Abstract
Surface reactivity of bioactive ceramics contributes in accelerating bone healing by anchoring osteoblast cells and the connection of the surrounding bone tissues. The presence of silicon (Si) in many biocompatible and bioactive materials has been shown to improve osteoblast cell adhesion, proliferation and bone regeneration due to its role in the mineralisation process around implants. In this study, the effects of Si-biphasic calcium phosphate (Si-BCP) on bioactivity and adhesion of human osteoblast (hFOB) as an in vitro model have been investigated. Si-BCP was synthesised using calcium hydroxide (Ca(OH)2) and phosphoric acid (H3PO4) via wet synthesis technique at Ca/P ratio 1.60 of material precursors. SiO2 at 3 wt% based on total precursors was added into apatite slurry before proceeding with the spray drying process. Apatite powder derived from the spray drying process was pressed into discs with Ø 10 mm. Finally, the discs were sintered at atmospheric condition to obtain biphasic hydroxyapatite (HA) and tricalcium phosphate (TCP) peaks simultaneously and examined by XRD, AFM and SEM for its bioactivity evaluation. In vitro cell viability of L929 fibroblast and adhesion of hFOB cell were investigated via AlamarBlue® (AB) assay and SEM respectively. All results were compared with BCP without Si substitution. Results showed that the presence of Si affected the material’s surface and morphology, cell proliferation and cell adhesion. AFM and SEM of Si-BCP revealed a rougher surface compared to BCP. Bioactivity in simulated body fluid (SBF) was characterised by pH, weight gain and apatite mineralisation on the sample surface whereby the changes in surface morphology were evaluated using SEM. Immersion in SBF up to 21 days indicated significant changes in pH, weight gain and apatite formation. Cell viability has demonstrated no cytotoxic effect and denoted that Si-BCP promoted good initial cell adhesion and proliferation. These results suggest that Si-BCP’s surface roughness (164 nm) was significantly higher than BCP (88 nm), thus enhancing the adhesion and proliferation of the osteoblast.
Keywords: Surface roughness, Calcium phosphate, Bioactivity, Cell adhesion, Cytotoxicity
1. Introduction
Calcium phosphate ceramics (CPCs) are a class of tunable bioactive materials that have been widely used for bone tissue repair and augmentation. They possess surface properties that support osteoblast adhesion and proliferation (i.e. osteoconduction) and stimulate new bone formation (i.e. osteoinduction). More significantly, CPCs have been shown to promote bone growth in vivo, and recruit bone marrow stromal cells (BMSCs) to ectopic sites to induce bone formation (Samayedia et al., 2013).
In the field of bone tissue engineering, hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP) are the two most extensively researched biomaterials in the entire family of CPCs, which have been frequently used for the development of the scaffold. HA, an inorganic mineral found in natural bone, is osteoconductive but has a slow resorption rate in the physiological environment, while β-TCP has an optimum resorption rate but poor mechanical stability. On the other hand, the complimentary properties of the two bioceramics have inspired researchers to develop a biphasic (HA/β-TCP) system, equipped with mechanical stability and time dependent resorbability, which is suitable for bioactive scaffold application in tissue engineering (Choi et al., 2013).
Surface reactivity is one of the common characteristics of bone bioactive ceramics. It contributes to their bone bonding ability and their enhancing effect on bone tissue formation. During implantation, reactions occur on the material tissue interface, which leads to time-dependent changes in the surface characteristics of the implant material, and also in tissues (Mazon et al., 2015).
Several literatures reported on silicon (Si) based materials that have superior bioactivity than simply CPCs (Manchon et al., 2015, Alexis et al., 2007). The biocompatibility of these materials has led them to be investigated extensively for use as implant material in the human body to repair and replace diseased and damaged bone. Despite these properties, Si also acts as a regulating factor for the deposition of calcium (Ca) and phosphorus (P) in the bone tissue and also plays an ongoing role on maintaining the bone after their formation. Ionic substitution as some metallic ions such as Si have documented biological effect on regeneration and bone remodelling (Manchon et al., 2015). Moreover, Si ions released from Si-substituted calcium phosphates could positively influence osteoblast cells (Ahn et al., 2013).
To investigate the proliferation and adhesion of osteoblasts on CPCs, we have systematically conducted the culturing of human osteoblast cells on Si-biphasic calcium phosphate (Si-BCP). Evaluation using human osteoblast mimics human bone regeneration as compared to other studies using mouse fibroblast, marrow stromal cell and osteoblast-like cell (Boorungsiman et al., 2012, Zhina et al., 2015, Ye et al., 2013).
In this paper, our studies were focused on the in vitro bioactivity and cytocompatibility evaluations of Si-BCP and compared with unsubstituted biphasic calcium phosphate (BCP) discs. Moreover, the adhesion and proliferation of osteoblasts onto Si-BCP material surfaces were determined based on the surface roughness of Si-BCP compared with unsubstituted BCP. Evaluations include characterisation by SEM, AFM, immersion in simulated body fluid (SBF), pH and weight gain study, cell viability and adhesion of cell using fibroblast and osteoblast respectively.
2. Materials and methods
Calcium phosphate slurry was prepared using calcium hydroxide and phosphoric acid via wet synthesis technique. The mixture precursors were based on 1.60 Ca/P ratio. Phosphoric acid was added gradually into calcium hydroxide solution and stirred at 250 rpm using a mechanical stirrer. Temperature during reaction of the mixture was controlled between 80 and 85 °C and final pH of the suspension was set below 9.5. Silicon dioxide (SiO2) was added into the apatite slurry at 3 wt%. White gelatinous apatite slurry was obtained at the end of the process. This gelatinous apatite slurry then proceeded with the spray drying (GEA Niro; Mobile Minor 2000 H, Soeborg, Denmark) process to obtain dry apatite powder.
Prior to spray drying, the spray dryer inlet temperature is set to 280 °C and an outlet temperature of not more than 120 °C. The compressed air is turned on to −0.05 MPa to 0.3 MPa (−0.5 to 3 bar) and 50–70% air on the flow metre. This is followed by turning on the feed pump (Brand Watson Marlow 505s) in the range of 20–45 rpm. To spray dry, DDI water is slowly fed first into the atomizer until the required outlet temperature is stable ∼85–95 °C. Then the feed pump is switched from distiled water to the gelatinous apatite slurry. The air pressure is kept constant in order to obtain homogenous atomisation. Once the spray drying process is completed, the spray dried apatite powder is collected from the glass-jar collector at the bottom of the spray dryer. Apatite powder derived from the spray drying process was pressed into discs (Ø:10 mm; H:1 mm) and sintered at 950 °C in air condition to obtain silicon-biphasic calcium phosphate (Si-BCP). The samples were then characterised using an X-ray diffractometer (Bruker, D8 Advanced, Germany).
2.1. Microscopic investigation
The surface structure of Si-BCP and BCP discs was first observed using a scanning electron microscope, SEM (Leo, Germany) and subsequently the quantitative surface roughness was determined using, atomic force microscopy, AFM (Shimadzu Corporation, SPM-9500J2, Japan). The measurements for AFM were performed in tapping mode.
2.2. Bioactivity studies
Si-BCP and BCP discs were immersed in 30 ml of simulated body fluid (SBF) and placed in an incubator at 37 °C (Memmert BE 600, Germany) for various immersion times (7 d, 14 d and 21 d). The solution was replenished every other day to expose the materials to fresh solution.
The SBF with ion concentrations equivalent to human blood plasma was freshly prepared by dissolving reagent-grade chemicals of NaCl, NaHCO3, KCl, K2HPO4·3H2O, MgCl2·6H2O, CaCl2 and Na2SO4 into DDI. The solution was buffered at pH 7.4 with 1 M HCl and tris (hydroxymethyl) aminomethane at 37 °C (Kokubo and Takadama, 2006). The comparative amounts of ionic concentrations in mM of SBF and human blood plasma are listed in Table 1.
Table 1.
Ion concentrations of SBF and human blood plasma.
| Ion concentration (mM) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Mg2+ | Ca2+ | Cl− | HCO3− | HPO42− | SO42− | |
| SBF solution | 142.0 | 5.0 | 1.5 | 2.5 | 148.8 | 4.2 | 1.0 | 0 |
| Human blood plasma | 142.0 | 5.0 | 1.5 | 2.5 | 103.0 | 27.0 | 1.0 | 0.5 |
Materials were removed from SBF and dried overnight at room temperature prior to weight measurement and observation of apatite growth using SEM. Meanwhile, pH of the withdrawn SBF solutions was measured using a pH metre.
2.3. Cell culture
L929 mouse subcutaneous connective tissue fibroblast cells (Mus musculus, NCTC clone 929, CCL-1™, ATCC®, Manassas, VA, USA) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco® Life Technologies, Grand Island, NY, USA) containing penicillin/streptomycin (100/100U; Gibco® Life Technologies), L-glutamine (200 mM; Gibco® Life Technologies), and HEPES (Gibco® Life Technologies) and supplemented with 10% foetal bovine serum (Gibco® Life Technologies). They were incubated at 37 °C in a humidified atmosphere containing 5% CO2 (CO2 incubator; Binder GmbH, Tutlingen, Germany). The medium was changed every 3 d under aseptic conditions.
The hFOB 1.19 cells (Homo sapiens, CRL-11372™, ATCC®, Manassas, VA, USA) were cultured in Dulbecco’s Modified Eagle’s Medium/Ham’s Nutrient Mixture F12 (1:1 DME/F12 Modified; Sigma–Aldrich, St Louis, MO, USA) containing Geneticin® (Gibco® Life Technologies) and supplemented with 10% foetal bovine serum (Gibco® Life Technologies). They were incubated at 34 °C in a humidified atmosphere containing 5% CO2 (CO2 incubator; Nuaire, Plymouth, MN, USA). The medium was changed every 3 d under aseptic conditions.
2.4. Cell viability using AlamarBlue®
Si-BCP and BCP extracts with a weight-volume ratio 200 mg/ml were generated by immersing the materials in complete media for 24 h at 37 °C without agitation. The unexposed (negative control) was the extraction vehicle (complete media) without any material added. The pure extract (200 mg/ml) and 100, 50, and 25 mg/ml dilutions (prepared using complete media) of Si-BCP and BCP were added to fibroblast cells that had been seeded at 1 × 105 cells/ml in 24-multidish plates for 24 h. After a 24-h incubation period, cell viability was tested using the AlamarBlue® cell viability reagent (Invitrogen®, Life Technologies). The culture was stained with AlamarBlue® (1:10) and incubated for 4 h at 37 °C. After incubation, the stained culture was detected by absorbance at 570 nm using a Universal Microplate Reader (Bio-Tek Instruments, Winooski, VT, USA). Four replicates were performed for each treatment (ISO 10993-5, 2009).
2.5. Cell adhesion
Both disc materials Si-BCP and BCP were placed into 24-multidish plate. hFOB cells were seeded onto the materials using complete media with a density of 5 × 104 cells/ml. The multidish plates were incubated at 34 °C. After 3 days of incubation, cell adhesion was observed under a Scanning Electron Microscope (SEM; Leo, Germany) post fixation with 2.5% glutaraldehyde.
3. Results and discussion
3.1. Phase analysis
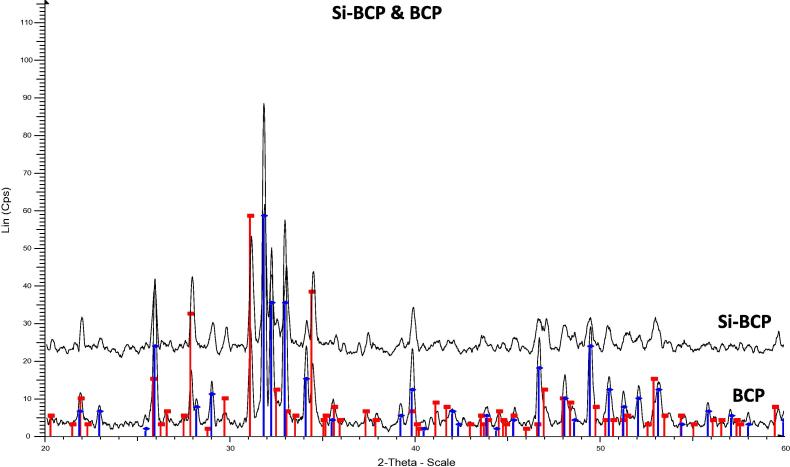
In order to determine the phase composition of the synthesised CaP, XRD analysis was conducted. Fig. 1 shows XRD patterns of Si-BCP and BCP. It is clearly shown that both materials have distinct peaks of HA and TCP phases. It suggested that both materials did not change into other phases during sintering because it’s stable at high temperature. However, due to poor crystallinity properties of Si which is also embedded in this mixture, it was not detected through this method.
Figure 1.
XRD pattern of Si-BCP and BCP.
3.2. Surface characteristics of Si-BCP
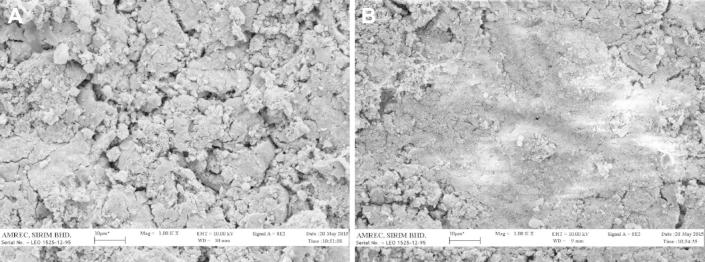
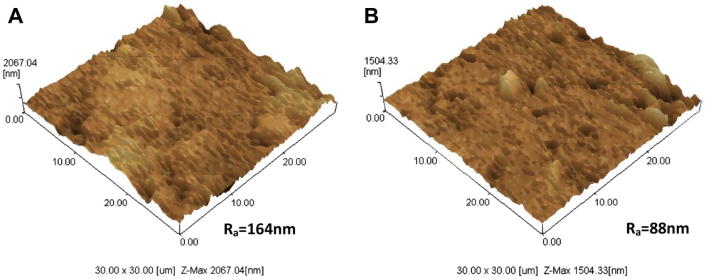
The Si-BCP and BCP materials were characterised to determine morphology of the discs using SEM (2D) and AFM (3D) prior to bioactivity and cell adhesion studies. The SEM microscopic images revealed significant differences in morphology of the Si-BCP and BCP materials (Fig. 2). The Si-BCP material exhibits a rougher surface compared to BCP. These results were corroborated with AFM surface topography images. The quantitative analysis of surface roughness (Ra) as illustrated in Fig. 3 showed that Ra of Si-BCP (164 nm) was significantly higher than BCP (88 nm). The presence of Si induced significant morphological changes of the material that enhanced the higher Ra.
Figure 2.
SEM images (1000×) of (A) Si-BCP and (B) BCP.
Figure 3.
AFM images of (A) Si-BCP and (B) BCP surface.
3.3. Bioactivity studies
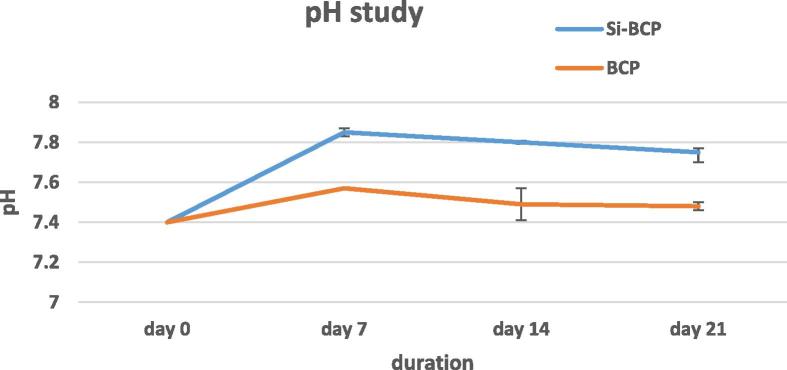
The in vitro bioactivity of a biomaterial can be easily predicted by using SBF, which possesses a similar ionic concentration as human blood plasma and, thus, it can simulate the microenvironment of a bone inside the body (Claudia et al., 2015, Kokubo and Takadama, 2006). Fig. 4 describes the pH of SBF at 7, 14 and 21 days. The starting pH of SBF was set to 7.4. The pH of both materials rapidly increased within 7 days of incubation followed by a slow decrease. The rapid increase of pH at day 7 mainly occurred at high concentrations of Ca ion into SBF solutions by dissolution. A fall of pH was observed at day 14 and 21. Release of the hydroxyl radical from the material surface chemistry might play a role in pH decrease at this stage. CaP compounds were precipitated spontaneously when the pH value of the physiological solution was over 7.4. During the prolonged immersion time, Ca and P ions are consumed largely due to the formation of abundant apatite; and simultaneously, OH− is consumed correspondingly. Thus the pH value of SBF decreases (Fan et al., 2009).
Figure 4.
pH study of Si-BCP and BCP. Data are expressed as mean ± standard deviation.
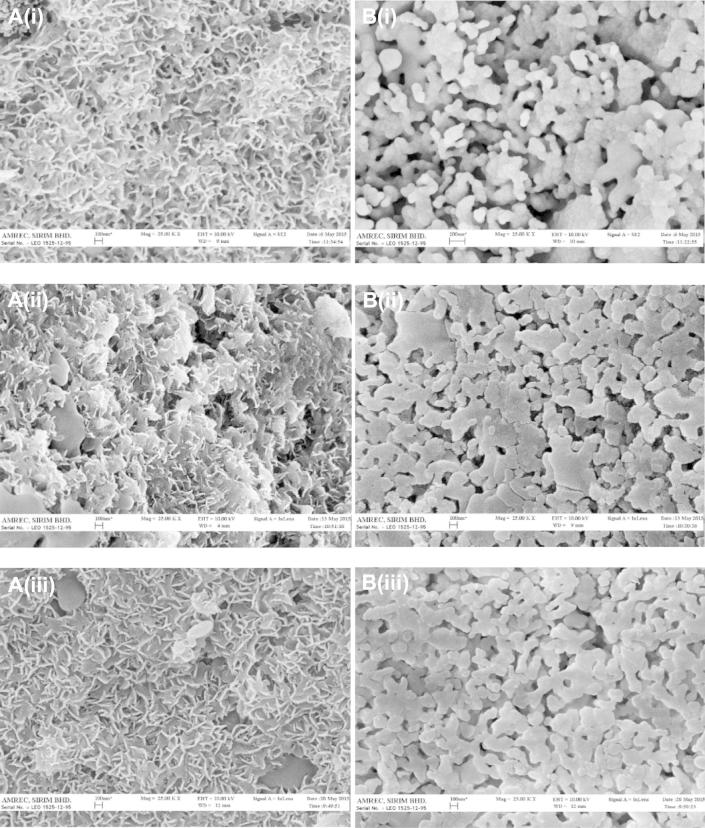
It has been reported that rapid nucleation was attributed to the rough surface morphology of the material after immersing in SBF. This rough surface of Si-BCP might create a favourable microenvironment where Ca and P ions tend to concentrate and react with the surface to form an apatite layer (Jyoti et al., 2010). Changes in surface morphology of the material surface after exposure to SBF at a pre-determined duration was examined using SEM. Fig. 5 showed the surface morphologies of the materials after various incubation periods in SBF. These images clearly confirmed apatite formation. After 7 days of incubation, Si-BCP showed confluent flaky-shaped apatite formation, however BCP showed confluent round-shaped apatite.
Figure 5.
Apatite appearances with different morphologies of materials (A) Si-BCP and (B) BCP after (i) 7 days (ii) 14 days and (iii) 21 days incubation in SBF respectively.
Surface bioactivity mainly refers to the precipitation and mineralisation of calcium phosphate on implants, which is affected by the physical and chemical properties of the surface (Zhang et al., 2013a). The formation and growth of apatite corresponding to the dynamic process follow as the material surfaces dissolve and the apatite layers precipitate on the surface. It is believed that apatite nucleation and growth occur with Ca and P consumption (Hejazi et al., 2015). Other studies also reported that OH− is very critical for surface bioactivity and it can improve the nucleation and growth of calcium phosphate. When the materials were soaked in the SBF, HA offered many OH− terminals that could absorb Ca2+ firstly, and then PO43−, which promoted the nucleation and growth of calcium phosphate (Zhang et al., 2013b).
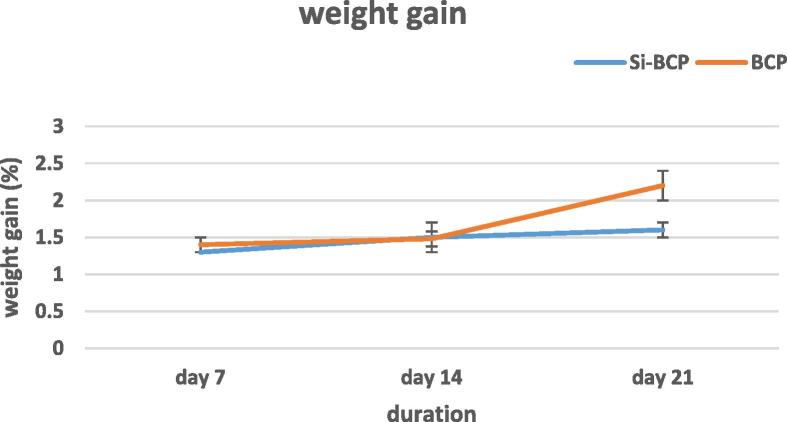
The dried material was weighed to determine whether the weight is increasing or decreasing after exposure to SBF at a pre-determined duration. Fig. 6 shows the weight gain (%) change of both materials immersed in SBF for different time durations. Both materials showed a weight gain with a varying rate of weight change. This variation is due to the differential dissolution and precipitation rates of Ca and P. Once HA nucleation starts, it is expected that there is a consistent weight gain (Karanjai et al., 2008). This result is in line with apatite formation on the material surface. The presence of apatite mineralisation caused the weight gain. Round-shaped apatite formation on BCP is heavier than flaky-shaped apatite on Si-BCP. The heavier apatite on the BCP surfaces is due to higher bioactivity and its apatite dense morphology characteristic. Spherical apatite obtained on the surface is because of the surface tension and adhesion force co-effect around the crystals (Fan et al., 2009).
Figure 6.
Weight gain study of Si-BCP and BCP. Data are expressed as mean ± standard deviation.
3.4. Cellular viability
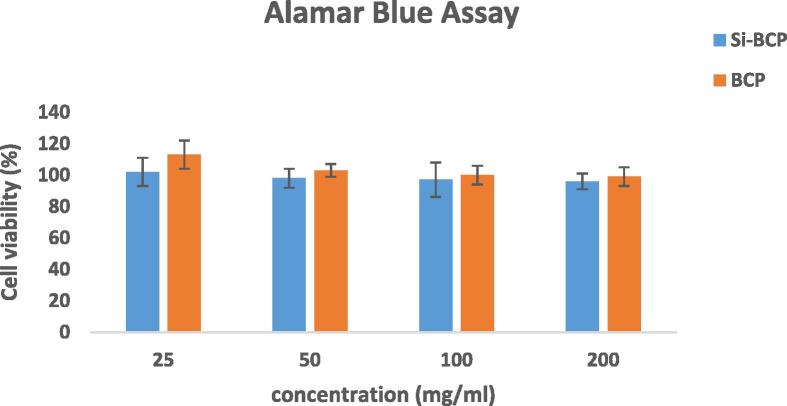
Cytotoxic response of the Si-BCP and BCP materials were assessed by the AB assay. Based on the cytotoxicity results as shown in Fig. 7, both materials showed no adverse effect on L929 cells on all concentration tested. Levels in the AB assay of both materials were more than 80% cell viability and cell growth increment. Baker et al. reported 50% cell viability and onward indicates that the material is non-cytotoxic, whereas less than 50% cell viability indicates that the material is cytotoxic (Baker et al., 2009). Cytotoxicity of bi-phasic calcium phosphate/PLLA biocomposite test showed L929 fibroblast could well differentiate and proliferate (Yang et al., 2010) and HA/PLA nanocomposite does not have a negative effect on the cell viability, morphology and proliferation on L929 fibroblastic cells (Wan et al., 2015).
Figure 7.
Cell viability of Si-BCP and BCP extract. Data are expressed as mean ± standard deviation.
3.5. Cellular adhesion on materials
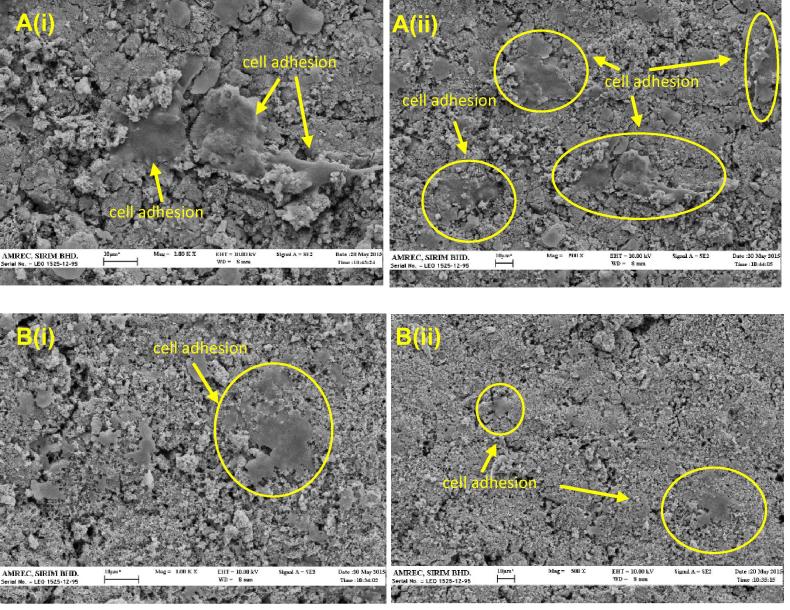
Both materials with excellent cell viability were further evaluated with cell adhesion study. Fig. 8 presents the morphology of the osteoblast cells cultured for 3 days on the surface of the Si-BCP and BCP. Difference in the response of the cells to the different surfaces was obvious. For the Si-BCP, many cells were observed and some of the cells seemed to be connected with each other in low magnification. At higher magnifications, adhered elongated morphological cells spread with numerous filamentous extensions indicating better attachment of osteoblast to the Si-BCP material. Low Si content of the BCP could enhance osteogenic gene activities in the cells. Ahn et al. hypothesised that silicon triggered an increase in the mass of the extracellular matrix, resulting in higher levels of bioactive osteogenic signalling molecules in close proximity to the cells and leading in turn to enhance osteogenic activity (Ahn et al., 2013). Meanwhile, for the BCP it is hard to distinguish between the cells and the matrix. Only a few cells were observed with flattened morphology. Xu et al. reported surface chemical properties of the biomaterials play a very important role in good surface bioactivity and cytocompatibility (Xu et al., 2009). Research has shown that mesoporous HA coating on β-TCP scaffold results in an increase in osteoblast adhesion (Ye et al., 2013), and nanoHA whisker/β-TCP/bioglass scaffold has been shown to stimulate cell proliferation and adhesion of osteoblast (Hu et al., 2012).
Figure 8.
SEM images of (A) Si-BCP and (B) BCP incubated 3 days with osteoblast at (i) 1000× and (ii) 500×. Yellow circles indicate osteoblast adhesion on materials.
4. Conclusion
The results imply that incorporation of Si has induced rapid nucleation towards apatite mineralisation. Moreover, our findings confirm the role of Si in enhancing osteoblast proliferation and adhesion mainly due to its rougher surfaces. Hence, it can be concluded that Si incorporated BCP has significant potential for bone regeneration applications.
Acknowledgements
This work was supported by Ministry of Science, Technology and Innovation (MOSTI) under grant 03-03-02-SF0261. We wish to thank SIRIM Berhad, En. Sahhidan Daud, En. Zuber Me and En. Ahmad Nizam Abdullah for the contribution in this study.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahn G., Lee J.Y., Seol D., Pyo S.G., Lee D. The effect of calcium phosphate cement-silica composite materials on proliferation and differentiation of pre-osteoblast cells. Mater. Lett. 2013;109:302–305. [Google Scholar]
- Alexis M.P., Joel W.R., Malcolm J.S., Michael S. Silicon substitution in the calcium phosphate bioceramic. Biomaterials. 2007;28:4023–4033. doi: 10.1016/j.biomaterials.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Baker M.A., Assis S.L., Higa O.Z., Costa I. Nanocomposite hydroxyapatite formation on Ti-13Nb-13Zr alloy exposed in a MEM cell culture medium and the effect of H2O2 addition. Acta Biomater. 2009;5(1):63–75. doi: 10.1016/j.actbio.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Boorungsiman S., Gentleman E., Carzaniga R., Evans N.D., Mccomb D.W., Porter A.E., Stevens M.M. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc. Natl. Acad. Sci. 2012;109(35):14170–14175. doi: 10.1073/pnas.1208916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y., Kwon J., Song D., Choi E.H., Lee Y., Kim K., Kim K. Surface modification of biphasic calcium phosphate scaffolds by non-thermal atmospheric pressure nitrogen and air plasma treatment for improving osteoblast attachment and proliferation. Thin Solid Films. 2013;547:235–240. [Google Scholar]
- Claudia T., Baltzar S., Jekabs G., Isabel I., Ana G., Daniel A., Maria V., Mattias E. Composition-dependent in vitro apatite formation at mesoporous bioactive glass-surfaces quantified by solid-state NMR and powder XRD. RSC Adv. 2015;5:86061–86071. [Google Scholar]
- Fan X., Chen J., Zou J., Wan Q., Zhou Z., Ruan J. Bone-like apatite formation on HA/316L stainless steel composite surface in simulated body fluid. Trans. Nonferrous Met. Soc. China. 2009;19:347–352. [Google Scholar]
- Hejazi M.S., Meratian M., Fathi M.H. Effect of alumina amount on the bioactivity of dense magnesium fluorapatite/alumina composite in simulated body fluid (SBF) using Taguchi method. J. Bioprocess Biotechnol. 2015;5:219. [Google Scholar]
- Hu H., Xu G., Zan Q., Liu J., Liu R., Shen Z., Ye X. In situ formation of nano-hydroxyapatite whisker reinforced porous β-TCP scaffolds. Microelectron. Eng. 2012;98:566–569. [Google Scholar]
- ISO 10993–5, 2009. Biological evaluation of medical devices – Part 5: tests for in vitro cytotoxicity.
- Jyoti M.A., Thai V.V., Min Y.K., Lee B.T., Song H.Y. In vitro bioactivity and biocompatibility of calcium phosphate cements using hydroxy-propyl-methyl-cellulose (HPMC) Appl. Surf. Sci. 2010;257:1533–1539. [Google Scholar]
- Karanjai M., Sundaresan R., Mohan T.R.R., Kashyap B.P. Evaluation of growth of calcium phosphate ceramics on sintered Ti-Ca-P composite. Mater. Sci. Eng., C. 2008;28:1401–1407. [Google Scholar]
- Kokubo T., Takadama H. How useful in SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–2915. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Manchon A., Alkhraisat M., Rueda-Rodriguez C., Torres J., Prados-Frutos J.C., Ewald A., Gbureck U., Cabrejos A. Silicon calcium phosphate ceramics as novel biomaterial to simulate the bone regenerative properties of autologous bone. J. Biomed. Mater. Res., Part A. 2015;103(2):479–488. doi: 10.1002/jbm.a.35196. [DOI] [PubMed] [Google Scholar]
- Mazon P., Garcia-Bernal D., Meseguer-Olmo L., Cragnolini F., Aza P.N.D. Human mesenchymal stem cell viability, proliferation and differentiation potential in response to ceramic chemistry and surface roughness. Ceram. Int. 2015;41(5):6631–6644. [Google Scholar]
- Samayedia S., Whittingtona A.R., Goldstein A.S. Calcium phosphate ceramics in bone tissue engineering: a review of properties and their influence on cell behaviour. Acta Biomater. 2013;9(9):8037–8045. doi: 10.1016/j.actbio.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Wan Y., Wu C., Xiong G., Zuo G., Jin J., Ren K., Zhu Y., Wang Z., Luo H. Mechanical properties and cytotoxicity of nanoplate-like hydroxyapatite/polylactide nanocomposite prepared by intercalation technique. J. Mech. Behav. Biomed. Mater. 2015;47:29–37. doi: 10.1016/j.jmbbm.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Xu L., Pan F., Yu G., Yang L., Zhang E., Ke Yang. In vitro and in vivo evaluation of the surface bioactivity of a calcium phosphate coated magnesium alloy. Biomaterials. 2009;30:1215–1523. doi: 10.1016/j.biomaterials.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Yang W., Yin G., Zhou D., Gu J., Li Y., Zhang H. Biocompatibility of surface-modified biphasic calcium phosphate/polyl-lactide biocomposite in vitro and in vivo. J. Mater. Sci. Technol. 2010;26(8):754–758. [Google Scholar]
- Ye X., Cai S., Xu G., Dou Y., Hu H., Ye X. Preparation and in vitro evaluation of mesoporous hydroxyapatite coated β-TCP porous scaffolds. Mater. Sci. Eng., C. 2013;33:5001–5007. doi: 10.1016/j.msec.2013.08.027. [DOI] [PubMed] [Google Scholar]
- Zhang J., Dai C., Wei J., Wen Z., Zhang S., Chen C. Degradable behaviour and bioactivity of micro-arc oxidized AZ91D Mg alloy with calcium phosphate/chitosan composite coating in m-SBF. Colloids Surf., B. 2013;111:179–187. doi: 10.1016/j.colsurfb.2013.05.040. [DOI] [PubMed] [Google Scholar]
- Zhang J., Dai C., Wei J., Wen Z., Zhang S., Lin L. Calcium phosphate/chitosan composite coating: effect of different concentrations of Mg2+ in the m-SBF on its bioactivity. Appl. Surf. Sci. 2013;280:256–262. [Google Scholar]
- Zhina H., Jhamah N., Javad M. Composite of porous starch-silk fibroin nanofiber-calcium phosphate for bone regeneration. Ceram. Int. 2015;41:10745–10754. [Google Scholar]