Abstract
Ginseng fruit saponins (GFS) extracted from the ginseng fruit are the bioactive triterpenoid saponin components. The aim of the present study was to develop a drug delivery system called proliposome using sodium deoxycholate (NaDC) as a bile salt to improve the oral bioavailability of GFS in rats. The liposomes of GFS were prepared by a conventional ethanol injection and formed the solid proliposomes (P-GFS) using spray drying method on mannitol carriers. The formulation of P-GFS was optimized using the response surface methodology. The physicochemical properties of liposome suspensions including encapsulation efficiency, in vitro drug release studies, particle size of the reconstituted liposome were tested. The solid state characterization studies using the method of Field emission-scanning electron microscope (FE-SEM), Fourier transform infrared (FT-IR) and Differential scanning colorimetric (DSC) were tested to study the molecular state of P-GFS and to indicate the interactions among the formulation ingredients. In vitro studies showed a delayed release of ginsenoside Re (GRe). In vivo studies were carried out in rats. The concentrations of GRe in plasma of rats and its pharmacokinetic behaviors after oral administration of GFS, Zhenyuan tablets (commercial dosage form of GFS) and P-GFS were studied using ultra performance liquid chromatography tandem mass spectrometry. It was founded that the GRe concentration time curves of GFS, Zhenyuan tablets and P-GFS were much more different in rats. Pharmacokinetic behaviors of P-GFS showed a second absorption peak on the concentration time curve. The pharmacokinetic parameters of GFS, Zhenyuan tablets, P-GFS in rats were separately listed as follows: T max 0.25 h, C max 474.96 ± 66.06 ng/ml and AUC0−∞ 733.32 ± 113.82 ng/ml h for GFS; T max 0.31 ± 0.043 h, C max 533.94 ± 106.54 ng/ml and AUC0−∞ 1151.38 ± 198.29 ng/ml h for Zhenyuan tablets; T max 0.5 h, C max 680.62 ± 138.051 ng/ml and AUC0−∞ 2082.49 ± 408.33 ng/ml h for the P-GFS. The bioavailability of P-GFS was nearly 284% and 181% of the GFS and Zhengyuan tablets respectively. In conclusion, the proliposomes significantly enhanced the drug bioavailability, absorption in the gastrointestinal tract and decreased its elimination time of GRe in rats and could be selectively applied for oral delivery of GFS.
Keywords: Ginseng fruit saponins, Proliposomes, Sodium deoxycholate, Oral bioavailability, Pharmacokinetics
1. Introduction
Ginseng (panax ginseng) is a highly used and valued Chinese herbal medicine to treat a variety of ailments for thousands of years. Saponins extracted from the ginseng plant are the active compounds and responsible for most of the pharmacological effects of ginseng. Ginseng fruit saponins (GFS) extracted from the berry of Ginseng possess higher ginsenoside contents than the roots and recently are a hotspot in treating many diseases (Yang et al., 2010). GFS are a group of the dammarane-type triterpene saponins. In previous publications, it had been proved that GFS showed a positive bioactivity effect in atherosclerosis, anti-diabetic and anti-obesity effects, diabetes associated cognitive deficits, anti-inflammatory activity, histamine and cytokine release, anti-stress effect, and systemic lupus erythematosus (Attele et al., 2002, Huo, 1984, Park et al., 2012, Yang and Zhang, 1986, Zhang and Jiang, 1981, Zhang et al., 1984). Ginsenoside Re (GRe) which contains the structural backbone of protopanaxatriol is the most important major bioactivity constituents of GFS and has been chosen as the character for evaluation of GFS (Lee et al., 2014). In particular, a previous study showed that ginsenoside Re in ginseng berry extracts showed a superior oral absorption of ginsenoside Re at equivalent ginsenoside Re dose to pure ginsenoside Re, indicating that GFS might be a good form for ginsenoside Re intake (Joo et al., 2010).
GFS is well-known as the BCS III (Biopharmaceutics Classification system) drugs with high solubility and low permeability. The poor oral bioavailability was observed for its low permeability and fast degradation and clearance in the gastrointestinal tract (Kim et al., 2013, Peng et al., 2012). To improve bioavailability of GFS, a variety of formulations such as nano particle, phospholipid complex, liposome have been used in previous studies (Ajazuddin and Saraf, 2010). Phospholipids have the characteristics of excellent biocompatibility and amphiphilicity. These unique properties make phospholipids most appropriate to be employed as important pharmaceutical excipients (Li et al., 2015). And the lipid-based drug delivery systems enhance the intestinal drug permeability and solubility (Larsen et al., 2011, Porter et al., 2008, Trevaskis et al., 2008). Liposomes can entrap both hydrophilic and hydrophobic agents, which could protect the entrapped agents from external destructive conditions, such as light, pH and enzymes (Chen, 2008).In addition to the property of liposome adhesion to and absorption into the intestinal epithelial cells, liposome as a novel method was used to improve the bioavailability of GFS. Considering the poor physicochemical stability problems of liposome including aggregation, fusion, oxidation and active agent’s leakage, one effective approach is used to formulate the aqueous liposome into solid proliposome defined as dry, free-flowing particles, containing water soluble carrier particles coated with phospholipids (Yanamandra et al., 2014). Once contacted with water, proliposome could immediately form a liposomal dispersion and be more uniform in size. The methods of preparing proliposome include single step spray drying method (Patil-Gadhe and Pokharkar, 2014), vacuum evaporation (Janga et al., 2012, Keon-Hyoung Song and Chang-Koo Shim, 2002), film deposition method (Janga et al., 2012), and fluidized bed method (Chen and Alli, 1987). The Spray drying method was used in this study considering the application in industries. Some studies focusing on proliposome surface charge and Carboxymethyl chitosan coated modification were reported to further improve the absorption of liposomes in the gastrointestinal tract (Bai et al., 2011, Janga et al., 2012). A liposome containing bile salts called bilosomes is widely studied and is a hotspot to biocompatibility compared with surface charge (Senior, 2001). Bilosomes have been applied to the oral immunization of peptide and protein antigens (Conacher et al., 2001) and could improve the integrity and stability of oral liposomes in gastrointestinal media (Hu et al., 2013). The enhanced oral absorption of bilosomes could be achieved by improving in vivo residence time, and permeation across the bio-membranes and trans-endocytosis internalization has been reported in a previous study (Niu et al., 2014). Sodium deoxycholate (NaDC) had been used as a bile salt to improve absorption and stability of vesical particles for the drug application dosages in oral delivery system (Gangadhar et al., 2014).
In the present study, the P-GFS containing sodium deoxycholate was firstly prepared and optimized to improve the oral bioavailability of the GFS. The physicochemical properties and the molecule state of the proliposomes were characterized by methods of FE-SEM, DSC and FT-IR. Particle size, encapsulation efficiency, stability evaluation, in vitro drug release and in vivo bioavailability studies in rats of P-GFS were also investigated to evaluate in this delivery system.
2. Materials and methods
2.1. Materials
Ginseng fruit saponins (GFS) were gifted from Bo Xiang Pharmaceutical Group (Ji Lin, China). Egg yolk phosphatidylcholine (EPC) with the purity over 80%, was purchased from the Guangzhou hanfang modern Chinese medicine research and development Co. Ltd. Ethyl acetate (EtOAc, 99.9%), acetonitrile (HPLC-grade), methanol (HPLC-grade), ethyl acetate, isopropanol, and phosphoric acid (HPLC-grade) were obtained from Sigma–Aldrich Co. (MO, USA). Sodium deoxycholate (NaDC), mannitol, anhydrous ethanol, hydrochloric acid, and other chemical reagents of analytical grade or better were obtained from Sinopharm Chemical Reagent Co. (Shanghai, China). The reference standards of ginsenoside Re, Salidroside (IS) were purchased from the National Institute for Food and Drug Control of China.
2.2. Preparation of P-GFS
The spray drying method was used in this study for the preparation of proliposomes. Briefly, egg yolk phosphatidylcholine (EPC) was dissolved in anhydrous ethanol to obtain lipid solution. GFS were added in water and heated to a temperature of 55 °C to obtain a transparent drug solution. The lipid and sodium deoxycholate (NaDC) solution was injected quickly into drug solution under stirring (600 r/min) for 10 min at 55 °C to obtain a liposome suspension containing NaDC. As the temperature was at 37 °C, manntiol was added to the suspension with stirring for 5 min in order to mix homogeneity completely before the spray drying. The spray dying relative parameters were set as follows: The inlet temperature was 130 °C and a feed rate was 400 ml/h.
2.3. Optimization of proliposomes
The single-factor tests were first carried out to ascertain the factors of the preparation methods of proliposomes. And then the main factors affecting the particle size and encapsulation efficiency (EE) of P-GFS were confirmed. Based on the result of single-factor tests, three factors which affected most namely the concentration of EPC (X1), the ratio of EPC and manntiol (X2), and the ratio of EPC and NaDC (X3) were selected to further optimize the composition of GFS proliposome (the ratio of drug and EPC concentration was 1:1). The selected factors were subjected to analysis by response surface methodology (RSM) with a three factor-three coded level Box-Behnken design (BBD). The range and the levels of experimental variables investigated in this study are presented in Table 1. In addition, several tests were done to validate the experiment design according to the optimal composition. Response surfaces describing the relationship between each factor and index were drawn according to the fitting equation. Finally, the optimized formula from the response surface drawing was applied to produce P-GFS and execute predictive analysis.
Table 1.
Coded and levels of the variables used in Box-Behnken design.
| Factors | Code | Range and levels |
||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| EPC concentration (mg/ml) | X1 | 1 | 2 | 3 |
| mannitol:EPC | X2 | 3:2 | 5:2 | 7:2 |
| NaDC:EPC | X3 | 1:8 | 1:4 | 1:2 |
The volume of the dissolution system was fixed at 400 ml.
2.4. HPLC analysis of ginsenoside Re
The quantitative ginsenoside Re analysis was performed using an HPLC System (SPD-10AVP HPLC, Shimadzu, Tokyo, Japan) with a ultraviolet detector. The separation and quantitation of ginsenoside Re were achieved using an HC-C18 column (150 mm × 2.8 mm, 3 μm; Agilent Corporation, Santa Clara, CA) with a mobile phase flow rate of 1.0 ml/min and a wavelength of 203 nm at 30 °C. The mobile phase consisted of water containing 0.04% (v/v) phosphoric acid and acetonitrile. The injection volume was 20 μl and the determination of ginsenoside Re was completed in 15 min and the peak time of ginsenoside Re was in 7.8 min. Excellent linearity was achieved with correlation coefficients greater than 0.999 for ginsenoside Re within the range of 0.5–100 μg/ml.
2.5. Assay and determination of entrapment efficiency
To access the concentration of ginsenoside Re encapsulated into the liposomes, the activated dialysis bags which were placed onto measurement beakers were used to separate the encapsulated and free ginsenoside Re. The liposome suspensions were prepared by adding water into proliposome powder of GFS and were placed into the dialysis bag for 12 h. An aliquot of 5 ml dissolution from the beaker was withdrawn and the amount of free ginsenoside Re (A) was measured by the HPLC method. The total amount of ginsenoside Re (I) was determined by adding methanol into the GFS. The amount of encapsulated GRe was got by the total amount of ginsenoside Re minusing the amount of free ginsenoside Re. The encapsulation efficiency (%) was calculated using the following equation:
To confirm the feasibility and accuracy of the method, an aliquot of 5 ml dissolution from the dialysis bag after 12 h was taken out for the HPLC determination as the ginsenoside Re encapsulated (B). The recovery of ginsenoside Re was obtained by calculating the ratio of experiment total amount (I = A + B) with the true usage (M) added in the test.
2.6. Measurement of particle size and zeta potential of the reconstituted liposome
The proliposome powders were hydrated with distilled water and agitated manually for 5 min and then the resultant liposome dispersion was used for the determination of particle size and zeta potential using a Nano particle and ZETA potentiometer (NANO ZS90, Malvern, US).
2.7. Solid state characterization
2.7.1. Field emission-scanning electron microscope (FE-SEM)
The surface morphology of mannitol, the pure drug, and proliposome powders was investigated by Field emission scanning electron microscope (FE-SEM) (JSM-6700F, JEOL, Japan). The Samples contacted with distilled water were first added to silicon chips and dried in room temperature. Then the samples were fixed on a brass stub using double-sided adhesive tape and obtained electric conduction by coating with a thin layer of gold. SEM images were recorded at 3.0 kV accelerating voltage.
2.7.2. Differential scanning colorimetry (DSC)
The DSC method was performed to evaluate the molecular state of the drug in the proliposome formulation by the METTLER DSC STARe System. (Mettler-Toledo, Germany). GFS, EPC, mannitol, NaDC, physical mixture and proliposomes with precise weight were placed flatly in aluminum crucibles (5.4mm × 2.0mm) for Thermal analysis and heated at a temperature range of 25–300 °C under a constant nitrogen gas flow of 150 ml /min with a heating rate of 10 °C per minute. A second blank aluminum crucible was sealed as the reference control.
2.7.3. Fourier transform infrared (FT-IR) spectroscopy
Infrared spectra were recorded by a FT-IR spectrophotometer (IR Prestige-21, SHIMADZU, Japan) for the analysis of an interaction effect between the GFS and mannitol. The transparent tablets of samples including GFS, EPC, NaDC, mannitol, physical mixture and the P-GFS were obtained by a conventional KBr pellet method. The KBr tablet spectrum was first collected under identical conditions as the background. Each spectrum was derived from 10 single averaged scans in the region of 400–4000 cm−1 at a spectral resolution of 4 cm−1.
2.8. In vitro release study
In vitro release study of P-GFS was performed using a dialysis bag (MW cut off 8000–12000). The dispersions of GFS and P-GFS containing equal amounts of GFS were placed into the dialysis bags immersing in 500 ml medium (pH = 1.2 HCl, pH = 6.8 phosphate buffer saline). The medium was stirred continuously at 300 rpm and kept at the temperature of 37 ± 0.5 °C. At different time intervals, an aliquot volume (1 ml) of dissolution was withdrawn and the same volume of fresh release medium was added into the measurement beakers to maintain constant volume. The samples were filtrated using a 0.45 μm cellulose nitrate membrane and then injected to the HPLC for analysis. The wavelength was fixed at 203 nm.
2.9. Oral bioavailability study
2.9.1. Animals
Male Wistar rats (n = 18, 260 ± 20 g) were purchased from the Laboratory Animal Center of Jilin University (Changchun, China). All the rats were housed at a temperature of 25 ± 2 °C, 12 h light/dark cycle with free access to water and food for 1 week before the experiment. The experimental protocol was approved by the Laboratory Animal Center of Jilin University (license No. SCXK-(JI) 2011-0003). Before drug administration, the rats were fasted over 12 h and had free access to water.
2.9.2. Pharmacokinetic study
The commercially available drug of GFS called Zhengyuan tablets was used to be the positive control compared with crude GFS and P-GFS. Eighteen rats were randomly divided into 3 groups. 3 ml of the distilled water was added into proliposomes, GFS and Zhenyuan tablets crushed and the suspensions were stirred for 5 min using a vortex. One group was given oral administration of GFS at a dose of 300 mg/kg and other groups were given the same way at a dose equivalent to 300 mg/kg of GFS. Approximately 0.5 ml blood samples obtained from the orbit were collected into heparinized centrifuge tubes at 0.083, 0.167, 0.333, 0.5, 1, 2, 4, 6, 8, 10, 12 and 24 h post-dose, and were immediately centrifuged at 10,000 rpm for 10 min. Plasma samples were withdrawn and stored at −20 °C until UPLC-MS/MS analysis.
2.9.3. Assay method
2.9.3.1. UPLC-MS/MS analysis of ginsenoside Re in plasma
A Waters Acquity UPLC system (Waters, Milford, MA, USA) coupled to a Waters Xevo TQ-S triple quadruple mass spectrometry with an electrospray ion source and Mass Lynx software version 4.1 (Waters, Milford, MA, USA) which was called Ultra performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) was used to quantify ginsenoside Re in rat plasma samples within 5 min. Separation of prepared samples was achieved using a Waters Acquity UPLC BEH C18 column (2.1 mm × 50 mm, 1.7 μ m) with a 0.2-mm filter (Waters, Milford, MA, USA) maintaining at 40 °C. The mobile phase consisted of acetonitrile (A) and water (B) at 0.4 ml/min. The gradient program is described as follows: 0–2 min, 5–70% A; 2–2.5 min, 70–5% A; 2.5–4.5 min, 5% A. The mass spectrometry was operated in the positive electrospray ionization (ESI+) mode. Salidroside was chosen as the internal standard. The ginsenoside Re and Salidroside were quantified independently at a m/z 945.5 → 637.5 (parent 945.5 and daughters 637.5) for ginsenoside Re and a m/z 299.1 → 119.0 (parent 299.1 and daughters 119.0) for salidroside. The voltages of the capillary and source were 2.8 kV and 60 V. The temperature of the source and desolvation were 150 °C and 500 °C, respectively. The gas flow of the cone and desolvation were 150 L/h and 1000 L/h, respectively. The cone voltages for ginsenoside Re and Salidroside were 50 V and 32 V respectively. The collision energy for ginsenoside Re and Salidroside were 38 eV and 13 eV respectively.
2.9.3.2. Sample preparation
100ul rat plasma samples were spiked with 100ul internal standard solutions (25 ng/ml Salidroside) and 100 μl of 4 mmol hydrochloric acid and then vortexed for 10 s. The hydrolyzation by adding hydrochloric acid lasted for 30 min at 80 °C in a water bath. 700 μl volume of ethyl acetate and n-butyl alcohol mixtures (1:1) was added into the mixture samples. After vortexing for 1 min and centrifugation at 12,000 rpm for 10 min, the supernatant organic solution was dried at 37 °C under a low speed of nitrogen stream. The residues were reconstituted by adding 100 μl of acetonitrile and 1 μl of the prepared samples were injected into the UPLC-MS/MS system for analysis.
2.10. Data analysis
For the pharmacokinetic study, the pharmacokinetic parameters of the maximum plasma concentration (Cmax), the time of the maximum concentrations (Tmax), the elimination half-time (T1/2), the area under concentration–time curve (AUC0−∞), and the elimination rate (Cl_F_obs) for GFS, Zhenyuan tablets and P-GFS were calculated through non-compartmental model analysis using the Drug and Statistics 3.0 program (DAS, life science college of Jilin university, Changchun, China). All the data were expressed as mean ± standard deviation (S.D.). The identification of significances between different groups was carried out with t-test. A p value < 0.05 was considered statistically significant.
2.11. Stability studies
The formulations stored at room temperature and in refrigerator (4 ± 2 °C) for a period of 90 days were formulated to study the stability of P-GFS. At definite time intervals (0, 10, 30, 60, and 90 days) samples were withdrawn and hydrated with distilled water to evaluate vesicle size and encapsulation efficiency and physical appearance of the P-GFS.
3. Results and discussion
3.1. Preparation of GFS proliposomes
The P-GFS were prepared successfully as described previously. Spray drying technology was chosen for preparation of P-GFS considering the further application to the pharmaceutical industry. The preparation conditions were optimized firstly using the single-factor tests. Anhydrous ethanol was chosen as the solvent to dissolve EPC and NaDC for its low toxicity, high dissolubility and the most important character that it’s easy to clear away. A previous study reported that ethanol could make the lipid distribution more uniform and efficient (Xu et al., 2009a). As the process of spray drying tends to destroy the membrane function of the phospholipid bilayer and causes a pronounced decrease in the encapsulation efficiency. It has been reported that mannitol, sorbitol and lactose are commonly used as drug carriers (Janga et al., 2012, Patil-Gadhe and Pokharkar, 2014, Xu et al., 2009b). The P-GFS had a viscous appearance and poor fluidity when sorbitol and lactose were used as the carriers in the preliminary experiment. Mannitol was widely used as dry powder carriers and it can overcome the drawbacks of sorbitol and lactose and ensure the quality of liposomes during production process and storage due to its homogeneous surface properties (Littringer et al., 2012). Mannitol carrier particles offer similar adhesion conditions to active particles attached to the surface. So mannitol was chosen as the carrier for its properties of high water dissolution and fluidity in preparing proliposomes powder.
Before the addition of carriers and spray drying, the formation of a liposome suspension was completed first. Instead of the classical cholesterol, sodium deoxycholate (NaDC) was selected as the bile salt surfactant to improve stability, permeability and bioavailability for the liposome in the gastrointestinal tract (Gangadhar et al., 2014). Incorporation of bile salt in liposomal formulation could stabilize the membrane against the detrimental effects of bile acids in the gastrointestinal tract and have better permeability characteristics for liposomes (Niu et al., 2014).
The injected temperature was fixed at 55 °C, higher than the phase transition temperature of EPC because the higher temperature could facilitate higher solubility and dispersibility of GFS in ethanol/water so as to form GFS more homogeneously.
3.2. Optimization of proliposomes and statistical analysis
There were 15 experimental runs for optimizing the three individual parameters in the Box-Behnken design (BBD) and the composition and entrapment efficiency (EE) of GRe according to the factorial design is shown in Table 2. The results showed that the EE ranged from 25.2% to 63.6%. The maximum EE value was found in conditions of X1 = 1.92123 mg/ml, X2 = 12:5 and X3 = 23:100. The values of regression coefficients were calculated, the response variable and the test variables were related by the following second-order polynomial equation:
Table 2.
Response surface central composite design and experimental encapsulation efficiency EE (%).
| No. | Levels of independent factors |
Response EE (%) | ||
|---|---|---|---|---|
| X1 | X2 | X3 | ||
| 1 | 1 | 3:2 | 1:4 | 42.4 |
| 2 | 1 | 7:2 | 1:4 | 40.1 |
| 3 | 3 | 3:2 | 1:4 | 35.2 |
| 4 | 3 | 7:2 | 1:4 | 30.0 |
| 5 | 2 | 3:2 | 1:8 | 42.2 |
| 6 | 2 | 3:2 | 1:2 | 46.7 |
| 7 | 2 | 7:2 | 1:8 | 44.2 |
| 8 | 2 | 7:2 | 1:2 | 25.2 |
| 9 | 1 | 5:2 | 1:8 | 40.0 |
| 10 | 3 | 5:2 | 1:8 | 51.0 |
| 11 | 1 | 5:2 | 1:2 | 46.0 |
| 12 | 3 | 5:2 | 1:2 | 27.6 |
| 13 | 2 | 5:2 | 1:4 | 62.4 |
| 14 | 2 | 5:2 | 1:4 | 63.6 |
| 15 | 2 | 5:2 | 1:4 | 61.2 |
The statistical significance of the regression model was checked by F-test and p value, and the analysis of variance (ANOVA) for the response surface quadratic model is shown in Table 3, in which the small p value for the model implied the model was statistically significant. The p value for the ‘lack of fit’ test was 0.12368, indicating the quadratic model was adequate. In Table 3, the interaction coefficients (X1X3, X2X3) were found to be significant (p < 0.01). The determination coefficient (R2 = 0.9830) revealed that the experimental results adequately fitted the equation selected (Ashraf et al., 2013a).
Table 3.
Estimated regression model of relationship between response variables (EE) and independent variables (X1, X2, X3).
| Source | df | Sum of squares | Mean square | F value | P-value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 9 | 1977.235 | 219.6928 | 32.13527 | 0.000672 | Significant |
| X1 | 1 | 76.26125 | 76.26125 | 11.15501 | 0.020554 | |
| X2 | 1 | 91.125 | 91.125 | 13.32919 | 0.014734 | |
| X3 | 1 | 127.2013 | 127.2013 | 18.60619 | 0.007617 | |
| X1X2 | 1 | 527.2708 | 527.2708 | 77.12583 | 0.000318 | |
| X1X3 | 1 | 2.1025 | 2.1025 | 0.30754 | 0.603084 | |
| X2X3 | 1 | 216.09 | 86.40 | 31.60828 | 0.002465 | |
| X12 | 1 | 675.4177 | 675.4177 | 98.79583 | 0.000176 | |
| X22 | 1 | 138.0625 | 138.0625 | 20.19491 | 0.006436 | |
| X32 | 1 | 319.3477 | 319.3477 | 46.71216 | 0.001023 | |
| Residual | 5 | 34.1825 | 6.8365 | |||
| Lack of fit | 3 | 31.3025 | 10.43417 | 7.245949 | 0.12368 | Not significant |
| Pure error | 2 | 2.88 | 1.44 | |||
| Cor. total | 14 | 2011.417 |
R2 = 0.9830, R2Adj = 0.9524, R2Pred = 0.9524.
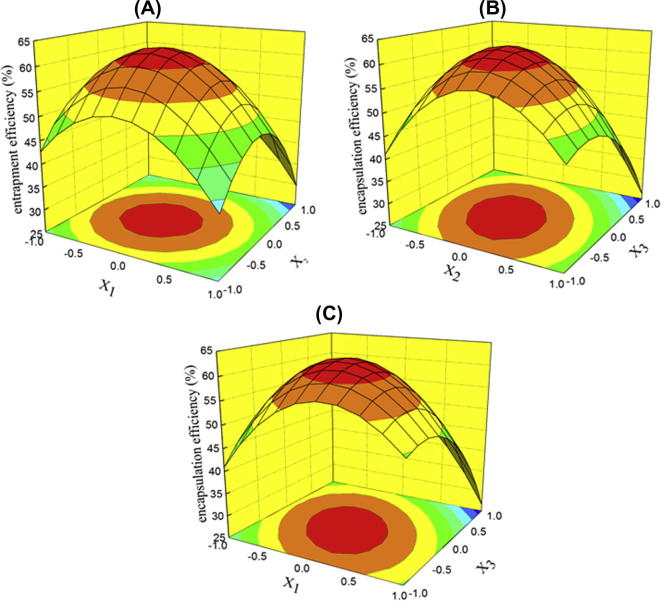
To better understand the predictive models of the results, three-dimensional graphs of the models, the response surface diagrams of the EE of GFS proliposomes are shown in Fig. 1. With an increase in the concentration of EPC, the ratio of mannitol to EPC and NaDC to EPC, the EE of GFS proliposomes ascended at first and descended later. It may be confirmed in Fig. 1A –C that the contribution of mannitol to the EE was less than to EPC and NaDC. The amount of NaDC also had an effect on the EE. From Fig. 1B and C, it may be seen that if the amount of NaDC was too high, the EE decreased. A reason for this result may be that the further increased amount of bile salt into liposomal lipid bilayers destroyed the vesicular structure (Andrieux et al., 2009).
Figure 1.
Response surface plot showing the effect of (A) concentration of EPC, (B) ratio of mannitol to EPC, and (C) ratio of NaDC to EPC.
The suitability of the model equation for predicting the optimum response values was tested using the recommended optimum conditions. The set of optimum composition, determined using the RSM optimization approach, was tested experimentally according to the model equation (GFS and EPC 2 mg/ml, mannitol: EPC = 12:5, NaDC: EPC = 23:100). Three batches of proliposomes were prepared according to the optimized composition and EEs of each batch were determined. The mean experimental EE was 62.37 ± 0.67 % (n = 3).
3.3. Measurement of particle size and zeta potential of the reconstituted liposomes
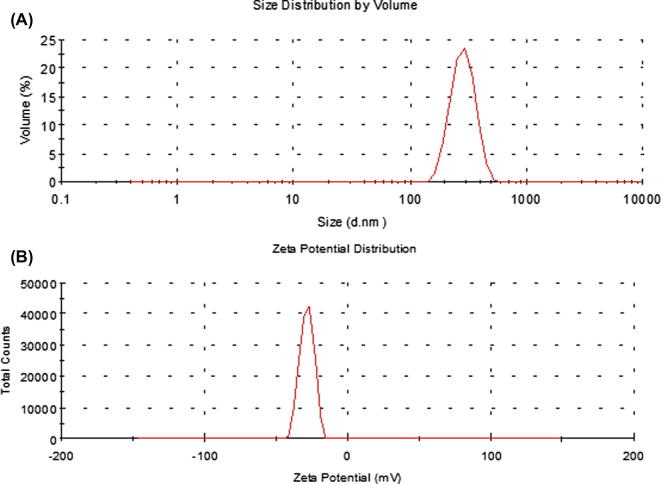
The particle size of the reconstituted liposomes showed a size range of 275.4 ± 10.74 nm (Fig. 2A) for the spherical particles which was further confirmed by FE-SEM (Fig. 3C). The zeta potential value was −28.6 ± 4.77 mV (Fig. 2B).
Figure 2.
Size distribution (A) and (B) zeta potential distribution of the reconstituted liposomes.
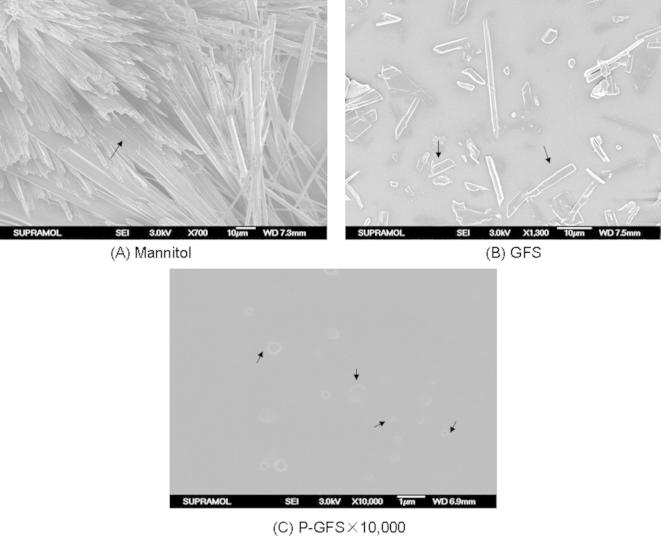
Figure 3.
Field emission-scanning electron microscope (FE-SEM) images of (A) mannitol, (B) GFS, (C) the reconstituted liposomes of P-GFS at ×10,000 magnification.
3.4. Solid state characterization
3.4.1. Field emission-scanning electron microscope (FE-SEM)
The surface morphology of mannitol, GFS and reconstituted liposomes were investigated by FE-SEM, and the images are represented in Fig. 3. The reconstituted solution prepared by adding distilled water into the powder of mannitol, GFS and P-GFS were all dried naturally on the silicon wafer. The typical crystalline structure of mannitol at ×700 magnification was obviously different from the surface of GFS and reconstituted liposomes. The hollow fiber structure (as the bold arrow marked) of mannitol powders (Fig. 3A) observed in the scanning electron microscope indicated that the mannitol was a better carrier and had no effect to the formation of reconstituted liposomes compared with Fig. 3B and C. GFS were encapsulated into the spherical lipid particles with a characteristic rough surface as the bold arrow marked in Fig. 3C compared with Fig. 3B. The images of FE-SEM showed that the GFS liposome could be successfully prepared by the proliposome contacted with water.
3.4.2. Differential scanning colorimetry (DSC)
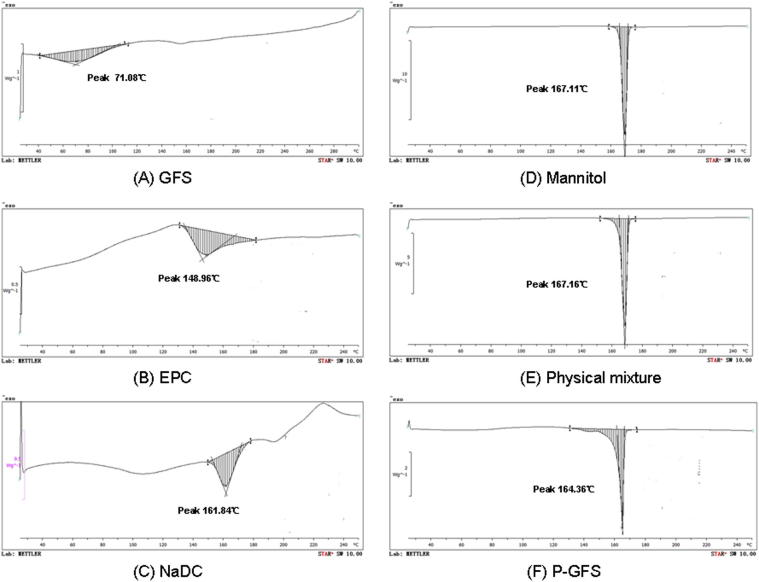
The molecular state of GFS in proliposome formulation was evaluated by performing the DSC analysis of GFS, EPC, NaDC, mannitol, physical mixture of GFS, EPC, NaDC, and mannitol, and proliposomes in Fig. 4. As is shown in Fig. 4A, GFS had an endothermal peak with a wide range because it is a mixture of compounds. It had an obvious peak value at 71.08 °C so the character DSC value of GFS was set at 71.08 °C. The DSC thermogram of EPC exhibited a peak value at 148.96 °C (Fig. 4B) .The formation of this peak might be due to the phase transition from gel-like state to liquid–crystal state (Singh et al., 2014). The DSC thermogram of mannitol showed an endothermal peak at 167.11 °C and the NaDC showed an outstanding endothermal peak at 161.84 °C respectively within the temperature range from 25 °C to 250 °C. The physical mixture showed an endothermal peak at 167.16 °C. Compared with the mannitol (the most important major component) peak (Fig. 4D), nearly no obvious change was found in the physical mixture (Fig. 4E). The endothermal peaks for the other ingredients were so small compared with mannitol that the thermogram only showed the peak of mannitol. What’s more, mannitol took the maximum weight of the physical mixture. Different from the physical mixture, the DSC thermogram of P-GFS (Fig. 4F) had a endothermal peak at 164.36 °C, a big peak move compared with the mannitol peak. These results possibly indicated that components in proliposomes had some interaction during proliposome formation resulting in mannitol peak migration, as previous researchers have suggested (Tan et al., 2012). It also could be speculated that there was no interaction among the excipients in the physical mixture (Ashraf et al., 2013b).
Figure 4.
DSC thermograms of (A) GFS, (B) EPC, (C) NaDC, (D) mannitol, (E) physical mixture and (F) P-GFS.
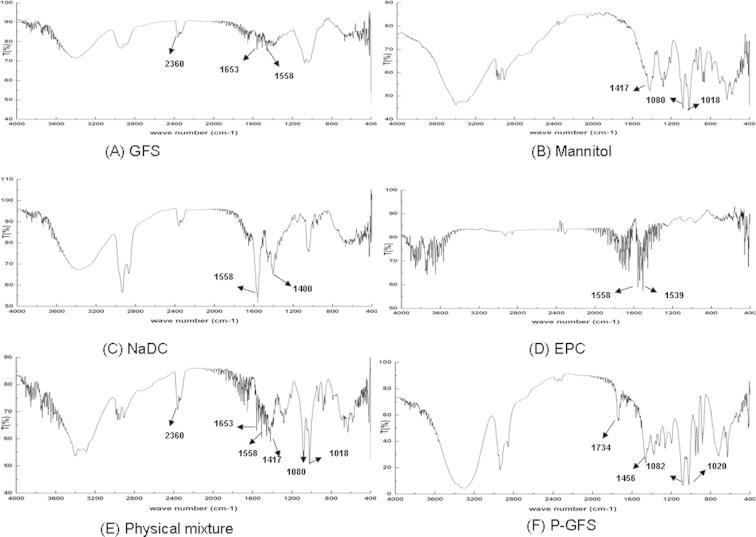
3.4.3. Fourier transform infrared (FT-IR) spectroscopy
The FT-IR spectra of GFS, mannitol, NaDC, EPC, physical mixture and P-GFS are shown in Fig. 5. The characteristic absorption peaks of GFS, mannitol, NaDC, EPC, physical mixture and proliposomes were as follows: 2360 cm−1, 1653 and 1558 cm−1 for GFS (Fig. 5A); 1417 cm−1, 1080 cm−1and 1018 cm−1 for mannitol (Fig. 5B); 1558 cm−1 and 1400 cm−1for NaDC (Fig. 5C); 1558 cm−1 and 1539 cm−1for EPC (Fig. 5D). The characteristic absorption peaks of all the ingredients could be seen in the physical mixture (Fig. 5E) indicating no molecular interaction effect among the ingredients. However, in the FT-IR spectra of proliposomes (Fig. 5F), the characteristic absorption peaks seen in the other samples were absent or shielded and new peaks at 1734 cm−1 and 1456 cm−1 were seen in the FT-IR spectra compared with physical mixture. The results indicated that some weak molecular interactions among proliposome ingredients had occurred in the preparation process of proliposomes. In the spectra of proliposomes, the characteristic absorption peaks of mannitol were still existing because the amount of mannitol was higher than the other ingredients in proliposomes.
Figure 5.
FT-IR spectra of (A) GFS, (B) mannitol, (C) NaDC, (D) EPC, (E) phyiscal mixture and (F) P-GFS.
3.5. In vitro release study
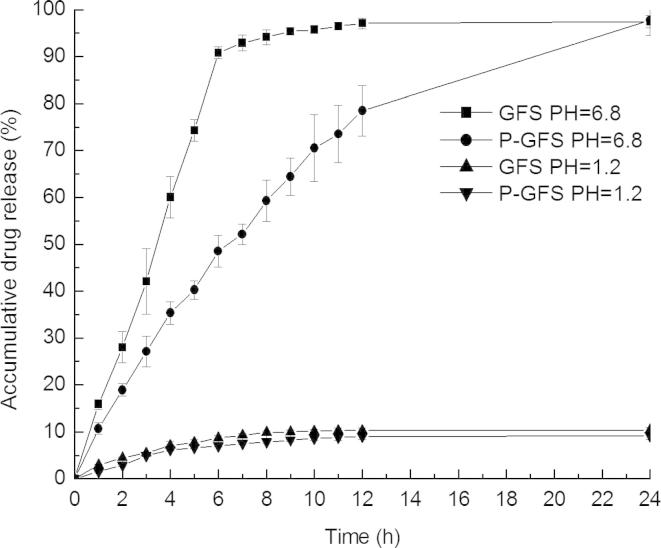
In vitro release profiles of GRe from the reconstituted liposomes solution of P-GFS and GFS solution in artificial gastric fluid (pH 1.2) and artificial intestinal fluid (pH 6.8) are shown in Fig. 6. Compared with control drug solution, GRe in the liposome solution had a sustained release at pH 6.8 while GRe in reconstituted liposomes and GFS solution had a low release at pH 1.2. In 12 h, only 10% of the GRe for GFS and P-GFS in pH = 1.2 was released compared with nearly 95% of G-Re for GFS and 70% for P-GFS in pH = 6.8. The results revealed that the pH and release medium had an effect on the release of GRe from liposomes. It could be speculated that the GRe was mainly absorbed in the intestinal tract. The formation of micelle of the GFS and reconstituted liposomes observed in pH = 1.2 may be contribution to the lower release of GRe (Khaskheli et al., 2015).
Figure 6.
In vitro release profiles of the reconstituted liposomes (P-GFS) and control drug solution (GFS) in artificial gastric fluid (pH 1.2) and intestinal fluid (pH 6.8) (mean ± SD, n = 3).
3.6. Pharmacokinetic study
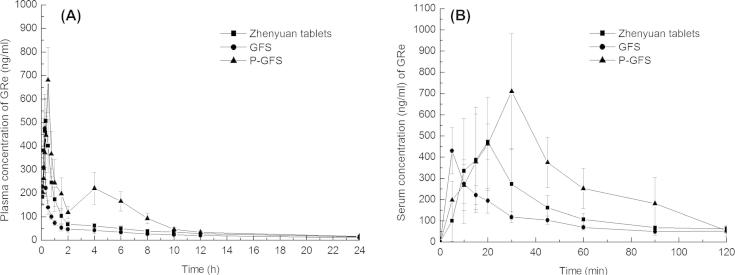
Pharmacokinetic study was carried out to compare the oral bioavailability of GFS, Zhenyuan tablets and proliposomes (P-GFS) by measuring the concentrations of GRe in rat plasma after oral administration. The mean plasma concentration–time profiles of GFS, Zhenyuan tablets and P-GFS are displayed in Fig. 7A and B. The main pharmacokinetic parameters of GFS, Zhenyuan tablets and proliposomes (P-GFS) are shown in Table 4. It was founded that the mean plasma concentration versus time profile of GFS, the commercial dosage form of GFS (Zhenyuan tablets) and P-GFS suspensions were much more different. Pharmacokinetic behaviors of P-GFS showed a second absorption peak on the concentration versus time profile and had a high maximum concentration and residence time. The pharmacokinetic parameters of GFS, Zhenyuan tablets and P-GFS in rats were respectively listed as follows: Tmax 0.25 h, Cmax 474.96 ± 66.06 ng/ml and AUC0−∞ 733.32 ± 113.82 ng/ml h for GFS; Tmax 0.31 ± 0.043 h, Cmax 533.94 ± 106.54 ng/ml and AUC0−∞ 1151.38 ± 198.29 ng/ml h for Zhenyuan tablets; Tmax 0.5 h, Cmax 680.62 ± 138.051 ng/ml and AUC0−∞ 2082.49 ± 408.33 ng/ml h for the P-GFS. The bioavailability of P-GFS was nearly 284% and 181% of the GFS and Zhengyuan tablets respectively.
Figure 7.
A and B represents separately mean GRe concentration–time profiles for 24 h and 2 h in rat plasma after oral administration of GFS, Zhenyuan tablets and P-GFS respectively (mean ± SD, n = 6).
Table 4.
Pharmacokinetics parameters of G-Re for GFS, Zhenyuan tablets and proliposomes (P-GFS) formulations in rats respectively (mean ± SD, n = 6).
| Formulations | Cmax (ng/ml) | Tmax (h) | T1/2 (h) | AUC0–∞ (ng/ml/h) | Cl_F_obs (ml/h) |
|---|---|---|---|---|---|
| GFS | 474.96 ± 66.06 | 0.25 ± 0.00 | 7.72 ± 1.61 | 733.32 ± 113.82 | 2667.53 ± 395.50 |
| Zhenyuan tablets | 533.94 ± 106.54 | 0.31 ± 0.043 | 10.15 ± 0.95 | 1151.38 ± 198.29 | 1760.44 ± 292.02 |
| P-GFS | 680.62 ± 138.051# | 0.5 ± 0.00∗ | 13.03 ± 1.30# | 2082.49 ± 408.33$ | 1043.87 ± 186.27$ |
∗,#,$ Indicates significant difference at p < 0.05, p < 0.01, and p < 0.001, respectively.
The values of Cmax, Tmax, T1/2, AUC0–∞ and Cl_F_obs (ml/h) for GRe in proliposomes showed obvious differences (p < 0.05). The higher values of Cmax and AUC0–∞ of GRe in proliposomes compared with control and Zhenyuan tablets obviously suggested that liposomes containing bile salt formulation could improve the bioavailability of GRe. The possible reasons for this are that liposomes containing sodium deoxycholate by facilitating transition from liposomes to mixed micelles could increase the stability and the permeability of GRe in the intestinal tract and protect the GRe degradation from enzyme as the previous study had showed (Porter et al., 2008, Senior, 2001, Song et al., 2005). In addition, the endocytosis by epithelial cells and the intestinal lymphatic transport reduce the first pass metabolism (Akbarzadeh et al., 2013, Hu et al., 2013, Ju et al., 2013, Porter et al., 2008). The double peaks’ curve of proliposome is also found in Fig. 7A and B. The encapsulation efficiency of GRe was about 63%, so the free GRe probably mainly contributed to the first peak. The second peak of the proliposome might also be caused by the absorption of GRe encapsulated in liposomes by the intestinal tract or the hepato-enteric circulation of GRe and reabsorbed in the intestinal tract (Khan et al., 2015).
3.7. Stability studies
The stability of the proliposome formulations was ascertained by monitoring the physical appearance, particle size and encapsulation efficiency after storage at room temperature (37 °C) and refrigerated temperature (4 °C) for a period of 90 days. At defined time intervals, the proliposomes were reconstituted to form liposome dispersion. As shown in Table 5 and Table 6, no dramatic change was observed in the physical appearance, particle size and encapsulation efficiency indicating the similar stability of the proliposome formulation stored at room temperature compared with the refrigerated temperature for a period of 3 months (Nasreen et al., 2015).
Table 5.
The stability of P-GFS in refrigerated temperature (4 ± 2 °C).
| Time (day) | 0 | 10 | 30 | 60 | 90 |
|---|---|---|---|---|---|
| Color | Light yellow | Light yellow | Light yellow | Light yellow | Light yellow |
| GRe assaying(mg/ml) ( ± s, n = 6) | 1.9745 ± 0.02 | 1.9439 ± 0.25 | 1.9722 ± 0.07 | 1.9581 ± 0.16 | 1.9468 ± 0.34 |
| Particle size analysis (nm) ( ± s, n = 6) | 278.1 ± 3.59 | 276.9 ± 5.23 | 273.9 ± 4.14 | 275.0 ± 1.09 | 276.6 ± 3.09 |
| Encapsulation efficiency (%) ( ± s, n = 6) | 71 ± 2.54 | 69 ± 3.16 | 68 ± 4.58 | 68 ± 5.49 | 66 ± 3.91 |
Table 6.
The stability of P-GFS in high temperature (37 ± 5 °C).
| Time(day) | 0 | 10 | 30 | 60 | 90 |
|---|---|---|---|---|---|
| Color | Light yellow | Light yellow | Light yellow | Light yellow | Light yellow |
| GRe assaying(mg/ml) ( ± s, n = 6) | 1.9627 ± 0.12 | 1.9702 ± 0.09 | 1.9488 ± 0.34 | 1.9576 ± 0.41 | 1.9630 ± 0.09 |
| Particle size analysis(nm) ( ± s, n = 6) | 279.6 ± 4.52 | 277.9 ± 3.98 | 275.3 ± 4.07 | 276.0 ± 2.43 | 277.1 ± 5.69 |
| Encapsulation efficiency (%) ( ± s, n = 6) | 69 ± 3.16 | 67 ± 4.56 | 66 ± 2.37 | 65 ± 1.68 | 65 ± 4.01 |
4. Conclusion
In this study, a drug delivery system of proliposome was prepared with optimum conditions for oral administration by the spray drying method. To obtain the highest entrapment efficiency, the best composition was set as follows: concentration of GFS and EPC was 2 mg/ml; ratio of mannitol to EPC was 12:5; ratio of NaDC to EPC was 23:100. The properties of reconstituted liposomes including entrapment efficiency, particle size and zeta potential were characterized and optimized. The results of FE-SEM visualization the formation and surface morphology of reconstituted liposomes and. the molecular state of proliposomes, and some weak interaction between the formulation ingredients, such as hydrogen bonds or van der Waals forces were discussed according to the diagrams of DSC, and FT-IR. The in vitro drug release study indicated that P-GFS has a controlled drug release profile. A further pharmacokinetic study suggested that proliposomes increased the oral availability of GFS. The controlled release and protection from the enzyme degradation of GRe and sodium deoxycholate may significantly decrease elimination of GFS and enhance absorption in the gastrointestinal tract. Moreover, the P-GFS had a good stability in the room temperature. In summary, this study offers a new formulation proliposome, to improve the stability of oral administration drugs that are subjected to degradation by the gastrointestinal enzyme which led a short half-life in vivo. Proliposome could be a potential formulation used for clinical application. However, further studies are needed to study the mechanism of the improved bioavailability and two peaks of the P-GFS in the plasma concentration–time profiles.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ajazuddin Saraf S. Applications of novel drug delivery system for herbal formulations. Fitoterapia. 2010;81:680–689. doi: 10.1016/j.fitote.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Akbarzadeh A., Rezaei-Sadabady R., Davaran S., Joo S.W., Zarghami N., Hanifehpour Y., Samiei M., Kouhi M., Nejati-Koshki K. Liposome: classification, preparation, and applications. Nanoscale Res. Lett. 2013;8 doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrieux K., Forte L., Lesieur S., Paternostre M., Ollivon M., Grabielle-Madelmont C. Solubilisation of dipalmitoylphosphatidylcholine bilayers by sodium taurocholate: a model to study the stability of liposomes in the gastrointestinal tract and their mechanism of interaction with a model bile salt. Eur. J. Pharm. Biopharm. 2009;71:346–355. doi: 10.1016/j.ejpb.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Ashraf M.A., Ullah S., Ahmad I., Qureshi A.K., Balkhair K.S., Rehman M.A. Green biocides, a promising technology: current and future applications. J. Sci. Food Agric. 2013;94(3):388–403. doi: 10.1002/jsfa.6371. [DOI] [PubMed] [Google Scholar]
- Ashraf M.A., Qureshi A.K., Gharibreza M.R., Rehman M.A., Ahmad I., Yusoff I. Intercationic effect on biosorbent efficacy. Desalin. Water Treat. 2013;52(7–9):1504–1513. [Google Scholar]
- Attele A.S., Zhou Y.P., Xie J.T., Wu J.A., Zhang L., Dey L., Pugh W., Rue P.A., Polonsky K.S., Yuan C.S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–1858. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- Bai C., Peng H., Xiong H., Liu Y., Zhao L., Xiao X. Carboxymethylchitosan-coated proliposomes containing coix seed oil: characterisation, stability and in vitro release evaluation. Food Chem. 2011;129:1695–1702. [Google Scholar]
- Chen M.L. Lipid excipients and delivery systems for pharmaceutical development: a regulatory perspective. Adv. Drug Deliv. Rev. 2008;60:768–777. doi: 10.1016/j.addr.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Chen C.M., Alli D. Use of fluidized bed in proliposome manufacturing. J. Pharm. Sci. 1987;76:419. doi: 10.1002/jps.2600760517. [DOI] [PubMed] [Google Scholar]
- Conacher M., Alexander J., Brewer J.M. Oral immunisation with peptide and protein antigens by formulation in lipid vesicles incorporating bile salts (bilosomes) Vaccine. 2001;19:2965–2974. doi: 10.1016/s0264-410x(00)00537-5. [DOI] [PubMed] [Google Scholar]
- Gangadhar K.N., Adhikari K., Srichana T. Synthesis and evaluation of sodium deoxycholate sulfate as a lipid drug carrier to enhance the solubility, stability and safety of an amphotericin B inhalation formulation. Int. J. Pharm. 2014;471:430–438. doi: 10.1016/j.ijpharm.2014.05.066. [DOI] [PubMed] [Google Scholar]
- Hu S., Niu M., Hu F., Lu Y., Qi J., Yin Z., Wu W. Integrity and stability of oral liposomes containing bile salts studied in simulated and ex vivo gastrointestinal media. Int. J. Pharm. 2013;441:693–700. doi: 10.1016/j.ijpharm.2012.10.025. [DOI] [PubMed] [Google Scholar]
- Huo Y.S. Anti-senility action of saponin in Panax ginseng fruit in 327 cases. Zhong xi yi jie he za zhi Chin. J. Modern Dev. Tradition. Med. Zhongguo Zhong xi yi jie he yan jiu hui (chou) Zhong yi yan jiu yuan, zhu ban. 1984;4:578–593. [PubMed] [Google Scholar]
- Janga K.Y., Jukanti R., Velpula A., Sunkavalli S., Bandari S., Kandadi P., Veerareddy P.R. Bioavailability enhancement of zaleplon via proliposomes: Role of surface charge. Eur. J. Pharma. Biopharma. Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2012;80:347–357. doi: 10.1016/j.ejpb.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Joo K.M., Lee J.H., Jeon H.Y., Park C.W., Hong D.K., Jeong H.J., Lee S.J., Lee S.Y., Lim K.M. Pharmacokinetic study of ginsenoside Re with pure ginsenoside Re and ginseng berry extracts in mouse using ultra performance liquid chromatography/mass spectrometric method. J. Pharm. Biomed. Anal. 2010;51:278–283. doi: 10.1016/j.jpba.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Ju J., He G., Duan Z., Zhao W., Liu Y., Zhang L., Li Y. Improvement of bilirubin adsorption capacity of cellulose acetate/polyethyleneimine membrane using sodium deoxycholate. Biochem. Eng. J. 2013;79:144–152. [Google Scholar]
- Keon-Hyoung Song S.-J.C., Shim Chang.-Koo. Preparation and evaluation of proliposomes containing salmon calcitonin. J. Control. Release. 2002 doi: 10.1016/s0168-3659(02)00238-9. [DOI] [PubMed] [Google Scholar]
- Khan A.M., Ahmad C.S., Farooq U., Mahmood K., Sarfraz M., Balkhair K.S., Ashraf M.A. Removal of metallic elements from industrial waste water through biomass and clay. Front. Life Sci. 2015;2015:1–8. < http://www.tandfonline.com/doi/full/10.1080/21553769.2015.1041187>. [Google Scholar]
- Khaskheli A.A., Talpur F.N., Ashraf M.A., Cebeci A., Jawaid S., Afridi H.I. Monitoring the Rhizopus oryzae lipase catalyzed hydrolysis of castor oil by ATR-FTIR spectroscopy. J Mol Catal B: Enzym. 2015;113:56–61. [Google Scholar]
- Kim U., Park M.H., Kim D.H., Yoo H.H. Metabolite profiling of ginsenoside Re in rat urine and faeces after oral administration. Food Chem. 2013;136:1364–1369. doi: 10.1016/j.foodchem.2012.09.050. [DOI] [PubMed] [Google Scholar]
- Larsen A.T., Sassene P., Mullertz A. In vitro lipolysis models as a tool for the characterization of oral lipid and surfactant based drug delivery systems. Int. J. Pharm. 2011;417:245–255. doi: 10.1016/j.ijpharm.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Lee S., Kim M.G., Ko S.K., Kim H.K., Leem K.H., Kim Y.J. Protective effect of ginsenoside Re on acute gastric mucosal lesion induced by compound 48/80. J. Ginseng Res. 2014;38:89–96. doi: 10.1016/j.jgr.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wang X., Zhang T., Wang C., Huang Z., Luo X., Deng Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015;10:81–98. [Google Scholar]
- Littringer E.M., Mescher A., Eckhard S., Schroettner H., Langes C., Fries M., Griesser U., Walzel P., Urbanetz N.A. Spray drying of mannitol as a drug carrier-the impact of process parameters on product properties. Drying Technol. 2012;30:114–124. [Google Scholar]
- Nasreen S., Rafique U., Ehrman S., Ashraf M.A. Hybrid mesoporous silicates: a distinct aspect to synthesis and application for decontamination of phenols. Saudi. J. Bio. Sci. 2015 doi: 10.1016/j.sjbs.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu M., Tan Y., Guan P., Hovgaard L., Lu Y., Qi J., Lian R., Li X., Wu W. Enhanced oral absorption of insulin-loaded liposomes containing bile salts: a mechanistic study. Int. J. Pharm. 2014;460:119–130. doi: 10.1016/j.ijpharm.2013.11.028. [DOI] [PubMed] [Google Scholar]
- Park E.-Y., Kim H.-J., Kim Y.-K., Park S.-U., Choi J.-E., Cha J.-Y., Jun H.-S. Increase in insulin secretion induced by panax ginseng berry extracts contributes to the amelioration of hyperglycemia in streptozotocin-induced diabetic mice. J. Ginseng Res. 2012;36:153–160. doi: 10.5142/jgr.2012.36.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil-Gadhe A., Pokharkar V. Single step spray drying method to develop proliposomes for inhalation: a systematic study based on quality by design approach. Pulm. Pharmacol. Ther. 2014;27:197–207. doi: 10.1016/j.pupt.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Peng D., Wang H., Qu C., Xie L., Wicks S.M., Xie J. Ginsenoside Re: its chemistry, metabolism and pharmacokinetics. Chin. Med. 2012;7:2. doi: 10.1186/1749-8546-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C.J., Pouton C.W., Cuine J.F., Charman W.N. Enhancing intestinal drug solubilisation using lipid-based delivery systems. Adv. Drug Deliv. Rev. 2008;60:673–691. doi: 10.1016/j.addr.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Senior K. Bilosomes: the answer to oral vaccine delivery? Drug Discovery Today. 2001;6:1031–1032. doi: 10.1016/s1359-6446(01)02010-4. [DOI] [PubMed] [Google Scholar]
- Singh C., Bhatt T.D., Gill M.S., Suresh S. Novel rifampicin-phospholipid complex for tubercular therapy: Synthesis, physicochemical characterization and in-vivo evaluation. Int. J. Pharm. 2014;460:220–227. doi: 10.1016/j.ijpharm.2013.10.043. [DOI] [PubMed] [Google Scholar]
- Song K.H., Chung S.J., Shim C.K. Enhanced intestinal absorption of salmon calcitonin (sCT) from proliposomes containing bile salts. J. Control. Release. 2005;106:298–308. doi: 10.1016/j.jconrel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Tan Q., Liu S., Chen X., Wu M., Wang H., Yin H., He D., Xiong H., Zhang J. Design and evaluation of a novel evodiamine-phospholipid complex for improved oral bioavailability. AAPS Pharmscitech. 2012;13:534–547. doi: 10.1208/s12249-012-9772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevaskis N.L., Charman W.N., Porter C.J.H. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv. Drug Deliv. Rev. 2008;60:702–716. doi: 10.1016/j.addr.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., He L., Nie S., Guan J., Zhang X., Yang X., Pan W. Optimized preparation of vinpocetine proliposomes by a novel method and in vivo evaluation of its pharmacokinetics in New Zealand rabbits. J. Control. Release. 2009;140:61–68. doi: 10.1016/j.jconrel.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Xu H., He L., Nie S., Guan J., Zhang X., Yang X., Pan W. Optimized preparation of vinpocetine proliposomes by a novel method and in vivo evaluation of its pharmacokinetics in New Zealand rabbits. J. Control. Release. 2009;140:61–68. doi: 10.1016/j.jconrel.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Yanamandra S., Venkatesan N., Kadajji V.G., Wang Z., Issar M., Betageri G.V. Proliposomes as a drug delivery system to decrease the hepatic first-pass metabolism: case study using a model drug. Eur. J. Pharm. Sci. 2014;64:26–36. doi: 10.1016/j.ejps.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Yang H.T., Zhang J.R. Treatment of systemic lupus erythematosus with saponin of ginseng fruit (SPGF): an immunological study. Zhong xi yi jie he za zhi Chin. J. Modern Dev. Tradition. Med. Zhongguo Zhong xi yi jie he yan jiu hui (chou), Zhong yi yan jiu yuan, zhu ban. 1986;6(157–159):131–152. [PubMed] [Google Scholar]
- Yang C.-Y., Wang J., Zhao Y., Shen L., Jiang X., Xie Z.-G., Liang N., Zhang L., Chen Z.-H. Anti-diabetic effects of Panax notoginseng saponins and its major anti-hyperglycemic components. J. Ethnopharmacol. 2010;130:231–236. doi: 10.1016/j.jep.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Zhang S.C., Jiang X.L. The anti-stress effect of saponins extracted from panax ginseng fruit and the hypophyseal-adrenal system (author’s transl) Yao xue xue bao Acta Pharm. Sinica. 1981;16:860–863. [PubMed] [Google Scholar]
- Zhang S.C., Ni G.C., Hu Z.H. Therapeutic and preventive effects of saponin of ginseng fruit on experimental gastric ulcers. J. Tradition. Chin. Med. 1984;4:45–50. Chung i tsa chih ying wen pan sponsored by All-China Association of Traditional Chinese Medicine Academy of Traditional Chinese Medicine. [PubMed] [Google Scholar]