Abstract
Cathodal transcranial direct current stimulation (c-tDCS) can reduce excitability of neurons in primary motor cortex (M1) and may facilitate motor recovery after stroke. However, little is known about the neurophysiological effects of tDCS on proximal upper limb function. We hypothesized that suppression of contralesional M1 (cM1) excitability would produce neurophysiological effects that depended on the severity of upper limb impairment. Twelve patients with varying upper limb impairment after subcortical stroke were assessed on clinical scales of upper limb spasticity, impairment, and function. Magnetic resonance imaging was used to determine lesion size and fractional anisotropy (FA) within the posterior limbs of the internal capsules indicative of corticospinal tract integrity. Excitability within paretic M1 biceps brachii representation was determined from motor-evoked potentials during selective isometric tasks, after cM1 sham stimulation and after c-tDCS. These neurophysiological data indicate that c-tDCS improved selective proximal upper limb control for mildly impaired patients and worsened it for moderate to severely impaired patients. The direction of the neurophysiological after effects of c-tDCS was strongly related to upper limb spasticity, impairment, function, and FA asymmetry between the posterior limbs of the internal capsules. These results indicate systematic variation of cM1 for proximal upper limb control after stroke and that suppression of cM1 excitability is not a “one size fits all” approach.
Keywords: corticospinal tract, ipsilateral pathways, magnetic resonance imaging, stroke prediction, transcranial direct current stimulation
Introduction
Six months after stroke, up to two-thirds of patients are unable to incorporate a weak hand into activities of daily living (Dobkin 2005). Following stroke there is often an imbalance in primary motor cortex (M1) excitability, with relative underexcitability in the stroke affected ipsilesional hemisphere and relative overexcitability in the contralesional hemisphere, and worse outcomes for patients with greater imbalance (Traversa et al. 1998). Rebalancing of cortical excitability in patients with stroke has been associated with improvement of upper limb function (Traversa et al. 1998; Shimizu et al. 2002; Murase et al. 2004; Stinear et al. 2008; Swayne et al. 2008) and can be promoted with noninvasive brain stimulation (Hummel and Cohen 2006). Transcranial direct current stimulation (tDCS) is a form of noninvasive brain stimulation that suppresses or facilitates M1 depending on the electrode polarity (Nitsche and Paulus 2000, 2001; Nitsche et al. 2003; Nitsche et al. 2005). Cathodal tDCS (c-tDCS) hyperpolarizes neurons and can be used to reduce the relative overexcitability of the contralesional hemisphere (Nowak et al. 2009).
Proximal upper limb muscles are innervated by projections from contralateral and ipsilateral motor cortex, and this bilateral pattern of organization has functional implications for adjuvants such as tDCS (Kuypers and Brinkman 1970; Turton et al. 1996; Lemon 2008). There is recent evidence in healthy adults that suppression of M1 can influence control of the ipsilateral proximal upper limb by reducing or increasing excitability of ipsilateral descending projections from noninvasive brain stimulation (Bradnam, Stinear, and Byblow 2010; McCambridge et al. 2011). However, upregulation of ipsilateral projections from contralesional M1 (cM1) may be an important functional adaptation in patients severely affected by stroke (Ward et al. 2006; Ward et al. 2007). Therefore, contralesional c-tDCS might not benefit this subgroup of patients. This might explain why cM1 suppression has had mixed effects on measures of paretic upper limb function in stroke patients to date. While some studies have shown positive effects on upper limb function (Fregni et al. 2005; Boggio et al. 2007; Grefkes et al. 2010; Kim et al. 2010), others have reported deleterious (Johansen-Berg et al. 2002; Murase et al. 2004; Lotze et al. 2006; Ackerley et al. 2010; Bestmann et al. 2010) or no effects (Talelli et al. 2007). These mixed findings indicate it is unlikely that there will be a “one size fits all” strategy for promoting upper limb function after stroke with noninvasive brain stimulation and that the extent to which the cM1 contributes to control of the paretic upper limb needs to be taken into account when selecting protocols for an individual patient. Therefore, the efficacy of contralesional c-tDCS may depend on whether patients are mildly or severely impaired (Schlaug et al. 2008).
This study examined the effects of c-tDCS of cM1 on paretic proximal upper limb muscle activation in patients with subcortical stroke. We hypothesized that because contralesional c-tDCS may suppress ipsilateral descending projections to proximal upper limb, after effects would depend on the relative contribution of cM1 to control of paretic proximal muscles. We predicted that for mildly impaired patients cM1 might interfere with control from the ipsilesional M1 at the level of the spinal cord. Therefore, suppressive tDCS of cM1 was expected to improve the control of the paretic proximal upper limb. Conversely, we predicted that for moderate to severely impaired patients control would be degraded because suppressing cM1 would downregulate ipsilateral compensatory pathways for proximal paretic upper limb control for these patients.
Materials and Methods
Participants
Twelve patients (9 males, mean age 64 ± 3.4 years, range 38–80 years) at least 6 weeks following subcortical cerebral infarction were studied (Table 1). A further 5 patients were assessed but did not meet the eligibility criteria (see Fig. 1) and were excluded (National Institutes of Health Stroke Scale [NIHSS] < 2 in 4 patients, severe upper limb paresis, 1 patient). Each of the 12 patients was age and gender matched with a healthy adult (mean age 63 ± 3.4 years, range 37 – 79 years, Edinburgh handedness score 0.93 ± 0.03) (Oldfield 1971). Informed consent was given by all participants in accordance with the Declaration of Helsinki. Ethical approval to carry out the study was granted by the regional ethics committee.
Table 1.
Participant characteristics at study inception
| Patient | Age (y) | Sex | Hem | Hand | Time (m) | NIHSS | FM | ARAT | ASH | Site of lesion | Lesion size (mm3) | FAAI | Age, healthy control (years) |
| 1 | 38 | F | R | R | 28 | 6 | 28 | 19 | 3 | MCA territory | 42341 | 0.48 | 37 |
| 2 | 57 | M | R | R | 7 | 6 | 30 | 29 | 4 | GP, Put | 1584 | 0.16 | 56 |
| 3 | 72 | M | R | R | 12 | 3 | 28 | 21 | 3 | x | x | x | 66 |
| 4 | 65 | F | R | R | 34 | 4 | 45 | 31 | 2 | MCA territory | 25236 | 0.36 | 66 |
| 5 | 57 | M | R | R | 8 | 5 | 36 | 25 | 2 | Pons | 537 | 0.13 | 57 |
| 6 | 70 | M | R | R | 3 | 7 | 14 | 19 | 1 | Pons | 1248 | 0.11 | 69 |
| 7 | 61 | M | L | L | 33 | 2 | 54 | 55 | 1 | Corona radiata | 59 | 0.05 | 56 |
| 8 | 80 | M | L | R | 2 | 2 | 64 | 57 | 0 | Corona radiata | 161 | 0.04 | 79 |
| 9 | 66 | M | L | R | 2 | 4 | 50 | 56 | 0 | Pons | 151 | 0.05 | 55 |
| 10 | 80 | M | L | R | 3 | 2 | 62 | 55 | 0 | Pons | 223 | 0.04 | 79 |
| 11 | 68 | F | R | L | 3 | 3 | 60 | 48 | 0 | MCA territory | 20797 | 0.10 | 69 |
| 12 | 54 | M | L | R | 3 | 3 | 56 | 53 | 0 | Superior Corona radiata | 10972 | 0.05 | 61 |
| Mean | 64 | 12 | 4 | 44 | 39 | 1 | 9392 | 0.14 | 63 |
Note: Age (years, y), Hem = hemisphere affected by stroke, Hand = hand dominance before stroke, time = time since stroke (months, m), NIHSS score = National Institutes of Health Stroke Scale (maximum 42), Fugl-Meyer (FM) upper limb score (maximum 66), ARAT = Action Research Arm Test, average of 2 preintervention sessions (maximum 57), ASH = modified Ashworth scale for BB spasticity (maximum 5). Affected structures: GP = globus pallidus, Put = putamen, MCA territory = middle cerebral artery territory, lesion size = brain volume affected by stroke (mm3), FAAI = fractional anisotropy asymmetry index calculated in the PLIC, x = no data collected.
Figure 1.
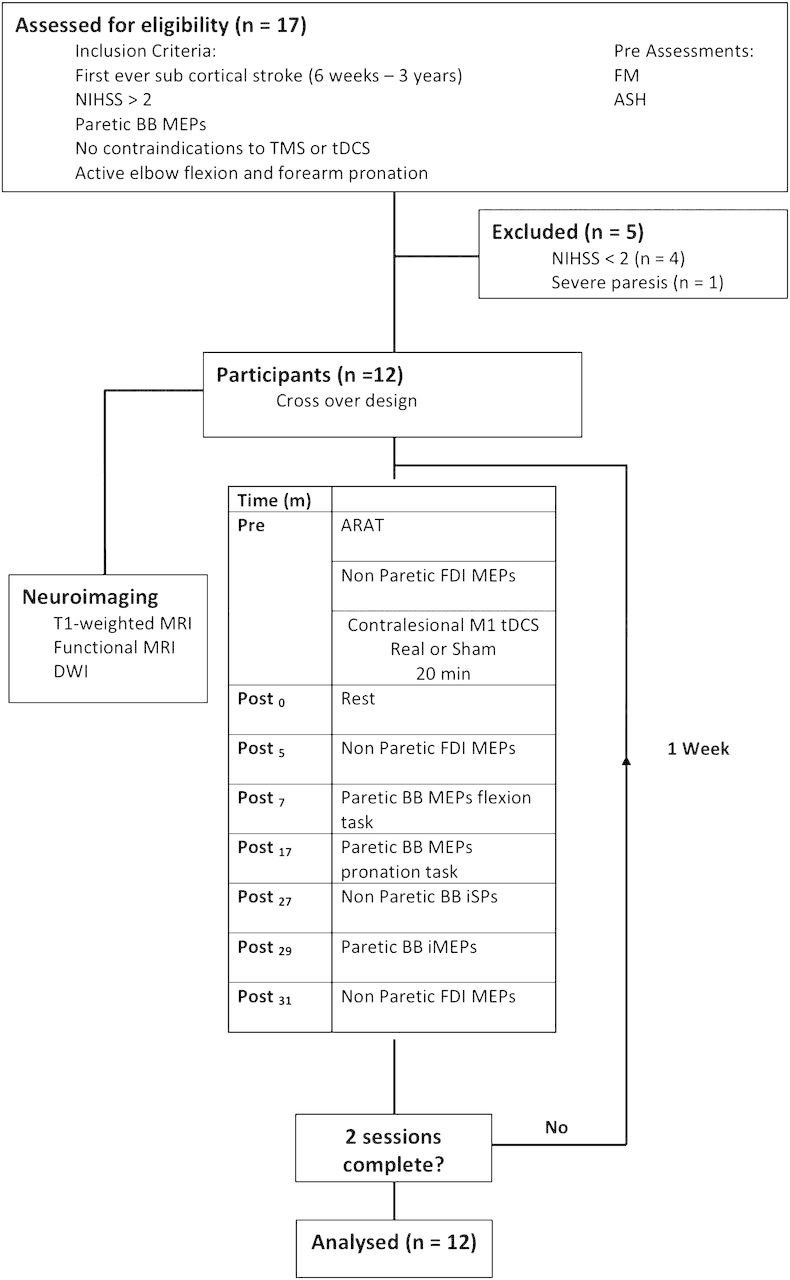
The experimental protocol for patients. Pre, post = before and after tDCS.
Experimental Design
The experimental protocol is outlined in Figure 1. Motor impairment and elbow flexor spasticity were assessed in the paretic upper limb using the NIHSS, Fugl-Meyer (FM), and modified Ashworth spasticity scales (ASHs) at an initial screening session. The NIHSS was developed by the National Institutes of Health to assess the severity of stroke. Scores range from 0 to 42, higher scores indicate greater deficiencies. The FM scale is a quantitative measurement of sensorimotor impairment, scored of a total of 66, higher scores reflecting less impairment (Brunnstrom 1966). The ASH is a clinical measure of muscle spasticity, scored of 5, higher scores indicate greater spasticity (Bohannon and Smith 1987). Patients attended 2 experimental sessions, separated by 1 week, in which they received either c-tDCS or sham tDCS. Action Research Arm Test (ARAT) scores and corticomotor excitability of biceps brachii (BB) muscle were assessed at each session. The ARAT is an observational assessment of paretic hand function after stroke and is scored of 57, higher scores reflect better motor function (Lyle 1981). On a separate day, patients had magnetic resonance imaging (MRI) brain scans performed to obtain structural and diffusion-weighted imaging (DWI). The healthy adult participants attended a single transcranial magnetic stimulation (TMS) session and did not receive tDCS or have an MRI brain scan.
Electromyography
Surface electromyography (EMG) was recorded from the BB and pronator teres (PT) in the nondominant limb of healthy participants with disposable adhesive electrodes (Ambu, Ballerup, Denmark) placed over the muscle bellies in a bipolar montage. In stroke patients, EMG was recorded from the BB and the PT in the paretic upper limb and from the BB and the first dorsal interosseus (FDI) of the nonparetic limb. EMG signals were amplified (CED 1902, Cambridge, UK), bandwidth filtered (2–1000 Hz), and sampled at 2 kHz (CED 1401, Cambridge, UK).
Motor Tasks
Participants performed separate blocks of brief isometric elbow flexion or forearm pronation while seated with their forearm constrained to prevent isotonic movement (Gerachshenko et al. 2008; Bradnam, Stinear, and Byblow 2010). Flexor and pronator contractions were paced with an auditory metronome set at a comfortable tempo for each individual (average 0.8 Hz, range 0.6–1 Hz). Participants were instructed to keep pace with the metronome as precisely as possible and to relax completely between contractions. Participants were not asked to produce a particular force but to concentrate on producing a short “burst” of EMG in time with the metronome. Verbal feedback by the experimenter who observed the EMG displayed on the computer screen assisted performance by each individual. Between 20 and 50 repetitions of each task were performed in blocks, with short rests as required. More impaired patients were subject to fatigue and timing errors. The same motor task was used to assess BB motor-evoked potentials (MEPs) in the nondominant arm of control participants who performed 50 repetitions per block paced at 1 Hz, with short rests as required. The aim of the procedure was to obtain at least 10 MEPs per condition/participant for averaging.
Transcranial Magnetic Stimulation
Single-pulse TMS was delivered with a figure of 8 coils (70 mm wing diameter) (MagStim Co., Whitland, Dyfed, Wales), positioned over M1 to induce a posterior to anterior directed current in the underlying brain. The “hotspot” for evoking contralateral MEPs in BB was located for both contralesional and ipsilesional M1 and marked on the scalp. Active motor threshold (AMT) was determined for ipsilesional M1, defined as the minimum stimulus intensity that elicited a 100 μV MEP in 4 of 8 trials during a paretic BB contraction.
Contralateral MEPs were obtained in the paretic BB before either rhythmic isometric forearm pronation or elbow flexion, that is, in the resting phase of the task just prior to voluntary contraction. The size of the BB MEP prior to pronation relative to flexion yields a selectivity ratio (SR). BB MEPs are typically smaller prior to pronation than flexion because BB is an antagonist to pronation. The SR therefore reflects the ability to suppress the antagonist and is normally lower in healthy adults than stroke patients (Gerachshenko et al. 2008).
Test stimulus intensity was set to 120% of the paretic BB AMT, except in 5 participants who had an AMT above 75% maximal stimulator output (MSO), in which case the test intensity was set to 90% MSO. TMS was timed to occur before the onset of muscle activity (i.e., when the muscle was at rest) during the paced elbow flexion and forearm pronation tasks, between 150 and 250 ms before every fifth metronome beat. For healthy adults, TMS of the nondominant M1 was applied at an intensity of 120% AMT during the same motor tasks and MEPs recorded in the nondominant BB.
The TMS protocol for the patients is summarized in Figure 1. Prior to tDCS and at Post5 and Post31, single-pulse TMS of cM1 was applied to elicit MEPs in the nonparetic FDI at rest. After tDCS, there was a 5-min consolidation period before FDI MEPs were collected at Post5. MEPs were then elicited for deriving SR (Post7) using the flexion and pronation tasks described above. At Post27, single-pulse TMS of ipsilesional M1 at 80% MSO was used to produce ipsilateral silent periods (iSPs) in the nonparetic BB, while patients maintained isometric elbow flexion at 20% MVC. The intensity was adjusted to produce a 1-mV MEP preintervention. Finally at Post29, TMS of cM1 at 80% MSO was used to produce ipsilateral MEPs (iMEPs) in the paretic BB, while patients maintained isometric paretic elbow flexion at 20% MVC. Sixteen responses were recorded for each measure described above.
Transcranial Direct Current Stimulation
An investigator blinded to all aspects of data collection performed tDCS and investigators responsible for data collection and analysis were blinded to the protocol until study completion. Contralesional c-tDCS was delivered with a constant current of 1 mA for 20 min using a Phoresor II stimulator (Model PM850, IOMED Inc., Utah) via two 35 cm2 saline soaked sponge electrodes, with the cathode positioned over the M1 BB hotspot and the anode over the contralateral forehead. This tDCS protocol has been used successfully in studies of stroke patients (Nowak et al. 2009). Sham tDCS was applied with the same configuration, but current intensity was ramped down to zero after 30 s (Gandiga et al. 2006). Following tDCS, patients sat quietly with eyes closed for 5 min to consolidate effects and avoid confounding after effects of stimulation by muscle activity (Huang et al. 2010). Session order was randomized.
Neuroimaging
A Siemens Magnetom Avanto 1.5-T MRI system was used to acquire high-resolution T1-weighted images with a 3D FLASH (fast low angled shot) sequence (time repetition [TR] = 11 ms, time echo [TE] = 4.94 ms, field of view [FOV] = 256 mm, voxel dimensions of 1 × 1 × 1 mm3). DWI was performed with a single shot diffusion-weighted spin echo pulse sequence (TR = 6601 ms, TE = 101 ms, FOV = 230 mm, voxel dimensions of 1.8 × 1.8 x 3 mm3), with diffusion gradients along 30 directions (b1 = 2000 s/mm2).
Data Analysis
Selectivity Ratio
BB MEPs for flexion and pronation tasks were rectified and the area (MEParea) calculated using the same latency window for each task in each individual. The root mean square EMG (rmsEMG) was calculated within a 100-ms prestimulus window and the time to EMG onset determined. Traces were discarded from each patient's data if rmsEMG was greater than 1 standard deviation (SD) from the mean rmsEMG for each task or if the agonist EMG onset was less than 70 ms or more than 250 ms after the stimulus. Trials in which MEParea was beyond 2 SDs of the mean were also discarded from further analysis. The remaining trials were averaged to obtain a measure of average BB MEParea for the pronation task and for the flexion task in order to compute the ratio (SR) of the 2 (Gerachshenko et al. 2008). The difference between sham and c-tDCS SR was calculated (ΔSR = SRsham − SRc-tDCS). A positive ΔSR indicates selective BB activation was improved by c-tDCS. In healthy participants, EMG data were analyzed as described previously and above, ensuring comparable rmsEMG between flexion and pronation trials for each individual (Bradnam, Stinear, and Byblow 2010).
iMEP and SP Area
iMEP area (iMEPAREA) was determined by rectifying and averaging traces from the paretic BB. iMEPAREA was calculated in a window 20- to 60-ms poststimulus after the same duration of prestimulus EMGAREA was subtracted (Bradnam, Stinear, Lewis, et al. 2010). iSP in the nonparetic BB was determined from the rectified and averaged trace. The iSPAREA relative to the mean of the prestimulus rmsEMG was calculated in a window between 30- and 70-ms poststimulus (Giovannelli et al. 2009).
Image Processing
Image processing was carried out using FSL (FMRIB Software Library, Oxford) (Smith et al. 2004; Woolrich et al. 2009). Structural T1-weighted images were skull stripped using the brain extraction tool (BET) (Smith 2002) and used to define the site and size of the brain lesion (Table 1 and Fig. 2). Lesion volume (mm3) was determined by manually tracing lesion masks in FSL-view and calculating mask volume using FSL-stats. DWIs were skull stripped using BET, corrected for motion and eddy currents using FDT (FMRIB's Diffusion Toolbox) to compute diffusion tensors, and coregistered to the patients T1-weighted image using FLIRT (Smith 2002). ROIs for the left and right posterior limb of the internal capsules (PLICs) were constructed from the JHU DTI-based white-matter labels atlas (Wakana et al. 2007; Hua et al. 2008; Mori et al. 2008). PLIC ROIs and the MNI template brain were registered to each patient's T1 using FLIRT (Jenkinson and Smith 2001; Jenkinson et al. 2002). A mean fractional anisotropy (FA) value was calculated for each PLIC using the statistical function in FSL (Smith et al. 2004; Woolrich et al. 2009). An FA asymmetry index was calculated as FAAI = (FAC − FAI)/(FAC + FAI), where FAC = FA in PLIC of contralesional hemisphere and FAI = FA in PLIC of ipsilesional hemisphere, yielding a value between −1.0 and +1.0 for each participant. Zero indicates symmetrical FA in the PLICs and negative and positive values relate to reduced FA in unaffected and affected PLIC, respectively (Stinear et al. 2007). FAAI values were calculated for 11 patients (Table 1).
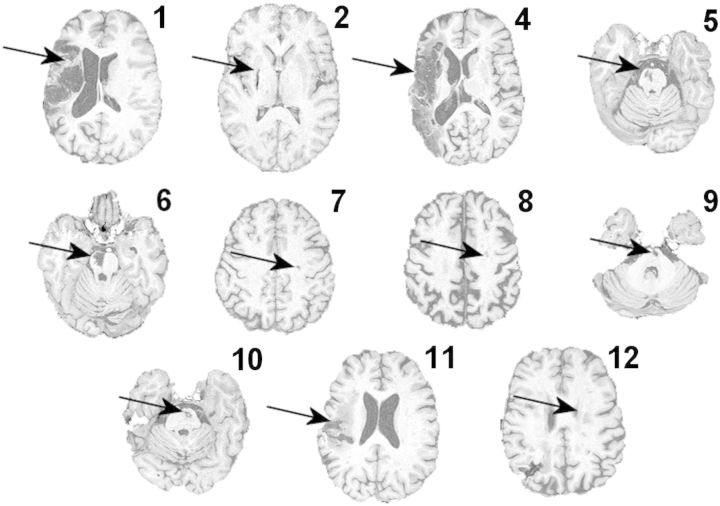
Figure 2.
Structural T1-weighted images in the axial plane are shown at the level of the lesion for each patient. Lesions are indicated by the arrows. Patient numbers correspond with Table 1. Note there is no T1-weighted image for patient 3.
Other Statistical Analyses
The SR was compared between patients after sham tDCS and healthy adults using a 2 sample 2-tailed t-test. To determine if SR was influenced by background muscle activity in patients, pr-stimulus rmsEMG and time to EMG burst onset were analyzed using a 2 Stimulation (c-tDCS, sham tDCS) × 2 Task (Flexion, Pronation) repeated measures analysis of variance (rmANOVA). As a manipulation check for suppressive effects of cM1 c-tDCS, nonparetic FDI MEP amplitude was measured before, and at 2 time points after, c-tDCS and sham tDCS. Postintervention FDI MEP amplitude was normalized to baseline for each patient. Normalized FDI MEP amplitude was analyzed using 2 Stimulation (c-tDCS, sham tDCS) × 2 Time (Post5, Post31) rmANOVA. FDI prestimulus rmsEMG was analyzed using a 2 Stimulation (c-tDCS, sham tDCS) × 3 Time (Pre, Post5, Post31) rmANOVA.
Regression analyses were performed to test the hypothesized relationship between the selective paretic BB activation (SR) and the effects of cM1 c-tDCS on selective paretic BB activation (ΔSR). Regressions were calculated separately for both SR and ΔSR with measures of time since stroke, NIHSS, ARAT, ASH, FM, FAAI, lesion size (log transformed), iMEPAREA, and iSPAREA.
SPSS software (SPSS Inc, Chicago, USA) was used for statistical analysis, and the level of significance was set to P < 0.05. rmANOVAs were tested for sphericity and corrected as required. Post hoc t-tests were used to explore main effects and interactions and were corrected for multiple comparisons (Rom 1990).
Results
There were no adverse events experienced by participants from the study procedures. Anatomical and DWI data were obtained for 11 of 12 patients with one patient unable to fit into the head coil.
Selectivity Ratio
Representative EMG traces for flexion and pronation tasks from 2 patients and 1 healthy adult are shown in Figure 3. Healthy participants completed the task without difficulty. The average burst onset times were 134 ± 8.9 and 122 ± 8.5 ms for flexion and pronation tasks, respectively. The average prestimulus rmsEMG values were 8 ± 2 and 7 ± 2 μV for flexion and pronation tasks, respectively. For patients, the range of number of trials retained for averaging in the c-tDCS session was 10–21 (flexion) and 10–19 (pronation) and for the Sham session 12–22 (flexion) and 10–18 (pronation), indicating that all subjects were eventually able to complete the required task. The average burst onset times for the c-tDCS session were 145 ± 8 (flexion) and 163 ± 10 ms (pronation) and for the Sham session 147 ± 12 (flexion) and 161 ± 11 ms (pronation), with no difference between Session or Task (all P > 0.07). The average prestimulus rmsEMG for the c-tDCS session was 15 ± 2 (flexion) and 13 ± 3 μV (pronation) and for the Sham session 16 ± 2 (flexion) and 15 ± 3 μV (pronation). There was no difference between Session and Task (all P > 0.16), indicating consistent prestimulus rmsEMG.
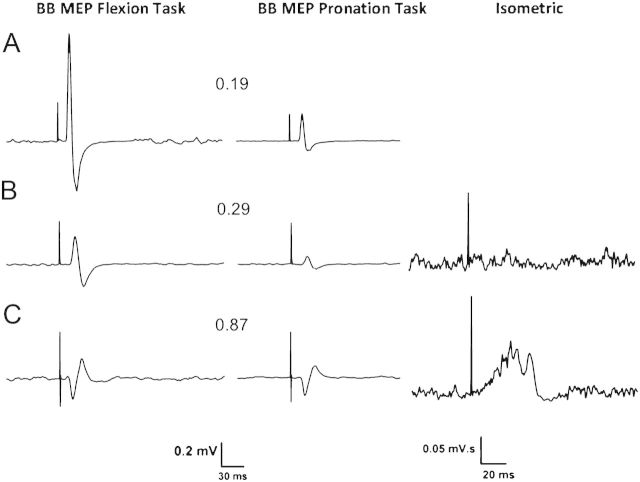
Figure 3.
Averaged paretic or nondominant BB MEPs from representative participants showing BB MEPs from the flexion and (left column), pronation tasks (middle column), and rectified BB iMEPs from the isometric task in patients (right column). Contralateral MEP traces are shown from (A) a healthy participant (#3, Table 2), (B) a mildly impaired patient (#9, Tables 1 and 3), and (C) a moderately impaired patient (#4). Ipsilateral (iMEP) traces are shown from a mildly impaired patient (#9) and a severely impaired patient (#1). The SR value is indicated between the BB MEP traces. Note the different calibration bars for MEPs and iMEPs.
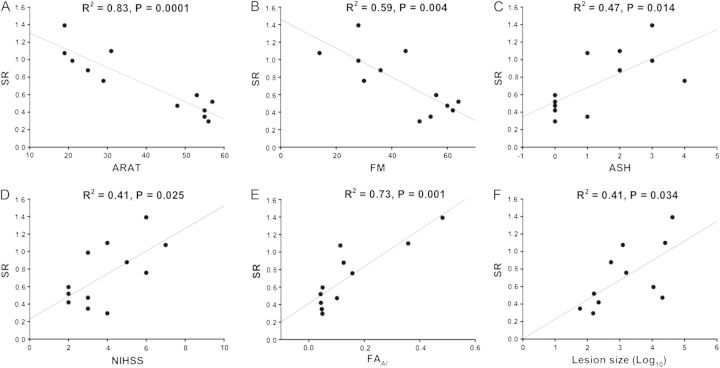
The SR reflects the ability to suppress BB prior to pronation, when it is an antagonist. SR data are presented in Table 2 for healthy controls and Table 3 for patients. As expected, SR was higher in patients than healthy adults (t11 = 4.38, P < 0.001) reflecting impaired BB suppression. SR correlated negatively with ARAT (R2 = 0.83, F1,11 = 47.81, P = 0.0001) and FM scores (R2 = 0.59, F1,11 = 14.37, P = 0.004). SR correlated positively with ASH (R2 = 0.47, F1,11 = 8.89, P = 0.014) and NIHSS (R2 = 0.41, F1,11 = 6.93, P = 0.025; Fig. 4A–D). SR correlated positively with FAAI (R2 = 0.73, F1,10 = 24.68, P = 0.001) and lesion size (R2 = 0.41, F1,10 = 6.21, P = 0.034; Fig. 4E,F). SR did not correlate with time since stroke (P > 0.1). Overall, higher SRs were associated with poorer clinical scores and a reduction in the integrity of ipsilesional white matter.
Table 2.
Healthy participants
| Stimulus intensity (%MSO) | BB MEP area (mV.ms) | SR | Handed | ||
| Flexion task | Pronation task | ||||
| Healthy adults | |||||
| 1 | 63 | 0.55 | 0.14 | 0.25 | 0.89 |
| 2 | 66 | 0.48 | 0.17 | 0.35 | 0.95 |
| 3 | 69 | 0.99 | 0.27 | 0.27 | 1.0 |
| 4 | 58 | 1.6 | 0.72 | 0.45 | 0.92 |
| 5 | 62 | 2.21 | 1.08 | 0.49 | 0.83 |
| 6 | 64 | 1.11 | 0.34 | 0.31 | 1.0 |
| 7 | 56 | 2.79 | 1.04 | 0.37 | 0.77 |
| 8 | 71 | 0.54 | 0.29 | 0.54 | 1.0 |
| 9 | 55 | 2.38 | 0.46 | 0.19 | 1.0 |
| 10 | 74 | 6.35 | 2.44 | 0.38 | 1.0 |
| 11 | 42 | 1.31 | 0.29 | 0.22 | 0.71 |
| 12 | 48 | 4.52 | 1.07 | 0.24 | 1.0 |
| Average | 60.7 ± 2.2 | 2.07 ± 0.5 | 0.69 ± 0.2 | 0.34 ± 0.03 | 0.93 ± 0.03 |
Note: Stimulus intensity, BB MEP area for each task, SRs, and handedness quotients are shown for healthy participants.
Table 3.
Stroke patients
| Sham tDCS | c-tDCS | ||||||||
| Stimulus intensity (%MSO) | BB MEP area (mV·ms) | SR | Stimulus intensity (%MSO) | BB MEP area (mV·ms) | SR | ΔSR | |||
| Flexion task | Pronation task | Flexion task | Pronation task | ||||||
| Patients | |||||||||
| 1 | 90 | 0.69 | 0.96 | 1.39 | 90 | 0.59 | 1.04 | 1.76 | −0.37 |
| 2 | 90 | 1.44 | 1.09 | 0.76 | 90 | 1.09 | 1.16 | 1.06 | −0.31 |
| 3 | 90 | 0.77 | 0.76 | 0.99 | 90 | 0.58 | 0.7 | 1.21 | −0.22 |
| 4 | 82 | 4.18 | 4.59 | 1.10 | 84 | 3.96 | 5.1 | 1.29 | −0.19 |
| 5 | 90 | 0.49 | 0.43 | 0.88 | 90 | 0.27 | 0.27 | 1.00 | −0.12 |
| 6 | 90 | 0.94 | 1.01 | 1.07 | 90 | 1.11 | 1.29 | 1.16 | −0.09 |
| 7 | 74 | 4.72 | 1.64 | 0.35 | 71 | 5.15 | 1.29 | 0.25 | 0.10 |
| 8 | 48 | 5.69 | 2.95 | 0.52 | 48 | 4.89 | 2.01 | 0.41 | 0.11 |
| 9 | 76 | 4.18 | 1.23 | 0.29 | 75 | 3.72 | 0.61 | 0.16 | 0.13 |
| 10 | 75 | 2.91 | 1.22 | 0.42 | 72 | 2.85 | 0.74 | 0.26 | 0.16 |
| 11 | 66 | 4.19 | 1.98 | 0.47 | 68 | 4.91 | 1.34 | 0.27 | 0.20 |
| 12 | 60 | 3.35 | 1.99 | 0.59 | 58 | 2.08 | 0.57 | 0.27 | 0.32 |
| Average | 77.6 ± 4.0 | 2.8 ± 0.5 | 1.7 ± 0.3 | 0.74 ± 0.1 | 77.2 ± 4.1 | 2.6 ± 0.5 | 1.3 ± 0.4 | 0.76 ± 0.2 | 0.02 ± 0.1 |
Note: Stimulus intensity, MEP area for each task, SR, and ΔSR for sham tDCS and c-tDCS are shown for each patient.
Figure 4.
Regressions with SR following sham tDCS. (A) SR and ARAT upper limb function score. Larger SRs were associated with lower ARAT scores, indicating lower paretic arm function. (B) SR and FM upper limb impairment score. Larger SRs were associated with low FM scores and greater upper limb impairment. (C) SR and ASH. Larger SRs were associated with greater spasticity in paretic BB. (D) SR and NIHSS. Larger SRs were associated with higher NIHSS scores, indicating greater upper limb impairment. (E) SR and FAAI. Larger SRs were associated with higher FAAI, indicating reduced corticospinal tract integrity from the ipsilesional hemisphere. (F) SR and lesion volume (log transformed). Larger SRs were associated with larger stroke lesions.
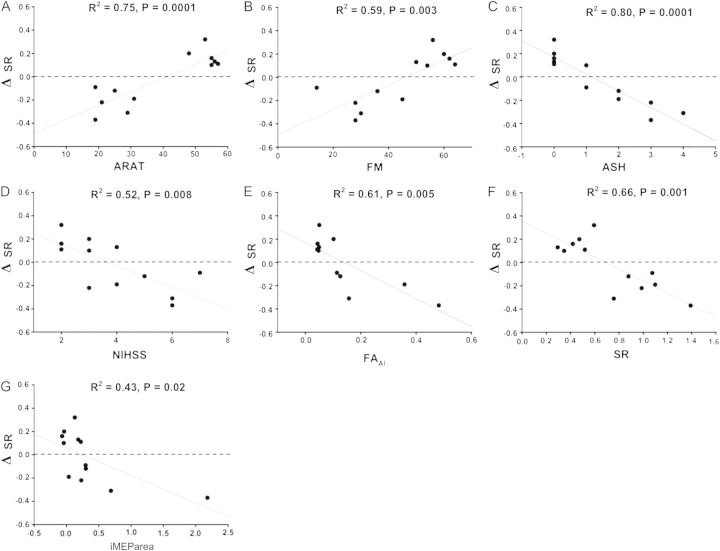
Effects of c-tDCS on SR (ΔSR)
The difference between SR measured after sham and after c-tDCS was calculated (ΔSR = SRsham − SRc-tDCS). As predicted, ΔSR was variable and ranged between −0.37 and 0.32 (Table 3). There was a positive correlation between ΔSR and ARAT (R2 = 0.75, F1,11 = 29.22, P = 0.0001) and between ΔSR and FM scores (R2 = 0.59, F1,11 = 15.72, P = 0.003; Fig. 5A,B). After c-tDCS, SR improved in patients with low ASH, and worsened in patients with ASH > 1, a negative correlation (R2 = 0.80, F1,11 = 61.65, P = 0.0001; Fig. 5C). There was a negative correlation between ΔSR and NIHSS (R2 = 0.52, F1,11 = 10.71, P = 0.008; Fig. 5D). Patients with worse clinical scores had negative ΔSR, indicating worsened control of paretic BB after suppression of cM1 with c-tDCS.
Figure 5.
Regressions with ΔSR. Positive ΔSR indicates improved selective muscle activation after contralesional c-tDCS and negative ΔSR indicates a worsening of selective muscle activation after contralesional c-tDCS. The dashed line represents no change, ΔSR = 0. (A) ΔSR and ARAT. SR improved for those with mildly impaired upper limb function worsened for those with ARAT scores < 40. (B) ΔSR and FM score. SR tended to improve for patients with mild upper limb impairment and worsen for those with FM < 50. (C) ΔSR and ASH. SR tended to improve for patients without elbow flexor spasticity and worsen for those with ASH > 1. (D) ΔSR and NIHSS. SR tended to worsen for patients with NIHSS of 5 or more. (E) ΔSR and FAAI. SR tended to improve for patients with good ipsilesional corticospinal tract integrity as indicated by FAAI < 0.11 but worsen for those with reduced ipsilesional corticospinal tract integrity. (F) ΔSR and SR. SR tended to improve in those with good initial selective muscle activation and worsen for those with poor initial selective muscle activation. (G) ΔSR and iMEParea. SR tended to improve for patients with small iMEPs in the paretic BB and worsen for those with large iMEPs. All other abbreviations as in text.
However, SR improved in patients with low FAAI and worsened in patients with high FAAI as indicated by a negative correlation (R2 = 0.61, F1,10 = 14.10, P = 0.005; Fig. 5E). Also, SR improved in patients with low baseline SR and worsened in patients with high baseline SR as indicated by the negative correlation (R2 = 0.66, F1,11 = 19.45, P = 0.001; Fig. 5F).
The excitability of the uncrossed corticomotor pathway from cM1 to paretic BB was examined by recording iMEPs. After c-tDCS, SR improved in patients with no iMEPs or small BB iMEPAREA and worsened in patients with larger BB iMEPAREA indicated by a negative correlation (R2 = 0.43, F1,11 = 7.46, P = 0.02; Fig. 5G). Overall, the ΔSR results indicate that clinical, structural, and neurophysiological measures can predict whether c-tDCS of cM1 will improve or degrade paretic upper limb control.
The iSP is indicative, at least in part, of transcallosal inhibition. There was no correlation between ΔSR and iSParea (P = 0.48). There was no relationship between ΔSR and time since stroke (P = 0.17). Similarly, there was no relationship between ΔSR and lesion volume (P = 0.34), indicating lesion size did not predict after effects of c-tDCS. Finally, neither lesion side nor handedness was associated with SR or ΔSR.
Nonparetic FDI MEPs
MEPs were recorded from nonparetic FDI to confirm that c-tDCS suppressed cM1 excitability. As expected, there was a main effect of stimulation (F1,11 = 9.22, P = 0.011) because MEPs were suppressed by c-tDCS in comparison to sham tDCS at 5- and 30-min poststimulation (P = 0.012 and P = 0.017, respectively). One sample t-tests indicated that after c-tDCS FDI MEP amplitude was reduced compared with baseline at 5 (−28 ± 0.8%, P = 0.004) and 30 (−35 ± 0.11%, P = 0.008) min poststimulation. FDI MEP amplitude was comparable prior to c-tDCS and sham stimulation (P = 0.29). EMG levels were consistent during MEP recording with no main effects or interactions for rmsEMG (all P > 0.12). Average rmsEMG values were pre: 11 ± 2 μV, post 1: 12 ± 1 μV, and post 2: 13 ± 1 μV. The results for the nonparetic FDI confirm cM1 excitability was suppressed by c-tDCS.
Discussion
In support of our hypothesis, suppressive tDCS of cM1 improved the control of the paretic proximal upper limb for mildly impaired patients and worsened control for moderate to severely impaired patients. Therefore, protocols for suppressing cM1 are not one size fits all but must be tailored to individual patients.
This is the first study to show that the effects of suppressing cM1 vary depending on the integrity of the white-matter tracts from the ipsilesional hemisphere innervating the paretic upper limb. Suppression of cM1 with c-tDCS worsened paretic upper limb control in patients who had greater impairment and worse damage to their ipsilesional white matter measured at the level of the PLIC. The relationship between ipsilesional white-matter disruption and motor impairment confirm earlier studies that examined paretic hand function after stroke (Ward et al. 2003; Jang et al. 2005; Stinear et al. 2007; Schaechter et al. 2009) and extend this relationship to the neurophysiological response in the paretic proximal upper limb, in response to contralesional c-tDCS. We speculate that significant disruption of ipsilesional motor pathways by stroke would result in greater excitability of the contralesional hemisphere as a compensatory response. In support, functional MRI studies of paretic hand function have found greater lateralization of cortical activity toward cM1 in patients with greater upper limb impairment (Cramer et al. 1997; Ward et al. 2006; Ward et al. 2007; Stinear et al. 2008). In turn, greater contralesional excitability would upregulate activity in the ipsilateral corticobulbospinal projections to the spinal cord, as measured by iMEPs. We found large iMEPs in the paretic BB of our severely impaired patients consistent with previous reports (Turton et al. 1996; Netz et al. 1997; Caramia et al. 2000; Trompetto et al. 2000; Alagona et al. 2001; Gerloff et al. 2006; Lewis and Perreault 2007), although to some extent the significant relationship with ΔSR was driven by the 2 most impaired patients. Although the effects of c-tDCS on direct ipsilateral projections to the spinal cord are inconclusive, the current findings indicate that suppression of cM1 may be contraindicated for patients with major disruption of the ipsilesional corticospinal tract, as this may result in downregulation of important compensatory activity.
Conversely, these results indicate that suppression of cM1 may be beneficial in patients with residual structural integrity of the ipsilesional hemisphere. These patients had less damage to ipsilesional motor pathways with lower FA asymmetry of the PLICs, better clinical scores, and more selective control of the paretic upper limb. cM1 may not therefore be required for compensation in these patients, and its ipsilateral projections may even interfere at the spinal level with the control of paretic proximal muscles by ipsilesional M1. The benefits of cM1 c-tDCS for paretic upper limb control in mildly impaired patients were unlikely to result from a reduction in transcallosal inhibition. This is because no correlation between changes in the SR following c-tDCS and the iSP was found. The iSP is considered, at least in part, to measure transcallosal inhibition across the corpus callosum (Chen 2004; Trompetto et al. 2004; Avanzino et al. 2007). In more severely affected patients, improvements in upper limb impairment have been associated with reduced transcallosal inhibition from the contralesional to the ipsilesional hemisphere (Harris-Love et al. 2011). However, there was no evidence for decreased transcallosal inhibition in the current study, perhaps because there was no repetitive motor training involved in the current study (Harris-Love et al. 2011). A more likely explanation is that c-tDCS suppressed ipsilateral corticomotor projections, thereby reducing interference with contralateral inputs at the spinal level for patients with mild upper limb impairment. Therefore, suppression of cM1 may be beneficial in patients with mild proximal upper limb weakness. This extends previous studies, which found that cM1 c-tDCS enhanced paretic hand function in mildly affected patients (Fregni et al. 2005; Boggio et al. 2007; Kim et al. 2010).
Although noninvasive brain stimulation may be used to promote balanced motor cortex excitability after stroke, there is still debate as to whether suppression of cM1 or facilitation of ipsilesional M1 is the more efficacious approach (Hummel and Cohen 2006; Hummel et al. 2008; Bolognini et al. 2009; Nowak et al. 2010). Differences in the contribution of cM1 to control of the paretic upper limb may explain why noninvasive brain stimulation has generally yielded mixed results in patients after stroke. For example, suppression of cM1 with repetitive TMS was found to degrade performance of a grip-lift task in stroke patients (Ackerley et al. 2010). Suppression of M1 using similar techniques can also impair ipsilateral upper limb function, motor learning, and skill retention in healthy adults (Chen et al. 1997; Carey et al. 2006; Bradnam, Stinear, and Byblow 2010). Future studies might well examine the effect of noninvasive brain stimulation on the control of distal and proximal muscles, in both the contralateral and the ipsilateral upper limb.
Neurophysiological and neuroimaging measures can be used to predict current functional status and functional recovery following stroke (Le Bihan et al. 2001; Stinear et al. 2007; Schaechter et al. 2009; Lindenberg et al. 2010; Radlinska et al. 2010; Zhu et al. 2010; Qiu et al. 2011) and may be useful to select patients who are suitable for specific protocols. In this study, measures of structural integrity of descending white-matter tracts predicted upper limb function, alongside clinical assessments. FA asymmetry measured in the PLICs predicted after effects of c-tDCS on SR, whereas lesion size did not. The finding that FA within the posterior limbs of the internal capsules can be used to predict upper limb function is in agreement with previous studies (Cramer et al. 2007; Stinear et al. 2007; Qiu et al. 2011; Riley et al. 2011) and reinforces the importance of the ipsilesional corticofugal pathways in stroke recovery. The present findings indicate FA asymmetry measures may also assist in selection of noninvasive brain stimulation protocols for individual stroke patients (Cramer 2010; Stinear 2010).
This study has several limitations that must be considered when interpreting the results. First, the stimulus intensity for tDCS cannot be individualized based on motor thresholds as for repetitive TMS (Priori et al. 2009) and may produce variable effects between individuals. Secondly, the cathode may hyperpolarize cortical areas adjacent to M1 (Nitsche et al. 2007). The dorsal premotor cortex may also have been modulated by c-tDCS (Boros et al. 2008). The contralesional premotor cortex is known to have a compensatory role in promoting upper limb function in more impaired patients (Johansen-Berg et al. 2002; Gerloff et al. 2006; Lotze et al. 2006; Ward et al. 2006; Bestmann et al. 2010). We cannot know if the decrement in paretic upper limb control in our moderate to severely affected patients was due to suppression of neurons within dorsal premotor cortex alongside those within M1. This may be problematic for selecting brain stimulation protocols to target the proximal paretic upper limb, as the representation of proximal muscles relative to distal muscles is greater in dorsal premotor cortex (Dum and Strick 1991). Further studies are needed to determine relative effects of “direct” dorsal premotor cortex tDCS with M1 tDCS on proximal paretic upper limb function. Third, the small number of participants may have influenced the neurophysiological results, which indicated that the effects of cM1 c-tDCS were mediated by ipsilateral rather than transcallosal projections. However, iMEP and iSP measures are variable and are not present in all healthy individuals or stroke patients (Chen et al. 2003; Talelli et al. 2006), making it difficult to precisely determine the pathways mediating effects on the ipsilateral side of the body. The effects of M1 tDCS on paretic arm control may be secondary to changes in transcallosal inhibition (Nowak et al. 2010). Further investigations are required to determine the relative effects of suppressive stimulation on ipsilateral corticomotor and transcallosal pathway excitability. Fourth, because the strength of stimulation was necessarily higher for patients than healthy control subjects, the extent of activation depth or spread between groups may have contributed in part to the difference in SR between groups. However, this would not affect ΔSR, the outcome measure of interest. A final limitation is the lack of outcome measures that specifically assess proximal paretic upper limb control. The ARAT was used in the current study, but primarily tests skills associated with using the hand and scores are not sensitive to improvements in proximal upper limb control (Lyle 1981). Development of a validated outcome measure specific to proximal upper limb function in stroke patients would improve future studies. Despite these limitations, this study has highlighted factors worthy of consideration before using noninvasive brain stimulation for rehabilitation after stroke.
There is unlikely to be a one size fits all treatment protocol when using noninvasive brain stimulation in stroke rehabilitation. We have shown that c-tDCS of cM1 may be beneficial for patients with mild impairment and contraindicated for patients with moderate to severe impairment. An important clinical aspect of the present study is that clinical measures of upper limb function and impairment, and spasticity in the paretic elbow flexors, may be useful for determining whether contralesional c-tDCS is contraindicated for an individual patient. Future experiments may identify how to individually prescribe tDCS as an adjuvant to therapy.
Funding
L.V.B. was supported by a University of Auckland Senior Health Research Scholarship.
Acknowledgments
The authors thank Suzanne Ackerley for performing the clinical assessments, Frederique Noten for assistance with data collection and preparation of figures, and Shailesh Kantak for insightful comments on the manuscript. The authors give special thanks to the patients for participating in the study and their families for supporting their participation. Conflict of Interest : None declared.
References
- Ackerley SJ, Stinear CM, Barber PA, Byblow WD. Combining theta burst stimulation with training after subcortical stroke. Stroke. 2010;41:1568–1572. doi: 10.1161/STROKEAHA.110.583278. [DOI] [PubMed] [Google Scholar]
- Alagona G, Delvaux V, Gerard P, De Pasqua V, Pennisi G, Delwaide PJ, Nicoletti F, Maertens de Noordhout A. Ipsilateral motor responses to focal transcranial magnetic stimulation in healthy subjects and acute-stroke patients. Stroke. 2001;32:1304–1309. doi: 10.1161/01.str.32.6.1304. [DOI] [PubMed] [Google Scholar]
- Avanzino L, Teo JT, Rothwell JC. Intracortical circuits modulate transcallosal inhibition in humans. J Physiol. 2007;583:99–114. doi: 10.1113/jphysiol.2007.134510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Swayne O, Blankenburg F, Ruff CC, Teo J, Weiskopf N, Driver J, Rothwell JC, Ward NS. The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS-fMRI. J Neurosci. 2010;30:11926–11937. doi: 10.1523/JNEUROSCI.5642-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25:123–129. [PubMed] [Google Scholar]
- Bohannon RW, Smith MB. Assessment of strength deficits in eight paretic upper extremity muscle groups of stroke patients with hemiplegia. Phys Ther. 1987;67:522–525. doi: 10.1093/ptj/67.4.522. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. J Neuroeng Rehabil. 2009;6:8. doi: 10.1186/1743-0003-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros K, Poreisz C, Munchau A, Paulus W, Nitsche MA. Premotor transcranial direct current stimulation (tDCS) affects primary motor excitability in humans. Eur J Neurosci. 2008;27:1292–1300. doi: 10.1111/j.1460-9568.2008.06090.x. [DOI] [PubMed] [Google Scholar]
- Bradnam LV, Stinear CM, Byblow WD. Theta burst stimulation of human primary motor cortex degrades selective muscle activation in the ipsilateral arm. J Neurophysiol. 2010;104:2594–2602. doi: 10.1152/jn.00365.2010. [DOI] [PubMed] [Google Scholar]
- Bradnam LV, Stinear CM, Lewis GN, Byblow WD. Task-dependent modulation of inputs to proximal upper limb following transcranial direct current stimulation of primary motor cortex. J Neurophysiol. 2010;103:2382–2389. doi: 10.1152/jn.01046.2009. [DOI] [PubMed] [Google Scholar]
- Brunnstrom S. Motor testing procedures in hemiplegia: based on sequential recovery stages. Phys Ther. 1966;46:357–375. doi: 10.1093/ptj/46.4.357. [DOI] [PubMed] [Google Scholar]
- Caramia MD, Palmieri MG, Giacomini P, Iani C, Dally L, Silvestrini M. Ipsilateral activation of the unaffected motor cortex in patients with hemiparetic stroke. Clin Neurophysiol. 2000;111:1990–1996. doi: 10.1016/s1388-2457(00)00430-2. [DOI] [PubMed] [Google Scholar]
- Carey JR, Fregni F, Pascual-Leone A. rTMS combined with motor learning training in healthy subjects. Restor Neurol Neurosci. 2006;24:191–199. [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154:1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Chen R, Gerloff C, Hallett M, Cohen LG. Involvement of the ipsilateral motor cortex in finger movements of different complexities. Ann Neurol. 1997;41:247–254. doi: 10.1002/ana.410410216. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Cramer SC. Stratifying patients with stroke in trials that target brain repair. Stroke. 2010;41:S114–S116. doi: 10.1161/STROKEAHA.110.595165. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, Kennedy DN, Finklestein SP, Rosen BR. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Parrish TB, Levy RM, Stebbins GT, Ruland SD, Lowry DW, Trouard TP, Squire SW, Weinand ME, Savage CR, et al. Predicting functional gains in a stroke trial. Stroke. 2007;38:2108–2114. doi: 10.1161/STROKEAHA.107.485631. [DOI] [PubMed] [Google Scholar]
- Dobkin BH. Clinical practice. Rehabilitation after stroke. N Engl J Med. 2005;352:1677–1684. doi: 10.1056/NEJMcp043511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJ, Lima MC, Rigonatti SP, Marcolin MA, Freedman SD, Nitsche MA, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16:1551–1555. doi: 10.1097/01.wnr.0000177010.44602.5e. [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Gerachshenko T, Rymer WZ, Stinear JW. Abnormal corticomotor excitability assessed in biceps brachii preceding pronator contraction post-stroke. Clin Neurophysiol. 2008;119:683–692. doi: 10.1016/j.clinph.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, Waldvogel D, Wittenberg GF, Ishii K, Cohen LG, et al. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129:791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- Giovannelli F, Borgheresi A, Balestrieri F, Zaccara G, Viggiano MP, Cincotta M, Ziemann U. Modulation of interhemispheric inhibition by volitional motor activity: an ipsilateral silent period study. J Physiol. 2009;587:5393–5410. doi: 10.1113/jphysiol.2009.175885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, Wang LE, Dafotakis M, Eickhoff SB, Fink GR. Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling. Neuroimage. 2010;50:233–242. doi: 10.1016/j.neuroimage.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Love ML, Morton SM, Perez MA, Cohen LG. Mechanisms of short-term training-induced reaching improvement in severely hemiparetic stroke patients: a TMS study. Neurorehabil Neural Repair. 2011;25:398–411. doi: 10.1177/1545968310395600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Lu CS, Chuang WL, Lin WY, Chen RS. Reversal of plasticity-like effects in the human motor cortex. J Physiol. 2010;588:3683–3693. doi: 10.1113/jphysiol.2010.191361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel FC, Celnik P, Pascual-Leone A, Frengi F, Byblow WD, Butefisch CM, Rothwell JC, Cohen L, Gerloff C. Controversy: noninvasive and invasive cortical stimulation show efficacy in treating stroke patients. Brain Stimul. 2008;1:370–382. doi: 10.1016/j.brs.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5:708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- Jang SH, Cho SH, Kim YH, Han BS, Byun WM, Son SM, Kim SH, Lee SJ. Diffusion anisotrophy in the early stages of stroke can predict motor outcome. Restor Neurol Neurosci. 2005;23:11–17. [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Lim JY, Kang EK, You DS, Oh MK, Oh BM, Paik NJ. Effect of transcranial direct current stimulation on motor recovery in patients with subacute stroke. Am J Phys Med Rehabil. 2010;89:879–886. doi: 10.1097/PHM.0b013e3181f70aa7. [DOI] [PubMed] [Google Scholar]
- Kuypers HG, Brinkman J. Precentral projections to different parts of the spinal intermediate zone in therhesus monkey. Brain Res. 1970;24:29–48. doi: 10.1016/0006-8993(70)90272-6. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Perreault EJ. Side of lesion influences bilateral activation in chronic, post-stroke hemiparesis. Clin Neurophysiol. 2007;118:2050–2062. doi: 10.1016/j.clinph.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010;74:280–287. doi: 10.1212/WNL.0b013e3181ccc6d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci. 2006;26:6096–6102. doi: 10.1523/JNEUROSCI.4564-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4:483–492. doi: 10.1097/00004356-198112000-00001. [DOI] [PubMed] [Google Scholar]
- McCambridge AB, Bradnam LV, Stinear CM, Byblow WD. Cathodal transcranial direct current stimulation of the primary motor cortex improves selective muscle activation in the ipsilateral arm. J Neurophysiol. 2011;105:2937–2942. doi: 10.1152/jn.00171.2011. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Netz J, Lammers T, Homberg V. Reorganization of motor output in the non-affected hemisphere after stroke. Brain. 1997;120(Pt 9):1579–1586. doi: 10.1093/brain/120.9.1579. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Doemkes S, Karakose T, Antal A, Liebetanz D, Lang N, Tergau F, Paulus W. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2007;97:3109–3117. doi: 10.1152/jn.01312.2006. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation—technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255–276. doi: 10.1016/s1567-424x(09)70230-2. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, et al. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568:291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak DA, Bosl K, Podubecka J, Carey JR. Noninvasive brain stimulation and motor recovery after stroke. Restor Neurol Neurosci. 2010;28:531–544. doi: 10.3233/RNN-2010-0552. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. 2009;23:641–656. doi: 10.1177/1545968309336661. [DOI] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009;2:241–245. doi: 10.1016/j.brs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Qiu M, Darling WG, Morecraft RJ, Ni CC, Rajendra J, Butler AJ. White matter integrity is a stronger predictor of motor function than BOLD response in patients with stroke. Neurorehabil Neural Repair. 2011;25:275–284. doi: 10.1177/1545968310389183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radlinska B, Ghinani S, Leppert IR, Minuk J, Pike GB, Thiel A. Diffusion tensor imaging, permanent pyramidal tract damage, and outcome in subcortical stroke. Neurology. 2010;75:1048–1054. doi: 10.1212/WNL.0b013e3181f39aa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JD, Le V, Der-Yeghiaian L, See J, Newton JM, Ward NS, Cramer SC. Anatomy of stroke injury predicts gains from therapy. Stroke. 2011;42:421–426. doi: 10.1161/STROKEAHA.110.599340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rom DM. A sequentially rejective test procedure based on a modified Bonferroni inequality. Biometrika. 1990;77:663–665. [Google Scholar]
- Schaechter JD, Fricker ZP, Perdue KL, Helmer KG, Vangel MG, Greve DN, Makris N. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp. 2009;30:3461–3474. doi: 10.1002/hbm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Renga V, Nair D. Transcranial direct current stimulation in stroke recovery. Arch Neurol. 2008;65:1571–1576. doi: 10.1001/archneur.65.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, Rossini PM. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125:1896–1907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol. 2010;9:1228–1232. doi: 10.1016/S1474-4422(10)70247-7. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Coxon JP, Fleming MK, Byblow WD. Priming the motor system enhances the effects of upper limb therapy in chronic stroke. Brain. 2008;131:1381–1390. doi: 10.1093/brain/awn051. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Swayne OB, Rothwell JC, Ward NS, Greenwood RJ. Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cereb Cortex. 2008;18:1909–1922. doi: 10.1093/cercor/bhm218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talelli P, Greenwood RJ, Rothwell JC. Arm function after stroke: neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin Neurophysiol. 2006;117:1641–1659. doi: 10.1016/j.clinph.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Talelli P, Greenwood RJ, Rothwell JC. Exploring Theta Burst Stimulation as an intervention to improve motor recovery in chronic stroke. Clin Neurophysiol. 2007;118:333–342. doi: 10.1016/j.clinph.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Pasqualetti P, Filippi M, Rossini PM. Follow-up of interhemispheric differences of motor evoked potentials from the ‘affected’ and ‘unaffected’ hemispheres in human stroke. Brain Res. 1998;803:1–8. doi: 10.1016/s0006-8993(98)00505-8. [DOI] [PubMed] [Google Scholar]
- Trompetto C, Assini A, Buccolieri A, Marchese R, Abbruzzese G. Motor recovery following stroke: a transcranial magnetic stimulation study. Clin Neurophysiol. 2000;111:1860–1867. doi: 10.1016/s1388-2457(00)00419-3. [DOI] [PubMed] [Google Scholar]
- Trompetto C, Bove M, Marinelli L, Avanzino L, Buccolieri A, Abbruzzese G. Suppression of the transcallosal motor output: a transcranial magnetic stimulation study in healthy subjects. Exp Brain Res. 2004;158:133–140. doi: 10.1007/s00221-004-1881-6. [DOI] [PubMed] [Google Scholar]
- Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101:316–328. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003;126:1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Frackowiak RS, Thompson AJ, Greenwood RJ, Rothwell JC. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur J Neurosci. 2007;25:1865–1873. doi: 10.1111/j.1460-9568.2007.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, Rothwell JC, Frackowiak RS. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129:809–819. doi: 10.1093/brain/awl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45:S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke. 2010;41:910–915. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]