Abstract
The aim of the present study was to characterize the endophytic bacterial strain designated MSR1 that was isolated from inside the non-nodulating roots of Medicago sativa after surface-sterilization. MSR1 was identified as Enterobacter cloacae using both 16S rDNA gene sequence analysis and API20E biochemical identification system (Biomerieux, France). Furthermore, this bacterium was characterized using API50CH kit (Biomerieux, France) and tested for antibacterial activities against some food borne pathogens. The results showed that E. cloacae consumed certain carbohydrates such as glycerol, d-xylose, d-maltose and esculin melibiose as a sole carbon source and certain amino acids such as arginine, tryptophan ornithine as nitrogen source. Furthermore, MSR1 possessed multiple plant-growth promoting characteristics; phosphate solubility, production of phytohormones acetoin and bioactive compounds. Inoculation of Pisum sativum with MSR1 significantly improved the growth parameters (the length and dry weight) of this economically important grain legume compared to the non-treated plants. To our knowledge, this is the first report addressing E. cloacae which exist in roots of alfalfa growing in Al-Ahsaa region. The results confirmed that E. cloacae exhibited traits for plant growth promoting and could be developed as an eco-friendly biofertilizer for P. sativum and probably for other important plant species in future.
Keywords: Bacterial endophytes, Enterobacter cloacae, Pisum sativum
1. Introduction
Bacterial endophytes are a group of soil bacterial that inhabit plant tissues and play a pivotal role in plant growth enhancement via direct and indirect mechanisms (Hallmann et al., 1997, Narula et al., 2013). Production of phytohormones, solubilization, of inorganic phosphate, nitrogen fixation and sequestration of iron are different direct ways of endophytic bacteria for plant growth-stimulation. Among the indirect ways are protection against pathogenic microorganisms and amelioration of ecological constraints such as drought, salinity and heavy metals (Rodríguez-Díaz et al., 2008, Dudeja et al., 2012, Rashid et al., 2012, Ji et al., 2013).
Certain Enterobacter spp. have been reported as plant-growth enhancers since they possess multiple growth-promoting activities (Kampfer et al., 2005, Deepa et al., 2010, Ramesh et al., 2014). Several endophytic bacteria with traits of plant growth-promoting activities have been isolated from different plant species. Examples are Enterobacter spp. from maize (McInroy and Kloepper, 1995); Enterobacter sakazakii and Enterobacter agglomerans from soybean (Kuklinsky-Sobral et al., 2004); Enterobacter cloacae from citrus plants, maize (Araújo et al., 2002, Hinton and Bacon, 1995); Enterobacter asburiae from sweet potato (Asis and Adachi, 2003). E. cloacae have been recently recovered from the soybean rhizosphere and enhanced significantly the growth of soybean-wheat (Ramesh et al., 2014).
Researchers have given a considerable attention to bacterial endophytes of root-nodules of Alfalfa (Medicago sativa L.) due to its significant role in increasing nitrogen input to soils (Gallego-Giraldo et al., 2014). These efforts revealed that root-nodules of alfalfa harbour diverse bacterial endophytes including the nodulating microsymbiont, Sinorhizobium meliloti (Young, 2003), in addition to non-nodulating strains of Endobacter medicaginis (Ramírez-Bahena et al., 2013) Micromonospora (Trujillo et al., 2010), and Brevibacillus choshinensis and Microbacterium trichothecenolyticum (Stajković et al., 2009). These bacterial species seem to share this ecological niche. A comprehensive study was conducted using cultural-independent methods that revealed a high taxonomic variability among bacterial communities associated with nodules, stem and leaves of M. sativa (Pini et al., 2012). They have found that members of Alphaproteobacteria are dominant in alfalfa tissues. However, little is known about the bacterial endophytes in roots of non-nodulating alfalfa plants growing in Al-Ahsaa region, Saudi Arabia. Therefore, the current work aimed at isolation and characterization using phenotypic and genotypic characteristics of bacterial endophyte isolated from surface-sterilized roots of alfalfa. In addition, inoculation of Pisum sativum with the isolated bacterium species was also assessed.
2. Materials and methods
2.1. Isolation of E. cloacae strain MSR1 from roots
2.1.1. Collection of Alfalfa plants
Alfalfa plants were uprooted along with the rhizosphere from farms of Al-Ahsaa city and brought immediately to the laboratory in sterile plastic bags. To remove soil particles, roots were washed under running tap water. Surface sterilization was carried out according to the method described previously (Vincent, 1970). E. cloacae were isolated from alfalfa roots by squeezing surface-sterilized roots between two sterilized glass slides and loopfuls of the exudates were streaked onto yeast-extract mannitol agar (YMA) (Vincent, 1970). The plates were then incubated at 30 °C for 48 h. Well isolated colonies were re-streaked on fresh agar plates and maintained in agar slants and stored at 4 °C for further use.
2.1.2. Morphological characteristics
The colonial characteristics (shape and margin) and pigmentation of MSR1 were determined. The shape of cells and Gram reaction were as described by Arora (2003).
2.1.3. Phenotypic characterization MSR1 using API20 and API50CH kits (Biomerieux, France)
Phenotypic characteristics of the strain MSR1 were investigated using the API20 and API50CH strip kits (Biomerieux, France) according to the manufacturer’s instructions. After inoculation, the strips were incubated at 30 °C and results were scored after 24 h hours for API20 and 48 for API50CH. Results of API20E were interpreted using the API 20E software Version 4.1 identification database.
2.1.4. Catalase test
MSR1 was tested for its ability produce catalase enzyme by adding drops of drops of hydrogen peroxide (5%) to an aliquot of an overnight MSR1 culture was smeared on a clean glass slide. Results were recorded as positive when gas bubbles were evolved within few seconds (Table 1).
Table 1.
Characteristics of strain MSR1.
| Characteristic | Result |
|---|---|
| Colony morphology | Rounded colony-entire edge |
| Pigmentation | White (yellowish) |
| Gram staining | Negative |
| Cells | Rod-shaped |
| NaCl tolerance (0–4%) | + |
| NaCl tolerance (5%) | − |
| ONPG | + |
| Arginine | + |
| Lysine | − |
| Ornithine | + |
| Citrate utilization | + |
| H2S production | − |
| Urea hydrolysis | − |
| Tryptophan | + |
| Indole production | + |
| Acetoin production | + |
| Charcoal gelatin | − |
| Glucose | + |
| Mannitol | + |
| Inositol | − |
| Sorbitol | + |
| Rhamnose | + |
| Sucrose | + |
| Melibiose | + |
| Amygdalin | + |
| Arabinose | + |
| Glycerol | + |
| d-Xylose | + |
| d-Maltose | + |
| Esculin | + |
| Methyl-α d-glucopyranoside | + |
| Catalase | + |
| Amylase | + |
| Phosphate solubilization | + |
| IAA production (μg ml−1) | 112 ± 6 |
| Antibacterial activity | |
| Listeria monocytogenes (ATCC 7644) | + |
| Escherichia coli (ATCC 25922) | − |
| Pseudomonas aeruginosa (ATCC 27853) | − |
| Salmonella enterica (ATCC 13076) | − |
| Staphylococcus aureus (ATCC 25923) | − |
| Staphylococcus saprophyticus (ATCC 15305) | − |
| Antibiotic resistance (R)/susceptibility (S) (mcg disc−1) | |
| Chloramphenicol 30 | S (1.8⁎ ± 0.08) |
| Cephradine 30 | R |
| Ampicillin sulbactam 10 | R |
| Erythromycin 15 | R |
| Tetracycline 30 | R |
| 16S r RNA gene sequence | 99% identity to Enterobacter cloacae |
| NCBI gene bank accession No. | KJ668861 |
Diameter of inhibition zone around the antibiotic disc.
2.1.5. Production of indole acetic acid (IAA)
The ability of the MSR1 strain to produce IAA was investigated using the method outlined previously (Gordon and Weber, 1951). MSR1 was grown in Bertani broth supplemented with 0.0 and 0.2 mg ml−1 of tryptophan and incubated in shaking incubator at 30 °C for 3 days. Then, the cells were harvested after centrifugation at 8000 rpm for 15 min then 1 ml of the supernatant was mixed vigorously with 2 ml of Salkowski’s reagent (150 ml of concentrated H2SO4, 250 ml of distilled H2O, 7.5 ml of 0.5 M FeCl3·6H2O) (Ehmann, 1977). The tubes were incubated in darkness at room temperature for 25 min. then the OD530 was measured and the quantity of the IAA produced was estimated from standard curve of IAA.
2.1.6. Phosphate solubilization
In order to determine whether or not MAR1 is phosphate solubilizer, the strain was streaked on Pikovskaya agar which supplemented with calcium triphosphate as the source of mineral phosphate. The plates were incubated at 30 °C after 72 h. Formation of a clear halo zone around the MAR1 colony was an indicator for a positive result (Pikovskaya, 1948).
2.1.7. Growth of MAR1 under different concentrations of NaCl
To determine the tolerance level to NaCl salinity, MAR1 grown on nutrient agar plates contained 0, 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10% NaCl. After 48 h of incubation at 30 °C, the plates were checked visually for bacterial growth. The experiment was done in triplicates.
2.1.8. Intrinsic antibiotic resistance
MSR1 was tested for susceptibility to five different antibiotics. An overnight-old culture of MSR1 was spread homogeneously on the NA plates and antibiotic discs of ampicillin sulbactam (SAM, 10 mcg) cephradine (CE, 30 mcg), chloramphenicol (C, 30 mcg), erythromycin (E, 15 mcg) and tetracycline (TE, 30 mcg) were placed on the plates under aseptic conditions (Table 1). After 24 h incubation period at 30 °C, the plated were checked for appearance of zones of inhibition around the antibiotic discs, indicating that the strain is sensitive to the antibiotic tested at the concentration used. The actual diameter of the zone of inhibition was calculated by subtracting the diameter of the disc from the total diameter. The experiment was carried out in triplicate.
2.1.9. Starch hydrolysis
MSR1 was checked for the ability to consume starch as carbon and energy source for its growth by streaking on starch agar plates. The plates were incubated at 30 °C for 24 h, then drops of iodine reagent were added to flood the plate and positive results were recorded when clear halos around the growing colonies appeared (Table 1).
2.1.10. Antibacterial activity
The antibacterial activity of the strain MSR1 was determined against six pathogens as reference bacteria; Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Salmonella enterica (ATCC 13076), Listeria monocytogenes (ATCC 7644), Staphylococcus aureus (ATCC 25923), and Staphylococcus saprophyticus, (ATCC 15305) (Table 1). A loopful of an overnight MSR1 culture was placed on the NA plates which were seeded with the test bacterial strains. After 72 h of incubation at 30 °C the plates were checked for formation of zones of inhibition around the MSR1 grown colonies (Table 1).
2.1.11. PCR amplification of the 16S rDNA
Template DNA was prepared from a single colony of MSR1 using InstaGene Matrix (Bio-Rad, USA) according to the manufacturer instructions. Amplification of the 16S rRNA gene was carried out using the universal primers; 27F 5′-AGA GTT TGA TCM TGG CTC AG-3′ and 1492R 5′-TACGGYTACCTTGTTACGACTT (Weisburg et al., 1991), in a total 20 μl of PCR reaction. The main PCR steps were programmed as follows: denaturation at 94 °C for 45 s, annealing at 55 °C for 60 s, and extension 72 °C for 60 s. in 30 amplification cycles, followed by a final extension step at 72 °C for 10 min. Genomic DNA from E. coli and PCR water were used as positive and negative controls, respectively. A Clean-up kit (Millipore) was used to purify the amplification products, according to manufacturer’s protocol.
2.1.12. 16S rDNA sequencing and construction of phylogenetic tree
Big Dye terminator cycle sequencing kit (Applied BioSystems, USA) was used for sequencing of the 16S rRNA gene. Sequencing products were resolved on an Applied Biosystems model 3730XL automated DNA sequencing system (Applied BioSystems, USA). The phylogenetic relationships tree among the MSR1 and several representative species from Enterobacteriaceae obtained as hits in BLASTN, was constructed using neighbour-joining (Saitou and Nei, 1987) as implemented in MEGA 5.02 (Tamura et al., 2011); bootstrap support (Felsenstein, 1985) were generated based on 1500 replicates. The evolutionary distances were computed using the Maximum Composite Likelihood method (Tamura et al., 2004). The 16s rRNA gene sequence of MSR1 was deposited in the NCBI database under the accession No. KJ668861 (Table 1).
2.1.13. Inoculation of P. sativum with MSR1 strain
The growth promotion of MSR1 on the growth was assessed by inoculation of P. sativum, an economically-important grain legume, with MSR1. Seeds of P. sativum were surface-sterilized as described and five healthy seeds planted per pot containing sterilized agricultural soils (Vincent, 1970). Seeds were then inoculated with MSR1 suspensions (2 ml of OD660 1.0) and untreated control plants to which 2 ml sterilized distilled water was used. After 15 days, the height, fresh and dry weights of the primary root and shoot were measured. The experiment was performed in triplicate. Significance between treatments was calculated after statistical analysis of data using Student’s t test.
3. Results
E. cloacae were isolated from inside surface-sterilized alfalfa roots and characterized using phenotypic traits and PCR-amplified 16S rRNA gene sequences (Table 1). Furthermore, the growth parameters (length of the primary root and number of secondary roots of and dry weight of roots) of P. sativum were significantly increased upon inoculation MSR1.
MSR1 exhibited a white, rounded colony with entire in edge. Gram staining showed that the cells of MSA1 are Gram-positive rod-shaped (Table 1).
3.1. Phenotypic characterization MSR1 using API20 and API50CH kits
The data of the phenotypic characteristics using the API 20E biochemical identification system (bioMérieux) are presented in Table 1. MSR1 consumed 75% (15 out of 20) of the different chemical substrates contained in the API20E strip. Generally, MSR1 fermented/oxidized all the carbohydrate tested except for the polyol carbohydrate, inositol (Table 1). Furthermore, MSR1 consumed ortho-nitrophenyl-β-galactoside (ONPG), Na pyruvate and the amino acids arginine ornithine but not lysine. In addition, MSR1 was unable to hydrolyse urea, charcoal gelatin or produce H2S from Na thiosulfate. Based on the results of API20E, MSR1 was identified as E. cloacae at a percentage of 95.1% using the relevant software Version 4.1 identification database. For further characterization, the strain MSR1 was tested for biochemical tests using API50CH (Table 1). Out of 50 different carbohydrates included in the API50CH strip, MAR1 was able to consume only 5 which represent 10% of different compounds as a sole source of carbon and energy. MAR1 utilized glycerol, d-xylose, d-maltose esculin and methyl-α d-glucopyranoside. As the majority of API 50CH tests for the strain MSR1were negative, they were therefore not shown.
3.2. Catalase test
As can be seen in Table 1, MSR1 was catalase positive as indicated by evolution of gas bubbles once hydrogen peroxide was added to the growing culture.
3.3. Production of indole acetic acid (IAA)
MSR1 produced IAA (112 μg ml−1) when the growth medium supplemented with tryptophan at a concentration of 0.2 μg ml−1 (Table 1). No detection of IAA was observed when MSR1 was grown on no-added tryptophan growth medium.
3.4. Phosphate solubility
MSR1 was tested for this characteristic using the qualitative method described earlier by Pikovskaya (1948) and the results presented in Table 1. A clear zone appeared around the colony of MSR1 grown on Pikovskaya agar supplemented with calcium triphosphate as a source of insoluble mineral phosphate.
3.5. Growth of MSR1 under different concentrations of NaCl
As presented in Table 1, MSR1 grew well at low levels of NaCl (1 and 2% NaCl) while at 3% NaCl, weak growth was observed. At 4% NaCl, MSR1 struggled to grow and after this concentration of the salt no growth appeared. Furthermore, MSR1 could grow under elevated levels of NaCl up to 8% after which no growth was observed.
3.6. Starch hydrolysis
The results of the starch hydrolysis test are presented in (Table 1). As can be seen, a halo zone was formed around the colonies of MSR1 upon addition of iodine reagent indicating its ability utilized starch as a carbon and energy source via alpha-amylase.
3.7. Intrinsic antibiotic resistance
Strain MSR1 exhibited intrinsic antibiotic resistance to all of the antibiotics tested with the exception of chloramphenicol (30 mcg) (Table 1). The diameter of the inhibition zone that formed around the chloramphenicol disc was 1.8 cm indicating that this antibiotic is effective against MSR1, compared to other antibiotics. On the other hand, MSR1 showed resistance to the following antibiotics: ampicillin sulbactam (10 mcg), erythromycin (15 mcg) cephradine (30 mcg), tetracycline (30 mcg) and erythromycin (15 mcg).
3.8. Antibacterial activity of MSR1
The results of the antibacterial activities of MSR1 against six pathogens are presented in Table 1. MSR1 exhibited an antibacterial activity against L. monocytogenes ATCC7644 as indicated by formation of the inhibition zone around the ground MSR1 colony. However, no zones of inhibitions were noticed around MSR1 colonies highlighting that no antibacterial activities against the rest of the bacterial strains were tested.
3.9. Sequences of the 16S rRNA gene
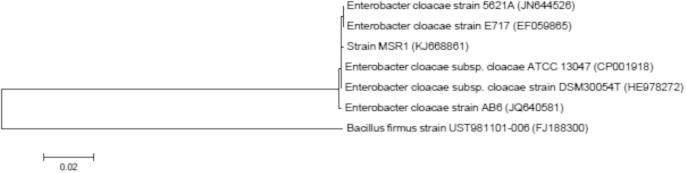
MSR1 exhibited 16SrRNA gene sequences of 99% homology with E. cloacae subsp. cloacae strain DSM 30054T (accession No. HE978272), E. cloacae strain 5621A (accession No. JN644526), E. cloacae strain AB6 (accession No. JQ640581) and E. cloacae strain E717 (accession No. EF059865) (Table 2). As expected there was a substantially low homology (81%) with Bacillus firmus strain UST981101-006 (accession No. FJ188300), after comparative sequence analysis using BLAST tools in NCBI. The 16S rDNA sequence-based phylogenetic tree analysis revealed that MSR1 belonged to the family Enterobacteriaceae and grouped with E. cloacae clade (Fig. 1).
Table 2.
16S rRNA gene sequence analysis and phylogenetic tree.
| Bacterial strain | Accession No. | Coverage % | Identity % |
|---|---|---|---|
| Enterobacter cloacae subsp. cloacae ATCC 13047 | CP001918 | 99 | 99 |
| Enterobacter cloacae subsp. cloacae strain DSM 30054T | (HE978272) | 99 | 99 |
| Enterobacter cloacae strain 5621A | (JN644526) | 99 | 99 |
| Enterobacter cloacae strain AB6 | (JQ640581) | 99 | 99 |
| Enterobacter cloacae strain E717 | EF059865) | 99 | 99 |
| Bacillus firmus strain UST981101-006 | (FJ188300) | 81 | 79 |
Figure 1.
Neighbor-joining tree based on 16S rDNA gene sequences showing relationships between strain MSR1 (accession No. KJ668861) and related species Enterobacter cloacae subsp. cloacae ATCC 13047 (accession No. CP001918), Enterobacter cloacae subsp. cloacae strain DSM 30054T (accession No. HE978272), Enterobacter cloacae strain 5621A (accession No. JN644526), Enterobacter cloacae strain AB6 (accession No. JQ640581), Enterobacter cloacae strain E717 (accession No. EF059865) and Bacillus firmus strain UST981101-006 (accession No. FJ188300). The percentage numbers above each branch indicate the 567 levels of bootstrap support (>50%) for the branch point based on 1.,500 568 resamplings. The bar represents 0.02 substitutions per site.
3.10. Effect of MSR1 inoculation on P. sativum
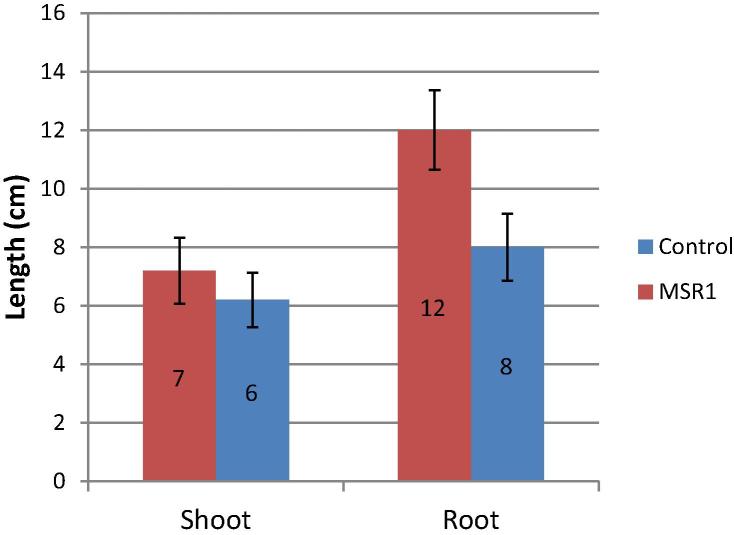
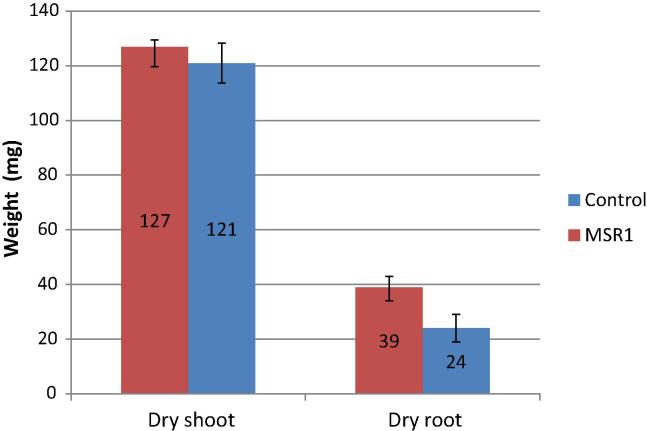
MSR1 was tested for its growth promotion activity for P. sativum and the results are presented in Figs. 2 and 3. MSR1 inoculation significantly improved the length of the primary, the number of secondary roots and root dry weight. The MSR1-inoculated roots of alfalfa reached a length of 12.5 cm compared to the control (8 cm) (Fig. 2). Likewise, the inoculation with MSR1 improved significantly the dry weight of the root per plant (39 mg plant−1) compared to the uninoculated control plants (24 mg plant−1), (P < 0.001) (Fig. 3). Furthermore, a significant increase in the number of lateral roots in P. sativum (20.57 cm) was noticed in MSR1-inoculated plants compared to the control ones (11 cm, respectively) (P < 0.01). No differences in the height or the dry weight of the shoot were observed between the treated and untreated plants (Figure 2, Figure 3). The positive effects of MSR1 on growth of P. sativum were more observed in the root system of this plant.
Figure 2.
Effects of MSR1 inoculation on length of root and shoot of Pisum sativum.
Figure 3.
Effects of MSR1 inoculation on dry weights of root and shoot of Pisum sativum.
4. Discussion
In the current study, E. cloacae were found to be in association with non-nodulating roots of alfalfa. This bacterium was also described phenotypically and genotypically and exhibited plant growth promotion when inoculated P. sativum (Table 1).
The commercially available simple tool, API 20E biochemical identification system (bioMérieux), was used to identify the MRS1 strain. This system is proved to be accurate for bacterial identification at the genus level and in some cases at the species level (Turcovský et al., 2011). In our case, the API20E provided identification of MSR1 as E. cloacae with a percentage of 95.1%. This finding was in agreement with those obtained from comparative 16rRNA gene sequences, indicating that both tools can provide accurate identification of Enterobacter spp.
Remarkably, MSR1 produced acetoin (the 3-hydroxy-2-butanone) (Table 1), an elicitor, which plays a pivotal role in induced systemic resistance. Elicitation of induced systemic resistance is mediated via certain metabolites produced by rhizobacteria resulted in enhancing the plant resistance against pathogens (Kloepper et al., 2004, Van Loon and Glick, 2004, Rudrappa et al., 2010). It has been observed that acetoin produced by Enterobacter aerogenes rendered maize plants more resistant against, Setosphaeria turcica, the Northern corn leaf blight fungus (D’Alessandro et al., 2014). Therefore, it can be concluded that the strain MSR1 via its ability to produce acetoin, could have a key role in induction of plant resistance against certain phytopathogens.
The findings that MSR1 was unable to utilize the majority carbohydrate in the API 50CH strips are line with the findings of other researchers studying members of Enterobacteriace; Xenorhabdus budapestensis, Xenorhabdus ehlersii, Xenorhabdus innexi and Xenorhabdus szentirmaii (Lengyel et al., 2005).
Catalase enzyme plays a major role in organism protection against toxic free radicals that are generated particularly under environmental stresses. Therefore, MSR1 expressing catalase activity could promote plant growth via an indirect way. These findings are in agreement with those obtained previously (Kravchenko et al., 2004, Bumunang and Babalola, 2014).
Production of the phytohormones is one of the common features of the plant growth-promoting bacteria, and MSR1 is not an exception. This finding is in consonance with those obtained by other researchers who studied E. cloacae isolated from different plant species (Deepa et al., 2010, Montanez et al., 2012, Ramesh et al., 2014).
IAA is a plant growth regulating hormone which controls plant cell division and root elongation (Kravchenko et al., 2004). Plant roots release nutrients that contain tryptophan which can be consumed by soil bacteria as a precursor for IAA production, a widespread trait among most plant growth-promoting bacteria.
Phosphate solubility is another common trait through which rhizobacteria could substantially enhance plant growth. The result that MSR1 had the ability to solubilize inorganic phosphate highlights that the strain secretes organic acids and phosphatases, which solubilize the insoluble phosphate. Consequently, the essential nutrient for plant growth, phosphorous, becomes available for roots to sustain plant growth. Our results run with those obtained by Li et al.,(2008) who reported that nodule endophytic bacteria of soybean grown in Heilongjiang province of China promote plant growth via phosphate dissolving ability. However, it is generally accepted that not all plant growth promoting bacteria are the phosphate solubilizers. It has been reported that rhizobacteria associated with the rhizosphere of Withania somnifera did not show phosphate solubilization ability (Rathaur et al., 2012).
MSR1 exhibited the ability to grow on growth medium containing as high NaCl levels as 4%. These results are in agreement with those obtained on other Bacillus strains which could tolerate elevated levels of NaCl (Dastager et al., 2014). Tolerance to salinity confers a selective advantage for rhizobacterial species populating the same soil localities suffering from elevated levels of salts.
Strain MSR1 exhibited intrinsic antibiotic resistance to all of the antibiotics tested with the exception of chloramphenicol. These results are in general agreement with those obtained by Ramesh et al. (2014) who studied the soybean endophytic bacterium, E. cloacae subsp. dissolvens MDSR9. Intrinsic resistance of rhizobacteria to antibiotics confers an ecological merit of survival in rhizospheric soils when they are applied as biofertilizers.
The antibacterial activity of the strain MSR1 against L. monocytogenes ATCC7644 provides an evidence that endophytic bacteria could have a role in out-competing human pathogenic bacteria. A further evidence for that, it has been reported that E. asburiae, which was isolated from the rhizosphere of Arabidopsis thaliana, out-competed human pathogens, S. enterica and the enterohemorrhagic E. coli (Cooley et al., 2003). Strains of Salmonella have been recorded as endophytic bacteria in alfalfa sprouts (Ponka et al., 1995).
Sequences of the 16S rRNA gene is a powerful and most commonly used tool in bacterial identification at both genus and specific levels and inferring phylogenetic relationships. MSR1 is identified as E. cloacae using the phenotypic-based API20E tests and verified using comparative sequence analysis of 16s rRNA gene, robusting the identification. Furthermore, the 16S rDNA sequence-based phylogenetic tree analysis revealed that MSR1 belonged to the family Enterobacteriaceae and grouped with E. cloacae clade (Fig. 1). 16srRNA gene sequence analyses were used in identification of many endophytic bacteria. As an example, Stajkovic et al. (2009) found that nodules of M. sativa growing in central Serbia harboured Bacillus megaterium.
These findings that MSR1 exhibited growth promotion activity for P. sativum are in general agreement with previous studies on rhizobacteria that substantially enhanced the plant growth. It has been reported that E. cloacae significantly enhanced the growth parameters of rice seedlings compared to the untreated ones (Suprapta et al., 2014). Similarly, rhizobacteria that are isolated from rhizosphere of one plant could improve the growth of another plant species. For example, growth enhancement of Phaseolus vulgaris and A. thaliana has been reported upon inoculation with B. megaterium, which was isolated from the rhizosphere of maize plants (López-Bucio et al., 2007). The growth promotion of P. sativum when inoculated with MSR1 can be explained by the multiple associative characteristics, phosphate solubility, nitrogen fixation, production of phytohormones and bioactive compounds, which this bacterium exhibits. The multifunctional plant growth promoting traits for rhizobacteria are clearly widespread features for this group of bacteria that could exert their positive effects via direct and indirect mechanisms.
To our knowledge, this is the first report addressing E. cloacae which exist in roots of alfalfa growing in Al-Ahsaa region. The results obtained in the current study confirmed that alfalfa roots harbour arrays of diverse bacteria. Furthermore, E. cloacae exhibited traits for plant growth promoting and could be developed as an eco-friendly biofertilizer for P. sativum and probably for other important plant species in future.
Acknowledgments
This work is partially supported by the Scientific Research Deanship, King Faisal University, Fund No. 145029. The authors are indebted to the Department of Life Sciences, King Faisal University for the facilities offered to carry out this work. Thanks should go to Mohamed Al-Farag for assistance in some experiments.
Footnotes
Peer review under responsibility of King Saud University.
References
- Araújo W.L., Marcon J., Maccheroni W., Jvan Elsas, Dvan Vuurde J.W.L. Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Appl. Environ. Microbiol. 2002;68:4906–4914. doi: 10.1128/AEM.68.10.4906-4914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora D.R. CBS Publisher; New Delhi: 2003. Text Book of Microbiology; pp. 4–48. [Google Scholar]
- Asis C.A., Adachi K. Isolation of endophytic diazotroph Pantoea agglomerans and nondiazotroph Enterobacter asburiae from sweet potato stem in Japan. Lett. Appl. Microbiol. 2003;38:19–23. doi: 10.1046/j.1472-765x.2003.01434.x. [DOI] [PubMed] [Google Scholar]
- Bumunang E.W., Babalola O.O. Characterization of Rhizobacteria from field grown genetically modified GM and non-GM maizes. Braz. Arch. Biol. Technol. 2014;57:1–8. [Google Scholar]
- Cooley M.B., Miller W.G., Mandrell R.E. Colonization of Arabidopsis thaliana with Salmonella enterica and enterohemorrhagic Escherichia coli O157, H7 and competition by Enterobacter asburiae. Appl. Environ. Microbiol. 2003;69:4915–4926. doi: 10.1128/AEM.69.8.4915-4926.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessandro M., Erb M., Ton J., Brandenburg A., Karlen D. Volatiles produced by soil-borne endophytic bacteria increase plant pathogen resistance and affect tritrophic interactions. Plant, Cell Environ. 2014;374:813–826. doi: 10.1111/pce.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastager S.G., Mawlankar R., Tang S.K., Srinivasan K., Ramana V. Bacillus enclensis sp. nov., isolated from sediment sample. Antonie Van Leeuwenhoek. 2014;105:199–206. doi: 10.1007/s10482-013-0066-3. [DOI] [PubMed] [Google Scholar]
- Deepa C.K., Dastager S.G., Pande A. Isolation and characterization of plant growth promoting bacteria from non-rhizospheric soil and their effect on cowpea Vigna unguiculata L. Walp. seedling growth. World J. Microbiol. Biotechnol. 2010;26:1233–1240. doi: 10.1007/s11274-009-0293-y. [DOI] [PubMed] [Google Scholar]
- Dudeja S.S., Giri R., Saini R., Suneja-Madan P., Kothe E. Interaction of endophytic microbes with legumes. J. Basic Microbiol. 2012;52:248–260. doi: 10.1002/jobm.201100063. [DOI] [PubMed] [Google Scholar]
- Ehmann A. The van Urk-Salkowski reagent – A sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J. Chromatogr. 1977;132:267–276. doi: 10.1016/s0021-9673(00)89300-0. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies. An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Gallego-Giraldo L., Bhattarai K., Pislariu C., Nakashima J., Jikamura Y. Lignin modification leads to increase nodule numbers in alfalfa Medicago sativa L. Plant Physiol. 2014 doi: 10.1104/pp.113.232421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S.A., Weber R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951;26:192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmann J., Quadt-Hallmann A., Mahaffee W.F., Kloepper J.W. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 1997;43:895–914. [Google Scholar]
- Hinton D.M., Bacon C.W. Enterobacter cloacae is an endophytic symbiont of corn. Mycopathologia. 1995;129:117–125. doi: 10.1007/BF01103471. [DOI] [PubMed] [Google Scholar]
- Ji S.H., Gururani M.A., Chun S.C. Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol. Res. 2013;169:83–98. doi: 10.1016/j.micres.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Kampfer P., Ruppel S., Remus R. Enterobacter radicincitans sp. nov., a plant growth promoting species of the family Enterobacteriaceae. Syst. Appl. Microbiol. 2005;28:213–221. doi: 10.1016/j.syapm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Kloepper J.W., Ryu C.M., Zhang S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology. 2004;94:1259–1266. doi: 10.1094/PHYTO.2004.94.11.1259. [DOI] [PubMed] [Google Scholar]
- Kravchenko L.V., Azarova T.S., Makarova N.M., Tikhonovich I.A. The effect of tryptophan present in plant root exudates on the phytostimulating activity of rhizobacteria. Microbiology. 2004;73:156–158. [Google Scholar]
- Kuklinsky-Sobral J., Araujo W.L., Mendes R., Geraldi I.O., Pizzirani-Kleiner A.A. Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion. Environ. Microbiol. 2004;6:1244–1251. doi: 10.1111/j.1462-2920.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- Lengyel K., Lang E., Fodor A., Szállás E., Schumann P., Stackebrandt E. Description of four novel species of Xenorhabdus, family Enterobacteriaceae Xenorhabdus budapestensis sp. nov., Xenorhabdus ehlersii sp. nov., Xenorhabdus innexi sp. nov., and Xenorhabdus szentirmaii sp. nov. Syst. Appl. Microbiol. 2005;282:115–122. doi: 10.1016/j.syapm.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Li J.H., Wang E.T., Chen W.F., Chen W.X. Genetic diversity and potential for promotion of plant growth detected in nodule endophytic bacteria of soybean grown in Heilongjiang province of China. Soil Biol. Biochem. 2008;40:238–246. [Google Scholar]
- López-Bucio J., Campos-Cuevas J.C., Hernández-Calderón E., Velásquez-Becerra C., Farías-Rodríguez R. Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol. Plant Microbe Interact. 2007;20:207–217. doi: 10.1094/MPMI-20-2-0207. [DOI] [PubMed] [Google Scholar]
- McInroy J.A., Kloepper J.W. Survey of indigenous bacterial endophytes from cotton and sweet corn. Plant Soil. 1995;173:337–342. [Google Scholar]
- Montanez A., Blanco A.R., Barlocco C., Beracochea M., Sicardi M. Characterization of cultivable putative endophytic plant growth promoting bacteria associated with maize cultivars Zea mays L. and their inoculation effects in vitro. Appl. Soil Ecol. 2012;58:21–28. [Google Scholar]
- Narula S., Anand R.C., Dudeja S.S. Beneficial traits of endophytic bacteria from field pea nodules and plant growth promotion of field pea. J. Food Legumes. 2013;26:73–79. [Google Scholar]
- Pikovskaya R.I. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Microbiologia. 1948;17:362–370. [Google Scholar]
- Pini F., Frascella A., Santopolo L., Bazzicalupo M., Biondi E.G. Exploring the plant-associated bacterial communities in Medicago sativa L. BMC Microbiol. 2012;12:78–87. doi: 10.1186/1471-2180-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponka A., Andersson Y., de Siitonen A., Jong B., Jahkola Salmonella in alfalfa sprouts. Lancet. 1995;345:462–463. doi: 10.1016/s0140-6736(95)90451-4. [DOI] [PubMed] [Google Scholar]
- Ramesh A., Sharma S.K., Sharma M.P., Yadav N., Joshi O.P. Plant growth-promoting traits in Enterobacter cloacae subsp. dissolvens MDSR9 isolated from soybean rhizosphere and its impact on growth and nutrition of soybean and wheat upon inoculation. Agric. Res. 2014;31:53–66. [Google Scholar]
- Ramírez-Bahena M.H., Tejedor C., Martín I., Velázquez E., Peix A. Enterobacter medicaginis gen. nov., sp. nov., isolated from alfalfa nodules in an acidic soil. Int. J. Syst. Evol. Microbiol. 2013;63:1760–1765. doi: 10.1099/ijs.0.041368-0. [DOI] [PubMed] [Google Scholar]
- Rashid S., Charles T.C., Glick B.R. Isolation and characterization of new plant growth-promoting bacterial endophytes. Appl. Soil Ecol. 2012;61:217–224. [Google Scholar]
- Rathaur P., Raja W., Ramteke P.W., John S.A. Effect of UV-B tolerant plant growth promoting rhizobacteria PGPR on seed germination and growth of Withania somnifera. Adv. Appl. Sci. Res. 2012;3:399–1404. [Google Scholar]
- Rodríguez-Díaz M., Rodelas Gonzalés B., Pozo Clemente C., Martínez-Toledo M.V., González-López J. A review on the taxonomy and possible screening traits of plant growth promoting rhizobacteria. In: Ahmad I., Pichtel J., Hayat S., editors. Plant-Bacteria Interactions, Strategies and Techniques to Promote Plant Growth. Wiley, V.C.H., Verlag, Gmb H., Co. KGaA; Germany: 2008. pp. 55–80. [Google Scholar]
- Rudrappa T. The rhizobacterial elicitor acetoin induces systemic resistance in Arabidopsis thaliana. Commun. Integr. Biol. 2010;3:130–138. doi: 10.4161/cib.3.2.10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method, A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Stajković O., De Meyer S., Miličić B., Willems A., Delić D. Isolation and characterization of endophytic non-rhizobial bacteria from root nodules of alfalfa Medicago sativa L. Bot. Serb. 2009;33:107–114. [Google Scholar]
- Suprapta D.N., Maulina N.M.I., Khalimi K. Effectiveness of Enterobacter cloacae to promote the growth and increase the yield of Rice. J. Biol. Agric. Health. 2014;41:44–50. [Google Scholar]
- Tamura K., Nei M., Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5, molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo M.E., AlonsoVega P., Rodríguez R., Carro L., Cerda E. The genus Micromonospora is widespread in legume root nodules, the example of Lupinus angustifolius. ISME J. 2010;4:1265–1281. doi: 10.1038/ismej.2010.55. [DOI] [PubMed] [Google Scholar]
- Turcovský I., Kuniková K., Drahovská H., Kaclíková E. Biochemical and molecular characterization of Cronobacter spp. formerly Enterobacter sakazakii isolated from foods. Antonie Van Leeuwenhoek. 2011;992:257–269. doi: 10.1007/s10482-010-9484-7. [DOI] [PubMed] [Google Scholar]
- Van Loon L.C., Glick B.R. Increased plant fitness by rhizobacteria. In: Sandermann H., editor. Molecular Ecotoxicology of Plants. Springer-Verlag; Berlin: 2004. pp. 177–205. [Google Scholar]
- Vincent J.M. Burgess and Son LTB; Oxford, United Kingdom: 1970. A manual for the practical study of root-nodule bacteria. [Google Scholar]
- Weisburg W.G., Barns S.M., Pelletier D.A., Lane D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991;17:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. The genus name Ensifer Casida 1982 takes priority over Sinorhizobium Chen et al., 1988, and Sinorhizobium morelense Wang et al., 2002. is a later synonym of Ensfer adhaerens Casida 1982. Is the combination ‘Sinorhizobium adhaerens’ Casida 1982 Willems et al. 2003 legitimate? Request for an opinion. Int. J. Syst. Evol. Microbiol. 2003;53:2107–2110. doi: 10.1099/ijs.0.02665-0. [DOI] [PubMed] [Google Scholar]