Abstract
Oscillatory activity is modulated by sensory stimulation but can also fluctuate in the absence of sensory input. Recent studies have demonstrated that such fluctuations of oscillatory activity can have substantial influence on the perception of subsequent stimuli. In the present study, we employed a simultaneity task in the somatosensory domain to study the role of prestimulus oscillatory activity on the temporal perception of 2 events. Subjects received electrical stimulations of the left and right index finger with varying stimulus onset asynchronies (SOAs) and reported their subjective perception of simultaneity, while brain activity was recorded with magnetoencephalography. With intermediate SOAs (30 and 45 ms), subjects frequently misperceived the stimulation as simultaneously. We compared neuronal oscillatory power in these conditions and found that power in the high beta band (∼20 to 40 Hz) in primary and secondary somatosensory cortex prior to the electrical stimulation predicted subjects' reports of simultaneity. Additionally, prestimulus alpha-band power influenced perception in the condition SOA 45 ms. Our results indicate that fluctuations of ongoing oscillatory activity in the beta and alpha bands shape subjective perception of physically identical stimulation.
Keywords: alpha, beta, MEG, oscillation, somatosensory
Introduction
Depending on the surrounding or internal brain states, physically identical sensory stimulation can be perceived quite differently. For example, subjective perception of ambiguous and bistable stimuli fluctuates over time despite identical and constant sensory input to the brain. Moreover, absolute detection thresholds for sensory perception can vary over time or over stimulus presentations. Several studies have shown that fluctuations of oscillatory neuronal activity can predict at least some of the perceptual variability. The state of oscillatory activity just prior to the onset of a stimulus influences whether the subsequent stimulation will be perceived, especially when stimuli are weak and near perceptual threshold. Among all frequency bands, alpha-band (∼8 to 12 Hz) activity has gained much attention in recent years. It has been shown that prestimulus alpha-band power and phase in human parietooccipital areas are correlated with conscious perception of visual stimuli (Thut et al. 2006; Hanslmayr et al. 2007; van Dijk et al. 2008; Mathewson et al. 2009; Mazaheri et al. 2009; Wyart and Tallon-Baudry 2009; Romei et al. 2010). Similarly in human somatosensory cortex, it has been shown that attentional or spontaneous fluctuations of prestimulus alpha-band activity influences perception of tactile stimuli. Linkenkaer-Hansen et al. (2004) showed that prestimulus amplitude of ongoing alpha and beta oscillations in human somatosensory cortex correlates with subjects' ability to detect a subsequent weak tactile stimulus, with intermediate levels of amplitudes showing the highest detection rates. Moreover, it has been shown that the phase of alpha oscillations before stimulus onset influences subsequent perception (Palva et al. 2005). Recent studies have demonstrated that cued attention to somatosensory stimuli modulates prestimulus alpha- and beta-band activity in human somatosensory cortex in a spatially (Jones et al. 2010; van Ede et al. 2010, 2011; Anderson and Ding 2011) and temporally specific way (van Ede et al. 2011). In addition, prestimulus alpha- and beta-band amplitudes modulate the amplitude of the early stimulus-evoked M50 component of the event-related field (ERF) (Jones et al. 2009; Anderson and Ding 2011) and are correlated to behavioral detection rates of subsequent stimuli (Linkenkaer-Hansen et al. 2004; Jones et al. 2010), similar to findings in human visual cortex for alpha-band amplitudes (van Dijk et al. 2008). In summary, these results are in line with the hypothesis that ongoing fluctuations of oscillatory neuronal synchronization in the prestimulus period modulates the gain of neuronal assemblies and thus facilitates subsequent processing of sensory stimulation (Fries 2005, 2009; van Dijk et al. 2008).
Similar to the perception of a single stimulus, simultaneous perception of 2 tactile stimuli shows a considerable variation. Perception of simultaneity is a powerful cue for determining whether 2 events define a single or multiple objects. Perception of the relative timing of 2 events tolerates a moderate degree of temporal delays between sensory stimulations. However, this tolerance of temporal delays introduces a substantial degree of variability. For example, when 2 tactile stimuli are presented with a stimulus onset asynchrony (SOA) of ∼30 to 70 ms, subjects show a considerable variation in their trial-by-trial responses when asked to judge whether the 2 stimulations were simultaneous or not, that is, asynchronously nonsimultaneously presented stimuli are frequently misperceived as simultaneous (Geffen et al. 2000; Kopinska and Harris 2004; Harrar and Harris 2005, 2008). The neurophysiological basis of this variability is not well understood.
In the present study, we used magnetoencephalography (MEG) to investigate the role of oscillatory neuronal activity for subjective perception of simultaneity and its variability. We employed a simultaneity task to study the role of prestimulus oscillatory activity for subjective perception. We focused on the somatosensory domain and compared conditions in which identical stimuli can lead to variable subjective perceptions on a trial-by-trial basis. These conditions offer an intriguing possibility to study the role of oscillatory neuronal synchronization under constant conditions of sensory stimulation (Rodriguez et al. 1999; Leopold et al. 2002).
Materials and Methods
Subjects
Twenty subjects participated in this study (24.9 ± 3.8 years [mean ± standard deviation], 7 males). None of the subjects had a known history of neurological disorders, and subjects gave written informed consent in accordance with the Declaration of Helsinki.
Paradigm and Stimuli
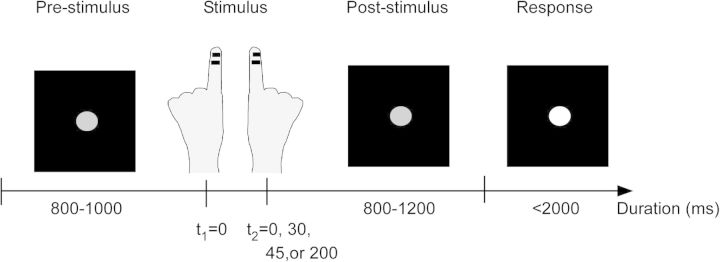
Subjects were seated comfortably with their head placed inside an MEG helmet and fixated a central gray dot on a screen positioned 60 cm in front of them. Each trial started with a decrease of luminance of the fixation dot, which served as the start cue (Fig. 1). After a randomized period of 800–1000 ms, subjects received short (0.3 ms) electrical pulses between the 2 distal joints of the left and right index finger to stimulate the cutaneous end branches of the digital nerves. The amplitude of the electric pulses was set to 60% of the individually determined subjective (mild) pain threshold level as measured prior to the recordings (mean amplitude 5.5 ± 0.7 mA). Notably, subsequent analyses were performed on within-subject levels, that is, we always compared conditions of identical stimulation amplitudes (for details, see Data Analysis). Stimulation of the fingers was applied with varying SOAs of ±200, ±45, ±30, or 0 ms with negative SOA indicating that stimulation was first on the left finger. The condition of 0 ms was presented twice as often as the other SOA. SOA were chosen based on behavioral pilot experiments to ensure a balanced distribution of difficulty levels. After another random period of 800–1200 ms, in which only the fixation dot was visible, the fixation dot increased luminance indicating the start of the response window. Subjects were asked to report whether they had perceived the stimulation as simultaneous or nonsimultaneous by button presses. Button configurations were balanced within and between subjects: Half of the subjects responded with the middle fingers of both hands and half of the subjects responded with the index and middle finger of one hand (5 with the right hand and 5 with the left hand). For each subject, the button configuration was switched blockwise, that is, allocation of response finger and subjective report was balanced within and across subjects. Subjects were instructed to respond within 2000 ms after presentation of response instructions and that response speed was not taken into account. If no response was given after 2000 ms or subjects responded before the presentation of the instructions, a warning was visually presented. The respective trial was discarded from analyses and repeated at the end of the block. Except the warning signal, no feedback was given, and subjects were naïve to the different SOA used. Five repetitions of each SOA (i.e., 40 trials) constituted one block with stimuli within one block presented in pseudorandom order. Each block was repeated 10 times with self-paced breaks of ∼2 min in between. Response instructions for each block were visually presented on the screen before the start of each block. The experimental run was controlled using Presentation software (Neurobehavioral Systems, Albany, NY). Subjects performed a training session of ∼5 min before the start of the MEG experiment.
Figure 1.
Schematic illustration of the paradigm. Subjects fixated a central gray dot throughout the entire trial. After 800–1000 ms, tactile stimulation was given to one index finger (right or left), followed by stimulation of the other finger after a randomized SOA (0, 30, 45, or 200 ms). After a jittered period (800–1200 ms), the luminance of the fixation dot increased, and subjects reported their subjective perception of simultaneity by pressing a button, upon which the next trial began (indicated by a luminance decrease of the fixation dot).
Data Acquisition and Analysis
Data Recording and Preprocessing
Neuromagnetic brain activity was continuously recorded using a 306-channel whole head MEG system (Neuromag Elekta Oy, Helsinki, Finland). Simultaneously, electrooculargram were recorded by placing electrodes above and below the left eye and on the outer sides of each eye. The data were recorded at a rate of 1000 Hz. Subjects' head position within the MEG helmet was registered by 4 coils placed at subjects' forehead and behind both ears. Individual full-brain high-resolution standard T1-weigthed structural magnetic resonance images (MRIs) were obtained from a 3-T MRI scanner (Siemens, Erlangen, Germany) and offline aligned with the MEG coordinate system using the coils and anatomical landmarks (nasion, left and right preauricular points).
MEG data were offline analyzed using FieldTrip (http://www.ru.nl/donders/fieldtrip), an open source matlab toolbox for neurophysiological data analysis (Oostenveld et al. 2011). Power line noise was removed from the continuous data using a discrete Fourier transformation of 10-s long signal periods to estimate the amplitudes and the phases of the 50, 100, and 150 Hz components. These components were subtracted from the continuous data as described earlier (Hoogenboom et al. 2006; van Ede et al. 2010, 2011; Lange et al. 2011). This was done separately for all 10-s periods around all periods of interest. Continuous data were segmented into trials, starting with the first appearance of the fixation dot and ending with appearance of instruction text. Artifacts caused by eye movements or muscle activity were removed using a semiautomatical algorithm, and the linear trend was removed from each trial.
Time–Frequency Analysis
Time–frequency representations (TFRs) were computed applying a Fourier transformation on adaptive sliding time windows containing 5 full cycles of the respective frequency f (Δt = 5/f), moved in steps of 25 ms (similar to Mazaheri et al. 2009; van Dijk et al. 2010; Haegens et al. 2011). Data segments were tapered with a single Hanning taper, resulting in a spectral smoothing of 1/Δt.
Next, we determined regions of interest in sensor space. We chose 4 sensors in the left and 4 sensors in the right hemisphere covering bilateral primary somatosensory cortex (SI) and 4 sensors in the left and 4 sensors in the right hemisphere covering secondary somatosensory cortex (SII) (Fig. 3). The choice of sensors was based on previous studies (Bauer et al. 2006; Haegens et al. 2010; Hagiwara et al. 2010; van Ede et al. 2010, 2011). This set of sensors defined the somatosensory region of interest for subsequent analyses for all subjects. The set of sensors in the left and in the right hemisphere were symmetrically distributed with respect to the midline of the sensor array (Fig. 3B,E,H,K).
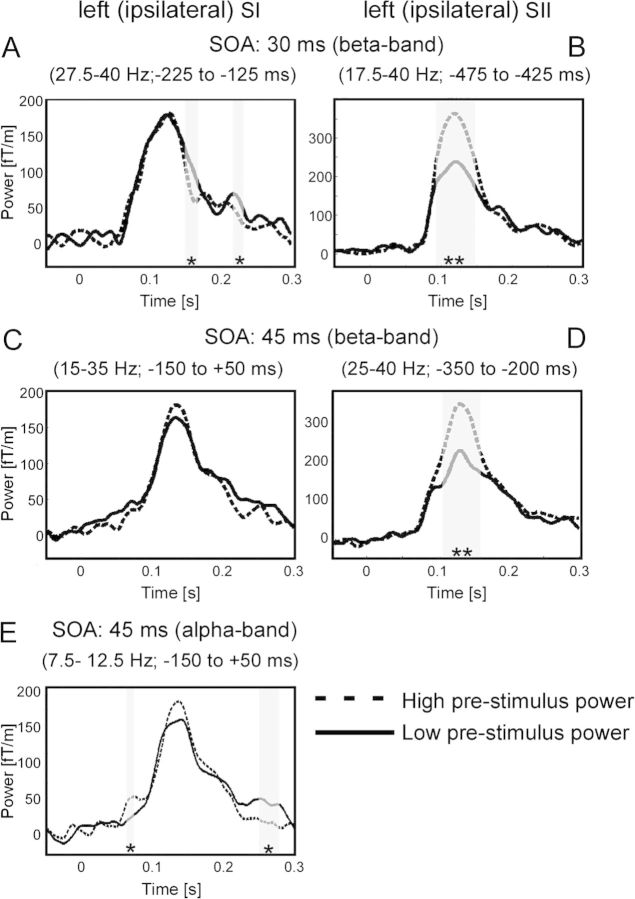
Figure 3.
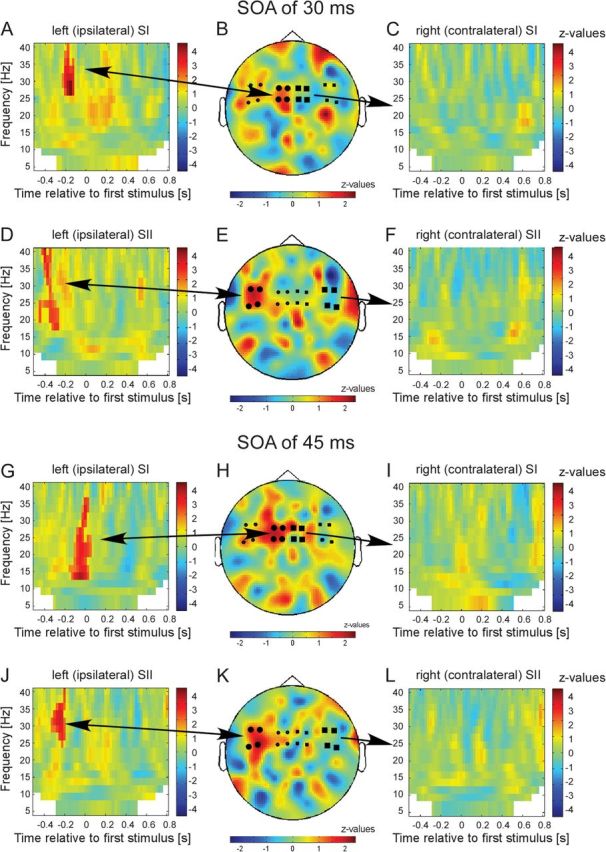
Results of the statistical comparison of trials with subjective simultaneity versus nonsimultaneity for conditions SOA 30 ms (A–F) and SOA 45 ms (G–L) for different sensor groups: (A) TFR for the 4 sensors over the left (ipsilateral) primary somatosensory cortex (SI) as indicated by the larger black circles in B. z values in nonsignificant pixels are lowered by 60% in order to highlight significant clusters. Color bars represent z values. Positive z values indicate higher power if subsequent stimulation was misperceived as simultaneously. (B) Topographical representation of the significant cluster as highlighted in A. Only time–frequency samples that correspond to the statistically significant time–frequency clusters in A were averaged to generate the topographical representation (for details, see Materials and Methods). (C) TFR for the 4 sensors over the right (contralateral) SI (as indicated by larger black squares). No significant clusters were found. (D) Same representation as in A but for 4 sensors over left (ipsilateral) secondary somatosensory cortex (SII). (E) Topographical representation for the significant cluster as highlighted in D. (F) TFR for the 4 sensors over the right (contralateral) SII (as indicated by larger black squares). (G–L) Same representation as in A–F but now for condition SOA of 45 ms.
For each subject separately, we sorted trials with respect to the SOA. Within each SOA-bin, we compared trials with reports of subjective simultaneity to trials in which the stimulation was perceived as nonsimultaneous. Thus, we compared 2 conditions with identical physical stimulation that only differed with respect to the subjective perception. To this end, we averaged spectral power over the sensors of interest (see above) for each perceptual condition and compared both conditions by independent samples t-tests. This comparison was done independently for each time–frequency sample and thus resulted in a time–frequency t-map for each subject. Note that this comparison is not an actual statistical test but serves as a normalization of interindividual differences. This comparison was done separately for sensors in the left and right SI and SII. Only conditions with SOA of 30 and 45 ms were included in the analysis as only these conditions revealed a reasonably high number of trials for both perceptual conditions (simultaneous and nonsimultaneous). Behavioral and neuromagnetic data revealed highly symmetrical patterns for positive and negative SOA (e.g., Fig. 2 for behavioral data), that is, no statistically significant differences were found when restricting the analyses to contra- or ipsilateral sites with respect to the site of the first stimulation. To increase statistical power, we pooled data regarding the site of the stimulation, that is, we report data in terms of contra- and ipsilateral to the site of the first stimulation. All t values of the time–frequency t-map were transformed to z values using SPM2 resulting in time–frequency z-maps (e.g., van Dijk et al. 2008; Mazaheri et al. 2009). For group-level statistics, we used the z-maps obtained for single subjects as inputs and determined their consistency across subjects. We used a nonparametric permutation approach that identifies clusters in time–frequency with significant changes. This effectively corrects for multiple comparisons (Maris and Oostenveld 2007; for details, see Lange et al. 2011). For statistical testing, the entire time window (−500 to 800 ms) was used. To generate topographical representations of statistically significant effects, we repeated the above-mentioned statistical comparison, but this time for each sensor independently, resulting in time–frequency z-maps for each sensor separately (instead of averaging over sensors). For each sensor, we averaged the z values over all individual time–frequency samples that correspond to the statistically significant time–frequency clusters in the above-mentioned analysis (as can be seen in, e.g., Fig. 3A,B). Finally, we plotted the averaged z values in a topographical representation (Fig. 3B,E,H,K).
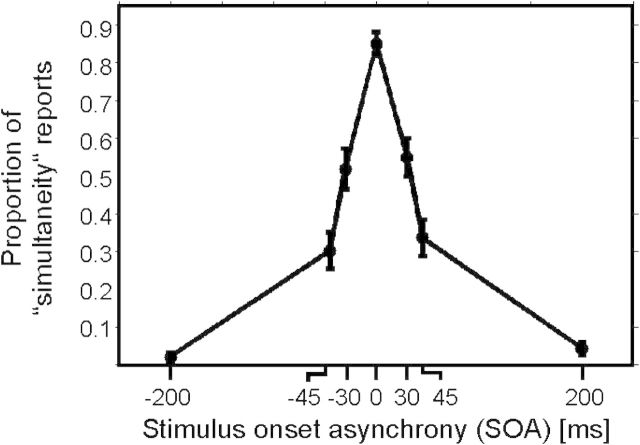
Figure 2.
Behavioral results presented as proportion of simultaneity reports depending on SOA of left and right index finger stimulations. Negative SOA indicate that stimulation was applied first to the left index finger. Data are presented as mean ± 1 SEM.
Correlation of Prestimulus Power and Detection Rates
Next, we aimed to further investigate the correlation of prestimulus power to perception of simultaneity. First, we averaged spectral power over time, frequency, and sensors. Sensors of interest were defined as mentioned above (left and right SI and SII). Time–frequency bands of interest were determined by the significant time–frequency clusters in the above-mentioned cluster-based statistical analysis on group level (Fig. 3A,D,G,J), resulting in 4 different time–frequency bands in the beta band. Since the significant clusters slightly differed in time and frequency for the different sensors of interest, time–frequency bands used to compute prestimulus power for the correlation analysis differed for each set of sensors of interest. The exact time–frequency bands for each analysis can be found in Figure 4.
Figure 4.
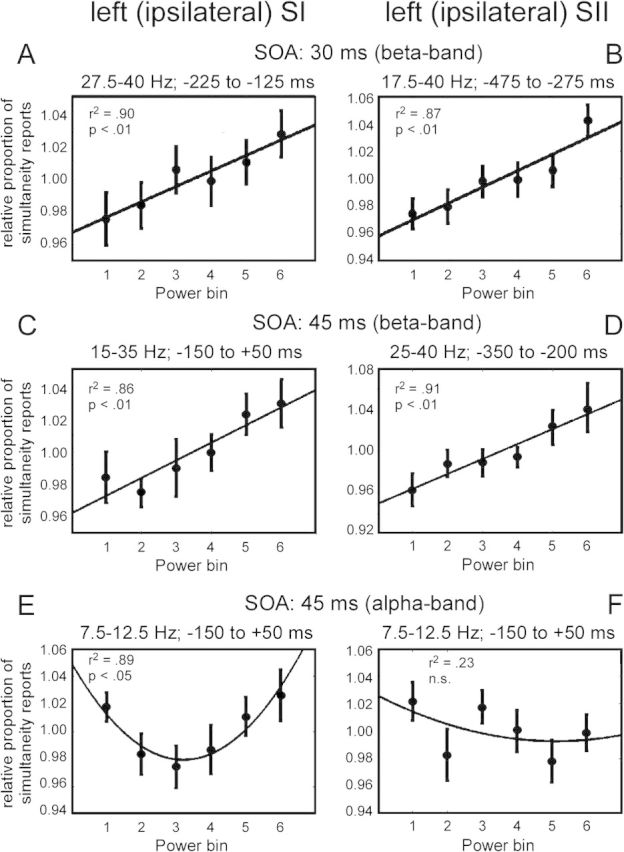
Regression analyses of the dependence of subjective perception on prestimulus oscillatory activity for the 4 significant clusters in the beta band (as shown in Fig. 3) and for the alpha band. The exact time–frequency bands to determine averaged prestimulus power bins are based on significant clusters in Figure 3 and are presented at the top of each figure. (A) Results for the significant cluster in the beta band for condition SOA 30 ms in sensors over ipsilateral SI (as highlighted in Fig. 3A). (B) Same analysis as in A but for the significant cluster in sensors over SII (as highlighted in Fig. 3D). (C–D) Same analysis as in A,B but for the significant clusters in the beta band for condition SOA 45 ms (as highlighted in Fig. 3G,J). For all regression analyses, a significant linear relationship was found (P < 0.01). (E) Same analysis for the alpha band for condition SOA 45 ms in sensors over ipsilateral SI. A significant quadratic relationship was found. (F) Same analyses as in E but for sensors over SII. No significant relationship was found.
Due to the relevance of prestimulus alpha-band power in somatosensory perception (Linkenkaer-Hansen et al. 2004; Jones et al. 2009, 2010; Anderson and Ding 2011; van Ede et al. 2011), we also included the alpha band into the analyses. The exact time–frequency bands used for each correlation analysis can be found in Figure 4. The averaging was done for each subject separately (with a common and fixed time–frequency–sensor triplet for all subjects, based on the group-level statistics). Subsequently, we sorted the single trials of each subject according to averaged power and divided all trials into 6 bins with equal number of trials. For each bin, we calculated the mean number of simultaneity reports and normalized the result for each subject. Finally, we computed the mean and standard error of the mean (SEM) over subjects and fitted linear and quadratic functions to the data to determine the best fit (Linkenkaer-Hansen et al. 2004; van Dijk et al. 2008; Jones et al. 2010).
Correlation of Prestimulus Power and ERFs
To study a potential relation between prestimulus alpha- and beta-band power and poststimulus ERFs (Jones et al. 2009, 2010; Anderson and Ding 2011), we correlated prestimulus power and ERFs in line with the above-mentioned analysis of prestimulus power and detection rates. To this end, we averaged power over time, frequency, and sensors. Sensors were chosen as defined above. Time–frequency bands were based on the significant clusters found in Figure 3A,D,G,J. Since the significant clusters slightly differed in time and frequency for the different sensors of interest, time–frequency bands used to compute prestimulus power differed for each set of sensors of interest. The exact time–frequency bands for each analysis can be found in Figure 5. Time–frequency bands were defined on group level, and the same time–frequency band was used for all subjects. Subsequently, we divided trials in 2 bins (low and high prestimulus alpha/beta power) and then computed the ERFs in the poststimulus period over the same sensors used for the power analyses (Jones et al. 2009, 2010). ERFs were computed by first applying a low-pass filter of 30 Hz, rectifying the signals by taking the root mean square of the signal in the time domain (e.g., Bauer et al. 2006; van Dijk et al. 2008; Mazaheri et al. 2009) and then averaging ERFs over trials and subjects. Statistical analysis was performed by applying dependent sample t-test between low and high power conditions for each time point.
Figure 5.
Dependence of poststimulus ERF amplitudes on prestimulus power for the 4 significant clusters in the beta band (as shown in Fig. 3) and for the alpha band. The exact time–frequency bands to determine averaged prestimulus power bins are based on significant clusters in Figure 3 and are presented at the top of each figure. (A) Results for the significant cluster in the beta band for condition SOA 30 ms in sensors over ipsilateral SI (as highlighted in Fig. 3A). (B) Same analysis as in A, but for the significant cluster in sensors over SII (as highlighted in Fig. 3D). (C–D) Same analysis as in A,B but for the significant clusters in the beta band for condition SOA 45 ms (as highlighted in Fig. 3G,J). (E) Same analysis for the alpha band for condition SOA 45 ms in sensors over ipsilateral SI. Significant differences (*P < 0.05, **P < 0.01) are indicated by gray shaded areas.
Source Reconstruction
To determine the cortical sources of the significant effects on sensor level, we applied an adaptive spatial filtering technique in the frequency domain (Gross et al. 2001).
The leadfield matrix was computed for grid points in a realistically shaped single-shell volume conduction model, derived from the individual subject's structural MRI (Nolte 2003). To this end, a regular 3D 1-cm grid in the Montreal Neurological Institute template brain was created, and each subject's structural MRI was linearly warped onto this template. The inverse of this warp was applied to the template grid, resulting in individual grids based on individual subject's volume conduction model. The individual source parameters estimated in this way were combined across subjects per grid position. We aimed to determine the sources for the statistically significant effects revealed on sensor level (Fig. 3). To this end, we computed cross-spectral density (CSD) matrices between all MEG sensor pairs from the Fourier transforms of the tapered data epochs at the frequency of interest for each subject separately. The data epoch and the frequency of interest were based on the significant time–frequency clusters of the above-mentioned group analysis on sensor level (Fig. 3A,D,G,J). Since the significant clusters differed in time and frequency for the different sensors of interest, time–frequency bands used for source reconstruction differed for each condition. The exact time–frequency bands for each analysis can be found in Figure 6. Common spatial filters for each subject were computed using the CSD between all MEG sensor pairs, averaged over all trials of a given condition for the respective subject (pooled over subjective perceptions). For each subject, the CSD matrices of single trials were then projected through those individual filters, providing single trial estimates of source power (Hoogenboom et al. 2010). Statistical testing on source level was performed in line with testing on sensor level (see above). Results were displayed on the template brain, and cortical sources were identified using the AFNI atlas (http://afni.nimh.nih.gov/afni), integrated into FieldTrip.
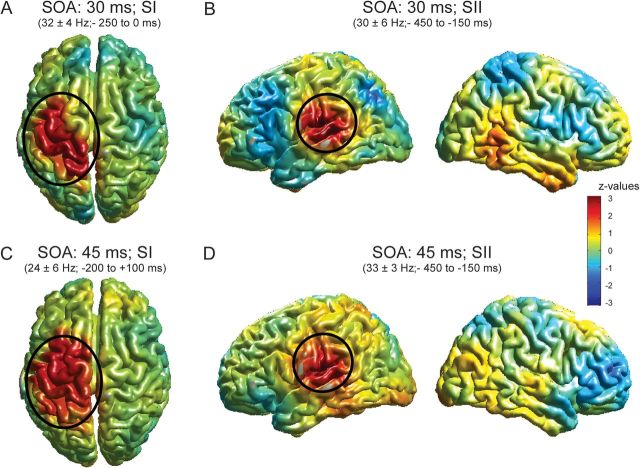
Figure 6.
Source analysis of significant clusters as found in Figure 3. The exact time–frequency bands used for source reconstruction are based on significant clusters in Figure 3 and are presented at the top of each figure. (A) Results for the significant cluster in the beta band for condition SOA 30 ms (as highlighted in Fig. 3A). z values in nonsignificant regions are lowered by 60% in order to highlight significant clusters. Additionally, significant clusters are highlighted by ovals. (B) Same as in A but for beta-band effect as highlighted in Figure 3D. Left column: view of the left hemisphere, right column: view of the right hemisphere. (C, D) Same as in A and B but for beta-band effect in SOA 45 ms (as highlighted in Fig. 3G,J). The color bar applies to all figures.
Results
Behavioral Results
Subjects were asked to report their subjective percept of simultaneity for electrical stimuli delivered to their left and right index finger with different SOAs. They made negligible errors for SOA of 0 and 200 ms (Fig. 2). However, intermediate SOA were perceived as simultaneous in some trials and as not simultaneous in other trials (SOA of −30 ms: 51.8 ± 5.5% (mean ± SEM) simultaneity reports; SOA of +30 ms: 54.9 ± 5.2%; SOA of −45 ms: 30.2 ± 4.8%; and SOA of +45 ms: 33.6 ± 4.8%).
Condition Contrasts
Next, we studied the role of oscillatory activity for the perception of simultaneity. Within each SOA we sorted trials with respect to subjects' perceptual reports. We compared spectral power between reports of simultaneity and reports of nonsimultaneity in sensors over sensorimotor areas.
For SOA of 30 ms, we found spectral power in sensors over ipsilateral primary somatosensory cortex (SI) to be statistically significantly enhanced in the frequency band 27.5–40 Hz if subjects perceived the stimulation erroneously as simultaneous. Notably, this effect occurred between −225 and −125 ms, that is, the effect appeared already before any electrical stimulation was delivered, and the effect was only present in ipsilateral sensors (Fig. 3A). In line with these findings, the topographical representation of this effect revealed a focus on sensors over ipsilateral SI (Fig. 3B). In sensors over secondary somatosensory cortex (SII), power was statistically significantly enhanced in the frequency band 17.5–40 Hz between −475 and −275 ms (Fig. 3D). The topographical representation revealed a focus over ipsilateral SII (Fig. 3E). No significant differences were observed in contralateral sensors (Fig. 3C,F).
For SOA of 45 ms, a similar finding was observed. In sensors over ipsilateral SI, oscillatory activity between 15–35 Hz and −150 to 50 ms was enhanced if subjects perceived the following stimulation erroneously as simultaneous (Fig. 3G,H). In sensors over ipsilateral SII, oscillatory activity between 25–40 Hz and −350 to −200 ms was significantly enhanced (Fig. 3J,K). Contralateral sensors did not show any significant differences (Fig. 3I,L).
Correlation of Prestimulus Power and Detection Rates
We found spectral power in alpha- and beta-frequency bands to be enhanced before and around the onset of stimulation, when subjects incorrectly perceived the 2 subsequent stimuli as simultaneous. To study the relation between subjective perception of stimuli and prestimulus oscillatory activity, we performed a correlation analysis. To this end, in each trial spectral power was averaged over sensors, time, and frequencies. Next, trials were sorted for spectral power and divided into 6 bins (Linkenkaer-Hansen et al. 2004; van Dijk et al. 2008; Jones et al. 2010). The perceptual reports were normalized per subject and then averaged over subjects. For the 4 cluster in the beta range in ipsilateral SI and SII (see Fig. 3A,D,G,J), we found a significant linear relationship between prestimulus power and subjects' perceptual reports in ipsilateral sensors for all conditions (SOA of 30 ms: SI: r2 = 0.90, P < 0.01; SII: r2 = 0.87, P < 0.01; SOA of 45 ms: SI: r2 = 0.86, P < 0.01; SII: r2 = 0.91, P < 0.01; Fig. 4A–D), that is, high prestimulus power was correlated with a high number of erroneous simultaneity reports. In contrast, we did not find a significant correlation in contralateral sensors (Supplementary Fig. S1). Additionally, we performed the same analysis for the alpha band in the condition SOA of 45 ms. We observed a quadratic relationship between subjective perception and prestimulus oscillatory activity in SI, with intermediate power bins showing the lowest probability of simultaneity reports (r2 = 0.89, P < 0.05; Fig. 4E). In other words, intermediate alpha amplitudes were associated with a more veridical perception of nonsimultaneity. No significant correlation was found in contralateral sensors (Supplementary Fig. S1).
Correlation of Prestimulus Power and ERFs
We additionally analyzed the correlation between prestimulus alpha-/beta-band activity and poststimulus ERFs (Jones et al. 2009, 2010; Anderson and Ding 2011).
First, we sorted trials in the condition SOA of 30 ms for power in ipsilateral sensors over SI in the time–frequency band between 27.5–40 Hz and −225 to −125 ms (i.e., the significant cluster in Fig. 3A). Trials with low prestimulus beta-band power revealed a significant increase of the ERFs between 150–168 and 216–232 ms (Fig. 5A). Trials with high prestimulus beta-band power in sensors over SII revealed increased ERFs between 93 and 148 ms (Fig. 5B).
For the condition SOA of 45 ms, we found no significant effects of prestimulus beta-band power on ERFs for sensors over SI (Fig. 5C). Sensors over SII revealed increased ERFs for trials with high beta-band power between 107 and 162 ms (Fig. 5D). Additionally, we sorted trials for prestimulus power in the alpha band. Sensors over SI revealed increased ERFs for trials with high prestimulus power between 64 and 75 ms. Furthermore, trials with low prestimulus power revealed increased ERFs between 250 and 278 ms (Fig. 5E).
Source Localization
To identify the cortical sources of the significant effects found in TFRs on sensor level (Fig. 3), we applied a beamforming approach. For both conditions (SOA of 30 and 45 ms), the comparatively late (∼−200 to 0 ms) significant components (Fig. 3A,G) revealed a significant source in ipsilateral sensorimotor areas with the peak located in ipsilateral SI (SOA of 30 ms: P < 0.05; SOA of 45 ms: P < 0.05, Fig. 6A,C). For both conditions, the earlier component (∼−450 to –250 ms) was located in ipsilateral SII (SOA of 30 ms: P < 0.05; SOA of 45 ms: P < 0.05, Fig. 6B,D).
Discussion
We studied the contribution of oscillatory neuronal activity to subjective perception of brief electrical stimuli applied bilaterally to the index fingers with varying SOAs. We were interested whether fluctuations of spectral power predict subjective perception. Crucially, the paradigm enabled us to study the role of neuronal oscillatory under conditions of constant physical stimulation while only the subjective perception was changed intrinsically.
When SOA was 30 or 45 ms, subjects frequently misperceived the stimulation as simultaneous. Erroneous perception of simultaneity was associated in both conditions (SOA 30 and 45 ms) with an increase of power in the beta band (∼20 to 30 Hz) in sensors over primary (SI) and secondary (SII) somatosensory cortex. The increase was evident in the cortical hemisphere ipsilateral to the site of the first stimulation but not in contralateral sites. Notably, this increase was visible before the onset of stimulation and the significant differences appeared earlier in sensors over SII than in sensors over SI. Source reconstruction confirmed a priori sensor selection by revealing significant cortical sources of the earlier effects (found in sensors presumably over SII at ∼−450 to −250 ms) in ipsilateral SII. The relatively later effects (∼−200 to 0 ms, observed in sensors presumably over SI) were located in ipsilateral sensorimotor areas with the peak located in SI. It should be noted that the source reconstruction was performed on prestimulus data, that is, in the absence of any stimulation. Without stimulation, absolute power levels have a smaller signal-to-noise ratio than power values in response to stimulation. Low signal-to-noise ratios naturally limit beamforming results by making also the sources noisier and thus spatially smeared. Furthermore, the observed significant effects are relatively short lived which also limits beamforming techniques. Despite these limitations and although the significant sources appear spatially smeared, their origins can be clearly assigned to SI and SII and are in good agreement in terms of location and quality with other findings of prestimulus power changes (Haegens et al. 2010, 2011; van Ede et al. 2011). In addition, the topographical representations imply weak activations in other cortical areas, presumably frontal and parietal areas (Fig. 3). However, none of these areas was found to be significantly activated in the source analysis. Reasons for the lack of significance might be that the effects in these areas were less strong than in the somatosensory areas or less consistent over subjects. Further studies need to unravel the contribution of nonsensory areas to the perception of simultaneity.
Notably, all reported effects were observed prior to onset of stimulation. We found prestimulus power in the beta (∼20 to 30 Hz) band in both ipsilateral SI and SII to be linearly correlated to perceptions of nonsimultaneity, that is, veridical perception was highest for low prestimulus amplitudes. In addition, alpha-band power in ipsilateral SI revealed a quadratic relation to perception of simultaneity for condition SOA of 45 ms, that is, veridical perception was highest for intermediate states of prestimulus alpha power.
One potential concern in the interpretation of the results might be that the effects are caused by motor preparation. It is well known that alpha- and beta-band power in sensorimotor cortex can be modulated by motor preparation and execution (e.g., Hari and Salmelin 1997). For several reasons, however, it is unlikely that our reported effects are related to motor preparation:
To minimize the influence of premovement power changes, we had included a jittered interval of 800–1200 ms after stimulus presentation before occurrence of the response cue. Subjects responded on average 539 ± 36 ms after the response cue. Thus, while subjects responded on average ∼1500 ms after stimulus presentation, significant differences in oscillatory activity were found ∼0 to 400 ms before stimulus presentation. In contrast, no significant differences were found in the poststimulus period prior to button presses. Consequently, we did not find any correlation between prestimulus power and reaction times (data not shown). Furthermore, response configurations were balanced across and within subjects so that the response hand and the site of the first stimulation were unrelated. Taken together, due to the setup and the timing of the significant effects, it is highly unlikely that the observed effects are due to motor preparation.
Recent studies investigated the influence of attention on prestimulus alpha- and beta-band power and their impact on tactile detection (Linkenkaer-Hansen et al. 2004; Jones et al. 2009, 2010; van Ede et al. 2010, 2011; Anderson and Ding 2011). These studies reported prestimulus effects to be lateralized contralaterally to the side of the stimulation. While in these studies, stimulation was applied unilaterally, and the side of stimulation was cued, we applied stimulation bilaterally, and the site of the first stimulation was unknown (i.e., randomized from trial to trial). Therefore, we did not expect attention to be lateralized. In line with this, we found prestimulus power modulations bilaterally rather than lateralized. In addition, fluctuations of prestimulus power modulations do significantly affect perception of subsequent stimuli and that these effects are lateralized with respect to the site of the first stimulation. While there are also poststimulus modulations of oscillatory activity in both hemispheres in response to bilateral stimulation, Figure 3 reveals that these modulations do not differ for the 2 subjective reports. In other words, poststimulus modulations of spectral power do not correlate with subjective perception of simultaneity.
In line with our findings, Jones et al. (2010) reported a linear relationship for veridical perception of tactile stimuli and prestimulus alpha-/beta-band power. While Jones et al. explicitly studied the effects in SI, we observed the effects in both, SI and SII. While we and Jones et al. found a linear relationship, Linkenkaer-Hansen et al. (2004) reported a quadratic relationship between prestimulus beta-band activity in sensorimotor areas and subjects' performance in a tactile detection task. A possible reason for these different findings of Linkenkaer-Hansen et al. might be that they used much broader time and frequency bands for their analyses.
In addition, studies reported that intermediate amplitudes of prestimulus alpha-band oscillations in sensorimotor areas were optimal for perception of weak tactile stimuli (Linkenkaer-Hansen et al. 2004; Zhang and Ding 2010). In line with these studies, we found a quadratic relationship between prestimulus alpha-band activity and simultaneity reports in sensors over SI, that is, veridical perception of simultaneity was highest for intermediate states of prestimulus alpha-band activity. In contrast, a linear relationship between prestimulus alpha-band activity and detection probabilities of tactile stimuli has been reported by Jones et al. (2010). Differences might be attributable to different tasks: While Jones et al. employed a cued attention task, subjects in our study were asked to report subjective simultaneity.
Several studies reported a correlation of prestimulus power and poststimulus ERFs (Jones et al. 2009, 2010; Zhang and Ding 2010; Anderson and Ding 2011). In line with previous studies (Jones et al. 2009, 2010; Anderson and Ding 2011), we found that trials with a high prestimulus power in the alpha band revealed increased ERFs between 64 and 75 ms in ipsilateral SI, which is likely to represent the early evoked M20/M50 component to electrical or mechanical stimulation. Note that the time scale is always presented relative to the presentation of the first stimulus, while the reported effects are always in the hemisphere contralateral to the site of the second stimulation. Due to this shift in stimulation parameters, we expect ERFs to be shifted by 30 or 45 ms, respectively. In their computational study, Jones et al. (2009) suggested that an increased M50 component might be caused by greater levels of recruited inhibition, subsequently decreasing the effect of excitatory cells. Notably, we found an increased early ERF component only in ipsilateral SI and only for the condition SOA of 45 ms, suggesting that the proposed inhibition processes induced by prestimulus alpha-band power influence only the (interhemispheric) processing of stimuli spaced 45 ms but not stimuli spaced 30 ms. We suggest that with higher prestimulus power, that is, with early inhibiting poststimulus processes, the second stimulus might be processed less efficiently, leading to a lower temporal precision and thus more incorrect reports in the perception of simultaneity.
In addition, we found that trials with low prestimulus beta-band power revealed a lower M100 peak (at ∼130 ms for SOA of 30 ms and at ∼145 ms for SOA of 45 ms, for discussion of the temporal shift of the M100 component, see above). Studies in human and nonhuman primates have demonstrated subsequent attenuation of ipsilateral somatosensory responses after contralateral tactile stimulation (Simões and Hari 1999; Simões et al. 2001; Hlushchuk and Hari 2006; Tommerdahl et al. 2006; Reed et al. 2011; Wühle et al. 2011) with the maximum attenuation for peaks at ∼100 ms (Simões et al. 2001; Wühle et al. 2011). Our results suggest that the attenuation is meditated by prestimulus states of the beta band. The correlation of beta-band power and ERFs was only found in sensors over ipsilateral SII but not in SI. Since SII receives input from both body sides and bilateral SI, it is a likely candidate for integration of bilateral sensory input. One potential explanation might be that the stronger attenuation of the M100 component reflects stronger interhemispheric interaction, which in turn is modulated by prestimulus states in the beta band.
The above-mentioned studies (Linkenkaer-Hansen et al. 2004; Jones et al. 2010) have argued that prestimulus alpha- and beta-band activity influences the perception and detection of tactile stimuli. In line with this hypothesis, we suggest that subjective perception of simultaneity strongly depends on the veridical perception of the second stimulus. If prestimulus alpha- and beta-band activity is at optimal states, the likelihood to detect the second stimulus is high. This in turn promotes veridical perception of the 2 stimuli as temporally separate. We report beta-band effects in SI and SII, while most previous studies reported prestimulus effects mainly in SI (Linkenkaer-Hansen et al. 2004; Jones et al. 2009, 2010; van Ede et al. 2010, 2011; Zhang and Ding 2010; Anderson and Ding 2011). One crucial difference is that we used bilateral stimulation, while the above-mentioned studies always used unilateral stimulation. Prestimulus activity in SII might therefore be relevant for bilateral integration of tactile stimuli or gating of information but less crucial for unilateral perception. However, it should be mentioned that prestimulus effects in the beta band have been reported also in SII before (Linkenkaer-Hansen et al. 2004). Another crucial difference is that previous studies explicitly or implicitly incorporated a spatial attention task where subjects had to direct attention to one body side. It might be possible that spatial attention is more strongly confined to SI, while bilateral interaction is more strongly relying on SI and SII.
Our main finding was that for both conditions (SOA of 30 and 45 ms) prestimulus beta-band activity was increased in SI and SII when stimulation was erroneously perceived as simultaneously. Several studies have reported involvement of beta-band oscillations in top-down modulations of attention or the perception of bistable stimuli (von Stein et al. 2000; Engel et al. 2001; Gross et al. 2004; Buschman and Miller 2007; Kranczioch et al. 2007; Pesaran et al. 2008; van Elswijk et al. 2010). In their computational study, Jones et al. (2010) suggested that prestimulus alpha-band activity modulates feedforward bottom-up processing, while beta-band activity reflects both feedforward and feedback modulations of cortical processes. Similarly, Engel and Fries (2010) suggested that beta-band activity plays a role in endogenous top-down modulation of cognitive processes. According to this hypothesis, low amplitudes of beta-band oscillations should promote bottom-up stimulus-driven processing, while high amplitudes should increase the threshold for the responses to novel unexpected stimuli. In line with this hypothesis, we suggest that fluctuations of prestimulus beta oscillations determine the threshold for detecting stimuli. An increase of beta activity impairs bottom-up processing, therefore renders distinct temporal detection of the first and the second stimulus more unlikely and thus biases (incorrect) simultaneous reports. Several studies also found interareal coherence mainly in the beta band (Gross et al. 2004; Kranczioch et al. 2007; Hipp et al. 2011). A recent study found increased prestimulus beta-band activity in superior temporal gyrus associated with the (incorrect) perception of the bistable McGurk illusion (Keil et al. 2011). We suggest that the perception of bistable stimuli (such as McGurk effect, attentional blink or our paradigm of simultaneity perception) is strongly influenced by ongoing network fluctuations in the beta band.
Similar to the attentional blink paradigm, in our paradigm the second of 2 subsequent stimuli is frequently misperceived. Both paradigms require thus a high temporal resolution of sensory perception. We propose that low states of beta oscillations prior to the sensory stimulation promote a processing of stimuli, while states of high beta amplitudes increase the threshold for sensory processing and make perception less accurate, especially for weak near-threshold stimuli (Engel and Fries 2010). In our case, less accurate (temporal) perception might bias simultaneity reports.
Prolonged SOA will lead to more veridical reports, that is, prolonged SOA will decrease the degree of ambiguity or bistability (Fig. 2). Subjective perception for prolonged SOA thus might be less influenced by small fluctuations of ongoing fluctuations of oscillatory activity. Additional components might thus be necessary to further increase perceptual threshold. One component might be inhibited bottom-up processing of sensory input in SI by alpha-band activity (Jones et al. 2009). In line with this hypothesis, we additionally found increased prestimulus alpha power for subjective perception of simultaneity in condition with SOA of 45 ms.
In summary, we found that prestimulus activity in the alpha and high beta band predicts the subjective perception of electrical simultaneity. We propose that states of prestimulus alpha- and beta-band activity determine perceptual detection thresholds for tactile and electrical stimuli (Engel and Fries 2010). Modulations in the beta band were found in SI and SII, while alpha-band modulations were found in SI. We suggest that these regions communicate in the respective frequency bands and thus control bottom-up and top-down information flow. The results mount on recent evidence and extend findings emphasizing the role of prestimulus oscillatory activity for perception.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Acknowledgments
Conflict of Interest : None declared.
References
- Anderson KL, Ding M. Attentional modulation of the somatosensory mu rhythm. Neuroscience. 2011;180:165–180. doi: 10.1016/j.neuroscience.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Bauer M, Oostenveld R, Peeters M, Fries P. Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J Neurosci. 2006;26:490–501. doi: 10.1523/JNEUROSCI.5228-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations-signalling the status quo? Curr Opin Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Geffen G, Rosa V, Luciano M. Sex differences in the perception of tactile simultaneity. Cortex. 2000;36:323–335. doi: 10.1016/s0010-9452(08)70844-x. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci U S A. 2001;98:694–699. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Schmitz F, Schnitzler I, Kessler K, Shapiro K, Hommel B, Schnitzler A. Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proc Natl Acad Sci U S A. 2004;101:13050–13055. doi: 10.1073/pnas.0404944101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Handel BF, Jensen O. Top-down controlled alpha band activity in somatosensory areas determines behavioral performance in a discrimination task. J Neurosci. 2011;31:5197–5204. doi: 10.1523/JNEUROSCI.5199-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Osipova D, Oostenveld R, Jensen O. Somatosensory working memory performance in humans depends on both engagement and disengagement of regions in a distributed network. Hum Brain Mapp. 2010;31:26–35. doi: 10.1002/hbm.20842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara K, Okamoto T, Shigeto H, Ogata K, Somehara Y, Matsushita T, Kira J, Tobimatsu S. Oscillatory gamma synchronization binds the primary and secondary somatosensory areas in humans. Neuroimage. 2010;51:412–420. doi: 10.1016/j.neuroimage.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Aslan A, Staudigl T, Klimesch W, Herrmann CS, Bauml KH. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage. 2007;37:1465–1473. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hari R, Salmelin R. Human cortical oscillations: a neuromagnetic view through the skull. Trends Neurosci. 1997;20:44–49. doi: 10.1016/S0166-2236(96)10065-5. [DOI] [PubMed] [Google Scholar]
- Harrar V, Harris LR. Simultaneity constancy: detecting events with touch and vision. Exp Brain Res. 2005;166:465–473. doi: 10.1007/s00221-005-2386-7. [DOI] [PubMed] [Google Scholar]
- Harrar V, Harris LR. The effect of exposure to asynchronous audio, visual, and tactile stimulus combinations on the perception of simultaneity. Exp Brain Res. 2008;186:517–524. doi: 10.1007/s00221-007-1253-0. [DOI] [PubMed] [Google Scholar]
- Hipp JF, Engel AK, Siegel M. Oscillatory synchronization in large-scale cortical networks predicts perception. Neuron. 2011;69:387–396. doi: 10.1016/j.neuron.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Hlushchuk Y, Hari R. Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. J Neurosci. 2006;26:5819–5824. doi: 10.1523/JNEUROSCI.5536-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenboom N, Schoffelen JM, Oostenveld R, Fries P. Visually induced gamma-band activity predicts speed of change detection in humans. Neuroimage. 2010;51:1162–1167. doi: 10.1016/j.neuroimage.2010.03.041. [DOI] [PubMed] [Google Scholar]
- Hoogenboom N, Schoffelen JM, Oostenveld R, Parkes LM, Fries P. Localizing human visual gamma-band activity in frequency, time and space. Neuroimage. 2006;29:764–773. doi: 10.1016/j.neuroimage.2005.08.043. [DOI] [PubMed] [Google Scholar]
- Jones SR, Kerr CE, Wan Q, Pritchett DL, Hamalainen M, Moore CI. Cued spatial attention drives functionally relevant modulation of the mu rhythm in primary somatosensory cortex. J Neurosci. 2010;30:13760–13765. doi: 10.1523/JNEUROSCI.2969-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Pritchett DL, Sikora MA, Stufflebeam SM, Hamalainen M, Moore CI. Quantitative analysis and biophysically realistic neural modeling of the MEG mu rhythm: rhythmogenesis and modulation of sensory-evoked responses. J Neurophysiol. 2009;102:3554–3572. doi: 10.1152/jn.00535.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil J, Muller N, Ihssen N, Weisz N. On the variability of the McGurk effect: audiovisual integration depends on prestimulus brain states. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr125. doi:10.1093/cercor/bhr125. [DOI] [PubMed] [Google Scholar]
- Kopinska A, Harris LR. Simultaneity constancy. Perception. 2004;33:1049–1060. doi: 10.1068/p5169. [DOI] [PubMed] [Google Scholar]
- Kranczioch C, Debener S, Maye A, Engel AK. Temporal dynamics of access to consciousness in the attentional blink. Neuroimage. 2007;37:947–955. doi: 10.1016/j.neuroimage.2007.05.044. [DOI] [PubMed] [Google Scholar]
- Lange J, Oostenveld R, Fries P. Perception of the touch-induced visual double-flash illusion correlates with changes of rhythmic neuronal activity in human visual and somatosensory areas. Neuroimage. 2011;54:1395–1405. doi: 10.1016/j.neuroimage.2010.09.031. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Wilke M, Maier A, Logothetis NK. Stable perception of visually ambiguous patterns. Nat Neurosci. 2002;5:605–609. doi: 10.1038/nn0602-851. [DOI] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K, Nikulin VV, Palva S, Ilmoniemi RJ, Palva JM. Prestimulus oscillations enhance psychophysical performance in humans. J Neurosci. 2004;24:10186–10190. doi: 10.1523/JNEUROSCI.2584-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. To see or not to see: prestimulus alpha phase predicts visual awareness. J Neurosci. 2009;29:2725–2732. doi: 10.1523/JNEUROSCI.3963-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri A, Nieuwenhuis IL, van DH, Jensen O. Prestimulus alpha and mu activity predicts failure to inhibit motor responses. Hum Brain Mapp. 2009;30:1791–1800. doi: 10.1002/hbm.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte G. The magnetic lead field theorem in the quasi-static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Phys Med Biol. 2003;21:3637–3652. doi: 10.1088/0031-9155/48/22/002. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S, Linkenkaer-Hansen K, Näätänen R, Palva JM. Early neural correlates of conscious somatosensory perception. J Neurosci. 2005;25:5248–5258. doi: 10.1523/JNEUROSCI.0141-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA. Free choice activates a decision circuit between frontal and parietal cortex. Nature. 2008;453:406–409. doi: 10.1038/nature06849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, Qi HX, Kaas JH. Spatiotemporal properties of neuron response suppression in owl monkey primary somatosensory cortex when stimuli are presented to both hands. J Neurosci. 2011;31:3589–3601. doi: 10.1523/JNEUROSCI.4310-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ. Perception's shadow: long-distance synchronization of human brain activity. Nature. 1999;397:430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- Romei V, Gross J, Thut G. On the role of prestimulus alpha rhythms over occipito-parietal areas in visual input regulation: correlation or causation? J Neurosci. 2010;30:8692–8697. doi: 10.1523/JNEUROSCI.0160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões C, Hari R. Relationship between responses to contra- and ipsilateral stimuli in the human second somatosensory cortex SII. Neuroimage. 1999;10:408–416. doi: 10.1006/nimg.1999.0476. [DOI] [PubMed] [Google Scholar]
- Simões C, Mertens M, Forss N, Jousmäki V, Lütkenhöner B, Hari R. Functional overlap of finger representations in human SI and SII cortices. J Neurophysiol. 2001;86:1661–1665. doi: 10.1152/jn.2001.86.4.1661. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommerdahl M, Simons SB, Chiu JS, Favorov O, Whitsel BL. Ipsilateral input modifies the primary somatosensory cortex response to contralateral skin flutter. J Neurosci. 2006;26:5970–5977. doi: 10.1523/JNEUROSCI.5270-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk H, Schoffelen JM, Oostenveld R, Jensen O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J Neurosci. 2008;28:1816–1823. doi: 10.1523/JNEUROSCI.1853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk H, van der Werf J, Mazaheri A, Medendorp WP, Jensen O. Modulations in oscillatory activity with amplitude asymmetry can produce cognitively relevant event-related responses. Proc Natl Acad Sci U S A. 2010;107:900–905. doi: 10.1073/pnas.0908821107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede F, de Lange F, Jensen O, Maris E. Orienting attention to an upcoming tactile event involves a spatially and temporally specific modulation of sensorimotor alpha- and beta-band oscillations. J Neurosci. 2011;31:2016–2024. doi: 10.1523/JNEUROSCI.5630-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede F, Jensen O, Maris E. Tactile expectation modulates pre-stimulus beta-band oscillations in human sensorimotor cortex. Neuroimage. 2010;51:867–876. doi: 10.1016/j.neuroimage.2010.02.053. [DOI] [PubMed] [Google Scholar]
- van Elswijk G, Maij F, Schoffelen JM, Overeem S, Stegeman DF, Fries P. Corticospinal beta-band synchronization entails rhythmic gain modulation. J Neurosci. 2010;30:4481–4488. doi: 10.1523/JNEUROSCI.2794-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stein A, Chiang C, König P. Top-down processing mediated by interareal synchronization. Proc Natl Acad Sci U S A. 2000;97:14748–14753. doi: 10.1073/pnas.97.26.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wühle A, Preissl H, Braun C. Cortical processing of near-threshold tactile stimuli in a paired-stimulus paradigm—an MEG study. Eur J Neurosci. 2011;34:641–651. doi: 10.1111/j.1460-9568.2011.07770.x. [DOI] [PubMed] [Google Scholar]
- Wyart V, Tallon-Baudry C. How ongoing fluctuations in human visual cortex predict perceptual awareness: baseline shift versus decision bias. J Neurosci. 2009;29:8715–8725. doi: 10.1523/JNEUROSCI.0962-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ding M. Detection of a weak somatosensory stimulus: role of the prestimulus mu rhythm and its top-down modulation. J Cogn Neurosci. 2010;22:307–322. doi: 10.1162/jocn.2009.21247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.