Abstract
Cassia italica Mill is an important medicinal plant within the family Fabaceae. Pot experiment was conducted to evaluate cadmium stress induced changes in physiological and biochemical attributes in C. italica with and without arbuscular mycorrhizal fungi (AMF). Cadmium stressed plant showed reduced chlorophyll pigment and protein content while AMF inoculation enhanced the chlorophyll and protein content considerably. AMF also ameliorated the cadmium stress induced reduction in total chlorophyll and protein contents by 19.30% and 38.29%, respectively. Cadmium stress enhanced lipid peroxidation while AMF inoculation reduced lipid peroxidation considerably. Increase in proline and phenol content was observed due to cadmium stress and AMF inoculation caused a further increase in proline and phenol content ensuring better growth under stressed conditions. AMF alone also enhanced proline and phenol content. Activity of antioxidant enzymes enhanced under cadmium treatment and AMF inoculation further enhanced their activity thereby strengthening the antioxidant system. Enhanced activities of antioxidants and increased accumulation of osmolytes help plants to avoid damaging impact of oxidative damage. The research has shown that AMF inoculation mitigated the negative impact of stress by reducing the lipid peroxidation and enhancing the antioxidant activity. The present study strongly supports employing AMF as the biological mean for enhancing the cadmium stress tolerance of C. italica.
Keywords: Cassia italic, AMF, Antioxidant enzymes, Proline, Phenol, Lipid peroxidation
1. Introduction
Cadmium stress is among the potential toxic heavy metals present in soil in low concentrations. Cadmium is highly mobile between soil–plant systems and therefore is quickly absorbed by the plants and hence transported to upper parts causing toxicity (Irfan et al., 2014). Processes including weathering of cadmium rich rocks, mining, smelting, over supplementation of phosphate fertilizers to agricultural soils and applying sewage sludge and metal polluted water for crop irrigation contribute to aggravate the situation by increasing the levels of cadmium (Zoffoli et al., 2013). Cadmium being a non essential metal is absorbed rapidly by plant roots and alters growth as well as developmental processes (Pagani et al., 2012). Cadmium accumulation promotes necrosis and chlorophyll destruction, perturbs nutrient uptake, carbon assimilation and enzyme activity (Singh and Prasad, 2014, Abd_Allah et al., 2015). Cadmium induced perturbation in enzyme activity is due to its high affinity toward the sulfhydryl group of enzymes (Wada et al., 2014).
Production and accumulation of reactive oxygen species (ROS) including O2-, H2O2 and OH- is increased manifolds on exposure to stresses (Wu et al., 2014, Abd_Allah et al., 2015). Although being a non-redox metal, cadmium initiates the over-production of ROS by interfering with the enzymes that are involved in the maintenance of redox homeostasis (Yan et al., 2013). Stress triggered enhancement in levels of toxic ROS leads to peroxidation of the membrane lipid thereby causing oxidative damage (Wu et al., 2014). Increased membrane lipid peroxidation leads to loss of their integrity resulting in membrane leakage. In addition, ROS also affects important cellular components like nucleic acids, proteins and chlorophylls through oxidation and ultimately results in perturbed cell functioning (Alqarawi et al., 2014, Abd_Allah et al., 2015, Ahmad et al., 2015). Plants have several indigenous defence mechanisms which are actively involved in mitigating the damage induced by stresses like heavy metals. Increase in synthesis and accumulation of osmotic constituents (Alqarawi et al., 2014), increased activity of antioxidant enzymes (Irfan et al., 2014, Abd_Allah et al., 2015) and efficient compartmentation of toxic metal ions into less sensitive cellular compartments like vacuoles (Liu et al., 2014) contribute to enhance stress tolerance. Moreover increased production of protective compounds, like metallothioneins and phytochelatins, which mediate chelation of toxic metals and metalloids therefore help in averting the stress effects (Sylwia et al., 2010). Moreover, enzymatic antioxidant system including superoxide dismutase [SOD], peroxidases [POD], catalase [CAT] and glutathione reductase [GR] also mediate the scavenging of toxic ROS hence help in preventing oxidative stress (Wu et al., 2014, Alqarawi et al., 2014, Abd_Allah et al., 2015).
Several plants form symbiotic associations with arbuscular mycorrhizal fungi (AMF). Studies have confirmed AMF as the best biological tool for improving plant growth under normal conditions and can also mitigate the adverse impacts of abiotic stresses on crop plants (Hashem et al., 2014; Alqarawi et al., 2014, Wu et al., 2014). AMF brings several morpho-physiological and biochemical changes in host plants that promote maintained plant growth and increased vigor. Modifications in root morphology induced by AMF colonization mediate increased water and mineral uptake (Ahanger et al., 2014, Wu et al., 2014). AMF has been reported to enhance the uptake of essential mineral nutrients like nitrogen phosphorous and potassium (Ahanger et al., 2014; Hashem et al., 2014; Alqarawi et al., 2014). The present study was carried to evaluate the impact of cadmium stress on growth and physio-biochemical parameters of Casssia italica Mill, and the role of AMF in ameliorating the adverse impact.
2. Materials and methods
2.1. Pot experiment setup and treatments
Healthy seeds were collected from mature pods of naturally grown C. indica Mill (Fig. 1 I-IV) found in the Arafat region, Holy Mecca, Saudi Arabia (Fig. 2). The seeds were geminated on blotter paper in petri dishes in a controlled growth chamber at 25 °C with a 16/8 h light/dark photoperiod and light intensity of 1500 μmol m−2 S−1. The blotter papers were wetted with full strength Hoagland’s solution for one week. After one week of germination, seedlings were transferred to pots containing peat and sand in the ratio of 1:1 (w/w) and were supplemented with Hoagland solution (100 mL pot−1) after every two days. The experiment was laid down in a factorial completely randomized design having three replicates for each treatment. After eight weeks of normal growth, cadmium stress was induced by supplementing Hoagland’s solution with 150 μM CdCl2. Pots receiving only Hoagland’s solution served as control. The arbuscular mycorrhizal fungi used in the present study contained a mixture of Funneliformis mosseae (syn. Glomus mosseae), Rhizophagus intraradices (syn. Glomus intraradices) and Claroideoglomus etunicatum (syn. Glomus etunicatum) as described by Hashem et al. (2014). The mycorrhizal inoculum was added to the experimental pots as 10 g of trap soil (approximately 100 spores/g trap soil, M = 80%). At the end of the incubation period, days plants were removed from the pots carefully and analyzed for several parameters.
Figure 1.
(I-IV). Naturally grown Casssia indica Mill and the mature pods taken for study.
Figure 2.
Map of Holy Mecca showing the location of Arafat area (arrow).
2.2. Determination of Cadmium (Cd) concentrations in plant
Dry shoot and root materials (0.1 g) were digested in a mixture of nitric acid and perchloric acid (4:1) using the hot block digestion procedure (overnight at 60 °C) according to the method of Jackson (1962) and described by Burd et al. (2000). After cooling, 1.0 ml of hydrogen peroxide (30%, v/v) was added to the digested sample and incubated for 2 h. The cadmium concentration was measured by an atomic absorption spectrophotometer (Perkin Elmer AA700, USA).
2.3. Photosynthetic pigments
Leaf samples (0.5 g) were extracted in 80% acetone as described by Arnon (1949). The optical densities of the supernatant were recorded at 480, 645 and 663 nm against a blank containing acetone (80%).
2.4. Estimation of proline
Free proline was estimated according to the method of (Bates et al., 1973). Leaf samples (0.5 g) were extracted in sulfosalicylic acid followed by centrifugation at 3000 g for 30 min. 2.0 ml of supernatant was mixed with an equal volume of acid ninhydrin solution [1.25 g ninhydrin, with 30 ml glacial acetic acid, and 20 ml of 6 M phosphoric acid] and glacial acetic acid. The samples were incubated at 100 °C for 10 min and the reaction was terminated by keeping the tubes in a container filled with ice. After cooling, proline was separated with 4 ml toluene and the optical density was measured at 520 nm. Toluene was used as blank.
2.5. Estimation of total protein content
Total protein content was estimated according to the method of Bradford (1976). Absorbance was recorded spectrophotometrically at 595 nm (Beckman 640 D, USA) using bovine serum albumin as a standard (10–100 μg ml−1).
2.6. Estimation of lipid peroxidation (malondialdehyde, MDA)
Lipid peroxidation was determined by measuring the amount of MDA produced by the thiobarbituric acid reaction as described by Heath and Packer (1968). Absorbance was recorded at 600 nm and 532 nm (1% [w/v] thiobarbituric acid in 20% trichloroacetic acid was used as blank). The concentration of MDA was calculated using an extinction coefficient of 155 mM cm−l.
2.7. Estimation of total phenolics
The total phenolics were extracted with 80% (v/v) acetone and estimated using sodium carbonate (20%) and Folin and Ciocalteu’s phenol reagent following Slinkard and Singleton (1977). Optical density of the mixture was read at 750 nm. Computation was done from the standard curve of pyrogallol.
2.8. Extraction and estimation of antioxidant enzyme
Fresh leaf samples (500 mg) were homogenized in 10 mL of chilled 50 mM phosphate buffer (pH 7.8). The extract was centrifuged at 15,000g for 15 min at 4 °C and the supernatant was used as an enzyme source. Superoxide dismutase (SOD, EC 1.15.1.1) activity was determined by the method of Van Rossum et al. (1997), by measuring the photoreduction of nitrobluetetrazolium at 560 nm. The reaction mixture contained 500 μL phosphate buffer (pH 7.8), 0.5 mL distilled H2O, 100 μL methionine, 50 μL NBT and 50 μL enzyme extract. One unit of SOD was defined as the amount of protein causing a 50% decrease of the SOD-inhibitable NBT reduction and activity was expressed as Unit mg−1 protein. The extraction of buffer for ascorbate peroxidase (APX) was supplemented with 2.0 mM ascorbate in addition to other ingredients. The method of Nakano and Asada (1981) was followed for the determination of APX activity. The decrease in absorbance was read at 265 nm and activity was expressed as EU mg−l protein. Method of Luck (1974) was used for the estimation of catalase (CAT, EC 1.11.1.6) activity. The reaction mixture contained 1.9 ml phosphate buffer (50 mM; pH 7.0) and 1 mL H2O2 (5.9 mM) and the reaction was initiated by adding 100 μL of the enzyme extract. Decrease in absorbance was measured at 240 nm for 2 min and activity was expressed as EU mg−1 protein. The activity of glutathione reductase (GR, EC 1.6.4.2) was determined in accordance with Carlberg and Mannervik (1985). The decrease in absorbance was read at 340 nm for 2 min and the activity of GR was calculated using the extinction coefficient of NADPH of 6.2 mM−1 cm−1 and expressed as EU mg−l protein.
2.9. Estimation of ion accumulation
Na+, K+, Mg2+, Mn and Ca2+ were estimated using atomic absorption spectrophotometer (Analyst 300, Perkin- Elmer, Germany) following Wolf (1982). In this method, powdered shoot (0.1 g) was digested in H2SO4/HNO3 mixture (1/5, v/v) for 24 h, followed by treatment with HNO3/HClO4 mixture (5/1, v/v). Calculation was done from the standard curve (10–100 μg/ml) of each mineral.
2.10. Statistical analysis
All experiments were repeated three times. Treatment means were statistically analyzed using Least Significant Difference (LSD) analysis of variance for a completely randomized design.
3. Results
A drastic decline in chlorophyll contents was observed due to cadmium stress (Table 1). Cadmium stress reduced chlorophyll a, chlorophyll b, total chlorophyll and carotenoid contents by 49.9%, 33.2%, 42.7% and 38.14% respectively. Inoculation of AMF not only enhanced these parameters but also ameliorated cadmium stress induced decline. Due to AMF chlorophyll a, chlorophyll b, total chlorophyll and carotenoid contents were enhanced by 11.74%, 21.4%, 19.30% and 39.5% respectively as compared to control (Table 1).
Table 1.
Effect of cadmium (150 μM) in the presence and absence of AMF on chlorophyll a (Ch a), chlorophyll b (Ch b), total chlorophyll, carotenoids (Carot) and total photosynthetic pigments (Total pig) in Cassia italica Mill. Data presented are mean ± SE (n = 5).
| Treatments | Ch a | Ch b | a/b | a + b | Carot | Total pig |
|---|---|---|---|---|---|---|
| Control | 1.643 | 1.013 | 1.621 | 2.65 | 0.506 | 3.16 |
| AMF | 1.836 | 1.23 | 1.493 | 3.06 | 0.706 | 3.77 |
| Cadmium | 0.823 | 0.676 | 1.216 | 1.5 | 0.313 | 1.81 |
| Cadmium + AMF | 1.023 | 0.876 | 1.167 | 1.9 | 0.41 | 2.31 |
| LSD at: 0.05 | 0.0593 | 0.0756 | 0.1497 | 0.0828 | 0.0352 | 0.1078 |
AMF: Arbuscular mycorrhizal fungi.
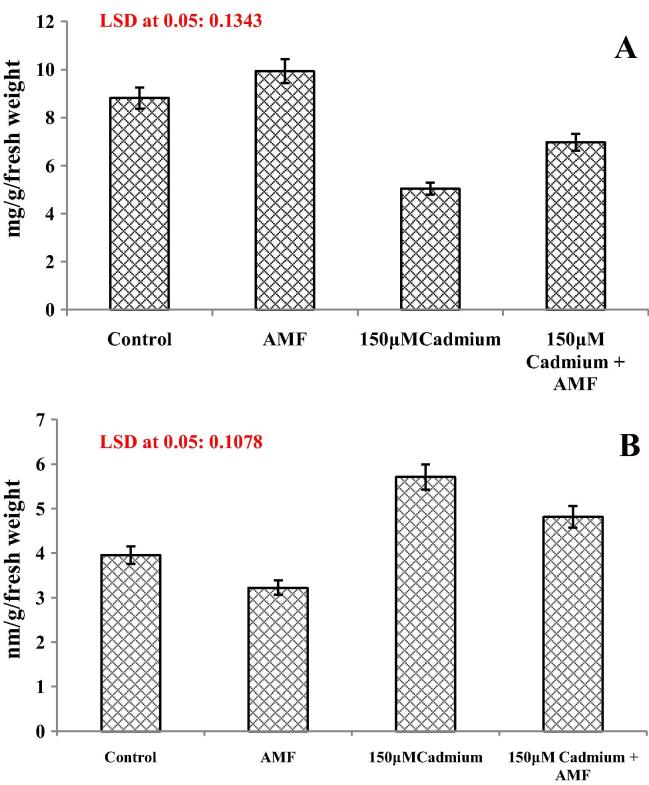
Total soluble protein decreased drastically due to cadmium stress and however AMF inoculation not only increased protein content but also ameliorated the cadmium induced reduction (Fig.3a). Relative to control AMF alone increased protein content by 12.7%. Cadmium stress reduced protein content by 42.7% while in AMF inoculated cadmium stressed (150 μM + AMF) plants reduction was only 20.8% (Figure3a). Lipid peroxidation measured in terms of MDA content increased in cadmium stressed (150 μM) plants and AMF colonized plants showed reduced MDA content (Fig.3b). AMF inoculation reduced cadmium stress induced lipid peroxidation to some extent. In comparison to control, MDA content decreased by 18.48% in AMF inoculated plants, however, cadmium stress enhanced MDA by 44.55% while AMF inoculated cadmium stressed plants showed only 23.03% as compared to control (Fig.3b).
Figure 3.
(A and B) Effect of cadmium (150 μM) in the presence and absence of AMF on (A) total soluble protein and (B) malonaldehyde content in Cassia italica Mill. Data presented are mean ± SE (n = 3).
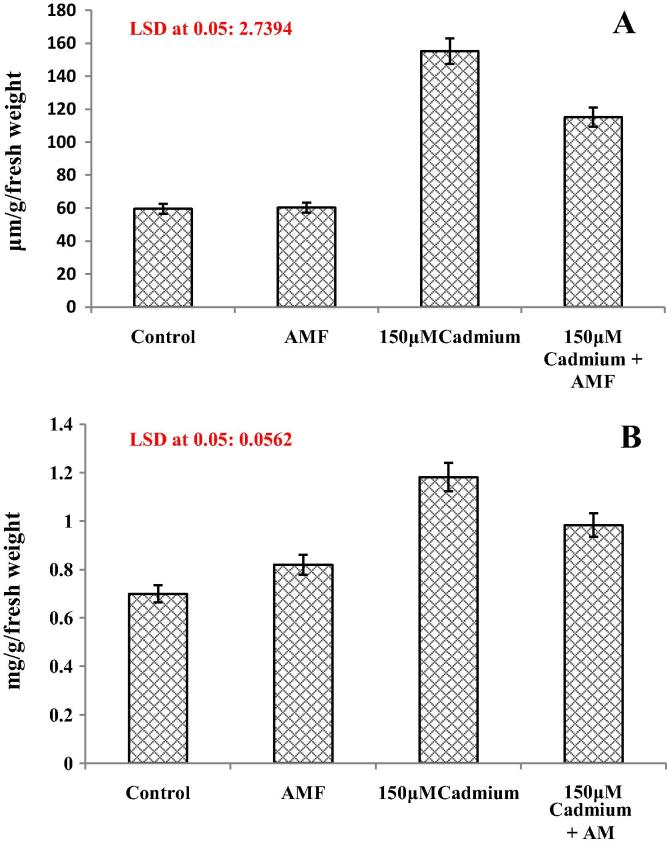
AMF inoculation enhanced proline content by 1.17% (Fig.4a). However a considerable increase in proline accumulation in C. italica pants subjected to cadmium stress was observed. Percent increase due to cadmium stress was 160.2%. However AMF inoculated cadmium stressed (150 μM + AMF) plants maintained less content as compared to cadmium stressed plants (Fig.4a).
Figure 4.
(A and B) Effect of cadmium (150 μM) in the presence and absence of AMF on (A) proline and (B) phenol contents in Cassia italica Mill. Data presented are mean ± SE (n = 3).
Total phenol content increased considerably in cadmium stressed plants. In comparison to control, AMF inoculated plants showed a 17.16% increase while cadmium stressed plants showed a 68.95% increase respectively (Fig.4b). However under cadmium stress AMF inoculation (150 μM + AMF) slightly reduced phenol content as compared to cadmium stressed plants. Percent increase in AMF inoculated cadmium stressed (150 μM + AMF) plants was 40.62% as compared to uninoculated control (Fig.4b).
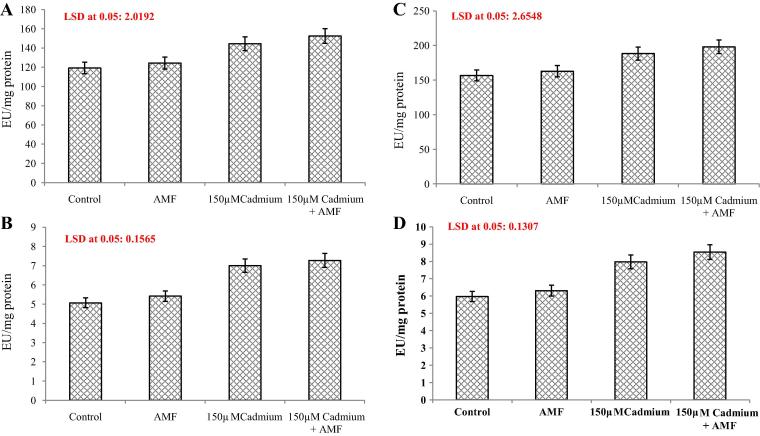
Activities of antioxidant enzymes enhanced considerably under cadmium stress (Fig.5A–D). In comparison to percent increase in the activity of SOD, APX, CAT and GR due to cadmium treatment (150 μM) was 21.1%, 38.1%, 20.1% and 38.52% respectively. AMF inoculation increased SOD, APX, CAT and GR activity by 4.2%, 6.7%, 3.8% and 5.69% respectively. However in combination with cadmium AMF caused (150 μM + AMF) a further enhancement in the activities of all antioxidants studied with the observed percent increase being 27.9%, 43.3%, 26.4% and 42.88% for SOD, APX, CAT and GR, respectively.
Figure 5.
(A–D) Effect of cadmium (150 μM)in the presence and absence of AMF on (A) superoxide dismutase [SOD]; (B) catalase [CAT]; (C) peroxidase [POD] and (D) glutathione reductase (GR) as (EU/mg protein) content in Cassia italica Mill. Data presented are mean ± SE (n = 3).
AMF inoculated plants showed reduced sodium content while increased content of potassium, magnesium, manganese, and calcium was observed (Table 2). Percent increase in potassium, magnesium, calcium and manganese due to AMF was observed as 3.21%, 2.91%, 13.3% and 9.5% respectively (Table 2). Cadmium stress increased sodium content by 38.8% while decreased potassium, magnesium, manganese, and calcium by 49.33%, 38.03%, 52.9% and 26.7% respectively. AMF inoculation mitigated the deleterious impact of cadmium on potassium, magnesium, manganese, and calcium uptake.
Table 2.
Effect of cadmium (150 μM) in the presence and absence of AMF on sodium, potassium, calcium, magnesium and manganese content in Cassia italica Mill. Data presented are mean ± SE (n = 5).
| Treatments | Na | K | Mg | Ca | Mn |
|---|---|---|---|---|---|
| Control (Non) | 180.13 | 732.43 | 290.22 | 133.16 | 32.25 |
| AMF | 154.01 | 755.97 | 298.69 | 145.83 | 36.57 |
| Cadmium | 250.19 | 371.06 | 179.84 | 97.59 | 15.17 |
| Cadmium + AMF | 214.84 | 491.94 | 211.54 | 106.80 | 22.97 |
| LSD at: 0.05 | 4.0161 | 5.0485 | 4.3062 | 4.4487 | 1.9838 |
AMF: Arbuscular mycorrhizal fungi.
AMF inoculation reduced the uptake of cadmium to a considerable extent. As compared to cadmium stressed plants AMF inoculated cadmium stressed plants maintained 37.8% less cadmium in shoots while a 35.29% increase was observed in roots (Table 3).
Table 3.
Effect of cadmium (150 μM) in the presence and absence of AMF cadmium uptake of Cassia italica Mill. Data presented are mean ± SE (n = 5).
| Treatments | Shoot (μg Cd/g dry weight) | Root (μg Cd/g dry weight) |
|---|---|---|
| Cadmium | 13.51 | 54.003 |
| Cadmium + AMF | 8.40 | 73.063 |
| LSD at: 0.05 | 1.3456 | 2.0325 |
AMF: Arbuscular mycorrhizal fungi.
4. Discussion
Cadmium stress drastically reduced chlorophyll contents. Our results of reduced chlorophyll pigments due to cadmium application support the findings of Chen et al., 2011, Liu et al., 2014 and Abd_Allah et al. (2015) for mustard; cotton and sunflower, respectively. In Abelmoschus esculentus and Cyamopsis tetragonoloba, Mangal et al. (2013) demonstrated reduced chlorophyll content due to cadmium stress. During stress chlorophyllase enzyme activity goes up resulting in altered biosynthesis of pigments as well as their degradation (Singh and Prasad, 2014, Abd_Allah et al., 2015). Enhancement in chlorophyll contents due to AMF inoculation is in corroboration with the findings of Malekzadeh et al. (2012) and Wu et al. (2014). In our study amelioration of cadmium stress induced the reduction of chlorophyll pigments due to AMF inoculation supporting the findings of Malekzadeh et al. (2012). AMF enhances the uptake of essential elements including magnesium which forms an important component of chlorophyll. AMF results in enhanced de novo synthesis of several proteins which are the important constituents of chlorophylls (Hashem et al., 2014, Abd_Allah et al., 2015).
Our results of increased MDA accumulation due to exposure of cadmium is in corroboration with the results of John et al. (2009) and Abd_Allah et al. (2015) in Brassica juncea and Helianthus annuus, respectively. Enhanced peroxidation of lipids measured in terms of MDA content is widely accepted as an important criteria for making the assessment of the severity of the stress induced oxidative damages (Wu et al., 2014). Increased peroxidation of membranes results in membrane instability. Exposure to stressful environmental conditions trigger an increase in peroxidation of membrane lipids ultimately resulting in loss of membrane integrity, thereby causing leakage of essential elements (Djebali et al., 2005). Increased membrane peroxidation during stress is ascribed to manifold enhancement in lipoxygenase activity thereby resulting in the rapid peroxidation of lipids as well as an increased accumulation of peroxides and hydroxyl radicals causing a further destruction of membranes and other important cellular components (Djebali et al., 2005). Under stress conditions production of toxic free radicals increases altering the cellular balance. Reduced peroxidation of membranes in AMF inoculated plants may be due to the possible role of AMF in phosphate uptake and antioxidant activity (Tang et al., 2009, Alqarawi et al., 2014). Increased activity of antioxidants mediate quick scavenging of reactive oxygen species and hence result in membrane protection (Alqarawi et al., 2014). Our results of reduced membrane peroxidation due to AMF inoculation are in confirmation with the findings of Hashem et al. (2014) for Vicia faba and Alqarawi et al. (2014) for Ephedra aphylla. Reduced accumulation of MDA in AMF inoculated plants may be due to the inhibition in the production of toxic free radicals. Reduced production of MDA due to AMF reported in our study support the protective role of AMF in protecting normal plant metabolism from the deleterious impact of ROS. Ling-Zhi et al. (2011) has also observed a positive role of AMF in growth maintenance under cadmium stress.
Plants exposed to adverse environmental condition show enhanced synthesis and accumulation of osmolytes which have an important role in maintaining growth under stressed conditions (Hashem et al., 2014, Alqarawi et al., 2014). Proline is among the important osmolytes involved in the maintenance of tissue water content. In our study an increase in proline accumulation due to cadmium stress was obvious and a further increase caused by AMF inoculation confirms the role of AMF in strengthening the stress tolerance mechanisms in plants. Under stress conditions activity of proline synthesizing enzymes is upregulated while its catabolism is lowered (Hashem et al., 2014). Proline and other osmolyte accumulation helps plants to maintain cellular water potential well below that of the soil solution. Our results of proline accumulation due to cadmium stress are in concurrence with results of Hayat et al., 2011, Irfan et al., 2014 and Abd_Allah et al. (2015) in Lycopersicon esculentum, Brassica juncea and Helianthus annus, respectively. Enhancement in proline in our study supports the role of proline in the maintenance of growth under stress conditions. Increase in proline due to AMF inoculation is in corroboration with the results of Shekoofeh et al. (2012). Similar to our results, in Ephedra aphylla Alqarawi et al. (2014) also observed increased proline accumulation due to AMF inoculation both under normal as well as stressed conditions. Further enhancement in proline accumulation in AMF inoculated plants support the potential role of AMF and proline in plants. Proline has a protective role for the protection of membranes and other cellular molecules like enzymes and also neutralizes toxic ROS therefore contributing to better growth under stress conditions (Irfan et al., 2014, Alqarawi et al., 2014). Proline does not interfere with the metabolic pathways rather it replaces water in these processes (Hashem et al., 2014, Abd_Allah et al., 2015).
Polyphenols include a broad group of plant secondary metabolites having an important role in plant defence against stresses. Phenols have antioxidant property and contribute to the cell wall formation and plant interactions with biotic as well as abiotic stresses (Vierheilig and Piche, 2002). Our results of increased phenol content due to AMF inoculation is in confirmation with other results on Ephedra aphylla (Alqarawi et al., 2014), Vicia faba (Hashem et al., 2014) and Helianthus annuus (Abd_Allah et al., 2015). Earlier increase in phenol content due to cadmium stress has been reported by Dudjak et al. (2004) in barley and Marquez-Garcıa et al. (2012) in Erica andevalensis. Recently Ahmad et al. (2015) also demonstrated increased phenol content in cadmium stressed Cannabis sativa plants. Phenols mitigate oxidative stress and increase membrane stability resulting in maintained plant growth (Khattab, 2007). Exposure to stressful conditions up regulates the activity of enzymes involved in biosynthesis of polyphenols resulting in efficient scavenging of toxic radicals and better growth performance of plants (Wada et al., 2014). In our results increase in phenols due to AMF inoculation confer the role AMF in enhancing the growth of plants under normal as well as stressed conditions.
Our results of increased activities of antioxidant enzymes due to cadmium stress corroborate with the findings of Abd_Allah et al., 2015 for Helianthus annuus; Irfan et al. (2014) for Brassica juncea and Alqarawi et al. (2014) for Ephedra aphylla. Enzymatic antioxidant defence system is constituted of several protective enzymes that mediate scavenging of toxic ROS from the plant cells. Enhanced activities of antioxidant enzymes result in quick scavenging of ROS therefore help in avoiding the oxidative stress (Alqarawi et al., 2014). AMF inoculation induced increment in antioxidant activities is in support of the findings of Ling-Zhi et al., 2011, Malekzadeh et al., 2012 and Alqarawi et al. (2014). Superoxide dismutase [SOD], catalase [CAT], ascorbate peroxidase [APX] and glutathione reductase [GR] are important antioxidant enzymes that protect plant cells from the toxic effects of ROS by mediating quick detoxification. SOD detoxifies superoxide radicals, the first line of defence so preventing stress induced cellular damage. Activity of SOD produces hydrogen peroxide (H2O2) that is converted into water either by catalase or ascorbate peroxidase (Wu et al., 2014, Abd_Allah et al., 2015). Glutathione reductase and ascorbate peroxidase are important enzymes of the ascorbate–glutathione cycle. Glutathione reductase [GR] catalyzes the conversion of oxidized glutathione [GSSG] to reduced form [GSH] and hence leading to maintenance of higher GSH/GSSG ratio (Noctor and Foyer, 1998, Rausch et al., 2007). In Ipomoea aquatica, earlier it has been demonstrated that antioxidant enzyme activities increased on exposure to cadmium stress and AMF inoculation further increased the activity resulting in quick ROS scavenging and hence averting oxidative stress for better stress adaptation (Bhaduri and Fulekar, 2012). Greater activities of antioxidant enzymes in C. italica due to cadmium stress and further increment as a result of AMF inoculation suggests the importance of AMF in mitigating the deleterious impacts of cadmium stress. Hence AMF can be potential approach for enhancing the tolerance level of C. italica.
Cadmium stress results in impeded uptake of essential mineral elements. In the present study cadmium reduced the uptake of important mineral elements like potassium, calcium, magnesium and manganese drastically. Our results of reduced mineral uptake under cadmium stress are in confirmation with the findings of Abd_Allah et al. (2015) for Helianthus annus. AMF not only reduced the deleterious effect of cadmium stress by reducing its uptake but also increased the uptake of other important mineral elements like potassium, calcium, manganese and magnesium significantly. Increase in sodium content in cadmium stressed plants further aggravated the problem while AF inoculation mitigated the uptake of sodium to some extent. Increased concentration of Na+ ions has a deleterious impact on growth and it disturbs the uptake of important ions like potassium and calcium mobility within the plant. Altered uptake of potassium results in reduction of Na/K ratio. Several reports confirm higher K/Na ratio as an important physiological attribute for mitigating stress induced deleterious changes (Alqarawi et al., 2014, Abd_Allah et al., 2015). Reduction in K/Na ratio stressed conditions enhances the susceptibility of plants while better exclusion of the deleterious ions contributes to maintained osmotic potential (Hashem et al., 2014). Potassium is an important macroelement involved in several processes including enzyme activation, stomatal movements and stress tolerance (Abd_Allah et al., 2015). During the present study reduction in Na and efficient uptake of other essential elements like potassium, calcium, magnesium and manganese content in AMF inoculated plant may be attributed to selective absorption of the deleterious Na ion. In addition increased Mg content in AMF treated plants has a direct effect on the chlorophyll pigments thereby resulting in enhanced pigment synthesis and hence photosynthesis. In Ephedra aphylla, Alqarawi et al. (2014) have also demonstrated the increased uptake of essential microelements in AMF inoculated stressed plants as compared to uninoculated counterparts. In addition to this an obvious increase in cadmium accumulation in cadmium treated plants was observed which was reduced to some extent by AMF inoculation particularly in shoots. Reduced accumulation of cadmium in shoots of AMF inoculated plants confer a role of AMF in the selective uptake of toxic ions.
5. Conclusion
In conclusion, cadmium stress affected growth and metabolism in C. italic, while increased lipid peroxidation and antioxidant enzyme activity. AMF inoculation mitigated the negative impact of stress by reducing the lipid peroxidation and enhancing the antioxidant activity. Present study strongly supports employing AMF as the biological mean for enhancing the cadmium stress tolerance of C. italica.
Acknowledgment
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at king Saud University for its funding this Research group NO (RG- 1435-014).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd_Allah E.F., Hashem Abeer, Alqarawi A.A., Alwathnani Hend A. Alleviation of adverse impact of cadmium stress in sunflower (Helianthus annuus L.) by arbuscular mycorrhizal fungi. Pak. J. Bot. 2015;47(2):785–795. [Google Scholar]
- Ahanger M.A., Hashem Abeer, Abd_Allah E.F., Ahmad P. 2014. Arbuscular mycorrhiza in crop improvement under environmental stress. In: Ahmad P., Rasool S. (Eds.), Emerging Technologies and Management of Crop Stress Tolerance, Volume 2. Pp 69–95.
- Ahmad A., Hadi F., Ali N. Effective phytoextraction of cadmium (cd) with increasing concentration of total phenolics and free proline in Cannabis sativa (L) plant under various treatments of fertilizers, plant growth regulators and sodium salt. Int. J. Phytoremed. 2015;17:56–65. doi: 10.1080/15226514.2013.828018. [DOI] [PubMed] [Google Scholar]
- Alqarawi A.A., Abd-Allah E.F., Abeer Hashem. Alleviation of salt-induced adverse impact via mycorrhizal fungi in Ephedra aphylla Forssk. J. Plant Interact. 2014;9(1):802–810. [Google Scholar]
- Arnon D.I. Copper enzymes in isolated chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates L.S., Waldren R.P., Teare I.D. Rapid determination of free proline for water stress studies. Plant Sci. 1973;39:205–207. [Google Scholar]
- Bhaduri A.M., Fulekar M.H. Assessment of arbuscular mycorrhizal fungi on the phytoremediation potential of Ipomoea aquatic on cadmium uptake. Biotechnology. 2012;2:193–198. [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantization of microgram quantities of protein using the principle of protein-dye binding. Anal. Biochem. 1976;72:248–259. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burd G.I., Dixon G.D., Glick B.R. A plant growth promoting bacterium that decreases heavy metal toxicity in plants. Can. J. Microbiol. 2000;46:237–245. doi: 10.1139/w99-143. [DOI] [PubMed] [Google Scholar]
- Carlberg I., Mannervik B. Glutathione reductase. Meth. Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- Chen X., Wang J., Shi Y., Zhao M.Q., Chi G.Y. Effects of cadmium on growth and photosynthetic activities in pakchoi and mustard. Bot. Stud. 2011;52:41–46. [Google Scholar]
- Djebali W., Zarrouk M., Brouquisse R., El Kahoui S., Limam F., Ghorbel M.H., Chaïbi W. Ultrastructure and lipid alterations induced by cadmium in tomato (Lycopersicon esculentum) chloroplast membranes. Plant Biol. 2005;7:358–368. doi: 10.1055/s-2005-837696. [DOI] [PubMed] [Google Scholar]
- Dudjak J., Lachman J., Miholova D., Kolihova D., Pivec V. Effect of cadmium on polyphenol content in young barley plants (Hordeum vulgare L.) Plant Soil Environ. 2004;50(11):471–477. [Google Scholar]
- Hashem A., Abd_Allah E.F., Alqarawi A.A., El-Didamony G., Alwhibi Mona S., Egamberdieva D., Ahmad P. Alleviation of adverse impact of salinity on faba bean (Vicia faba L.) by arbuscular mycorrhizal fungi. Pak. J. Bot. 2014;46(6):2003–2013. [Google Scholar]
- Hayat S., Hasan S.A., Ahmad A. Growth, nitrate reductase activity and antioxidant system in cadmium stressed tomato (Lycopersicon esculentum Mill) cultivars. Biotech. Agron. Soc. Environ. 2011;15:401–414. [Google Scholar]
- Heath R.L., Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125(1):189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Irfan M., Ahmad A., Hayat S. Effect of cadmium on the growth and antioxidant enzymes in two varieties of Brassica juncea. Saudi J. Bio. Sci. 2014;21:125–131. doi: 10.1016/j.sjbs.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M.L. Prentice Hall; New York (NY): 1962. Soil Chemical Analysis. p. 263-268. [Google Scholar]
- John R., Ahmad P., Gadgil K., Sharma S. Cadmium and lead-induced changes in lipid peroxidation, antioxidative enzymes and metal accumulation in Brassica juncea L. at three different growth stages. Arch. Agron. Soil Sci. 2009;55(4):395–405. [Google Scholar]
- Khattab H. Role of glutathione and polyadenylic acid on the oxidative defense systems of two different cultivars of canola seedlings grown under saline conditions. Aus. J. Basic App. Sci. 2007;1:323–334. [Google Scholar]
- Ling-Zhi L., Zong-Qiang G., Yu-Long Z., Pei-Jun L. Cadmium accumulation and physiology of marigold (Tagetes erecta L.) as affected by arbuscular mycorrhizal fungi. Pedosphere. 2011;21(3):319–327. [Google Scholar]
- Liu L., Sun H., Chen J., Zhang Y., Li D., Li C. Effects of cadmium (Cd) on seedling growth traits and photosynthesis parameters in cotton. P. Omic J. 2014;7(4):284–290. [Google Scholar]
- Luck H. Catalase. In: Bergmeyer J., Grabi M., editors. vol. II. Academic Press; New York: 1974. pp. 885–890. (Methods of Enzymatic Analysis). [Google Scholar]
- Malekzadeh P., Farshian S.H., Ordubadi B. Interaction of arbuscular mycorrhizal fungus (Glomus intraradices and Glomus etunicatum) with tomato plants grown under copper toxicity. Afr. J. Biotech. 2012;11(46):10555–10567. [Google Scholar]
- Mangal M., Agarwal M., Bhargava D. Effect of cadmium and zinc on growth and biochemical parameters of selected vegetables. J. Pharm. Phytochem. 2013;2(1):106–114. [Google Scholar]
- Marquez-Garcıa B., Fernandez-Recamales M.A., Cordoba F. Effects of Cadmium on Phenolic Composition and antioxidant activities of Erica andevalensis. J. Bot. 2012 [Google Scholar]
- Nakano Y., Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Noctor G., Foyer C.H. Ascorbate and glutathione: keeping active oxygen under control. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Pagani M.A., Tomas M., Carrillo J., Bofill R., Capdevila M., Atrian S., Andreo C.S. The response of the different soybean metallothionein isoforms to cadmium intoxication. J. Inorganic Biochem. 2012;117:306–315. doi: 10.1016/j.jinorgbio.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Rausch T., Gromes R., Liedschulle V., Muller I., Bogs J., Galovic V., Wachter A. Novel insight into the regulation of GSH biosynthesis in higher plants. Plant Biol. 2007;9:565–572. doi: 10.1055/s-2007-965580. [DOI] [PubMed] [Google Scholar]
- Shekoofeh E., Sepideh H., Roya R. Role of mycorrhizal fungi and salicylic acid in salinity tolerance of Ocimum basilicum resistance to salinity. Afr. J. Biotech. 2012;11(9):2223–2235. [Google Scholar]
- Singh A., Prasad S.M. Effect of agro-industrial waste amendment on Cd uptake in Amaranthus caudatus grown under contaminated soil: an oxidative biomarker response. Ecotoxicol. Environ. Saf. 2014;100:105–113. doi: 10.1016/j.ecoenv.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Slinkard K., Singleton V.L. Total phenol analyses, automation and comparison with manual methods. Am. J. Enol. Vitic. 1977;28:49–55. [Google Scholar]
- Sylwia W., Anna R.S., Ewa B., Stephan C., Maria A.D. The role of subcellular distribution of cadmium and phytochelatins in the generation of distinct phenotypes of AtPCS1 and CePCS3 expressing tobacco. J. Plant Physiol. 2010;167:981–988. doi: 10.1016/j.jplph.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Tang M., Chen H., Huang J.C., Tian Z.Q. AM fungi effects on the growth and physiology of Zea mays seedlings under diesel stress. Soil Bio. Biochem. 2009;41:936–940. [Google Scholar]
- Van Rossum M.W.P.C., Alberda M., Van der Plas L.H.W. Role of oxidative damage in tulip bulb scale micropropagation. Plant Sci. 1997;130:207–216. [Google Scholar]
- Vierheilig H., Piche Y. 2002. Signalling in arbuscular mycorrhiza: facts and hypotheses. In: Buslig B., Manthey j. (Ed.) Flavonoids in cell functions. New York: Kluwer Academic. 23–39. [DOI] [PubMed]
- Wada K.C., Mizuuchi K., Koshio A., Kaneko K., Mitsui T., Takeno K. Stress enhances the gene expression and enzyme activity of phenylalanine ammonia-lyase and the endogenous content of salicylic acid to induce flowering in pharbitis. J. Plant Physiol. 2014;171:895–902. doi: 10.1016/j.jplph.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Wolf B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Comm. Soil Sci. Plant Anal. 1982;13:1035–1059. [Google Scholar]
- Wu Q.S., Zou Y.N., Abd_Allah E.F. 2014. Mycorrhizal Association and ROS in Plants. In: P. Ahmad (Ed): Oxidative Damage to Plants. 2014, doi: 10.1016/B978-0-12-799963-0.00015-0 © Elsevier Inc., All rights reserved.
- Yan L., Li X., He M., Zeng F. Behavior of native species Arrhenatherum Elatius (Poaceae) and Sonchus Transcaspicus (Asteraceae) exposed to a heavy metal-polluted field: plant metal concentration, phytotoxicity, and detoxification responses. Int. J. Phytorem. 2013;15:924–937. doi: 10.1080/15226514.2012.735288. [DOI] [PubMed] [Google Scholar]
- Zoffoli H.J., do Amaral-Sobrinho N.M., Zonta E., Luisi M.V., Marcon G., Tolon Becerra A. Inputs of heavy metals due to agrochemical use in tobacco fields in Brazil’s southern region. Environ. Monit. Assess. 2013;185:2423–2437. doi: 10.1007/s10661-012-2721-y. [DOI] [PubMed] [Google Scholar]