Abstract
Increasing lack of potable water in arid countries leads to the use of treated wastewater for crop production. However, the use of inappropriate irrigation practices could result in a serious contamination risk to plants, soils, and groundwater with sewage water. This research was initiated in view to the increasing danger of vegetable crops and groundwater contamination with pathogenic bacteria due to wastewater land application. The research was designed to study: (1) the effect of treated wastewater irrigation on the yield and microbial contamination of the radish plant under field conditions; (2) contamination of the agricultural soil profile with fecal coliform bacteria. Effluent from a domestic wastewater treatment plant (100%) in Jeddah city, Saudi Arabia, was diluted to 80% and 40% with the groundwater of the experimental site constituting three different water qualities plus groundwater as control. Radish plant was grown in two consecutive seasons under two drip irrigation systems and four irrigation water qualities. Upon harvesting, plant weight per ha, total bacterial, fecal coliform, fecal streptococci were detected per 100 g of dry matter and compared with the control. The soil profile was also sampled at an equal distance of 3 cm from soil surface for fecal coliform detection. The results indicated that the yield increased significantly under the subsurface irrigation system and the control water quality compared to surface irrigation system and other water qualities. There was a considerable drop in the count of all bacteria species under the subsurface irrigation system compared to surface irrigation. The bacterial count/g of the plant shoot system increased as the percentage of wastewater in the irrigation water increased. Most of the fecal coliform bacteria were deposited in the first few centimeters below the column inlet and the profile exponentially decreased with increasing depth.
Keywords: Coliform, Contamination, Escherichia coli, Subsurface irrigation, Soil profile, Wastewater
1. Introduction
About 1.2 million people in developing countries live in water scarce areas and by 2025, the number is expected to increase to 1.8 million due to the lack of undependable policies or suitable management strategy for reuse of treated wastewater in crop production (FAO, 2007). In the arid areas, there is an increasing need for water which resulted in the emergence of wastewater application in agriculture to reduce the demand on freshwater resources. Approximately 70% of treated wastewater is used for agriculture (Cytryn, 2010) and may have detrimental environmental and health effects. Transmissions of intestinal nematode (Ascaris lumbricoides and Trichuris trichiura), pathogenic bacteria and diseases, including diarrhea, dysentery, typhoid and cholera to farmer working in the wastewater irrigated fields and/or vegetable consumers were the major risks of the use of treated wastewater (WHO, 1989).
Determination of different microbial pathogen numbers in a partially treated wastewater samples is imperative and can allow an effective assessment of the treatment process. There are always worries and precautions about the reverse effect of treated wastewater use in the irrigation of edible crops (Toze, 2005). Thus, international and local organizations have concerns about putting standards for reuse of treated wastewater in agriculture. Plant production of corn, potato, lettuce, olive trees and alfalfa irrigation with treated wastewater was increased compared to plants irrigated with natural water resources which may be due to the presence of plant nutrients (mainly nitrogen and phosphorus) in the treated wastewater but the risk due to the presence of some pathogens is still under consideration (Mandi and Abissy, 2000, Kouraa et al., 2002, Munir and Mohammad, 2004, Lopez et al., 2006).
Fecal coli bacteria are present in human and animal feces and are relatively harmless to humans’ intestines and Escherichia coli is the most common species of fecal coli bacteria. Furthermore, species of the genus Streptococcus cause pneumonia, ear infection and meningitis. Like fecal coli bacteria, fecal streptococci are applied as indicators of water pollution and have been used for many years to determine the quality and safety of water for irrigation and human consumption (WHO, 1989, Ashraf, 2015.). In treated wastewater, detection of these bacteria means a failure in the disinfection process but their absence does not necessarily guarantee the absence of pathogens. In practice, the desire goal is to obtain zero fecal coliform in water to be used for irrigation of raw eaten crops. To prevent transmission of many diseases, efficient treated wastewaters that comply with the microbiological quality guidelines should be used for crop irrigation.
The fate and transport of bacteria in soils is essential for assessing the risks for groundwater contamination by land-applied wastewater. Contaminant transport experiments can be very useful tools for the quantitative assessment of microbial transport in soils and to explicate the important factors and processes that control microbial transport. Many investigators have examined the transport of microorganisms in soil after land application of wastewater. These studies focused on the removal of bacteria (Schaub and Sorber, 1977, Smith et al., 1985) or viruses (Schaub and Sorber, 1977, Lance et al., 1982, Lance and Gerba, 1984) under conditions of saturated or unsaturated flow in soil columns or in field tests. Many others have investigated bacterial transport through soils (Aislabie et al., 2001, McLeod et al., 2003, Guber et al., 2005). Some have modeled bacteria transport through undisturbed soils (McGechan and Vinten, 2003, Pang et al., 2008). Transport of fecal coliform has been reported in many studies as well (McCoy and Hagedorn, 1980, Jamieson et al., 2002, Unc and Goss, 2003). Contamination of groundwater resources with fecal bacteria or viruses from wastewater poses a threat to the portability and the use of water resources (Crane and Moore, 1984, Kadam et al., 2008).
In the western region of Saudi Arabia, many local farmers use treated and untreated wastewater to grow vegetable crops under no regulation or legislation. The main objective of this research was to exploit and identify microbiological risks associated with the use of treated domestic wastewater in the irrigation of a radish plant under field condition as well as the contamination of the soil profile exposed to wastewater application containing fecal coliform bacteria. To achieve this aim, chemical analysis and some pathogenic bacteria were investigated in the effluent of the wastewater treatment plant, which was used to irrigate radish plant for two successive seasons under two drip irrigation systems. Transport experiment of wastewater through soil column was also investigated to quantify the distribution of fecal coliform.
2. Material and methods
2.1. Experimental design
The experimental site (1 km × 1 km in size) which is the Agricultural Research Station of King Abdulaziz University, Saudi Arabia is located at Hada Al-Sham village; about110 km northeast of Jeddah city on the mainstream of Usfan basin; or about 72 km east of the red sea coast. The soil of the experimental site was classified as sandy loam, pH 7.9, EC 2.4 dS/m, and organic matter 0.55%, heavy metals (mg/kg): Pb 0.9, Cd 0.001, Cr 0.001, and Ni 0.03. Ground water is the main source of irrigation in this region. During this study, the temperature ranged from 30 to 45 °C.
White Radish plant (Raphanus sativus L. cv. Smart Selection Indian) was grown under two different drip irrigation systems and four water qualities. Seeds were obtained from the local markets of Jeddah, washed and soaked in 2% Na hypochlorite for four hours. A split block design arranged in strips subplots with two irrigation systems (surface and subsurface- drip irrigation) and four different treated wastewater qualities (100%, 60% and 40% in addition to groundwater as control) was used. The sub plot area was 2 m × 3 m with four replicates. Fertilizers were applied and irrigation water requirements were calculated and scheduled (Balkhair et al., 2014, Ashraf et al., 2014).
2.2. Irrigation water quality
Bani-Malik WWTP is one of typical plants of Jeddah city. The treated wastewater was used as an irrigation water source for all experiments. The plant located at the center of the city; received its wastewater from neighboring households covering several districts. It treated water up to the secondary stage. Samples of the treated wastewater were collected from the effluent of Bani-Malik WWTP for chemical and biological analysis. Private trucks were rented twice a week to transport treated wastewater from wastewater treatment plant to two reservoirs, connected to four different storage tanks in the field site. Four different irrigation water qualities were prepared and used in this experiment where the effluent of Bani-Malik WWTP was diluted to 40% (40% wastewater and 60% natural groundwater) and 80% in water tanks with a capacity of 2000 gallons. These two dilutions plus raw effluent and local groundwater (control) constitute four different water qualities. Associated tanks and pipelines were installed for each water quality and each irrigation system.
2.3. Collection and analysis of water and plant samples
Fifty treated wastewater samples (one-liter volume), from the effluent of the wastewater treatment plant, were collected for two successive years (at least five samples/time), in a wide mouth sterile glass bottles. Samples were immediately transferred in ice tank to the Lab. Physical and chemical characteristics were determined as described before (Rabah et al., 2007, APHA, 1992, River Watch Network, 1991, USEPA, 1985).
The growth of the plants irrigated with different concentrations of treated wastewater was determined after 4 months. The plants of each treatment were collected in clean plastic bags and transferred directly to the Microbiological lab under refrigeration conditions. The vegetative weight of three random guarded plants for each treatment was determined and recorded as mean fresh weight/plant (g/plant) and total weight/ hectare (t/ha) were calculated for each treatment. Five plants of each treatment were used for the different bacterial counts per gram of the examined plant.
The obtained data were statistically analyzed using the analysis of variance procedures and mean separation under the criteria of Least Significant Difference (LSD) test (Steel and Torrie, 2000, El-Nakhlawy, 2010, Balkhair and Ashraf, 2015).
2.4. Microbiological examination
The total viable bacterial counts in water sample (CFU/100 ml) were determined using Microfil S filtration devices (0.22-μm pore size; Millipore, Billerica, MA) which were used to filter 100 ml of each water sample. The filters were placed on heterotrophic tryptic soy agar plates and incubated at 35 °C before quantifying the CFU/100 ml. Similarly, CFU/g dry weight of the radish shoot system was calculated (ICMSF, 1987).
Total coliforms in water were counted using multiple tube fermentation (MTF) procedures (APHA, 2005). On the other hand, MPN of fecal streptococci was determined using azide dextrose broth at 37 °C for 48 h. Positive tube was indicated by dense turbidity and confirmed using ethyl violet azide dextrose broth incubated at 37 °C for 24 h. The formation of purple color at the bottom of the tube confirmed the presence of fecal streptococci.
Salmonellae group was detected and enumerated by MPN technique (El-Lathy et al., 2009) on Rappaport–Vassiliadis (RV) broth, then streaked on bismuth sulfite agar (ISO, 2000, APHA, 2005). Total Vibrios was detected and enumerated by MPN technique on alkaline peptone broth (pH 8.4) then streaked on thiosulfate citrate bile sucrose agar according to Koch (1994) and APHA (2005). Identification of Salmonella and V. cholera was carried out according to Robert and Noel (1981) and APHA (2005).
Detection and enumeration of E. coli were determined using membrane filter procedure and mTEC Agar (Difco 0334) as described by EPA (2002). Colonies confirmation was determined using Urea Substrate Medium (Urea 2.0 g, phenol red 0.01 g and 100 ml distilled water). Counts were determined from the equation:
| (1) |
| (2) |
MPN of Pseudomonas aeruginosa was determined using three replicate tubes containing l-asparagine broth medium, double and single strengths were used for 10, 1 and 0.1 ml, respectively. Positive tubes were scored as green fluorescent pigment due to the growth of P. aeruginosa (Yehia and Sabae, 2011). Detection of Listeria group was carried out by MPN and by direct detection on Listeria selective broth and agar according to Fenlon (1985).
2.5. Leachate experiment
Undisturbed soil sample was collected from the upper 43 cm at Hada AL-Sham field experimental site. A plexiglass column with inner diameter of 10 cm and height of 50 cm was filled with the soil gradually to maintain soil’s bulk density. Transport experiment was conducted by allowing wastewater-containing known count of fecal bacteria to flow from the top of the soil column at a rate equivalent to the irrigation rate of radish plant. The experiment lasted for six hours, when finished; the soil column was cut into slices of 2 cm at the first 8 cm from soil surface and slices of 4 cm along the rest of the column for coliform detection. The data are presented as normalized concentration No/Nt (number of bacteria retained in the column at specified depth, No, divided by the number in a unit volume of the input wastewater-containing bacteria, Nt) per gram of dry soil and are plotted as a function of the distance from the column inlet.
3. Results and discussion
Water quality is a concern of many countries in the world due to its limited supply and increasing demand due to population increase. Hence, there is a need to encourage people to use treated wastewater for irrigation in arid and semi-arid areas. Water delivered to the consumer or used for irrigation purposes should meet the high requirements of modern hygiene and it should at least be free from pathogenic organisms and toxic substances (Vozmaya, 1983, Abdo, 2005). Thus, attention has been paid for monitoring and assessing the microbiological quality of treated wastewater all over the world (Lindskog and Lindskog, 1988, Fernandez-Alvars et al., 1991). Wastewater represents the main source of water pollution in different parts of the world, e.g. Egypt (Yehia and Sabae, 2011); Poland (Niewolak, 2000); Nigeria (Akaninwor et al., 2007) and Brazil (Gunkel et al., 2007). A powerful monitoring program is needed to provide reliable information about the current water quality before its use in a large scale in agriculture; therefore, the present study was conducted not only to explore the impact of the wastewater quality use, but also to assess the water quality, physico-chemical and the microbial flora of the effluent of Bani-Malik WWTP. The chemical analysis of Bani-Malik WWTP is presented in Table 1. The mean water temperature and pH value were 40 °C and 7.45, respectively while electrical conductivity of treated wastewater and groundwater samples were 2.15 dS/m and 3.9 dS/m respectively. It is a known fact that high temperature enhances the growth and development of human pathogens. Studies indicated a positive correlation between water temperature and total coliform, fecal coliform and P. aeruginosa (Abdo et al., 2010). pH plays an important role in the solubility of the metal hydroxides and the kinetics of the oxidation and hydrolysis processes, where metallic ions tend to precipitate as hydroxides at high pH values. Generally, the obtained pH values fall within the World Health Organization standard limits 7.0–8.5 (DWAF, 1996, WHO, 1984, WHO, 1989).
Table 1.
Chemical and biological analysis of treated wastewater along with standards of the Ministry of Waters and Electricity, Food and Agriculture Organization, and local groundwater (control).
| Parameter | Bani-Malik Effluent WWTP | LGW∗∗∗ | MWE∗ | FAO∗∗ |
|---|---|---|---|---|
| pH | 7.45 | 7.89 | 6.0–8.4 | 6–9 |
| EC (dS/m) | 2.15 | 3.510 | 3.900 | 0–3 |
| SAR | 7.27 | 13 | 0–15 | |
| TDS (mg/l) | 764.55 | 1612 | 2500 | 0–2000 |
| SS (mg/l) | 130.55 | 0 | 10 | – |
| COD (mg/l) | 17.14 | 10.0 | – | – |
| BOD (mg/l) | 2.8 | 0.8 | ND | – |
| NH4+(mg/l) | 33.31 | 0 | 5 | 0–5 |
| NO3− (mg/l) | 23.48 | 0 | 10 | 0–8 |
| P3− (mg/l) | 6.56 | 0 | – | 0–2 |
| Ca ++ (mg/l) | 61.15 | 54.28 | 200 | 400 |
| Mg++ (mg/l) | 11.51 | 27.66 | 150 | 60 |
| Na+ (mg/l) | 43.53 | 83.76 | – | 900 |
| K+ (mg/l) | 6.5 | 3.89 | – | 0–2 |
| Fe++ (mg/l) | 1.58 | 0.018 | 5 | 5 |
| Zn++ (mg/l) | 0.096 | 0.001 | 4 | 2 |
| Mn++ (mg/l) | 0.096 | 0.002 | 0.2 | 0.2 |
| Cu++ (mg/l) | 0.050 | 0.079 | 0.4 | 0.2 |
| Pb+++ (mg/l) | 0.019 | 0.004 | 0.1 | 5 |
| Cd++ (mg/l) | 0.0091 | 0.0001 | 0.01 | 0.2 |
| Cr++ (mg/l) | 0.014 | 0.029 | 0.1 | 0.1 |
| Ni++ (mg/l) | 0.032 | 0.006 | 0.2 | 0.2 |
EC = electrical conductivity (dS/m), COD = chemical oxygen demand (mg/l), BOD = biological oxygen demand (mg/l), *MWE = Ministry of Waters and Electricity, **FAO = Food & Agriculture Organization (1985), ***LGW = local groundwater, TDS: total dissolved solids, SAR: sodium adsorption ratio.
The stages of wastewater treatment were evaluated through determination of ammonia; pH; BOD; COD, chloride; conductivity, suspended solids; total dissolved solids; fats; nitrate, nitrite, total nitrogen; phosphate and total phosphorus (Howard et al., 2004). Electrical conductivity was 2.15 dS/m and higher value was found in the groundwater sample (3.9 dS/m). EC were positively correlated with anion concentrations and total bacterial counts (Abdo et al., 2010). COD values were 10 and 17 mg/l for the treated wastewater and groundwater, respectively. A similar situation was recognized in Jordan, where two monitoring wastewater treatment stations were studied for their water quality before and after stations upgrade (Al-Omari et al., 2013). On the other hand, biochemical oxygen demand (BOD) of Bani-Malik WWTP was 2.8 mg/l and the suspended solids (SS) were 130.5 mg/l. BOD was primarily affected by the presence of microorganisms (APHA, 1992). The NH4+, NO3− and P3− concentrations were 33.31, 23.48 and 6.56 mg/l, respectively. It was well known that wastewater contained many nutrients and ions especially nitrate (NO3−), stimulating plant growth (Magesan, 2001), but when wastewater is irrigated beyond the assimilation capacity of the soil–plant system, it can provide a source of readily leachable nutrient or contaminant (Magesan and Wang, 2003).
Excessive release of heavy metals into the environment has posed a great problem worldwide to many life forms and do not degrade into harmless products (Gupta et al., 2001). The ions of Zn++, Mn++, Cu++, Pb+++, Cd++, Cr++ and Ni++ were detected in the tested wastewater with high variability (Table 1). Comparing the mean metal concentrations of heavy metals with the Saudi standards for reclaimed wastewater reuse and with FAO guidelines for interpretation of water quality for irrigation showed that the Bani-Malik WWTP meets the required standards and guidelines of heavy metal concentration for reused water in irrigation. Lower levels of heavy metals may be due to the fact that Bani-Malik WWTP employed the highest rate of a combination of domestic and industrial wastewaters. It was well known that Cu++ Pb++, Mg++, Mn++, and Zn++ ions get into the aquatic ecosystem through runoffs from agricultural lands (Hutton and Symon, 1986, Pate et al., 2001, Umar and Opaluwa, 2010). Higher quantities of Pb++, Zn++, Fe++, Cd++, Cu++, Mn++ and Hg++ in treated waste water were detected by Opaluwa et al. (2012).
In addition to harmful heavy metals, many biological agents pose serious health threats, such as a risk of transmitting intestinal nematodes and bacterial infections especially to consumers and farm workers. The most common manifestation of waterborne illness is gastrointestinal upset (nausea, vomiting, and diarrhea) and in susceptible individuals such as infants, the elderly and immune compromised individuals, the effects may be more severe, chronic or even fatal (Health Canada, 2006). Heterotrophic, total coliform (TC); fecal coliform (FC); total streptococci were determined per 100 ml of the treated wastewater (TWW) and the groundwater (GW) samples. The results were compared to the standard recommended by FAO (Table 2). Higher counts of TC, FC, TS, E. coil and P. aeruoginosa were recorded for TWW compared with GW but the count of TC and FC was slightly lower than that recommended by WHO, 1989. The presence of these bacteria indicates insufficient water treatment and presence of E. coli is a much more certain indication of fecal contamination. Data from a source with known contamination showed that TC and FC tests were the most reliable contamination indicators, but in some cases, TC bacteria may not be of fecal origin and positive TC test result should be followed by FC, E. coil, or enterococci tests to confirm contamination (Atherholt et al., 2003). In a studied wastewater treatment plant in Poland, contamination with E. coli, Klebsiella pneumoniae and Pantoea agglomerans was recorded (Luczkiewicz et al., 2010).
Table 2.
Bacterial counts of Bani-Malik treated wastewater effluent compared to local groundwater (LGW) and WHO standard.
| Bacterial count/ 100 ml | Bani-Malik Effluent | LGW⁎ | WHO⁎⁎ |
|---|---|---|---|
| Total viable bacterial count | 1.1 × 103 | 2.4 × 102 | ND |
| Total coliform | 962 | 240 | 1000 |
| Fecal coliform | 240 | 0.0 | <1000 |
| Fecal Streptococci | 35–65 | 0.0 | ND |
| E. coli | 60 | 0.0 | ND |
| P. aeuroginosa | 20 | 0.0 | ND |
| Salmonella | 4 | 0.0 | ND |
| Total Vibrio | 4 | ND | ND |
| Listeria group | 2 | ND | ND |
| Nematode (egg/l) | 3 | ND | 1 |
ND: not detected.
LGW = local groundwater.
WHO: World Health Organization (1985).
There was no fecal coliform guideline for restricted irrigation due to the lack of evidence of bacterial infection risk to farm workers and nearby residents. An evidence of enteric infections in farming families in direct contact with partially treated wastewater and in populations living nearby sprinkler irrigated fields, when the water quality exceeds 106 FC/100 ml, suggested that a fecal coliform guideline should be added (WHO, 2001). However, where adults and school-aged rural children are in direct contact with the partially treated wastewater originating in an urban area, there may still be at risk of diarrheal disease at a level of 103–104 FC/100 ml and reduced guideline level of 103 FC/100 ml would be safer and also reduce the risks of epidemic infections (WHO, 2001).
Results presented in Table 3 showed that the vegetative weight/plant under subsurface drip was much higher than that of surface drip irrigation in the first season, while the results of the second season were totally the opposite. This clear difference in the yield between the two seasons may be attributed to the different planting dates indicating two different climatic conditions. Results also showed no significant difference in the vegetative yield (t/ha) between the two irrigation systems in the first season (values are nearly the same), while in the second season, surface drip irrigation system showed higher yield than that of the subsurface. The results of wastewater treatments clearly indicated that the vegetative weight (t/ha) and the vegetative weight (g/ha) were significantly higher in the 2012 season than that of 2011. The highest yields were obtained from raw effluent (wastewater before dilution) followed by the control (GW), 80%, and 40% treatments. This high yield could be attributed to the increase in the absorption of macro and micro nutrients from the TWW. Several researchers attributed the increase in crop production to the increase in the nutrient availability (Mandi and Abissy, 2000, Noor et al., 2014, Munir and Mohammad, 2004, Lopez et al., 2006, Adhikari et al., 2011).
Table 3.
Yield of white radish plant grown during 2011 and 2012 seasons under the effects of two irrigation systems and four treated wastewater qualities.
| Yield components |
||||
|---|---|---|---|---|
| Treatment | Vegetative weight (g/plant) |
Vegetative weight (t/ha) |
||
| 2011 | 2012 | 2011 | 2012 | |
| Irrigation system | ||||
| Surface | 256.00 b | 520.25 a | 24.683 a | 46.829 a |
| Subsurface | 275.88 a | 461.45 b | 24.818 a | 41.408 b |
| Wastewater (%) | ||||
| 0.0 | 268.87 bc | 582.63 a | 24.200 bc | 52.475 a |
| 40 | 232.12c | 450.88 b | 20.913 cd | 40.225 a |
| 80 | 256.25 bc | 375.63 c | 23.021 cd | 33.800 bc |
| 100 | 317.625 ab | 598.13 a | 28.575 ab | 53.838 a |
*Means followed by the same letter(s) are not significantly different according to LSD test at p ⩽ 0.05.
Table 4 presents the means of all investigated bacterial categories, associated with the radish shoot system under the two irrigation systems and four water qualities. In general all categories except total bacteria count were not affected by seasons for a given irrigation system. However, there is a clear impact of irrigation system on all categories except the total bacteria count in the second season. Apparently, there was a considerable drop in the counts of all bacterial categories under the subsurface irrigation system compared to surface irrigation system. Results of wastewater impact on bacteria count were as expected, as the percentage of wastewater increased the bacterial count increased. This relation is true for all studies categories. The consequence of different wastewater qualities was clear in all bacteria categories except the total bacteria count. In other words, there is always a clear cut of significance for each individual wastewater quality. Accordingly, no two different water qualities share a single significant level. The situation is completely different in the case of the total bacteria count. The results of this category indicate that there is no significant difference under the impact of 100%, 80%, and 40% treated wastewater qualities in the first season. It also indicates that there is no significant difference between wastewater qualities of 80%, 40% and ground water, though; there is a significant difference between undiluted wastewater quality 100% and groundwater. Similar comparisons can be drawn from the results of the second season where there is a slight change in the order of impact.
Table 4.
Mean of bacterial counts (count/100 g) of white radish shoot system under the effect of two irrigation systems and four wastewater quality during 2011 and 2012 seasons.
| Bacterial count |
||||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | Total count |
Total coliform |
Fecal coliform |
Total streptococcus |
||||
| 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | |
| Irrigation system | ||||||||
| Surface | 1405 a⁎ | 1584 a | 71 a | 81.9 a | 15.4 a | 22.3 a | 7.17 a | 11.3 a |
| Subsurface | 1124 b | 1446 a | 15.2 b | 7.5 b | 5.3 b | 4.9 b | 2.6 b | 3.2 b |
| Wastewater (%) | ||||||||
| 0 | 1033 b | 1350 c | 3.7 d | 2.9 d | 0.5 d | 0.82 d | 0.66 d | 0.33 d |
| 40 | 1222 ab | 1445 bc | 27.7 c | 32.5 c | 5.3 c | 11.7 c | 3.5 c | 7.2 c |
| 80 | 1315 ab | 1598 ab | 61.3 b | 50.5 b | 14.2 b | 18.3 b | 6.7 b | 9.8 b |
| 100 | 1488 a | 1667 a | 79.3 a | 92.7 a | 21.5 a | 23.6 a | 8.7 a | 11.7 a |
Means followed by the same letter(s) are not significantly different according to LSD test at p ⩽ 0.05.
Table 5 showed the means of bacterial content under the effects of the interaction between irrigation systems and wastewater quality. This table was provided due to the existence of interaction effect between irrigation systems and wastewater quality. The impact of interaction occurs only in the total bacteria count of the second season and in the total streptococcus of the first season. Except total bacteria count, there is always a high reduction in bacteria count under the subsurface irrigation system compared to surface irrigation system. This kind of results was expected simply because of the plant exposure to irrigation water. Moreover, the reduction in bacterial count was clear in the second growing season. This reduction may be justified by the climatic variations between the two seasons as well as the application of irrigation water. In fact, there is nothing specific about total bacteria count; however, its deviated behavior can be contributed to the fact that the surrounding environment may host hundreds of bacteria species. The obtained results are partially substantiated the unrestricted wastewater reuse and suggest the need for more studies on crop contamination caused by climatic conditions. In contrast, Tyrrel et al. (2004) reported that concerns may be expressed to salad vegetables, which may become contaminated with pathogens as a result of crop irrigation using poor quality water sources and there are fears that consumers may be put at risk if irrigation water quality is not controlled. To increase product quality and to protect consumer confidence, alternative and potentially more expensive water sources, e.g. public mains supply can be used.
Table 5.
Mean of bacterial contents (count/100 g) under the effects of the interaction between irrigation systems and wastewater quality during 2011 and 2012 seasons.
| Wastewater (%) | Bacterial count |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total count |
Total coliform |
Fecal coliform |
Total streptococcus |
||||||
| 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | ||
| Surface | 0 | 1400.0 | 1633.3 | 1.467 | 2.50 | 0.00 | 0.633 | 1.25 | 0.471 |
| 40 | 1343.3 | 1550.0 | 43.75 | 59.25 | 6.90 | 20.00 | 7.25 | 1.250 | |
| 80 | 1433.3 | 1586.7 | 104.00 | 92.25 | 22.25 | 31.75 | 9.25 | 0.942 | |
| 100 | 1443.3 | 1566.2 | 134.25 | 173.25 | 32.25 | 37.00 | 11.00 | 2.494 | |
| Subsurface | 0 | 666.7 | 1066.8 | 6.00 | 3.75 | 1.00 | 1.00 | 0.00 | 0.00 |
| 40 | 1100.0 | 1340.0 | 11.75 | 5.75 | 3.75 | 3.40 | 0.00 | 0.047 | |
| 80 | 1196.8 | 1746.5 | 18.75 | 8.25 | 6.00 | 4.99 | 4.00 | 0.00 | |
| 100 | 1533.3 | 1630.0 | 24.25 | 12.75 | 10.75 | 10.15 | 6.25 | 0.967 | |
| LCD (0.05) | NS | 163.56 | NS | NS | NS | NS | 1.169 | NS | |
Table 6 showed the analysis of variance of four bacterial contents under the effects of irrigation systems, wastewater quality and their interaction in both seasons 2011 and 2012. Results indicated that the effect of irrigation systems was not significant in all investigated categories except total bacteria count where both seasons were altered at a confidence of more than 95%. The total bacterial count was also affected by wastewater quality in the second season only while the impact on all other categories was not significant. As the interaction between irrigation systems and wastewater qualities is concerned, a significant effect was obtained on both total bacteria count and total streptococcus while other categories were not affected. In a similar experiment, lettuces and radishes were grown under drip and furrow techniques and irrigated with waste stabilization pond effluent and with effluent diluted with clean water, crop contamination levels of E. coli were 103–104 cell/100 g (fresh weight) for radishes and lettuces respectively, but salmonellae were always absent (Bastos and Mara, 1995). However, after rainfall event, salmonellae were isolated from lettuce surfaces and numbers of E. coli increased.
Table 6.
Analysis of variance of bacterial content (cell/100 g) in white radish crop shoot system under the effects of irrigation systems and wastewater quality and their interaction during 2011 and 2012 seasons.
| Source of variation | df | MS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bacterial count | |||||||||
| Total Count |
Total coliform |
Fecal coliform |
Total streptococcus |
||||||
| 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | ||
| Replicate | 3 | 65,286 | 20,633 | 48.1 | 452 | 7.9 | 16.48 | 0.485 | 1.63 |
| Irrigation system (IS) | 1 | 630,935∗ | 153,088∗ | 24,819 NS | 44,305 NS | 809 NS | 2431.8 NS | 168.6 NS | 532.5 NS |
| Wastewater quality (SW) | 3 | 287,796 NS | 165,526∗ | 9154 NS | 11,302 NS | 697 NS | 770.9 NS | 99.05 NS | 197 NS |
| IS × SW | 3 | 230,420 NS | 212,184∗∗ | 5344 NS | 9132 NS | 228.6 NS | 327.5 NS | 11.4∗∗ | 50.9 NS |
NS: not significant at p ⩽ 0.05, ∗,∗∗: Significant at p ⩽ 0.05 and p ⩽ 0.001, respectively.
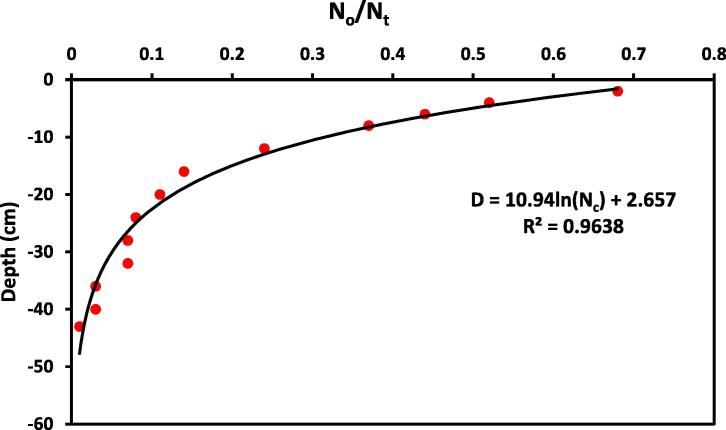
The observed and fitted spatial distributions of bacteria retained in the columns are shown in Fig. 1. The retention profile is plotted as normalized concentration Nc = No/Nt (number of bacteria recovered in the soil, No, divided by the total number of bacteria injected into the column, Nt) per gram of dry soil as a function of distance (D) from the column inlet. Most of the cells were retained close to the soil column inlet, and the rate of deposition decreased with depth. A logarithmic function has been fitted to the observed data. Hence an exponential relationship between Nc and D was found as: with a coefficient of determination, R2 = 0.964.
Figure 1.
Detected normalized concentration Nc (number of bacteria retained in the soil column at specified depth, No, divided by the number in a unit volume of the input bacterial, Nt) per gram of dry soil and fitted logarithmic function versus the distance from the column inlet.
About 65% (from the total count in the column) of the bacteria was retained in the top 10 cm of the soil column. However the total retained count in the soil column was 68% compared to outflow of 24%. These observations can be attributed to bacteria straining. Some researchers have recognized straining as a potentially important mechanism for bacteria deposition (Bradford et al., 2004, Gargiulo et al., 2008, Firouzi et al., 2015). Bacteria retention in soils occurs by attachment and straining mechanisms. In many experiments, it has been observed that attachment is an important mechanism influencing bacterial retention in porous media. The straining mechanism involves the physical blocking of movement through pores smaller than the bacteria (Stevik et al., 2004). The above percentages of retained versus outflow are most likely to change significantly if the flow system was under saturated condition. Hence more outflow counts may be detected due to the driving force of soil water potential.
4. Conclusion
Subsurface drip irrigation system used in this study was successful in reducing the contamination of all studied bacteria categories compared to surface drip system. Moreover, for a given irrigation system, all bacteria categories except the total bacteria count were not affected by the seasons. Conversely, all bacteria categories except the total bacteria count in the second season were significantly affected by the irrigation systems. On the other hand, the interaction between irrigation systems and wastewater quality analysis was useful in determining the affected bacterial categories, whereas both total bacteria count and total streptococcus were significantly affected.
The soil column experiment was a useful tool in mapping the spatial distribution of fecal coliform along the soil profile. Most of the fecal coliform bacteria were retained in the first few centimeters below the column inlet and the profile exponentially decreased with increasing depth. The specified irrigation rate created an unsaturated flow condition, hence, low outflow rates which in turn promote less threat to the underline flow system. The study also supports the fact that as the content of wastewater in the irrigation water increases the bacterial count increases in the growing plant. It also partially substantiated the unrestricted wastewater reuse and suggests the need for more studies on crop contamination caused by other factors like climatic conditions. The treatment technique that is used in the studied wastewater treatment plant (Bani-Malik) must be revised to ensure the reduction of emerging bacterial pathogens to non-detectable levels or to levels that have not been associated with human health risk.
Acknowledgments
This work was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under Grant No. (155-008-D1434). The authors, therefore, acknowledge with thanks DSR technical and financial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdo M.H. Physico-chemical characteristics of Abu Za’baal Ponds, Egypt. Egypt. J. Aquat. Res. 2005;31(2):1–15. [Google Scholar]
- Abdo M.H., Sabae S.Z., Haroon B.M., Refaat B.M., Mohammed A.S. Physico-chemical characteristics, microbial assessment and antibiotic susceptibility of pathogenic bacteria of Ismailia canal water, River Nile, Egypt. J. Am. Sci. 2010;6(5):234–250. [Google Scholar]
- Adhikari P., Shukla M.K., Mexal J.J. Treated wastewater application in southern New Mexico: effect on soil chemical properties and surface vegetation coverage. Appl. Environ. Soil Sci. 2011;46:105–116. [Google Scholar]
- Aislabie J.M., Fraser R.H., Smith J., McLeod M. Leaching of bacterial indicators through four New Zealand soils. Aust. J. Soil Res. 2001;39:1397–1406. [Google Scholar]
- Akaninwor J.O., Anosike E.O., Egwim O. Effect of indomie industrial effluent discharge on microbial properties of new Calabar River. Sci. Res. Essay. 2007;2(1):1–5. [Google Scholar]
- Al-Omari A., Al-Houri Z., Al-Weshah R. Impact of the As Samra wastewater treatment plant upgrade on the water quality (COD, electrical conductivity, TP, TN) of the Zarqa River. Water Sci. Technol. 2013;67(7):1455–1464. doi: 10.2166/wst.2013.686. [DOI] [PubMed] [Google Scholar]
- APHA . eighteenth ed. American Public Health Association; Washington, D.C.: 1992. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- APHA, American Public Health Association. 2005. Standard Methods for the Examination of Water and Wastewater, twenty first ed. Washington, D.C.
- Ashraf M.A. Persistent organic pollutants (POPs): a global issue, a global challenge. Environ. Sci. Pollut. Res. 2015 doi: 10.1007/s11356-015-5225-9. [DOI] [PubMed] [Google Scholar]
- Ashraf M.A., Ahmad M., Aqib S., Balkhair K.S., Bakar N.K.A. Chemical species of metallic elements in the aquatic environment of an ex-mining catchment. Water Environ. Res. 2014;86(8):77–728. doi: 10.2175/106143014x13975035525825. [DOI] [PubMed] [Google Scholar]
- Atherholt T., Feerst E., Hovendon K., Jae R., Joseph D. Evaluation of indicators of fecal contamination in groundwater. Am. Water Works Assoc. 2003;95(10):119–131. [Google Scholar]
- Balkhair K.S., Ashraf M.A. Field accumulation risks of heavy metals in a soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi J. Biol. Sci. 2015 doi: 10.1016/j.sjbs.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkhair K.S., Ismail S.M., El-Nakhlawy F.S., Al-Solaimani S.G. Impact of wastewater irrigation on the yield and quality of white radish under arid environment. Life Sci. J. 2014;11(4):246–254. [Google Scholar]
- Bastos R.K.X., Mara D.D. The bacterial quality of salad crops drip and furrow irrigated with waste stabilization pond effluent: an evaluation of the who guidelines. Water Sci. Technol. 1995;31(12):425–430. [Google Scholar]
- Bradford S.A., Bettahar M., Šimůnek J., van Genuchten M.Th. Straining and attachment of colloids in physically heterogeneous porous media. Vadose Zone J. 2004;3:384–394. [Google Scholar]
- Canada Health. Water Quality and Health Bureau, Healthy Environments and Consumer Safety Branch, Health Canada; Ottawa, Ontario: 2006. Guidelines for Canadian drinking water quality: guideline technical document — bacterial waterborne pathogens — current and emerging organisms of concern. [Google Scholar]
- Crane S.R., Moore J.A. Bacterial pollution of groundwater: a review. Water Air Soil Pollut. 1984;22:67–83. [Google Scholar]
- Cytryn E. Effect of treated wastewater irrigation on antibiotic resistance in agricultural soil bacteria. Environ. Health Problem. 2010 < http://www.ehf.org.il/en/grant/effect-treated-wastewater-irrigation-antibiotic-resistance-agricultural-soil-bacteria-dr-eddie>. [Google Scholar]
- DWAF, 1996. Department of Water Affairs and Forestry, South African water quality guidelines, Aquatic ecosystems, Pretoria, South Africa, vol. 7.
- El-Lathy M.A., El-Taweel G.E., El-Sonosy M.W., Samhan F.A., Moussa T.A.A. Determination of pathogenic bacteria in wastewater using conventional and PCR techniques. Environ. Biotechnol. 2009;5(2):73–80. [Google Scholar]
- El-Nakhlawy F.S. Sci. Pub. Center, King Abdulaziz University, Jeddah, Saudi Arabia; 2010. Statistical Design and Analysis in the Scientific Research. [Google Scholar]
- EPA, 2002. Method 1103.1: Escherichia coli (E. coli) in water by membrane filtration using membrane-Thermotolerant Escherichia coli Agar (mTEC), Office of Water Environmental Protection, Washington DC, Agency 20460.
- FAO, 2007. Coping with water scarcity. Challenge of the twenty-first century. UN-Water, <https://www.google.com/search?q=Coping+with+water+scarcity.+Challenge+of+the+twenty-first+century.+UN-Water%2C+FAO%2C+2007&ie=utf-8&oe=utf-8> (accessed February 5, 2015).
- Fenlon D.R. Wild birds and silage as reservoir of Listeria in the agricultural environment. J. Bacteriol. 1985;59:443–452. doi: 10.1111/j.1365-2672.1985.tb03357.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Alvars R.M., Carballo-Cuervo S., Dela Rosa Jorge M.C., Rodriquez-de ececay J. The influence of agricultural run-off on bacterial population in rivers. Appl. Bacteriol. 1991;70:437–442. doi: 10.1111/j.1365-2672.1991.tb02961.x. [DOI] [PubMed] [Google Scholar]
- Ahmad Farrokhian Firouzi, Mehdi Homaee, Erwin Klumpp, Roy Kasteel, Wolfgang Tappe. Bacteria transport and retention in intact calcareous soil columns under saturated flow conditions. J. Hydrol. Hydromech. 2015;63(2):102–109. [Google Scholar]
- Gargiulo G., Bradford S.A., Simunek J., Ustohal P., Vereecken H., Klumpp E. Bacteria transport and deposition under unsaturated flow conditions: the role of water content and bacteria surface hydrophobicity. Vadose Zone J. 2008;7(2):406–419. [Google Scholar]
- Guber A.K., Shelton D.R., Pachepsky Ya.A. Transport and retention of manure-borne coliforms in undisturbed soil columns. Vadose Zone J. 2005;4:828–837. [Google Scholar]
- Gunkel G., Kosmol J., Sobral M., Rohn H., Montenegro S., Aureliano J. Sugar cane industry as a source of water pollution-case study on the situation in Ipojuca River, pernambuco, Brazil. Water Air Soil Pollut. 2007;180(1–4):261–269. [Google Scholar]
- Gupta V.K., Gupta M., Sharma S. Process development for the removal of lead and chromium from aqueous solution using red mud – an aluminum industry waste. Water Res. 2001;35(5):1125–1134. doi: 10.1016/s0043-1354(00)00389-4. [DOI] [PubMed] [Google Scholar]
- Howard I., Espigares E., Lardelli P., Martín J.L., Espigares M. Evaluation of microbiological and physicochemical indicators for wastewater treatment. Environ. Toxicol. 2004;19:241–249. doi: 10.1002/tox.20016. [DOI] [PubMed] [Google Scholar]
- Hutton M., Symon C. The quantities of cadmium, lead, mercury and arsenic entering the UK environment from human activities. Sci. Total Environ. 1986;59:129–150. doi: 10.1016/0048-9697(86)90018-5. [DOI] [PubMed] [Google Scholar]
- ICMSF . second ed. University of Toronto; 1987. International Commission on Microbiological Specification for Foods: Microorganisms in Foods. 1. Their Significance and Methods of Enumeration. [Google Scholar]
- ISO, International Organization of Standardization 2000. Water quality. Detection and enumeration of Salmonella. International Organization for Standardization, Geneva, first ed. Stage: 90.92 TC 147/SC4 ICS 07100.20.
- Jamieson R.C., Gordon R.J., Sharples K.E., Stratton G.W., Madani A. Movement and persistence of fecal bacteria in agricultural soils and subsurface drainage water: a review. Can. Biosyst. Eng. 2002;44:1–9. [Google Scholar]
- Kadam A.M., Oza G.H., Nemade P.D., Shankar H.S. Pathogen removal from municipal wastewater in constructed soil filter. Ecol. Eng. 2008;33(1):37–44. [Google Scholar]
- Koch A.L. Growth measurement. In: Gerhardt P., Murray R.G.E., Wood W.A., Kriegz N.R., editors. Methods for General and Molecular Biology. American Society for Microbiology; Washington, D.C.: 1994. pp. 248–277. [Google Scholar]
- Kouraa A., Fethi F., Fahde A., Lahlou A., Ouazzani N. Reuse of urban sewage water treated by combined stabilization pond system in Benslimane (Morocco) Urban Water. 2002;4:373–378. [Google Scholar]
- Lance J.C., Gerba C.P. Virus movement in soil during saturated and unsaturated flow. Appl. Environ. Microbiol. 1984;47:335–337. doi: 10.1128/aem.47.2.335-337.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance J.C., Gerba C.P., Wang D.S. Comparative movement of different enteroviruses in soil columns. J. Environ. Qual. 1982;11:341–351. [Google Scholar]
- Lindskog R.U., Lindskog P.A. Bacteriological contamination of water in rural areas: an intervention study from Malawi. J. Trop. Med. Hyg. 1988;91:1–7. [PubMed] [Google Scholar]
- Lopez A., Pollice A., Lonigro A., Masi S., Palese A.M., Cirelli G.L., Toscano A., Passino R. Agricultural sewage water reuse in Southern Italy. Desalination. 2006;187:323–334. [Google Scholar]
- Luczkiewicz A., Fudala-Ksiazek S., Jankowska K., Quant B., Olańczuk-Neyman K. Diversity of fecal coliforms and their antimicrobial resistance patterns in wastewater treatment model plant. Water Sci. Technol. 2010;61(6):1383–1392. doi: 10.2166/wst.2010.015. [DOI] [PubMed] [Google Scholar]
- Magesan G.N. Changes in soil physical properties after irrigation of two forested soils with municipal wastewater. N. Z. J. For. Sci. 2001;31:188–195. [Google Scholar]
- Magesan G.N., Wang H. Application of municipal and industrial residuals in New Zealand forests: an overview. Aust. J. Soil Res. 2003;41:557–569. [Google Scholar]
- Mandi, L., Abissy M., 2000. Utilization of Arundo donax and Typha latifolia for heavy metals removal from urban sewage water and reuse of treated sewage water for alfalfa irrigation. In: Third International Symposium on Sewage Water, Reclamation, Recycling and Reuse, Paris, France. pp. 158–165.
- McCoy E.L., Hagedorn C. Transport of resistant-labeled Escherichia coli through a transition between two soils in a topographic sequence. J. Environ. Qual. 1980;9:686–691. [Google Scholar]
- McGechan M.B., Vinten A.J.A. Simulation of transport through soil of E. coli derived from livestock slurry using the MACRO model. Soil Use Manage. 2003;19(4):321–330. [Google Scholar]
- McLeod M., Aislabie J., Ryburn J., McGill A., Taylor M. Microbial and chemical tracer movement through two Southland soils, New Zealand. Aust. J. Soil Res. 2003;41:1163–1169. [Google Scholar]
- Munir M., Mohammad A. Forage yield and nutrient uptake as influenced by secondary treated sewage water. J. Plant Nutr. 2004;27:351–365. [Google Scholar]
- Niewolak S. Bacteriological monitoring of River water quality in the north area of wigry national park. Polish J. Environ. Stud. 2000;9(4):291–299. [Google Scholar]
- Noor M.J., Sultana S., Fatma S., Ahmad M., Zafar M., Sarfraz M., Balkhyour M.A., Safi S.Z., Ashraf M.A. Estimation of Anticipated Performance Index and Air Pollution Tolerance Index and of vegetation around the marble industrial areas of Potwar region: bioindicators of plant pollution response. Environ. Geochem. Health. 2014;2014:1–18. doi: 10.1007/s10653-014-9657-9. [DOI] [PubMed] [Google Scholar]
- Opaluwa O.D., Aremu M.O., Ogbo L.O., Magaji J.I., Odiba I.E., Ekpo E.R. Assessment of heavy metals in water, fish and sediments from UKE Stream, Nasarawa State, Nigeria. Curr. World Environ. 2012;7(2):213–220. [Google Scholar]
- Pang L., Mcleod M., Aislabie J., Simunek J., Close M., Hector R. Modeling transport of microbes in ten undisturbed soils under effluent irrigation. Vadose Zone J. 2008;7(1):97–111. [Google Scholar]
- Pate K.P., Pandy R.M., George L. Heavy metal content of different effluents water around major industrial cities of Guryurat. J. Indian Soc. Soil Sci. 2001;59(1):89–94. [Google Scholar]
- Rabah S., Azab E., Aly M. Studies on bacterioplankton and inhibitory strains of actinomycetes in lake Bardawil, Egypt. World J. Microbiol. Biotechnol. 2007;23(2) [Google Scholar]
- River Watch Network. 1991. Escherichia coli (E. coli) membrane filter procedure (adapted from USEPA Method 1103.1, 1985), Montpelier, VT.
- Robert M.S., Noel R.K. Manual of Methods for General Bacteriology. American Society for Microbiology; Washington, D.C: 1981. General characterization; pp. 409–433. [Google Scholar]
- Schaub S.A., Sorber C.A. Virus and bacteria removal from wastewater by rapid infiltration through soil. Appl. Environ. Microbiol. 1977;33:609–619. doi: 10.1128/aem.33.3.609-619.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.S., Thomas G.W., White R.E., Ritonga D. Transport of Escherichia coli through intact and disturbed soil columns. J. Environ. Qual. 1985;14:87–91. [Google Scholar]
- Steel R.G., Torrie J.H. third ed. Mc. Graw Hill; NY, USA: 2000. Principles and Procedures of Statistics. [Google Scholar]
- Stevik T.K., Ausland A.K., Hanssen J.F. Retention and removal of pathogenic bacteria in wastewater percolating through porous media: a review. Water Res. 2004;38:1355–1367. doi: 10.1016/j.watres.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Toze S. Reuse of effluent water-benefits and risks. Agric. Water Manag. 2005;80:147–159. [Google Scholar]
- Tyrrel, S.F., Knox, J.W., Burton, C.H., Weatherhead, E.K., 2004. Assuring the microbiological quality of water used to irrigate salad crops: an assessment of the options available Final Report to Horticultural Development Council by Institute of Water & Environment, Cranfield University.
- Umar M.A., Opaluwa O.D. Evaluation of heavy metals in sediments of Rafin Mallam stream in Keffi, Nasarawa State. Int. J. Chem. 2010;20(2):99–103. [Google Scholar]
- Unc A., Goss M.J. Movement of faecal bacteria through the vadose zone. Water Air Soil Pollut. 2003;149:327–337. [Google Scholar]
- USEPA, 1985. Test methods for Escherichia coli and enterococci in water by the membrane filter procedure (Method #1103.1). EPA 600/4-85-076. U.S.
- Vozmaya N.F. Mir Publishers; 1983. Chemistry of Water & Microbiology. 2 pervy rizhsky perevlok, 1–110 GSP, Moscow, 19820 USSR. [Google Scholar]
- WHO Guideline for Drinking Water Quality Recommendation. World Health Organization, Geneva. 1984;1:130. [Google Scholar]
- WHO, 1989. Health guidelines for the use of wastewater in agriculture and aquaculture. Report of a WHO Scientific Group. Geneva, (Technical Report Series, No. 778). [PubMed]
- WHO, 2001. Water Quality: Guidelines, Standards and Health. Edited by Lorna Fewtrell and Jamie Bartram. Published by IWA Publishing, London, UK. ISBN: 1900222280 <http://www.who.int/water_sanitation_health/dwq/whoiwa/en/> (accessed May 2, 2015).
- Yehia H.M., Sabae S.Z. Microbial pollution of water in El-Salam canal, Egypt. Am Eurasian J. Agric. Environ. Sci. 2011;11(2):305–309. [Google Scholar]