Abstract
The cytotoxic and antioxidant properties of lipophilic compounds extracted from different parts of four Chenopodium L. (Chenopodium album, Chenopodium hybridum, Chenopodium rubrum and Chenopodium urbicum) species were evaluated. The highest phenolic content was found in herb and seeds of all examined plants. Large amounts of free polyphenols were observed in herb extracts of C. album (3.36 mg/g DW), seeds of C. urbicum (3.87 mg/g DW) and roots of C. urbicum (1.52 mg/g DW). The cytotoxic activities of the extracts were assessed against human lung carcinoma A-549 and ovarian carcinoma TOV-112D and normal human fibroblast cell lines. Our study demonstrated that the extracts from the herb of C. rubrum and C. urbicum had the best antioxidant effect of all the extracts analyzed. Most of the extracts tested exhibited low cytotoxicity. However, the extracts from herb and seeds of C. album and C. hybridum showed the significant antiproliferative effect on the TOV-112 cell line.
It can be concluded that antioxidant activity and phenolic composition differ mainly between plant parts and are quite similar between the plants, when the same plant part is analyzed. Thus, the Chenopodium extracts could be used as a readily accessible source of natural antioxidants, and may be used in the pharmaceutical industry and for food supplements production.
Keywords: Chenopodium, Antioxidant activity, Cytotoxic activity, Polyphenols
1. Introduction
Chenopodium genus includes herbaceous, strongly fragrant annual plants and is widely spread worldwide, mainly in the moderate and subtropical zone (El-Sayed et al., 1989). In Poland, there are 30 species of Chenopodium. The chemical composition of Chenopodium has not been fully known. Recently, four species, i.e. Chenopodium ambrosioides L., Chenopodium album L., Chenopodium rubrum L. and Chenopodium quinoa Willd. attracted special attention. The plants belonging to Chenopodium are known to be a rich source of flavonoids (mainly kaempferol and quercetin glucosides), phenolic acids and terpenoids (Gohar and Elmazar, 1997, Repo-Carrasco-Valencia et al., 2010). Moreover, the leaves of Chenopodium are rich in carotenoids and their seeds in proteins and fats (Bhargava et al., 2009).
Many species of Chenopodium were reported to possess numerous medicinal properties used in folk medicine. Modern pharmaceutical research has also confirmed potent antipruritic, antibacterial antifungal and anticancer activities of these plants (Bhargava et al., 2009, Khoobchandani et al., 2009, Baldi and Choudhary, 2013, Gawlik-Dziki et al., 2013, Miranda et al., 2014).
These healing and usable advantages of plants from Chenopodium directed our attention to native species of this genus. The available studies of antioxidant activities in this genus are not numerous and primarily regard C. quinoa, C. album and C. ambrosioides (Alvarez-Jubete et al., 2010).
Several assays have been frequently used to estimate antioxidant capacities in plants and their medicinal and food products. Most of the antioxidant potential of plants results from the redox properties of phenolic compounds. Antioxidant effects of polyphenols are exerted through different mechanisms. They act as reducers, have an ability to scavenge free radicals and chelate metal ions – cofactors of enzymes catalyzing oxidative reactions, inhibit oxidases, terminate radical chain reactions and stabilize free radicals (Gawlik-Dziki, 2008, Rice-Evans et al., 1997). The content of phenolic compounds depends on plant species and on environmental conditions. Many medicinal herbs exhibiting good antioxidant activities have been employed as the source of natural antioxidants. The effectiveness of plant extracts and natural compounds of high antioxidant activity in prevention of many cancer types is well known but the use of antioxidant agents in adjunctive cancer therapy is still controversial because of conflicting findings.
The aim of this paper was to evaluate the antioxidant and cytotoxic properties of crude methanolic extracts from different parts of C. album, Chenopodium hybridum, C. rubrum and Chenopodium urbicum.
2. Materials and methods
2.1. Chemicals and reagents
All chemical reagents used in the experiment were purchased from various commercial suppliers and were of the highest purity available. Folin–Ciocalteu reagent, 2,2-diphenyl-1-picryl-hydrazyl (DPPH•), linoleic acid, ferrozine (3-(2-pyridyl)-5,6-bis (4-phenyl-sulfonic acid)-1,2,4-triazine) were purchased from Sigma–Aldrich (St-Louis, USA). Reference compounds of gallic acid and quercetin were purchased from ROTH. All the other chemicals were of analytical grade and purchased from Polish Reagents (POCH, Gliwice, Poland). Cytotoxic activities of the examined fractions were determined using the BrdU Labeling and Detection Kit III measuring cell proliferation (Roche Diagnostics GMbH, Mannheim Germany).
2.2. Plant material
The herb, roots and seeds of C. album, C. hybridum, C. rubrum and C. urbicum were examined. All species were collected in June (herb) and July (seeds, roots) 2009 from their natural environments in the Lublin region of eastern Poland. The identity of plants was confirmed by Prof. Tadeusz Krzaczek and voucher specimens were deposited at the Herbarium of Department of Pharmaceutical Botany, Faculty of Pharmacy, Medical University of Lublin. The herbs, roots and seeds were dried in normal conditions and adequately fragmented according to the 6th Polish Pharmacopoeia (2002).
2.3. Extraction and hydrolysis methods
To estimate total phenolics and flavonoids, the dried samples (2.5 g) were extracted under reflux with methanol. Crude methanol extracts were brought to dryness in vacuo and re-dissolved in hot distilled water. After decanting were placed in 50 ml volumetric flasks and stored in the dark in a freezer. Moreover, acidic hydrolysis (TPCA) was preformed. The samples (5 g) were heated with 60 ml of 1.2 N hydrochloric acid in 50% methanol. The mixtures were centrifuged, evaporated in vacuo and water residues were placed in 50 ml volumetric flasks and filled up to the mark with distilled water.
For antioxidant and cytotoxic assays, portions (10 g) of each plant material were macerated with 70% ethanol for 3 days at room temperature. The extracts were evaporated to dryness, weighed and stored in a freezer at −20 °C. The freshly prepared solutions of dry extracts were used in the study.
2.4. Total phenolic content (TPC)
The amount of total phenolics was determined before and after hydrolysis of plant material using the Singleton and Rossi (1965) colorimetric method with some modifications. The absorbance was measured at 660 nm (Spectrophotometer UV–VIS, Evolution 300, Thermo-Finnigan, Italy). The results were expressed as mg of gallic acid equivalent (GAE) per 1 g of dry weight (DW).
2.5. Total flavonoid content (TFC)
Total flavonoids were evaluated according to the method described by Lamaison and Carret (1990). The absorbance was measured at 394 nm. Finally, the total flavonoid content was expressed as mg of quercetin equivalent (QE) per 1 g of DW.
2.6. In vitro assay of cytotoxic activity
The cytotoxic activity of extracts was determined using ELISA test with colorimetric detection (Roche’s test, BrdU – 5-bromo-2′-deoxyuridine kit III). Their anticancer activities against ovarian (TOV-112D ATCC CRL-11731 Human ovary primary malignant adenocarcinoma, endometrioid carcinoma cell line) and lung cancer (A549 ECACC 86012804 Human lung epithelial cell line derived from a 58-year-old Caucasian male) cell lines were tested. Normal human skin fibroblast cells in vitro (FS primary cell line isolated from the skin of a 25-year-old female 5th passage) were included in the cytotoxicity test as a control group.
Cancer cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 1% of 2 mM l-glutamine, 50 IU/ml penicillin and 50 mg/ml streptomycin. The human fibroblast cell, which is a non-cancerous cell line, was grown in an incubator with 10% CO2 at 37 °C in DMEM containing 10% fetal bovine serum (Sigma) and 1% 10.000 U penicillin plus 10 mg/ml streptomycin. According to their growth profiles, the optimal plating density of each cell line was determined. The appropriate extract dilutions (100 μg/ml) were added. The cytotoxic effects were determined 24, 48 and 72 h after administration. The end-point determinations were performed with 5-bromo-2-deoxy-uridine (BrdU) labeling and the detection kit III using an ELISA reader (Genesys 20, Thermo Spectronic, Madison, WI, USA). The growth percentage was evaluated spectrophotometrically versus the untreated controls using the cell viability assay. Moreover, a coefficient of cell proliferation inhibition (GI) or coefficient of cell proliferation stimulation (GS) was evaluated.
2.7. Free radical scavenging activity
To determine the antioxidant activity of plant extracts, the method based on the reduction of methanolic solution of colored free radical DPPH• was used. The changes in color from deep-violet to light-yellow were measured at 515 nm in a UV/visible light spectrophotometer (Thermo Evolution 300). Radical scavenging activity was measured according to the Brand-Williams et al. (1995) method. Antioxidant activity was expressed as EC50 (efficient concentration): the amount of dry extract (mg of DW) needed to obtain 50% activity per 1.0 ml of the initial solution.
2.8. Inhibition of linoleic acid peroxidation (LPO)
The antioxidant activity was also determined as the degree of inhibition on the hemoglobin-catalyzed peroxidation of linoleic acid according to Kuo et al. (1999). The hydroxyperoxide formed was assayed according to the ferric thiocyanate method with mixing with 0.02 M FeCl2 followed by 30% ammonium thiocyanate. The absorbance of sample (As) was measured at 480 nm. The absorbance of blank (A0) was obtained without hemoglobin to the reaction mixture; the absorbance of control (A100) was determined without the sample added to the mixture. Thus, the antioxidative activity of the sample was calculated according to the formula:
Antioxidant activity was expressed as EC50 (efficient concentration): the amount of dry extract (mg of DW) needed to obtain 50% activity per 1.0 ml of the initial solution.
2.9. Metal chelating activity (CHEL)
The chelating power was determined by the method described by Guo et al. (2001). Absorbance was measured spectrophotometrically at 562 nm. The percentage of inhibition of ferrozine-Fe2+ complex formation was calculated according to the following formula:
where: Ac – absorbance of the blank, Ap – absorbance in the presence of the test sample.
Antioxidant activity was expressed as EC50 (efficient concentration): the amount of dry extract (mg of DW) needed to obtain 50% activity per 1.0 ml of the initial solution.
2.10. Reducing power (RED)
Reducing power was determined according to Oyaizu (1984). The absorbance at 700 nm was measured. Increased absorbance of the reaction mixture indicated increased reducing power. Activity was expressed as quercetin equivalent (QE). Antioxidant activity was expressed as EC50 (efficient concentration): quercetin equivalent (μg/ml) needed to obtain 50% activity per 1.0 ml of the initial solution.
2.11. Statistical analysis
Experimental data were shown as means ± S.D. for biochemical assays. Statistical significance was estimated through Tukey’s test for the data obtained from three independent samples of each extract in three parallel experiments (n = 9).
Besides the classical pairwise correlation, we applied the scaled principal component analysis. It is a multivariate technique, which projects the multivariate data onto the two-dimensional plane. Investigating the position of samples one can discuss their similarity, whereas inspecting the projection of the variable axes (denoted as arrows) one can easily see the complex intercorrelations between all the variables. The plane for projection is situated by the algorithm to preserve maximum possible information during the dimension reduction, i.e. the plane coordinates are the one and only possible way to explain maximum possible overall variance. Moreover, the axes are always orthogonal, so no information is observed in several dimensions simultaneously.
Statistical tests were performed using Statistica 6.0 software (StatSoft, Inc., Tulsa, USA).
3. Results and discussion
The TPC contents in the extracts are shown in Table 1. The contents were in the range from 1.01 ± 0.43 to 3.92 ± 0.38 GAE mg/g DW before hydrolysis and from 5.68 ± 1.32 to 12.39 ± 0.7 GAE mg/g DW after hydrolysis. The highest TPC content was found in herb and seeds of all examined plants. The large amounts of free polyphenols were observed in herb extracts of C. album (3.36 mg/g DW), seeds of C. urbicum (3.87 mg/g DW) and roots of C. urbicum (1.52 mg/g DW). The comparative analysis of TPC content before and after the hydrolysis demonstrated the highest differences in C. urbicum seeds (from 3.87 to 12.52 mg/g DW), in C. urbicum root (from 1.58 to 9.73 mg/g DW) and in C. rubrum herb (from 3.36 to 8.87 mg/g). Generally, all the extracts were found to be quite rich in phenols. The TFC contents were also high, especially in the samples obtained from all herbs and ranged from 0.68 mg QE/g DW in C. rubrum root to 7.8 mg QE/g DW in C. rubrum herb of dry weight (Table 1). The highest content of flavonoids was demonstrated in the samples obtained from the herb of all examined species (4.2–7.8 mg QE/g DW).
Table 1.
Total phenolic (TPC), phenolic after acidic hydrolysis (TPCA) and flavonoid content (TF) in C. album, C. hybridum, C. rubrum and C. urbicum expressed as mg GAE or mg QE per 1 g of dry plant material, respectively.
| Sample | Total phenolic content [mg GAE/g DW] | Total phenolic content after acidic hydrolysis [mg GAE/g DW] | Total flavonoid content [mg QE/g DW] |
|---|---|---|---|
| C. album herb | 2.95 ± 0.25a⁎ | 7.26 ± 1.64a | 6.20 ± 0.28a |
| C. hybridum herb | 2.95 ± 0.58a | 7.41 ± 2.69a | 7.20 ± 0.65b |
| C. rubrum herb | 3.92 ± 0.38b | 9.91 ± 2.42b | 7.80 ± 0.43b |
| C. urbicum herb | 3.36 ± 1.43b | 8.87 ± 0.57b | 4.20 ± 1.62c |
| C. album root | 1.01 ± 0.43c | 5.68 ± 1.32c | 1.22 ± 0.19d |
| C. hybridum root | 1.22 ± 0.14c | 6.62 ± 2.26d | 0.70 ± 0.58e |
| C. rubrum root | 1.47 ± 0.58c | 6.24 ± 1.7d | 0.68 ± 0.14e |
| C. urbicum root | 1.58 ± 1.14c | 9.73 ± 0.99e | 0.74 ± 0.23e |
| C. album seeds | 1.89 ± 0.65c | 8.09 ± 1.59b | 1.60 ± 0.96d |
| C. hybridum seeds | 3.72 ± 0.16b | 10.56 ± 1.53b | 2.60 ± 0.2f |
| C. rubrum seeds | 3.66 ± 1.63b | 8.22 ± 0.55e | 1.74 ± 1.09d |
| C. urbicum seeds | 3.87 ± 0.43b | 12.39 ± 0.7e | 3.20 ± 0.46f |
Mean values followed by different superscripts (a–f) in a column are significantly different (P < 0.05).
According to the literature reports, plants from Chenopodiaceae have quite high content of polyphenols, especially of flavonoids (Repo-Carrasco-Valencia et al., 2010), e.g. bitter quinoa (C. quinoa) seeds contain 86.4 mg GAE/10 g DW and sweet quinoa – 77.2 mg GAE/10 g DW (Dini et al., 2010). Nsimba et al. (2008) who examined two ecotypes of C. quinoa, have shown that the Bolivia Altiplano type of this plant contains 94.3 mg/g whereas the Japan sea-level type – 148.0 mg/g polyphenols (expressed as mg tannic acid equivalent/g DW). Otherwise, Dasgupta and De (2007) have reported that leaves of C. album contain 44.2 μg GAE/mg of total phenolics and 9.53 μg CE/mg of flavonoids in dry plant material. In the recent study, the total phenol content of quinoa was found to be 25 mg GAE/100 g of dry-weight basis. The samples of canihua (C. pallidicaule) had higher total phenol content – 413 mg GAE/100 g DW. Our results are comparable with those obtained by other authors. Our study revealed that Polish wildly growing species from Chenopodium genus were a rich source of polyphenols.
The results of the cytotoxic measurements are summarized in Table 2. The analysis of ethanolic extracts demonstrated that some of Chenopodium species could suppress cancerous cells multiplying in vitro. Unfortunately, there properties are often accompanied by high toxicity in skin fibroblasts. The extract from the C. album herb (at the dose of 0.1 mg/cm3 after 48 h incubation) caused inhibition about the 95% of accumulating cells of crayfish of the smuggling ovary to endometrium. These data are similar to those obtained from C. hybridum (0.1 mg/cm3). However, toxicity of both these extracts to skin fibroblasts was also high. The mortality of cells after 72 h amounted to 95% (C. album) and 90% (C. hybridum), respectively. In C. album (0.2 mg/cm3) seeds we observed lower cytotoxic activity toward cells of metastatic ovarian carcinoma (55%). Moreover, 30% activity of this extract to human cells of pulmonary carcinoma was demonstrated, which was likely to exert cytopathic effects on skin fibroblasts (65%).
Table 2.
The cytotoxic activity of different parts of C. album, C. hybridum, C. rubrum, C. urbicum.
| Sample | Concentration of dry material [mg/cm3] | Concentration of dry extract [mg/cm3] | Time of incubation [h] | Skin’s fibroblasts | A 549 GI GS (%) | TOV-112D GI GS (%) |
|---|---|---|---|---|---|---|
| Control (human skin fibroblasts) | 24 | 0 | 0 | 0 | ||
| 48 | 0 | 0 | 0 | |||
| 72 | 0 | 0 | 0 | |||
| C. album herb | 1.12 | 0.2 | 24 | 0 | 15 | 50 |
| 48 | 25C | 15 | 95C | |||
| 72 | 70C | 15 | 95 | |||
| C. album root | 2.35 | 0.2 | 24 | 0 | 10 | 0 |
| 48 | 0 | 50 | 5 | |||
| 72 | 0 | 50 | 5 | |||
| C. album seeds | 2.00 | 0.1 | 24 | 5C | 5 | 25 |
| 48 | 15C | 25 | 55 | |||
| 72 | 65C | 30 | 55 | |||
| C. hybridum herb | 1.00 | 0.2 | 24 | 15 | 0 | 5 |
| 48 | 80 | 0 | 95 | |||
| 72 | 90 | 0 | 95 | |||
| C. hybridum root | 1.54 | 0.1 | 24 | 0 | 0 | 5 |
| 48 | 0 | 5 | 5 | |||
| 72 | 0 | 5 | 5 | |||
| C. hybridum seeds | 1.50 | 0.1 | 24 | 0 | 10C | 5 |
| 48 | 0 | 10 | 55 | |||
| 72 | 15 | 20 | 50 | |||
| C. rubrum herb | 0.82 | 0.2 | 24 | 90C | 0 | 0 |
| 48 | 90 | 5 | 5 | |||
| 72 | 100 | 5 | 5C | |||
| C. rubrum root | 1.08 | 0.2 | 24 | 5 | 0 | 0 |
| 48 | 40 | 5 | 0 | |||
| 72 | 50 | 5 | 0 | |||
| C. rubrum seeds | 1.00 | 0.1 | 24 | 90C | 5C | 10 |
| 48 | 95 | 5 | 5 | |||
| 72 | 100 | 5 | 0 | |||
| C. urbicum herb | 0.87 | 0.2 | 24 | 10 | 0 | 0 |
| 48 | 25 | 0 | 5 | |||
| 72 | 60C | 0 | 5 | |||
| C. urbicum root | 0.91 | 0.1 | 24 | 0 | 5 | 0 |
| 48 | 10 | 10 | 5 | |||
| 72 | 10 | 10 | 5 | |||
| C. urbicum seeds | 0.67 | 0.1 | 24 | 5 | 0 | 5 |
| 48 | 5 | 5 | 5 | |||
| 72 | 5 | 5 | 10C | |||
Furthermore, the correlation coefficient (R2) between the cytotoxic activity and total phenolic content of the plants studied was determined. No correlation was found for herbs and roots. R2 between the cytotoxic capacities and the phenolic contents in seeds was 0.569 and this value suggested their influence on this direction of action. These results may indicate the synergistic effect of phenolic compounds on cancer cell properties. A similar conclusion has been drawn from the observations of a potent anti-tumoral effect of hydroalcoholic extract from the leaves of C. quinoa (Gawlik-Dziki et al., 2013). The published data on cytotoxic and anticancer properties of Chenopodiaceae species are limited. According to the literature, C. ambrosioides is the most active in inhibiting polymerization of human chromosomes, hence the highest potential for inhibition of cancer cell formation (Potawale et al., 2008). Nascimento et al. (2006) suggested that C. ambrosioides had a potent anti-tumoral effect, which was evident at small doses and even when the treatment was initiated two days after tumor implantation.
The anticancer activity of whole plant extracts is a result of many various activities, among which one of crucial roles plays antioxidant capacity. To measure the antioxidant potential of raw materials, it is necessary to use many different methods and to consider the content and composition of phenolic compounds in extracts. For instance, some other soluble compounds, such as simple carbohydrates or amino acids, may be present in extracts and interfere with the antioxidant test results or with determinations of total phenolics. A number of studies dealt with a correlation between the structure of polyphenolics and their antioxidant activity (Rice-Evans et al., 1996), yet due to diverse methods used, a correlation was not explicitly confirmed.
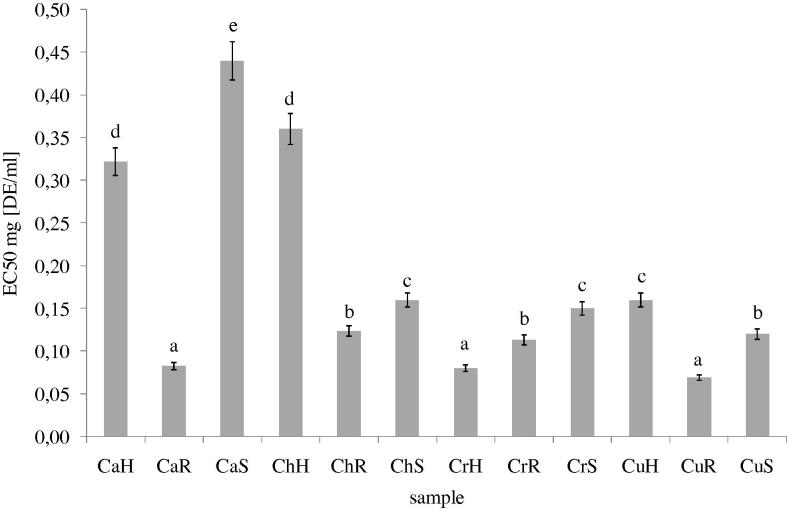
Antioxidant activity of hydroalcoholic extracts from different parts of Chenopodium was determined using four different methods. Taking into account antiradical activity it may be concluded that this activity is dependent both on genus and part of plant. The highest antiradical activity was determined for CaR, CrH and CuR, whereas the lowest for CaS, CaH and ChH. In general, a good source of antiradical compounds was all parts of Cr and Cu (Fig. 1).
Figure 1.
DPPH• radicals scavenging capacity of methanolic extracts from different parts of C. album, C.hybridum, C. rubrum and C. urbicum. CaR (C. album root); CrR (C. rubrum root); CaS (C. album seed); CaH (C. album herb); ChH (C. hybridum herb); CuH (C. urbicum herb); CrH (C. rubrum herb); CrS (C. rubrum seed); ChS (C. hybridum seed); CuS (C. urbicum seed); ChR (C. hybridum root); CuR (C. urbicum root). Bars having different letters are significantly different (p < 0.05).
The results of various studies can be difficult to compare due to different experimental conditions used (Hirose et al., 2010). The value obtained for Chenopodium species in our research could be compared to the other herbs and spices analyzed in other studies e.g. Mariutti et al. (2008). This indicated the medium of antioxidant activity of Chenopodium among the other plants.
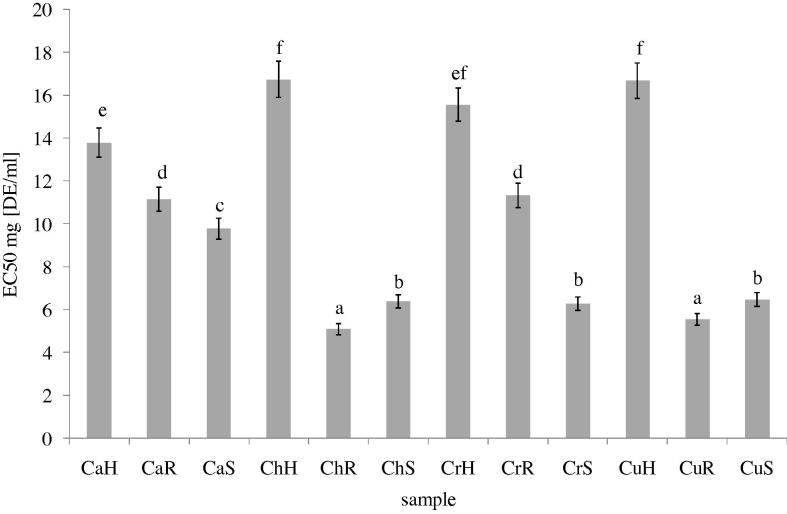
The roots of C. hybridum, are the most active extract interfering with the formation of ferrous and ferrozine complexes, which suggests their chelating activity and ability to capture ferrous ions before ferrozine. Regardless of plant species the lowest chelating activity was determined for extracts obtained from herb. The highest activity was determined for extracts obtained for ChR and CuR. Slightly lower activity was found for samples obtained from ChS, CrS and CuS (Fig. 2).
Figure 2.
Metal chelating activity of methanolic extracts from different parts of C. album, C. hybridum, C. rubrum and C. urbicum. Explanatory notes as in Fig 1. Bars having different letters are significantly different (p < 0.05).
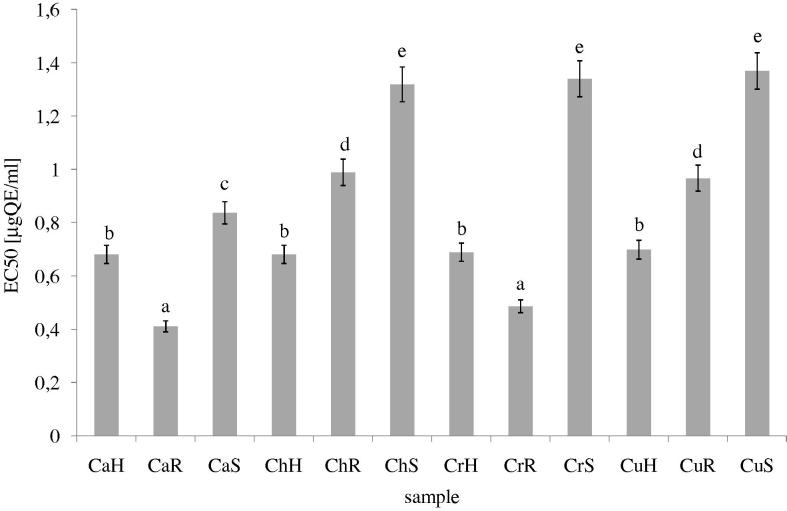
Fig. 3 shows the reductive abilities of study extracts. In our study, the reducing power depended on the examined part of plant.
Figure 3.
Reducing power of methanolic extracts from different parts of C. album, C. hybridum, C. rubrum and C. urbicum. Explanatory notes as in Fig. 1. Bars having different letters are significantly different (p < 0.05).
The highest activities were observed in seed samples. As shown in Fig. 3, ChS and CrS and CuS extracts exhibited high reducing power suggesting its strong electron donating capacity.
The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity (Nadaroğlu et al., 2007). The reducing power of extracts increases similarly to the total antioxidant activity.
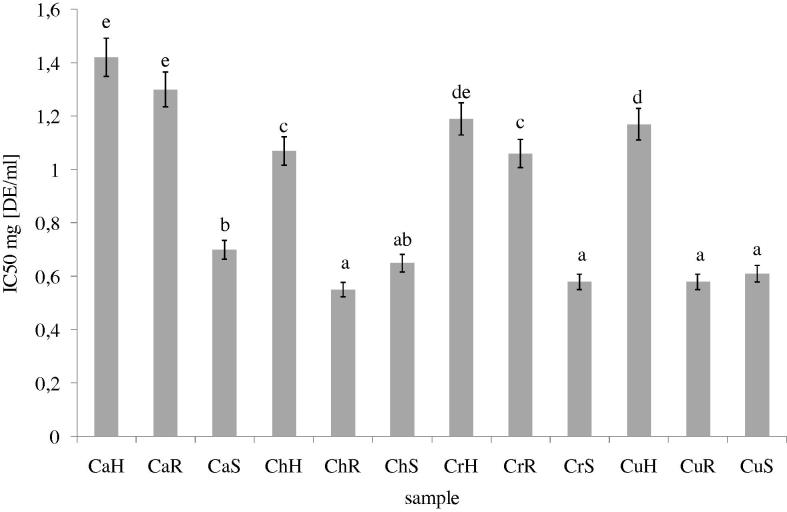
As in the case of chelating power, the lowest LPO activity was determined for extracts obtained from herb. The highest ability to inhibit lipids peroxidation was determined in the case of extracts from seeds (all species) and ChR (Fig. 4).
Figure 4.
Ability of lipid peroxidation inhibition of methanolic extracts from different parts of C. album, C. hybridum, C. rubrum and C. urbicum. Explanatory notes as in Fig. 1. Bars having different letters are significantly different (p < 0.05).
Numerous studies have been conducted on the relationship between the antioxidant activity and content of polyphenols in plant extracts. The results of these studies are often contradictory. Some researchers found a correlation between the concentration of polyphenols and antioxidant activity (Nowak and Gawlik-Dziki, 2007, Velioglu et al., 1998), while the others failed to demonstrate such a correlation. Gazzani et al. (1998) and Kähkönen et al. (1999) did not find a positive correlation between the content of phenolic compounds and antioxidant activity of plant extracts. According to them, phenolic compounds react differentially with the Folin–Ciocalteu reagent; therefore, the antioxidant activity of extracts should not be determined based on the total content of polyphenols.
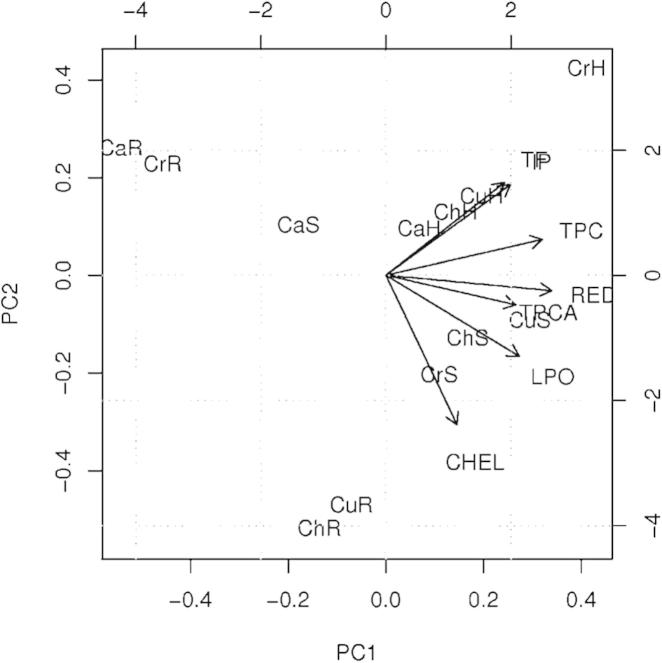
Our work attempted to demonstrate the mutual influence of different mechanisms of antioxidant protection. Besides the classical pairwise correlation (Table 3), we applied the scaled principal component analysis (Fig. 5).
Table 3.
Pearson’s correlation coefficients between the determined parameters in all study samples.
| Parameter | TPC | TPCA | TF | DPPH | CHEL | RED | LPO |
|---|---|---|---|---|---|---|---|
| TPC | X | 0.7 | 0.63 | 0.69 | 0.16 | 0.88 | 0.5 |
| TPCA | 0.7 | X | 0.21 | 0.51 | 0.39 | 0.62 | 0.49 |
| TF | 0.63 | 0.21 | X | 0.67 | 0.12 | 0.67 | 0.37 |
| DPPH | 0.69 | 0.51 | 0.67 | X | 0.11 | 0.55 | 0.31 |
| CHEL | 0.16 | 0.39 | 0.12 | 0.11 | X | 0.46 | 0.68 |
| RED | 0.88 | 0.62 | 0.67 | 0.55 | 0.46 | X | 0.79 |
| LPO | 0.5 | 0.49 | 0.37 | 0.31 | 0.68 | 0.79 | X |
TPC – total phenolic content, TPCA – total phenolic content after hydrolysis, TF – total flavonoid content, DPPH – ability to free radicals scavenging, CHEL – metal chelating activity, RED – reducing power, LPO – inhibition of linoleic acid peroxidation.
Figure 5.
The score plot of the first two principal components of scaled PCA with corresponding loading vectors.
The main correlations observed between chemical composition and the antioxidant activity, are:
-
(a)
A very strong correlation between total flavonoid content and free radical scavenging activity.
-
(b)
A quite strong correlation between reducing power, total phenolic content and total phenolic content after hydrolysis.
Inhibition of linoleic acid peroxidation and metal chelating activity are weakly correlated with the content of the compounds investigated.
The plant samples form several clusters:
-
1.
CaR (C. album root), CrR (C. rubrum root)
-
2.
CaS (C. album seed)
-
3.
CaH (C. album herb), ChH (C. hybridum herb), CuH (C. urbicum herb)
-
4.
CrH (C. rubrum herb)
-
5.
CrS (C. rubrum seed), ChS (C. hybridum seed), CuS (C. urbicum seed)
-
6.
ChR (C. hybridum root), CuR (C. urbicum root)
These clusters are grouped mainly against the plant part, and not species. Therefore, it can be concluded that antioxidant activity and phenolic composition differ mainly between plant parts and are quite similar between the plants, when the same plant part is analyzed.
4. Conclusions
The extracts from all examined species of Chenopodium contained significant amounts of phenols and flavonoids, which play a major regulatory role in oxidation. We confirmed that the extracts demonstrate multidirectional biological activity, such as anticancer and antioxidant abilities.
The findings of this study revealed that the Chenopodium extracts could be used as a readily accessible source of natural antioxidants, and may be used for food supplements production and in the pharmaceutical industry. Further research into purification and identification of active compounds is required to widen the knowledge about the protective mechanisms involved and possible future applications.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alvarez-Jubete L., Wijngaard H., Arendt E.K., Gallagher E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010;119:770–778. [Google Scholar]
- Baldi A., Choudhary N.K. In vitro antioxidant and hepatoprotective potential of Chenopodium album extract. Int. J. Green Pharm. 2013;7(1):50–56. [Google Scholar]
- Bhargava A., Shukla S., Kumar R., Ohri D. Metroglyph analysis of morphological variation in Chenopodium spp. World J. Agric. Sci. 2009;5(1):117–120. [Google Scholar]
- Brand-Williams W., Cuvelier E., Berset C.M. Use of free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995;28:25–30. [Google Scholar]
- Dasgupta N., De B. Antioxidant activity of some leafy vegetables of India: a comparative study. Food Chem. 2007;101:471–474. [Google Scholar]
- Dini I., Tenore G.C., Dini A. Antioxidant compound contents and antioxidant activity before and after cooking in sweet and bitter Chenopodium quinoa seeds. LWT Food Sci. Technol. 2010;43:447–451. [Google Scholar]
- El-Sayed A.M., Al-Yahya M.A., Hassan M.M. Chemical composition and antimicrobial activity of the essential oil of Chenopodium botrys growing in Saudi Arabia. Int. J. Crude Drug Res. 1989;27:185–188. [Google Scholar]
- Gawlik-Dziki U. Effect of hydrothermal treatment on the antioxidant properties of broccoli (Brassica oleracea var. botrytis italic) florets. Food Chem. 2008;109:393–401. doi: 10.1016/j.foodchem.2007.12.058. [DOI] [PubMed] [Google Scholar]
- Gawlik-Dziki U., Świeca M., Sułkowski M., Dziki D., Baraniak B., Czyz J. Antioxidant and anticancer activities of Chenopodium quinoa leaves extracts – in vitro study. Food Chem. Toxicol. 2013;57:154–160. doi: 10.1016/j.fct.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Gazzani G., Papetti A., Massolini G., Daglia M. Anti- and prooxidant activity of water soluble components of some common diet vegetables and effect of thermal treatment. J. Agric. Food Chem. 1998;46:4118–4122. [Google Scholar]
- Gohar A.A., Elmazar M.A. Isolation of hypotensive flavonoids from Chenopodium species growing in Egypt. Phytochem. Res. 1997;11:564–567. [Google Scholar]
- Guo J.T., Lee H.L., Chiang S.H., Lin H.I., Chang C.Y. Antioxidant properties of the extracts from different parts of broccoli in Taiwan. J. Food Drug Anal. 2001;9:96–101. [Google Scholar]
- Hirose Y., Fujta T., Ishii T., Ueno N. Antioxidative properties and flavonoids composition of Chenopodium quinoa seeds cultivated in Japan. Food Chem. 2010;119:1300–1306. [Google Scholar]
- Kähkönen M.P., Hopia A.T., Vuorela H.J., Rauha J.-P., Pihlaja K., Kujala T.S., Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Khoobchandani M., Ojeswi B.K., Sharma B., Srivastava M. Chenopodium album prevents progression of cell growth and enhances cell toxicity in human breast cancer cell lines. Oxid. Med. Cell. Longev. 2009;2:160–165. doi: 10.4161/oxim.2.3.8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J.-M., Yeh D.-B., Pan B. Rapid photometric assay evaluating antioxidative activity in edible part material. J. Agric. Food Chem. 1999;47:3206–3209. doi: 10.1021/jf981351o. [DOI] [PubMed] [Google Scholar]
- Lamaison J.L.C., Carret A. Teneurs en principaux flavonoids des fleurs de Crataegus monogyna Jacq et de Crataegus laevigata (Piret DC) en fonction de la vegetation. Plantes Médicinales Phytothérapie. 1990;25:12–16. [Google Scholar]
- Mariutti L.R.B.M., Baretto G.P.M., Bragagnolo N., Mercadante A.Z. Free radical scavenging activity of ethanolic extracts from herbs and spices commercialized in Brazil. Braz. Arch. Biol. Technol. 2008;51(6):1225–1232. [Google Scholar]
- Miranda M., Delatorre-Herrera J., Vega-Gálvez A., Jorquera E., Quispe-Fuentes I., Martínez E. Antimicrobial potential and phytochemical content of six diverse sources of quinoa seeds (Chenopodium quinoa Willd.) Agric. Sci. 2014;5:1015–1024. [Google Scholar]
- Nadaroğlu H., Demir Y., Demir N. Antioxidant and radical scavenging properties of Iris germanica. Pharm. Chem. J. 2007;41(8):409–415. [Google Scholar]
- Nascimento F.R.F., Cruz G.V.B., Pereira P.V.S., Maciel M.C.G., Silva L.A., Azevedo A.P., Barroqueiro E.S., Guerra R.N. Ascitic and solid Ehrlich tumor inhibition by Chenopodium ambrosioides L. treatment. Life Sci. 2006;78:2650–2653. doi: 10.1016/j.lfs.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Nowak R., Gawlik-Dziki U. Polyphenols of Rosa L. leaves extracts and their radical scavenging activity. Z. Naturforsch. 2007;62c:32–38. doi: 10.1515/znc-2007-1-206. [DOI] [PubMed] [Google Scholar]
- Nsimba R.Y., Kikuzaki H., Konishi Y. Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chem. 2008;106:760–766. [Google Scholar]
- Oyaizu M. Studies on products of browning reaction – antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1984;44:307–315. [Google Scholar]
- Polish Pharmacopoeia VI, 2002. Warszawa: PTF.
- Potawale S.E., Luniya K.P., Mantri R.A., Mehta U.K., Wassem M.D., Sadiq M.D., Veta l.Y.D., Deshmukh R.S. Chenopodium ambrosioides: an ethnopharmacological review. Pharmacology online. 2008;2:272–286. [Google Scholar]
- Repo-Carrasco-Valencia R., Hellström Pihlava J.M., Mattila P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), Kañiwa (Chenopodium pallidicaule) and Kiwicha (Amaranthus caudatus) Food Chem. 2010;120:128–133. [Google Scholar]
- Rice-Evans C.A., Miller N.J., Paganga G. Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Rad. Biol. Med. 1996;20(7):933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Rice-Evans C.A., Miller N.J., Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2(4):152–159. [Google Scholar]
- Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- Velioglu Y.S., Mazza G., Gao L., Oomach B.D. Antioxidant activity and total phenolics of selected fruits, vegetables and grain products. J. Agric. Food Chem. 1998;46:4113–4117. [Google Scholar]