Abstract
Activation of the thrombotic and complement systems is the main recognition and effector mechanisms in the multiple adverse biological responses triggered when biomaterials or therapeutic cells come into blood contact. We have created a surface which is auto-protective to human innate immunity by combining three fundamentally different strategies, all developed by us previously, which have been shown to induce substantial, but incomplete hemocompatibility when used separately. In summary, we have conjugated a factor H–binding peptide; and an ADP-degrading enzyme; using a PEG linker on both material and cellular surfaces. When exposed to human whole blood, factor H was specifically recruited to the modified surfaces and inhibited complement attack. In addition, activation of platelets and coagulation was efficiently attenuated, by degrading ADP. Thus, by inhibiting thromboinflammation using a multicomponent approach, we have created a hybrid surface with the potential to greatly reduce incompatibility reactions involving biomaterials and transplantation.
Keywords: Surface modification, poly(ethylene glycol) (PEG), Factor H-binding peptide, Apyrase, Complement, Coagulation
1. Introduction
In modern medicine, artificial materials frequently come into contact with blood and other tissue fluids (e.g., in extracorporeal circulation devices used for hemodialysis, hemofiltration, cardiopulmonary bypass (CPB), extracorporeal membrane oxygenation (ECMO), and plasmapheresis). This contact induces a sequence of events involving protein adsorption; inflammatory reactions, including activation of the complement and coagulation systems; and adhesion of immunocompetent cells to the exposed surfaces. These events result in serious thromboinflammatory incompatibility reactions targeting the implanted materials [1], [2]. For example, for hemodialysis patients, the risk of myocardial infarction is 5–10 times higher than for healthy individuals. Chronic whole-body inflammation triggered by hemodialysis likely contributes to arteriosclerosis in uremic patients and is associated with a significantly decreased life expectancy. Also, CPB and ECMO procedures, which have increased over the past decade as a result of vascular bypass surgery and long-lasting infections affecting the lungs (e.g., swine influenza, severe acute respiratory syndrome, SARS), are associated with side effects related to contact between blood and material surfaces that result in cellular and humoral defense reactions known as the systemic inflammatory response syndrome (SIRS). In addition, small implants within the bloodstream react with the cascade systems of the blood; instead of systemic reactions, these interactions produce other deleterious effects: Vascular stents elicit fibrosis, restenosis, and thrombosis at the implantation site, and cardiac aids and pumps can trigger thrombotic reactions, leading to emboli.
Simultaneous activation of innate immunity and the thrombotic cascade also occurs during the transplantation of cells, such as islets of Langerhans [3], [4], mesenchymal stem/stromal cells (MSC), and hepatocytes [5]. Graft loss results in part from a thromboinflammatory instant blood-mediated inflammatory reaction (IBMIR). This reaction consists of an innate immune attack triggered by activation of the complement and coagulation systems, followed by rapid binding of activated platelets and infiltration of polymorphonuclear leukocytes (PMNs) [3], [4]. The corresponding reactions in whole-organ transplantation are ischemia-reperfusion injury and xenogenic/allogeneic antibody-mediated rejection, the major mediator of cell damage during transplantation [6], which is also triggered by complement activation and thrombotic reactions. Thus, it is important to make surfaces of artificial materials and transplanted cells inert against activation of innate immunity and the thrombotic cascade by regulating thromboinflammation.
Our group has studied the regulation of the coagulation/platelet and complement systems on biomaterial surfaces using various approaches. Nilsson et al. successfully immobilized an ADP-degrading apyrase on substrate surfaces that inhibits both platelet activation and platelet-dependent activation of the coagulation system [7]. Recently, we also identified peptides with high affinity for various domains of human factor H, an abundant plasma protein that regulates complement activation both in solution and on self-surfaces [8]. One of these peptides (5C6) recruited factor H without interfering with its regulatory function, since it bound to a region of this regulator that does not interact with the C3 convertase [8].
We now describe the creation of a combined surface coating that is autoregulatory against thromboinflammation. This surface modification with 5C6 and apyrase can be applied onto substrates (artificial materials) and cells. An amphiphilic polymer, poly(ethylene glycol)-conjugated phospholipid (PEG-lipid) co-immobilizes 5C6 and apyrase on the cell surface, as outlined in Fig. 1 . The PEG-lipid derivatives can be immobilized to cell membranes by hydrophobic interactions with the lipid bilayer membranes without either cytotoxicity or a volume increase [9], [10]. So far we have previously studied the effect of surface modification of living cells and islets with PEG-lipid derivatives on graft survival during cell transplantation [9]. The other end of the PEG derivative facing the fluid phase can be functionalized to allow binding of peptides, proteins, or oligonucleotides [10]. The PEG-lipid derivative itself is able to suppress coagulation and the inflammatory reactions of the IBMIR to a certain extent [11]. In this paper, we performed various assays to evaluate 5C6 and apyrase function: detection of complement-activation markers on the modified surfaces, hemolytic assays of the complement alternative pathway (AP), and xenogeneic biocompatibility assays of adherent porcine aortic endothelial cells (PAECs) in contact with human whole blood.
Fig. 1.
Surface modification of substrate and cell surfaces with 5C6 and apyrase. (a) Immobilization of biotinylated peptide onto polystyrene surfaces via avidin. (b) Immobilization of thiolated peptide and apyrase onto glass surfaces via maleimide-conjugated PEG. Thiolated factor H-binding peptide 5C6 and apyrase are conjugated to the end of PEG chains via a thiol-maleimide reaction. (c) Chemical structure of 5C6-conjugated PEG-lipid for surface modification of cells (erythrocytes, endothelial cells). (d) Schematic representation of a cell surface modified with 5C6 and apyrase, which are co-immobilized on cell surfaces by incorporation into the lipid bilayer membrane. Factor H is recruited to the surface by 5C6 from human blood to impair complement activation, and apyrase degrades ADP to suppress platelet and coagulation activation.
2. Materials and methods
2.1. Preparation of factor H and peptides (5C6 and Sc)
2.1.1. Biotinylation of factor H
Human factor H was prepared from human serum [12]. Biotinylation of factor H was performed using biotinamidohexanoic acid N-hydroxysuccinimide ester (Sigma–Aldrich, Inc., St. Louis, USA). A solution of factor H (300 μg/mL in PBS) was mixed with biotinamidohexanoic acid N-hydroxysuccinimide ester (1.76 mM in DMSO) for 30 min at room temperature (RT), followed by dialysis against PBS at 4 °C overnight.
2.1.2. Synthesis of factor H-binding peptide or control peptide
Analogs of the factor H-binding peptide 5C6 (ASSSRCTYSHWCSH) were prepared using solid phase peptide synthesis (SPPS) and cyclized via oxidation of its cysteine residues as described previously [8]. For surface attachment, the sequence was expended at the C-terminus by a short spacer group ([PEG3]2) followed by either a free cysteine (5C6-Cys) or a lysine residue, to which a biotin moiety was attached during SPPS (5C6-biotin). An inactive, linear control peptide based on a scrambled 5C6 sequence (Sc; YSSSWAHASTRASH) was prepared and modified as described above (Sc-Cys, Sc-biotin). Identity and purity of each peptide were verified by HPLC and mass spectrometry (the purity for each peptide is >95%).
2.2. Preparation of 5C6-immobilized substrate surfaces
2.2.1. Peptide immobilization on the polystyrene surface
Polystyrene microtiter plates (Maxisorp, Nunc, Roskilde, Denmark) were coated with avidin (Sigma–Aldrich Inc, 10 μg/mL) by incubation overnight at 4 °C. The surface was then blocked by incubation with 0.05% (v/v) Tween 20 in PBS for 30 min. Biotinylated peptides (5C6-biotin, Sc-biotin, 2 μg/mL, in PBS) were subsequently bound to the avidin layer by incubation for 60 min at RT, and the surface was extensively washed with PBS.
2.2.2. Peptide immobilization on glass surfaces
Glass slides were washed with 2-propanol (Sigma–Aldrich, Inc.) and pure water, then immersed in 5% 3-aminopropyl triethoxysilane (APTES, Sigma–Aldrich, Inc.) in toluene solution for 1 h at RT. After washes with ethanol and pure water, they were dried in vacuo at 80°C overnight, resulting in APTES-coated glass slides. α-N-hydroxysuccinimidyl-ω-maleimidyl poly(ethylene glycol) (Mal-PEG-NHS, PEG: 5 kDa, NOF Corporation, Tokyo, Japan) was dissolved in dichloromethane (2 mg/mL, Sigma–Aldrich Inc) and added to the APTES-coated glass slides, which were incubated for 4 days at RT with gentle shaking and then sequentially washed with dichloromethane, ethanol, and pure water.
The Mal-PEG-modified surfaces were exposed to a solution of 5C6-Cys or Sc-Cys at various concentrations (15.6–1000 μg/mL in PBS) for 12 h at 4 °C to conjugate the peptide to the end of the PEG chain through a thiol-maleimide reaction. The peptide-immobilized surfaces were used for further experiments after being washed with PBS.
2.3. Functional evaluation of 5C6-immobilized substrate surfaces
2.3.1. Binding of factor H on the peptide-immobilized surface
Binding of factor H to 5C6- and Sc-immobilized polystyrene surfaces was examined by incubating lepirudin-anticoagulated plasma (50 μg/mL; Shering AG, Saksa, Germany) in a dilution series for various periods of time at 37 °C. Plasma was diluted 1:3, then in further three-fold dilutions in veronal-buffered saline, pH 7.4, containing 0.15 mM Ca2+ and 0.5 mM Mg2+ (VBS2+) and incubated for 60 min with agitation. Plasma diluted 1:1000 in VBS2+ was also incubated for 0–60 min. Binding of factor H was detected with sheep anti-factor H (The Binding Site Group, Ltd., Birmingham, UK) diluted 1:2000 in PBS-Tween 20 with 1% BSA, then with peroxidase (HRP)-conjugated rabbit anti-sheep Ig (Dako, Glostrup, Denmark) diluted 1:500 in PBS-Tween 20 with 1% BSA. Binding was evaluated by incubation with TMB + substrate chromogen (Dako), and the absorbance was read at 450 nm.
To examine the binding of factor H to peptide-immobilized glass surfaces (peptide concentrations, 15.6–1000 μg/mL), the surfaces were blocked with BSA (10 mg/mL) for 1 h at RT, then incubated with biotin-factor H (0–10 μg/mL in PBS) for 1 h at RT. After washing with PBS, HRP-conjugated streptavidin (GE Healthcare, Uppsala, Sweden) was added for 5 min, followed by sequential incubation with TMB + substrate chromogen (Dako).
The bound factor H was further characterized by western blot analysis. Lepirudin plasma, native or diluted 1:25 or 1:1000 in PBS, was incubated on 5C6- and Sc-immobilized polystyrene surfaces for 5 or 100 min. After extensive washing, the bound proteins were eluted using electrophoresis sample buffer containing 2% SDS, and the samples were subjected to SDS-PAGE under reducing conditions, followed by western blot analysis using sheep anti-factor H (The Binding Site) diluted 1:500 and HRP-conjugated rabbit anti-sheep IgG (Dako) diluted 1:500.
2.3.2. Evaluation of complement activation on peptide-surfaces in plasma and whole blood
Blood was drawn from healthy volunteers who had received no medication for at least 10 days prior to blood collection. Blood was collected in 11-mL vacutainer tubes and anticoagulated with lepirudin at a final concentration of 50 μg/mL. Complement activation was examined by incubating whole blood on 5C6- and Sc-immobilized microtiter plates. Blood with EDTA (10 mM), Mg2+/EGTA (10 mM, mixed with 2.5 mM Mg2+), or PBS (5%, for volume correction) was incubated on the peptide-immobilized surfaces (100 μL/well) at 37 °C for 60 min. Activation was interrupted by the addition of EDTA (10 mM final concentration). An aliquot of whole blood was used for CD11b analysis, and the remaining blood was centrifuged (3000 g, 20 min) to obtain plasma and stored at −80 °C until further analyzed. The microtiter plates were washed extensively with PBS-Tween 20 and incubated with rabbit anti-C3c (Dako, diluted 1:1000) and then with HRP-conjugated swine anti-IgG (1:500) and TMB + substrate chromogen (Dako).
2.3.3. Granulocyte expression of CD11b
Expression of CD11b on granulocytes after exposure to the peptide surfaces was analyzed with monoclonal mouse anti-CD11b-RPE (mAb; Dako, clone 2LPM19c) according to the manufacturer's protocol: In brief, after incubation of the whole blood on the peptide surface for 60 min at 37 °C, the antibody (1 μg/100 μL) was added and incubated for 30 min. Erythrocytes were subsequently lysed in AKC solution (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) by incubation for 10 min, and leukocytes were isolated by centrifugation (400 g, 5 min). The pellet was washed once with PBS (with 2% BSA), resuspended in 1% paraformaldehyde in PBS (Apoteket, Gothenburg, Sweden), and analyzed on a Cell Lab Quanta SC flow cytometer (Beckman Coulter, Miami, FL, USA). Granulocytes were gated on size and granularity, and fluorescent intensity was recorded on 5000 gated cells.
2.4. Functional evaluation of 5C6 on erythrocytes
2.4.1. Preparation of 5C6-conjugated PEG-lipid and PEG-lipid derivatives
Methoxyl-PEG-conjugated phospholipid (MeO-PEG-lipid), Mal-PEG-conjugated phospholipid (Mal-PEG-lipid) and biotin-PEG-conjugated phospholipid (biotin-PEG-lipid) were synthesized as previously described [9]. A solution of 5C6 or Sc (40 μL, 10 mg/mL in PBS) was mixed with Mal-PEG-lipid (360 μL, 28 mg/mL in PBS) to conjugate the peptide at the end of the PEG chain (to yield 5C6-PEG-lipid and Sc-PEG-lipid, respectively).
Biotin-PEG-lipid and FITC-labeled streptavidin (GE Healthcare) were used in order to confirm incorporation of PEG-lipid derivatives into rabbit erythrocyte surfaces. Rabbit erythrocytes (Håtunaholm laboratories, Sweden), 1% in PBS, were mixed with biotin-PEG-lipid (500 μg/mL in PBS) for 30 min at RT, FITC-streptavidin (1:10 diluted in PBS) was added and incubated for 5 min. After a wash with PBS, the erythrocytes were examined using confocal laser scanning microscopy (LSM510 META, Carl Zeiss, Jena, Germany).
2.4.2. Surface modification of rabbit erythrocytes with 5C6-conjugated PEG-lipid
Hemolytic assays of the AP were performed using rabbit erythrocytes [13], [14], which were modified with 5C6-PEG-lipid or Sc-PEG-lipid to examine the effect of peptide incorporation into cell membranes. Mixtures of MeO-PEG-lipid and 5C6-PEG-lipid or MeO-PEG-lipid and Sc-PEG-lipid (molar ratios of 100:0, 99:1, 98:2, 95:5, 90:10, 80:20, 50:50, 20:80, 0:100) were used for the surface modification of rabbit erythrocytes. The total concentrations of PEG-lipid were 0.01, 0.1, 0.5, 2.5, and 12.5 mg/mL, respectively. First, rabbit erythrocytes were washed with PBS until the supernatant was clear. A solution of PEG-lipid mixture was added to the erythrocytes and incubated for 30 min at RT with gentle mixing, followed by washing with Mg2+/EGTA buffer (8 mM EGTA, 2 mM MgSO4, 1 g/L gelatin in veronal-buffered saline, pH 7.5) on ice three times. A suspension of 1% rabbit erythrocytes (50 μL) was mixed with 1/8-diluted human serum in Mg2+/EGTA buffer (100 μL) and shaken for 1 h at 37 °C. The supernatant was then collected by centrifugation, and the absorbance of the supernatants at 405 nm was measured to calculate the percentage of erythrocytes lysed.
After incubation with human serum, the erythrocytes were collected and incubated with 1/5 diluted anti-human factor H antibody for 30 min at RT. After washing with PBS, they were incubated with 1/1000 diluted Alexa488-labeled anti-sheep IgG (Invitrogen, Carlsbad, CA, USA) for analysis by confocal laser scanning microscopy and FACS (BD LSRII, FACSCalibur, BD Biosciences, San Jose, CA, USA).
2.5. Immobilization of apyrase on erythrocytes
2.5.1. Immobilization of apyrase on erythrocytes with Mal-PEG-lipid
Apyrase was immobilized on the surface of erythrocytes (human and rabbit) using Mal-PEG-lipid as previously reported [15]. Thiol groups were introduced into the apyrase by thiolation using Traut's reagent (Thermo Fisher Scientific, Waltham, MA, USA): Apyrase solution (10 mg/mL, 400 μL, from potato, Sigma–Aldrich Inc) was mixed with Traut's reagent (10 mg/mL, 66 μL). The solution was incubated with gentle mixing at RT for 1 h. Thiolated apyrase (apyrase-SH) was purified using a spin column (Thermo Fisher Scientific). In order to visually examine the immobilization of apyrase on the erythrocyte surface, we used Alexa 488-labeled apyrase-SH. Prior to thiolation with Traut's reagent, apyrase was labeled with Alexa Fluor® 488 by using a labeling kit (Invitrogen) according to the manufacturer's protocol.
Erythrocytes (5 × 104–5 × 105 cells) were incubated in a solution of Mal-PEG-lipid (100 μL, 10 mg/mL in PBS) for 30 min at RT and then washed three times with cold PBS to obtain erythrocytes modified with Mal-PEG-lipid (Mal-PEG-erythrocyte). To obtain apyrase-erythrocytes, Mal-PEG-erythrocytes were mixed with 50 μL of apyrase-SH solution (600 μg/mL in PBS) and left for 30 min at RT, then washed with cold PBS three times.
2.5.2. Functional evaluation of apyrase on erythrocytes
ATP assay: Rabbit apyrase-erythrocytes (1 × 104, 1 × 105 and 5×105 cells/mL) were suspended in PBS containing 2 μM ATP and incubated for 10, 30, or 60 min, then centrifuged. The ATP concentration in the supernatant was determined with a luciferase-based ATP kit (BioThema, Handen, Sweden) according to the manufacturer's protocol.
2.5.3. Platelet aggregation testing by aggregometry
Blood was drawn into Vacuette®-evacuated collection tubes containing citrate (Greiner Bio-One GmbH, Kremsmuenster, Austria) and centrifuged at 180 g for 10 min at 22 °C to prepare platelet-rich plasma (PRP). Apyrase-erythrocytes or erythrocytes (5 ×105 cells in 225 μL PRP) were mixed with PRP at 37 °C, and a solution of ADP (5 μM) was added to the PRP and the light transmittance monitored by a Multiplate® aggregometer (Dynabyte Medical, Munich, Germany).
2.5.4. Slide-chamber experiments using whole blood
The slide-chamber model developed by our group [16] was used to evaluate the function of the peptide on substrate and cell surfaces in human whole blood. The chambers and blood-collection materials were coated with heparin according to the Corline method (Corline System AB, Uppsala, Sweden). Whole blood from seven healthy donors was collected into heparin-coated tubes containing soluble heparin (Leo Pharma A/S, Ballerup, Denmark) at a final concentration of 0.05 IU heparin/mL. Human apyrase-erythrocytes were mixed with the whole blood to a final concentration of 10%. Blood (1.45 mL) was added to each well of the slide chambers, which were covered by poly(vinyl chloride) plates. In addition, blood mixed with PEG-erythrocytes (10% final concentration), with apyrase alone (250 μg/mL), and without additives were used as controls. The chamber was rotated vertically at 22 rpm for 60 min in a 37 °C water bath. Then 1.2 mL of blood was collected from each well and mixed with EDTA-K3 solution at a final concentration of 10 mM, and the platelet concentration was analyzed in a Coulter AcT 5diff® hematology analyzer (Coulter Corporation, Miami, FL, USA). The blood was then centrifuged at 3000 g for 25 min at 4 °C, and plasma was collected and stored at −70 °C until further analysis by enzyme immunoassay (EIA) for C3a, sC5b-9, and thrombin–antithrombin complexes (TAT).
2.6. Co-immobilization of apyrase and 5C6 on glass surfaces
2.6.1. Immobilization of apyrase and 5C6 onto substrates
A mixed solution of 5C6-Cys or Sc-Cys (125 μg/mL) and apyrase-SH (250 μg/mL) was incubated on Mal-PEG-modified substrates for 12 h at 4 °C to immobilize both the peptide and apyrase. The treated surface was used for further experiments after washing with PBS. The apyrase activity on the surface was monitored by the ATP assay described above.
2.6.2. Slide chamber experiments using whole blood
Whole blood from seven healthy donors was collected into heparin-coated tubes without any soluble heparin and then incubated in the slide chambers as described above.
2.7. Co-immobilization of apyrase and 5C6 on PAECs (xenogeneic setting)
2.7.1. Surface modification of PAEC cells with 5C6-PEG-lipid and MeO-PEG-lipid
Complement activation was measured after exposure of PAECs to human whole blood in the presence and absence of 5C6-PEG-lipid in order to assess the function of the peptides. Here a mixture of MeO-PEG-lipid and 5C6-PEG-lipid (molar ratios 100:0, 98:2, 95:5, 90:10, and 80:20, total concentrations 0.5 and 5 mg/mL) was used for the surface modification of PAECs. PAECs (a kind gift from Prof Lena Claesson-Welsh) were cultured in F-12 + GlutaMAX™ (Invitrogen) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in 5% CO2. PAECs (3 × 106 cells) were collected by centrifugation (100×g, 5 min) after incubation with trypsin/EDTA (Invitrogen) for 3 min at 37 °C. Cells were then seeded onto APTES-coated glass slides and cultured in the same medium for 2 days. In order to examine the incorporation of PEG-lipid derivatives onto the surface of adherent PAECs, we used biotin-PEG-lipid and FITC-labeled streptavidin as described above. After PAECs were exposed to biotin-PEG-lipid (0.5 mg/mL in medium) for 30 min at RT, FITC-streptavidin (1:10 diluted in PBS) was added and incubated for 5 min. After a PBS wash, the PAECs were analyzed by confocal laser scanning microscopy. Adherent PAECs were exposed to the mixture of MeO-PEG-lipid and 5C6-PEG-lipid for 20 min at RT, followed by washing with medium, then used for slide chamber experiments. The viability assay was performed by trypan blue exclusion test (viability: >95% for PAECs after surface modification).
2.7.2. Surface modification of PAEC cells with peptide and apyrase
Cells were seeded onto APTES-coated glass slides and cultured in culture medium for 2 days. The PAECs were then incubated in peptide-PEG-lipid and Mal-PEG-lipid mixtures (total PEG-lipid: 5 mg/mL, peptide-PEG-lipid: 10 mol%) for 20 min at RT, followed by washing with medium and incubation in a solution of apyrase-SH (250 μg/mL) for 20 min at RT. These PAECs were used for the slide-chamber experiments after washing with medium. To prepare apyrase-immobilized PAECs, PAECs were incubated in a solution of apyrase-SH (250 μg/mL) after being treated with Mal-PEG-lipid (5 mg/mL) for 20 min at RT.
2.7.3. Slide-chamber experiments using whole blood
Whole blood from six healthy donors was collected into heparin-coated tubes containing soluble heparin to final concentrations of 0.2 or 1.0 IU heparin/mL. After the wells of the chambers were filled with blood (1.65 mL), they were covered by glass slides whose surfaces had been covered with PAECs prepared as described above. The chamber was rotated vertically at 22 rpm for 30 min in a 37 °C water bath, followed by platelet counting and collection of plasma as described above.
After exposure of the PAEC-coated glass slides to whole blood, they were washed with PBS and then immersed in 4% paraformaldehyde (Apoteket) and incubated with anti-human factor H antibody (diluted 1:10) for 30 min at RT. After a PBS wash, they were incubated with Alexa488-labeled anti-sheep IgG, then examined by confocal laser scanning microscopy.
2.7.4. Measurement of C3a, sC5b-9, C5a, and TAT in plasma
C3a, C5a, sC5b-9, and TAT in plasma were measured by conventional sandwich EIAs. For soluble C3a, plasma was diluted 1:500 to 1:6000 in working buffer (PBS containing 0.05% Tween 20, 10 mg/mL BSA, and 10 mM EDTA). As previously reported [17], C3a was captured by anti-human C3a mAb 4SD17.3 and detected by biotinylated polyclonal rabbit anti-C3a antibody and HRP-conjugated streptavidin. Zymosan-activated serum, calibrated against purified C3a, served as a standard. Values were expressed as ng/mL. C5a was analyzed with a commercial kit (HyCult Biotechnology, Uden, The Netherlands) according to their protocol. Samples were diluted 1:5-1:25, and values were expressed as ng/mL.
For sC5b-9, plasma was diluted 1:2-1:50 in working buffer. sC5b-9 was captured by anti-human C5b-9 mAb aE11 (Diatec Monoclonals AS, Oslo, Norway) and detected with anti-human C5 polyclonal rabbit antibody (Dako) and HRP-conjugated anti-rabbit IgG (Dako). Zymosan-activated serum containing 6 × 104 AU/mL served as a standard. Values were expressed as AU/mL. For TAT, plasma was diluted 1:20 in normal citrate-phosphate-dextrose plasma. TAT was captured by anti-human thrombin mAb and detected by HRP-coupled anti-human antithrombin mAb (Enzyme Research Laboratories, South Bend, IN, USA). A standard prepared by diluting pooled human serum in normal citrate-phosphate-dextrose plasma was used. Values were expressed as μg/L.
2.7.5. Statistical analysis
Results are presented as means ±SEM. Data plotting and statistical analysis were performed using Prism version 5.0d for Macintosh software (Graphpad, San Diego, CA, USA). Differences between means of two groups were statistically evaluated using the paired Student's t-test or repeated measures one-way ANOVA with Dunnett's post hoc-test when more than two groups were being compared to a control. Data were assumed to be normally distributed.
3. Results
3.1. Properties of 5C6-immobilized surfaces in lepirudin-plasma
Factor H-specific (5C6) and scrambled control (Sc) peptides were immobilized onto polystyrene and glass surfaces through biotin/avidin reactions and covalent thiol-maleimide bonding via the PEG chain, respectively (Fig. 1a,b). The modified surfaces were compared regarding factor H binding, both from lepirudin plasma and in purified form. 5C6 coated surfaces bound significantly higher amounts compared to Sc, and the binding was dependent on peptide density (Supplemental Fig. 1a–e). The modified surfaces were incubated with lepirudin-anticoagulated whole blood and complement activation (generation of C3a, C5a, sC5b-9) and granulocyte activation (expression of CD11b) was monitored. Coating with 5C6 significantly reduced all complement activation makers when compared to the control and lowered granulocyte activation via the AP (Supplemental Fig. 2a–e). Taken together, these results indicate that factor H, captured by 5C6 immobilized on surfaces, can suppress complement activation in human whole blood.
3.1.1. Effect of erythrocyte bound 5C6 on hemolytic assays
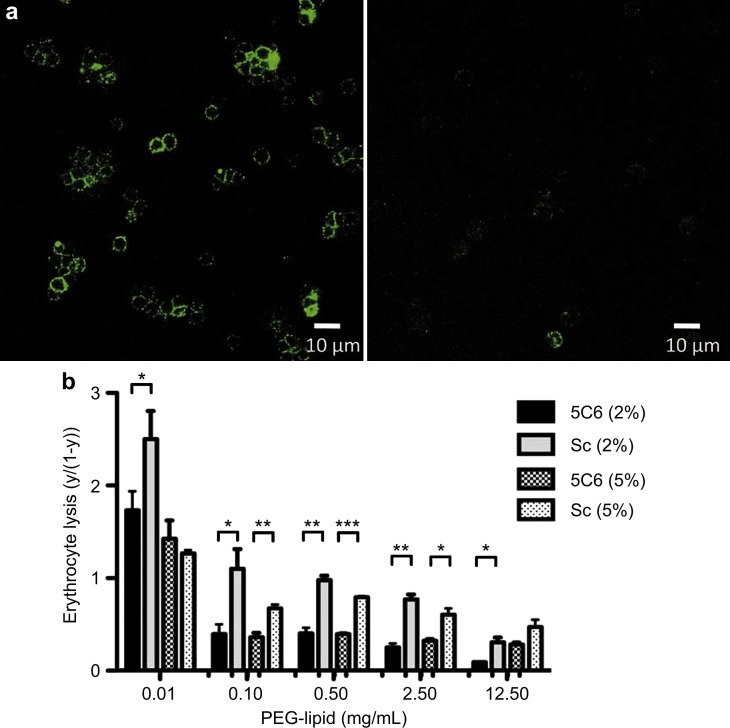
Next, 5C6 was incorporated onto the erythrocyte surface by using a PEG-lipid derivative that could be anchored to the cell membrane through hydrophobic interactions (Fig. 1c,d) [9], [10]. The PEG-lipid derivative on erythrocytes was detected using fluorescent streptavidin and biotin-PEG-lipid (Supplemental Fig. 3a). The modified erythrocytes were then incubated with human serum and subjected to immunostaining for factor H, followed by FACS analysis (Fig. 2 a, Supplemental Fig. 3b). Confocal microscopy revealed fluorescent staining on the erythrocyte surfaces modified with 5C6-PEG-lipid/MeO-PEG-lipid but not on those treated with Sc-PEG-lipid/MeO-PEG-lipid; and FACS analysis yielded similar results. Next, we performed hemolytic assays to examine the effect of 5C6 on complement activation via the AP, by incubating rabbit erythrocytes with human serum [13], [14]. The optimal concentration of 5C6 to coat the erythrocyte surfaces was determined by mixing inert MeO-PEG-lipid with 5C6-PEG-lipid at different proportions. Under uninhibited conditions, approximately 99% of the rabbit erythrocytes were hemolyzed after incubation with human serum. However, the lysis (calculated as y/(1-y), where y is the fraction lysed [18]) was suppressed by surface modification with PEG-lipid and was further reduced in the presence of 5C6, as compared to Sc-modified groups (Fig. 2b, Supplemental Fig. 3c). As the PEG-lipid concentration increased, the difference between 5C6-immobilized erythrocyte and Sc-immobilized erythrocyte became smaller as seen in Fig. 2b. The effect of PEG can be also seen clearly as the surface density of PEG is larger. Under optimal conditions, 5C6 was able to specifically recruit functional factor H from human serum and suppress AP-mediated complement activation on the erythrocyte surface.
Fig. 2.
Factor H binding and inhibition of complement activation on 5C6- and Sc-immobilized rabbit erythrocyte surfaces. (a) Detection of factor H by immunofluoresence staining on rabbit erythrocyte surfaces modified with 5C6 (left) and scrambled control peptide (Sc; right) after incubation in human serum. Representative pictures taken by confocal laser-scanning microscopy. (b) Hemolytic assays with various concentrations of PEG-lipid with 2% or 5% peptide content (n = 3). Erythrocyte lysis was calculated as y/(1-y), where y is the fraction lysed. Data shown are means ± SEM. Student's t-test was used to compare 5C6 and Sc. *, p < 0.05, **, p < 0.01, ***, p < 0.001.
3.2. Effect of 5C6 on complement activation on the surface of PAECs
PAECs were cultured on APTES-coated glass slides to confluence, and 5C6 was incorporated into the PAECs using PEG-lipid, and then exposed to blood containing 1.0 IU heparin/mL in a slide chamber model [16]. Fluorescence could be observed at the periphery of all adherent cells after treatment with biotin-PEG-lipid and FITC-streptavidin, with no cell detachment from the substrate surface (Supplemental Fig. 4a).
PAECs were treated with a mixture of 5C6-PEG-lipid and MeO-PEG-lipid (total PEG-lipid concentrations: 0.5 or 5 mg/mL) and exposed to human whole blood. After exposure to blood, the PAECs were subjected to immunostaining for factor H (Supplemental Fig. 4b), which could be detected on the PAEC surfaces modified with 5C6-PEG-lipid/MeO-PEG-lipid. The generation of C3a and sC5b-9 by PAEC surfaces modified with MeO-PEG-lipid was generally lower when compared to the control PAECs, indicating that the PEG coating by itself protected the cell surfaces from complement activation. However, a significantly greater reduction of C3a and sC5b-9 markers was seen in the presence of 5C6 on the PAEC surfaces, with an optimal effect at peptide concentrations of 2–10% (Supplemental Fig. 4c,d). In contrast, the platelet count did not decrease in either control PAECs or PAECs modified with PEG-lipid, and no differences were seen in TAT due to the presence of heparin, which can suppress coagulation (Supplemental Fig. 4e,f). Collectively, these results indicate that 5C6 was able to specifically recruit factor H from human blood, thereby suppressing complement activation on the PAEC surface.
3.3. Effect of apyrase on coagulation on the surface of erythrocytes
Next we immobilized apyrase on erythrocytes by using Mal-PEG-lipid and Alexa 488-apyrase-SH, as shown in Fig. 1d. Fluorescence was clearly observed at the periphery of erythrocytes treated with Mal-PEG-lipid and Alexa 488-apyrase-SH, but not those treated with Alexa 488-apyrase-SH alone (Fig. 3 a). No morphological changes were observed after immobilization of apyrase.
Fig. 3.
Activity of apyrase immobilized to erythrocyte surfaces. (a) Immobilization of Alexa 488-labeled apyrase on rabbit erythrocytes using surface modification with PEG-lipid (left). The control consisted of erythrocytes mixed with Alexa 488-labled apyrase (right). Representative pictures taken by confocal laser-scanning microscopy. (b) ATPase activity of apyrase immobilized on erythrocytes (n = 3). Data shown are means ± SEM. Repeated measures one-way ANOVA with Dunnett's post hoc-test was used to compare all groups with apyrase-negative erytrocytes. ***, p < 0.001. (c) ADP-induced platelet aggregation in PRP in the presence of apyrase-immobilized human erythrocytes, as monitored by aggregometry.
The effect of the erythrocyte-bound apyrase on ATP decay and ADP-induced platelet aggregation was then tested. Apyrase-immobilized erythrocytes degraded exogenously added ATP in a dose-dependent manner (Fig. 3b). In addition, ADP-induced platelet aggregation was effectively suppressed in the presence of apyrase-erythrocytes, whereas no suppression was seen in non-modified erythrocytes (Fig. 3c). These results indicate that apyrase had been immobilized with retained activity.
When human whole blood was rotated in the slide chamber, the platelet count gradually decreased with time, reflecting coagulation and platelet activation. There was less decrease in platelet count in whole blood mixed with apyrase-erythrocytes and apyrase, whereas PEG-erythrocytes showed a reduction similar to that of whole blood (Supplemental Fig. 5a) with TAT generation following a similar trend (Supplemental Fig. 5b). In contrast, there were no differences in complement activation markers among the groups (Supplemental Fig. 5c,d), indicating that the immobilized apyrase did not affect complement. These results demonstrate that in whole blood, apyrase immobilized on cell surfaces is able to inhibit platelet activation as well as suppressing coagulation activation.
3.4. Effect of co-immobilized 5C6/apyrase on the activation of complement and coagulation in whole blood
5C6 and apyrase were co-immobilized onto the substrate surfaces to investigate their combined effect. We initially examined the ATPase activity of 5C6/apyrase surfaces to ensure that the apyrase was not inhibited by the presence of 5C6 (Supplemental Fig. 6). When the co-immobilized substrate surfaces were exposed to blood in the slide chamber, the platelet consumption was significantly lower for 5C6/apyrase-, Sc/apyrase-, and apyrase-immobilized surfaces than for the control PEG surface (Fig. 4 a). TAT levels showed similar trends, indicating that co-immobilized apyrase can suppress activation of platelets and coagulation (Fig. 4b). Markers of complement activation (C3a, sC5b-9) were significantly reduced on the 5C6/apyrase surface when compared to the Sc/apyrase, apyrase, and PEG surfaces (Fig. 4c, d), indicating that complement activation was suppressed on the co-immobilized 5C6 surfaces.
Fig. 4.
Attenuation of platelet, coagulation, and complement activation on 5C6/apyrase-co-immobilized glass substrate surfaces in whole blood. Whole blood without heparin was incubated on PEG-coated, apyrase-immobilized, 5C6/apyrase, or Sc/apyrase co-immobilized substrate surfaces for 60 min at 37 °C, (n = 6). The figures show (a) platelet consumption and generation of (b) TAT, (c) C3a, and (d) sC5b-9. Data shown are means ± SEM. Repeated measures one-way ANOVA with Dunnett's post hoc-test was used to compare all groups versus PEG (a–b) and apyrase-5C6 (c–d)*, p < 0.05, **, p < 0.01, ***, p < 0.001.
Finally, the cell surfaces of PAECs were modified with both peptide and apyrase using Mal-PEG-lipid (Fig. 1d), and the adherent PAECs were tested in the slide-chamber model. Both platelet consumption and TAT levels were significantly lower for 5C6/apyrase-, Sc/apyrase- and apyrase-modified cell surfaces than for the control PAEC surfaces (Fig. 5 a,b). In addition, C3a and sC5b-9 were significantly reduced on the 5C6/apyrase modified cell surfaces when compared to the other groups (Fig. 5c,d), and factor H could be detected by immunostaining on the PAEC surfaces modified with 5C6 and apyrase (Fig. 5e). Collectively, these results show that factor H is recruited to the surfaces of substrates, and that PAEC surfaces, modified with 5C6/apyrase, can suppress both coagulation and complement activation in whole blood.
Fig. 5.
Attenuation of platelet, coagulation, and complement activation on 5C6/apyrase-co-immobilized PAECs in whole blood. Whole blood (0.2 IU/mL heparin) was incubated on control PAECs, or apyrase-immobilized, 5C6/apyrase, or Sc/apyrase-co-immobilized PAECs for 30 min at 37 °C (n = 6). The figures show (a) platelet consumption and generation of (b) TAT, (c) C3a, and (d) sC5b-9. (e) Immunofluorescence staining of factor H on PAEC surfaces modified with 5C6/apyrase (left), Sc/apyrase (middle), or apyrase (right) after incubation in whole blood. Representative pictures taken by confocal laser-scanning microscopy. Repeated measures one-way ANOVA with Dunnett's post hoc-test was used to compare all groups versus control (a–b) and apyrase/5C6 (c–d), **, p < 0.01, ***, p < 0.001.
4. Discussion
In this paper, we have demonstrated in two human whole-blood models that activation of platelets, coagulation and complement systems can be controlled simultaneously by co-immobilizing the factor H-binding peptide 5C6 and apyrase on both biomaterial and cell surfaces.
The complement system is controlled by regulators of complement activation (RCA), which mainly regulate classical pathway (CP) and AP C3 convertases. In addition to plasma membrane regulators (MCP, DAF, and CD59), the plasma proteins, C4-binding protein (C4BP) and factor H also regulate surface-bound CP and the AP convertases, respectively. Both these fluid-phase regulators are recruited to host cells by binding to cell-surface glyocosaminoglycans [19], [20] to regulate complement activation through the CP and AP, respectively. The concept of exploiting those complement-regulatory effects on foreign surfaces has been demonstrated by our group [21]: Engberg et al. have used streptococcal M protein-derived peptides to specifically recruit human C4BP to substrate surfaces, thereby reducing complement activation via the CP and suggesting RCA-binding peptides as promising regulators of complement activation both on cell and material surfaces. Several studies have indicated that complement activation on material surfaces is initiated through the CP [22], [23]. However, the AP C3 convertase is of major importance because of its ability to amplify the reaction on the surface [24], [25], [26], thereby triggering various inflammatory reactions. Therefore, it seems of major importance to regulate the AP C3 convertase in order to avoid complement attack.
We earlier examined the possibility to suppress complement activation through the AP by covalent immobilization of factor H on substrate surfaces [12], [27]. Although this approach is promising, it has limitations: First, the protein binds randomly to the surface, so that only a fraction of the protein maintains an active conformation. Second, large amounts of factor H are needed to coat the surface. In our approach, 5C6-coated cells and materials specifically recruit factor H from host blood, thereby mimicking the host surface and protecting themselves from attack by complement [28], [29]. Coating with a small peptide also allows us to avoid the costly and cumbersome preparation and immobilization of recombinant proteins. By using 5C6, we can avoid random binding of the protein and instead obtain a consistent orientation and configuration of the proteins: The optimal surface density of 5C6 for hemolytic assays was found to be at a maximum of 10%, indicating spatial requirements of the interaction between factor H and C3b. Presumably, since factor H has a functional domain regions at each of its termini, access to C3b may be limited by steric hindrance at higher densities.
Platelets are intimately involved in the incompatibility processes that occur on foreign surfaces and are directly activated by surfaces [30]. Not only are platelets closely connected to the coagulation cascade, but they can also interact with leukocytes and trigger activation of complement, thereby acting as an important hub that mediates crosstalk between these components [30], [31]. When platelets are activated, a multi-step process begins that involves adhesion, aggregation, contraction, and secretion. Released ADP is essential for recruiting and aggregating platelets; therefore, it is an obvious target for platelet inhibition [32]. In order to regulate platelet activation, vascular ADP is continuously degraded under homeostatic conditions by CD39 (NTPDase-1, EC 3.6.1.5), an enzyme present on human endothelial cells [33]. In the present study, we used the homologous potato apyrase as a prototype enzyme to obtain proof-of-concept that this group of enzymes can be a valuable tool for attenuating thrombotic reactions on material and cell surfaces.
Recent studies have shown that NTPDase-1 (CD39) is both anti-inflammatory and immunosuppressive, and that extracellular adenosine signaling modulates neutrophil accumulation, particularly during conditions of acute injury (e.g., ambient hypoxia) [34], [35], [36], [37]. In such conditions, extracellular adenosine is mainly derived from the enzymatic phosphohydrolysis of precursor nucleotides to adenosine [38], [39]. This two-step enzymatic process involves the ecto-apyrase CD39 (converting ATP/ADP to AMP) and the ecto-5 nucleotidase CD73 (converting AMP to adenosine). Both enzymes have been implicated in attenuating acute injury and inflammation in models of ambient hypoxia [34], cyclic mechanical stretch [40], and bleomycin-induced lung injury [41]. Studies of NTPDase1-deficient mice have identified an important role for this enzyme in controlling acute immune responses [42]. NTPDase1-KO mice demonstrate exacerbated inflammation in experimental models of hypoxia, endotoxin-induced lung inflammation, and colitis [34], [40]. Similarly, humans carrying a single specific point mutation in the NTPDase1 gene have recently been shown to be more susceptible to inflammatory bowel disease [40]. These studies suggest that the use of CD39 would also have an anti-inflammatory and immunosuppressive effect in cell and whole-organ transplantation.
5. Conclusions
In summary, our combined coating consists of three major components for protection of the underlying surface: 1) the PEG linker, 2) the factor H-binding peptide 5C6, and 3) the ADP-degrading apyrase. Factor H was specifically recruited to the 5C6-modified surfaces and inhibited complement attack when they were exposed to human whole blood. In addition, activation of platelets and coagulation was efficiently attenuated, by degrading ADP. Thus, by inhibiting thromboinflammation using a multicomponent approach, we have created a hybrid surface with the potential to greatly reduce incompatibility reactions involving biomaterials and therapeutic cells.
Acknowledgments
This work was supported by grants from the Swedish Research Council (VR) 2009-4675, 2009-4462, 90293501, A0290401, and A0290402 and Stem Therapy; the Swedish Research Council; and the Swedish Research Council/SSF/Vinnova contract grant number 60761701; the Japan Society for the Promotion of Science grant number 442-H22; the Novo Nordisk Foundation; National Institutes of Health grants EB003968, AI068730, GM062134, and AI030040; and faculty grants from the Linnæus University, Sweden.
J.D.L is the inventor of the factor H-binding peptide 5C6 and is the founder of Amyndas Biotherapeuticas, which performs clinical development of 5C6 for various indications. None of the other authors have conflicts of interest.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.biomaterials.2012.10.040.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplementary Fig. 1.
Factor H binding on 5C6 and Sc immobilized onto substrate surfaces. Factor H specific 5C6 and scrambled control (Sc) peptides were immobilized onto polystyrene and glass surfaces (Fig. 1a,b): Biotinylated peptides (5C6-biotin, Sc-biotin) were bound to avidin adsorbed onto polystyrene microtiter plates (a, b, e) and peptides carrying cysteine (5C6-Cys, Sc-Cys) were immobilized on Mal-PEG-modified glass surfaces (c, d). Mal-PEG was conjugated to the amine groups of APTES-coated glass surfaces through amide bonding, and peptides were further conjugated to the maleimide groups at the end of PEG molecules. (a, b) Factor H binding (detected by EIA) to 5C6 or Sc-immobilized polystyrene surfaces from lepirudin-anticoagulated plasma. Data shown are means ± SEM, (n = 3): time course in (a) undiluted plasma and (b) serially diluted plasma. (c) Purified factor H binding to glass surfaces coated with 5C6, Sc, and non-peptide-PEG (n = 3). (d) Binding of purified factor H to glass surfaces coated with 5C6 in increasing density (n = 3). (e) Western blot analysis of factor H eluted from 5C6- (lanes 1, 3, 5) and Sc-immobilized (2, 4, 6) polystyrene surfaces after incubation with lepirudin-plasma 1:1 (1, 2), 1:25 (3, 4), or 1:1000 (5, 6). Factor H is seen as a band of 150 kDa, while no proteins with lower molecular weight (e.g., FHL-1) were detected. The binding of purified factor H could be clearly detected on 5C6-coated surfaces, as compared to the Sc- and non-peptide-PEG control surfaces. Factor H binding was dependent on the surface density of the 5C6 peptide.
Supplementary Fig. 2.
Attenuation of complement activation in lepirudin-anticoagulated whole blood by 5C6 immobilized on polystyrene. 5C6 or Sc were immobilized on the surface of polystyrene microtiter plates and lepirudin-anticoagulated blood with EDTA (10 mM), Mg2+/EGTA (10 mM EGTA, 2.5 mM Mg2+), or PBS (5%, for volume correction) was incubated in the wells at 37 °C for 60 min. Material-induced complement activation was monitored as (a) surface-bound C3-fragments (n = 8) and fluid phase activation markers (b) C3a (n = 8), (c) C5a (n = 5), and (d) sC5b-9 (n = 8) measured by EIA; (e) expression of CD11b on granulocytes (n = 5) measured by FACS. Data shown are means ± SEM. Paired student's t-test was used to compare 5C6 and Sc, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Significant reduction of all complement activation markers were seen on the 5C6-immobilized surfaces compared to the Sc-immobilized surfaces in both the absence and presence of Mg2+/EGTA. When the CP was abolished with EGTA, granulocyte activation was significantly decreased, whereas no clear difference was seen in native whole blood.
Supplementary Fig. 3.
Factor H binding and subsequent inhibition of complement-mediated lysis of 5C6-coated rabbit erythrocytes. Incorporation of 5C6 to rabbit erythrocyte surfaces was achieved by using a PEG-lipid derivative that could be anchored to the cell membrane through hydrophobic interactions. (a) Surface modification of rabbit erythrocytes on an APTES-glass surface with fluorescent PEG-lipid (without peptides), showing a strong fluorescence on all erythrocyte surfaces, indicating that ligands can be incorporated via PEG-lipid without causing cell damage. (b) Detection of factor H by FACS on rabbit erythrocyte surfaces modified with 5C6 and Sc using PEG-lipid after incubation in human serum. (c) Hemolytic assay of 5C6- and Sc-immobilized rabbit erythrocytes in human serum, used to determine the optimal concentration of 5C6 for coating erythrocyte surfaces. The ratio between 5C6-PEG-lipid and inert MeO-PEG-lipid was varied from 0 to 50%. (Under these conditions, in the absence of complement inhibitors, approximately 99% of the rabbit erythrocytes were hemolyzed after incubation with human serum.) Erythrocyte lysis was calculated as y/(1-y), where y is the fraction lysed. Data shown are means ± SEM (n = 3). Student's t-test was used to compare 5C6 and Sc, *p < 0.05, **p < 0.01, ***p < 0.001. 5C6, which was anchored to erythrocytes by a PEG-lipid, with an optimal effect at peptide concentrations of 1–5%, could protect the cells against complement-mediated lysis.
Supplementary Fig. 4.
Factor H binding and inhibition of complement activation by 5C6- immobilization on PAEC surfaces. PAECs were cultured on APTES-coated glass slides to confluence, and 5C6 and Sc were incorporated into the PAECs using PEG-lipid (0.5 or 5 mg/mL; Fig. 1c, d) and then exposed to blood containing 1.0 IU heparin/mL. Control: PAECs without any modification with PEG-lipid. (a) Detection of biotin-PEG-lipid by FITC-streptavidin at the periphery of all adherent cells with no cell detachment from the substrate surface. (b) Immunofluorescence staining of factor H on PAEC surfaces modified with 5C6 (left) and Sc (right) after incubation in human whole blood. Representative pictures taken by confocal laser-scanning microscopy. (c, d) Generation of C3a and sC5b-9 and (e, f) platelet consumption and TAT generation in human whole blood (1.0 IU/mL heparin) after incubation on 5C6 immobilized-PAEC surfaces at 37 °C for 30 min. The surface of the PAECs was modified with various proportions between 5C6 and PEG-lipid in a total concentration of 0.5 mg/mL (c, e) or 5 mg/mL (d, f) (n = 6). Data shown are means ± SEM. Repeated measures one-way ANOVA with Dunnett's post hoc-test was used to compare all groups with peptide negative PAECs *p < 0.05, **p < 0.01, ***p < 0.001. It is possible to incorporate 5C6 onto the surface of PAECs adhering to glass slides without causing cell damage. The generation of C3a and sC5b-9 on PAECs was suppressed in the surfaces modified with MeO-PEG-lipid when compared to the control PAECs, indicating that the PEG protected the cell surfaces from complement activation. Moreover, a greater reduction of C3a and sC5b-9 was seen in the presence of 5C6 on the PAEC surfaces, with an optimal effect at peptide concentrations of 2–10%.
Supplementary Fig. 5.
Attenuation of platelet and coagulation activation on apyrase-immobilized cell surfaces. Apyrase was immobilized on human erythrocytes using Mal-PEG-lipid. Ten percent of apyrase-coated erythrocytes, PEG-coated erythrocytes or soluble apyrase (250 μg/mL) were added to whole blood (0.05 IU/mL heparin) and incubated for 60 min at 37 °C in a slide chamber model. (a) Platelet consumption, (b) generation of TAT, (c) C3a, (d) sC5b-9. Data shown are means ± SEM (n = 7). Repeated measures one-way ANOVA with Dunnett's post hoc-test was used to compare all groups with control blood (d-g) ***, p < 0.001. When human whole blood was rotated in the slide chamber, the platelet count gradually decreased with time reflecting coagulation and platelet activation, with less decrease in platelet count in whole blood mixed with apyrase-erythrocytes or soluble apyrase, whereas PEG-erythrocytes showed a reduction similar to that of whole blood. TAT levels showed a similar tendency, with significantly lower levels for apyrase-erythrocytes and apyrase compared to PEG-erythrocytes and controls. No differences were observed in complement activation markers among the groups, indicating that the immobilized apyrase did not affect complement.
Optimization of 5C6/apyrase-co-immobilized glass substrate surfaces and cell surface. 5C6/apyrase co-immobilized substrate surface and cell surface were prepared as described in Materials and methods (2.6, 2.7). 5C6 and apyrase were co-immobilized on the surface with different concentrations (apyrase: 0–250 μg/mL, 5C6: 0, 125 μg/mL). (a, c) ATP assay and (b, d) binding of purified factor H were examined to see the activity of apyrase and 5C6 on these combined surfaces of substrate (a, b) and rabbit erythrocyte (c, d). For co-immobilization of 5C6 and apyrase onto the surfaces, the ATP degradation activity was almost the same as apyrase-immobilized surface (a, c), indicating that immobilized the activity of apyrase was not inhibited by the presence of 5C6. Also, the amount of bound factor H was almost the same between 5C6/apyrase co-immobilized surface and 5C6 surface (b, d), indicating that the binding ability of 5C6 is not inhibited by the presence of apyrase on the surface.
References
- 1.Ratner B.D. The catastrophe revisited: blood compatibility in the 21st Century. Biomaterials. 2007;28:5144–5147. doi: 10.1016/j.biomaterials.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nilsson B., Korsgren O., Lambris J.D., Ekdahl K.N. Can cells and biomaterials in therapeutic medicine be shielded from innate immune recognition? Trends Immunol. 2010;31:32–38. doi: 10.1016/j.it.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennet W., Sundberg B., Groth C.G., Brendel M.D., Brandhorst D., Brandhorst H. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48:1907–1914. doi: 10.2337/diabetes.48.10.1907. [DOI] [PubMed] [Google Scholar]
- 4.Moberg L., Johansson H., Lukinius A., Berne C., Foss A., Kallen R. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360:2039–2045. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 5.Gustafson E.K., Elgue G., Hughes R.D., Mitry R.R., Sanchez J., Haglund U. The instant blood-mediated inflammatory reaction characterized in hepatocyte transplantation. Transplantation. 2011;91:632–638. doi: 10.1097/TP.0b013e31820ae459. [DOI] [PubMed] [Google Scholar]
- 6.Diepenhorst G.M., van Gulik T.M., Hack C.E. Complement-mediated ischemia-reperfusion injury: lessons learned from animal and clinical studies. Ann Surg. 2009;249:889–899. doi: 10.1097/SLA.0b013e3181a38f45. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson P.H., Engberg A.E., Back J., Faxalv L., Lindahl T.L., Nilsson B. The creation of an antithrombotic surface by apyrase immobilization. Biomaterials. 2010;31:4484–4491. doi: 10.1016/j.biomaterials.2010.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y.Q., Qu H., Sfyroera G., Tzekou A., Kay B.K., Nilsson B. Protection of nonself surfaces from complement attack by factor H-binding peptides: implications for therapeutic medicine. J Immunol. 2011;186:4269–4277. doi: 10.4049/jimmunol.1003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teramura Y., Kaneda Y., Iwata H. Islet-encapsulation in ultra-thin layer-by-layer membranes of poly(vinyl alcohol) anchored to poly(ethylene glycol)-lipids in the cell membrane. Biomaterials. 2007;28:4818–4825. doi: 10.1016/j.biomaterials.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 10.Teramura Y., Iwata H. Cell surface modification with polymers for biomedical studies. Soft Matter. 2010;6:1081–1091. [Google Scholar]
- 11.Teramura Y., Iwata H. Improvement of graft survival by surface modification with poly(ethylene glycol)-lipid and urokinase in intraportal islet transplantation. Transplantation. 2011;91:271–278. doi: 10.1097/tp.0b013e3182034fa4. [DOI] [PubMed] [Google Scholar]
- 12.Andersson J., Larsson R., Richter R., Ekdahl K.N., Nilsson B. Binding of a model regulator of complement activation (RCA) to a biomaterial surface: surface-bound factor H inhibits complement activation. Biomaterials. 2001;22:2435–2443. doi: 10.1016/s0142-9612(00)00431-2. [DOI] [PubMed] [Google Scholar]
- 13.Platts-Mills T.A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974;113:348–358. [PubMed] [Google Scholar]
- 14.Nilsson U.R., Nilsson B. Simplified assays of hemolytic activity of the classical and alternative complement pathways. J Immunol Methods. 1984;72:49–59. doi: 10.1016/0022-1759(84)90432-0. [DOI] [PubMed] [Google Scholar]
- 15.Luan N.M., Teramura Y., Iwata H. Immobilization of soluble complement receptor 1 on islets. Biomaterials. 2011 doi: 10.1016/j.biomaterials.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Hong J., Nilsson Ekdahl K., Reynolds H., Larsson R., Nilsson B. A new in vitro model to study interaction between whole blood and biomaterials. Studies of platelet and coagulation activation and the effect of aspirin. Biomaterials. 1999;20:603–611. doi: 10.1016/s0142-9612(98)00210-5. [DOI] [PubMed] [Google Scholar]
- 17.Ekdahl K.N., Nilsson B., Pekna M., Nilsson U.R. Generation of IC3 at the interface between blood and gas. Scand J Immunol. 1992;35:85–91. doi: 10.1111/j.1365-3083.1992.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 18.Jackson R.W., Basinger S.F., Werth J.M. Use of a logistic function in expressing kinetic hemolysis data. Infect Immun. 1970;1:142–145. doi: 10.1128/iai.1.2.142-145.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hessing M., Vlooswijk R.A., Hackeng T.M., Kanters D., Bouma B.N. The localization of heparin-binding fragments on human C4b-binding protein. J Immunol. 1990;144:204–208. [PubMed] [Google Scholar]
- 20.Schmidt C.Q., Herbert A.P., Hocking H.G., Uhrin D., Barlow P.N. Translational mini-review series on complement factor H: structural and functional correlations for factor H. Clin Exp Immunol. 2008;151:14–24. doi: 10.1111/j.1365-2249.2007.03553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engberg A.E., Sandholm K., Bexborn F., Persson J., Nilsson B., Lindahl G. Inhibition of complement activation on a model biomaterial surface by streptococcal M protein-derived peptides. Biomaterials. 2009;30:2653–2659. doi: 10.1016/j.biomaterials.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsson U.R., Storm K.E., Elwing H., Nilsson B. Conformational epitopes of C3 reflecting its mode of binding to an artificial polymer surface. Mol Immunol. 1993;30:211–219. doi: 10.1016/0161-5890(93)90050-l. [DOI] [PubMed] [Google Scholar]
- 23.Andersson J., Ekdahl K.N., Lambris J.D., Nilsson B. Binding of C3 fragments on top of adsorbed plasma proteins during complement activation on a model biomaterial surface. Biomaterials. 2005;26:1477–1485. doi: 10.1016/j.biomaterials.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Tengvall P., Askendal A., Lundstrom I. Temporal studies on the deposition of complement on human colostrum IgA and serum IgG immobilized on methylated silicon. J Biomed Mater Res. 1997;35:81–92. doi: 10.1002/(sici)1097-4636(199704)35:1<81::aid-jbm8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 25.Andersson J., Ekdahl K.N., Larsson R., Nilsson U.R., Nilsson B. C3 adsorbed to a polymer surface can form an initiating alternative pathway convertase. J Immunol. 2002;168:5786–5791. doi: 10.4049/jimmunol.168.11.5786. [DOI] [PubMed] [Google Scholar]
- 26.Murakami Y., Iwata H., Kitano E., Kitamura H., Ikada Y. Interaction of poly(styrene sulfonic acid) with the alternative pathway of the serum complement system. J Biomater Sci Polym Ed. 2005;16:381–395. doi: 10.1163/1568562053654095. [DOI] [PubMed] [Google Scholar]
- 27.Andersson J., Bexborn F., Klinth J., Nilsson B., Ekdahl K.N. Surface-attached PEO in the form of activated Pluronic with immobilized factor H reduces both coagulation and complement activation in a whole-blood model. J Biomed Mater Res A. 2006;76:25–34. doi: 10.1002/jbm.a.30377. [DOI] [PubMed] [Google Scholar]
- 28.Wu J., Wu Y.Q., Ricklin D., Janssen B.J., Lambris J.D., Gros P. Structure of complement fragment C3b-factor H and implications for host protection by complement regulators. Nat Immunol. 2009;10:728–733. doi: 10.1038/ni.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serruto D., Rappuoli R., Scarselli M., Gros P., van Strijp J.A. Molecular mechanisms of complement evasion: learning from staphylococci and meningococci. Nat Rev Microbiol. 2010;8:393–399. doi: 10.1038/nrmicro2366. [DOI] [PubMed] [Google Scholar]
- 30.Markiewski M.M., Nilsson B., Ekdahl K.N., Mollnes T.E., Lambris J.D. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28:184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Hamad O.A., Ekdahl K.N., Nilsson P.H., Andersson J., Magotti P., Lambris J.D. Complement activation triggered by chondroitin sulfate released by thrombin receptor-activated platelets. J Thromb Haemost. 2008;6:1413–1421. doi: 10.1111/j.1538-7836.2008.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cattaneo M., Gachet C. ADP receptors and clinical bleeding disorders. Arterioscler Thromb Vasc Biol. 1999;19:2281–2285. doi: 10.1161/01.atv.19.10.2281. [DOI] [PubMed] [Google Scholar]
- 33.Marcus A.J., Broekman M.J., Drosopoulos J.H., Islam N., Alyonycheva T.N., Safier L.B. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest. 1997;99:1351–1360. doi: 10.1172/JCI119294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eltzschig H.K., Thompson L.F., Karhausen J., Cotta R.J., Ibla J.C., Robson S.C. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 35.Grenz A., Osswald H., Eckle T., Yang D., Zhang H., Tran Z.V. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reutershan J., Cagnina R.E., Chang D., Linden J., Ley K. Therapeutic anti-inflammatory effects of myeloid cell adenosine receptor A2a stimulation in lipopolysaccharide-induced lung injury. J Immunol. 2007;179:1254–1263. doi: 10.4049/jimmunol.179.2.1254. [DOI] [PubMed] [Google Scholar]
- 37.Van Linden A., Eltzschig H.K. Role of pulmonary adenosine during hypoxia: extracellular generation, signaling and metabolism by surface adenosine deaminase/CD26. Expert Opin Biol Ther. 2007;7:1437–1447. doi: 10.1517/14712598.7.9.1437. [DOI] [PubMed] [Google Scholar]
- 38.Linden J. Adenosine in tissue protection and tissue regeneration. Mol Pharmacol. 2005;67:1385–1387. doi: 10.1124/mol.105.011783. [DOI] [PubMed] [Google Scholar]
- 39.Fredholm B. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 40.Eckle T., Fullbier L., Wehrmann M., Khoury J., Mittelbronn M., Ibla J. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol. 2007;178:8127–8137. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 41.Volmer J.B., Thompson L.F., Blackburn M.R. Ecto-5'-nucleotidase (CD73)-mediated adenosine production is tissue protective in a model of bleomycin-induced lung injury. J Immunol. 2006;176:4449–4458. doi: 10.4049/jimmunol.176.7.4449. [DOI] [PubMed] [Google Scholar]
- 42.Eltzschig H.K., Ibla J.C., Furuta G.T., Leonard M.O., Jacobson K.A., Enjyoji K. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Optimization of 5C6/apyrase-co-immobilized glass substrate surfaces and cell surface. 5C6/apyrase co-immobilized substrate surface and cell surface were prepared as described in Materials and methods (2.6, 2.7). 5C6 and apyrase were co-immobilized on the surface with different concentrations (apyrase: 0–250 μg/mL, 5C6: 0, 125 μg/mL). (a, c) ATP assay and (b, d) binding of purified factor H were examined to see the activity of apyrase and 5C6 on these combined surfaces of substrate (a, b) and rabbit erythrocyte (c, d). For co-immobilization of 5C6 and apyrase onto the surfaces, the ATP degradation activity was almost the same as apyrase-immobilized surface (a, c), indicating that immobilized the activity of apyrase was not inhibited by the presence of 5C6. Also, the amount of bound factor H was almost the same between 5C6/apyrase co-immobilized surface and 5C6 surface (b, d), indicating that the binding ability of 5C6 is not inhibited by the presence of apyrase on the surface.