Abstract
Because many words are typically used in the context of their referent objects and actions, distributed cortical circuits for these words may bind information about their form with perceptual and motor aspects of their meaning. Previous work has demonstrated such semantic grounding for sensorimotor, visual, auditory, and olfactory knowledge linked to words, which is manifest in activation of the corresponding areas of the cortex. Here, we explore the brain basis of gustatory semantic links of words whose meaning is primarily related to taste. In a blocked functional magnetic resonance imaging design, Spanish taste words and control words matched for a range of factors (including valence, arousal, imageability, frequency of use, number of letters and syllables) were presented to 59 right-handed participants in a passive reading task. Whereas all the words activated the left inferior frontal (BA44/45) and the posterior middle and superior temporal gyri (BA21/22), taste-related words produced a significantly stronger activation in these same areas and also in the anterior insula, frontal operculum, lateral orbitofrontal gyrus, and thalamus among others. As these areas comprise primary and secondary gustatory cortices, we conclude that the meaning of taste words is grounded in distributed cortical circuits reaching into areas that process taste sensations.
Keywords: fMRI, gustatory cortex, language, reading, semantic network, taste
Introduction
Understanding the way the meaning of words is represented and processed in the human brain still represents one of the main challenges in cognitive neuroscience. A neurobiological perspective on language mechanisms suggests that words are processed by distributed cortical circuits with topographies that reflect aspects of their referential meaning (Pulvermüller 2001, Pulvermüller, Hauk, et al. 2005; Martin 2007; Barsalou 2008; Pulvermüller and Fadiga 2010). These neural webs unify neurons in perisylvian areas by storing word form information and neurons in the more widespread cortical areas critically involved in processing perceptual and/or motor information about word meaning. Since word forms frequently co-occur with nonlinguistic information, for example, the visual perception of specific objects, sounds, odors, tastes, or body movements, Hebbian learning implies that the neuronal representations of these words will include coactivated neuronal systems, involving the specific sensory and motor information related to semantically relevant perceptions and actions. In the very same way the question “What does the word bear mean?” can be answered by showing a picture of that animal, the neuron circuit for this word form should become linked semantically to neuronal populations in the visual system.
Neuropsychological and neuroimaging studies have provided overwhelming evidence for such meaning-related differential topographies. For example, the processing of object-related nouns versus action verbs and of names of animals versus tools are affected differentially by focal brain damage (Warrington and Shallice 1984; Miceli et al. 1988; Damasio and Tranel 1993; Daniele et al. 1994; Humphreys and Forde 2001; Neininger and Pulvermüller 2003; Bak et al. 2006; Gainotti 2008; Pulvermüller et al. 2010). Consistently with these lesion studies, neuroimaging studies have found differential activation of brain areas when action- or perceptually-related words are processed (Damasio et al. 1996; Martin et al. 1996; Moore and Price 1999; Pulvermüller et al. 1999; Martin and Chao 2001). Evidence has accumulated, even for quite precisely defined categorical distinctions between semantic categories such as action word subtypes. The results of behavioral (Pulvermüller et al. 2001; Glenberg et al. 2008; Dalla Volta et al. 2009) neurophysiological (electroencephalography and magnetoencephalography [MEG], Pulvermüller et al. 2000, Pulvermüller, Shtyrov, et al. 2005; Shtyrov et al. 2004), neuroimaging (Hauk et al. 2004; Kemmerer et al. 2008), and transcranial magnetic stimulation experiments (Buccino et al. 2005; Pulvermüller, Hauk, et al. 2005) reveal that the comprehension of action words, which semantically relate to different body parts, automatically activates the motor and premotor cortices somatotopically. Moreover, they show that motor cortex stimulation or lesion, for example, the arm or leg motor cortex, has a specific causal influence on the processing of action words, for example, arm- versus leg-related action words (such as “pick” and “kick”). Words that relate to different kinds of objects and convey different kinds of semantic information about visual features, for example, shape versus color, activate different sections of the temporal cortex (Moscoso del Prado Martin et al. 2006; Pulvermüller and Hauk 2006). Sound-related words (e.g., “telephone”) activate the superior temporal cortex more strongly than control items (Kiefer et al. 2008). Even odor-related words spark the primary and secondary olfactory cortices (González et al. 2006). These converging results provide strong evidence for a role of sensorimotor systems in the processing of word meaning (Martin 2007; Barsalou 2008; Pulvermüller and Fadiga 2010). However, the sensorimotor account is still restricted to specific sensory modalities. In order to make strong general statements, the remaining sensory modality, namely gustation, needs to be explored. Our study aims to bridge this gap by investigating brain correlates of the words semantically related to taste.
Several neuroimaging studies have identified specific cortical regions in the human brain that respond to gustatory stimuli, which evoke taste sensations (Kobayakawa et al. 1996, 1999; Faurion et al. 1998, 1999; Cerf-Ducastel et al. 2001; O’Doherty et al. 2001; De Araujo et al. 2003; Frank et al. 2003; Small et al. 2003; Onoda et al. 2005; Haase et al. 2006). These studies identified the anterior insula and the frontal operculum as the primary gustatory cortices (PGCs) and also the orbitofrontal cortex (OFC) as the secondary (or higher order) gustatory cortex (SGC) (Small et al. 2007). Therefore, the specific hypothesis motivating the present research was that, in a passive reading task, taste-related words would activate these primary and secondary taste-processing areas (anterior insula, frontal operculum, and OFC) more strongly than matched control words.
Materials and Methods
Subjects
Fifty-nine right-handed (Oldfield 1971) native Spanish speakers (29 females; mean age 22.51 (3.90), range 17–37) volunteered to enroll as students at the Universitat Jaume I (years of education = 14.47 ± 1.92) to participate in an functional magnetic resonance imaging (fMRI) study. Participants had normal or corrected-to-normal visual acuity and no history of neurological of psychiatric disorders. They were paid for their participation. Ethical approval was obtained from the Universitat Jaume I Ethics Committee, and each subject signed a written informed consent prior to participation.
Stimuli and fMRI Design
We applied a block design paradigm with 3 conditions: 2 activation and 1 baseline conditions. The activation stimuli consisted of 2 different lists of 50 visually presented words (concrete nouns) selected according to their gustatory connotations. These 2 word sets were selected from a previous norming study in which 18 subjects, who did not participate in the fMRI experiment, were asked to rate a pool of concrete nouns according to whether or not their meaning related to gustatory information. Subjects were asked whether the words referred to objects with a strong taste, and they used a scale ranging from 1 (no or a very weak gustatory semantic link) to 7 (a very strong gustatory link). One list (taste-related words, TW) included words with pronounced gustatory meaning defined in this manner, which were presented during the TW condition; the other list (control words, CW) included words with no or very weak gustatory semantic links, which were assigned to the CW condition (mean scores [standard deviation, SD] were 5.70 (0.48) vs. 1.25 (0.43), respectively, t98 = 48.02, P < 0.0001) (see Supplementary material). In order to minimize any physical or psycholinguistic differences that could influence the hemodynamic response, both the TW and the CW lists were matched for a range of psycholinguistic and semantic variables (see below and see Table 1).
Table 1.
The psycholinguistic and semantic variables of stimuli
| Variables | Taste words | Control words |
| Word frequencya | 60.8 (121.9) | 60.3 (129.5) (NS) |
| Number of letters | 5.96 (1.88) | 5.76 (1.67) (NS) |
| Number of syllables | 2.46 (0.76) | 2.48 (0.64) (NS) |
| Valence (1–9 scale) | 6.09 (1.18) | 5.68 (1.14) (NS) |
| Arousal (1–9 scale) | 4.97 (0.52) | 4.71 (1.13) (NS) |
| Imageability (1–7 scale) | 6.05 (0.70) | 5.87 (0.67) (NS) |
| Gustatory (1–7 scale) | 6.04 (0.60) | 1.42 (0.43)** |
| Olfactory (1–7 scale) | 5.21 (0.90) | 1.75 (0.72)** |
| Vision (1–7 scale) | 5.91 (1.25) | 5.68 (1.15)* |
| Action (1–7 scale) | 3.56 (1.60) | 3.92 (1.07) (NS) |
Note: Mean (SD) of the ratings and values of the taste words and control words used in the experiment. NS: nonsignificant difference.
occurrences per 5 million according to the LEXESP corpus (Sebastián-Gallés et al. 2000).
*Significant difference at P = 0.01 (two tails); **Significant difference at P = 0.001 (two tails).
The baseline (B) stimuli used in 50 baseline trials consisted of strings of hash marks, one for each trial (for example, #####), that matched the length of the words used in the other 2 lists (for similar methods, see Hauk et al. 2004). Note that, unlike the English context where the hash, pound, or number sign (#) is meaningful to an extent, this sign has no meaning in the Spanish context.
Stimuli were presented in 20-s blocks, and each included 10 stimuli of 1 of the 3 conditions: TW, CW, or hash marks. The block sequence was pseudorandomized unpredictably. Each stimulus lasted 150 ms with a stimulus onset asynchrony of 2000 ms. A full paradigm lasted 320 s, including one additional baseline block at the end to allow the blood oxygen level–dependent (BOLD) response to settle to the baseline. Participants were instructed to silently read each word or to simply look at the screen in the baseline condition.
Semantic Rating of Stimulus Words
After the fMRI experiment, 41 subjects (21 female; mean age = 23.12 (4.23); years of education = 14.63 (1.98)) completed a questionnaire in which they were asked to rate the meaning of all the word stimuli used in the experiment. Subjects rated the relevance of gustatory, olfactory, action, and visual features for the meaning of these words on a scale from 1 to 7. The words appeared pseudorandomly in 4 different questionnaires, with a maximum of 3 items from the same category in direct succession. The questions used to obtain semantic ratings were:
Gustation: “Does the meaning of this word relate to a taste you can detect with your mouth and tongue?”
Olfaction: “Does the meaning of this word relate to an odor or smell you can sense with your nose?”
Action: “Does the meaning of this word relate to an action you can perform by moving any part of your body?”
Vision: “Does the meaning of this word relate to an object you can perceive with your eyes?”
In the questionnaire study, subjects also taking part in the fMRI experiment were presented with typical examples of the stimulus materials. The semantic rating scores were used to study the association between these ratings and the differences in language use they indicate and the brain activation to gustatory-related words. To this end, the subjects’ mean ratings were correlated with the percentage of signal change in a priori regions of interest (ROIs, see below).
Behavioral Data Analysis
Behavioral data analyses were performed using standard statistics software (PASWm Inc., Chicago, IL). Below, the data are expressed as mean and SD, unless otherwise indicated. The primary analysis for the statistical differences between the subjective ratings on the relevance meaning of gustatory and control words was tested using repeated measures of within-subject two-way analyses of variance (ANOVAs); design: word sets (TW, CW) × rating score (gustatory, olfactory, vision, action). Post hoc t-test comparisons between levels of interest were assumed significant at a two-tailed P < 0.05.
fMRI Acquisition
Images were acquired on a 1.5-T scanner (Avanto; Siemens). Twenty oblique transverse slices covering the whole brain were acquired using a T2*-weighted (time repetition [TR] = 2000 ms; echo time = 40 ms; flip angle 80°; Field of View [FOV] = 125.6 × 125.6; matrix size = 64 × 64 voxels; in-plane spatial resolution = 3.94 × 3.94 mm, 5-mm thick, 1-mm skip, and 1 interleaved). Anatomical scans were also obtained using contiguous 1-mm sagittal images across the entire brain with a T1-weighted fast field echo sequence (time echo = 4.2 ms, TR = 11.3 ms, flip angle = 90°; FOV = 231 × 264 mm; matrix = 224 × 256 × 176 voxels).
Image Data Analysis
Preprocessing
The neuroimaging data were preprocessed and analyzed with statistical parametric mapping using the SPM5 software (Wellcome Department of Cognitive Neurology, London) implemented in Matlab (v.7.0; Mathworks Inc. Sherborn, MA). Functional images were realigned with a two-pass procedure in which functional volumes were registered to the first volume in the series in a first step and to the mean image of all the realigned volumes in a second step. Next, the anatomical scans from each subject were coregistered to the mean image and segmented in to the gray and white matter partitions. Normalization parameters were extracted from the segmentation of each subject's anatomical T1-weighted scan and applied to their corresponding functional scans (voxel size resampled to 3 × 3 × 3 mm3, template provided by the Montreal Neurological Institute [MNI]). Finally, all the images were smoothed with a Gaussian kernel of 6-mm full-width at half-maximum.
Statistical Analyses
Statistical parametric maps were generated with a general linear model defined for each subject with a boxcar function, convolved with the hemodynamic response function and its temporal derivative. Furthermore, 6 covariates were added to each subject's model corresponding to their motion parameters derived from the realignment of functional volumes. To determine activation probability that was specific to each condition per subject and voxel, pair-wise contrasts for each pair of conditions were defined at an initially fixed effect level. Hypothesis testing at the random effects level was conducted by one-sample t-tests in which the parameter estimates images from the contrasts between main conditions (TW > CW) were included from each subject. At this second level, all the comparisons were thresholded at a significance level of P < 0.01 and were corrected for multiple comparisons across the entire brain at both the voxel (false discovery rate [FDR]) and the cluster levels (P < 0.05, Familywise Error corrected).
ROI Analysis
In addition to the whole-brain analyses, ROI analyses were performed to test for condition and laterality effects, besides studying subjective gustatory ratings association with the brain activation in these regions. For a priori ROIs, each subject's average percentage signal change value was extracted for each condition on a single-subject basis. ROI definition was based on anatomical regions (e.g., structures, gyri, Brodmann's areas) defined with the Anatomical Automatic Labeling (AAL) atlas (Tzourio-Mazoyer et al. 2002) as implemented in the WFU PickAtlas toolbox (Maldjian et al. 2003) for SPM5; afterward, these ROIs were masked with the conjunction's mask of all the images used in the fMRI model analysis. The a priori selected AAL ROIs were defined based on the previous anatomical regions involved in gustatory and semantic processing. Therefore, the anatomical ROI definition included: the frontal operculum (Small et al. 2004; Pulvermüller et al. 2009; Kemmerer and González-Castillo 2010), insula (Small et al. 2004), lateral OFC (Small et al. 2003, 2004, 2007; Goldberg et al. 2006b), amygdale (González et al. 2006), inferior frontal cortex (Bookheimer 2002), inferior temporal cortex, supplementary motor area, and premotor cortex (Hauk et al. 2004; Kemmerer et al. 2008; Pulvermüller et al. 2009). Furthermore, for a detailed analysis of the inferior frontal cortex, we subdivided it into its opercular, triangular, and orbital cytoarchitectonic portions by means of the predefined ROI based on Brodmann's areas 44 (pars opercularis), 45 (triangularis), and 47 (orbitalis). All these ROIs were defined for each hemisphere separately, and the percentage signal change was extracted using Marsbar (Brett et al. 2002).
To test for word category differences and the hemispheric specificity of activations in ROIs, a Condition (TW, CW) × Hemisphere (Left, Right) repeated-measures ANOVA was performed on the percent signal change values from each ROI. Furthermore, each subject's average percentage signal change values for the TW condition alone, obtained from the subtraction of the TW minus the CW conditions’ percentage signal change, were plotted against the individual subject's average gustatory semantic rating score of the words from the TW condition, and the correlation was calculated in an exploratory analysis to test how subjective gustatory ratings related to brain activation in ROIs. Thus, gustatory word-related scores correlated with the extracted percentage signal change from all 10 ROIs from each hemisphere by applying Pearson's correlation. Given the number of variables and the number of pair-wise correlations, we applied a Bonferroni correction to all correlation results. Given the sample size (n = 41) and the number of tests (10 ROIs by 2 hemispheres) and 20 possible correlations with the gustatory word-related scores, any positive or negative correlation over r40 = ±0.46 (two-tailed P < 0.05, Bonferroni corrected) was considered significant (Meinert 1986; Busatto et al. 1997; Sankoh et al. 1997; Curtin and Schulz 1998).
Results
Behavioral Results
The within-subjects two-way ANOVA word set (TW, CW) × rating (gustatory, olfactory, visual, action) indicated a significant interaction of word sets and rating scores (F1,3 = 249.9, P < 0.001). Post hoc paired t-tests showed that subjects rated the 2 word categories, TW and CW, differently in their gustatory (t40 = 39.30; P < 0.001), olfactory (t40 = 24.74; P < 0.001), and visual (t40 = 2.55; P < 0.015) semantic links; however, the semantic action ratings did not differ between the TW and the CW categories (P > 0.1). The main post hoc analysis results were replicated using nonparametric tests (P < 0.05). Table 1 lists all the means and SDs of the semantic ratings.
Imaging Results
Whole-Brain Analysis
Figure 1A shows the contrast of the BOLD signal in the TW versus the CW conditions. The TW condition shows that activation increased in the left frontal operculum (BA 47), the lateral OFC (BA11), anterior insula (BA13), middle temporal gyrus (BA21), angular gyri (BA 39), dorsal posterior cingulate (BA 31), mesial prefrontal cortex (BA9/10) and middle prefrontal cortex (BA8), and premotor cortex (BA6), cuneus (BA 18/19) and precuneus (BA18). Activation also peaked in the midbrain structures below the hypothalamus, such as the subthalamic nucleus and the substantia nigra. The MNI coordinates referring to these structures are reported in Table 2. Opposite contrasts, which looked for significantly higher activations in the CW condition than in the TW condition, showed no significant differences.
Figure 1.
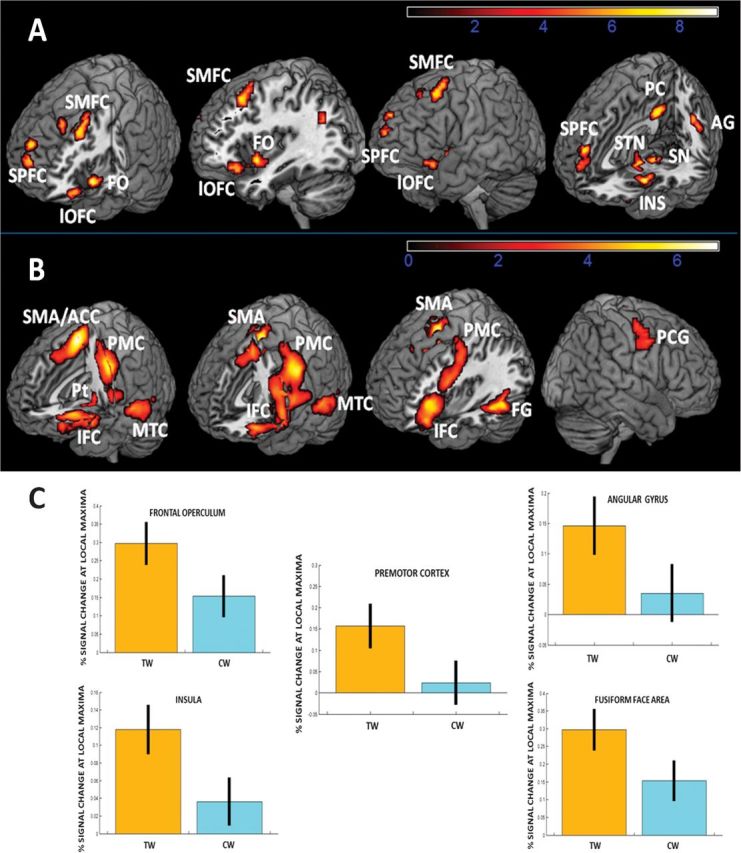
Brain activation when comparing the taste word versus the control word conditions (A) and the control word versus the baseline conditions (B) at the threshold (P < 0.01, FDR-corrected at the single voxel level and P < 0.05, Familywise Error-corrected at the cluster level). Color bars represent the T-values scale for each contrast. Left is left in all the brain images. (C) Represents percentage signal change in brain local maxima for those regions showing higher activation when comparing the TW versus CW conditions. ACC, anterior cingulated cortex; AG, angular gyrus; CW, control words; FG, fusiform gyrus; FO, frontal operculum; IFC, inferior frontal cortex; lOFC, lateral orbitofrontal cortex; MTC, middle temporal cortex; PC, posterior cingulate; PCG, precentral gyrus; PMC, premotor cortex; Pt, putamen; SMA, supplementary motor area; SMFC, superior/middle frontal cortex; SN, substantia nigra; SPFC, superior prefrontal cortex; STN, subthalamic nucleus; TW, tasty words.
Table 2.
Brain activations when comparing the BOLD signal between the TW and CW conditions
| Contrast | Brain area | Brodmann area | Hemisphere | x | y | z | T-score | #-voxels |
| TW > CW | ||||||||
| Insula | 13 | L | −36 | 6 | −9 | 6.89 | 41 | |
| Frontal operculum/lateral OFC | 11/47 | L | −39 | 33 | −15 | 4.67 | 19 | |
| Superior temporal gyrus/angular gyrus | 39 | L | −33 | −60 | 30 | 4.70 | 23 | |
| Posterior cingulate | 31 | B | −3 | −36 | 33 | 5.87 | 53 | |
| Precentral/middle frontal gyrus | 6/8 | L | −36 | 21 | 48 | 5.50 | 48 | |
| Superior prefrontal cortex | 9/10 | L | −3 | 57 | 30 | 5.49 | 54 | |
| Superior/middle frontal gyrus | 8 | L | −18 | 30 | 51 | 4.60 | 11 | |
| Cuneus | 18/19 | B | 0 | −87 | 15 | 4.80 | 27 | |
| Cuneus/precuneus | 18 | R | 15 | −75 | 18 | 5.14 | 22 | |
| Substantia nigra | L | −12 | −24 | −9 | 5.56 | 20 | ||
| Subthalamic nucleus/substantia nigra/Thalamus | L | −9 | −12 | −9 | 5.26 | 25 |
Note: L = left; R = right; B = activation extends bilaterally and the local maxima reported at corresponding hemisphere. MNI coordinates.
Figure 1B illustrates the contrast of the parameter estimates for the contrast CW versus the baseline. The CW condition shows that activation increased in the left inferior frontal cortex, including Broca's area, and premotor cortices (BA 6/44/45/47), supplementary motor area and adjacent anterior cingulate (BA6/32), left fusiform and middle temporal gyrus (BA37/22 including the visual word form area), putamen, globus pallidus, and thalamus. All these activations were left lateralized, except the activation in the right prefrontal cortex (BA6; see Table 3).
Table 3.
Brain activations during the control words condition reported as the main local maxima for each cluster
| CW > B | Brain area | H | BA | x | y | z | T-score | k |
| Supplementary motor area | L | 6 | −3 | 3 | 60 | 9.03 | 341 | |
| Anterior cingulate | L | 32 | −9 | 21 | 48 | 4.52 | ||
| Fusiform gyrus | L | 37 | −45 | −51 | −18 | 8.00 | 334 | |
| L | 37 | −42 | −42 | −18 | 6.42 | |||
| Middle temporal | L | 22 | −60 | −33 | 3 | 5.86 | ||
| Premotor cortex | L | 6 | −51 | −6 | 45 | 8.00 | 1312 | |
| Broca's area | L | 44/45 | −36 | 30 | 9 | 7.67 | ||
| Inferior frontal cortex | L | 47 | −36 | 33 | −9 | 6.68 | ||
| Precentral gyrus | R | 6 | 54 | 0 | 42 | 4.81 | 65 | |
| Putamen | L | −24 | −9 | 6 | 4.95 | 54 | ||
| Lateral globus pallidus | L | −21 | −6 | −3 | 4.38 |
ROI Analysis Results
The ANOVA analyses on each individual ROI show the main effects of condition in the amygdalae (F1,40 = 9.68; P < 0.005) and in BA47 (F1,40 = 4.79; P < 0.05) with a stronger activation to taste compared with control words. The main effects of hemisphere were present in the inferior temporal gyrus around the visual word form area (F1,40 = 6.84; P < 0.05), the frontal operculum (F1,40 = 4.115; P < 0.05), and in both the anterior and the posterior parts of Broca's area versus their right homotopic loci, BA44 (F1,40 = 6.94; P < 0.05) and BA45 (F1,40 = 4.99; P < 0.05). In all of these contrasts, activation was stronger in the left language dominant hemisphere than in the homotopic area of the right hemisphere. It is important to note that the lateral OFC, as a main center of gustatory processing, shows an interaction effect (F1,40 = 4.94; P < 0.05) due to stronger taste word activation if compared with the control items in the left hemisphere (but not in the right). Table 4 offers further details of the ROI comparisons, including the means and SDs for each condition in each ROI.
Table 4.
Mean (SD) of the percentage signal change for the TW and CW conditions (TW–CW) for each ROI in the left and right hemispheres
| ROI | Condition | Left hemisphere | Right hemisphere |
| Amygdala | TW** | 0.34 (0.69) | 0.16 (0.79) |
| CW | 0.19 (0.71) | 0.10 (0.78) | |
| Orbitofrontal | TW | 0.33 (0.64)** | 0.23 (0.55) |
| CW | 0.24 (0.65) | 0.24 (0.51) | |
| Operculum | TW | 0.28 (0.58)* | 0.18 (0.57) |
| CW | 0.27 (0.55) | 0.17 (0.51) | |
| Insula | TW | 0.04 (0.51) | −0.03 (0.51) |
| CW | 0.05 (0.48) | −0.04 (0.47) | |
| Inferior temporal | TW | 0.19 (0.52)* | −0.01 (0.64) |
| CW | 0.14 (0.49) | −0.06 (0.59) | |
| Premotor | TW | 0.11 (0.58) | 0.17 (0.49) |
| CW | 0.16 (0.63) | 0.14 (0.46) | |
| SMA | TW | −0.08 (0.83) | −0.03 (0.77) |
| CW | −0.09 (0.78) | −0.06 (0.71) | |
| BA44 | TW | 0.36 (0.46)* | 0.18 (0.61) |
| CW | 0.32 (0.46) | 0.18 (0.55) | |
| BA45 | TW | 0.39 (0.55)* | 0.20 (0.63) |
| CW | 0.36 (0.54) | 0.22 (0.56) | |
| BA47 | TW* | 0.31 (0.65) | 0.19 (0.47) |
| CW | 0.25 (0.64) | 0.14 (0.47) |
Note: The location of the signs provides information about the higher intensity in the direction of the differences. TW: taste word condition; CW: control word condition; BA: Brodmann's area; SMA: supplementary motor area.
*significant condition/lateralization effect at P < 0.05; **significant condition/lateralization effect P < 0.005; */* significant interaction effect P < 0.05.
The main analysis results were reinvestigated using nonparametric tests. All the ROI tests were repeated using the Kolmogorov–Smirnov test. All the ROI differences found by parametric testing could be replicated at P < 0.05.
Finally, the correlation analysis of the mean ratings of the gustatory semantics of taste words and the ROI-specific activation differences between the TW and the CW conditions failed to reveal any significant correlation surpassing the conservative corrected threshold of r > 0.46 (P < 0.05 Bonferroni corrected; n = 41). No significant correlations between brain activation strength and semantic ratings were observed in these gustatory areas or in the other ROIs examined.
Discussion
The hypothesis being tested was that the passive reading of taste words, with strong gustatory semantic links, would activate the primary and secondary sensory gustatory regions of the human brain, particularly the anterior insula, the frontal operculum, and the OFC. Our results fully confirm this hypothesis. In these 3 regions, which are known as the main hub for taste processing, the BOLD signal obtained while participants read taste-related words (TW) was significantly stronger than when they read control words (CW) with no or very weak taste associations. The ROI analysis further confirms that taste words elicited a relatively stronger activation in the left lateral OFC when compared with the control items. Together, these results strongly support the proposal that the semantic circuits of taste-related words include neuron populations in gustatory cortical areas.
Most previous studies using fMRI and positron emission tomography support the role of both the frontal operculum and the anterior insula as primary gustatory areas (PGAs) in humans, which has therefore been argued to be the homolog of the PGA in the monkey (for a discussion, see Onoda et al. 2005; Small 2006). This putative human PGA is not exactly concordant with that identified by MEG in the shortest latency after gustatory stimulation, which was located in a more posterior region, the transitional cortex between the insula and the parietal operculum (Kobayakawa et al. 1999, 2005; Onoda et al. 2005). However, given the well-known limitations of MEG source localization (Hämäläinen 1995), due to fundamental issues related to the inverse problem, and the weak signals reaching the MEG sensors from deep sources (such as the insula), caution is required when interpreting these results. Using salt and saccharine as stimuli, Kobayakawa et al. (1999) estimated the sources of an early MEG response between the parietal operculum and the posterior insular cortex, while those of subsequent activations were attributed to other areas, which is consistent with the fMRI literature, especially concerning the frontal operculum and the anterior insula. The results of the present work suggest that these areas become active not only in perception but also in the reprocessing of taste information, which is characteristic of the processing of taste-related word meaning. Similarly to the gustatory system, the human OFC is crucial for olfaction, and it also plays a role in representing and processing taste, flavor, and food reward (see the review of Small et al. 2007). According to the literature, the main contribution of the OFC to gustatory processing is thought to be encoding the affective value of taste stimuli (O’Doherty et al. 2001; Small et al. 2003, 2007). Likewise, the OFC also encodes retronasal olfaction (Small et al. 2005) and oral somatosensory stimulation, both of which always occur in conjunction with the sensation of taste during eating (De Araujo et al. 2003; Small et al. 2007). We will discuss below the possible relationship between olfactory and gustatory semantic links.
Goldberg et al. (2006b) studied the role of sensory brain regions in semantic decisions according to tactile, gustatory, auditory, and visual knowledge. During the experiment, participants were asked to determine whether a specific word item possessed a given property of 1 of 4 sensory modalities, including color (green), sound (loud), touch (soft), or taste (sweet). Therefore, it was an explicit judgment task that forced subjects to consult their mental representations of sensory information. Their fMRI results showed that the predicted sensory brain regions were activated by the 4 sensory comparison tasks, suggesting that “the brain's sensory mechanisms might support not only the perceptual encoding of visual, auditory, tactile, and gustatory experiences but also semantic decisions which reference that knowledge” (p. 4917). Specifically, gustatory decisions were associated with increased activity in the left OFC. This pattern was consistent with a previous finding of the same authors (Goldberg et al. 2006a) when a different decision task, based on the knowledge of semantic categories, was used (e.g., fruit) in which flavor properties were necessary and relevant. Our results significantly extend these results by showing that an explicit comparison task is not necessary to achieve the activation of the PGCs and SGCs; the passive reading of taste words is sufficient to accomplish this activation.
The psycholinguistic evaluation of the stimulus words used in the present study demonstrates that a range of variables, especially valence, arousal, imageability, frequency of use, number of letters and syllables, were a priori matched between the 2 word types examined. Although the most pronounced (TW = 6.04 (0.60) vs. CW = 1.42 (0.43)) and clear-cut (t40 = 39.31; P < 0.001) difference between the word groups was found in the gustatory semantic links, these stimulus groups also significantly differed in terms of their rated olfactory associations and their visual semantic links. One may therefore argue that olfactory and visual semantic links can, in principle, provide an alternative account of the activation differences in the left anterior insula, the frontal operculum, and the OFC. This may be the case of the left OFC, which has been seen to become active to olfactory words according to previous study of González et al. (2006); moreover, and generally, the prefrontal areas anterior to Broca's area have sometimes been seen to be active in semantic tasks (Bookheimer 2002). However, according to the background found in the literature and to our results, activation in the anterior insula and the frontal operculum seem to be more specific to the word category under study herein (gustatory words) and may, therefore, be a true reflection of taste processing in semantic access. In particular, a previous work into the visual semantic links (Hauk et al. 2008) did not note any activity in these primary gustatory regions. In this sense, the relationship between the taste-related activation and the gustatory semantic ratings of the taste relatedness of words shown by the present work may further strengthen the case for the specificity of these activations to the processing of semantic information about taste. It is likely that the absence of significant correlations in our present data was due to the selection of gustatory words with extreme gustatory semantic ratings (e.g., the mean range between 5 and 7), which reduced the variability, thus working against significance of potential correlations. Therefore, at present, activation of these primary gustatory regions may be attributed to gustatory semantic links; thus, further research using correlation and regression analysis on differential semantic associations of words with variable semantic characteristics may further strengthen these results and, specially, may document specificity of the activation to gustatory semantic processes.
fMRI studies, like the one reported here, are always correlational and cannot, therefore, address the crucial question of whether the activated regions are also necessary for the process under study, that of accessing gustatory semantic information linked to words. Lesions in the anterior insula have been reported to lead to deficits in processing information about specific types of emotions, especially the inability to recognize a facial expression of disgust (Calder et al. 2000). Note that a link between the anterior insula and the disgust processing, as a special case of gustatory processing, can be explained by this region's known status as PGC. If semantic circuits for taste-related words include neurons in the anterior insula and the frontal operculum, a lesion in these structures may, therefore, produce a category-specific deficit in processing these words. Further research is necessary to test the new prediction as a result of the present work, that of a lesion in the anterior insula in the left hemisphere leading to a processing deficit for taste-related words.
Our results are not consistent with a link between anterior insular activation and word-evoked emotion processing as affective-emotional stimulus properties had been matched carefully between taste and control words.
Activation Outside the Gustatory System
Other brain areas were selectively activated while reading taste-related words (Table 2). Some of these areas belong to the subcortical gustatory processing system (Small 2006; Small et al. 2007). The human gustatory pathway, which is assumed to be the equivalent to that in the monkey (Small 2006; Small et al. 2007), starts with cranial nerves VII, IX, and X, reaches the nucleus of the solitary tract from where second-order gustatory fibers project to the ventral posterior medial nucleus of the thalamus and on to the cortex. FMRI studies have reported activation to gustatory stimuli in the thalamus (e.g., Cerf-Ducastel et al. 2001; Haase et al. 2006). Other structures that the present study finds are specifically active to taste words have also previously been shown to be active in association with taste processing, including the precentral gyrus (Kobayakawa et al. 1999; Haase et al. 2006), the cingulate gyrus (Cerf-Ducastel et al. 2001; O’Doherty et al. 2001; Haase et al. 2006), the angular gyrus (Cerf-Ducastel et al. 2001), and the substantia nigra (Small et al. 2003).
Since it is possible that all the corresponding activations noted in the present work are related, to an extent, to the processing of gustatory information and to other information linked semantically to our present stimulus words, it may also be worthwhile to explore alternative accounts. For example, the activation of the precentral gyrus, which includes premotor cortex (BA6; Fig. 1C), may be related to motor or action knowledge relating to gustatory experiences. Note that this may be an intrinsic link, as, when experiencing strong taste, we involuntarily respond by moving our face, hence tasting is inevitably related to complex face, lips, mouth movements. The observed precentral activation, which was stronger to TW than to CW, may also be a brain correlate of the implicit action knowledge linked to TW-related meaning. Thus, similar located precentral activation has been related to masticatory activity (Byrd et al. 2009; Iida et al. 2010) and even to the retrieval of complex action plans (Wise and Murray 2000; Schluter et al. 2001; Rushworth et al. 2003; O’Shea et al. 2007). Otherwise, the similar ratings of the semantic action relationship of the 2 word groups investigated may explain the lack of functional differences in more posteriorly located premotor regions, such as the premotor face area (Pulvermüller et al. 2006). Previous studies have related activation in the anterior bank of the precentral gyrus to the naming of actions and tools (Grabowski et al. 1998), suggesting a role in the retrieval of words denoting actions or objects with characteristic actions. Therefore, TW-related higher activation in the premotor association cortex may stem from subjects’ accumulated verbal and motor experiences related to tasty words. It may reflect implicit action knowledge immanent to taste words; but these semantic action links cannot be evidenced in the semantic rating because the memory of taste related to the words may dominate rather than the (secondary) knowledge of how one would respond when experiencing that taste.
The amygdale showed a ROI condition effect that was not present in the voxel-wise statistical tests, and that is probably the result of the more liberal threshold and its discrete structural volume in the atlas definition (Maldjian et al. 2003; Poldrack 2007), increasing the probability of colocalization errors across subjects after normalization and reducing the statistical significance at voxel level (Salmond et al. 2002). The amygdala's condition effect was reported in an early study by our group into olfactory words processing (González et al. 2006), and it has been related to taste stimuli and their hedonic processing (O’Doherty et al. 2001, 2002; Small et al. 2003, 2008). In contrast, in the present study, we controlled that words included in both the TW and the CW conditions so they were equated by valence and arousal effects, which likely reduced the contrast activation of the amygdala during the TW semantic processing.
Sensorimotor Circuits as a Cortical Basis for Semantics
As already mentioned in the Introduction, evidence for this theoretical framework has been obtained in recent years, especially from action words that relate semantically to different body parts (Pulvermüller et al. 2001, Pulvermüller, Hauk, et al. 2005; Pulvermüller, Shtyrov, et al. 2005; Hauk et al. 2004; Hauk and Pulvermüller 2004). The data suggest that the comprehension of these words activates the motor and premotor cortices somatotopically. For example, reading a leg-related verb such as “to kick” activates classical language areas, as well as the motor regions involved in leg/foot movement. Processing of mouth- (e.g., “to kiss”) and hand-related (“to pick”) words activates the regions involved in mouth and hand movements, respectively. Besides, a similar somatotopic pattern has recently been observed for abstract meaning processing, especially those idioms including arm- and leg-related action words (Boulenger et al. 2009). A wide range of studies supports the idea that the perceptual information associated with the reference of a word is important for its neural representation. Martin et al. (1995) and Simmons et al. (2007) found that processing color words activate a region overlapping the area involved in perception of color, thus providing evidence that conceptual knowledge is grounded in modality-specific brain systems (Simmons et al. 2007). Similarly, words that are semantically related to sounds (e.g., telephone) activate superior temporal auditory areas, even if the stimuli are presented in a written form (Kiefer et al. 2008). In a previous fMRI study (González et al. 2006), we observed that reading words with strong olfactory associations in their meaning activates olfactory regions. In the experiment, subjects read words such as “canela” (cinnamon), “alcanfor” (camphor), “fétido” (fetid), etc. Obviously, they were not exposed to any olfactory stimulation during the neuroimaging session. The results of the present work extend this pattern to the semantic gustatory domain: participants read words such as “sal” (salt), miel (“honey”), or uva (“grape”) but, again, they were not exposed to any gustatory stimulation.
These data and others suggest that word meaning is not confined to just meaning-specific brain regions in some left perisylvian areas; instead, it seems likely that semantic representations are distributed systematically throughout the brain. Additional cortical areas, which are critically involved in processing perceptual and motor information of the semantic reference, possibly contribute to the processing of word meaning. In this framework, sensorimotor circuits play a key role as a cortical basis for language processing (Pulvermüller and Fadiga 2010). Activation of gustatory brain regions when a subject processes words with gustatory semantic attributes is consistent with this viewpoint. It seems that gustatory information may be interwoven with the neuronal representation of such words. Further research may extend the involvement of sensory modal-specific systems to the comprehension or processing of sentence meaning, as in the motor system (Boulenger et al. 2009). Finally, these results may provide us a clearer answer as to why we salivate when we talk about food and when we are hungry.
In conclusion, our data show that reading words with gustatory meaning activate, along with the general left-perisylvian language areas, those brain regions sparked by gustatory stimuli, which are involved in gustatory perception. This pattern is coherent with the theoretical perspective (Pulvermüller 1999, 2001, Pulvermüller, Shtyrov, et al. 2005; Pulvermüller and Fadiga 2010) of viewing words as being cortically organized into neural distributed assemblies with different topographies that reflect aspects of their references. These cortical circuits include neurons in sensory systems that play a key role in perception, and, as we can now state, in perception through “all” the major sensory modalities (visual, auditory, somatosensory, olfactory, and, as shown here, gustatory) (Barsalou 1999, 2008; Pulvermüller 1999; Martin 2007; Pulvermüller and Fadiga 2010).
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
This work was supported by the CONSOLIDER-INGENIO 2010 Program of the Spanish Ministry of Science and Education (grant number CSD2007-00012), by research grants of the Spanish Ministry of Science and Innovation (grant numbers PSI 2009-10067 and PSI2010-20168), and the Medical Research Council (UK) (grant number U1055.04.003.00001.01).
Supplementary Material
Acknowledgments
Conflict of Interest : None declared.
References
- Bak TH, Yancopoulou D, Nestor PJ, Xuereb JH, Spillantini MG, Pulvermüller F, Hodges JR. Clinical, imaging and pathological correlates of a hereditary deficit in verb and action processing. Brain. 2006;129(Pt 2):321–332. doi: 10.1093/brain/awh701. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Perceptual symbol systems. Behav Brain Sci. 1999;22(4):577–609. doi: 10.1017/s0140525x99002149. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Grounded cognition. Annu Rev Psychol. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Boulenger V, Hauk O, Pulvermüller F. Grasping ideas with the motor system: semantic somatotopy in idiom comprehension. Cereb Cortex. 2009;19(8):1905–1914. doi: 10.1093/cercor/bhn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox. 2002 Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, Sendai, Japan. Available on CD-ROM in Neuroimage, Vol. 16, No 2. San Diego (CA). Publisher name: Elsevier. [Google Scholar]
- Buccino G, Riggio L, Melli G, Binkofski F, Gallese V, Rizzolatti G. Listening to action-related sentences modulates the activity of the motor system: a combined TMS and behavioral study. Brain Res Cogn Brain Res. 2005;24(3):355–363. doi: 10.1016/j.cogbrainres.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Busatto GF, Pilowsky LS, Costa DC, Ell PJ, David AS, Lucey JV, Kerwin RW. Correlation between reduced in vivo benzodiazepine receptor binding and severity of psychotic symptoms in schizophrenia. Am J Psychiatry. 1997;154:56–63. doi: 10.1176/ajp.154.1.56. [DOI] [PubMed] [Google Scholar]
- Byrd KE, Romito LM, Dzemidzic M, Wong D, Talavage TM. fMRI study of brain activity elicited by oral parafunctional movements. J Oral Rehabil. 2009;36:356–361. doi: 10.1111/j.1365-2842.2009.01947.x. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Manes F, Antoun N, Young AW. Impaired recognition and experience of disgust following brain injury. Nat Neurosci. 2000;3(11):1077–1078. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Van de Moortele P-F, MacLeod P, Le Bihan D, Faurion A. Interaction of gustatory and lingual somatosensory perceptions at the cortical level in the human: a functional magnetic resonance imaging study. Chem Senses. 2001;26:371–383. doi: 10.1093/chemse/26.4.371. [DOI] [PubMed] [Google Scholar]
- Curtin F, Schulz P. Multiple correlation and Bonferroni’s correction. Biol Psychiatry. 1998;44:775–777. doi: 10.1016/s0006-3223(98)00043-2. [DOI] [PubMed] [Google Scholar]
- Dalla Volta R, Gianelli C, Campione GC, Gentilucci M. Action word understanding and overt motor behavior. Exp Brain Res. 2009;196(3):403–412. doi: 10.1007/s00221-009-1864-8. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D. Nouns and verbs are retrieved with differently distributed neural systems. Proc Natl Acad Sci U S A. 1993;90:4957–4960. doi: 10.1073/pnas.90.11.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- Daniele A, Giustolisi I, Silveri MC, Colosimo C, Gainotti G. Evidence for a possible neuroanatomical basis for lexical processing of nouns and verbs. Neuropsychologia. 1994;32:1325–1341. doi: 10.1016/0028-3932(94)00066-2. [DOI] [PubMed] [Google Scholar]
- De Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci. 2003;18:2059–2068. doi: 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- Faurion A, Cerf B, Le Bihan D, Pillias AM. fMRI study of taste cortical areas in humans. Ann N Y Acad Sci. 1998;855:535–545. doi: 10.1111/j.1749-6632.1998.tb10623.x. [DOI] [PubMed] [Google Scholar]
- Faurion A, Cerf B, Van De Moortele PF, Lobel E, MacLeod P, Le Bihan D. Human taste cortical areas studied with functional magnetic resonance imaging: evidence of functional lateralization related to handedness. Neurosci Lett. 1999;277:183–192. doi: 10.1016/s0304-3940(99)00881-2. [DOI] [PubMed] [Google Scholar]
- Frank GK, Kaye WH, Carter CS, Brooks S, May C, Fissell K, Stenger VA. The evaluation of brain activity in response to taste stimuli—a pilot study and method for central taste activation as assessed by event-related fMRI. J Neurosci Methods. 2003;30:99–105. doi: 10.1016/s0165-0270(03)00240-1. [DOI] [PubMed] [Google Scholar]
- Gainotti G. Chapter 9 Disorders of semantic memory. Handb Clin Neurol. 2008;88:203–223. doi: 10.1016/S0072-9752(07)88009-2. [DOI] [PubMed] [Google Scholar]
- Glenberg AM, Sato M, Cattaneo L. Use-induced motor plasticity affects the processing of abstract and concrete language. Curr Biol. 2008;18(7):R290–R291. doi: 10.1016/j.cub.2008.02.036. [DOI] [PubMed] [Google Scholar]
- Goldberg RF, Perfetti CA, Schneider W. Distinct and common cortical activation for multimodal semantic categories. Cogn Affect Behav Neurosci. 2006;6:214–222. doi: 10.3758/cabn.6.3.214. [DOI] [PubMed] [Google Scholar]
- Goldberg RF, Perfetti CA, Scheneider W. Perceptual knowledge retrieval activates sensory brain regions. J Neurosci. 2006;26:4917–4921. doi: 10.1523/JNEUROSCI.5389-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González J, Barrós-Loscertales A, Pulvermüller F, Meseguer V, Sanjuán A, Belloch V, Ávila C. Reading cinnamon activates olfactory brain regions. Neuroimage. 2006;15:906–912. doi: 10.1016/j.neuroimage.2006.03.037. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Damasio AR. Premotor and prefrontal correlates of category-related lexical retrieval. Neuroimage. 1998;7(3):232–243. doi: 10.1006/nimg.1998.0324. [DOI] [PubMed] [Google Scholar]
- Haase L, Cerf-Ducastel B, Buracas G, Murphy C. On-line psychophysical data acquisition and event-related fMRI protocol optimized for the investigation of brain activation in response to gustatory stimuli. J Neurosci Methods. 2006;159:98–107. doi: 10.1016/j.jneumeth.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Hämäläinen MS. Functional localization based on measurements with a whole-head magnetometer system. Brain Topogr. 1995;7:283–289. doi: 10.1007/BF01195254. [DOI] [PubMed] [Google Scholar]
- Hauk O, Davis MH, Kherif F, Pulvermüller F. Imagery or meaning? Evidence for a semantic origin of category-specific brain activity in metabolic imaging. Eur J Neurosci. 2008;27(7):1856–1866. doi: 10.1111/j.1460-9568.2008.06143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermüller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41:301–307. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Hauk O, Pulvermüller F. Neurophysiological distinction of action words in the fronto-central cortex. Hum Brain Mapp. 2004;21:191–201. doi: 10.1002/hbm.10157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GW, Forde EM. Hierarchies, similarity, and interactivity in object recognition: category-specific neuropsychological deficits. Behav Brain Sci. 2001;24:453–509. [PubMed] [Google Scholar]
- Iida T, Kato M, Komiyama O, Suzuki H, Asano T, Kuroki T, Kaneda T, Svensson P, Kwara M. Comparison of cerebral activity during teeth clenching and fist clenching: a functional magnetic resonance imaging study. Eur J Oral Sci. 2010;118:635–641. doi: 10.1111/j.1600-0722.2010.00784.x. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Castillo JG, Talavage T, Patterson S, Wiley C. Neuro-anatomical distribution of five semantic components of verbs: evidence from fMRI. Brain Lang. 2008;107(1):16–43. doi: 10.1016/j.bandl.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Gonzalez-Castillo J. The Two-Level Theory of verb meaning: an approach to integrating the semantics of action with the mirror neuron system. Brain Lang. 2010;112(1):54–76. doi: 10.1016/j.bandl.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M, Sim EJ, Herrnberger B, Grothe J, Hoenig K. The sound of concepts: four markers for a link between auditory and conceptual brain systems. J Neurosci. 2008;28(47):12224–12230. doi: 10.1523/JNEUROSCI.3579-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayakawa T, Endo H, Ayabe-Kanamura S, Kumagai T, Yamaguchi Y, Kikuchi Y, Takeda T, Saito S, Ogawa H. The primary gustatory area in human cerebral cortex studied by magnetoencephalography. Neurosci Lett. 1996;212:155–158. doi: 10.1016/0304-3940(96)12798-1. [DOI] [PubMed] [Google Scholar]
- Kobayakawa T, Ogawa H, Kaneda H, Ayabe-Kanamura S, Endo H, Saito S. Spatio-temporal analysis of cortical activity evoked by gustatory stimulation in humans. Chem Senses. 1999;24:201–209. doi: 10.1093/chemse/24.2.201. [DOI] [PubMed] [Google Scholar]
- Kobayakawa T, Wakita M, Saito S, Gotow N, Sakai N, Ogawa H. Location of the primary gustatory area in humans and its properties, studied by magnetoencephalography. Chem Senses. 2005;30(1):i226–i227. doi: 10.1093/chemse/bjh196. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraf RA, Burdette JB. An automated method for neuro-anatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: structure and processes. Curr Opin Neurobiol. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG. Discrete cortical regions associated with knowledge of color and knowledge of action. Science. 1995;270:102–105. doi: 10.1126/science.270.5233.102. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379:649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- Meinert CL. Clinical trials. design, conduct and analysis. New York: Oxford University Press; 1986. p. 214. [Google Scholar]
- Miceli G, Siveri C, Nocentini U, Caramazza A. Patterns of dissociation in comprehension and production of nouns and verbs. Aphasiology. 1988;2:351–358. [Google Scholar]
- Moore CJ, Price CJ. A functional neuroimaging study of the variables that generate category-specific object processing differences. Brain. 1999;122:943–962. doi: 10.1093/brain/122.5.943. [DOI] [PubMed] [Google Scholar]
- Moscoso del Prado Martín F, Hauk O, Pulvermüller F. Category specificity in the processing of color-related and form-related words: an ERP study. Neuroimage. 2006;29:29–37. doi: 10.1016/j.neuroimage.2005.07.055. [DOI] [PubMed] [Google Scholar]
- Neininger B, Pulvermüller F. Word-category specific deficits after lesions in the right hemisphere. Neuropsychologia. 2003;41(1):53–70. doi: 10.1016/s0028-3932(02)00126-4. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85:1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Onoda K, Kobayakawa T, Ikeda M, Saito S, Kido A. Laterality of human primary gustatory cortex studied by MEG. Chem Senses. 2005;30:657–666. doi: 10.1093/chemse/bji059. [DOI] [PubMed] [Google Scholar]
- O’Shea J, Sebastian C, Boorman ED, Johansen-Berg H, Rushworth MF. Functional specificity of human premotor-motor cortical interactions during action selection. Eur J Neurosci. 2007;26:2085–2095. doi: 10.1111/j.1460-9568.2007.05795.x. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Soc Cogn Affect Neurosci. 2007;2(1):67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F. Words in the brain's language. Behav Brain Sci. 1999;22(2):253–279. [PubMed] [Google Scholar]
- Pulvermüller F. Brain reflections of words and their meaning. Trends Cogn Sci. 2001;5:517–524. doi: 10.1016/s1364-6613(00)01803-9. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F. Brain mechanisms linking language and action. Nat Rev Neurosci. 2005;6:576–582. doi: 10.1038/nrn1706. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Cooper-Pye E, Dine C, Hauk O, Nestor P, Patterson K. The word processing deficit in semantic dementia: all categories are equal but some categories are more equal than others. J Cogn Neurosci. 2010;22(9):2027–2041. doi: 10.1162/jocn.2009.21339. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Fadiga L. Active perception: sensoriomotor circuits as a cortical basis for language. Nat Rev Neurosci. 2010;11(5):351–360. doi: 10.1038/nrn2811. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Härle M, Hummel F. Neurophysiological distinction of verb categories. Neuroreport. 2000;11:2789–2793. doi: 10.1097/00001756-200008210-00036. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Härle M, Hummel F. Walking or talking? Behavioral and neurophysiological correlates of action verbs processing. Brain Lang. 2001;78:143–168. doi: 10.1006/brln.2000.2390. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Hauk O. Category-specific conceptual processing of color and form in left fronto-temporal cortex. Cereb Cortex. 2006;16:1193–1201. doi: 10.1093/cercor/bhj060. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Hauk O, Nikulin VV, Ilmoniemi RJ. Functional links between motor and language systems. Eur J Neurosci. 2005;21:793–797. doi: 10.1111/j.1460-9568.2005.03900.x. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Huss M, Kherif F, Moscoso del Prado Martin F, Hauk O, Shtyrov Y. Motor cortex maps articulatory features of speech sounds. Proc Natl Acad Sci U S A. 2006;103(20):7865–7870. doi: 10.1073/pnas.0509989103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F, Kherif F, Hauk O, Mohr B, Nimmo-Smith I. Distributed cell assemblies for general lexical and category-specific semantic processing as revealed by fMRI cluster analysis. Hum Brain Mapp. 2009;30(12):3837–3850. doi: 10.1002/hbm.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F, Lutzenberger W, Preissl H. Nouns and verbs in the intact brain: evidence from event-related potentials and high frequency cortical responses. Cereb Cortex. 1999;9:498–508. doi: 10.1093/cercor/9.5.497. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Shtyrov Y, Ilmoniemi RJ. Brain signatures of meaning access in action word recognition. J Cogn Neurosci. 2005;17(6):884–892. doi: 10.1162/0898929054021111. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Johansen-Berg H, Göbel SM, Devlin JT. The left parietal and premotor cortices: motor attention and selection. Neuroimage. 2003;20:89–100. doi: 10.1016/j.neuroimage.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Ashburner J, Vargha-Khadem F, Connelly A, Gadian DG, Friston KJ. The precision of anatomical normalization in the medial temporal lobe using spatial basis functions. Neuroimage. 2002;17(1):507–512. doi: 10.1006/nimg.2002.1191. [DOI] [PubMed] [Google Scholar]
- Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustments methods in clinical trials. Stat Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Krams M, Rushworth MF, Passingham RE. Cerebral dominance for action in the human brain: the selection of actions. Neuropsychologia. 2001;39:105–113. doi: 10.1016/s0028-3932(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Sebastián-Gallés N, Martí MA, Carreiras M, Cuetos F. LEXESP: una base de datos informatizada del español. Barcelona (Spain): Universitat de Barcelona; 2000. [Google Scholar]
- Shtyrov Y, Hauk O, Pulvermüller F. Distributed neuronal networks for encoding category-specific semantic information: the mismatch negativity to action words. Eur J Neurosci. 2004;19(4):1083–1092. doi: 10.1111/j.0953-816x.2004.03126.x. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Ramjee V, Beauchamp MS, McRae K, Martin A, Barsalou LW. A common neural substrate for perceiving and knowing about color. Neuropsychologia. 2007;45(12):2802–2810. doi: 10.1016/j.neuropsychologia.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM. Central gustatory processing in humans. In: Hummel T, Welge-Lüssen A, editors. Taste and smell. An update. Adv otorhinolaryngol. Basel (Switzerland): Karger; 2006. pp. 191–220. [DOI] [PubMed] [Google Scholar]
- Small DM, Bender G, Veldhuizen MG, Rudenga K, Nachtigal D, Felsted J. The role of the human orbitofrontal cortex in taste and flavour processing. Ann NY Acad Sci. 2007;1121:136–151. doi: 10.1196/annals.1401.002. [DOI] [PubMed] [Google Scholar]
- Small DM, Gerber JC, Mak YE, Hummel T. Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron. 2005;47:593–603. doi: 10.1016/j.neuron.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gestation. Neuron. 2003;39:701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F. Separable substrates for anticipatory and consummatory food chemosensation. Neuron. 2008;57(5):786–797. doi: 10.1016/j.neuron.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Voss J, Mak YE, Simmons KB, Parrish T, Gitelman D. Sperience-dependent neural integration of taste and smell in the human brain. J Neurophysiol. 2004;92(3):1892–1903. doi: 10.1152/jn.00050.2004. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labelling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Category specific semantic impairments. Brain. 1984;107(3):829–853. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- Wise SP, Murray EA. Arbitrary associations between antecedents and actions. Trends Neurosci. 2000;23:271–276. doi: 10.1016/s0166-2236(00)01570-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


