Abstract
It is always a challenge to determine the total cellulase activity efficiently without reducing accuracy. The most common total cellulase activity assay is the filter paper assay (FPA) established by the International Union of Pure and Applied Chemistry (IUPAC). A new procedure to measure the FPA with microplate-based assay was studied in this work, which followed the main idea of IUPAC to dilute cellulase preparation to get fixed glucose release. FPAs of six cellulase preparations were determined with the microplate-based assay. It is shown that FPAs of cellulase Youtell, RCconc, R-10, Lerkam, Yishui and Sinopharm were 67.9, 46.0, 46.1, 27.4, 7.6 and 8.0 IU/ml respectively. There was no significant difference at the 95% confidence level between the FPA determined with IUPAC and the microplate-based assay. It could be concluded that the FPA could be determined by the microplate-based assay with the same accuracy and much more efficiency compared with that by IUPAC.
Keywords: Cellulase, Filter paper assay, IUPAC, Microplate-based assay
1. Introduction
Lignocelluloses are the most abundant renewable bioresource in the world. The cellulase, which has been widely used in the food processing, is the critical enzyme which could catalyze cellulose to oligosaccharides and glucose (Patindol et al., 2007, Kapasakalidis et al., 2009, Renouard et al., 2010, Abbès et al., 2011). In fact, the cellulase is a system consisting of endoglucanases, exoglucanases, and β-d-glucosidases, all of which hydrolyze crystalline cellulose synergically. The cellulase activities are always measured using insoluble cellulose. The heterogeneity of insoluble cellulose and the complexity of the cellulase system cause formidable problems in measuring total cellulase activity (Mullings, 1985, Criquet, 2002, Helbert et al., 2003, Eveleigh et al., 2009, Farnet et al., 2010, Dashtban et al., 2010). The most common total cellulase activity assay is the filter paper assay (FPA) using Whatman No. 1. filter paper as the substrate, which was established and published by the International Union of Pure and Applied Chemistry (IUPAC) (Zhang et al., 2006, Batool et al., 2015). The main idea of the IUPAC method is that cellulase must be diluted until the amount of product plotted against cellulase concentration is reasonably linear. The assay requires a fixed amount (2 mg) of glucose released from a 50-mg filter paper (1 × 6 cm). A series of cellulase dilution solutions is required to achieve a fixed degree of hydrolysis (Ghose, 1987, Butt et al., 2015).
Though the IUPAC method is accepted worldwide, there are still some shortcomings for FPA assays, such as labor-intensiveness, low-throughput, and requiring a large quantity of substrate, cellulase and chemicals. Several methods were developed for the purpose of high-throughput cellulase activity screening these years (Boyer et al., 2002, Goddard and Reymond, 2004, Xiao et al., 2005, Kasana et al., 2008, King et al., 2008, Peralta et al., 2008). Decker found that replacing the filter paper with Solka-floc, Sigmacell-20, Avicel and cotton linters, the assay for a rather similar substrate in hydrolytic properties to the filter paper could be automated on a Cyberlabs C400 robotics deck (Ashraf et al., 2013, Decker et al., 2003). Berlin used the disk made from yellow poplar to estimate the hydrolysis of cellulose to glucose in a 96-well microplate. The assay shows considerable time and cost benefits over the standard assay (Berlin et al., 2005). Chundawat developed a procedure with the 96-well Biomass Conversion Research Lab microplate method for the high-throughput assay of cellulase. The entire procedure was automated using a robotic pipetting workstation (Chundawat et al., 2008). The above works are all developed for high-throughput screening, so the preciseness of the method was not given prior consideration (Kiyani et al., 2014, Khaskheli et al., 2015).
Xiao et al. (Xiao et al., 2004) developed a high-throughput assay to determine cellulase activity called the microplate-based filter paper assay (MFPA). In the MFPA, cellulase (20 μl) is added to microplate wells containing pieces of filter paper with a diameter of 7 mm and sodium citrate buffer. After incubation for 60 min at 50 °C, DNS reaction is used to measure the amount of glucose released. The MFPA can be used to rapidly assay a large number of samples. Despite the reactions being performed in different vessels, parts of the procedures of the IUPAC assay and MFPA are similar, such as hydrolysis of filter paper by cellulase followed by DNS reaction. However, there are two distinctions between the IUPAC assay and MFPA. First, the amount of cellulase that could release ∼2 mg or ∼128 μg of glucose is used in the MFPA, while exactly 2 mg of glucose is required in the IUPAC assay. Second, the IUPAC assay estimates the concentration of enzyme that would have released exactly 2.0 mg of glucose by plotting glucose liberated against enzyme concentration, while one hydrolysis point at about 2 mg or 128 μg of glucose released is determined to calculate the FPA of cellulase in the MFPA. The hydrolysis of filter paper catalyzed by cellulase is not a linear reaction. Therefore, the amount of glucose released needs to be fixed to guarantee accuracy for different operators. The principles of the IUPAC assay achieve a fixed amount of glucose release. In this work, we combine the principles of the IUPAC assay and the reaction system of the MFPA. The FPA of six cellulase preparations are measured following the concept of the IUPAC assay, in which the cellulase is diluted to determine the hydrolysis of a fixed amount of glucose, in the reaction system of the MFPA. The goal of this research is to evaluate the possibility to substitute the standard IUPAC assay with a microplate-based approach for batch assays to determine the FPA of cellulase samples to increase efficiency without sacrificing accuracy.
2. Materials and methods
2.1. Substrates
Whatman No. 1 filter paper was cut into 1.0 × 6.0 cm strips with a paper cutter for the IUPAC and into circles with a diameter of 5.0 mm with an office paper punch for the 96-well microplate assays.
2.2. Cellulases
Cellulases from six different manufacturers are Cellulase Youtell, Cellulase RCconc, Cellulase Lerkam, Cellulase Yishui, Cellulase R-10, and Cellulase Sinopharm.
2.3. Determination of filter paper activity
The IUPAC method was performed essentially according to the procedure prepared by Ghose (Ghose, 1987).
At first, 1 ml of 0.05 M Na-citrate buffer, pH 4.8 and 1 × 6 cm filter paper were added to a test tube. Then, 0.5 ml of diluted cellulase was added to the tube. At least two dilutions must be made of each cellulase sample. One dilution should release slightly more and the other one slightly less than 2.0 mg of glucose. The tubes were incubated at 50 °C for exactly 60 min. At the end of the incubation, each tube was removed from the 50 °C bath and the cellulase reaction was stopped by immediately adding 3.0 ml of DNS reagent. All tubes were boiled for exactly 5.0 min in a vigorously boiling water bath. Finally, after the colored solution was diluted with 20 ml of H2O, the absorbance at 540 nm was measured.
The microplate-based assay was performed in a 96-well PCR plate. The cellulase preparations were diluted gradiently to get a fixed amount of 80 μg glucose released between two dilutions. Then, the FPA was calculated with the Formula B.
The other part of the assay follows MFPA process basically. A 20 μl aliquot of each diluted cellulase was added into wells containing a 7-mm diameter filter paper disk and 40 μl of 50 mM Na-citrate buffer, pH 4.8. After 60 min of incubation at 50 °C, 120 μl of DNS was added into each reaction and incubated at 95° for 5 min. All incubations were performed in a Peltier Thermal Cycler (MJ Research, PTC-200). Finally, a 36-μl aliquot of each sample was transferred to the wells of a flat-bottomed plate containing 160 μl of H2O, and the absorbance at 540 nm was measured.
2.4. Calculations
2.4.1. IUPAC
Estimation of the concentration of cellulase which would have released exactly 2.0 mg of glucose by means of a plot of glucose liberated against cellulase concentration was done. To find the required cellulase concentration, two data points were taken, which are very close to 2.0 mg, and a straight line was drawn between them to find the cellulase dilution that would produce exactly 2.0 mg glucose equivalents of reducing sugar. Then, the dilutions were translated into concentrations:
FPA was calculated as
| (A) |
2.4.2. Microplate-based assay
Relative to the amount of glucose equivalents expected to be produced in IUPAC, the absolute amount of glucose released in the microplate-based assay at the critical dilution is 80 μg. The estimated amount of cellulase which releases 80 μg glucose contains 0.37 units, the same as that of IUPAC. So the FPA could be calculated as
| (B) |
3. Results and discussion
3.1. Measurement of FPA with IUPAC method
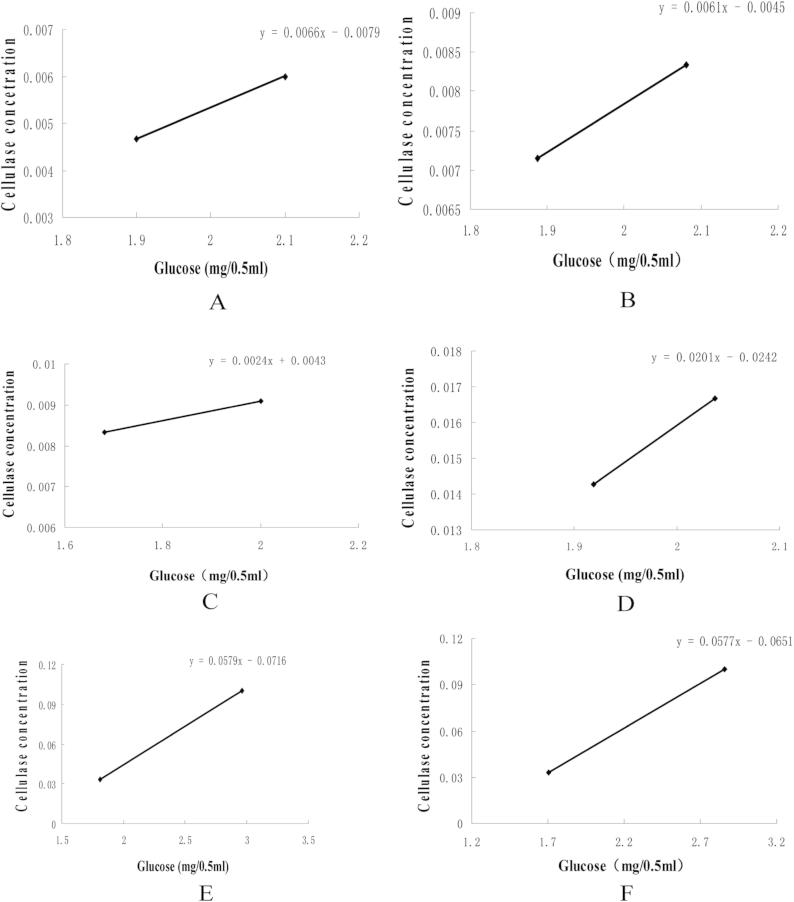
FPAs of six commercial cellulases were measured with the IUPAC method in this work. After each cellulase was diluted gradiently, the Whatman No. 1. filter paper was hydrolyzed with diluted cellulase in a 1.5 ml reaction system. Then the concentrations of cellulase which released exactly 2.0 mg of glucose were determinated by plotting glucose liberated against cellulase concentration. The FPA of each cellulase preparation was measured fourteen times. The activities of six cellulase preparations were obtained with the Formula A. The results of one series of cellulase preparation are shown in Fig. 1 and Table 1. The results of other thirteen parallel samples are not shown here. It is shown that 2 mg of glucose could be obtained by plotting glucose against cellulase concentrations at two suitable dilutions. The cellulase Youtell has the highest FPA (69.9 IU), while the cellulase Sinopharm has the lowest FPA (7.7 IU/ml). The average activities of fourteen parallel tests of six cellulase preparations are shown in Table 3. It is shown that the activities of six cellulases ranged from 7 IU/ml to 70 IU/ml, which cover the scope of PDA of common commercial cellulases.
Fig. 1.
The curves for fixing the cellulase concentration that produce 2 mg of glucose release with IUPAC. (A) Youtell, (B) RCconc, (C) R-10, (D) Lerkam, (E) Yishui, (F) Sinopharm. ∗Only a series of results within 14 parallel tests of each cellulase are shown in Figs.1 and 2 and Table 1, Table 2.
Table 1.
A series of FPAs of six cellulase preparations with the IUPAC method.
| Cellulase dilution | Cellulase concentration | FPA (IU/ml) | |
|---|---|---|---|
| Youtell | 189 | 0.0053 | 69.9 |
| RCconc | 130 | 0.0077 | 48.1 |
| R-10 | 109 | 0.0092 | 40.7 |
| Lerkam | 63 | 0.0159 | 23.3 |
| Yishui | 24 | 0.0442 | 8.4 |
| Sinopharm | 21 | 0.0476 | 7.7 |
Table 3.
Comparison of FPAs of fourteen parallel tests determined with IUPAC and microplate-based assay.
| Sample | FPA with IUPAC (IU/ml) | PFA with microplate-based assay (IU/ml) | t-Value |
|---|---|---|---|
| Youtell | 67.4 ± 2.6 | 67.9 ± 3.1 | 0.46 |
| RCconc | 45.7 ± 0.9 | 46.0 ± 1.6 | 0.61 |
| R-10 | 46.4 ± 1.5 | 46.1 ± 1.8 | 0.48 |
| Lerkam | 26.9 ± 2.0 | 27.4 ± 1.9 | 0.68 |
| Yishui | 7.0 ± 1.2 | 7.6 ± 1.1 | 1.38 |
| Sinopharm | 8.9 ± 1.3 | 8.0 ± 1.0 | 2.05 |
T-test: data obtained from the microplate-based assay were compared with those obtained from IUPAC among six cellulase preparations. The calculated t-values which are below the critical t-value of 2.06 at the 95% confidence level and DF = 26.
3.2. Measurement of FPA with microplate-based assay
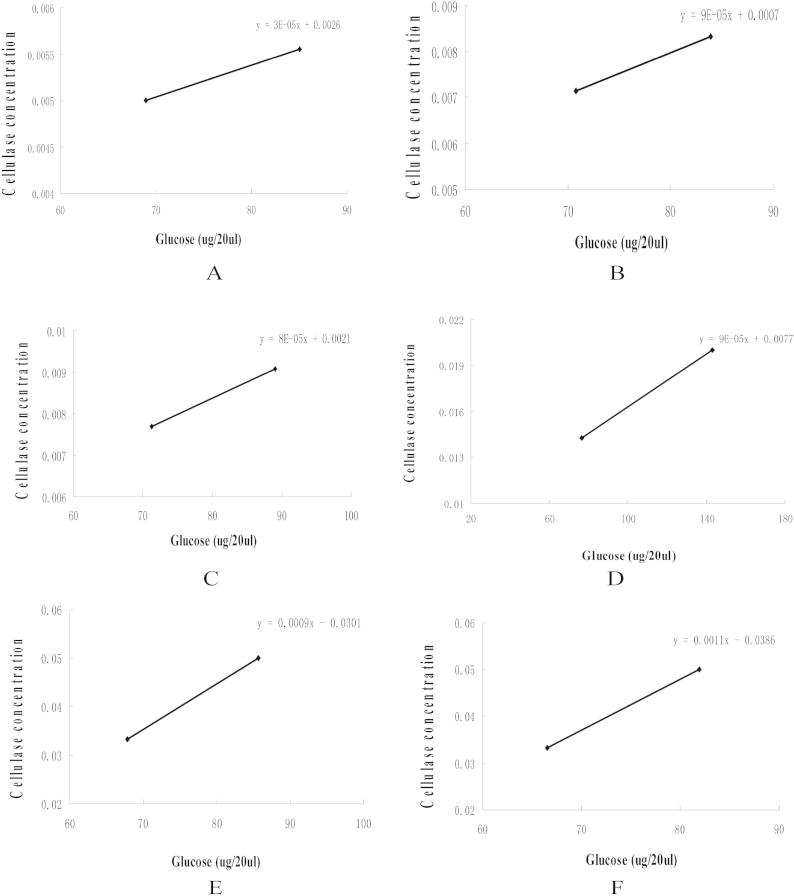
After the cellulase preparations were diluted gradiently in microplate, the FPA were measured with microplate-based assay. The amount of cellulase releasing 80 μg of glucose in the well of microplate was fixed. Each FPA of six cellulases was measured fourteen times. PDAs of a series of six cellulase preparations are shown in Fig. 2 and Table 2. The average activities of six cellulase preparations are shown in Table 3. It is shown the cellulase concentration releasing exactly 80 μg glucose could be obtained by means of plotting glucose against cellulase concentration. Formula B, which is different from that in Xiao’s work, was applied to calculate FPA. Formula B is similar to that of IUPAC (Formula A) because they come from the same idea to dilute gradiently and fix a certain amount of glucose release (Naureen et al., 2014).
Fig. 2.
The curves for fixing the cellulase concentration that produce 80 μg of glucose with the microplate-based assay. (A) Youtell, (B) RCconc, (C) R-10, (D) Lerkam, (E) Yishui, (F) Sinopharm.
Table 2.
A series of FPAs of six cellulase preparations with the microplate-based assay.
| Cellulase dilution | Cellulase concentration | FPA (IU/ml) | |
|---|---|---|---|
| Youtell | 179 | 0.0056 | 69.8 |
| RCconc | 122 | 0.0082 | 47.4 |
| R-10 | 128 | 0.0078 | 44 |
| Lerkam | 77 | 0.01299 | 24.6 |
| Yishui | 16 | 0.0625 | 8.2 |
| Sinopharm | 21 | 0.0476 | 7.7 |
3.3. Comparison of the IUPAC and microplate-based assay
A T-test is performed on the data shown in Table 3. It is shown that there was no significant difference at the 95% confidence level between FPAs of six cellulases determined with IUPAC and microplate-based assay. It could be concluded that the FPA of cellulase could be determined accurately with microplate-based assay same as that with IUPAC in a wide range (7–70 IU/ml). However, in the case of measurement of a large amount of cellulase preparations, there would be a huge amount of solution delivery work. It is labor-intensive with the IUPAC method because the cellulase or buffer solutions would have to be delivered again and again among the tubes. For the microplate-based assay, the solution could be delivered easily with a multichannel pipettor. For example, it needs 96 times to deliver the buffer or diluted solution into 96 tubes in the IUPAC method, while it costs only 8 times to deliver the same solution into 96 wells in the microplate-based assay. It is much more efficient for solution delivery, which leads to higher deficiency to measure FPA in the microplate-based assay (Surhio et al., 2014).
4. Conclusion
The procedure and calculation for measuring the FPA with microplate-based assay were studied in this work. The FPAs of six cellulases were determined following the idea of IUPAC in microplate. The microplate-based assay has the same accuracy and much more efficiency compared with that of IUPAC. It would be an alternative method to measure the FPA of cellulase with the microplate-based assay in future.
Acknowledgments
This project was supported by the Jilin Province Development and Reform Commission (2011003-1).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbès F., Bouaziz M.A., Blecker C., Masmoudi M., Attia H., Besbes S. Date syrup: effect of hydrolytic enzymes (pectinase/cellulase) on physicochemical characteristics, sensory and functional properties. LWT – Food. Sci. Technol. 2011;44:1827–1834. [Google Scholar]
- Ashraf M.A., Ullah S., Ahmad I., Qureshi A.K., Balkhair K.S., Rehman M.A. Green biocides, a promising technology: current and future applications. J. Sci. Food. Agric. 2013;94(3):388–403. doi: 10.1002/jsfa.6371. [DOI] [PubMed] [Google Scholar]
- Batool S., Khalid A., Chowdury A.J.K., Sarfraz M., Balkhair K.S., Ashraf M.A. Impacts of azo dye on ammonium oxidation process and ammonia oxidizing soil bacteria. RSC Adv. 2015;5:34812–34820. [Google Scholar]
- Berlin A., Maximenko V., Bura R., Kang K., Gilkes N., Saddler J. A rapid microassay to evaluate enzymatic hydrolysis of lignocellulosic substrates. Biotechnol. Bioeng. 2005;93:880–886. doi: 10.1002/bit.20783. [DOI] [PubMed] [Google Scholar]
- Boyer V., Fort S., Frandsen T.P., Schein M., Cottaz S., Driguez H. Chemoenzymatic synthesis of a bifunctionalized cellohexaoside as a specific substrate for the sensitive assay of cellulase by fluorescence quenching. Chem. Eur. J. 2002;8:1389–1394. doi: 10.1002/1521-3765(20020315)8:6<1389::aid-chem1389>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Butt M.A., Ahmad M., Fatima A., Sultana S., Zafar M., Yaseen G., Ashraf M.A., Shinwari Z.K., Kayani S. Ethnomedicinal uses of plants for the treatment of snake and scorpion bite in Northern Pakistan. J. Ethnopharmacol. 2015;1:1–14. doi: 10.1016/j.jep.2015.03.045. [DOI] [PubMed] [Google Scholar]
- Chundawat S.P.S., Balan V., Dale B.E. High-throughput microplate technique for enzymatic hydrolysis of lignocellulosic biomass. Biotechnol. Bioeng. 2008;99:1281–1294. doi: 10.1002/bit.21805. [DOI] [PubMed] [Google Scholar]
- Criquet S. Measurement and characterization of cellulase activity in sclerophyllous forest litter. J. Microbiol. Methods. 2002;50:165–173. doi: 10.1016/s0167-7012(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Dashtban M., Maki M., Leung K.T., Mao C., Qin W. Cellulase activities in biomass conversion: measurement methods and comparison. Crit. Rev. Biotechnol. 2010;30:302–309. doi: 10.3109/07388551.2010.490938. [DOI] [PubMed] [Google Scholar]
- Decker S.R., Adney W.S., Jennings E., Vinzant T.B., Himmel M.E. Automated filter paper assay for determination of cellulase activity. Appl. Biochem. Biotechnol. 2003;107:689–706. doi: 10.1385/abab:107:1-3:689. [DOI] [PubMed] [Google Scholar]
- Eveleigh D.E., Mandels M., Andreotti R., Roche C. Measurement of saccharifying cellulase. Biotechnol. Biofuels. 2009;2:1–8. doi: 10.1186/1754-6834-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnet A.M., Qasemian L., Guiral D., Ferre E. A modified method based on arsenomolybdate complex to quantify cellulase activities: application to litters. Pedobiologia. 2010;53:159–160. [Google Scholar]
- Ghose T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987;59:257–268. [Google Scholar]
- Goddard J.P., Reymond J.L. Enzyme assays for high-throughput screening. Curr. Opin. Biotechnol. 2004;15:314–322. doi: 10.1016/j.copbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Helbert W., Chanzy H., Husum T.L., Schlein M., Ernst S. Fluorescent cellulose microfibrils as substrate for the detection of cellulase activity. Biomacromolecules. 2003;4:481–487. doi: 10.1021/bm020076i. [DOI] [PubMed] [Google Scholar]
- Kapasakalidis P.G., Rastall R.A., Gordon M.H. Effect of a cellulase treatment on extraction of antioxidant phenols from black currant (Ribes nigrum L.) Pomace. J. Agric. Food Chem. 2009;57:4342–4351. doi: 10.1021/jf8029176. [DOI] [PubMed] [Google Scholar]
- Kasana R.C., Salwan R., Dhar H., Dutt S., Gulati A. A rapid and easy method for the detection of microbial cellulases on agar plates using gram’s iodine. Curr. Microbiol. 2008;57:03–507. doi: 10.1007/s00284-008-9276-8. [DOI] [PubMed] [Google Scholar]
- Khaskheli A.A., Talpur F.N., Ashraf M.A., Cebeci A., Jawaid S., Afridi H.I. Monitoring the Rhizopus oryzae lipase catalyzed hydrolysis of castor oil by ATR-FTIR spectroscopy. J. Mol. Catal. B Enzym. 2015;113:56–61. [Google Scholar]
- King B.C., Donnelly M.K., Bergstrom G.C., Walker L.P., Gibson D.M. An optimized microplate assay system for quantitative evaluation of plant cell wall-degrading enzyme activity of fungal culture extracts. Biotechnol. Bioeng. 2008;102:1033–1044. doi: 10.1002/bit.22151. [DOI] [PubMed] [Google Scholar]
- Kiyani S., Ahmad M., Zafar M.A., Sultana S., Khan M.P.Z., Ashraf M.A., Hussain J., Yaseen G. Ethnobotanical uses of medicinal plants for respiratory disorders among the inhabitants of Gallies-Abbottabad, Northern Pakistan. J. Ethnopharmacol. 2014;156:47–60. doi: 10.1016/j.jep.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Mullings R. Measurement of saccharification by cellulases. Enzyme Microb. Technol. 1985;7:586–591. [Google Scholar]
- Naureen R., Tariq M., Yusoff I., Choudhury A.J.K., Ashraf M.A. Synthesis, spectroscopic and chromatographic studies of sunflower oil biodiesel using optimized base catalyzed methanolysis. Saudi. J. Biol. Sci. 2014;22:322–339. doi: 10.1016/j.sjbs.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patindol J., Wang L., Wang Y.J. Cellulase-assisted extraction of oligosaccharides from defatted rice bran. J. Food Sci. 2007;72:421–516. doi: 10.1111/j.1750-3841.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- Peralta P., Carter B.T., Lin H., Tao H.Y., Cornish V.W. High-throughput selection for cellulase catalysts using chemical complementation. J. Am. Chem. Soc. 2008;130:17446–17452. doi: 10.1021/ja8055744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renouard S., Hano C., Corbin C., Fliniaux O., Lopez T., Montguillon J. Cellulase-assisted release of secoisolariciresinol from extracts of flax (Linum usitatissimum) hulls and whole seeds. Food Chem. 2010;122:679–687. [Google Scholar]
- Surhio M.A., Talpur F.N., Nizamani S.M., Amin F., Bong C.W., Lee C.W., Ashraf M.A., Shahd M.R. Complete degradation of dimethyl phthalate by biochemical cooperation of the Bacillus thuringiensis strain isolated from cotton field soil. RSC Adv. 2014;4:55960–55966. [Google Scholar]
- Xiao Z.Z., Storms R., Tsang A. Microplate-based filter paper assay to measure total cellulase activity. Biotechnol. Bioeng. 2004;88:832–837. doi: 10.1002/bit.20286. [DOI] [PubMed] [Google Scholar]
- Xiao Z.Z., Storms R., Tsang A. Microplate-based carboxymethylcellulose assay for endoglucanase activity. Anal. Biochem. 2005;342:176–178. doi: 10.1016/j.ab.2005.01.052. [DOI] [PubMed] [Google Scholar]
- Zhang P.Y.H., Himmel M.E., Mielenz J.R. Outlook for cellulase improvement: screening and selection strategies. Biotechnol. Adv. 2006;24:452–481. doi: 10.1016/j.biotechadv.2006.03.003. [DOI] [PubMed] [Google Scholar]