Abstract
Dopamine (DA) signaling in the medial prefrontal cortex (mPFC) plays a critical role in the processing of emotional information and memory encoding. Activation of DA D4 receptors within the prelimbic (PLC) division of the mPFC bidirectionally modulates emotional memory by strongly potentiating the salience of normally nonsalient emotional memories but blocking the acquisition of suprathreshold emotionally salient fear memories. Previous in vitro studies have shown that activation of cortical DA D4 receptors can bidirectionally modulate levels of α-calcium calmodulin–dependent kinase II (α-CaMKII), a molecule essential for learning and memory. Using an olfactory fear conditioning procedure in rats combined with microinfusions into the mPFC, we examined the potential role of D4 receptor–mediated control of emotional memory salience through signaling via CaMKII, cAMP/protein kinase A (PKA), and protein phosphatase-1 (PP1) signaling. We report that CaMKII blockade prevents the ability of intra-mPFC DA D4 receptor activation to potentiate the salience of subthreshold fear memory. In contrast, blockade of either cAMP/PKA or PP1 signaling pathways rescued the blockade of suprathreshold fear memory via intra-mPFC D4 receptor activation. Our results demonstrate that modulation of emotional memory salience via intra-mPFC DA D4 receptor transmission depends upon downstream signaling via CaMKII, cAMP/PKA, and PP1 substrates.
Keywords: CaMKII, dopamine, D4, emotion, memory, prefrontal cortex, schizophrenia
Introduction
Functional disturbances in frontal cortical dopamine (DA) transmission and calcium calmodulin–dependent kinase II (CaMKII) signaling have been implicated in the pathophysiology of neuropsychiatric disorders such as schizophrenia and attention deficit hyperactivity disorder (ADHD) (Frankland et al. 2008; Lauzon and Laviolette 2010). Indeed, DAergic receptor–mediated modulation of the α and β isoforms of CaMKII has been shown to be functionally important in the context of animal models of psychosis (Greenstein et al. 2007; Yamasaki et al. 2008; Novak and Seeman 2010). However, the mechanisms by which DA and CaMKII signaling pathways may interact at the systems level to modulate emotional processing are not currently understood. The DA D4 receptor subtype is highly expressed in medial prefrontal cortex (mPFC) (Ariano et al. 1997; De Almeida and Mengod 2010) and plays a crucial modulatory role during the acquisition (encoding) of fear memory (Laviolette et al. 2005). Using an olfactory fear conditioning procedure, we have demonstrated that activation of D4 receptors within the prelimbic cortex (PLC) division of the mPFC potentiates the emotional salience of subthreshold fear conditioning stimuli during acquisition but not expression (recall) phases of emotional learning (Lauzon et al. 2009). In addition, activation of PLC D4 receptors blocks the acquisition of emotionally salient suprathreshold fear memories, demonstrating a fundamental role for D4 receptor signaling during the acquisition of emotional memory (Lauzon et al. 2009; Lauzon and Laviolette 2010).
Previous work by Gu et al. (2004, 2006) have shown that activation of cortical D4 receptors dynamically and bidirectionally modulate levels of α-CaMKII, a molecule essential for regulating several postsynaptic targets involved in learning and memory (Malenka and Nicoll 1999; Frankland et al. 2001; Gu and Yan 2004; Gu et al. 2006). Specifically, they reported that D4 receptor activation strongly increases α-CaMKII activity, resulting from stimulation of phospholipase-C which in turn increases intracellular Ca2+ through inositol-1,4,5-triphospate. Interestingly, the D4 receptor–mediated elevation in α-CaMKII activity was only observed during spontaneously low levels of neuronal activity within the mPFC. In contrast, during high neuronal activation in the mPFC, activation of D4 receptors led to a decrease in α-CaMKII activity, a process mediated via decreased protein kinase A (PKA) which in turn increased protein phosphotase-1 (PP1) levels, ultimately resulting in dephosphorylation and reduced activity of α-CaMKII.
We hypothesized that intra-PLC D4 receptor signaling may control the salience of emotional memory by coregulating CaMKII levels. Specifically, using an olfactory fear conditioning assay in rats, we tested whether intra-PLC D4 receptor activation may amplify the emotional salience of normally nonsalient fear stimuli via upregulation of CaMKII levels. Furthermore, we examined whether D4 receptor–mediated blockade of suprathreshold emotional fear memory may depend upon indirect modulation of CaMKII signaling via actions on the PP1 and the cAMP/PKA signaling pathways. We report that blockade of CaMKII signaling by either direct inhibition of CaMKII or blockade of CaMKII autophosphorylation prevents the ability of intra-PLC D4 receptor activation to potentiate emotional memory. In contrast, activation of cAMP/PKA signaling or inhibition of PP1 rescues D4 receptor–mediated blockade of suprathreshold emotional fear memory acquisition, demonstrating bidirectional control of fear memory acquisition and salience via distinct signaling pathways within the PLC.
Materials and Methods
Animals and Surgery
All procedures were performed in accordance with the Canadian Council on Animal Care and approved by the University of Western Ontario's Council on Animal Care. Male Sprague Dawley rats (300–350 gm; Charles River) were anesthetized with a ketamine (80 mg/ml)–xylazine (6 mg/kg) mixture intraperitoneally and placed in a stereotaxic device. Stereotaxic coordinates were based upon the atlas of Paxinos and Watson (1996). Two stainless steel guide cannulae (22 gauge) were implanted into the PLC division of the mPFC using the following stereotaxic coordinates (15° angle; in mm from bregma): anteroposterior (AP) +2.9, lateral (LAT) ±1.9, ventral V −3.0 from the dural surface (see Fig. 1). Jeweler's screws and dental acrylic were used to secure the cannulae.
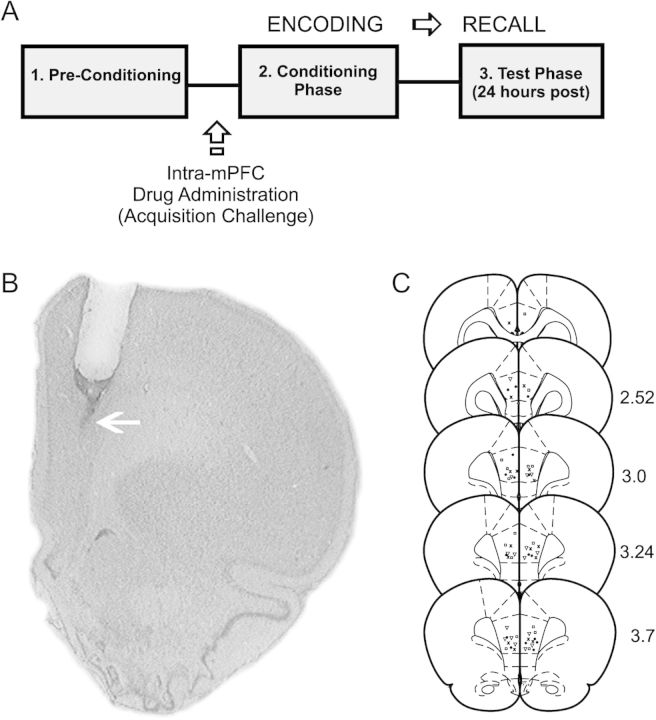
Figure 1.
Histological analysis of intra-mPFC microinjection sites and experimental protocol summary. (A) Microphotograph of a representative injector placement within the mPFC (white arrow represents injector tip location). (s) Schematic representation showing experimental associative fear conditioning assay and timeline for examining the acquisition (encoding) of associative olfactory fear memory. (C) Schematic illustration showing representative bilateral placements of microinjectors. For illustrative clarity, only a subset of experimental groups is presented. Symbols represent separate experimental groups; ▿, D4 agonist PD 168077; 50 ng/0.5 μL versus acquisition of subthreshold emotional stimuli; ×, D4 agonist PD 168077 50 ng/0.5 μL versus acquisition of supra-threshold emotional stimuli;  , Saline versus acquisition of subthreshold emotional stimuli; •, Saline versus acquisition of suprathreshold emotional stimuli.
, Saline versus acquisition of subthreshold emotional stimuli; •, Saline versus acquisition of suprathreshold emotional stimuli.
Olfactory Fear Conditioning
Olfactory fear conditioning took place in 1 of 2 distinct environments, counterbalanced within groups: environment A was a 30″ × 30″ Plexiglas box with black stripes on a white background, whereas environment B had black dots on a white background with a grid shock floor. Testing 24 h later took place in 1 of 2 alternate environments, in which animals had not previously received electric shock, counterbalanced within groups. On day 1 (habituation phase), animals were habituated to a random combination of shock environment A or B and test environment A or B in a counterbalanced order for 30 min in each environment. On day 2 (conditioning phase), animals were returned to the conditioning room and placed in the previously assigned shock environment. During conditioning, one of the odors (almond or peppermint) was presented to the animal for 19 s and a footshock was delivered (0.4 mA or 0.8 mA) for 1 s. These 2 different levels of footshock (0.4 mA and 0.8 mA) correspond to subthreshold and suprathreshold levels of fear conditioning stimuli, as reported previously (Laviolette and Grace 2006). One hundred and twenty seconds later, the alternate odor was presented for 20 s (CS−) in the absence of footshock. This cycle was repeated 5 times. On the following day (test phase), rats were returned to the test room and placed in the previously assigned test environment. Before odor presentation, the rat was allowed to explore the environment for 1 min and baseline levels of freezing and exploratory behavior were observed. Odors (CS+ or CS−) were presented for 5 min each to the animal in a counterbalanced order, and the amount of time freezing was recorded. Freezing behavior was defined as complete immobility with the exception of respiratory-related movement. We also analyzed exploratory behavior in response to presentations of CS+ or CS− odors, as described previously (Lauzon et al. 2009). Exploratory behavior was scored as follows, with a score assigned for every min of each of the 5 min during the CS+ or CS− odor presentations: 0, no locomotion; 1, ambulation across 1 side of the testing chamber; 2, ambulation across 2 sides; 3, exploration of the full perimeter of the testing chamber; and 4, exploration of the center and entire perimeter of the test chamber. Experimental procedures and timeline for examining olfactory fear memory acquisition are summarized schematically in Figure 1A.
Drug Administration
All drugs were dissolved in physiological saline, with pH adjusted to 7.4. Bilateral intra-mPFC microinjection of either saline vehicle, the selective D4 agonist PD 168077 (Tocris) (50 ng/0.5 μL), the selective CaMKII inhibitors; Autocamtide-2-related inhibitory peptide, AIP (Tocris, 5–50 ng/0.5 μL) and KN-62 (Tocris, 2.5–250 ng/0.5 μL), the cAMP analog and protein kinase activator 8-CPT-cAMP (BioMol, 50–500 ng/0.5 μL), or the selective PP1 inhibitor tautomycetin (Tocris, 10–200 ng/0.5 μL) were microinfused immediately prior to the olfactory conditioning procedure.
Histology
Following experimental completion rats were perfused, brains were removed, and stored in a formalin/25% sucrose solution for 24 h. Brains were sectioned into 40 μm coronal slices, mounted, and stained using cresyl violet to allow for histological analysis of the site of injection. The majority of mPFC placements were localized within the boundaries of the prelimbic cortical area (PLC). Rats found to have misplaced guide cannulae were excluded from behavioral analysis.
Data Analysis
Data were analyzed with one-, two-, or three-way analysis of variance (ANOVA) where appropriate or Student's t-tests. Post hoc analyses were performed with Fisher’s least significant difference and Newman–Keuls tests.
Results
Histological Analysis
Histological analysis revealed microinfusion injector cannulae placements to be bilaterally localized within the anatomical boundaries of PLC region of the mPFC, as determined by the atlas of Paxinos and Watson (1996). In Figure 1B, we present a microphotograph showing a typical representative injector placement within the PLC. In Figure 1C, we present a schematic illustration showing representative intra-PLC bilateral cannulae placements along the rostrocaudal axis of the PLC.
Intra-PLC Inhibition of CaMKII Blocks DA D4-Mediated Potentiation of Subthreshold Fear Stimuli
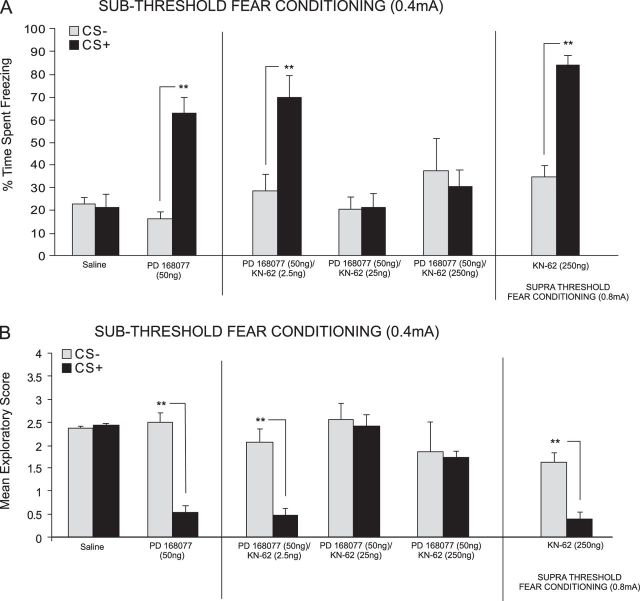
We first examined the effects CaMKII inhibition on D4-mediated potentiation of subthreshold fear stimuli by simultaneously activating DA D4 receptors and inhibiting CaMKII in the mPFC during the subthreshold (0.4 mA) fear conditioning procedure. We performed bilateral intra-mPFC microinfusions of either saline, a specific DA D4 agonist; PD 168077 (50 ng/0.5 μL) alone, and coadministered the selective CaMKII inhibitor; KN-62 (2.5, 25, or 250 ng/0.5 μL) or AIP (5 or 50 ng/0.5 μL) immediately prior to fear conditioning training. KN-62 inhibits CaMKII activity by binding directly to the calmodulin-binding site of the enzyme (Sumi et al. 1991; Hidaka and Yokokura 1996). In contrast, AIP decreases CaMKII activity by binding to the autophosphorylation site, independently of Ca2+/calmodulin activity (Ishida et al. 1995; Yang et al. 2004). Examining the behavioral effects of coadministration with KN-62, ANOVA revealed a significant interaction between group and treatment (F5,89 = 7.43, P < 0.0001) on times spent freezing to either CS+ or CS− cue presentations at testing. Post hoc analysis illustrated a potentiation in associative fear conditioning expression by both PD 168077 50 ng/0.5 μL alone (n = 7, P < 0.01) and when coadministered with the lowest dose of KN-62, 2.5 ng/0.5 μL (n = 8, P < 0.01) demonstrated by a significantly greater time spent freezing to the CS+ compared with the CS− (Fig. 2A). In contrast, post hoc analysis revealed no significant difference between freezing time to the CS+ compared with the CS− for the saline (n = 8, P > 0.05) control group or animals receiving coadministration of PD 168077 with the higher doses of KN-62, 25 ng/0.5 μL (n = 8; P > 0.05), and 250 ng/0.5 μL (n = 8, P > 0.05) (Fig. 2A). To determine if intra-mPFC KN 62 produced any learning/inhibition on acquisition of fear memory, we included a control experiment wherein rats received the highest effective dose of KN-62 (250 ng/0.5 μL) alone during suprathreshold fear conditioning. Post hoc analysis revealed that KN-62, 250 ng/0.5 μL (n = 8, P < 0.01) alone did not influence the acquisition of suprathreshold fear memory, demonstrated by a significantly greater time spent freezing to the CS+ compared with the CS− (Fig. 2A, right side). Similarly, ANOVA of spontaneous exploratory behavior during presentations of either CS+ or CS− revealed a significant interaction between group and treatment (F5,89 = 4.98, P = 0.001). Post hoc analysis revealed rats treated with either PD 168077 50 ng/0.5 μL alone (n = 7, P < 0.01) or PD 168077 50 ng/0.5 μL coadministered with the lowest dose of KN-62, 2.5 ng/0.5 μL (n = 8; P < 0.01) displayed significantly decreased exploratory behavior in response to the CS+ relative to the CS− (Fig. 2B). In contrast, post hoc analysis revealed no significant difference in exploratory behavior observed in response to the CS+ compared with the CS− for the saline (n = 8, P > 0.05) control group or animals receiving coadministration of PD 168077 (50 ng/0.5 μL) with the higher doses of KN-62, 25 ng/0.5 μL (n = 8, P > 0.05), and 250 ng/0.5 μL (n = 8, P > 0.05) (Fig. 2B). To further investigate the effect of CaMKII inhibition on acquisition of associative learning, we included a control experiment (n = 8) wherein rats received the highest dose of KN-62 (250 ng/0.5 μL) alone during the subthreshold fear conditioning procedure. Post hoc analysis revealed that KN-62 (250 ng/0.5 μL, n = 8, P < 0.01) alone produced a potentiation in associative learning demonstrated by significantly decreased exploratory behavior in response to the CS+ relative to the CS− (Fig. 2B, right side).
Figure 2.
Effects of intra-mPFC DA D4 receptor and CaMKII inhibition during fear-conditioning. (A) Saline controls displayed no significant difference in time spent freezing in response to CS+ vs. CS− presentations following subthreshold (0.4 mA; see Materials and Methods) fear conditioning. In contrast, intra-PLC PD 168077 (50 ng/0.5 μL) significantly potentiated the acquisition of subthreshold fear memory. Coadministration with the CaMKII inhibitor KN-62 (2.5–250 ng/0.5 μL dose dependently attenuates the effects of D4 receptor activation. In contrast, intra-PLC KN-62 (250 ng/0.5 μL) alone did not have a significant effect on the acquisition of suprathreshold (0.8 mA) fear memory. (B) Analysis of exploratory scores revealed that saline controls showed no CS+-related exploratory behavior suppression. In contrast, PD 168077 (50 ng/0.5 μL) significantly potentiated CS+-related suppression of exploratory behavior, which was dose dependently blocked by coadministration of KN-62 (2.5–250 ng/0.5 μL). Bars represent mean ± standard error of the mean for this and all subsequent figures.
Blockade of Intra-mPFC CaMKII Autophosphorylation Blocks D4 Receptor–Mediated Emotional Memory Potentiation
Next, we examined the possible effects of simultaneously activating DA D4 receptors and inhibiting CaMKII in the mPFC during the subthreshold (0.4 mA) fear conditioning procedure. We performed bilateral intra-mPFC microinfusions of either saline, a specific DA D4 agonist; PD 168077 (50 ng/0.5 μL) alone, or coadministered with specific CaMKII inhibitor; AIP (5 or 50 ng/0.5 μL) immediately prior to fear conditioning, to determine the effects of CaMKII inhibition on D4 receptor–mediated potentiation of emotional learning during the acquisition phase (encoding) of this associative information. ANOVA revealed a significant interaction between group and treatment (F3,51 = 8.11, P = < 0.01) on time spent freezing to either CS+ or CS− cue presentations at testing. Post hoc analysis illustrated a potentiation in associative fear conditioning expression by both PD 168077 50 ng/0.5 μL alone (n = 7, P < 0.01) and when coadministered with the lowest dose of AIP, 5 ng/0.5 μL (n = 8, P < 0.01) demonstrated by a significantly greater time spent freezing to the CS+ compared with the CS− (Fig. 3A). In contrast, post hoc analysis revealed no significant difference between freezing time to the CS+ compared to the CS− for the saline (n = 8, P > 0.05) control group or animals receiving coadministration of PD 168077 with the highest dose of AIP, 50 ng/0.5 μL (n = 8, P > 0.05) (Fig. 3A).
Figure 3.
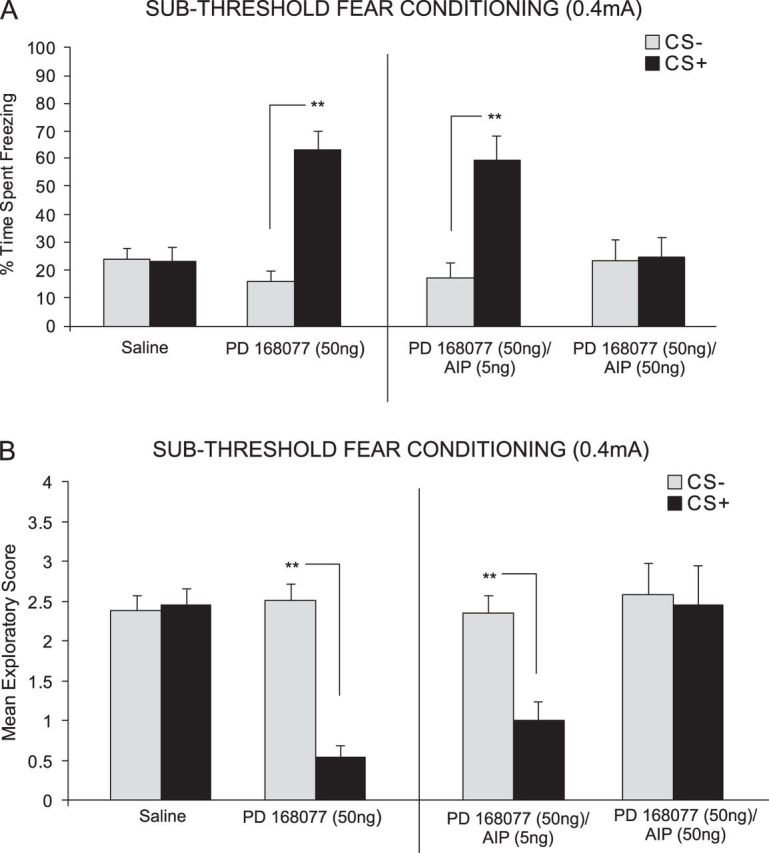
Effects of intra-PLC D4 receptor activation and blockade of CaMKII autophosphorylation on fear-conditioning. (A) Saline controls showed no significant CS+-related freezing behavior. In contrast, PD 168077 (50 ng/0.5 μL) significantly potentiated the acquisition of subthreshold olfactory fear memory, demonstrated by significantly greater CS+ freezing behavior. This effect was dose dependently blocked by coadministration of the CaMKII autophosphorylation inhibitor AIP (5–50 ng/0.5 μL). (B) Analysis of fear memory-related exploratory scores revealed that saline control rats showed no associative suppression of spontaneous exploratory behavior. In contrast, PD 168077 (50 ng/0.5 μL) significantly potentiated the acquisition of subthreshold (0.4 mA footshock) fear memory, demonstrated by significant suppression of CS+-related exploratory scores. This effect was dose dependently blocked by coadministration of AIP (5–50 ng/0.5 μL).
ANOVA of exploratory behavior scores during CS+/CS− presentations revealed a significant interaction between group and treatment (F3,51 = 5.16, P ≤ 0.01). Post hoc analysis revealed rats treated with either PD 168077 50 ng/0.5 μL alone (n = 7, P < 0.01) or PD 168077 50 ng/0.5 μL coadministered with the lowest dose of AIP, 5 ng/0.5 μL (n = 8; P < 0.01) displayed significantly decreased exploratory behavior in response to the CS+ relative to the CS− (Fig. 3B). In contrast, post hoc analysis revealed there was no significant difference in exploratory behavior observed in response to the CS+ compared to the CS− for the saline (n = 8, P > 0.05) control group or animals receiving coadministration of PD 168077 5 ng/0.5 μL with the highest dose of AIP, 50 ng/0.5 μL (n = 8, P > 0.05) (Fig. 3A).
Intra-mPFC Inhibition of PP1 Rescues DA D4-Mediated Blockade of Emotionally Salient Fear Stimuli
We next examined the possible effects of simultaneously activating DA D4 receptors and inhibiting PP1 in the mPFC during suprathreshold (0.8 mA) fear conditioning. We performed bilateral intra-mPFC microinfusions of either saline, a specific DA D4 agonist; PD 168077 (50 ng/0.5 μL) alone, or coadministered with a specific PP1 inhibitor; tautomycetin (10, 100, or 200 ng/0.5 μL) immediately prior to fear conditioning, to determine the effects of PP1 inhibition on D4 receptor–mediated block of suprathreshold fear memory acquisition. ANOVA revealed a significant main effect of treatment (F4,68 = 6.80, P < 0.0001) on time spent freezing to either CS+ or CS− cue presentations at testing. Post hoc analysis illustrated an attenuation of associative fear conditioning acquisition by both PD 168077 50 ng/0.5 μL alone (n = 7, P > 0.05) and when coadministered with the lower doses of tautomycetin, 10 ng/0.5 μL (n = 8, P > 0.05) and 100 ng/0.5 μL (n = 8, P > 0.05) demonstrated by no significant difference in the time spent freezing to the CS+ compared to the CS− (Fig. 4A). In contrast, post hoc analysis revealed a significant difference between freezing time to the CS+ compared to the CS− for the saline (n = 7, P< 0.05) control group and animals receiving coadministration of PD 168077 50 ng/0.5 μL with the highest dose of tautomycetin, 200 ng/0.5 μL (n = 8, P < 0.05) (Fig. 4A). To further investigate the effect of PP1 inhibition activation on acquisition of associative learning, we included a control experiment (n = 7) wherein rats received the highest dose of tautomycetin (200 ng/0.5 μL) alone during suprathreshold fear conditioning. Post hoc analysis revealed that tautomycetin 200 ng/0.5 μL (t6 = 2.18, P < 0.05) alone did not block suprathreshold acquisition in associative learning demonstrated by a significantly greater time spent freezing to the CS+ compared to the CS− (Fig. 4A, right side)
Figure 4.
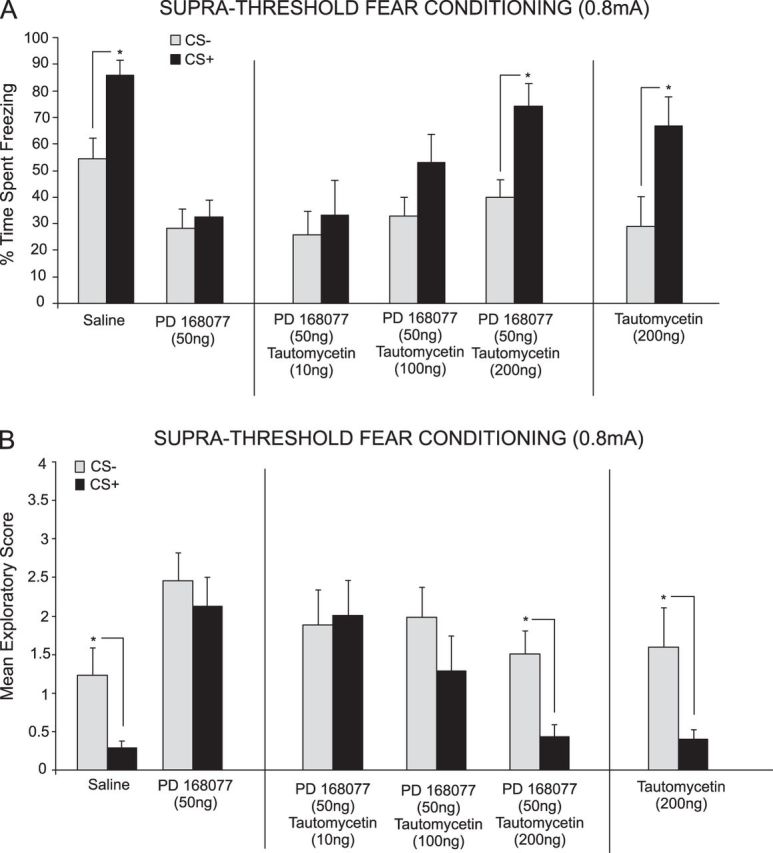
Inhibition of PP1 rescues DA D4 receptor–mediated blockade of suprathreshold fear memory acquisition. (A) Saline controls showed significant freezing to CS+ presentations after conditioning with a suprathreshold footshock (0.8 mA). In contrast, intra-PLC PD 168077 (50 ng/0.5 μL) blocked the acquisition of suprathreshold fear memory. This effect was dose dependently reversed by coadministration of tautomycetin (10–200 ng/0.5 μL). In a separate control experiment, intra-PLC tautomycetin (200 ng/0.5 μL) alone did not influence the acquisition of suprathreshold fear memory. (B) Saline controls showed significant suppression of exploratory behavior in response to CS+ presentations. In contrast, intra-PLC PD 168077 (50 ng/0.5 μL) blocked this effect. D4-mediated blockade of suprathreshold fear memory acquisition was dose dependently reversed by tautomycetin (10–200 ng/0.5 μL). In a separate control experiment, intra-PLC tautomycetin (200 ng/0.5 μL) alone did not affect the acquisition of suprathreshold fear memory.
ANOVA of exploratory behavior scores during CS+/CS− presentations revealed a significant main effect of treatment (F4,68 = 6.27, P < 0.001). Post hoc analysis revealed rats treated with either PD 168077 50 ng/0.5 μL alone (n = 7, P> 0.05) or PD 168077 50 ng/0.5 μL coadministered with the lower doses of tautomycetin, 10 ng/0.5 μL (n = 8, P > 0.05) and tautomycetin 100 ng/0.5 μL (n = 8, P > 0.05) displayed no significant difference exploratory behavior in response to the CS+ relative to the CS− (Fig. 4B). In contrast, post hoc analysis revealed a significant difference in exploratory behavior in response to the CS+ compared to the CS− for the saline (n = 8, P < 0.05) control group and animals receiving coadministration of PD 168077 50 ng/0.5 μL with the highest dose of tautomycetin, 200 ng/0.5 μL (n = 8, P < 0.05) (Fig. 4B). To further investigate the effect of PP1 inhibition on acquisition of associative learning, we included a control experiment (n = 7) wherein rats received the highest dose of tautomycetin (200 ng/0.5 μL) alone during the suprathreshold fear conditioning procedure. Statistical analysis revealed that tautomycetin (200 ng/0.5 μL) (t6 = 2.29, P < 0.05) alone did not block suprathreshold acquisition in associative learning demonstrated by a significantly decreased exploratory behavior in response to the CS+ relative to the CS− (Fig. 4B, right side).
Activation of Protein Kinase A Rescues DA D4-Mediated Blockade of Suprathreshold Fear Memory Acquisition
We next performed bilateral intra-PLC microinfusions of either saline, PD 168077 (50 ng/0.5 μL) alone, coadministration with the PKA activator; 8-CPT-cAMP (50 ng or 500 ng/0.5 μL), or 8-CPT-cAMP alone (control, 500 ng/0.5 μL) to examine the effects of intra-PLC PKA activation on D4 receptor–mediated block of suprathreshold fear memory acquisition. ANOVA revealed a significant effect of treatment (F3,61 = 9.23, P < 0.0001) on time spent freezing to either CS+ or CS− cue presentations at testing. Post hoc analysis illustrated an attenuation of associative fear conditioning acquisition by both PD 168077 50 ng/0.5 μL alone (n = 7, P > 0.05) and when coadministered with the lowest dose of 8-CPT-cAMP, 50 ng/0.5 μL (n = 8, P > 0.05) demonstrated by no significant difference in the time spent freezing to the CS+ compared to the CS− (Fig. 5A). In contrast, post hoc analysis revealed a significant difference between freezing time to the CS+ compared to the CS− for the saline (n = 7, P < 0.05) control group and animals receiving coadministration of PD 168077 50 ng/0.5 μL with the highest dose of 8-CPT-cAMP, 500 ng/0.5 μL (n = 8, P < 0.05) (Fig. 5A). To further investigate the effect of PKA activation on acquisition of associative learning, we included a control experiment (n = 8) wherein rats received the highest dose of 8-CPT-cAMP, (500 ng/0.5 μL) alone during suprathreshold fear conditioning. Statistical analysis revealed that 8-CPT-cAMP 500 ng/0.5 μL (t7 = 3.34, P ≤ 0.05) alone did not block suprathreshold fear memory acquisition demonstrated by significantly greater times spent freezing to the CS+ relative to the CS− (t7 = 3.34, P ≤ 0.05; Fig. 5A, right side).
Figure 5.
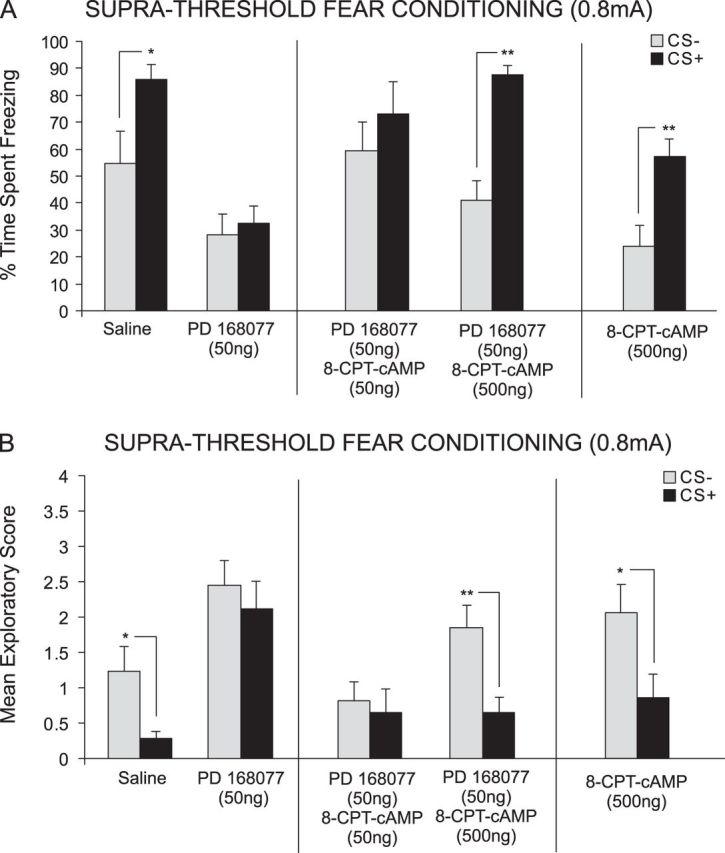
Activation of cAMP/PKA signaling rescues D4 receptor–mediated blockade of suprathreshold fear memory. (A) Saline controls showed significant CS+-related freezing after conditioning with suprathreshold footshock (0.8 mA). However, intra-PLC PD 168077 (50 ng/0.5 μL) blocked acquisition of suprathreshold fear memory. This effect was dose dependently reversed by coadministration of the cAMP/PKA activator 8-CPT-cAMP (50–500 ng/0.5 μL). In a separate control experiment, intra-PLC 8-CPT-cAMP (500 ng/0.5 μL) alone did not influence the acquisition of suprathreshold fear memory. (B) Similarly, exploratory behavior analyses demonstrated significant CS+-related attenuation in exploratory behavior in saline controls. In contrast, intra-PLC PD 168077 (50 ng/0.5 μL) administration blocked this effect. D4 receptor–mediated blockade of suprathreshold fear memory acquisition was dose dependently reversed by coadministration of 8-CPT-cAMP (50–500 ng/0.5 μL). Intra-PLC 8-CPT-cAMP (500 ng/0.5 μL) alone did not have any significant effect on fear-related exploratory scores.
ANOVA of spontaneous exploratory behavior during presentations of either CS+ or CS− revealed a significant effect of treatment (F3,61 = 11.18, P < 0.0001). Post hoc analysis revealed rats treated with either PD 168077 50 ng/0.5 μL alone (n = 7; P > 0.05) or PD 168077 50 ng/0.5 μL coadministered with the lowest dose of 8-CPT-cAMP 50 ng/0.5 μL (n = 8, P > 0.05) displayed no significant difference exploratory behavior in response to the CS+ relative to the CS− (Fig. 5B). In contrast, post hoc analysis revealed there was a significant difference in exploratory behavior observed in response to the CS+ compared to the CS− for the saline (n = 8, P < 0.05) control group and animals receiving coadministration of PD 168077 50 ng with the highest dose of 8-CPT-cAMP 500 ng/0.5 μL (n = 8, P < 0.01) (Fig. 5B). To investigate the effect of PKA activation on acquisition of associative learning, we included a control experiment (n = 8) wherein rats received the highest dose of 8-CPT-cAMP (500 ng/0.5 μL) alone during the suprathreshold fear conditioning procedure. Post hoc analysis revealed that 8-CPT-cAMP 500 ng (t7 = 2.30, P < 0.037) alone did not block suprathreshold acquisition in associative learning demonstrated by a significantly decreased exploratory behavior in response to the CS+ relative to the CS− (Fig. 5B, right side).
Discussion
DA D4 receptor transmission within the mPFC is an important modulator of emotional memory acquisition, in single neurons and at the behavioral level. Blockade of DA D4 receptor transmission either systemically or directly within the PLC division of the mPFC prevents the acquisition, but not the expression, of associative fear memory (Laviolette et al. 2005; Lauzon et al. 2009). In addition, pharmacological activation of DA D4 receptors in the PLC dose dependently potentiates the emotional salience of normally nonsalient subthreshold fear conditioning stimuli (footshock) and blocks the acquisition of emotionally salient (suprathreshold) associative fear memories (Lauzon et al. 2009). Thus, DA D4 receptor transmission within the mPFC can bidirectionally modulate fear memory acquisition by regulating the emotional salience of these memories; potentiating nonsalient emotional memory while blunting the acquisition of highly salient emotional memories. However, the downstream molecular events involved in D4-mediated modulation of emotional memory salience have not previously been identified.
Within the mPFC, CaMKII signaling is bidirectionally modulated by D4 receptor-specific activation (Gu and Yan 2004; Gu et al. 2006). Using in vitro culture preparations of mPFC neurons, Gu and Yan (2004) demonstrated that during low levels of mPFC neuronal activity, D4 receptor activation strongly increased α-CaMKII activity. In contrast, during high levels of neuronal activation, D4 receptor activation strongly attenuated α-CaMKII activity. Taken together, these studies provide a functional molecular framework whereby D4 receptor activation may modulate memory-related CaMKII signaling within the PFC, depending upon baseline levels of neuronal activity within mPFC networks. Given previous evidence that emotionally salient events can strongly activate prefrontal cortical networks (Surguladze et al. 2011; Dickie et al. 2011; Dresler et al. 2011), we hypothesized that the D4-mediated bidirectional modulation of α-CaMKII may be related to the ability of D4 receptor activation to modulate the behavioral acquisition of emotional associative memories, depending upon the baseline emotional salience (sub vs. suprathreshold footshock levels) of the fear conditioning stimulus. Specifically, we proposed that the observed potentiation of normally subthreshold emotional stimuli may be related to the ability of D4 receptor activation to increase endogenous levels of CaMKII during low levels of neuronal activity (corresponding to exposure to subthreshold nonsalient footshock stimuli). Such an increase in mPFC CaMKII levels was predicted to potentiate the strength of associative fear memory.
CaMKII Bidirectionally Regulates Fear Memory Salience via DA D4 Transmission in the mPFC
Our results demonstrate that intra-PLC inhibition of CaMKII signaling, dose dependently blocked the ability of D4 receptor activation to potentiate normally subthreshold olfactory fear memories. This effect was observed with both a direct inhibitor of CaMKII signaling via KN-62, which blocks the calmodulin-binding site; or with AIP, which blocks autophosphorylation of CaMKII, independently of Ca2+/calmodulin activity. Given the ability of D4 receptor activation to increase CaMKII during low neuronal activation these results suggest that CaMKII activity downstream of the D4 receptors is a probable molecular mechanism underlying D4-mediated potentiation of subthreshold fear stimuli. It is important to note that neither of the CaMKII inhibitors used in the present study are specific enough to differentiate between the β and the α isoforms of CaMKII, and future studies will be required to more precisely characterize the precise CaMKII substrate linked to D4 receptor–mediated modulation of emotional memory, in vivo. Interestingly, administration of CaMKII inhibitors alone failed to modulate fear memory acquisition, suggesting that intra-mPFC blockade of CaMKII in and of itself is not sufficient to prevent the acquisition of fear memory or produce nonspecific impairments in learning or memory. Rather, consistent with in vitro evidence, intra-mPFC CaMKII signaling appears to depend upon coactivation of D4 receptor substrates for the modulation of emotional memory salience.
To investigate the bidirectional nature of the relationship between D4 receptor activation and α-CaMKII, we administered a PKA activator during D4-mediated block of acquisition of emotionally salient fear stimuli. During high levels of mPFC neuronal activity, D4 activation initiates an inhibitory downstream cascade resulting in increased PKA activity which in turn increases PP1-mediated dephosphorylation of α-CaMKII (Gu and Yan 2004). We hypothesized that this inhibition of CaMKII activity may be responsible for our observed blockade of suprathreshold fear memory acquisition. Currently, there is no direct or specific pharmacological method to activate CaMKII in vivo. Thus, we targeted indirect components of D4 receptor–mediated control of CaMKII in vivo by either pharmacologically increasing PKA activity or inhibiting PP1 activity. As predicted by previous in vitro studies, our behavioral results demonstrate that pharmacological activation of PKA or inhibition of PP1 can dose dependently rescue D4-mediated blockade of suprathreshold fear memory acquisition. Taken together with the KN-62 and AIP findings, regulation of CaMKII activity provides strong evidence of a specific molecular mechanism through which the D4 receptors exert their bidirectional effect during the acquisition phase of emotional learning.
Functionally, D4 receptor–mediated modulation of α-CaMKII within the PLC may elucidate a molecular mechanism underlying abnormalities in emotional processing, learning, and memory observed in neuropsychiatric disorders such as schizophrenia, Post-Traumatic Stress Disorder, Attention-Deficit Hyperactivity Disorder, and addiction (Lauzon and Laviolette 2010). Various lines of evidence suggest that an optimal level of D4 receptor signaling within the mPFC is required for adaptive emotional learning, memory, and the development of appropriate adaptive behavioral and psychological responses. Specifically, whereas hyperstimulation of D4 receptor transmission within the PLC may abnormally amplify the emotional salience of subthreshold sensory experiences; hyperstimulation of these same D4 receptor substrates in the context of emotionally salient experiences may pathologically blunt the emotional salience of these memories leading to suboptimal emotional processing and memory encoding, as previously suggested (Lauzon et al. 2009; Lauzon and Laviolette 2010). Our findings demonstrate that D4 receptor transmission exerts bidirectional modulation of emotional memory salience through interactions with CaMKII signaling pathways. A functional role for intra-PLC CaMKII signaling during emotional memory formation is consistent with previous evidence implicating CaMKII as a critical mediator of associative memory formation in PFC networks (Frankland et al. 2001). Indeed, CaMKII is highly expressed in the mammalian forebrain and plays a critical role in modulating various postsynaptic elements important for synaptic plasticity, learning, and memory (Malenka and Nicoll 1999; Frankland et al. 2001). Interestingly, the genetic locus of CaMKII is found on 5q32, a genomic region that has been associated with schizophrenia in several clinical studies (Paunio et al. 2001; Sklar et al. 2004). In addition, postmortem studies have revealed that CaMKII-β and α−isoforms are abnormally expressed in prefrontal cortical areas in individuals with schizophrenia and linked to schizophrenia-related behavioral abnormalities in rodent models (Novak et al. 2006; Novak and Seeman 2010; Seeman et al. 1993). The present findings thus add to a growing body of evidence linking CaMKII signaling to the aberrant emotional regulation present in schizophrenia-related psychoses and provides a potential DA receptor-specific substrate through which CaMKII signaling may control emotional salience processing and memory formation.
Recent in vitro work by Yuen and Yan (2011) has demonstrated that D4 receptor–mediated modulation of CaMKII activity is tightly linked to AMPA receptor synaptic activity within the mPFC. Thus, during low mPFC neuronal activity levels, D4-mediated activation of CaMKII results in a potentiation in AMPA receptor synaptic efficacy whereas during high neuronal activity levels, D4 receptor activity decreases CaMKII levels concomitant with decreased AMPA receptor signaling. Given the essential role of AMPA-mediated glutamatergic signaling in learning and memory-related synaptic plasticity, this may provide another potential mechanism by which D4 signaling within the PLC may control emotional memory formation and processing via CaMKII modulation. The present results demonstrate an important role for D4 receptor–mediated modulation of CaMKII, cAMP/PKA, and PP1 directly within the mPFC, controlling the salience of emotional memory acquisition. Interestingly, previous evidence from clinical trials has largely demonstrated a lack of therapeutic efficacy for D4 receptor specific antagonists in the treatment of schizophrenia-related psychopathology (Kramer et al. 1997; Corrigan et al. 2004). Nevertheless, the highly effective atypical antipsychotic, clozapine, demonstrates high affinity for the D4 receptor subtype relative to other D2-like receptors (Govindaiah et al. 2010). The present results suggest that modulation of downstream molecular targets of intra-PLC D4 receptor substrates, such as CaMKII, may serve as potential pharmacotherapeutic targets in the context of aberrant D4 receptor signaling in the context of dysregulated emotional processing and memory encoding.
Funding
Canadian Institutes of Health Research, the Ontario Mental Health Foundation, and an Natural Sciences and Engineering Research Council of Canada fellowship to N.M.L.
Acknowledgments
Conflict of Interest : None declared.
References
- Ariano MA, Wang J, Noblett KL, Larson ER, Sibley DR. Cellular distribution of the rat D4 dopamine receptor protein in the CNS using anti-receptor antisera. Brain Res. 1997;752:26–34. doi: 10.1016/s0006-8993(96)01422-9. [DOI] [PubMed] [Google Scholar]
- Corrigan MH, Gallen CC, Bonura ML, Merchant KM. Effectiveness of the selective D4 antagonist sonepiprazole in schizophrenia: a placebo controlled trial. Biol Psychiatry. 2004;55:445–451. doi: 10.1016/j.biopsych.2003.10.004. [DOI] [PubMed] [Google Scholar]
- De Almeida J, Mengod G. D2 and D4 dopamine receptor mRNA distribution in pyramidal neurons and GABAergic subpopulations in monkey prefrontal cortex: implications for schizophrenia treatment. Neuroscience. 2010;170:1133–1139. doi: 10.1016/j.neuroscience.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Dickie EW, Brunet A, Akerib V, Armony JL. Neural correlates of recovery from post-traumatic stress disorder: a longitudinal fMRI investigation of memory encoding. Neuropsychologia. 2011;49:1771–1778. doi: 10.1016/j.neuropsychologia.2011.02.055. [DOI] [PubMed] [Google Scholar]
- Dresler T, Hahn T, Plichta MM, Ernst LH, Tupak SV, Ehlis AC, Warrings B, Deckert K, Fallgater AJ. Neural correlates of spontaneous panic attacks. J Neural Transm. 2011;118:263–269. doi: 10.1007/s00702-010-0540-2. [DOI] [PubMed] [Google Scholar]
- Frankland PW, O’brien C, Ohon M, Kirkwood A, Silva AJ. Alpha-CAMKII-dependent plasticity in the cortex is required for permanent memory. Nature. 2001;411:309–313. doi: 10.1038/35077089. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Sakaguchi M, Arruda-Carvalho M. Starting at the endophenotype: a role for alpha-CaMKII in schizophrenia? Mol Brain. 2008;6:1–3. doi: 10.1186/1756-6606-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaiah G, Wang T, Gillette MU, Crandall SR, Cox CL. Regulation of inhibitory synapses by presynaptic D4 dopamine receptors in thalamus. J Neurophysiol. 2010;104:2757–2765. doi: 10.1152/jn.00361.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein R, Novak G, Seeman P. Amphetamine sensitization elevates CaMKIIβ mRNA. Synapse. 2007;61:827–834. doi: 10.1002/syn.20429. [DOI] [PubMed] [Google Scholar]
- Gu Z, Jiang Q, Yuen EY, Yan Z. Activation of dopamine D4 receptors induces synaptic translocation of Ca2+/calmodulin-dependent protein kinase II in cultured prefrontal cortical neurons. Mol Pharmacol. 2006;69:813–822. doi: 10.1124/mol.105.018853. [DOI] [PubMed] [Google Scholar]
- Gu Z, Yan Z. Bidirectional regulation of Ca2+/calmodulin-dependent protein kinase II activity by dopamine D4 receptors in prefrontal cortex. Mol Pharmacol. 2004;66:948–955. doi: 10.1124/mol.104.001404. [DOI] [PubMed] [Google Scholar]
- Hidaka H, Yokokura H. Molecular and cellular pharmacology of a calcium/calmodulin-dependent protein kinase II (CaM kinase II) inhibitor, KN-62, and proposal of CaM kinase phosphorylation cascades. Adv Pharmacol. 1996;36:193–219. doi: 10.1016/s1054-3589(08)60583-9. [DOI] [PubMed] [Google Scholar]
- Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Last B, Getson A, Reines SA. The effects of a selective D4 dopamine receptor antagonist (L-745-870) in acutely psychotic inpatients with schizophrenia. Arch Gen Psychiatry. 1997;54:567–572. doi: 10.1001/archpsyc.1997.01830180085011. [DOI] [PubMed] [Google Scholar]
- Lauzon NM, Bishop SF, Laviolette SR. Dopamine D1 versus D4 receptors differentially modulate the encoding of salient versus nonsalient emotional information in the medial prefrontal cortex. J Neurosci. 2009;29:4836–4845. doi: 10.1523/JNEUROSCI.0178-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzon NM, Laviolette SR. Dopamine D4-receptor modulation of cortical neuronal network activity and emotional processing: implications for neuropsychiatric disorders. Behav Brain Res. 2010;208:12–22. doi: 10.1016/j.bbr.2009.11.037. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. Cannabinoids potentiate emotional learning plasticity in neurons of the medical prefrontal cortex through basolateral amygdala inputs. J Neurosci. 2006;26:6458–6468. doi: 10.1523/JNEUROSCI.0707-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285:325–334. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Novak G, Seeman P. Hyperactive mice show elevated D2 high receptors, a model for schizophrenia: calcium/calmodulin-dependent kinase II alpha knockouts. Synapse. 2010;64:794–800. doi: 10.1002/syn.20786. [DOI] [PubMed] [Google Scholar]
- Novak G, Seeman P, Tallerico T. Increased expression of calcium/calmodulin-dependent protein kinase IIb in frontal cortex in schizophrenia and depression. Synapse. 2006;59:61–68. doi: 10.1002/syn.20211. [DOI] [PubMed] [Google Scholar]
- Paunio T, Ekelund J, Varilo T, Parker A, Hovatta I, Turunen JS, Rinard K, Foti A, Terwilliger JD, Juvonen H, et al. Genome-wide scan in a nationwide study sample of schizophrenia families in Finland reveals susceptibility loci on chromosome 2q and 5q. Hum Mol Genet. 2001;10:3037–3048. doi: 10.1093/hmg/10.26.3037. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed. San Diego (CA): Academic; 1996. [DOI] [PubMed] [Google Scholar]
- Seeman P, Guan HC, Van Tol HHM. Dopamine D4 receptors elevated in schizophrenia. Nature. 1993;365:441–445. doi: 10.1038/365441a0. [DOI] [PubMed] [Google Scholar]
- Sklar P, Pato MT, Kirby A, Petryshen TL, Medeiros H, Carvalho C, Macedo A, Dourado A, Coelho I, Valente J, et al. Genome-wide scan in Portuguese island families identifies 5q31-5q35 as a susceptibility locus for schizophrenia and psychosis. Mol Psychiatry. 2004;9:213–218. doi: 10.1038/sj.mp.4001418. [DOI] [PubMed] [Google Scholar]
- Sumi M, Kiuch K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. The newly synthesized selective Ca2+/calmodulin-dependent protein kinase II inhibitor KN 93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun. 1991;181:968–975. doi: 10.1016/0006-291x(91)92031-e. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Chu EM, Marshall N, Evans A, Anilkumar AP, Timehin C, McDonale C, Ecker C, Philips ML, David AS. Emotion processing in schizophrenia: fMRI study of patients treated with risperidone long-acting injections or conventional depot medication. J Psychopharmacol. 2011;25:722–733. doi: 10.1177/0269881110363316. [DOI] [PubMed] [Google Scholar]
- Yamasaki N, Maekawa M, Kobayashi K, Kajii Y, Maeda J, Soma M, Takao K, Tanda K, Ohira K, Toyama K, et al. Alpha-CaMKII deficiency causes immature dentate gyrus, a novel candidate phenotype of psychiatric disorders. Mol Brain. 2008;6:1–21. doi: 10.1186/1756-6606-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HW, Hu XD, Zhang HM, Xin WJ, Li MT, Zhang T, Zhou LJ, Liu XG. Roles of CaMKII, PKA and PKC in the induction and maintenance of LTP of C-fiber-evoked field potentials in rat dorsal spinal horn. J Neurophysiol. 2004;91:1122–1133. doi: 10.1152/jn.00735.2003. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Yan Z. Cellular mechanisms for dopamine D4 receptor-induced homeostatic regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. J Biol Chem. 2011;286:24957–24965. doi: 10.1074/jbc.M111.221416. [DOI] [PMC free article] [PubMed] [Google Scholar]