Abstract
Type 2 diabetes imposes a large and growing burden on the public’s health. This burden, combined with the growing evidence for primary prevention from randomized controlled trials of structured lifestyle programs leads to recommendations to include caloric reduction, increased physical activity and specific assistance to patients in problem solving to achieve modest weight loss as well as pharmacotherapy. These recommendations demand exploration of new ways to implement such primary prevention strategies through more integrated community organization, medical practice and policy. The US experience with control of tobacco use and high blood pressure offers valuable lessons for policy, such as taxation on products, and for practice in a variety of settings, such as coordination of referrals for lifestyle supports. We acknowledge also some notable exceptions to their generalizability. This paper presents possible actions proposed by an expert panel, summarized in Table 1 as recommendations for immediate action, strategic action and research. The collaboration of primary care and public health systems will be required to make many of these recommendations a reality. This paper also provides information on the progress made in recent years by the Division of Diabetes Translation at the US Centers for Disease Control and Prevention (CDC) to implement or facilitate such integration of primary care and public health for primary prevention.
Keywords: Behavior, community, diabetes, lifestyle, primary care, primary prevention
Diabetes epidemic
Diabetes has emerged as a major public health problem in the 21st century. In the USA, an estimated 26 million people (8% of the entire population) have diabetes; 7 million of them are not even aware that they have the disease.1 Over the last several decades, diabetes prevalence has increased 5- to 7-fold in the USA. In the USA, ∼1.9 million new cases were diagnosed in adults in 2010.1 For Americans born in the year 2000, the lifetime risk of developing diabetes is ∼40% among females and 30% among males.2 Diabetes is a major cause of blindness, kidney failure, cardiovascular disease, reductions in quality of life and premature death. In addition to causing much human suffering, it imparts major economic burdens, costing an estimated annual $174 billion in the USA alone, and an increasing burden on medical care systems and resources everywhere.1
Why primary prevention and integration of primary care with public health?
Effective management is essential for reducing morbidity and premature mortality related to diabetes and the tools for treating diabetes are stronger than ever before.3–6 Primary prevention, however, is highly attractive as a complementary and integrated strategy for Type 2 diabetes for several reasons (Fig. 1). Firstly, the immense public health burden imposed by diabetes justifies action at the population level. Secondly, currently available treatments, while valuable, are costly, convey risks of harmful side effects (e.g. hypoglycemia), still have limited efficacy and are less likely to be effective for persons who have problems accessing medical care or adhering to self-care regimens. Thirdly, prevention of Type 2 diabetes by lifestyle modification is likely to produce beneficial other effects (e.g. reduction in risk of hypertension, hyperlipidemia, heart disease and certain cancers). Fourthly, most of the determinants of caloric intake, weight management and physical activity are beyond the reach or influence of medical care practitioners by themselves and are likely to be more amenable to public health efforts. Fifthly, since racial/ethnic and socio-economic disparities are the result of several factors, it is logical to suggest that integration of primary care and public health interventions will be needed to address these disparities.7 Finally, a variety of primary prevention strategies, including both lifestyle modification and pharmacotherapy for those at high risk, have been rigorously tested in randomized controlled trials (RCTs) and proven efficacious.8 Translation studies have been conducted in a variety of settings to provide guidance on cost-effective implementation of proven interventions.9,10 However, these interventions need to be implemented on a large scale if they are to reduce the growing incidence of Type 2 diabetes. Although less easily tested in controlled trials, public health strategies that produce mutually supportive changes in behavior, policies and environments at the community level may be more cost-effective with greater reach for mass intervention and can create synergy with structured lifestyle and pharmacotherapy interventions tailored to high-risk individuals.11
Figure 1.
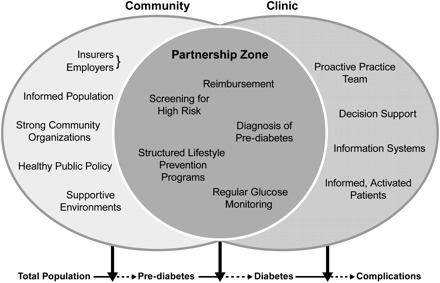
Prevention of Type 2 diabetes: The community-clinic partnership model; provided by Centers for Disease Control and Prevention, Division of Diabetes Translation. Elements in the clinical component are adapted from the Chronic Care Model, MacColl Institute for Health care Innovation. The elements listed in this figure are not intended to be all-inclusive but to provide information on the kinds of elements contributed by each sector and shared across sectors
For these reasons, primary care medicine needs the support of public health or community interventions for primary prevention. Public health also needs the support of the medical care system. Assessing risk status, discussing risk and referring to a proven community-based prevention program is a critical role for the primary care practitioner. For many people, specific encouragement by their health care practitioner is a key factor in taking action to improve their health.12
Evidence for prevention from efficacy trials
RCTs of structured lifestyle modification have consistently demonstrated that caloric reduction plus increased physical activity leading to modest weight loss reduces the risk of incident Type 2 diabetes in adults at high risk by 50–70%.13 Most relevant for the US population, but generalizable to other countries, is the Diabetes Prevention Program (DPP) research trial led by the US National Institutes of Health (NIH).8 DPP recruited 3234 middle-aged overweight or obese adults with impaired glucose tolerance, a high-risk state for Type 2 diabetes. Participants were randomly assigned to one of three conditions: (i) a lifestyle intervention that employed behavioral counseling to promote caloric reduction and physical activity; (ii) metformin, a widely used anti-diabetic medication known to improve insulin sensitivity and (iii) a placebo for metformin (i.e. a control group). The DPP lifestyle intervention produced an initial weight loss of ∼6% of body weight after 12 months, diminishing to ∼4% after 3 years. The intervention also significantly increased self-reported physical activity (equivalent to brisk walking) from 100 to 190 minutes per week. The effect on diabetes incidence was impressive: compared to their counterparts in the placebo group, DPP participants in the lifestyle intervention group enjoyed a 58% reduction in incident diabetes over 4 years. This benefit was highly robust; it was observed in men and women across race and ethnic groups and it was even stronger at older ages.
RCTs have also generally shown pharmacotherapy to be effective for the primary prevention of Type 2 diabetes in high-risk adults, although few of those agents currently available on the market have been the subject of more than one trial. The DPP proved that metformin was safe and effective for primary prevention, producing a 31% reduction in diabetes risk. Protective effects of similar magnitude in adults with impaired glucose tolerance have been observed for acarbose14 and orlistat15—oral agents not absorbed from the gastrointestinal tract that inhibit absorption of carbohydrates and fats, respectively. Stronger effects have been observed for troglitazone, the first of the thiazoledenediones, or TZDs, a class of insulin-sensitizing agents.16 However, troglitazone was pulled from the market because of hepatotoxicity. Newer TZDs with little hepatotoxicity are available but have not been as rigorously tested for primary prevention. Most recently, pioglitazone reduced the risk of conversion of impaired glucose tolerance to Type 2 diabetes mellitus by 72% but was associated with significant weight gain and edema.17
Lessons from blood pressure control
Hypertension is well established as a risk factor for heart disease, stroke, kidney failure and a wide array of other vascular diseases. In turn, obesity and sodium intake are major modifiable risk factors for high blood pressure. In lean economically isolated (Yanomamo and Xingu Indians of Brazil and rural populations in Kenya and Papua New Guinea) populations who consume fresh foods because there are few processed foods, high blood pressure is uncommon, and the age-related rise in blood pressure so common in the industrialized world is absent.18 In the 1970s and 1980s, evidence from RCTs began to accumulate, indicating that pharmacotherapy agents could prevent vascular complications in hypertensive adults but that their effectiveness depended on detection of elevated blood pressures, behavioral changes and maintenance over time, much as with diabetes. Since the early 1970s and in recent years, trials have proved the efficacy of lifestyle modification to reduce blood pressure and prevent hypertension in adults at high risk.19 In combination with increased community detection programs and compelling epidemiologic data, these trials of primary and secondary prevention led to a series of strong national recommendations for the prevention, detection and treatment of high blood pressure in the USA to enhance the National High Blood Pressure Education Program.20 Coincident with these developments, many countries enjoyed historical declines in cardiovascular mortality. However, despite the impressive evidence base, the availability of a large pharmacologic armamentarium and a formidable degree of scientific consensus, prevention, detection and treatment of high blood pressure remains suboptimal.
This story holds several possible lessons for public health strategies aimed at primary prevention of Type 2 diabetes. Firstly, mere dissemination of knowledge about health benefits of lifestyle change is not sufficient: salt intake grew despite warnings that it contributed to hypertension risk. Secondly, drug therapy can play a major role if the drugs are strong and safe, if wedded to a campaign of awareness and detection and if accompanied by systematic interventions to ensure access and adherence to prescribed regimens. Thirdly, limited long-term data on lifestyle modification has contributed to challenges in achieving policy change. Finally, while the food industry responded with some low-sodium food options, these have thus far been insufficient to promote change in overall salt intake. Although the 2005 Dietary Guidelines for Americans urged reduced sodium in the diet, it was not until 2010 that the Dietary Guidelines featured substantially reduced sodium intake as a priority over dietary fat or trans-fat reduction.
Lessons from tobacco control
Heart disease-, cancer-, stroke- and chronic lung disease-related deaths have been declining thanks to a combination of medical and public health developments in the last third of the 20th century.21 On the medical side, developments in pharmaceutical agents and other improved treatments have been notable, as described for hypertension above. Among the most notable developments on the public health side have been the acceleration of reductions in tobacco consumption and exposure of non-smokers to secondhand smoke following the implementation of statewide tobacco control programs and policies in California, Massachusetts, Florida, Arizona, Oregon and Mississippi.22 The prevalence of smoking declined for most age groups and all race/ethnicity groups in California and both youth smoking and aggregate cigarette sales have declined significantly and independently in proportion to tobacco control program expenditures in states that followed California’s lead.23–25 Cardiovascular disease rates improved swiftly after smoking prevalence declined and rebounded when the California program support was cut back.26 Rates of chronic lung disease and bronchial cancer responded as well, although with greater lag times. These improvements were especially noticeable in California, with lung cancer rate declines four times greater than in the rest of the USA.27
The tobacco control story conveys several possible lessons. Firstly, success in health behavior change was achieved largely by coordinated public health policy, regulatory, mass media and environmental control means. Although individually tailored programs of behavior change and pharmacotherapy to reduce nicotine dependence were certainly available, their reach and efficiency were far less than policies that prevented initiation of smoking and prevented smoking in public places.28 The strategies that appear to have been most effective related to the intermediate targets of (i) changing social norms of public behavior, (ii) restraining the advertising by industries that market tobacco products and (iii) increasing the cost of the behavior through pricing strategies. Secondly, the public health approach generally produced successful behavior change across boundaries of age, sex, race and ethnicity (the per cent decline in smoking among African-American and Hispanic males and females were greater in California between 1990 and 2005 than in non-Hispanic Whites);29 although many disparities in smoking prevalence persist across strata of socio-economic status. Thirdly, it demonstrates the potential that lies outside the health care system for empowering individuals, organizations and communities to self-manage behavior change.30–35 Fourthly, strength of infrastructure—including number and training of personnel, level of funding and presence of coalitions—was directly related to policy change and reductions in smoking rates.35 Finally, the tobacco control story highlights the role of communities and states as laboratories for innovating and evaluating policy change.36
Although these ‘lessons’ have served as guides, if not inspiration, in other public health initiatives, their generalizations to physical activity and nutrition must acknowledge that the issues in changing these more pervasive behaviors and associated lifestyles, commercial interest, environmental and media influences will bring other, often more complex, issues into play.30–34 Indeed, with cutbacks in the state funding of their comprehensive tobacco control programs and relative magnitude of tobacco industry spending, there are no guarantees that the advances in tobacco control can be sustained.
Proposed actions to help translate diabetes prevention into practice
A multidisciplinary panel of US experts selected by Centers for Disease Control and Prevention (CDC) leadership at the time reviewed various issues related to diabetes prevention in a 2-day meeting. An international group selected and convened by their Dutch hosts at the Heelsum symposium in 2010 further examined the comparative issues of primary care and community collaboration on weight management across various nations. Both groups represented those disciplines and professions seen by their organizers as having leadership roles in primary prevention of Type 2 diabetes, especially weight management, in various sectors and populations. The first panel enumerated specific action items to help speed translation of proven diabetes prevention strategies into practice. The panel avoided explicit prioritization since the expert consensus was that priorities would differ across settings. Rather, the goal was to offer a comprehensive menu of possibilities that cut across disciplines and settings. The possible array of action items is summarized in Table 1. For ease of presentation, the action items in Table 1 were grouped into a 2 × 3 classification scheme based on primary locus of action (Health Care System versus Public Health Settings) and type of action (Immediate Action versus Strategic Action versus Research). These classifications, however, are not to imply choices that need to be made between the categories. They should be approached as potentially synergistic or at least additive, interventions and policies.
Table 1.
Proposed action items
| Setting | Immediate action | Strategic action | Research |
| Health care system | Measures | Financing | Technology |
| Define uniform diagnostic criteria, high-risk state, outcome targets | Support reimbursement for proven prevention programs | Identify affordable technologies for measuring glycemia | |
| Screening, diagnosis and referral | Policy | Promote studies that link different clinical and community environments | |
| Assess risk and discuss risk status with patients | Identify policies that support sustainability and reach of proven prevention programs | Research mobile technologies that help increase reach of proven prevention programs | |
| Conduct prediabetes diagnostic test, as appropriate | Advocate for insurers and Medicare/Medicaid to pay for proven prevention programs | Assessment, evaluation and surveillance | |
| Refer patients with prediabetes to proven prevention program | Involve CMS to identify codes and reimbursement policies for diabetes prevention | Support national surveillance system for prediabetes | |
| Intervention delivery | Partnerships | Evaluate strategies to increase identification of those at high risk and participation in proven prevention programs | |
| Determine role of drugs/medications | Support the National Diabetes Prevention Program | Intervention delivery | |
| Support the National Diabetes Prevention Program | Establish multi-sectorial partnerships to advocate policy and environmental change and its evaluation | Research ways to increase sustainability and reach of effective prevention programs | |
| Support delivery of proven prevention programs in convenient locations | Encourage companies that advertise products that support unhealthy eating and sedentary behaviors to change products to healthier options | Research effective prevention strategies for those at lower risk (do not yet have prediabetes) | |
| Educate primary health providers about proven prevention strategies | Support natural experiments and opportunistic evaluations of policy and environmental changes, especially those that include the clinical sector | ||
| Monitor glycemic status and cardiovascular risk factors in high-risk patients | Research pharmaceutical agents that are currently under investigation for prevention efficacy | ||
| Demonstration of best practices | |||
| Lead by example | |||
| Encourage institutions to adopt a ‘healthy environment’ (e.g. no convenience machines, provide activity breaks, encourage walking, integrate health clinics with gyms and fitness centers) | |||
| Facilitate linkages between sectors that influence behavior and risk | |||
| Provide feedback to primary health provider and medical records | |||
| Support effective interactive technologies, e.g. websites) to provide support for lifestyle improvements | |||
| Public health | Intervention delivery | Policy | Technology |
| Support implementation of the National Diabetes Prevention Program | Identify policies that support sustainability and reach of proven prevention programs | Promote studies that link different clinical and community environments | |
| Support delivery of proven prevention programs in convenient locations | Explore life and health insurance incentives for healthy lifestyle and prevention | Research mobile technologies that help increase reach of proven prevention programs | |
| Support training of workforce to deliver proven prevention programs | Organize local, statewide and Federal multi-agency task forces to identify and prioritize actions government can take to contribute to diabetes prevention, healthy eating and physical activity | Assessment, evaluation and surveillance | |
| Conduct quality assurance of prevention programs—certification program | Identify policies that improve worksite, school and community environments to support healthy lifestyles | Conduct national surveillance system for prediabetes | |
| Health communication | Explore strategies to eliminate ads for calorie-dense foods aimed at children and decrease screen time | Evaluate marketing strategies for healthy eating and physical activity that have been used effectively for tobacco control | |
| Provide messages and tools to increase knowledge of diabetes risk | Partnerships | Conduct opinion polling about potential policy changes to encourage healthy eating and physical activity | |
| Provide messages to high-risk population and health care professionals to increase uptake of proven prevention programs | Develop collaborative relationships and partnership among federal, state, local and private institutions to facilitate implementation of proven prevention programs for those at high risk and population-wide health promotion strategies | Evaluate ways to diffuse effective health promotion interventions for adults and youth | |
| Partnerships | Intervention delivery | ||
| Develop collaborative relationships and partnership among federal, state, local and private institutions to facilitate implementation of proven prevention programs for those at high risk and population-wide health promotion strategies | Research ways to increase sustainability and reach of effective prevention programs | ||
| Support natural experiments and opportunistic evaluations of policy and environmental changes | |||
| Conduct economic studies of proposed intervention programs and policies | |||
| Systematic review of neighborhood and environmental studies and their impact on diabetes and other health-related outcomes |
In the health care system, ‘immediate actions’ might be designed to enhance risk identification and stratification, counseling on risk reduction, referral to proven community-based prevention programs and pharmacotherapy. Such improvements might be accelerated by better adherence to practice guidelines, changes in reimbursement for preventive services and promotion of continuing medical education related to diabetes prevention. ‘Strategic action’ might include greater changes in reimbursement for preventive services such as reimbursement to medical practitioners for referral and reimbursement to a community-based organization and encouraging state governments to undertake and evaluate broadly based model prevention programs for whole populations as ‘natural experiments’. ‘Research’ might focus on information technology designed to detect individuals at risk, facilitate behavioral counseling and cost-effectiveness evaluations and better link clinics to community settings.
In public health settings, immediate actions might include public dissemination of prevention messages via the mass media, Internet and care providers as well as policies to fund implementation of evidence-based programs and regulations in schools, worksites, community organizations and health care settings.37,38 Strategic action might promote new partnerships to guide interventions to improve physical activity and/or diet, to facilitate sustainable funding for evidence-based lifestyle programs and to create health-promoting after-school environments for young people and health-promoting work environments for adults. Research might focus on improved diabetes surveillance, Internet-based structured lifestyle programs and interventions aimed at youth at home and at school.
The scale of the diabetes epidemic and the contribution of common risk factors to other chronic diseases will require interventions to address environmental and policy determinants of physical activity and diet such as community design, transportation policy, park and recreation policy and funding, school physical education laws and availability and cost of healthful foods.39–41 Strategic action might include the establishment of multi-sector partnerships to advocate policy and environmental change and their evaluation. Research in this area might include opinion polling about support for potential policy changes among policy makers and the public, systematic evaluation of community interventions, cost-effectiveness studies and natural experiments to identify the most practicable areas of policy and environmental change for intervention. This research requires interdisciplinary collaboration with epidemiologists, urban planners, social scientists, policy experts and others.
The panel recognized that this classification scheme in Table 1 was in some ways artificial: many of the action items spanned horizontally, linking the health care system with the public health setting, as suggested by Figure 1; others spanned vertically, incorporating ongoing research into clinical and public health action. Recognizing the importance of such cross-cutting themes, in the following paragraphs, the authors highlight a few.
Quality of care/quality improvement
Although we know the general principles that guide primary prevention of Type 2 diabetes from the DPP research trial and obesity trials, knowledge alone will not translate into improved care. The Institute of Medicine’s landmark report on Crossing the Quality Chasm demonstrated that the quality of care will not improve from information alone.42
Efforts to improve quality of care in various jurisdictions have emphasized the measurement of evidence-based processes of care and outcomes as the first step in both internally and externally driven quality improvement efforts.43 For the health care organization and provider, such data allow self-assessment and direct attention to areas that need quality improvement. Externally, such data have been used for public reporting, which in theory can improve care by allowing health care purchasers and consumers to choose high-quality providers and provide an incentive for providers to improve their care. In addition, growing efforts by private payers in the USA—and, more recently, the US Centers for Medicare and Medicaid Services (CMS)—to reward high-quality care with increased reimbursement—so-called ‘pay for performance’—provides an additional financial incentive.
Thus, prevention and control of conditions like Type 2 diabetes seems to call for incorporating process-of-care and outcome-performance measures into both governmental quality improvement efforts to measure quality of care and private efforts such as the US National Committee on Quality Assurance’s HEDIS measures that assess the quality of care in managed care organizations. The first line of assessment would logically relate to patient screening and counseling on risk factors predicting subsequent development of diabetes.
Persons from minority groups are at higher risk for diabetes and have sometimes received inferior care and had worse outcomes.7 Therefore, quality improvement efforts will need to take special care to ensure that no incentives exist for unintended consequences. For example, reforms should not pay less to organizations providing care for particularly challenging patients, such as those with advanced disease or multiple co-morbidities.
Issues related to youth
American estimates of diagnosed diabetes prevalence for people <20years from the SEARCH for Diabetes in Youth Study were 0.26%.44 A consensus panel convened by the American Diabetes Association and the American Academy of Pediatrics recommended that testing for Type 2 diabetes be performed every 2 years in the context of a health care visit for overweight or obese children aged 10 years and older in the presence of other risk factors.45,46
With regard to primary prevention, most experts agree that clinicians should prescribe and support lifestyle (diet and activity) modification for the entire family as well as for the patient, in an age-appropriate manner and as the prerequisite for all overweight and obesity treatments for children and adolescents. One study showed the long-term benefits of family-based lifestyle interventions in the clinical setting on weight and risk factors in obese youth.47 However, few clinics have the resources or expertise to offer evidence-based lifestyle interventions, so funding and training are needed, in addition to greater collaboration, referral and integration with community and public health resources.
While some school-based interventions have produced sustained improvements in physical activity or diet,48 most have focused on one element of lifestyle change and offer little evidence of having substantially improved body mass index (BMI) or other diabetes-related outcomes.49 The HEALTHY study (part of the STOPP-T2D study funded by the NIH) reduced the per cent of middle-school students with BMI >95th percentile (obese) but did not show a significant effect on reducing the percentage of >85th percentile (overweight and obesity combined).50 Other benefits of the trial were observed, including reduced fasting insulin levels and waist circumference. Community-based interventions are proliferating, although there is little consistency in the data on outcomes.51,52 As we await further public health interventions, with more consistent outcome data, interventions delivered in child care, preschools, schools, communities, medical settings, etc. should continue to focus on improving energy balance in youth.
Translating diabetes prevention to the public health sector
The very promising but intensive and costly lifestyle intervention of the DPP research trial was designed for efficacy and not for sustainable delivery by a community organization.8,53,54 The design of effective ‘real world’ models for implementing the DPP lifestyle intervention requires a collaborative effort that balances fidelity to the DPP design with additional incentives, communications and organizational elements that predispose, enable and reinforce behavioral changes in both practitioners and patients and that optimize reach, adoption and implementation and effectiveness, minimize cost and improve sustainability for capable community partners.55–61
Two major barriers that have prevented translation of DPP findings to the growing population of people who might benefit are the cost and the one-on-one intervention format.62 For the DPP to extend into the public health sector, it is necessary to demonstrate effective strategies to: (i) identify persons in community or clinical settings with risk characteristics similar to the DPP study population and (ii) optimize referral and participation in lifestyle interventions modeled after the DPP but adapted for broad-scale delivery in community settings.
Several translation studies and demonstration projects have been conducted to address these issues. These studies illustrate the bridge we seek to build between more strictly clinical, RCT-based interventions and the community-level interventions required to achieve greater reach and cost-effectiveness.9,10,63 For example, two studies supported by the NIH evaluated the feasibility and effectiveness of training Y (formerly called YMCA) wellness instructors to deliver a group-based adaptation of the DPP lifestyle intervention in Y facilities.9,64 The first of these studies used a social marketing approach to recruit persons with diabetes risk factors within the communities served by specific Y facilities to a free screening event in which each individual’s risk status is ascertained using a combination of the American Diabetes Association risk assessment tool and capillary glucose values.9 Persons identified as having increased risk for abnormal glucose metabolism were offered access free of charge to the adapted DPP lifestyle intervention at the Y and were followed prospectively to determine the effectiveness of the new program to achieve the meaningful changes in weight and cardiovascular risk-factor levels that translated into improved health outcomes in the DPP clinical trial. This study showed a 6% weight loss over 1 year which is in line with the weight loss achieved in the DPP research trial.
The second study focused on the feasibility and effectiveness of a clinic-based strategy to identify, counsel and refer patients for participation in a community-based lifestyle intervention.64 Practices in this study are using a two-stage screening procedure in which adult patients with two or more diabetes risk factors are encouraged to attend a formal prediabetes-test visit that involves both fasting and 2-hour post-challenge glucose tests using a capillary blood sample. This approach was chosen because capillary samples offer reasonable sensitivity and specificity for discriminating normal from impaired glucose tolerance (i.e. the primary DPP risk characteristic),65,66 are considerably more feasible to perform at a high volume in busy primary care settings and offer the advantage of immediate feedback to patients about their risk levels. This study is evaluating not only the feasibility of this overall screening approach but also different strategies to optimize participation by high-risk patients, who are offered brief advice followed by referral to a community-based DPP lifestyle intervention at either a nearby Y facility or a DPP study research site.64
Economic modeling studies suggest that, even if these adapted interventions proved only half as effective as the original DPP approach, they are still likely to be cost-effective and could be financed in a way that achieves short-term return on investment for a private health insurer offering the program.67 Moreover, the studies illustrated the kind of novel partnerships among a health system, an academic institution and a community-based organization that are especially appealing for translation.
Based on its own examination of these experiments and other pilot work they conducted, the CDC’s Division of Diabetes Translation (CDC/DDT) began plans to carry out the National Diabetes Prevention Program in 2009 and was authorized in the 2010 Affordable Care Act to oversee and manage this program. CDC/DDT is taking a strategic approach to creating the National Diabetes Prevention Program. This approach includes the core elements of: (i) Training—helping train the workforce that can implement the program cost effectively. To do this, CDC/DDT has established the Diabetes Training and Technical Assistance Center at Emory University. (ii) Program Recognition—setting standards that will help assure program quality and consistency which are necessary components for effectiveness and reimbursement. (iii) Intervention sites—supporting the start-up of sites easily accessible in the community that will deliver the intervention and working with third-party payers to initiate and sustain intervention funding. (iv) Health Marketing—raising awareness among both health care providers and high-risk populations to increase referral and use of the program.68 The inaugural participating organizations working with CDC/DDT in the National Diabetes Prevention Program are the Y’s delivering the 16-session core and monthly post-core sessions (total 1-year program) and United Health Group which includes United Health Care, a third-party payer, that is providing payment for the intervention on a pay for performance basis. As the CDC program recognition is implemented, more organizations will be involved in delivering the intervention and additional third-party payers will participate.
A promising model is being tested by Finland for nationwide dissemination of a highly beneficial and cost-effective approach to diabetes prevention.54–56 Other European models are being tested. In Germany, an implementation process is underway that includes the pay for performance medical care concept and clear implications for quality management with incentives for patient coordination, patient education, referral, plus structural and public health integration.57
Creating and funding an infrastructure for promoting healthful eating and active living
Although some evidence has accrued about effective interventions to improve physical activity and eating behaviors,49 there is limited infrastructure or funding in place to apply this knowledge by widely implementing evidence-based interventions or to generate more practice-based evidence from innovations in the absence of much controlled-trial evidence of what works. Because of extensive promotion of foods of low nutritional quality and products like cars, television programs and computer games that enable people to be sedentary, a major counterforce may be needed to promote healthful habits. There are models for multi-sectoral physical activity and/or nutrition coalitions led by governments of other countries, notably Australia,34 and in the Healthy Eating-Active Living Convergence Partnership in the USA, which includes the Robert Wood Johnson Foundation, the WK Kellogg Foundation, Kaiser-Permanente, CDC, the California Endowment and Nemours Health and Prevention Services (www.rwjf.org). The US National Physical Activity Plan which is a multi-sector plan developed with input from several groups serves as a useful guide for improving physical activity.69 Partnerships and collaborations could be expanded.33,70–73. The largest efforts of this type are CDC’s Communities Putting Prevention to Work, which is supporting 39 communities to implement policy, environmental and system changes to prevent obesity and address tobacco use74 and Community Transformation grants to reduce chronic diseases, promote healthy lifestyles, reduce disparities and control health care spending.75 Evaluations are underway to document lessons from these experiences. Multi-sectoral coalitions are prioritizing promising interventions guided by available research, advocating for changes in policies and participating in surveillance to evaluate effects. Such groups are promoting improved interventions across a variety of settings, including health care, private industry, multiple government agencies and education.
Tobacco taxes and legal settlements with the tobacco industry have provided financing for tobacco control activities in a few states, but few comparable funding sources have been mobilized for promoting physical activity and healthful eating. New taxes on foods and beverages have been proposed, but public support is uncertain. Parking fines have been dedicated in part to support mass transit in some cities. Willingness-to-pay studies and public opinion polling will be needed to identify acceptable funding options that might include a combination of dedicated taxes and fees, government support from general funds, voluntary contributions from industries and philanthropic contributions. The 2010 Affordable Care Act’s health promotion and prevention fund that will support obesity prevention through ‘community transformation grants’ on an ongoing basis.75 This funding source should allow community initiatives to gain strength and innovations to be demonstrated and evaluated.
Finally, the worksite promises to be a cost-effective settings and infrastructures for community-clinical interface, insofar as employers are often engaged both in the insuring of clinical care and in the promotion and protection of health in the employee population and environments.76–78 This and other community settings offer opportunities for the generation of practice-based evidence to complement and extend the necessarily limited evidence-based practices from controlled trials.46
Conclusions
The growing epidemics of overweight, obesity and Type 2 diabetes demand urgent and coordinated attention. Primary prevention of Type 2 diabetes is a logical strategy in light of the scale and the cost of ongoing medical treatment for the diabetes epidemic and the inevitable increases in diabetes incidence with the overweight and obesity epidemics. Behavioral strategies tailored to high-risk individuals have proven effective in RCTs. Adaptation of these evidence-based strategies for use in community settings has been under investigation with promising results now being taken to scale and evaluated by CDC/DDT and partners. Given the size of the diabetes epidemic and the number of people at high risk approaches aimed exclusively at individual behavior change in clinical settings will likely prove inadequate for diabetes control at the population level. Improvements in policy and the environmental factors summarized in Table 1 would predispose, enable and reinforce more healthful diets and more active lifestyles for widespread and sustained behavior changes. These will require development of infrastructure, environmental and policy changes and ongoing funding of a multilevel, multidisciplinary approach and an experimental attitude at the state and local levels to allow public health researchers to evaluate the ingredients of successful innovations that constitute natural experiments in diabetes prevention.
Declaration
Funding: The convening of the panel and preparation of this report were supported by the Division of Diabetes Translation of the National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, US Department of Health and Human Services.
Ethical approval: none
Conflict of interest: none.
Acknowledgments
The authors wish to acknowledge Namratha Swamy, PhD, Jennifer Jones, Henry Wong, PhD, and Lauren Hess of Danya International, Inc. for their support in the coordination of the expert panel and preparation of the manuscript. We are indebted also to Dean Schillinger, MD, University of California at San Francisco and Chief, California Diabetes Program, California Department of Public Health. The content is solely the responsibility of the authors and does not necessarily represent the official position of the Centers for Disease Control and Prevention.
Planning, Coordinating and Writing Group
FLB, MD, Johns Hopkins University (Co-Chair); LWG, DrPH, University of California at San Francisco (Co-Chair); Marshall Chin, MD, MPH, University of Chicago; Ana Diez-Roux, MD, PhD, MPH, University of Michigan School of Public Health; Michael Engelgau, MD, MS, CDC; Russell Glasgow, PhD, Kaiser Permanente Colorado; Francine R Kaufman, MD, Children’s Hospital Los Angeles; David G Marrero, PhD, Indiana University School of Medicine; James F Sallis, PhD, San Diego State University and Desmond Williams, MD, PhD, CDC.
Expert panel
FLB, MD, Johns Hopkins University; Marshall Chin, MD, MPH, University of Chicago; Ana Diez-Roux, MD, PhD, MPH, University of Michigan School of Public Health; Michael Engelgau, MD, MS, CDC; Russell Glasgow, PhD, Kaiser Permanente Colorado; LWG, DrPH, University of California at San Francisco; Francine R Kaufman, MD, Children’s Hospital Los Angeles; David G Marrero, PhD, Indiana University School of Medicine; Robert E Ratner, MD, FACP; James F Sallis, PhD, San Diego State University and Desmond Williams, MD, PhD, CDC.
Other attendees
Kelly Acton, MD, MPH, Indian Health Service (IHS); Ana Alfaro-Correa, ScD, MA, CDC; Bill Dietz, MD, PhD, CDC; Linda Geiss, MS, CDC; Dawne Hood, CDC; Laura Kettel Kahn, PhD, CDC; Maurice Martin, PhD, MEd, CDC; Kathy Rufo, MPH, CDC; Dawn Satterfield, RN, PhD, CDC; Peter Savage, MD, NIH; Mary Vernon Smiley, MD, MPH, CDC; Russ Sniegowski, MPH, CDC; Frank Vinicor, MD, MPH, CDC; Edward Weiss, MD, CDC; Julie Will, PhD, CDC; Desmond Williams, MD, PhD, CDC and David Williamson, PhD, CDC.
References
- 1.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. http://www.cdc.gov/diabetes/pubs/factsheet05.htm. (accessed on 3 April 2011) [Google Scholar]
- 2.Narayan KM, Boyle JP, Thomson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–90. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM, Cleary PA, Backlund JC, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes: the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study research group. N Engl J Med. 2005;353:2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 6.Gaede P, Vedal P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–93. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 7.Agency for Healthcare Research and Quality. 2010 National Health Care Disparities Report. Rockville, MD: U.S. Department of Health and Human Services, Agency for Healthcare Research and Quality; AHRQ Pub. No. 11–0005, 2011. www.ahrq.gov/qual/qrdr10.htm. (accessed on 9 October 2011) [Google Scholar]
- 8.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the diabetes prevention program into the community. The DEPLOY Pilot Study. Am J Prev Med. 2008;35:357–63. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amundson JW, Butcher MK, Gohdes D, et al. Translating the diabetes prevention program into practice in the general community: findings from the Montana cardiovascular disease and diabetes prevention program. Diabetes Educ. 2009;35:209–23. doi: 10.1177/0145721709333269. [DOI] [PubMed] [Google Scholar]
- 11.Heath GW, Brownson RC, Kruger J, et al. The effectiveness of urban design and land use and transport policies and practices to increase physical activity: a systematic review. J Phys Act Health. 2006;3(suppl 1):S55–76. doi: 10.1123/jpah.3.s1.s55. [DOI] [PubMed] [Google Scholar]
- 12.Glanz K. Progress in dietary behavior change. Am J Health Promot. 1999;14:112–17. doi: 10.4278/0890-1171-14.2.112. [DOI] [PubMed] [Google Scholar]
- 13.Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. A statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Am J Clin Nutr. 2004;80:257–63. doi: 10.1093/ajcn/80.2.257. Review. [DOI] [PubMed] [Google Scholar]
- 14.Chiasson JL, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290:486–94. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 15.Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155–61. doi: 10.2337/diacare.27.1.155. . Erratum in: Diabetes Care. 2004; 27: 856. [DOI] [PubMed] [Google Scholar]
- 16.Knowler WC, Hamman RF, Edelstein SL, et al. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes. 2005;54:1150–6. doi: 10.2337/diabetes.54.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFronzo RA, Tripathy D, Schwenke DC, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364:1104–15. doi: 10.1056/NEJMoa1010949. [DOI] [PubMed] [Google Scholar]
- 18.Carvalho JJ, Baruzzi RG, Howard PF, et al. Blood pressure in four remote populations in the INTERSALT study. Hypertension. 1989;14:238–46. doi: 10.1161/01.hyp.14.3.238. [DOI] [PubMed] [Google Scholar]
- 19.Whelton PK, Appel LJ, Espeland MA, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 1998;279:839–846. doi: 10.1001/jama.279.11.839. [DOI] [PubMed] [Google Scholar]
- 20.National Heart, Lung, and Blood Institute. National High Blood Pressure Education Program: Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: National Institutes of Health; 1991. Publication NIH 03–5233. [PubMed] [Google Scholar]
- 21.Ford ES, Capewell S. Proportion of the decline in cardiovascular mortality disease due to prevention versus treatment: public health versus clinical care. Annu Rev Public Health. 2011;32:5–22. doi: 10.1146/annurev-publhealth-031210-101211. [DOI] [PubMed] [Google Scholar]
- 22.Eriksen MP, Green LW, Husten CG, Pederson LL, Pechacek TF. Thank you for not smoking: The public health response to tobacco-related mortality in the United States. In: Ward JW, Warren C, editors. Silent Victories: The History and Practice of Public Health in the Twentieth-Century America. Oxford, UK: Oxford University Press; 2007. pp. 423–36. [Google Scholar]
- 23.Rogers T. The California Tobacco Control Program: introduction to 20-year retrospective. Tobacco Control. 2010;19(suppl. 1):i1–i2. doi: 10.1136/tc.2010.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tauras JA, Chaloupka FJ, Farrelly MC, et al. State tobacco control spending and youth smoking. Am J Public Health. 2005;95:338–44. doi: 10.2105/AJPH.2004.039727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farrelly MC, Pechacek TF, Thomas KY, Nelson D. The impact of tobacco control programs on adult smoking. Am J Public Health. 2008;98:304–9. doi: 10.2105/AJPH.2006.106377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fichtenberg CM, Glantz SA. Association of the California Tobacco Control Program with declines in cigarette consumption and mortality from heart disease. N Engl J Med. 2000;343:1772–7. doi: 10.1056/NEJM200012143432406. [DOI] [PubMed] [Google Scholar]
- 27.California Department of Health Services. California Tobacco Control Update 2006: The Social Norm Change Approach. Sacramento, CA: California Department of Health Services; 2006. [Google Scholar]
- 28.Dorfman L, Wilbur P, Lingas EO, Woodruff K, Wallack L. Accelerating Policy on Nutrition: Lessons from Tobacco, Alcohol, Firearms, and Traffic Safety. Berkeley, CA: Berkeley Media Studies Group; 2005. [Google Scholar]
- 29.Roesler A, Burns D. The quarter that changed the world. Tobac Control. 2010;19(suppl 1):i3–15. doi: 10.1136/tc.2009.030809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daynard RA. Lessons from tobacco control for the obesity control movement. J Public Health Policy. 2003;24:291–5. [PubMed] [Google Scholar]
- 31.Mercer SL, Green LW, Rosenthal AC, et al. Possible lessons from the tobacco experience for obesity control. Am J Clin Nutr. 2003;77:1073S–82S. doi: 10.1093/ajcn/77.4.1073S. [DOI] [PubMed] [Google Scholar]
- 32.Green LW, Mercer SL, Rosenthal AC, Dietz WC, Husten CC. Proceedings of the International Union for Nutrition Science, Vienna: IUNS, 2001. Modern Aspects of Nutrition—Present Knowledge and Future Perspectives, in the Book Series Forum Nutrition (Formerly Bibliotheca Nutritio et Dieta) Vol. 56. Basel, Switzerland: S. Karger Publishers; 2003. Possible lessons for physician counseling on obesity from the progress in smoking cessation in primary care. [PubMed] [Google Scholar]
- 33.Eriksen MP. Koplan JP, Liverman CT, Kraak VI, (eds). Preventing Childhood Obesity. Washington, DC: National Academies Press; 2005. Lessons learned from public health efforts and their relevance to preventing childhood obesity. . Appendix D. [PubMed] [Google Scholar]
- 34.Green LW, Nathan R, Mercer S. The health of health promotion in public policy: drawing inspiration from the tobacco control movement. Health Prom J Austr. 2001;12:12–8. [Google Scholar]
- 35.Stillman FA, Hartman AM, Graubard BI, et al. Evaluation of the American Stop Smoking Intervention Study (ASSIST): a report of outcomes. J Natl Cancer Inst. 2003;95:1681–91. doi: 10.1093/jnci/djg098. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Best Practices for Comprehensive Tobacco Control Programs—2007. 2nd edn. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2007. . www.cdc.gov/tobacco/stateandcommunity/best_practices/index.htm (accessed on 29 May 2011) [Google Scholar]
- 37.Green LW, Orleans CT, Ottoson JM, et al. Inferring strategies for disseminating physical activity policies, programs, and practices from the successes of tobacco control. Am J Prev Med. 2006;31(suppl 4):S66–81. doi: 10.1016/j.amepre.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 38.Task Force on Community Preventive Services. The Guide to Community Preventive Services: What Works to Promote Health? Oxford, UK: Oxford University Press; 2005. . For updates to systematic reviews of evidence-based preventive interventions to promote population health, http://www.thecommunityguide.org. [Google Scholar]
- 39.Sallis JF, Cervero RB, Ascher W, et al. An ecological approach to creating active living communities. Annu Rev Public Health. 2006;27:297–322. doi: 10.1146/annurev.publhealth.27.021405.102100. [DOI] [PubMed] [Google Scholar]
- 40.Diez Roux A. Residential environments and cardiovascular health. J Urban Health. 2003;80:569–89. doi: 10.1093/jurban/jtg065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.French SA, Story M, Jeffery RW. Environmental influences on eating and physical activity. Annu Rev Public Health. 2001;22:309–35. doi: 10.1146/annurev.publhealth.22.1.309. [DOI] [PubMed] [Google Scholar]
- 42.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 43.Wennberg JE, Gittelson A. Variations in medical care among small areas. Sci Am. 1982;29(S1):13–23. doi: 10.1038/scientificamerican0482-120. [DOI] [PubMed] [Google Scholar]
- 44.SEARCH for Diabetes in Youth Study Group. Liese AD, D'Agostino RB, Jr, et al. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510–8. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- 45.American Diabetes Association. Type 2 diabetes in children and adolescents: consensus statement. Diabetes Care. 2000;23:381–9. doi: 10.2337/diacare.23.3.381. [DOI] [PubMed] [Google Scholar]
- 46.Rosenbloom AL, Silverstein JH, Amemiya S, Zeitler P, Klingensmith GJ. Type 2 diabetes mellitus in the child and adolescent. Pediatr Diabetes. 2008;9:512–26. doi: 10.1111/j.1399-5448.2008.00429.x. [DOI] [PubMed] [Google Scholar]
- 47.Monzavi R, Dreimane D, Geffner ME, et al. Improvement in risk factors for metabolic syndrome and insulin resistance in overweight youth who are treated with lifestyle intervention. J Pediatr. 2006;117:e1111–8. doi: 10.1542/peds.2005-1532. [DOI] [PubMed] [Google Scholar]
- 48.Hoelscher DM, Feldman HA, Johnson CC, et al. School-based health education programs can be maintained over time: results from the CATCH Institutionalization study. Prev Med. 2004;38:594–606. doi: 10.1016/j.ypmed.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 49.Institute of Medicine. Bridging the Evidence Gap in Obesity Prevention: A Framework to Inform Decision Making. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 50.HEALTHY Study Group. Foster GD, Linder B, et al. A school-based intervention for diabetes risk reduction. N Engl J Med. 2010;363:443–53. doi: 10.1056/NEJMoa1001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Economos CD, Hyatt RR, Goldberg JP, et al. A community intervention reduces BMI z-score in children: shape up Somerville first year results. Obesity. 2007;15:1325–36. doi: 10.1038/oby.2007.155. [DOI] [PubMed] [Google Scholar]
- 52.Garfield SA, Malozowski S, Chin MH, et al. Considerations for diabetes translational research in real-world settings. Diabetes Care. 2003;26:2670–4. doi: 10.2337/diacare.26.9.2670. [DOI] [PubMed] [Google Scholar]
- 53.Glasgow RE. Translating research to practice: lessons learned, areas for improvement, and future directions. Diabetes Care. 2003;26:2451–6. doi: 10.2337/diacare.26.8.2451. [DOI] [PubMed] [Google Scholar]
- 54.Lindström J, Peltonen M, Eriksson JG, et al. Determinants for the effectiveness of lifestyle intervention in the Finnish Diabetes Prevention Study. Diabetes Care. 2008;31:857–62. doi: 10.2337/dc07-2162. [DOI] [PubMed] [Google Scholar]
- 55.Saaristo T, Moilanen L, Korpi-Hyovaiti E, et al. Lifestyle intervention for prevention of type 2 diabetes in primary health care: one-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D) Diabetes Care. 2010;33:2146–51. doi: 10.2337/dc10-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saaristo T, Peltonen M, Keinänen-Kiukaanniemi S, et al. National Type 2 Diabetes Programme in Finland: FIN-D2D. Int J Circumpolar Health. 2007;66:101–12. doi: 10.3402/ijch.v66i2.18239. [DOI] [PubMed] [Google Scholar]
- 57.Rothe U, Muller G, Schwarz PE, et al. Evaluation of a diabetes management system based on practice guidelines, integrated care, and continuous quality management in a Federal State of Germany: a population-based approach to health care research. Diabetes Care. 2008;31:863–8. doi: 10.2337/dc07-0858. [DOI] [PubMed] [Google Scholar]
- 58.Schwarz PE. Public health implications: translation into diabetes prevention initiatives—four-level public health concept. Med Clin North Am. 2011;95:397–407. doi: 10.1016/j.mcna.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 59.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371:1783–9. doi: 10.1016/S0140-6736(08)60766-7. [DOI] [PubMed] [Google Scholar]
- 60.Glasgow RE, Emmons KM. How can we increase translation of research into practice? Types of evidence needed. Annu Rev Public Health. 2007;28:413–33. doi: 10.1146/annurev.publhealth.28.021406.144145. [DOI] [PubMed] [Google Scholar]
- 61.Green LW, Glasgow R. Evaluating the relevance, generalization, and applicability of research: issues in external validity and translation methodology. Eval Health Prof. 2006;29:126–53. doi: 10.1177/0163278705284445. [DOI] [PubMed] [Google Scholar]
- 62.Herman WH, Brandle M, Zhang P, et al. Costs associated with the primary prevention of type 2 diabetes mellitus in the diabetes prevention program. Diabetes Care. 2003;26:36–47. doi: 10.2337/diacare.26.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marrero DG. The prevention of type 2 diabetes: an overview. J Diabetes Sci Technol. 2009;3:756–60. doi: 10.1177/193229680900300423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ackermann RT. Description of an integrated framework for building linkages among primary care clinics and community organizations for the prevention of type 2 diabetes: emerging themes from the CC-Link study. Chronic Illn. 2010;6:89–100. doi: 10.1177/1742395310364857. http://www.ncbi.nlm.nih.gov/pubmed/20484325. [DOI] [PubMed] [Google Scholar]
- 65.Rolka DB, Narayan KM, Thompson TJ, et al. Performance of recommended screening tests for undiagnosed diabetes and dysglycemia. Diabetes Care. 2001;24:1899–903. doi: 10.2337/diacare.24.11.1899. [DOI] [PubMed] [Google Scholar]
- 66.Kruijshoop M, Feskens EJ, Blaak EE, de Bruin TW. Validation of capillary glucose measurements to detect glucose intolerance or type 2 diabetes mellitus in the general population. Clin Chim Acta. 2004;341:33–40. doi: 10.1016/j.cccn.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 67.Ackermann RT, Marrero DG, Hicks KA, et al. An evaluation of cost sharing to finance a diet and physical activity intervention to prevent diabetes. Diabetes Care. 2006;29:1237–41. doi: 10.2337/dc05-1709. [DOI] [PubMed] [Google Scholar]
- 68. Centers for Disease Control and Prevention, US Dept of Health and Human Services. See www.cdc.gov/diabetes (accessed on 27 December 2011 )
- 69. National Physical Activity Plan. Make the Move. www.physicalactivityplan.org (accessed on 27 December 2011)
- 70.Green LW. In praise of partnerships; caveats on coalitions. Health Promot Pract. 2000;1:64–65. [Google Scholar]
- 71.Sallis JF, Glanz K. The physical activity environment and food environments: toward solutions to the obesity epidemic. Milbank Quart. 2009;87:123–54. doi: 10.1111/j.1468-0009.2009.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samuels SE. Project LEAN–lessons learned from a national social marketing campaign. Public Health Rep. 1993;108:45–53. [PMC free article] [PubMed] [Google Scholar]
- 73.Samuels SE, Green LW, Tarlov AR. Project LEAN. Am J Public Health. 1989;79:350. doi: 10.2105/ajph.79.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Centers for Disease Control and Prevention. Communities Putting Prevention to Work. www.cdc.gov/CommunitiesPuttingPreventiontoWork/. (accessed on 27 December 2011) and http://www.hhs.gov/recovery/programs/cppw/factsheet.html (accessed on 27 December 2011)
- 75. Centers for Disease Control and Prevention. Community Transformation Grants (CTGs). http://www.cdc.gov/communitytransformation/. (accessed on 27 December 2011)
- 76.Maes L, Van Cauwenberghe E, Van Lippevelde W, et al. Effectiveness of workplace interventions in Europe promoting healthy eating: a systematic review. Eur J Public Health. 2011 doi: 10.1093/eurpub/ckr098. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 77.Engbers LH, van Poppel MN, Chin A Paw MJ, van Mechelen W. Worksite health promotion programs with environmental changes: a systematic review. Am J Prev Med. 2005;29:61–70. doi: 10.1016/j.amepre.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 78.Pelletier KR. A review and analysis of the clinical- and cost-effectiveness studies of comprehensive health promotion and disease management programs at the worksite: 1998–2000 update. Am J Health Promot. 2001;16(2):107–16. doi: 10.4278/0890-1171-16.2.107. [DOI] [PubMed] [Google Scholar]


