Abstract
Postinfectious bronchiolitis obliterans (PIBO) is an irreversible obstructive lung disease characterized by subepithelial inflammation and fibrotic narrowing of the bronchioles after lower respiratory tract infection during childhood, especially early childhood. Although diagnosis of PIBO should be confirmed by histopathology, it is generally based on history and clinical findings. Irreversible airway obstruction is demonstrated by decreased forced expiratory volume in 1 second with an absent bronchodilator response, and by mosaic perfusion, air trapping, and/or bronchiectasis on computed tomography images. However, lung function tests using spirometry are not feasible in young children, and most cases of PIBO develop during early childhood. Further studies focused on obtaining serial measurements of lung function in infants and toddlers with a risk of bronchiolitis obliterans (BO) after lower respiratory tract infection are therefore needed. Although an optimal treatment for PIBO has not been established, corticosteroids have been used to target the inflammatory component. Other treatment modalities for BO after lung transplantation or hematopoietic stem cell transplantation have been studied in clinical trials, and the results can be extrapolated for the treatment of PIBO. Lung transplantation remains the final option for children with PIBO who have progressed to end-stage lung disease.
Keywords: Bronchiolitis obliterans, Post-infectious, Child, Inflammation, Fibrosis
Introduction
Bronchiolitis obliterans (BO) is an irreversible obstructive lung disease characterized by subepithelial inflammation and fibrotic narrowing of the bronchioles1). Although histological classification of BO includes cryptogenic organizing pneumonia and obliterative or constrictive bronchiolitis, the term "BO" is interchangeable with constrictive bronchiolitis in the pediatric clinical field because most BO cases show pathological findings of constrictive bronchiolitis. The final finding of fibrosis in BO is caused by injury and subsequent inflammation of small airways by diverse insults such as infection, rejection, the graft-versus-host immune response, autoimmunity, and various chemicals.
In the pediatric clinical field, three main categories of BO are generally encountered: (1) postinfectious BO (PIBO), (2) BO after hematopoietic stem cell transplantation (HSCT), and (3) BO after lung transplantation (LT). These three categories all exhibit irreversible airway obstruction of small airways and related respiratory symptoms. However, in regards to pathophysiology, diagnosis, treatment, and prognosis, PIBO is somewhat different from the others. BO after HSCT or LT is diagnosed based on a decline in lung function measured serially, and is treated based on alloimmunity, including rejection and the graft-versus-host immune response. Therefore, treatment of BO after HSCT or LT may require more potent immunosuppressants than treatment of PIBO. This review focuses on the existing knowledge of the diagnosis and treatment of BO after HSCT or LT as well as PIBO, and how our current understanding of these diseases can be applied to inform better diagnosis and treatment of PIBO.
Epidemiology
The development of PIBO has been associated with adenovirus (serotype 3, 7, and 21), measles virus, influenza, and Mycoplasma pneumoniae infection2,3,4,5,6,7). Although the prevalence of PIBO has not been estimated accurately, 0.6% of 3,141 autopsies and lung biopsies performed at a single center were diagnosed as BO, and most of these cases were PIBO8). The prevalence of BO after HSCT among cases with allogeneic HSCT is 2%-6%9,10,11,12). The prevalence of BO after LT was markedly higher, up to 35% within 5 years posttransplant13), than the prevalence of PIBO and BO after HSCT.
The prognosis of PIBO seems to be better than that of BO after HSCT or LT. Children with PIBO are heterogeneous in terms of the causative organism and age at injury. Pediatric pulmonologists can identify the cause and the time of injury only through retrospective review at the time of diagnosis. There are a few reports about the clinical course of PIBO that retrospectively collected information about the period before the diagnosis as well as prospectively collected information after diagnosis13,14). The clinical course was reported to vary across 3.5 years of follow-up, in which 22.6% of cases went into remission, 67.7% had persistence of respiratory symptoms and signs, and 9.7% died13). Kim et al.5) reported similar findings in Korea, where 25% of children with PIBO caused by adenovirus or M. pneumoniae showed improvement of disease state at the time of diagnosis. The subjects included in these two studies were recruited from tertiary hospitals; therefore, mild cases of PIBO might not have been included, and the prognosis of PIBO may be better than indicated by these hospital-based studies. In contrast, children with BO after HSCT have a poor prognosis, with a 5-year survival rate of 45%-59% versus 76%-77.5% in those without BO after HSCT15,16). The 5-year survival rate of children with LT was about 50%, and BO was responsible for 48% of deaths in patients more than 5 years post-transplantation17).
Pathogenesis
The pathogenesis of BO has not been fully elucidated. There is limited information about the pathogenesis of PIBO in particular, because the causative organisms of PIBO are diverse, and the duration from the time of insult to the examination of histology and bronchoalveolar lavage (BAL) fluid cannot be accurately evaluated. Some pathological findings of PIBO, such as a variable degree of chronic inflammation and fibrosis in bronchioles, have been consistent across studies18). In children with postviral BO, the cellular infiltrate in the lung was mainly composed of CD3+ T cells with a predominance of the CD8+ T-cell subtype18). Koh et al.6) also observed increased CD8+ T cells and a decreased CD4/CD8 ratio in the BAL and biopsy specimens of children with BO who had a history of measles pneumonia during an outbreak in 2000-2001. These two studies identified a predominant role of CD8+ T cells in the development of BO after viral infection. T cells are known to play a key role in the development of various inflammatory diseases.
Koh et al.6) demonstrated a marked increase in neutrophils and interleukin (IL) 8 in the BAL of children with PIBO, suggesting that this might be an effector cell in PIBO pathogenesis. Neutrophils were also predominant in the BAL of patients with BO after LT19). Furthermore, the degree of neutrophil elevation in BAL correlated with the stage of BO after LT20). Based on BAL studies of matrix metalloproteinases, reactive oxygen species, and defensins21,22,23,24,25,26), Kennedy et al.19) recently proposed that neutrophils play a central role in the pathogenesis of BO after LT. They proposed that epithelial cells injured by diverse posttransplant insults release IL-8 and other proinflammatory cytokines, resulting in the recruitment of inflammatory cells, including neutrophils. They further suggested that these neutrophils produce matrix metalloproteinases, defensins, and reactive oxygen species, resulting in matrix degradation, collagen deposition, fibroblast proliferation, and ultimately peribronchial fibrosis19).
The pathogenesis of BO after HSCT and LT is associated with alloreactivity, i.e., allograft rejection in lung transplant recipients or graft-versus-host disease in allo-stem cell transplantation. T-cell depletion prevented the development of BO after allogeneic HSCT27). This therefore indicates that T cells play a central role in the process of alloreactivity. Recently, Th17 cell-mediated autoimmunity to type V collagen, which is involved in tissue remodeling, was observed in patients with LT28). In an animal model of BO after LT, IL-17 expression was increased in the allograft, whereas peripheral Tregs were decreased29). Considering that IL-17 induces IL-8 secretion, Th17 cells could be responsible for the observed neutrophilia in the BAL of patients with BO after LT.
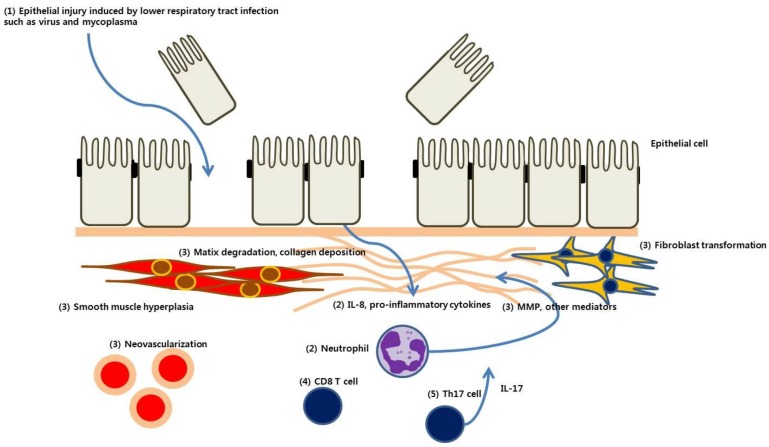
The pathogenesis of PIBO, as presumed from evidence related to PIBO and BO after LT or HSCT, is summarized in Fig. 1.
Fig. 1. Pathogenesis of postinfectious bronchiolitis obliterans. (1) Epithelial injury is induced by lower respiratory tract infection with microorganisms such as virus or mycoplasma. (2) Epithelial cells release interleukin (IL) 8 and other proinflammatory mediators, which recruit neutrophils and other inflammatory cells to the small airway. (3) Matrix metalloproteinase (MMP) and profibrotic cytokines and mediators are released from those cells, resulting in matrix degradation, collagen deposition, fibroblast proliferation, and ultimately, peribronchial fibrosis. (4) CD8+ T cells play a predominant role in epithelial injury and chronic inflammation after viral infection. (5) Th17 cells are involved in tissue remodeling, and IL-17 induces IL-8 secretion, which is related to airway neutrophilia.
The duration of inflammation after the insult in children with PIBO
Understanding how long inflammation lasts after the development of PIBO is important to inform the decision of how long patients with BO should be medicated with immunosuppressants, which are a common treatment due to the chronic inflammation. A relatively long-term follow-up study for PIBO by Cazzato et al.30) showed persistent inflammation in the BAL of children with PIBO, wherein the median disease duration at the time of BAL assessment was 3.7 years (range, 0.1-9.6 years). However, this study included only a small number of subjects and included subjects with PIBO of unknown etiology. They also showed a decline in lung function including forced expiratory volume in 1 second (FEV1; percent predicted), forced expiratory flow at 25%-75% of vital capacity (FEF25%-75%; predicted), and FEV1/forced vital capacity (FVC) ratio over a follow-up period of 10.2 years (interquartile range, 3.2-12.0 years). The authors suggested that the decline in lung function reflected progression of the disease over time and may be caused by ongoing inflammation, a contention that was supported by BAL findings. However, Zhang et al.13) showed no deterioration of lung function in children with PIBO over a relatively short follow-up period. The study by Cazzato et al.30) might have included severe cases that were followed for a long duration in hospital. Moreover, only studied a small number of subjects (n=11) were studied, therefore the results cannot be extrapolated to the general PIBO population.
A recent follow-up study of lung function in children with PIBO over a period of 35 months (range, 24-82 months) reported increased FVC and stable FEV1 over time, resulting in a decline in the FEV1/FVC ratio, which suggests dysanaptic growth31). In addition, the authors reported persistent neutrophilic inflammation several years after the onset of disease in the absence of overt infection, as assessed by microbiological analysis. However, these BAL findings may be influenced by recent respiratory infection that may no longer be present at the time of BAL sampling. Moreover, children with PIBO may be more susceptible to respiratory infection than children without PIBO. Chronic bacterial colonization should also be considered as a potential cause of these results because cases with PIBO are frequently complicated by bronchiectasis. However, if the finding of persistent neutrophilic inflammation several years after the onset of disease in these patients is accepted, the factors that may drive this must be considered. One potential factor is the presence of T cells against a certain peribronchial antigen to which the patient is exposed after initial injury, as suggested by the pathophysiology of BO after HSCT. However, there have been no PIBO studies that address this issue.
It is currently not known how long inflammation lasts after the initial insult in children with PIBO. Some patients with PIBO may have persistent inflammation, resulting in progressive decline of lung function. Others may have inflammation for a short duration after the insult, but impaired development of airways with conserved normal lung growth.
Diagnosis
Although a diagnosis of PIBO should be confirmed by histopathology, most pediatric pulmonologists diagnose PIBO based on history and clinical findings according to the following criteria: (1) acute severe respiratory infection during childhood, especially early childhood; (2) persistent airway obstruction after initial insult and unresponsiveness to systemic steroids and bronchodilators, as demonstrated by clinical symptoms and signs, and a lung function test, if it can be performed; (3) mosaic perfusion, air trapping, and/or bronchiectasis in chest computed tomography; and (4) exclusion of other chronic lung diseases such as severe asthma, bronchopulmonary dysplasia, chronic aspiration, primary ciliary dyskinesia, cystic fibrosis, immunodeficiency, and alpha-1-antitrypsin deficiency (Table 1). Recent studies of clinical prediction rules to diagnose PIBO in children found that typical clinical history, adenovirus infection, and high-resolution computed tomography with mosaic perfusion were highly predictable variables32).
Table 1. Diagnosis of postinfectious bronchiolitis obliterans.
| History of lower respiratory infection, particularly adenovirus, mycoplasma, or measles. |
| Persistent airway obstruction symptoms and signs, or recurrent airway obstruction symptoms and signs in a mild form. |
| Sign of obstruction: FEV1/FVC <0.8 or FEV1 percent predicted <80%. |
| Irreversible airway obstruction demonstrated by lung function test: absent BDR, but positive BDR in some patients. |
| CT (inspiration and expiration): mosaic perfusion, air trapping, and/or bronchiectasis. |
| Exclusion of other chronic lung disease (asthma, BPD, chronic aspiration, PCD, cystic fibrosis, and immunodeficiency). |
| Postinfectious bronchiolitis obliterans is clinically diagnosed when all of the above criteria are met. |
FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; BDR, bronchodilator response; BPD, bronchopulmonary dysplasia; CT, computed tomography; PCD, primary ciliary dyskinesia.
The diagnosis criteria for BO after LT were revised in 200133). The revised criteria use the term bronchiolitis obliterans syndrome (BOS), which is defined by a change in pulmonary function and does not necessarily require histological confirmation. A diagnosis of potential BOS (BOS 0-P) was added to the previous staging system to enable early detection of BOS, and is defined as a 10%-19% decrease in FEV1 and/or ≥25% decrease in FEF25%-75% from baseline. FEF25%-75% was more sensitive than FEV1 at detecting small airway obstructions, and thus may be useful for diagnosing and monitoring the progression of PIBO. However, FEF25%-75% did not demonstrate good reproducibility (coefficient of variation, 10%)34).
Chien et al.35) proposed a modification of the US National Institute of Health consensus definition of BOS in patients with BOS after HSCT. The proposed criteria are slightly different from the criteria for BOS after LT. To diagnose BO after HSCT, other chronic graft-versus-host diseases with restrictive lung disease, such as scleroderma, bronchiolitis obliterans organizing pneumonia, and myositis, should be ruled out. Therefore, reduced FEV1 alone is not sufficient to represent airway obstruction and FEV1/vital capacity should be included in the criteria. In addition, FVC could decrease due to early collapse of the airway during a forced expiratory maneuver, and thus FEV1/slow vital capacity may be a better marker of obstruction35).
However, lung function tests using spirometry are not feasible in young children, and most cases of PIBO develop during early childhood. The data reported by studies using spirometry has not provided a diagnostic value in children at risk of PIBO but demonstrates long-term lung function in children with established PIBO, including airway and lung development. Further studies focused on obtaining serial measures of lung function in infants and toddlers with a risk of BO after lower respiratory tract infection are therefore needed.
Exhaled nitric oxide (eNO) was increased in patients with BOS after LT36), and served as a predictive value for future BOS37). However, eNO was also increased in patients with respiratory infection after LT, indicating that it is a nonspecific marker of airway inflammation37). In contrast to the results in BOS after LT, eNO was lower in stem cell recipients with BO than in stem cell recipients without BO15). eNO measurement might have been performed in the fibrotic phase in this study, or the pathophysiology of BOS after HSCT might be different from that of BOS after LT. However, there is no information on the role of eNO in the diagnosis and monitoring of children with PIBO.
Treatment
Although the optimal treatment of PIBO has not been established, corticosteroids have been used to combat the inflammatory component. Systemic steroids can be used rather than inhaled steroids in consideration of the obliteration of the small airways. Prolonged oral steroid therapy over a period of 2 months to 2 years was applied to about 70% of children with PIBO in a relatively long-term observational study13). Some studies have used pulse therapy with methylprednisolone (30 mg/kg/day) for 3 days per month to treat PIBO, and this strategy is expected to have fewer side effects compared to daily oral steroids5,38). Systemic steroids should be given in the early period of the disease, before fibrosis is established. By the time a diagnosis of PIBO is made, the small airways might already be obliterated with fibrosis. Furthermore, after the start of systemic steroids to treat PIBO, the question arises as to how long inflammation lasts after the development of PIBO. Since the answer to this question is unknown, it is difficult to know when to start and finish systemic steroid therapy. Although the timing of the decision to treat the disease and the duration of small airway inflammation differs between BO after HSCT and PIBO, seven of nine patients after HSCT receiving up to six cycles of methylprednisolone pulse therapy were clinically stable without further decline of lung function39).
Other immunosuppressants, including methotrexate, azathioprine, cyclophosphamide, thalidomide, imatinib, and etanercept, have been tested in patients with BOS after LT or HSCT. Although most studies are retrospective, case series, or case-control designed, the results are not promising40,41,42,43,44), and there are no reports of the use of these drugs in children with PIBO.
Although, theoretically, a bronchodilator response should be absent in children with fixed airway obstruction such as in PIBO, a positive bronchodilator response ranging from 10% to 42.9% was present in children with PIBO13,30,45). The use of a bronchodilator beta-2-agonist should be applied on an individual basis according to the bronchodilator response. Recently, tiotropium bromide, an anticholinergic agent was shown to improve airway obstruction and air trapping for 24 hours in children with PIBO46). In addition, it has been suggested that azithromycin, a macrolide antibiotic with anti-inflammatory properties, may be beneficial for the progression of BOS after LT or HSCT, however, the results are controversial47,48,49,50). In children with PIBO, azithromycin has been used clinically without supporting evidence31).
LT remains the final option for children with BO after LT or HSCT who have progressed to end-stage lung disease. Of 31 transplanted cases with diffuse lung disease, except for cystic fibrosis and pulmonary vascular disease, in a two hospital-based study, two were children with PIBO51). LT should be considered for children with PIBO who have progressed to end-stage lung disease.
Conclusions
PIBO is an irreversible obstructive lung disease that is characterized by subepithelial inflammation and fibrotic narrowing of the bronchioles after lower respiratory tract infection during childhood, especially early childhood. Although diagnosis of PIBO should be confirmed by histopathology, it is generally based on history and clinical findings. The optimal treatment for PIBO has not been established, but corticosteroids have been used with the aim of reducing the inflammatory component. LT remains the final option for children with PIBO who have progressed to end-stage lung disease.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Barker AF, Bergeron A, Rom WN, Hertz MI. Obliterative bronchiolitis. N Engl J Med. 2014;370:1820–1828. doi: 10.1056/NEJMra1204664. [DOI] [PubMed] [Google Scholar]
- 2.Spigelblatt L, Rosenfeld R. Hyperlucent lung: long-term complication of adenovirus type 7 pneumonia. Can Med Assoc J. 1983;128:47–49. [PMC free article] [PubMed] [Google Scholar]
- 3.Becroft DM. Bronchiolitis obliterans, bronchiectasis, and other sequelae of adenovirus type 21 infection in young children. J Clin Pathol. 1971;24:72–82. doi: 10.1136/jcp.24.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbert FA, Wilkinson D, Burchak E, Morgante O. Adenovirus type 3 pneumonia causing lung damage in childhood. Can Med Assoc J. 1977;116:274–276. [PMC free article] [PubMed] [Google Scholar]
- 5.Kim CK, Kim SW, Kim JS, Koh YY, Cohen AH, Deterding RR, et al. Bronchiolitis obliterans in the 1990s in Korea and the United States. Chest. 2001;120:1101–1106. doi: 10.1378/chest.120.4.1101. [DOI] [PubMed] [Google Scholar]
- 6.Koh YY, Jung da E, Koh JY, Kim JY, Yoo Y, Kim CK. Bronchoalveolar cellularity and interleukin-8 levels in measles bronchiolitis obliterans. Chest. 2007;131:1454–1460. doi: 10.1378/chest.06-0188. [DOI] [PubMed] [Google Scholar]
- 7.Laraya-Cuasay LR, DeForest A, Huff D, Lischner H, Huang NN. Chronic pulmonary complications of early influenza virus infection in children. Am Rev Respir Dis. 1977;116:617–625. doi: 10.1164/arrd.1977.116.4.617. [DOI] [PubMed] [Google Scholar]
- 8.Hardy KA, Schidlow DV, Zaeri N. Obliterative bronchiolitis in children. Chest. 1988;93:460–466. doi: 10.1378/chest.93.3.460. [DOI] [PubMed] [Google Scholar]
- 9.Dudek AZ, Mahaseth H, DeFor TE, Weisdorf DJ. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biol Blood Marrow Transplant. 2003;9:657–666. doi: 10.1016/s1083-8791(03)00242-8. [DOI] [PubMed] [Google Scholar]
- 10.Holland HK, Wingard JR, Beschorner WE, Saral R, Santos GW. Bronchiolitis obliterans in bone marrow transplantation and its relationship to chronic graft-v-host disease and low serum IgG. Blood. 1988;72:621–627. [PubMed] [Google Scholar]
- 11.Santo Tomas LH, Loberiza FR, Jr, Klein JP, Layde PM, Lipchik RJ, Rizzo JD, et al. Risk factors for bronchiolitis obliterans in allogeneic hematopoietic stem-cell transplantation for leukemia. Chest. 2005;128:153–161. doi: 10.1378/chest.128.1.153. [DOI] [PubMed] [Google Scholar]
- 12.Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1072–1078. doi: 10.1016/j.bbmt.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Irion K, Kozakewich H, Reid L, Camargo JJ, da Silva Porto N, et al. Clinical course of postinfectious bronchiolitis obliterans. Pediatr Pulmonol. 2000;29:341–350. doi: 10.1002/(sici)1099-0496(200005)29:5<341::aid-ppul2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Yalcin E, Dogru D, Haliloglu M, Ozcelik U, Kiper N, Gocmen A. Postinfectious bronchiolitis obliterans in children: clinical and radiological profile and prognostic factors. Respiration. 2003;70:371–375. doi: 10.1159/000072900. [DOI] [PubMed] [Google Scholar]
- 15.Ditschkowski M, Elmaagacli AH, Koldehoff M, Gromke T, Trenschel R, Beelen DW. Bronchiolitis obliterans after allogeneic hematopoietic SCT: further insight--new perspectives? Bone Marrow Transplant. 2013;48:1224–1229. doi: 10.1038/bmt.2013.17. [DOI] [PubMed] [Google Scholar]
- 16.Nakaseko C, Ozawa S, Sakaida E, Sakai M, Kanda Y, Oshima K, et al. Incidence, risk factors and outcomes of bronchiolitis obliterans after allogeneic stem cell transplantation. Int J Hematol. 2011;93:375–382. doi: 10.1007/s12185-011-0809-8. [DOI] [PubMed] [Google Scholar]
- 17.Benden C, Goldfarb SB, Edwards LB, Kucheryavaya AY, Christie JD, Dipchand AI, et al. The registry of the International Society for Heart and Lung Transplantation: seventeenth official pediatric lung and heart-lung transplantation report--2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33:1025–1033. doi: 10.1016/j.healun.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Mauad T, Dolhnikoff M Sao Paulo Bronchiolitis Obliterans Study Group. Histology of childhood bronchiolitis obliterans. Pediatr Pulmonol. 2002;33:466–474. doi: 10.1002/ppul.10097. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy VE, Todd JL, Palmer SM. Bronchoalveolar lavage as a tool to predict, diagnose and understand bronchiolitis obliterans syndrome. Am J Transplant. 2013;13:552–561. doi: 10.1111/ajt.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devouassoux G, Drouet C, Pin I, Brambilla C, Brambilla E, Colle PE, et al. Alveolar neutrophilia is a predictor for the bronchiolitis obliterans syndrome, and increases with degree of severity. Transpl Immunol. 2002;10:303–310. doi: 10.1016/s0966-3274(02)00074-6. [DOI] [PubMed] [Google Scholar]
- 21.Hubner RH, Meffert S, Mundt U, Bottcher H, Freitag S, El Mokhtari NE, et al. Matrix metalloproteinase-9 in bronchiolitis obliterans syndrome after lung transplantation. Eur Respir J. 2005;25:494–501. doi: 10.1183/09031936.05.00091804. [DOI] [PubMed] [Google Scholar]
- 22.Riise GC, Ericson P, Bozinovski S, Yoshihara S, Anderson GP, Lindén A. Increased net gelatinase but not serine protease activity in bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2010;29:800–807. doi: 10.1016/j.healun.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Behr J, Maier K, Braun B, Schwaiblmair M, Vogelmeier C. Evidence for oxidative stress in bronchiolitis obliterans syndrome after lung and heart-lung transplantation. The Munich Lung Transplant Group. Transplantation. 2000;69:1856–1860. doi: 10.1097/00007890-200005150-00020. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch J, Elssner A, Mazur G, Maier KL, Bittmann I, Behr J, et al. Bronchiolitis obliterans syndrome after (heart-)lung transplantation. Impaired antiprotease defense and increased oxidant activity. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1640–1646. doi: 10.1164/ajrccm.160.5.9902012. [DOI] [PubMed] [Google Scholar]
- 25.Riise GC, Williams A, Kjellstrom C, Schersten H, Andersson BA, Kelly FJ. Bronchiolitis obliterans syndrome in lung transplant recipients is associated with increased neutrophil activity and decreased antioxidant status in the lung. Eur Respir J. 1998;12:82–88. doi: 10.1183/09031936.98.12010082. [DOI] [PubMed] [Google Scholar]
- 26.Nelsestuen GL, Martinez MB, Hertz MI, Savik K, Wendt CH. Proteomic identification of human neutrophil alpha-defensins in chronic lung allograft rejection. Proteomics. 2005;5:1705–1713. doi: 10.1002/pmic.200401036. [DOI] [PubMed] [Google Scholar]
- 27.Ditschkowski M, Elmaagacli AH, Trenschel R, Peceny R, Koldehoff M, Schulte C, et al. T-cell depletion prevents from bronchiolitis obliterans and bronchiolitis obliterans with organizing pneumonia after allogeneic hematopoietic stem cell transplantation with related donors. Haematologica. 2007;92:558–561. doi: 10.3324/haematol.10710. [DOI] [PubMed] [Google Scholar]
- 28.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagiri T, Inoue M, Morii E, Minami M, Sawabata N, Utsumi T, et al. Local IL-17 production and a decrease in peripheral blood regulatory T cells in an animal model of bronchiolitis obliterans. Transplantation. 2010;89:1312–1319. doi: 10.1097/TP.0b013e3181d8ea16. [DOI] [PubMed] [Google Scholar]
- 30.Cazzato S, Poletti V, Bernardi F, Loroni L, Bertelli L, Colonna S, et al. Airway inflammation and lung function decline in childhood post-infectious bronchiolitis obliterans. Pediatr Pulmonol. 2008;43:381–390. doi: 10.1002/ppul.20784. [DOI] [PubMed] [Google Scholar]
- 31.Mosquera RA, Hashmi SS, Pacheco SE, Reverdin A, Chevallier J, Colasurdo GN. Dysanaptic growth of lung and airway in children with post-infectious bronchiolitis obliterans. Clin Respir J. 2014;8:63–71. doi: 10.1111/crj.12037. [DOI] [PubMed] [Google Scholar]
- 32.Colom AJ, Teper AM. Clinical prediction rule to diagnose post-infectious bronchiolitis obliterans in children. Pediatr Pulmonol. 2009;44:1065–1069. doi: 10.1002/ppul.21080. [DOI] [PubMed] [Google Scholar]
- 33.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 34.Hutchison AA, Erben A, McLennan LA, Landau LI, Phelan PD. Intrasubject variability of pulmonary function testing in healthy children. Thorax. 1981;36:370–377. doi: 10.1136/thx.36.5.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chien JW, Duncan S, Williams KM, Pavletic SZ. Bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation-an increasingly recognized manifestation of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16(1 Suppl):S106–S114. doi: 10.1016/j.bbmt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher AJ, Gabbay E, Small T, Doig S, Dark JH, Corris PA. Cross sectional study of exhaled nitric oxide levels following lung transplantation. Thorax. 1998;53:454–458. doi: 10.1136/thx.53.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neurohr C, Huppmann P, Leuschner S, von Wulffen W, Meis T, Leuchte H, et al. Usefulness of exhaled nitric oxide to guide risk stratification for bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2011;11:129–137. doi: 10.1111/j.1600-6143.2010.03327.x. [DOI] [PubMed] [Google Scholar]
- 38.Fischer GB, Sarria EE, Mattiello R, Mocelin HT, Castro-Rodriguez JA. Post infectious bronchiolitis obliterans in children. Paediatr Respir Rev. 2010;11:233–239. doi: 10.1016/j.prrv.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Ratjen F, Rjabko O, Kremens B. High-dose corticosteroid therapy for bronchiolitis obliterans after bone marrow transplantation in children. Bone Marrow Transplant. 2005;36:135–138. doi: 10.1038/sj.bmt.1705026. [DOI] [PubMed] [Google Scholar]
- 40.Glanville AR, Baldwin JC, Burke CM, Theodore J, Robin ED. Obliterative bronchiolitis after heart-lung transplantation: apparent arrest by augmented immunosuppression. Ann Intern Med. 1987;107:300–304. doi: 10.7326/0003-4819-107-2-300. [DOI] [PubMed] [Google Scholar]
- 41.Dusmet M, Maurer J, Winton T, Kesten S. Methotrexate can halt the progression of bronchiolitis obliterans syndrome in lung transplant recipients. J Heart Lung Transplant. 1996;15:948–954. [PubMed] [Google Scholar]
- 42.Browne PV, Weisdorf DJ, DeFor T, Miller WJ, Davies SM, Filipovich A, et al. Response to thalidomide therapy in refractory chronic graft-versus-host disease. Bone Marrow Transplant. 2000;26:865–869. doi: 10.1038/sj.bmt.1702626. [DOI] [PubMed] [Google Scholar]
- 43.Stadler M, Ahlborn R, Kamal H, Diedrich H, Buchholz S, Eder M, et al. Limited efficacy of imatinib in severe pulmonary chronic graft-versus-host disease. Blood. 2009;114:3718–3719. doi: 10.1182/blood-2009-07-231159. [DOI] [PubMed] [Google Scholar]
- 44.Busca A, Locatelli F, Marmont F, Ceretto C, Falda M. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2007;82:45–52. doi: 10.1002/ajh.20752. [DOI] [PubMed] [Google Scholar]
- 45.Aguerre V, Castanos C, Pena HG, Grenoville M, Murtagh P. Postinfectious bronchiolitis obliterans in children: clinical and pulmonary function findings. Pediatr Pulmonol. 2010;45:1180–1185. doi: 10.1002/ppul.21304. [DOI] [PubMed] [Google Scholar]
- 46.Teixeira MF, Rodrigues JC, Leone C, Adde FV. Acute bronchodilator responsiveness to tiotropium in postinfectious bronchiolitis obliterans in children. Chest. 2013;144:974–980. doi: 10.1378/chest.12-2280. [DOI] [PubMed] [Google Scholar]
- 47.Lam DC, Lam B, Wong MK, Lu C, Au WY, Tse EW, et al. Effects of azithromycin in bronchiolitis obliterans syndrome after hematopoietic SCT--a randomized double-blinded placebo-controlled study. Bone Marrow Transplant. 2011;46:1551–1556. doi: 10.1038/bmt.2011.1. [DOI] [PubMed] [Google Scholar]
- 48.Gerhardt SG, McDyer JF, Girgis RE, Conte JV, Yang SC, Orens JB. Maintenance azithromycin therapy for bronchiolitis obliterans syndrome: results of a pilot study. Am J Respir Crit Care Med. 2003;168:121–125. doi: 10.1164/rccm.200212-1424BC. [DOI] [PubMed] [Google Scholar]
- 49.Yates B, Murphy DM, Forrest IA, Ward C, Rutherford RM, Fisher AJ, et al. Azithromycin reverses airflow obstruction in established bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2005;172:772–775. doi: 10.1164/rccm.200411-1537OC. [DOI] [PubMed] [Google Scholar]
- 50.Khalid M, Al Saghir A, Saleemi S, Al Dammas S, Zeitouni M, Al Mobeireek A, et al. Azithromycin in bronchiolitis obliterans complicating bone marrow transplantation: a preliminary study. Eur Respir J. 2005;25:490–493. doi: 10.1183/09031936.05.00020804. [DOI] [PubMed] [Google Scholar]
- 51.Rama JA, Fan LL, Faro A, Elidemir O, Morales DL, Heinle JS, et al. Lung transplantation for childhood diffuse lung disease. Pediatr Pulmonol. 2013;48:490–496. doi: 10.1002/ppul.22634. [DOI] [PubMed] [Google Scholar]