Abstract
A new and rapid protocol for optimum callus production and complete plant regeneration has been assessed in Malaysian upland rice (Oryza sativa) cv. Panderas. The effect of plant growth regulator (PGR) on the regeneration frequency of Malaysian upland rice (cv. Panderas) was investigated. Mature seeds were used as a starting material for callus induction experiment using various concentrations of 2,4-D and NAA. Optimal callus induction frequency at 90% was obtained on MS media containing 2,4-D (3 mg L−1) and NAA (2 mg L−1) after 6 weeks while no significant difference was seen on tryptophan and glutamine parameters. Embryogenic callus was recorded as compact, globular and light yellowish in color. The embryogenic callus morphology was further confirmed with scanning electron microscopy (SEM) analysis. For regeneration, induced calli were treated with various concentrations of Kin (0.5–1.5 mg L−1), BAP, NAA and 0.5 mg L−1 of TDZ. The result showed that the maximum regeneration frequency (100%) was achieved on MS medium containing BAP (0.5 mg L−1), Kin (1.5 mg L−1), NAA (0.5 mg L−1) and TDZ (0.5 mg L−1) within four weeks. Developed shoots were successfully rooted on half strength MS free hormone medium and later transferred into a pot containing soil for acclimatization. This cutting-edge finding is unique over the other existing publishable data due to the good regeneration response by producing a large number of shoots.
Abbreviations: 2,4-D, 2,4-dichlorophenoxyacetic acid; NAA, naphthaleneacetic acid; Kin, kinetin; MS, Murashige and Skoog; BAP, benzylaminopurine; TDZ, thidiazuron
Keywords: Upland rice, Regeneration frequency, BAP, Kinetin, TDZ
1. Introduction
Rice is a strategic crop in the Asian region especially Malaysia and it is essential to maintain a domestic production level for food security reasons. Upland rice is one of the special rice types grown on limited irrigation conditions. Malaysian upland rice normally is cultivated in Sabah and Sarawak by the rural communities for low-scale production. Upland rice yield in Malaysia is recorded ranging from 0.46 to 1.1 tonnes per hectare (Sohrabi et al., 2013). Efforts were made by Sohrabi et al. (2012) to study about 50 Malaysian upland rice varieties for genetic diversity, showing that various upland rice cultivars need to be improvised for crop improvement purposes. Research on upland rice improvement has gained little attention due to the unstable grain yields. Furthermore, the low yield of upland rice grain is commonly because of the poor agronomy management practices and lack of the application of nutrient management in the upland rice cultivation (Hanafi et al., 2009). In addition, a lot of strategies need to be devised to improve upland rice productivity via the conventional breeding approach along with recent achievements in genetic improvement to balance the world consumption of rice. Although Malaysian agricultural mostly depended on wetland rice varieties such as MR219 and MR220 it is still insufficient to support the demand of domestic consumption. To date, over a half of the population of this world would totally depend on rice as a staple food and that demand for rice production is still increasing everyday. Malaysia typically imported 1031.4 thousand metric tonnes of rice from different countries due to insufficient supply to feed its blooming population (DOA, 2011). For this reason, rice transformation using the biotechnology approach needs to be applied to address both biotic and abiotic factors that hinder the rice production globally. However, an effective and robust tissue culture protocol system in upland rice was limited and relatively low and varied among genotypes tested (Geng et al., 2008). Thus, the most viable option that is still feasibleis through optimization of several parameters in rice genetic improvement program. Numerous previous reports in line with the goals have been published such as genotype, type of explant used, desiccation treatment, carbon sources and plant growth regulators (PGRs) in medium (Ali et al., 2004, Saharan et al., 2004, Lin and Zhang, 2005, Ikram-Ul-Haq et al., 2009, Feng et al., 2011). Apart from modification in plant growth regulators used, amino acids such as tryptophan and sorbitol also showed positive effects on rice callus induction (Chowdhry et al., 1993, Shahsavari et al., 2010), while glutamine has been recommended by the researcher in rice tissue culture (Ge et al., 2006, Shahsavari, 2011). The selection of embryogenic callus induction was critical before any regeneration study took place. Numerous studies on SEM analysis of good quality of rice callus were performed (Bevitori et al., 2014, Narciso and Hatorri, 2010, Vega et al., 2009) as visual observation may misjudge the callus morphology and appearance. Therefore, SEM was used to identify the embryogenic rice callus appearance in this study as a pre-requisite for the successful application of the plant regeneration approach. Shahsavari (2010) found that application of various parameters resulted in 31–68% regeneration frequency in the Selasi cultivar of Malaysian upland rice.
Until now, there are several reports in upland rice micropropagation such as those reported by Geng et al., 2008, Shahsavari et al., 2010, Shahsavari, 2010 and Zhao et al. (2011). Several reports have shown that the exogenous application of plant growth regulators such as kinetin (Kin), benzylaminopurine (BAP), and naphthalene acetic acid (NAA) with addition of thidiazuron (TDZ) could improve regeneration frequency in upland rice (Ge et al., 2006, Zhao et al., 2011). Other than that, sorbitol or maltose has been shown to have a promotive effect on regeneration of upland rice cultivar (Feng et al., 2011, Geng et al., 2008, Shahsavari et al., 2010). However, TDZ has been shown to improve regeneration of Handao 297 Chinese upland rice cultivar upto 81.2% (Zhao et al., 2011). Dey et al. (2012) also concluded that the addition of TDZ into the regeneration medium significantly enhanced the proliferation of multiple shoots using the shoot apex in rice (Oryza sativa).In this present study, the modified regeneration medium was applied to regulate the initiation of multiple shoots from each scutellum derived calli while the other carbon source and gelling agent were standardized to promote fast growth. To our best knowledge, this protocol for high frequency plant regeneration is still lacking in the other Malaysian upland rice cultivars using embryogenic callus cultures, hence the novelty of this study.
The purpose of this study aimed to ascertain high quality embryogenic calli from mature seeds using optimal concentration of 2,4 dichlorophenoxyacetic acid (2,4-D) and naphthaleneacetic acid (NAA) and amino acid concentrations as well as their morphological variations under SEM. Keeping in view the above statements, an attempt was made to establish an improvement regeneration protocol for Malaysian upland rice genotype (Oryza sativa) cv. Panderas.
2. Materials and methods
2.1. Establishment of aseptic explants
Manually dehusked seeds of upland rice, Panderas cultivar were obtained from Panderas village, Pahang, Malaysia. The seeds were surface sterilized with 70% alcohol (v/v) for one minute and followed by immersion of 100% commercial bleach plus a drop of Tween-20 (Sigma–Aldrich, USA) for 30 min at 120 rpm shaking speed. Seeds were rinsed several times thoroughly using sterile distilled water before blotted dry on sterile tissue paper prior to callus induction.
2.2. Callus induction
Sterilized seeds were cultured on a basal callus induction medium consisting of MS salts (Murashige and Skoog, 1962) and B5 vitamins (Gamborg et al., 1968) fortified with 2,4-D (1–4 mg L−1) and NAA (0–4 mg L−1) either alone or in combination, 30 g L−1 sucrose, 0.5 g L−1 casein hydrolysate and 0.4% (w/v) gelrite. Subsequently, the influence of various concentrations (0, 25, 50, 100, 150 mg L−1) of amino acids (tryptophan and glutamine) on callus induction performance was carried out to optimize the best induced callus media. This optimal basal MS medium was designated as callus induction media (CIM) in this experiment. Each treatment contained 10 seeds with 3 replicates and the experiments were repeated twice. Sterilized seeds were incubated in the dark condition at 25–27 °C for 21 days and after 3 weeks of culture, the whole proliferated scutellum-derived calli were transferred into fresh CIM for another subsequent 3 weeks at the same condition. To confirm embryogenic characteristics, matured embryos callus were chosen and prepared for scanning electron microscopy (SEM) observations.
2.3. Scanning electron microscopy (SEM)
Embryogenic callus and non embryogenic callus were fixed in 3% buffered glutaraldehyde (0.1 M phosphate buffer, pH 7.2) for 2 h at room temperature. Later, the calluses were dehydrated through a gradient ethanol series [30% (v/v), 50% (v/v), 70% (v/v), 80% (v/v), 90% (v/v)] and 95% (v/v) for 15 min each and 100% ethanol twice for 15 min. The samples were dried and coated with gold (JEOL 780174712) and viewed under scanning electron microscopy (JEOL JSM 6390LV).
2.4. Plant Regeneration and acclimatization
High quality embryogenic calluses were selected and placed on regeneration media (RM) supplemented with a combination of Kin (0.5, 1.0, 1.5 mg L−1), BAP (0.5, 1.0, 1.5 mg L−1), NAA (0.5, 1.0, 1.5 mg L−1) plus TDZ (0.5 mg L−1), 0.5 g L−1 glutamine, 30 g L−1 maltose (Zuraida et al., 2011) and solidified with 4 g L−1 gelrite. Every treatment contained three replications with four clumps of callus each. All cultures were then incubated under 16/8 h (light/dark) photoperiod at 27 °C for 4 weeks. To facilitate root production, regenerated in vitro plantlets were transferred to half strength basal MS medium free hormone for 7 days. The in vitro plantlets with healthy roots were transplanted into the soil for acclimatization. Gelrite residue that was entrapped on the root system was washed under distilled water to minimize fungal attack. Regenerated plantlets were grown and maintained till maturity.
2.5. Statistical analysis
Callus induction frequency was determined after 3 weeks of culture based on the number of seeds producing good quality callus. Regeneration frequency was evaluated based on regenerated plantlets produced per embryogenic callus divided by the number of callus inoculated. Each experiment was conducted in three replicates in completely randomized block design. Data were analyzed using analysis of variance (ANOVA) and differences of means were evaluated by Duncan’s multiple range test (DMRT) of SPSS statistical package ver16.
3. Results
3.1. Effect of plant growth regulators on callus induction
The present study showed calluses protruded from the scutellum region and were clearly visible after seven days of culture. Compact, light yellowish in color and globular embryogenic callus were obtained after 21 days (Fig. 3b). Based on Fig. 1, all treatments produced embryogenic callus ranging from 6% to 90%. The result showed that the increment of both 2,4-D and NAA caused a reduction in callus percentage. Appearance of callus was dark yellow in color and turned to brownish when cultured on MS medium containing high auxin (T20). Among the different concentrations of 2,4-D and NAA tested, MS media containing 3 mg L−1 (2,4-D) and 2 mg L−1 (NAA) (T13) produced the highest percentage of callus induction with satisfactory embryogenic characteristics. The lowest callus induction (6%) was obtained on the MS media consisting of 4 mg L−1 (2,4-D) and 4 mg L−1 (NAA) which was found to be non embryogenic calluses that are pointed, rough and rhizogenic. It was observed that callus induction for all the media tested was statistically significant. In addition, the maximum callus induction percentage (70% and 90% respectively) was recorded at 3 mg L−1 (2,4-D) with a combination of NAA ranging from 1 to 2 mg L−1 (Fig. 1). The optimal media with the highest callus percentage (90%) showing compact, globular and light yellowish color was MS media composed of 3 mg L−1 2,4-D and 2 mg L−1 NAA (T13).
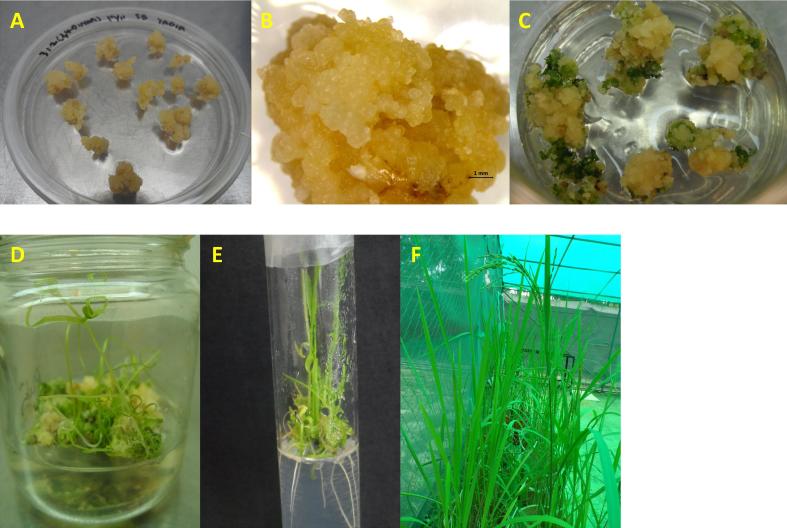
Figure 3.
Plant regeneration through somatic embryogenesis of Malaysian upland rice cv. Panderas. (A) Embryogenic calli after 2 weeks of subsequent subculture on callus induction medium (B) An embryogenic callus from mature seeds after 4 weeks culture, scale bar ± 1 mm (C) Embryogenic callus showing green spots after 14 days culture (D) Shoot regeneration after 4 weeks of culture on regeneration medium (E) In vitro regenerated rice plantlets on hormone free MS medium for rooting (F) A fertile regenerated rice plant at 3 months growth.
Figure 1.
Effect of different combinations of 2,4-D and NAA concentrations on the callus induction percentage after 6 weeks culture. 2,4-D:NAA; T1 (1:0); T2 (1:1); T3 (1:2); T4 (1:3); T5 (1:4); T6 (2:0); T7 (2:1); T8 (2:2); T9 (2:3); T10 (2:4); T11 (3:0); T12 (3:1); T13 (3:2); T14 (3:3); T15 (3:4); T16 (4:0); T17 (4:1); T18 (4:2); T19 (4:3); T20 (4:4). Columns represent means for replicates (N = 3) with error bars showing standard errors.
3.2. Effect of tryptophan and glutamine on callus induction
The present study showed that the increment of tryptophan concentration had decreased the frequency of callus formation and fresh weight except for the concentration of 25 mg L−1 of tryptophan. Related to that, the study showed 25 mg L−1 of tryptophan in MSB5 media containing 3 mg L−1 2,4-D and 2 mg L−1 NAA revealed the highest frequency of callus induction (97.5%) with 72 mg of average fresh weight but no significant difference (P > 0.05) at the third week. Furthermore, at the sixth week, the callus fresh weight increased three fold while the percentage of callus induction was maintained in all treatments and showed a significant difference (P < 0.05). However, the lowest frequency of callus induction was 77.5% recorded on MSB5 media supplemented with 3 mg L−1 2,4-D, 2 mg L−1 NAA and 150 mg L−1 tryptophan but the result was proven to be significantly different (P < 0.05) with the other treatments. The lowest percentage of callus induction and fresh weight were significantly different at both third and sixth week respectively (Table 1).
Table 1.
Effect of tryptophan and glutamine on the callus induction frequencies from mature seed explants on MSB5 media after 6 weeks.
| Amino acid | Concentration (mg L−1) | Callus induction frequencies (%) | Fresh weight (mg) |
|---|---|---|---|
| Tryptophan | 0 | 97.5 ± 2.5a | 210.5 ± 2.5a |
| 25 | 97.5 ± 2.5a | 216.0 ± 1.0a | |
| 50 | 90.0 ± 0.0a | 199.0 ± 1.0a | |
| 100 | 87.5 ± 2.5a | 191.5 ± 3.5a | |
| 150 | 77.5 ± 2.5b | 182.0 ± 4.0b | |
| Glutamine | 0 | 97.5 ± 2.5a | 210.5 ± 2.5a |
| 25 | 85.0 ± 0.0a | 172.5 ± 2.5b | |
| 50 | 90.0 ± 0.0a | 187.0 ± 1.0b | |
| 100 | 72.5 ± 2.5a | 153.0 ± 2.0b | |
| 150 | 65.0 ± 0.0a | 153.0 ± 2.0b | |
Values are means of replicates ± SD. Means on the same column with different superscripts are significantly different (P < 0.05). The bold values show the highest frequencies.
In this present study, the addition of several concentrations of glutamine into MSB5 medium containing 3 mg L−1 2,4-D and 2 mg L−1 NAA does not lead to any significant response among the treatments. This study showed that control treatment had the highest percentage of callus induction (97.5%) with average of fresh weight (210.5 mg) compared to other treatment containing glutamine (Table 1). The percentage of callus induction and callus fresh weight was decreased as the glutamine concentration increased except for the 50 mg L−1 concentration at both third and sixth weeks. In line with that, it was found that 50 mg L−1 glutamine showed the highest frequency of callus induction (87.5%) which was statistically significant for glutamine treatment while callus fresh weight was significantly different respectively at the third week. Callus fresh weight was found to increase three folds with significant difference (P < 0.05) between different concentrations despite frequency of callus induction recorded not being significantly different (P > 0.05) at the sixth week.
The above results showed that inclusion of tryptophan and glutamine has not given any significant changes in terms of percentage compared to the control treatment (without tryptophan and glutamine). Whereas, in the medium containing 25 mg L−1 tryptophan highest fresh weight induced callus (215 mg) was recorded after 6 weeks. The callus obtained was further analyzed through SEM for embryogenic structure.
The morphology of embryogenic calli was observed as compact and globular-shaped (Fig. 2a) compared to unorganized and sheet shaped structures in non embryogenic callus (Fig. 2b). The presence of extracellular matrix surface network (ECMSN) in SEM analyzed callus which consisted of fibrillar and membranous network was also observed in both figures shown (Fig. 2c and d). This indicates that the ECMSN provided an active role during plant cell morphogenesis as this feature induced the somatic embryogenesis and organogenesis. The callus with embryogenic structure was then selected for in vitro regeneration studies.
Figure 2.
Scanning of electron micrographs on embryogenic (A) and non embryogenic callus (B) of upland rice cv. Panderas. (C) Globular and dome like structures of embryogenic callus are present with the fibrillar network (white asterisks) and (D) membranous features (red asterisks).
3.3. Effect of different concentrations of PGR’s on the regeneration frequencies
Our preliminary findings showed that addition of TDZ to regeneration media comprising the lowest PGR concentration tested did improve the regeneration frequency from 33% to 66%. Therefore, 0.5 mg L−1 TDZ was added in the regeneration media for subsequent studies. Optimal regeneration percentage (100%) was recorded in the MS medium composed of 1.5 mg L−1 (Kin), 0.5 mg L−1 (BAP), 0.5 mg L−1 (NAA) and 0.5 mg L−1 (TDZ). The green spot first emerged on day seven and proliferated after 14 days of culture which later developed into shoot buds. However, treatment without TDZ (control) showed a delay of green spot appearance after 30 days and eventually developed into a plantlet.
Based on total concentration of cytokinin (BAP + Kin) of 1.0 mg L−1 tested, no significant differences (P > 0.05) of regeneration frequency between RM2 (66.6%), RM5 (53.3%) and RM6 (40%) were observed. However, total cytokinin of 2.0 mg L−1 of RM4 and RM7 showed significant differences (P < 0.05). The regeneration frequencies were lower in the medium containing total concentration of cytokinins (BAP + Kin) of 1.0–1.5 mg L−1 regardless of the NAA level in the medium: RM1 (33.3%), RM2 (66.6%), RM5 (53.3%) and RM6 (40%). The regeneration frequency between RM4 and RM1 were significantly different (P < 0.05). The average number of regenerated shoots (10–12 shoots) for each clump was five times higher in media consisting of TDZ compared to media without TDZ. The regeneration frequency of RM3 and RM4 was significantly higher (P < 0.05) than other media. The inclusion of TDZ in the media showed no sign of callus browning and accelerated early morphogenesis of shoot development. After four weeks, in vitro plantlets over 10 cm in height were developed and later transferred onto half strength MS media without growth regulators for root development (Fig. 3e). The plantlets showing healthy and normal growth were transferred into the soil (Fig. 3f).
4. Discussion
Many studies revealed the presence of synthetic auxin, 2,4-D was an important catapult factor for successful rice callus induction (Lin and Zhang, 2005, Karthikeyan et al., 2009, Joyia and Khan, 2013) but other researchers used 2,4-D combined with BAP (Sahoo et al., 2011) or NAA (Bano et al., 2005). Further, the use of 2,4-D was observed as inevitable for micropropagation through calluses. However, our studies showed that the combination of 2,4-D (3 mg L−1) and NAA (2 mg L−1) induced better callus induction frequency (90%). Our finding was also in contrast to previous reports on other Malaysian upland rice grown on MSB5 media consisting of 2,4-D only (Shahsavari, 2010). Nevertheless, our finding was also in agreement with Ali et al. (2004) who observed that 2,4-D combined with BAP or NAA gave better response to callus induction but in wetland rice. Trejo-Tapia et al. (2002) also suggested in their finding that a combination of auxin (NAA and 2,4-D) was a better alternative rather than using the single auxin. Castillo et al. (1998) also reported supplementation of 2,4-D alone or in combination into callus induction media enhanced callus induction. Since the same hormonal composition is not suitable for all rice varieties, the modifying media were diversified to overcome the genotypic influence for particular rice varieties. In fact, NAA function was reported to stimulate the frequency of embryogenesis in the initial culture stage of rice while Endress (1994) suggested 2,4-D could promote DNA hypermethylation in a pre-embryonic phase which was responsible to preserve the cell in highly mitotic mode. In our findings, high concentration of 2,4-D (4 mg L−1) caused the callus become brown and low in quality and not favorable for in vitro regeneration. This might be due to that high dose concentration of 2,4-D could induce a suppressive effect on callusing and in vitro regeneration through the effect of the remaining 2,4-D residues on re-differentiation in the mitotic stage (Rueb et al., 1994). Similar reduction in quantity of callus induction with increasing concentration of 2,4-D was reported in indica rice (Ramesh et al., 2009) and indica rice variety PAU 201 (Wani et al., 2011). Based on our findings, we suggested that a combination of 2,4-D and NAA produced a high percentage of callus formation for upland rice.
Our findings on tryptophan addition showed a slight increase in callus induction frequency. Previous studies have shown that the addition of tryptophan in callus induction medium could not increase frequency but help to accelerate the formation of embryogenic calluses (Chowdhry et al., 1993, Shahsavari, 2011). However, we could not spot any positive effect of glutamine addition on callus induction frequency as the increment of glutamine supplementation resulted in low measurements compared to the control. It is advisable to use a combination of various amino acids (glutamine, asparagine and arginine) as Zuraida et al. (2011) did to promote the improvement of somatic embryogenesis of wetland rice. In our observations, non embryogenic callus showing necrosis or browning was not selected for regeneration.
Sahoo et al. (2011) reported that characteristics of the embryogenic callus was yellowish color and had a similar morphological outlook in our studies. With higher concentrations of both auxins used (2,4-D and NAA), the callus percentage declined in parallel with the observation of a brown callus. According to Lee et al. (2002), the induction of embryogenic callus in terms of number, color, size and morphology varied between rice genotypes and depended on the composition of media used, type of explants and interaction between these factors. Visarada et al. (2002) also reported four types of calluses based on morphology; type I – white or cream color compact organized callus, type II – yellow organized callus, type III – yellow or brown unorganized callus, and type IV – rhizogenic callus. The globular surfaces can be smooth or hairy in texture and be colored either cream or white. The present study revealed that callus induction of upland rice may respond differently according to different treatments of PGR. Moreover, we select mature seeds as source of explant because they are available throughout the year and amenable to transformation via callus culture. Furthermore, scutellum derived calli from mature seeds are the suitable starting materials of undifferentiated cells for in vitro regeneration into fertile crops.
Since the selection of embryogenic callus through visual observation may misjudge the structures, further investigation with SEM analysis was performed. Numerous studies have shown the morphology observation through SEM in rice cultivars (Sangduen and Klamsomboon, 2001, Vega et al., 2009). Our SEM analysis pattern was in lieu with the findings of Narciso and Hatorri (2010). The extracellular matrix surface network (ECMSN) that was visible in the cell composition of the callus has been extensively discussed by Bevitori et al. (2014) and Pilarska et al. (2014). Previous reports on similar structures have been published by Xu et al. (2011) in banana, Lai et al. (2011) in pennyworts and Pilarska et al. (2013) in clover. The structural arrangement of ECMSN that covered the callus surface has its critical role in cell-to-cell interaction as well as in embryogenic competence. Other authors suggested that ECMSN was formed following the stress response to in vitro plant culture to protect the covering callus against external factors (Dubois et al., 1992, Popielarska-Konieczna et al., 2008).
Many auxins and cytokinins in the regeneration media are known to promote rice regeneration frequency in the recalcitrant genotype (Rueb et al., 1994, Lee et al., 2002). Lee et al. (2002) reported that the highest regeneration frequency on MS medium containing NAA (2.0 mg L−1) and Kin at a range of 1.0–4.0 mg L−1. Auxins and cytokinins may interact through synergistic, antagonistic and additive mechanisms relying on inoculated tissue culture conditions that promote developmental decision toward callogenesis and shoot formation. Combination of cytokinins such as TDZ and BAP with other auxins has been tested in immature embryos from other crops such as sorghum (Pola et al., 2007, Kishore et al., 2006) and Easter lily (Nhut et al., 2006). Zhao et al. (2011) stated that the highest regeneration frequency (81.2%) of upland rice callus was recorded with a combination of BAP (0.5 mg L−1), Kin (0.5 mg L−1) Zeatin (1.0 mg L−1), TDZ (0.5 mg L−1), NAA (0.5 mg L−1), IAA (0.15 mg L−1) and IBA (0.15 mg L−1) respectively. Our regeneration medium (RM2) was formulated from Zhao et al. (2011) except for Zeatin, IBA and IAA and showed reduced regeneration percentage (66.6%). Therefore, we optimized Kin concentration up to 1.5 mg L−1 and found that the best regeneration percentage was achieved at 100% (Table 2). The present study also observed the auxin (NAA) and cytokinin (BAP, Kin and TDZ) ratio is crucial to in vitro regeneration response from undifferentiated tissue. Moreover, Lee and Huang (2014) suggested the balance between auxin and cytokinin played a major role in the initiation of regeneration of induced calli. Both cytokinins and auxins are thought to influence the cell cycle and morphogenic competence in plant growth (Jones et al., 2010). Unbalanced concentration between auxin and cytokinin (RM5) resulted in a decrease in regeneration frequency as the development of multiple shoot proliferation appeared to be suppressed (Table 2). From our studies, inclusion of TDZ in regeneration media increased the shoot number per callus (Table 2; Fig. 3d) compared to treatment without TDZ. This result was similar to Zhao et al. (2011) findings that discovered the number of regenerated plantlets were higher when cultured on 0.5 mg L−1 TDZ. TDZ has proven to be more effective in accelerating the shoot formation of upland rice cultivars. Dey et al. (2012) found very promising results that application of TDZ helped for the initiation of shoot proliferation from the shoot apices of rice by regenerating a maximum of 10.3 shoots per explant. This result indicated that TDZ addition will assist the number of shoots from embryogenic calluses that enhance the regeneration potential of callus.
Table 2.
Effect of different concentrations of PGRs on the regeneration frequencies of calli on MS media.
| Treatment | PGR (mg L−1) |
No of calluses inoculated | Regeneration frequency (%) | |||
|---|---|---|---|---|---|---|
| BAP | Kinetin | NAA | TDZ | |||
| RM1 | 0.5 | 0.5 | 0.5 | 0 | 12 | 33.3 ± 14.43ab |
| RM2 | 0.5 | 0.5 | 0.5 | 0.5 | 12 | 66.6 ± 38.18ab |
| RM3 | 0.5 | 1.0 | 0.5 | 0.5 | 12 | 93.3 ± 11.54b |
| RM4 | 0.5 | 1.5 | 0.5 | 0.5 | 12 | 100 ± 0.00b |
| RM5 | 0.5 | 0.5 | 1.0 | 0.5 | 12 | 53.3 ± 50.33ab |
| RM6 | 0.5 | 0.5 | 1.5 | 0.5 | 12 | 40 ± 52.91ab |
| RM7 | 1.5 | 0.5 | 0.5 | 0.5 | 12 | 91.6 ± 14.43ab |
Values are means of replicates ± SD. Means on the same column with the different superscripts are significantly different (P < 0.05). RM is defined as regeneration media. Total cytokinin refers to total amount of BAP + Kin concentration. BAP: benzylaminopurine; NAA: naphthaleneacetic acid; TDZ: thidiazuron. The bold values show the highest frequencies of plant regeneration.
Besides modifying the PGRs, 4 g L−1 gelrite was used as a gelling agent in the present study and may contribute to the good quality of regenerated upland rice callus as stated by Shahsavari (2011). Moreover, gelrite was proven better compared to other gelling agents in rice culture as it provided more water stress for somatic embryogenesis improvement (Rafique et al., 2011). Apart from tissue culture of upland rice, Swarna and Mahsuri varieties of wetland rice callus have been highly regenerated as reported by Pravin et al. (2011). Joyia and Khan (2013) also reported that regeneration response of Pakistan rice was better when using gelrite by producing the greater number of green shoots. Our result was found in agreement with all the mentioned studies that the use of gelrite produced good regenerated calluses. It was accepted that the choice of the carbon source plays a dominant role in determining good callogenesis and enhanced regeneration.
In conclusion, it was observed that the correct concentration of PGR combination (BAP, Kin, NAA and TDZ) has significantly enhanced the regeneration frequencies of this local upland rice cultivar. Thus, this experiment showed an efficient protocol for in vitro plantlet micropropagation production from mature seeds of upland rice cv. Panderas as readily available explants within a short period of time (one month) while maintaining the embryogenic features compared to the conventional regeneration system that required five months of cultivation. This rapid and reliable protocol may provide an opportunity in further genetic transformation studies by incorporation of important agronomic traits.
Acknowledgement
The authors wish to express their greatest gratitude and appreciation to the Universiti Teknologi Malaysia for providing financial support through a research grant (Q.J130000.2444.00G065) under Fundamental Research Grant Scheme Fund. Thanks are also due to Plant Tissue Culture Laboratory staff, Faculty of Biosciences and Medical Engineering for their technical assistance.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Abd Rahman Jabir Mohd Din, Email: jabir@ibd.utm.my.
Alina Wagiran, Email: alina@fbb.utm.my.
References
- Ali S., Xue Q., Zhang X. Assessment of various factors involved in the tissue culture system in rice. Rice Sci. 2004;11:345–349. [Google Scholar]
- Bano S., Jabeen M., Rahim F., Ilahi I. Callus induction and regeneration in seed explants of rice (Oryza sativa vc. Swat II) Pak. J. Bot. 2005;37:829–883. [Google Scholar]
- Bevitori R., Popielarska-Konieczna M., dos Santos E.M., Grossi-de-Sá M.F., Petrofeza R. Morpho-anatomical characterization of mature embryo-derived callus of rice (Oryza sativa L.) suitable for transformation. Protoplasma. 2014;251:545–554. doi: 10.1007/s00709-013-0553-4. [DOI] [PubMed] [Google Scholar]
- Castillo A.M., Egana B., Sanz J.M., Cistue L. Somatic embryogenesis and plant regeneration from barley cultivars grown in Spain. Plant Cell Rep. 1998;17:902–906. doi: 10.1007/s002990050506. [DOI] [PubMed] [Google Scholar]
- Chowdhry C.N., Tyagi A.K., Maheshwari N., Maheshwari S.C. Effect of L-proline and L-tryptophan on somatic embryogenesis and plantlet regeneration of rice (Oryza sativa L. cv. Pusa 169) Plant Cell Tissue Organ Cult. 1993;32:357–361. [Google Scholar]
- Dey M., Bakshi S., Galiba G., Sahoo L., Panda S.K. Development of a genotype independent and transformation amenable regeneration system from shoot apex in rice (Oryza sativa spp. indica) using TDZ. 3 Biotechnology. 2012;2:233–240. [Google Scholar]
- DOA, 2011. Paddy statistics of Malaysia. Ministry of Agriculture and Agro-based Industry. p. 4. Available at: <http://www.doa.gov.my/c/document_library/get_file?uuid=8b3bb7ed-4363-4471-b760-6f528e6273dc&groupId=38371> (accessed 21 May 2014).
- Dubois T., Dubois J., Guedira M., Diop A., Vasseur J. SEM characterization of an extracellular matrix arpund somatic proembryos in roots of Cichorium. Ann. Bot. 1992;70:119–124. [Google Scholar]
- Endress R. Springer-Verlag; Berlin, Alemania, Germany: 1994. Plant Cell Biotechnology. p. 353. [Google Scholar]
- Feng X., Zhao P., Hao J., Hu J., Kang D., Wang H. Effect of sorbitol on expression of genes involved in regeneration of upland rice (Oryza sativa L.) Plant Cell Tissue Organ Cult. 2011;106:455–463. [Google Scholar]
- Gamborg O.L., Miller R.A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968;50:151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Ge X.J., Chu Z.H., Lin Y.J., Wang S.P. A tissue culture system for different germplasms of indica rice. Plant Cell Rep. 2006;25:392–402. doi: 10.1007/s00299-005-0100-7. [DOI] [PubMed] [Google Scholar]
- Geng P.P., La H.G., Wang H.Q., Stevens E.J.C. Effect of sorbitol concentration on regeneration of embryogenic callus in upland rice varieties (Oryza sativa L.) Plant Cell Tissue Organ Cult. 2008;92:303–313. [Google Scholar]
- Hanafi M.M., Hartinie A., Shukor J., Mahmud T.M.M. Upland rice varieties in Malaysia: agronomic and soil physic-chemical characteristics. Pertanika J. Trop. Agric. Sci. 2009;32:225–246. [Google Scholar]
- Ikram-Ul-Haq, Chang-Xing Z., Mukhtar Z., Jaleel C.A., Azooz M.M. Effect of physical desiccation on plant regeneration efficiency in rice (Oryza sativa L.) variety super Basmati. J. Plant Physiol. 2009;166:1568–1575. doi: 10.1016/j.jplph.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Jones B., Gunnera S.A., Petersson S.V., Tarkowski P., Graham N., May S., Dolezal K., Sanberg G., Ljungb K. Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell. 2010;22:2956–2969. doi: 10.1105/tpc.110.074856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyia F.A., Khan M.S. Scutellum-derived callus-based efficient and reproducible regeneration system for elite varieties of indica rice in Pakistan. Int. J. Agric. Biol. 2013;15:27–33. [Google Scholar]
- Karthikeyan A., Pandian S.T.K., Ramesh M. High frequency plant regeneration from embryogenic callus of a popular indica rice (Oryza sativa L.) Phys. Mol. Biol. Plants. 2009;15:371–375. doi: 10.1007/s12298-009-0042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore S.N., Visarada K.B.R.S., Aravinda Lakshmi Y., Pashupatinath E., Rao S.V., Seetharama N. In vitro culture methods in sorghum with shoot tips the explants material. Plant Cell Rep. 2006;25:174–182. doi: 10.1007/s00299-005-0044-y. [DOI] [PubMed] [Google Scholar]
- Lai K.S., Yusoff K., Maziah M. Extracellular matrix as the early structural marker for Centella asiatica embryogenic tissues. Biol. Plant. 2011;55:549–553. [Google Scholar]
- Lee S., Huang W. Osmotic stress stimulates shoot organogenesis in callus of rice (Oryza sativa L.) via auxin signaling and carbohydrate metabolism regulation. Plant Growth Regul. 2014;73:193–204. [Google Scholar]
- Lee K.S., Jeon H.S., Kim M.Y. Optimization of a mature embryos-based in vitro culture system for high frequency somatic embryogenic callus induction and plant regeneration form japonica rice cultivar. Plant Cell Tissue Organ Cult. 2002;71:9–13. [Google Scholar]
- Lin Y.J., Zhang Q.F. Optimising the tissue culture conditions for high efficiency transformation of indica rice. Plant Cell Rep. 2005;23:540–547. doi: 10.1007/s00299-004-0843-6. [DOI] [PubMed] [Google Scholar]
- Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tabacco tissue culture. Physiol. Plant. 1962;15:473–497. [Google Scholar]
- Narciso J.O., Hatorri K. Genotypic differences in morphology and ultrastructure of callus derived from selected rice varieties. Philip. Sci. Lett. 2010;3:59–65. [Google Scholar]
- Nhut D.T., Hahn N.T.M., Tuan P.Q., Nguyet T.M., Tram N.T.H., Chinch N.C., Nguyen N.H., Vinh D.N. Liquid culture as a positive condition to induce and enhance quality and quantity of somatic embryogenesis of Lilium longiflorum. Sci. Hortic. 2006;110:93–97. [Google Scholar]
- Pilarska M., Knox J.P., Konieczny R. Arabinogalactan-protein and pectin epitopes in relation to an extracellular matrix surface network and somatic embryogenesis and callogenesis in Trifolium nigrescens. Plant Cell Tissue Organ Cult. 2013;115:35–44. [Google Scholar]
- Pilarska M., Popielarska-Konieczna M., Ślesak H., Kozieradzka-Kiszkurno M., Goralski G., Konieczny R., Bohdanowicz J., Kuta E. Extracellular matrix surface network is associated with non-morphogenic calli of Helianthus tuberosus cv. Albik produced from various explants. Acta Soc. Bot. Pol. 2014;1:67–73. [Google Scholar]
- Pola S., Saradamani N., Ramana T. Enhanced shoot regeneration in tissue culture studies of sorghum bicolor. J. Agric. Technol. 2007;3:275–286. [Google Scholar]
- Popielarska-Konieczna M., Kozieradzka-Kiszkurno M., Świerczyńska J., Goralski G., Ślesak H., Bohdanowicz J. Are extracellular matrix surface network components involved in signaling and protective function. Plant Signal. Behav. 2008;3:707–709. doi: 10.4161/psb.3.9.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravin J.P., Dudharem M.S., Saluja T., Sarawgi A.K., Saxena R., Girish C. Assessment of critical factors influencing callus induction, in vitro regeneration and selection of bombarded indica rice genotypes. J. Agric. Biotechnol. Sustain. Dev. 2011;3:44–59. [Google Scholar]
- Rafique M.Z., Rashid H., Chaudhary M.F., Chaudhry Z., Cheema N.M. Study on callogenesis and organogenesis in local cultivars of rice (Oryza sativa L.) Pakistan J. Bot. 2011;43:191–203. [Google Scholar]
- Ramesh M., Murugiah V., Gupta A.K. Efficient in vitro plant regeneration via leaf base segments of indica rice (Oryza sativa L.) Indian J. Exp. Biol. 2009;47:68–74. [PubMed] [Google Scholar]
- Rueb S., Leneman M., Schilperoort R.A., Hensgens L.A.M. Efficient plant regeneration through somatic embryogenesis from callus induced on mature rice embryos (Oryza sativa L.) Plant Cell Tissue Organ Cult. 1994;36:259–264. [Google Scholar]
- Saharan V., Yadav R.C., Yadav N.R., Chapagain B.P. High frequency plant regeneration from dessicated callus of indica rice (Oryza sativa L.) Afr. J. Biotechnol. 2004;3:256–259. [Google Scholar]
- Sahoo K.K., Tripathi A.K., Pareek A., Sopory S.K., Singla-Pareek S.L. An improved protocol for efficient transformation and regeneration of diverse indica rice cultivars. Plant Methods. 2011;7:49–59. doi: 10.1186/1746-4811-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangduen N., Klamsomboon P. Histological and scanning electron observation on embryogenic and non-embryogenic callus of aromatic Thai rice (Oryza sativa L. cv. Khao Daw Mali 105) Kasetsart J. 2001;35:427–432. [Google Scholar]
- Shahsavari E. Evaluation and optimizations of media on the tissue culture system of upland rice. Int. J. Agric. Biol. 2010;12:537–540. [Google Scholar]
- Shahsavari E. Impact of tryptophan and glutamine on tissue culture of upland rice. Plant Soil Environ. 2011;57:7–10. [Google Scholar]
- Shahsavari E., Maheran A.A., Siti Nor Akmar A., Hanafi M.M. The effect of plant growth regulators on optimization of tissue culture system in Malaysian upland rice. Afr. J. Biotechnol. 2010;9:2089–2094. [Google Scholar]
- Sohrabi M., Rafii M.Y., Hanafi M.M., Siti Nor Akmar A., Latif M.A. Genetic diversity of upland rice germplasm in Malaysia revealed by quantitative traits. Sci. World J. 2012 doi: 10.1100/2012/416291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi M., Rafii M.Y., Hanafi M.M., Latif M.A. Genetic divergence of Malaysian upland rices revealed by microsatellite markers. Plant Omics J. 2013;6:175–182. [Google Scholar]
- Trejo-Tapia G., Amaya U.M., Morales G.S., Sanchez A.J., Bonfil B.M., Rondriguez-Monroy M., Jimenez-Aparicio A. The effect of cold pretreatment, auxins and carbon source on anther culture of rice. Plant Cell Tissue Organ Cult. 2002;71:41–46. [Google Scholar]
- Vega R., Vasquez N., Espinoza A.M., Gatica A.M., Marta V. Histology of somatic embryogenesis in rice (Oryza sativa cv.5272) Rev. Biol. Trop. 2009;57:141–150. [Google Scholar]
- Visarada K.B.R.S., Sailaja M., Sarma N.P. Effect of callus induction media on morphology of embryogenic callus in rice genotypes. Biol. Plant. 2002;45:495–502. [Google Scholar]
- Wani S.H., Sanghera G.S., Gosal S.S. An efficient and reproducible method for regeneration of whole plants from mature seeds of a high yielding Indica rice (Oryza sativa L.) variety PAU 201. N. Biotechnol. 2011;4:418–422. doi: 10.1016/j.nbt.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Xu C., Zhao L., Pan X., Śamaj J. Developmental localization and methylesterification of pection epitopes during somatic embryogenesis of banana (Musa ssp. AAA) PLoS ONE. 2011;6(8):1–12. doi: 10.1371/journal.pone.0022992. e22992, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Zheng S., Ling H. An regeneration system and Agrobacterium-mediated transformation of Chinese upland rice cultivar Handao297. Plant Cell Tissue Organ Cult. 2011;106:475–483. [Google Scholar]
- Zuraida A.R., Naziah B., Zamri Z., Sreeramanan S. Efficient plant regeneration of Malaysian indica rice MR 219 and 232 via somatic embryogenesis system. Acta Physiol. Plant. 2011;33:1913–1921. [Google Scholar]