biloba leaves. Curr. Drug Metab. 2012;13:494–509. doi: 10.2174/1389200211209050494. [DOI] [PubMed] [Google Scholar]
- Liu H.-F., Yang J.-L., Du F.-F., Gao X.-M., Ma X.-T., Huang Y.-H. Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug Metab. Dispos. 2009;37:2290–2298. doi: 10.1124/dmd.109.029819. [DOI] [PubMed] [Google Scholar]
- Lu J., Li Y., Hu D., Chen X., Liu Y., Wang Y., Ashraf M.A., Zhao Y. One-step synthesis of interpenetrating network hydrogels: environment sensitivities and drug delivery properties. Saudi J. Biol. Sci. 2016;23:S22–S31. doi: 10.1016/j.sjbs.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naureen R., Tariq M., Yusoff I., Choudhury A.J.K., Ashraf M.A. Synthesis, spectroscopic and chromatographic studies of sunflower oil biodiesel using optimized base catalyzed methanolysis. Saudi J. Biol. Sci. 2014;22:322–339. doi: 10.1016/j.sjbs.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor M.J., Ahmad M., Ashraf M.A., Zafar M., Sultana S. A review of the pollen analysis of South Asian honey to identify the bee floras of the region. Palynology. 2015;2015:1–12. [Google Scholar]
- Ou H.C., Lee W.J., Lee I.T., Chiu T.H., Tsai K.L., Lin C.Y. Ginkgo biloba extract attenuates oxLDL-induced oxidative functional damages in endothelial cells. J. Appl. Physiol. 2009;106:1674–1685. doi: 10.1152/japplphysiol.91415.2008. [DOI] [PubMed] [Google Scholar]
- Qureshi T., Memon N., Memon S.Q., Ashraf M.A. Decontamination of ofloxacin: optimization of removal process onto sawdust using response surface methodology. Desalin. Water Treat. 2015;2015:1–9. [Google Scholar]
- Rangel-Ordóñez L., Nöldner M., Schubert-Zsilavecz M., Wurglics M. Plasma levels and distribution of flavonoids in rat brain after single and repeated doses of standardized Ginkgo biloba extract EGb761. Planta Med. 2010;76:1683–1690. doi: 10.1055/s-0030-1249962. [DOI] [PubMed] [Google Scholar]
- Safi S.Z., Batumalaie K., Mansor M., Chinna K., Mohan S., Karimian H., Qvist R., Ashraf M.A., Yan G.O.S. Glutamine treatment attenuates hyperglycemia-induced mitochondrial stress and apoptosis in umbilical vein endothelial cells. Clinics. 2015;70:8. doi: 10.6061/clinics/2015(08)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safi S.Z., Qvist R., Chinna K., Ashraf M.A., Darishiani Paramasivam., Ismail I.S. Gene expression profiling of the peripheral blood mononuclear cells of offspring of one type 2 diabetic parent. Int. J. Diabetes Dev. Ctries. 2015;2015:1–8. [Google Scholar]
- Saleem S., Zhuang H., Biswal S., Christen Y., Doré S. Ginkgo biloba extract neuroprotective action is dependent on heme oxygenase 1 in ischemic reperfusion brain injury. Stroke. 2008;39:3389–3396. doi: 10.1161/STROKEAHA.108.523480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surhio M.A., Talpur F.N., Nizamani S.M., Amin F., Bong C.W., Lee C.W., Ashraf M.A., Shahid M.R. Complete degradation of dimethyl phthalate by biochemical cooperation of the Bacillus thuringiensis strain isolated from cotton field soil. RSC Adv. 2014;4:55960–55966. [Google Scholar]
- Wu Y.-Z., Li S.-Q., Cui W., Zu X.-G., Wang F.-F., Du J. Ginkgo biloba extract improves coronary blood flow in patients with coronary artery disease: role of endothelial-dependent vasodilation. Planta Med. 2007;73:624–628. doi: 10.1055/s-2007-981536. [DOI] [PubMed] [Google Scholar]
- Yoshitake T., Yoshitake S., Kehr J. The Ginkgo biloba extract EGb761 and its main constituent flavonoids and ginkgolides increase extracellular dopamine levels in the rat prefrontal cortex. Br. J. Pharmacol. 2010;159:659–668. doi: 10.1111/j.1476-5381.2009.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Sun Y., Li C. Simultaneous determination of ginkgo flavonoids and terpenoids in plasma: ammonium formate in LC mobile phase enhancing electrospray ionization efficiency and capacity. J. Am. Soc. Mass Spectrom. 2008;19:445–449. doi: 10.1016/j.jasms.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Zhou L., Meng Q.-J., Qian T., Yang Z.-Q. Ginkgo biloba extract enhances glucose tolerance in hyperinsulinism-induced hepatic cells. J. Nat. Med. 2011;65:50–56. doi: 10.1007/s11418-010-0456-z. [DOI] [PubMed] [Google Scholar]
- Zhou W., Chai H., Lin P.H., Lumsden A.B., Yao Q.-Z., Chen C.-Y. Clinical use and molecular mechanisms of action of extract of Ginkgo biloba leaves in cardiovascular diseases. Cardiovasc. Drug Rev. 2004;22:309–319. doi: 10.1111/j.1527-3466.2004.tb00148.x. [DOI] [PubMed] [Google Scholar]