Abstract
People show consistent differences in their cognitive and emotional responses to environmental cues, manifesting, for example, as variability in social reward processing and novelty-seeking behavior. However, the neurobiological foundation of human temperament and personality is poorly understood. A likely hypothesis is that personality traits rely on the integrity and function of distributed neurocircuitry. In this diffusion tensor imaging (DTI) study, this hypothesis was tested by examining the associations between reward dependence (RD) and novelty seeking (NS), as measured by Cloninger’s Temperament and Character Inventory, and fractional anisotropy (FA) and mean diffusivity (MD) as DTI-derived indices of white matter (WM) microstructure across the brain. The results supported the hypothesis. RD was associated with WM architecture coherence as indicated by a negative correlation between RD and FA in frontally distributed areas including pathways connecting important constituents of reward-related neurocircuitry. The associations between RD and FA could not be explained by age, sex, alcohol consumption, or trait anxiety. In contrast, no effects were observed for NS. These findings support the theory that WM fiber tract properties modulate individual differences in social reward processing.
Keywords: addiction, diffusion tensor imaging, neuroimaging, personality, Temperament and Character Inventory
Introduction
Structural properties of brain white matter (WM) fiber bundles facilitate and constrain the functional efficiency of specialized cortical circuits and modulate cognitive and emotional functions at the core of human personality. The multilevel and cumulative nature of human personality suggest that the integrity of widespread WM pathways is of importance. Recent methodological advances in structural neuroimaging have prompted a boost in research investigating the structural correlates of individual differences in personality traits (O'Gorman et al. 2006; Cohen et al. 2009; Westlye et al. 2011). However, since only a few and mostly relatively small-scale studies have investigated the relationships between individual differences in personality traits and measures of WM connectivity (Volpe et al. 2008; Kim and Whalen 2009; Camara et al. 2010; Jung et al. 2010; Takeuchi et al. 2010; Westlye et al. 2011; Xu and Potenza 2011; Xu et al. 2011), these putative associations have remained elusive.
Diffusion tensor imaging (DTI) is sensitive to direction and degree of water displacement in biological tissues (Beaulieu 2002; Le Bihan 2003). Water diffusion in brain parenchyma is restricted by, for example, cytoskeletal axonal elements including plasma membrane, microtubules, and myelin sheaths (Black and Baas 1989; Brady 1991; Beaulieu 2002), and DTI thus represents an imaging method putatively highly sensitive to the microstructural architecture of the human brain WM.
Documenting associations between WM connectivity and variability in reward processing and novelty seeking (NS) behavior might provide a window into the neural mechanisms underlying an array of normal social behaviors but also increase our understanding of the extremes along the behavioral continuum, including manifestations of drug addiction, pathological gambling, and risk-taking behavior.
One previous study has explored the associations between NS and reward dependence (RD), as measured by the Temperament and Character Inventory (TCI) (Cloninger et al. 1993) and DTI-derived measures of structural connectivity. Using probabilistic tractography, Cohen et al. (2009) linked individual differences in NS and RD to the structural connectivity profile of two different corticostriatal networks. The amount of projections from the hippocampus and amygdala to the striatum correlated with NS, whereas the relative connectivity strength between the striatum and prefrontal regions was associated with RD. While this study provided novel and important results, the sample size was small (n = 20), and large-scale studies with more statistical power are needed.
Since personality characteristics are highly multidimensional comprising a plethora of social, cognitive, emotional, and behavioral characteristics, it is likely that various cerebral circuits mediate the interindividual variability in these traits. The main aim of this study was to test the relationships between NS and RD as assessed by TCI (Cloninger et al. 1993) and WM integrity in a large sample comprising 263 healthy adults. Specifically, we tested for associations between NS and RD, respectively, and DTI-derived indices of WM microstructural properties including fractional anisotropy (FA) (Pierpaoli and Basser 1996) and mean diffusivity (MD) by means of tract-based spatial statistics (TBSS) (Smith et al. 2006).
Whereas NS, comprising dimensions of impulsiveness, exploratory excitability, curiosity, and disorderliness represent the personality trait most widely linked to the dopamine (DA) system (Cloninger et al. 1993; Noble et al. 1998; Hansenne et al. 2002; Zald et al. 2008; Bodi et al. 2009), less is known about the neural basis of social reward dependence, which reflects the propensity of an individual to react strongly to reinforcements and maintain behavior previously associated with rewards or relief of punishment. Recent neuroimaging studies including magnetic resonance imaging (MRI), functional MRI (fMRI), DTI, and positron emission tomography suggest that brain systems subserving the processing of social reward information includes ventral and dorsal striatum and pre- and orbitofrontal cortical regions (King-Casas et al. 2005; Izuma et al. 2008; Schreckenberger et al. 2008; Cohen et al. 2009; Gardini et al. 2009; Lebreton et al. 2009; Van Schuerbeek et al. 2010). In addition, the intrinsic social features of RD also suggest involvement of limbic and temporal areas subserving various aspects of social behavior and cognition (Myers 1972; Rankin et al. 2006; Zahn et al. 2007). Thus, whereas multiple cerebral circuits are likely to be associated with interindividual variability in NS and RD, we hypothesized strongest and most reliable relationships in networks connecting frontal, striatal, temporal, and limbic regions of the brain.
Materials and Methods
Participants
The sample was drawn from an ongoing longitudinal research project (Fjell et al. 2008; Westlye et al. 2009) coordinated by the Center for the Study of Human Cognition, Department of Psychology, University of Oslo, Norway. Volunteers were recruited by newspaper advertisements and underwent a health screening interview before enrollment to ensure that the study sample represented a healthy population. Participants were required to be right-handed native Norwegian speakers older than 20 years, normal or corrected to normal vision and hearing, and be free of neurological injuries or diseases known to affect nervous system functioning, including previous symptoms of brain infarct or stroke, neurodegenerative disorders, and traumatic brain injury with subsequent loss of consciousness or amnesia. Individuals were excluded if they 1) reported any previous or current psychiatric diagnosis or 2) had received any psychological or pharmacological treatment for psychiatric disease within the last 2 years. The health screening interview was administered by phone at enrollment and repeated at the time of the first assessment. In addition, all eligible participants were assessed for symptoms of depression using the Beck Depression Inventory (BDI) (Beck et al. 1987), and participants scoring above 16 (aggregate consistent with a mild depression) were excluded. Self-reported weekly alcohol consumption (in standard units) was recorded and used to test and control for effects of alcohol use on the relationships between temperament traits and DTI.
MRI scans were examined by a neuroradiologist and deemed free of significant anomalies. One participant was excluded based on the radiological evaluation. Complete data sets including DTI and TCI were available from 263 participants (150 females) ranging 20–85 years of age (mean: 50 years, standard deviation [SD] = 17.3 years). All participants scored >26 on Mini Mental State Examination (Folstein et al. 1975) indicating a nondemented sample. Mean full-scale IQ (FIQ) as measured by Wechsler Abbreviated Scale of Intelligence (Wechsler 1999) was 114.7 (range: 92–145, SD = 8.8). The study was approved by the Regional Ethical Committee of Southern Norway (REK-Sør) and conducted in accordance with the Helsinki Declaration. Written informed consent was obtained from all participants prior to the examinations.
Temperament and Character Inventory
TCI consists of 240 items comprising 7 dimensions of personality, thereof 4 temperament scales (NS, Harm Avoidance, Reward Dependence, and Persistence), 3 character scales (Self-directedness, Cooperativeness, and Self-transcendence), and 25 subscales. The items are rated with a dichotomous “true” or “false” scale. We have recently reported associations between the anxiety-related temperament trait harm avoidance and DTI measures (Westlye et al. 2011), and now we focus on RD and NS, assumed to be stable temperamental traits with high heritability (Cloninger 1987). RD describes the tendency to react and depend strongly or weakly to reinforcements, and the maintenance of behavior previously associated with rewards or relief of punishment. High RD individuals tend to be eager to help and please others, persistent, sympathetic, sentimental, sensitive to praise, and social cues. Low RD individuals are described as socially detached, practical, tough minded, emotionally cool, and independent. NS reflect the intensity and patterns in the responses to novelty and cues for reward and are manifested as exploratory activity in pursuit of potential rewards. Whereas novelty seekers tend to be impulsive, exploratory, curious, easily bored, disorderly, and quick tempered, low NS individuals are typically slow tempered, reflective, reserved, loyal, systematic, and orderly (Cloninger 1986, 1987).
MRI Acquisition
MRI data were collected using a 12-channel head coil on a 1.5-T Siemens Avanto scanner (Siemens Medical Solutions, Erlangen, Germany). For diffusion-weighted imaging, a single-shot twice-refocused spin-echo echo planar imaging pulse sequence with 30 diffusion sensitized gradient directions and the following parameters was used: repetition time/echo time = 8200 ms/82 ms, b value = 700 s/mm2, voxel size = 2.0 × 2.0 × 2.0 mm, and 64 axial slices. The sequence was repeated in 2 successive runs with 10 b = 0 and 30 diffusion-weighted images collected per run. Acquisition time was 11 min 21 s.
DTI Analysis
Image analyses and tensor calculations were performed using FSL (http://www.fmrib.ox.ac.uk/fsl/index.html) (Smith et al. 2004; Woolrich et al. 2009). First, each concatenated volume was affine registered to the first T2-weighted b = 0 volume using FLIRT (Jenkinson and Smith 2001) correcting for motion between scans and residual eddy-current distortions present in the diffusion-weighted images. In order to preserve the orientational information after motion correction, we reoriented each volume’s B matrix by applying the corresponding transformation matrix from the motion correction procedure. After removal of nonbrain tissue (Smith 2002), FA, eigenvector, and eigenvalue maps were computed. MD was defined as the mean of the 3 eigenvalues ([λ1 + λ2 + λ3]/3) and radial diffusivity as the mean of the second and third eigenvalue ([λ2 + λ3]/2). Axial diffusivity (AD) was defined as the largest eigenvalues (λ1). It should be noted that the nomenclature (“mean,” “radial,” and “axial” diffusivity) pertains to the eigenvalues of the diffusion tensor and not necessarily to the underlying brain tissue (Wheeler-Kingshott and Cercignani 2009). Next, all individuals’ FA volumes were skeletonized and transformed into a common space as employed in TBSS (Smith et al. 2006, 2007). Briefly, all volumes were nonlinearly warped to the FMRIB58_FA template by use of local deformation procedures performed by FNIRT (Andersson et al. 2007a, 2007b), a nonlinear registration toolkit using a b-spline representation of the registration warp field (Rueckert et al. 1999). The common template used in the present study is a high-resolution average of 58 FA volumes from healthy male and female subjects aged 20–50 years. Although we previously have demonstrated excellent native-to-standard warping across subjects in a life span sample (Westlye et al. 2010), all warped FA volumes were inspected for accuracy. Next, a mean FA volume of all subjects was generated and thinned to create a mean FA skeleton representing the centers of all common tracts. We thresholded and binarized the mean skeleton at FA > 0.2 to reduce the likelihood of partial voluming at the boundaries between tissue classes, yielding a mask of 127 694 WM voxels.
Individual FA values were warped onto this mean skeleton mask by searching perpendicular from the skeleton for maximum FA. Using maximum FA from the center of the tracts further minimizes partial voluming (Smith et al. 2006). The resulting tract invariant skeletons for each participant were fed into voxelwise permutation-based cross-subject statistics. Similar warping and analyses were employed on AD, MD, and radial diffusivity data, yielding AD, MD, and radial diffusivity skeletons sampled from voxels with FA > 0.2.
Statistical Analysis
The relationships between NS, RD, harm avoidance and FIQ, BDI, alcohol consumption, and age were tested using Pearson correlations. Main effects of sex on the various measures were tested using independent samples t-tests.
Voxelwise analyses of DTI data were carried out using nonparametric permutation-based inference (Nichols and Holmes 2002) as implemented in the randomize tool in FSL. Linear effects of NS and RD on FA and MD, respectively, were tested with general linear models (GLMs) allowing age and sex to covary. Threshold-free cluster enhancement (TFCE) (Smith and Nichols 2009) was used for statistical inference. Five thousand permutations were performed for each contrast. Statistical P value maps were thresholded at P < 0.0125, corrected for multiple comparisons across space using resampling, and further adjusted according to a Bonferroni correction with a factor of 4 (NS, RD × FA, MD).
In order to estimate statistically unique effect sizes of the various covariates, we performed linear regressions with individual mean FA values from significant voxels generated in the voxelwise analyses as dependent variable, temperament trait (NS or RD), age, and sex as covariates. Diffusivity along the principal axis of the tensor (AD) and the mean along the 2 minor axes (radial diffusivity) provide biologically meaningful and complementary measures of the microstructural tissue architecture. Thus, in order to explore the underlying sources of the effects on FA/MD, we tested the association between temperament traits and mean axial/radial diffusivity in voxels with observed statistically significant effects on FA and/or MD.
Since associations between RD, NS, and WM microstructure may be modulated by secondary variables including substance abuse, we included weekly alcohol consumption as an additional covariate in the above described regressions. The difference in percent explained variance (Δ% variance) when including alcohol in the model was estimated. In addition, since temperament traits are related and we recently published relations between harm avoidance and DTI parameters in the same sample (Westlye et al. 2011), additional analyses including harm avoidance as a covariate were performed in order to estimate the potential influence of this factor on the associations between RD/NS and DTI.
Age is a powerful predictor of WM microstructure (Westlye et al. 2010). In order to rule out that the main results were primarily driven by the oldest subjects in which the aging-related processes are more prominent, we split the main sample at the median age (53 years) and reanalyzed data from the youngest half (131 individuals, 76 females). Mean (SD) age of the youngest subsample was 35.6 (10.7) years, and mean (SD) scores on NS were 0.52 (0.16) and RD 0.62 (0.16), compared with 0.48 (0.16) and 0.63 (0.17), respectively, in the total sample. Age was still included as a covariate.
Results
TCI Scales, Age, Sex, IQ, and BDI
Table 1 summarizes the sample characteristics of NS and RD scores from TCI, age, FIQ, alcohol consumption, and BDI per decade and in total. The TCI scores in the present study are similar to those obtained in a previous study of 300 adults (Cloninger et al. 1993). Average TCI scores were 0.63 ± 0.17 for RD and 0.48 ± 0.16 for NS. Comparisons between genders revealed that females (0.67 ± 0.15) scored higher than males (0.57 ± 0.17, t = 4.63, P < 0.001) on RD. There was a trend toward a larger alcohol consumption in males (P = 0.052), whereas there was no differences between genders on NS, age, BDI, or FIQ.
Table 1.
Sample descriptive for age groups and the total sample
| Age group (years) | N | Age mean years (SD) | Females n (%) | RD mean (SD) | NS mean (SD) | Education mean (SD) | MMSE mean (SD) | FIQ mean (SD) | BDI mean (SD) | Alcohol mean (SD) |
| 20–29 | 48 | 23.9 ± 2.5 | 25 ± 52 | 0.69 ± 0.13 | 0.57 ± 0.15 | 15.5 ± 1.9a | n.a. | 112.9 ± 7.0 | 3.5 ± 3.2 | 4.2 ± 4.1 |
| 30–39 | 32 | 34.8 ± 2.8 | 20 ± 63 | 0.66 ± 0.16 | 0.48 ± 0.16 | 17.2 ± 2.4 | 29.4 ± 0.7 | 115.6 ± 8.2 | 3.7 ± 3.7 | 3.5 ± 3.7 |
| 40–49 | 32 | 45.1 ± 3.1 | 20 ± 63 | 0.61 ± 0.18 | 0.50 ± 0.18 | 15.4 ± 2.1 | 29.4 ± 0.6 | 115.6 ± 7.4 | 3.6 ± 4.2 | 3.7 ± 2.6 |
| 50–59 | 67 | 54.2 ± 2.7 | 39 ± 58 | 0.60 ± 0.18 | 0.49 ± 0.14 | 15.3 ± 2.2 | 29.2 ± 0.8 | 113.5 ± 7.2 | 4.1 ± 3.2 | 5.3 ± 3.8 |
| 60–69 | 46 | 64.1 ± 2.8 | 28 ± 61 | 0.61 ± 0.18 | 0.48 ± 0.14 | 16.3 ± 3.4 | 29.2 ± 0.7 | 113.7 ± 10.8 | 4.7 ± 3.9 | 4.7 ± 4.8 |
| 70–79 | 27 | 72.7 ± 2.4 | 14 ± 52 | 0.60 ± 0.12 | 0.41 ± 0.11 | 15.7 ± 3.1 | 28.8 ± 1.2 | 117.7 ± 11.0 | 5.9 ± 4.3 | 6.1 ± 5.9 |
| 80–89 | 11 | 81.9 ± 1.7 | 4 ± 36 | 0.55 ± 0.16 | 0.27 ± 0.09 | 15.1 ± 2.5 | 28.5 ± 0.8 | 121.6 ± 11.7 | 6.9 ± 4.4 | 5.2 ± 3.7 |
| Total | 263 | 50.0 ± 17.3 | 150 ± 57 | 0.63 ± 0.17 | 0.48 ± 0.16 | 15.7 ± 2.6 | 29.2 ± 0.8b | 114.7 ± 8.8 | 4.4 ± 3.8c | 4.7 ± 4.2d |
Note: RD and NS are TCI mean scale scores between 0 (true) and 1 (false). MMSE: Mini Mental Stale Examination. n.a., not applicable.
Many subjects in the 20–30 years group were still attending college or university at the time of assessment. Completed years of education at the time of assessment are used in the present study.
MMSE not available for participants below 30 years of age. In total, MMSE scores were available for 210 of 215 subjects above 30 years of age.
BDI scores were available for 260 participants.
Weekly alcohol consumption (in standard units) was available for 253 participants.
Relations between the temperament traits and subject characteristics were investigated with Pearson’s correlations and are shown in Table 2. RD was moderately positively correlated with NS (r = 0.17, P < 0.01) and negatively with age (r = −0.21, P < 0.001). NS correlated negatively with age (r = −0.33, P < 0.001), harm avoidance (r = −0.25, P < 0.01), and BDI (r = −0.16, P < 0.01) and positively with alcohol consumption (r = 0.21, P < 0.001).
Table 2.
Correlations between reward dependency, NS, age, BDI, trait anxiety, FIQ, and alcohol consumption
| RD | NS | |
| Age | −0.21*** | −0.33*** |
| BDIa | −0.08 | −0.16** |
| FIQ | −0.01 | −0.08 |
| RD | 1 | 0.17** |
| NS | 0.17** | 1 |
| HA | 0.09 | −0.25*** |
| Alcoholb | −0.06 | 0.21*** |
Note: RD, reward dependence; HA, harm avoidance.
Based on 260 participants.
Based on 253 participants.
**P < 0.01; ***P < 0.001.
Relationships between Temperament Traits and WM Microstructure
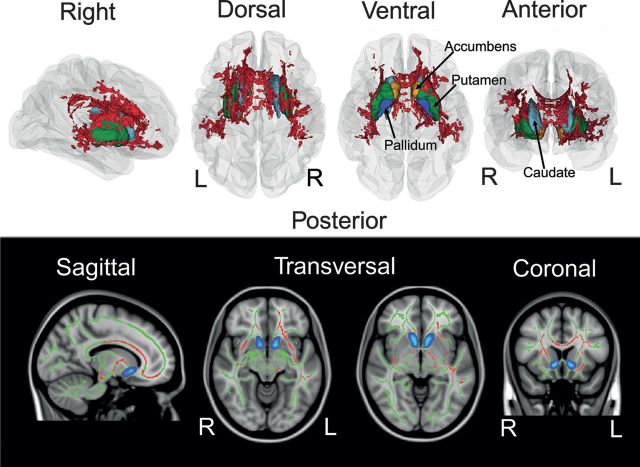
Relations between NS and RD and WM indices were investigated voxelwise across the TBSS skeleton using GLMs allowing age and sex to covary. Figure 1 maps the spatial distribution of significant (P < 0.0125, corrected) voxels showing negative linear effects of RD on FA across the skeleton, indicating lower FA with higher RD in 8.3% of the skeleton voxels. Post hoc regression revealed that RD accounted for 10.2% of the variance in mean FA across these voxels (t = −5.44). No significant association was found between RD and MD. In the same set of voxels, we found that age accounted for 33.3% (t = −11.36) and sex for 1.7% (t = 2.10) of the variance in FA. No significant association was found between NS and DTI.
Figure 1.
Reward dependence and WM microstructure. The spatial distribution of voxels showing significant relationships between reward dependence (RD) and FA. Linear effects of RD were tested voxelwise with GLMs while covarying for age and sex. Significant effects (P < 0.0125, fully corrected for multiple comparisons across space and Bonferroni adjusted) are shown as 3D renderings in right, dorsal, ventral, and anterior view on a reconstructed glass cerebrum. Red illustrates voxels showing negative associations with RD and FA (white panel). Striatal structures are illustrated for anatomical reference. The lower (black) panel depicts the same effects superimposed on sagittal, transversal, and coronal slices of a Montreal Neurological Institute (MNI) template brain. Red color indicates significant negative associations between RD and FA. Green areas are the nonsignificant remains of the skeleton. Nucleus accumbens is shown in green for reference. Briefly, associations between RD and FA are predominantly expressed in anterior regions and in the WM surrounding the corpus striatum central to reward processing. Portrayed sections are sagittal (x-coordinate, 103), transversal (z-coordinate, 62 and 76), and coronal (y-coordinate, 142) referring to the MNI coordinate (millimeter) system. R indicates right; L, left.
Post hoc analyses to untangle the diffusivity patterns accounting for the association between RD and FA revealed a positive association between RD and radial diffusivity (t = 4.23, P < 0.001), indicating increased diffusivity perpendicular to fiber orientation with higher RD. In contrast, AD was not related to RD (t = 0.28, P = 0.78).
Linear regressions exploring possible modulating effects of alcohol consumption and harm avoidance on the relationship between RD and mean FA across the voxels showing main effects of RD were investigated. Harm avoidance was negatively related to mean FA in the extracted voxels (t = −2.78, p = 0.006). Importantly, however, including harm avoidance in the statistical model slightly increased the amount of variance of FA explained by RD from 10.2% to 10.3%. Alcohol was not associated with FA. Adding alcohol as a covariate in the statistical model increased percentage variance of FA explained by RD by 0.7% from 10.3% to 11%. Thus, covarying for harm avoidance and weekly alcohol consumption had minimal influence on the associations between RD and FA.
Median Split Analysis
In order to rule out the possibility that aging-related processes disproportionally influenced the association between RD and FA, we performed additional analysis on the youngest cohort by splitting the total sample at median age. TBSS revealed widespread associations (P < 0.05, corrected for multiple comparisons across space) between RD and FA, particularly in anterior brain regions closely corresponding to the effect sites observed in the total sample. Effects are typically pronounced in WM wrapping the corpus striatum and limbic structures central to reward processing and social cognition.
Discussion
Whereas neuroscientists have begun to use DTI to examining the structural correlates of various psychiatric and neurological disorders (Taylor et al. 2004), few studies have examined the structural underpinnings of normal variations in personality characteristics using DTI. The present study provides a link between normal variations in social reward dependency and WM microstructure in a large healthy sample. Specifically, we found that increased RD was associated with decreased FA primarily in anterior regions, and these effects were generally independent of age, sex, and alcohol consumption. Furthermore, we observed that the associations with FA were mainly explained by radial diffusivity, suggesting that structural properties primarily providing hindrance of diffusion perpendicular to the primary axis of the diffusion tensor are driving the effects. No associations were found between NS and DTI indices. The implications of these novel findings will be discussed below.
Cohen et al. (2009) demonstrated an intriguing link between personality characteristics and the WM connectivity profiles of 2 different corticostriatal networks. The relative amount of structural projections reaching the striatum when seeding probabilistic tractography from the hippocampus and amygdala correlated with NS, whereas the same relative measure of connectivity strength between the striatum and prefrontal regions was associated with RD. Cohen et al. (2009) found no associations between the personality characteristics and FA in the connecting pathways, which suggest that the observed relations might be explained by the relative volume of the various striatal subdivisions rather than the strength of the axonal pathways. In contrast to the Cohen et al. (2009) study, our findings strongly suggest that RD is linked to WM microstructure. The discrepancy between studies is likely due to a major difference in statistical power related to sample size. To our knowledge, this is the first large-scale investigation of associations between variability in RD and NS and DTI-derived indices of WM integrity. Associations between FA and RD were profound in the anterior parts of the corpus callosum connecting the left and right frontal hemispheres, in fibers running within the internal capsule connecting brain stem and thalamic nuclei with the frontal lobes, and in the corticostriatal fiber system of the external capsule (Wakana et al. 2004; Schmahmann and Pandya 2006; Martino et al. 2010).
Although the relationships between RD and FA were predominantly distributed in anterior brain areas and in fibers surrounding the corpus striatum, our findings point to effects of WM integrity in anatomically widespread areas rather than limited to highly specific neurocircuits. RD comprises cognitive, emotional, behavioral, and social facets, including learning and maintaining behavior previously associated with signals of reward. In particular, this is true for verbal signals related to social approval, sentiment, and emotional support (Cloninger et al. 1993). Processing of rewarding stimuli are known to recruit DA neurons of the ventral tegmental area and substantia nigra, which project to the striatum, nucleus accumbens, and frontal cortex, which are involved in motivation- and goal-directed behavior (Di Chiara and Imperato 1988; Robbins and Everitt 1996; Wise 1996; Schultz et al. 1997). Our findings of an association between RD and FA in tracts encompassing striatal and frontal regions are consistent with this notion. However, the multifaceted character of RD suggests that subserving WM circuits are distributed beyond a confined frontostriatal network. Amygdala and its connections are critical for processing of emotionally charged stimuli and receive sensory input from the major sensory systems and connect to, for example, the thalamus and higher order association areas of the cortex, modulating emotional responses (LeDoux 2000). Moreover, functional imaging, patient, and lesion studies converge on a key role of the temporal pole in social behavior and cognition (Myers 1972; Rankin et al. 2006; Zahn et al. 2007). Hence, the notion that multiple brain regions are implicated in complex temperament traits supports the distributed nature of the relations between RD and microstructural WM measures.
Very similar associations between RD and FA were observed in a younger subcohort, suggesting that age-related decline in WM integrity was not causing the associations. Furthermore, these findings suggest that the association between a personality profile dependent upon social reward and WM microstructure is established relatively early in life. This is coherent with the notion that temperament and personality are highly heritable dimensions which in close interactions with the environment emerge early in life and show stability over time (Costa et al. 1987).
In the present study, the associations with RD and FA were mainly explained by radial diffusivity. These findings points to alterations in axonal density, membrane integrity, and/or myelination as candidate mechanisms underlying RD differences (Beaulieu 2002; Song et al. 2002, 2003). However, the neurobiological underpinnings of diffusion properties in the brain are complex, and such interpretations are likely to be oversimplifications of the underlying tissue properties.
We recently reported that the anxiety-related temperament trait harm avoidance from the TCI scale is associated with reduced WM integrity in major WM tracts (Westlye et al. 2011), implying that structural connectivity modulates anxiety-related aspects of personality. Whereas anxiety-related personality traits are associated with and represent an important predisposing factor for depression and anxiety-related disorders (Farmer et al. 2003), the association between RD and psychopathology is less consistent (Svrakic et al. 1993; Bulik et al. 1995; Howard et al. 1997; Richman and Frueh 1997; Kim et al. 2006; Tikkanen et al. 2007; Conrad et al. 2009), and high RD might even protect against the development of depression (Farmer et al. 2003). Thus, although our results indicate decreased WM microstructure with increased RD, further studies are needed to explore the implications of these findings for the understanding of neuropsychiatric disorders related to altered reward processing.
An association between RD and brain structure is supported by morphometric MRI studies. Despite inconsistency across studies (Iidaka et al. 2006; Gardini et al. 2009; Lebreton et al. 2009; Van Schuerbeek et al. 2010), negative correlations between RD and gray matter density of frontostriatal, limbic, and temporal areas have been reported in the most powered studies (Gardini et al. 2009; Van Schuerbeek et al. 2010). These findings provide evidence of a negative relation between RD and gray matter volume in areas implicated in motivation and reward processing (dorsal striatum, prefrontal, and orbitofrontal cortical regions) (Leon and Shadlen 1999; Rolls 2000; Tricomi et al. 2004; Roberts 2006; Balleine et al. 2007) and social cognition (posterior cingulate and superior temporal cortex) (Zahn et al. 2007; Danziger et al. 2009; Lombardo et al. 2010). Our observations of decreasing frontal WM integrity with increasing RD are in line with the gray matter morphometry literature but extends previous findings by emphasizing the importance of the microstructural integrity of the subjacent WM in motivation and reward-seeking behavior (Di Chiara and Imperato 1988; Robbins and Everitt 1996; Wise 1996; Schultz et al. 1997). It is possible that variability in WM structure particularly in tracts encompassing frontal areas modulates the functioning of networks involved in reward processing. Conceivably, reduced structural integrity in the WM tissue feeding these networks may affect the sensitivity to rewards and subsequently manifest in a highly reward dependent personality profile as measured by the TCI.
Individual differences in the sensitivity or reactivity of mesocorticolimbic pathways have been strongly implicated in drug addiction and obesity (Wang et al. 2004; Kreek et al. 2005). Addiction appears to correlate with a decrease in DA functioning expressed as reduced D2 receptor level and DA release (Volkow et al. 2004, 2008; Melis et al. 2005) as well as reduced glucose metabolism in prefrontal regions and the cingulate gyrus (Volkow et al. 1993, 2001, 2007). This hypodopaminergic state is assumed to reflect a primary cause of drug-seeking behavior (Bowirrat and Oscar-Berman 2005; Melis et al. 2005). Speculatively, our findings of decreased WM connectivity in these regions in high RD individuals could reflect a structural correlate of the reduced activation in mesolimbic and prefrontal regions in at-risk subjects and therefore constitute a structural imaging biomarker or a susceptibility factor for addictive and compulsive propensities. More studies are necessary to test and bring this notion further.
RD was significantly higher in females than males, which is in line with normative and clinical samples across cultures (Cloninger et al. 1991; Takeuchi et al. 1993; Farmer et al. 2003; Gardini et al. 2009). It has been proposed that this reflects sex differences in noradrenergic systems (Cloninger 1987). Whether such variability is reflected in WM microstructure of noradrenergic circuits or circuitry of other catecholamines is uncertain. However, we found that the relations between RD and the DTI indices were not significantly influenced by sex, suggesting that common neurocircuitry is responsible for individual differences in RD across genders.
Conclusion
This study demonstrates significant associations between WM microstructure and social reward dependence predominantly in anterior regions of the brain, including frontostriatal and frontolimbic circuits known to play a critical role in the processing of reinforcements and social rewards. In general, we found negative associations between RD and FA, indicating that higher RD is associated with decreased microstructural integrity of the brain WM. These novel findings represent a step toward an understanding of the brain structural foundations for complex reward-related personality traits. Our results thus provide a putative structural mechanism for variability in RD, and the relations between RD and WM integrity could be a useful starting point for further studies exploring the neurobiological underpinnings of human personality as well as in the quest for neurobiological risk factors and correlates of reward-related disorders including drug addiction, pathological gambling, and risk-taking behavior.
Funding
Research Council of Norway (grant 204966 to L.T.W., 177404 and 186092 to K.B.W., and 175066 and 189507 to A.M.F.); University of Oslo (to K.B.W. and A.M.F.).
Acknowledgments
We thank Paulina Due-Tønnesen for neuroradiological evaluations. Conflict of Interest: None declared.
References
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation. 2007 FMRIB technical report TR07JA2. Oxford, UK: Available from: www.fmrib.ox.ac.uk/analysis/techrep. [cited 2007 June 28] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. 2007 FMRIB technical report TR07JA1. Oxford, UK: Available from: www.fmrib.ox.ac.uk/analysis/techrep. [cited 2007 June 28] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck depression inventory scoring manual. New York: The Psychological Corporation; 1987. [Google Scholar]
- Black MM, Baas PW. The basis of polarity in neurons. Trends Neurosci. 1989;12:211–214. doi: 10.1016/0166-2236(89)90124-0. [DOI] [PubMed] [Google Scholar]
- Bodi N, Keri S, Nagy H, Moustafa A, Myers CE, Daw N, Dibo G, Takats A, Bereczki D, Gluck MA. Reward-learning and the novelty-seeking personality: a between- and within-subjects study of the effects of dopamine agonists on young Parkinson's patients. Brain. 2009;132:2385–2395. doi: 10.1093/brain/awp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- Brady ST. Molecular motors in the nervous system. Neuron. 1991;7:521–533. doi: 10.1016/0896-6273(91)90365-7. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Weltzin TE, Kaye WH. Temperament in eating disorders. Int J Eat Disord. 1995;17:251–261. doi: 10.1002/1098-108x(199504)17:3<251::aid-eat2260170306>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Camara E, Rodriguez-Fornells A, Munte TF. Microstructural brain differences predict functional hemodynamic responses in a reward processing task. J Neurosci. 2010;30:11398–11402. doi: 10.1523/JNEUROSCI.0111-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR. A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatr Dev. 1986;4:167–226. [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants: a proposal. Arch Gen Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM. The Tridimensional Personality Questionnaire: U.S. normative data. Psychol Rep. 1991;69:1047–1057. doi: 10.2466/pr0.1991.69.3.1047. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Arch Gen Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Schoene-Bake JC, Elger CE, Weber B. Connectivity-based segregation of the human striatum predicts personality characteristics. Nat Neurosci. 2009;12:32–34. doi: 10.1038/nn.2228. [DOI] [PubMed] [Google Scholar]
- Conrad R, Wegener I, Imbierowicz K, Liedtke R, Geiser F. Alexithymia, temperament and character as predictors of psychopathology in patients with major depression. Psychiatry Res. 2009;165:137–144. doi: 10.1016/j.psychres.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, Zonderman AB, McCrae RR, Cornoni-Huntley J, Locke BZ, Barbano HE. Longitudinal analyses of psychological well-being in a national sample: stability of mean levels. J Gerontol. 1987;42:50–55. doi: 10.1093/geronj/42.1.50. [DOI] [PubMed] [Google Scholar]
- Danziger N, Faillenot I, Peyron R. Can we share a pain we never felt? Neural correlates of empathy in patients with congenital insensitivity to pain. Neuron. 2009;61:203–212. doi: 10.1016/j.neuron.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer A, Mahmood A, Redman K, Harris T, Sadler S, McGuffin P. A sib-pair study of the Temperament and Character Inventory scales in major depression. Arch Gen Psychiatry. 2003;60:490–496. doi: 10.1001/archpsyc.60.5.490. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Greve DN, Fischl B, Benner T, van der Kouwe AJ, Salat D, Bjørnerud A, Due-Tønnessen P, Walhovd KB. The relationship between diffusion tensor imaging and volumetry as measures of white matter properties. Neuroimage. 2008;42:1654–1668. doi: 10.1016/j.neuroimage.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gardini S, Cloninger CR, Venneri A. Individual differences in personality traits reflect structural variance in specific brain regions. Brain Res Bull. 2009;79:265–270. doi: 10.1016/j.brainresbull.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Hansenne M, Pinto E, Pitchot W, Reggers J, Scantamburlo G, Moor M, Ansseau M. Further evidence on the relationship between dopamine and novelty seeking: a neuroendocrine study. Pers Individ Dif. 2002;33:967–977. [Google Scholar]
- Howard MO, Kivlahan D, Walker RD. Cloninger's tridimensional theory of personality and psychopathology: applications to substance use disorders. J Stud Alcohol. 1997;58:48–66. doi: 10.15288/jsa.1997.58.48. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Matsumoto A, Ozaki N, Suzuki T, Iwata N, Yamamoto Y, Okada T, Sadato N. Volume of left amygdala subregion predicted temperamental trait of harm avoidance in female young subjects. A voxel-based morphometry study. Brain Res. 2006;1125:85–93. doi: 10.1016/j.brainres.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jung RE, Grazioplene R, Caprihan A, Chavez RS, Haier RJ. White matter integrity, creativity, and psychopathology: disentangling constructs with diffusion tensor imaging. PLoS One. 2010;5:e9818. doi: 10.1371/journal.pone.0009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lee SJ, Yune SK, Sung YH, Bae SC, Chung A, Kim J, Lyoo IK. The relationship between the biogenetic temperament and character and psychopathology in adolescents. Psychopathology. 2006;39:80–86. doi: 10.1159/000090597. [DOI] [PubMed] [Google Scholar]
- King-Casas B, Tomlin D, Anen C, Camerer CF, Quartz SR, Montague PR. Getting to know you: reputation and trust in a two-person economic exchange. Science. 2005;308:78–83. doi: 10.1126/science.1108062. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Lebreton M, Barnes A, Miettunen J, Peltonen L, Ridler K, Veijola J, Tanskanen P, Suckling J, Jarvelin MR, Jones PB, et al. The brain structural disposition to social interaction. Eur J Neurosci. 2009;29:2247–2252. doi: 10.1111/j.1460-9568.2009.06782.x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leon MI, Shadlen MN. Effect of expected reward magnitude on the response of neurons in the dorsolateral prefrontal cortex of the macaque. Neuron. 1999;24:415–425. doi: 10.1016/s0896-6273(00)80854-5. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek SA, Suckling J, Baron-Cohen S, Consortium MA. Shared neural circuits for mentalizing about the self and others. J Cogn Neurosci. 2010;22:1623–1635. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- Martino J, Brogna C, Robles SG, Vergani F, Duffau H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2010;46:691–699. doi: 10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Melis M, Spiga S, Diana M. The dopamine hypothesis of drug addiction: hypodopaminergic state. Int Rev Neurobiol. 2005;63:101–154. doi: 10.1016/S0074-7742(05)63005-X. [DOI] [PubMed] [Google Scholar]
- Myers RE. Role of prefrontal and anterior temporal cortex in social behavior and affect in monkeys. Acta Neurobiol Exp (Wars) 1972;32:567–579. [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EP, Ozkaragoz TZ, Ritchie TL, Zhang X, Belin TR, Sparkes RS. D2 and D4 dopamine receptor polymorphisms and personality. Am J Med Genet. 1998;81:257–267. [PubMed] [Google Scholar]
- O'Gorman RL, Kumari V, Williams SC, Zelaya FO, Connor SE, Alsop DC, Gray JA. Personality factors correlate with regional cerebral perfusion. Neuroimage. 2006;31:489–495. doi: 10.1016/j.neuroimage.2005.12.048. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, Miller BL. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129:2945–2956. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman H, Frueh BC. Personality and PTSD II: personality assessment of PTSD-diagnosed Vietnam veterans using the cloninger tridimensional personality questionnaire (TPQ) Depress Anxiety. 1997;6:70–77. doi: 10.1002/(sici)1520-6394(1997)6:2<70::aid-da3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Roberts AC. Primate orbitofrontal cortex and adaptive behaviour. Trends Cogn Sci. 2006;10:83–90. doi: 10.1016/j.tics.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- Schreckenberger M, Klega A, Grunder G, Buchholz HG, Scheurich A, Schirrmacher R, Schirrmacher E, Muller C, Henriksen G, Bartenstein P. Opioid receptor PET reveals the psychobiologic correlates of reward processing. J Nucl Med. 2008;49:1257–1261. doi: 10.2967/jnumed.108.050849. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;1(Suppl 23):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, Robson MD, Jones DK, Klein JC, Bartsch AJ, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc. 2007;2:499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Svrakic DM, Whitehead C, Przybeck TR, Cloninger CR. Differential diagnosis of personality disorders by the seven-factor model of temperament and character. Arch Gen Psychiatry. 1993;50:991–999. doi: 10.1001/archpsyc.1993.01820240075009. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R. White matter structures associated with creativity: evidence from diffusion tensor imaging. Neuroimage. 2010;51:11–18. doi: 10.1016/j.neuroimage.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Yoshino A, Kato M, Ono Y, Kitamura T. Reliability and validity of the Japanese version of the Tridimensional Personality Questionnaire among university students. Compr Psychiatry. 1993;34:273–279. doi: 10.1016/0010-440x(93)90010-2. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Hsu E, Krishnan KR, MacFall JR. Diffusion tensor imaging: background, potential, and utility in psychiatric research. Biol Psychiatry. 2004;55:201–207. doi: 10.1016/j.biopsych.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Tikkanen R, Holi M, Lindberg N, Virkkunen M. Tridimensional Personality Questionnaire data on alcoholic violent offenders: specific connections to severe impulsive cluster B personality disorders and violent criminality. BMC Psychiatry. 2007;7:1–6. doi: 10.1186/1471-244X-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–292. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Van Schuerbeek P, Baeken C, De Raedt R, De Mey J, Luypaert R. Individual differences in local gray and white matter volumes reflect differences in temperament and character: a voxel-based morphometry study in healthy young females. Brain Res. 2010;31:32–42. doi: 10.1016/j.brainres.2010.11.073. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe U, Federspiel A, Mucci A, Dierks T, Frank A, Wahlund LO, Galderisi S, Maj M. Cerebral connectivity and psychotic personality traits. A diffusion tensor imaging study. Eur Arch Psychiatry Clin Neurosci. 2008;258:292–299. doi: 10.1007/s00406-007-0796-1. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio (TX): Psychological Corporation; 1999. [Google Scholar]
- Westlye LT, Bjørnebekk A, Grydeland H, Fjell AM, Walhovd KB. Linking an anxiety-related personality trait to brain white matter microstructure: diffusion tensor imaging and harm avoidance. Arch Gen Psychiatry. 2011;68:369–377. doi: 10.1001/archgenpsychiatry.2011.24. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Bjørnerud A, Due-Tønnessen P, Fjell AM. Error-related negativity is mediated by fractional anisotropy in the posterior cingulate gyrus—a study combining diffusion tensor imaging and electrophysiology in healthy adults. Cereb Cortex. 2009;19:293–304. doi: 10.1093/cercor/bhn084. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due-Tonnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM. Life-span changes of the human brain White matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20:2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Wheeler-Kingshott CA, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45:S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Xu H, Potenza MN. White matter integrity and five-factor personality measures in healthy adults. Neuroimage. 2012;59:800–807. doi: 10.1016/j.neuroimage.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kober H, Carroll KM, Rounsaville BJ, Pearlson GD, Potenza MN. White matter integrity and behavioral activation in healthy subjects. Hum Brain Mapp. 2012 doi: 10.1002/hbm.21275. doi: 10.1002/hbm.21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci U S A. 2007;104:6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, Shelby ES, Smith CE, McHugo M, Kessler RM. Midbrain dopamine receptor availability is inversely associated with novelty-seeking traits in humans. J Neurosci. 2008;28:14372–14378. doi: 10.1523/JNEUROSCI.2423-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]