Abstract
Cystathionine β-synthase (CBS) is a key enzyme in human (patho)physiology with a central role in hydrogen sulfide metabolism. The enzyme is composed of a pyridoxal 5′-phosphate-binding catalytic domain, flanked by the following two domains: a heme-binding N-terminal domain and a regulatory C-terminal domain binding S-adenosyl-l-methionine (AdoMet). CO or NO• binding at the ferrous heme negatively modulates the enzyme activity. Conversely, AdoMet binding stimulates CBS activity. Here, we provide experimental evidence for a functional communication between the two domains. We report that AdoMet binding significantly enhances CBS inhibition by CO. Consistently, we observed increased affinity (∼5-fold) and faster association (∼10-fold) of CO to the ferrous heme at physiological AdoMet concentrations. NO• binding to reduced CBS was also enhanced by AdoMet, although to a lesser extent (∼2-fold higher affinity) as compared with CO. Importantly, CO and NO• binding was unchanged by AdoMet in a truncated form of CBS lacking the C-terminal regulatory domain. These unprecedented observations demonstrate that CBS activation by AdoMet puzzlingly sensitizes the enzyme toward inhibition by exogenous ligands, like CO and NO•. This further supports the notion that CBS regulation is a complex process, involving the concerted action of multiple physiologically relevant effectors.
Keywords: allosteric regulation, carbon monoxide, heme, hydrogen sulfide, nitric oxide, S-adenosylmethionine, signal transduction
Introduction
Cystathionine β-synthase (CBS)3 is a key enzyme in the trans-sulfuration pathway of methionine metabolism, a pathway leading to cysteine and, ultimately, glutathione synthesis (Fig. 1) (1). Besides this role, CBS accomplishes two important functions: (i) it represents one of the major enzymatic sources of hydrogen sulfide (H2S), and (ii) it contributes to homocysteine (Hcy) homeostasis. Cystathionine γ-lyase, the enzyme that catalyzes cystathionine degradation downstream of CBS in the trans-sulfuration pathway, is also able to catalyze alternative reactions leading to H2S production (2).
FIGURE 1.
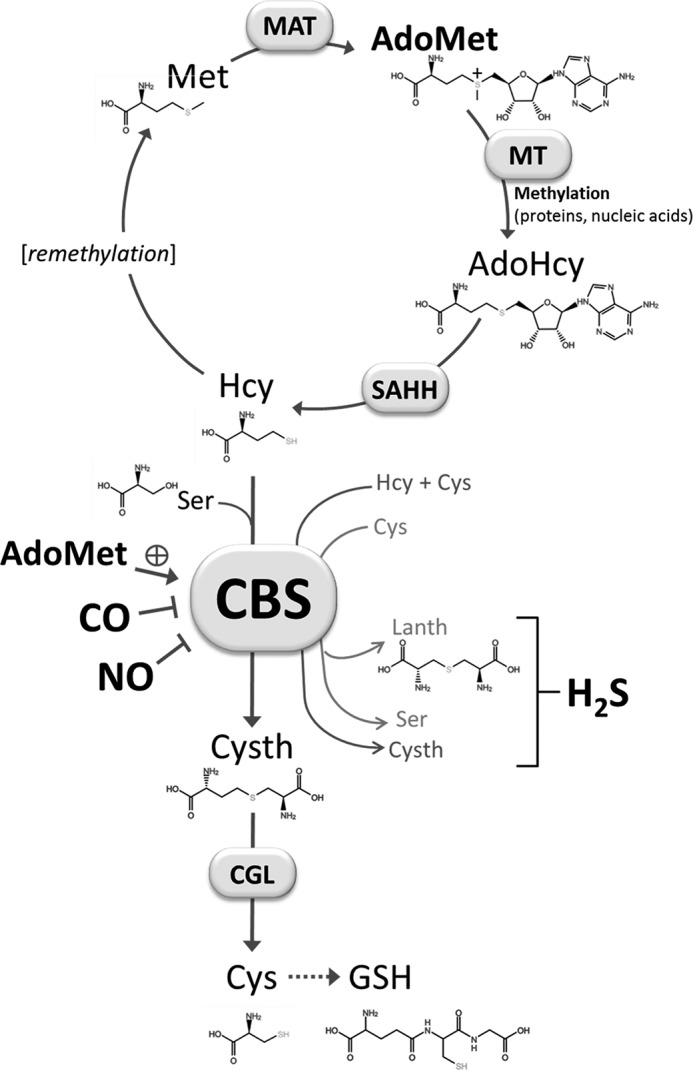
CBS and the trans-sulfuration pathway of the methionine cycle. CBS catalyzes the condensation of homocysteine (Hcy) with cysteine (Cys) or serine (Ser) forming cystathionine (Cysth) and either H2S or H2O. CBS can also catalyze the conversion of Cys into Ser or lanthionine (Lanth) with concomitant production of H2S. CBS is allosterically activated by AdoMet and inhibited by NO• and CO. Met, methionine; MAT, methionine adenosyltransferase; MT, methyltransferases (involved in methylation reactions); SAHH, S-adenosyl-l-homocysteine hydrolase; CGL, cystathionine γ-lyase; GSH, glutathione.
Together with NO• and CO, H2S has been recognized as a “gasotransmitter” implicated in the regulation of many physiological processes (3–5), such as vasodilation, cellular stress response, inflammation, apoptosis, and energy metabolism (5–7). Altered H2S metabolism associated with CBS has been proposed to be a key event in multiple human diseases, such as colorectal and ovarian cancer (8, 9). This led to the recognition of CBS as an important target for the discovery of inhibitors with therapeutic potential (10).
Human CBS can assemble as a homotetramer, although there is evidence for other oligomeric arrangements (11–13). Each 63-kDa monomer is composed of a central pyridoxal 5′-phosphate (PLP)-binding catalytic domain, flanked by the following two domains: the b-type heme-binding N-terminal domain and the regulatory C-terminal domain binding S-adenosyl-l-methionine (AdoMet) (11–13). The heme-binding domain is considered an evolutionary hallmark, because it is only found in CBS from multicellular organisms (13). Both in the ferric and ferrous state, the heme iron is low spin and hexa-coordinated, being axially bound to His-65 and Cys-52 (human CBS numbering). With the heme in the ferric state, the enzyme is fully active, whereas heme reduction was reported to promote a slow (>20 min at 37 °C) enzyme inactivation (14). Despite its low reduction potential (−350 mV), the CBS heme has been shown to be enzymatically reduced by methionine synthase reductase at the expense of NADPH oxidation (15, 16). The ability of the CBS ferrous heme to bind gaseous ligands like CO and NO•, resulting in enzyme inhibition, makes it a remarkable redox-sensitive sensor, which confers to the H2S-synthesizing CBS a central role in the interplay between the three gasotransmitters (15, 17–20).
Several observations support the (patho)physiological relevance of the heme-based regulation of CBS by CO (21). As an example, in a mouse model of brain hypoxia, cerebral vasodilation has been reported to be associated with CBS regulation by CO (22). Heme oxygenase, a CO-generating O2 sensor, is indeed down-regulated in hypoxia, and the reduced CO levels have been shown to result in de-repression of CBS-catalyzed H2S generation, leading to vasodilation of the precapillary arterioles (22). Experimental evidence also supports a physiological role of NO• in the regulation of CBS (23).
Contrary to the heme-based negative regulation, AdoMet binding to the C-terminal domain (also known as Bateman module) stimulates CBS activity by 2–5-fold (24), and it was reported to stabilize the enzyme against proteolysis (25, 26). AdoMet is not only an intermediate in the methionine cycle, but importantly it is also the universal methyl donor in methylation reactions in human metabolism. CBS regulation by AdoMet has been recognized historically as physiologically relevant (24). Defects in this regulatory mechanism are currently considered as playing a role in the pathogenesis of classic homocystinuria (27, 28), the inherited error of metabolism associated with CBS deficiency. More recently, AdoMet has been shown to markedly modulate the proliferation of colorectal cancer cells as compared with non-tumorigenic cells (29).
An increasing number of structural and functional data have been reported over the past few years to elucidate the molecular mechanism of CBS activation by AdoMet (11, 12, 26, 30, 31). Two AdoMet-binding sites with different affinity and distinct functional and structural roles have been proposed (26). The crystallographic structure of the full-length human CBS with all three domains shows that in the absence of AdoMet the C-terminal domain reduces the substrate accessibility to the PLP site in the catalytic core (11). Conversely, the structure obtained in the presence of AdoMet shows that, upon AdoMet binding, two C-terminal domains from adjacent monomers associate, thereby increasing the active site accessibility and in turn the enzyme activity (12). This model is further supported by the observation that the truncated C-terminal regulatory domain (lacking the first 405 residues composing the N-terminal and catalytic domains) assembles as a dimer in the presence of AdoMet (31).
Herein, we provide experimental evidence for a functional communication between the AdoMet-binding domain and the heme moiety in human CBS. We report that AdoMet at physiological concentrations, without affecting the redox properties of the heme sensor, enhances its ability to bind the gasotransmitters CO and NO•, thereby sensitizing CBS toward inhibition. These unprecedented observations provide new insights into the mechanism of CBS and its complex in vivo regulation.
Experimental Procedures
Expression and Purification of CBS
Recombinant full-length human CBS was produced and purified as described previously (19). Site-directed mutagenesis was employed to generate a vector expressing a CBS truncated form devoid of the C-terminal 143 residues and thus lacking the AdoMet-binding domain. The 1227G>A mutant (cDNA numbering) carrying a premature stop codon at position 409 was generated from the full-length CBS-expressing pET28b-based vector, using the XL QuikChange kit (Agilent) and the primers 5′-GAAGAAGCCCTGGTGATGGCACCTCCGTG (forward) and 5′-CACGGAGGTGCCATCACCAGGGCTTCTTC (reverse). The resulting pET28b-ΔhCBS construct was used to transform Escherichia coli BL21(DE3) Rosetta cells. Cells were grown in Luria-Bertani (LB) medium supplemented with 25 μg/ml kanamycin (NZYTech) and 34 μg/ml chloramphenicol (NZYTech) at 37 °C and 140 rpm. At A600 nm = 0.4–0.5, protein expression was induced by addition of 100 μm isopropyl β-d-thiogalactopyranoside (NZYTech), followed by supplementation with 37.5 mg/liter δ-aminolevulinic acid (Sigma). After a 16-h incubation at 25 °C and 120 rpm, cells were harvested by centrifugation and resuspended in 50 mm potassium phosphate, 300 mm KCl, 10% glycerol, pH 7.0 (buffer A, 10 ml was used to resuspend cells from 1 liter of culture), containing 1 mm phenylmethylsulfonyl fluoride (Merck), 1 mg/ml lysozyme (Applichem), and deoxyribonuclease I (Applichem). Following a 30-min incubation on ice, cells were lysed by sonication and centrifuged at 8200 × g for 10 min at 4 °C. The cleared supernatant was supplemented with 10 mm imidazole and loaded at 2.5 ml/min onto a HisTrap FF crude 5-ml column (GE Healthcare) previously equilibrated with buffer A containing 10 mm imidazole (buffer B). Protein purification was carried out in an ÅKTA Prime fast performance liquid chromatography system (GE Healthcare). After washing the column with 15 column volumes of buffer B at 5 ml/min, the protein was eluted with 20-column volumes of linear gradient up to 500 mm imidazole. Pooled fractions were concentrated with an Amicon Ultra-15 centrifugal filter unit with an Ultracel-30 membrane (Millipore), and the protein was further purified by size exclusion chromatography, using a HiLoad 26/600 Superdex S200 column (GE Healthcare) previously equilibrated with buffer A containing 20 μm PLP (buffer C). The protein was loaded and eluted with buffer C at 0.5 ml/min. The purity of the isolated protein was assessed by SDS-PAGE. Protein concentration was assessed by the Bradford method (32), and the concentration of the ferric heme in the isolated protein was determined using ϵ428 nm = 92,700 m−1 cm−1 (33). Unless otherwise stated, the experiments were carried out in buffer A.
H2S Synthesis by CBS
H2S production by recombinant human CBS was measured at 37 °C in buffer A supplemented with 100 μm EDTA. The reaction was carried out in a thermostated cuvette under stirring. Anaerobic conditions were ensured by adding glucose (3 mm), glucose oxidase (4 units/ml), catalase (13 μg/ml), and SOD (6 units/ml) to nitrogen-equilibrated buffer and adding 300 μl of mineral oil on top of the 1-ml reaction mixture. Briefly, CBS (1.3 μm) was incubated for ∼10 min with PLP (50 μm), Hcy (400 μm), and AdoMet (0 or 500 μm) and reduced with sodium dithionite (225 μm) prior to the addition of CO (from 0 to 50 μm). Afterward, the reaction was triggered by addition of cysteine (10 mm), and the H2S produced by AdoMet-bound or AdoMet-free CBS was measured in an Agilent Cary-60 spectrophotometer by the lead acetate assay (34) and the methylene blue (35) method, respectively. Because of its higher sensitivity, the latter method proved more appropriate to measure the relatively slow H2S production catalyzed by the reduced AdoMet-free protein, particularly in the presence of CO. Importantly, both methods were internally calibrated, and data were normalized with reference to the activity measured in the absence of CO.
CO and NO• Affinity for CBS
Anaerobic titrations of CBS with CO and NO• were monitored at 20 °C by UV-visible absorption spectroscopy in an Agilent Cary-60 spectrophotometer, using a rubber-cap sealed quartz cuvette, made anaerobic by nitrogen flushing prior to liquid transfer. CBS (1.0–2.3 or 1.0–2.9 μm in heme for full-length or truncated CBS, respectively) was flushed with nitrogen and transferred anaerobically into the cuvette. Glucose oxidase (4 units/ml), catalase (13 μg/ml), SOD (60 units/ml), and glucose (3 mm) were added to scavenge contaminant oxygen, hydrogen peroxide, and superoxide anion. CBS was reduced with sodium dithionite (final concentration from 11.3 to 45 μm), diluted from a 45 mm stock solution (quantitated using ϵ314 nm = 8043 m−1 cm−1 (36)). CO stock solutions were prepared by equilibrating thoroughly degassed buffer A with the pure gas at 1 atm, yielding 1 mm CO at 20 °C. NO• stock solutions were prepared by equilibrating degassed ultra-pure water with authentic NO• gas at 1 atm, and kept on ice protected from light. After each CO or NO• addition with gas-tight Hamilton syringes, spectra were recorded, and the absorption changes were visually inspected in real time. When no more changes were observed, a new addition was immediately made.
In agreement with Refs. 17, 19, 37, two apparent Kd values (Kd1 and Kd2) had to be assumed to satisfactorily fit the CO data. The Kd1 and Kd2 values were determined by fitting the data to Equation 1,
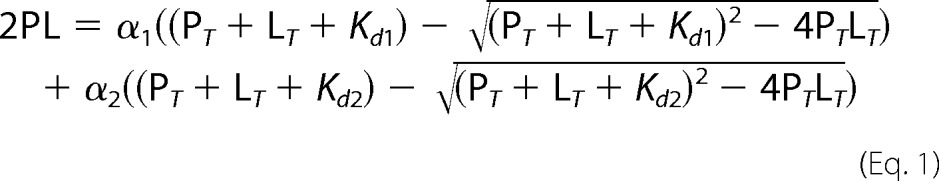 |
where PL is the concentration of CO-bound CBS; PT and LT are the total concentrations of CBS and CO, respectively, and α1 and α2 are the protein fractions binding CO at higher (Kd1) and lower (Kd2) affinities, respectively. For NO• data fitting, a single Kd value was employed as in Ref.19, according to Equation 2,
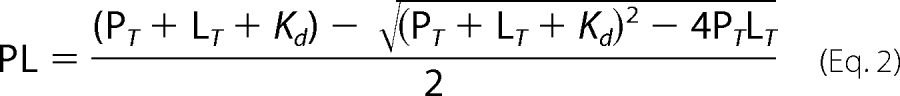 |
Stopped-flow Measurements
Stopped-flow experiments were carried out in a thermostated instrument (DX.17MV, Applied Photophysics, Leatherhead, UK), equipped with a photodiode-array (light path, 1 cm). To avoid light artifacts, the intensity of the white-light incident beam was decreased, and a filter cutting UV light at λ <360 nm was used. Absorption spectra were recorded with an acquisition time of 10 ms per spectrum according to a logarithmic time scale. All reactions were investigated at 25 °C in buffer A. CBS was flushed with nitrogen, and glucose oxidase (4 units/ml), catalase (13 μg/ml), SOD (6 units/ml), and glucose (2 mm) were added to scavenge oxygen, hydrogen peroxide, and superoxide anion. The protein was then placed on ice, protected from light to prevent possible damaging photoreactions. CBS was reduced by addition of 90 μm sodium dithionite and, when indicated, incubated with AdoMet (or S-adenosyl-l-homocysteine (AdoHcy)) for ≥10 min. Reduced CBS in the absence or presence of AdoMet (or AdoHcy) was finally mixed in the stopped-flow apparatus with solutions containing CO, NO•, or O2, and the spectra were recorded over time.
Spectral Data Analysis
Data were analyzed with the software MATLAB (Mathworks, South Natick, MA). Global fit analysis of the spectral data were performed by singular value decomposition analysis combined with curve fitting (38).
Results
AdoMet Enhances CBS Inhibition by CO
The H2S-producing activity of ferrous CBS at increasing CO concentrations was measured using Hcy and cysteine as substrates, before and after incubating the protein with AdoMet. As shown in Fig. 2, AdoMet binding to CBS markedly enhanced CO inhibition, lowering the apparent Ki value for CO from 9.5 ± 1.0 μm (Fig. 2, solid symbols) to 0.7 ± 0.1 μm (open symbols). Differently from AdoMet, AdoHcy did not enhance CO inhibition; accordingly, in control experiments, addition of 10 μm CO yielded only 40–50% inhibition of CBS regardless of the presence or absence of 500 μm AdoHcy.
FIGURE 2.
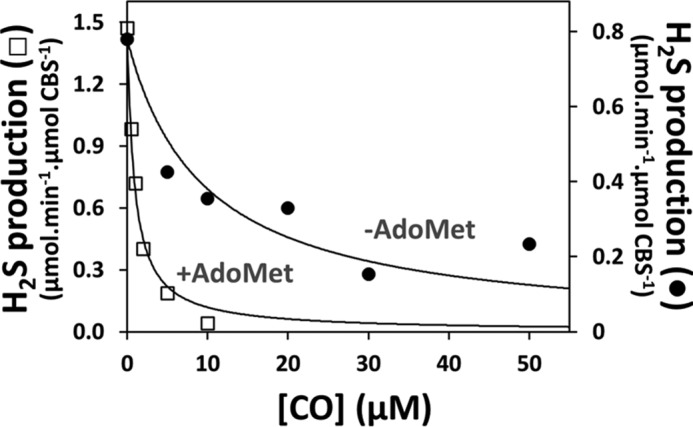
AdoMet enhances CO inhibition of H2S production by CBS. H2S-producing activity of CBS was measured at 37 °C at the indicated CO concentrations in the absence (Ki(CO) = 9.5 ± 1.0 μm) or presence of 500 μm AdoMet (Ki(CO) = 0.7 ± 0.1 μm). Reaction buffer consisted of 50 mm KPi, 300 mm KCl, 10% glycerol, 100 μm EDTA, pH 7.0. Values are expressed as micromoles of H2S/min/μmol of CBS.
AdoMet Enhances CBS Affinity for CO
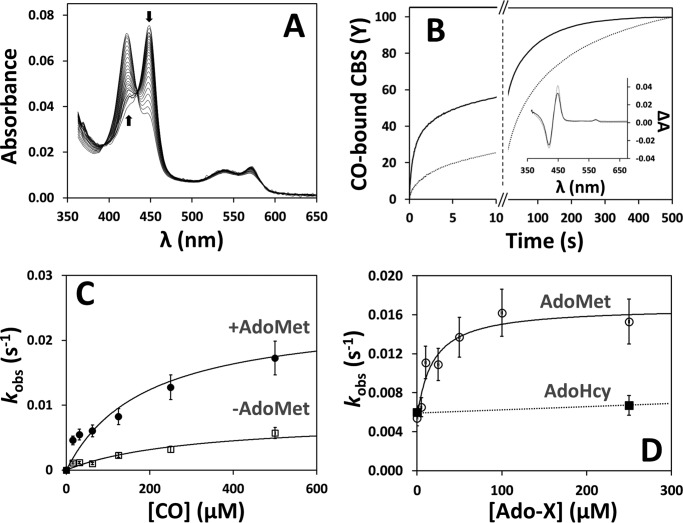
The effect of AdoMet on the affinity of ferrous CBS for CO was investigated by anaerobic CO titrations of the enzyme before and after incubation with AdoMet, monitored by UV-visible absorption spectroscopy (Fig. 3). CO binding to AdoMet-free or AdoMet-bound CBS resulted in a shift of the Soret band from 449 nm (reduced CBS) to 422 nm (CO-bound CBS) (Fig. 3A). Interestingly, as compared with the AdoMet-free enzyme, global fit of the spectral data revealed a significantly higher CO affinity of AdoMet-bound CBS (Fig. 3B), consistent with its higher sensitivity toward CO inhibition. In agreement with the literature (17, 19, 37), the titration profiles were satisfactorily fitted only by assuming two Kd values. Whereas 90% of AdoMet-bound CBS displayed higher CO affinity (Kd1 = 4.5 ± 1.1 μm) and only 10% lower affinity (Kd2 = 34 ± 3 μm), the vast majority (80%) of the AdoMet-free CBS displayed lower affinity for CO (Kd2 = 45 ± 16 μm) and only 20% higher affinity (Kd1 = 0.7 ± 0.6 μm). Overall, binding of AdoMet to CBS resulted in an ∼5-fold decrease (from 29 ± 4 to 6 ± 1 μm) of the C50 value for CO binding.
FIGURE 3.
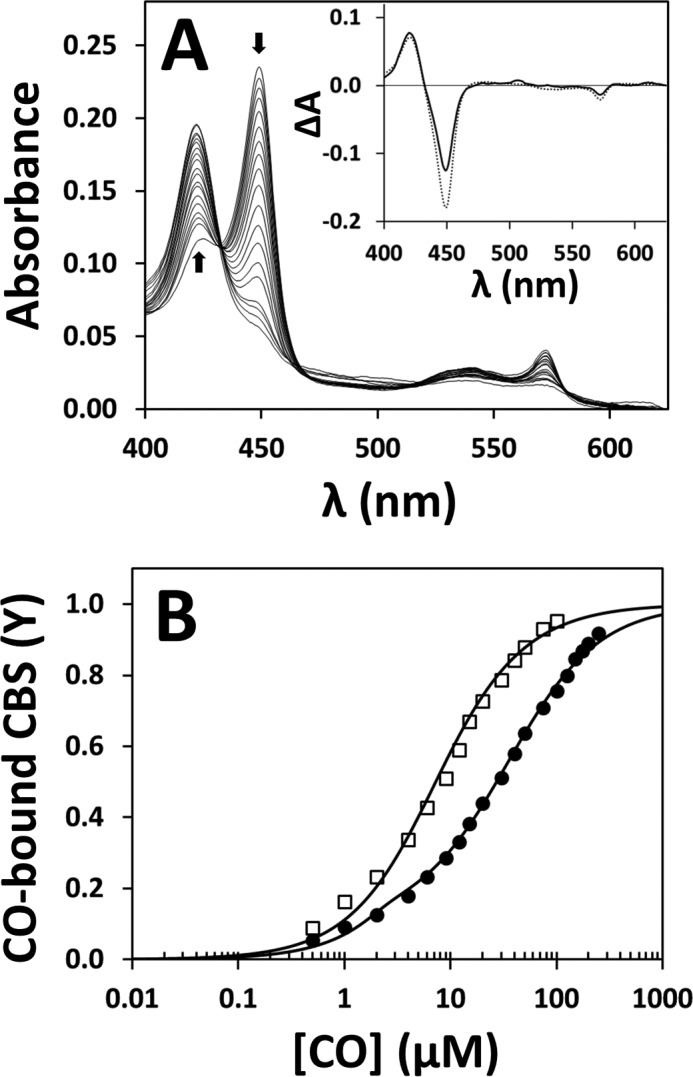
AdoMet enhances the affinity of CBS for CO. A, absorption spectra collected during anaerobic titration of reduced CBS (2.2 μm in heme) with CO, in the absence of AdoMet. Arrows depict the direction of absorption changes. T = 20 °C. Inset, optical transitions detected on CO binding to CBS in the presence (solid line) or absence (dotted line) of 500 μm AdoMet. B, titration profiles obtained by global fit of the spectral data acquired in the absence (solid circles) or presence (open squares) of 500 μm AdoMet. Data were fitted according to Equation 1, yielding the following: Kd1 = 4.5 ± 1.1 μm (90%) and Kd2 = 34 ± 3 μm (10%) for AdoMet-bound CBS (C50 = 6 ± 1 μm), and Kd1 = 0.7 ± 0.6 μm (20%) and Kd2 = 45 ± 16 μm (80%) for AdoMet-free CBS (C50 = 29 ± 4 μm).
AdoMet Enhances the Kinetics of CO Binding to CBS
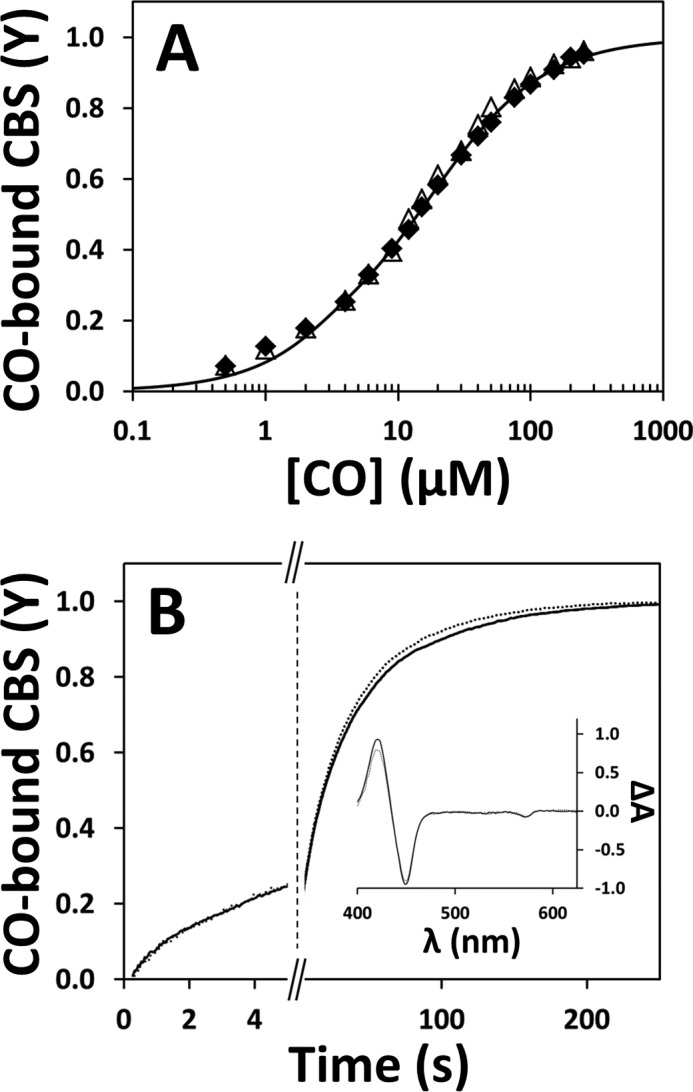
The higher CO affinity displayed by AdoMet-bound CBS prompted us to investigate by time-resolved absorption spectroscopy the effect of AdoMet on the kinetics of CO binding (Fig. 4). When CO (1 mm before mixing) was stopped-flow mixed with either the AdoMet-free or the AdoMet-bound ferrous CBS, the 449-nm Soret band shifted to 422 nm (Fig. 4A). Global fit analysis of the kinetic data revealed that both AdoMet-free and AdoMet-bound CBS react with CO according to biphasic time courses (Fig. 4B), with a major slow phase accounting for ∼60 and ∼80% of the overall amplitude in the presence or absence of AdoMet, respectively. Interestingly, in agreement with the increased affinity, CO binding to AdoMet-bound CBS (Fig. 4B, solid line) proceeded at higher rates compared with the AdoMet-free enzyme (Fig. 4B, dotted line), with AdoMet causing an ∼10-fold decrease of the reaction half-time (from 55 to 5.5 s). No effect on the CO binding kinetics was observed in control experiments carried out with AdoHcy (250 μm after mixing) in place of AdoMet (Fig. 4D).
FIGURE 4.
AdoMet elicits faster CO binding to CBS. A, spectra collected over 500 s after stopped-flow mixing 1 mm CO with reduced CBS (1.5 μm in heme) at 25 °C in 50 mm potassium phosphate, 300 mm KCl, 10% glycerol, pH 7.0, containing 2 mm glucose, 4 units/ml glucose oxidase, 13 μg/ml catalase, and 6 units/ml SOD. Arrows depict the direction of absorption changes. B, reaction time courses measured in the absence (dotted line) or presence of AdoMet (500 μm before mixing; solid line). Fitted rate constants (% reaction amplitude) are as follows: k1 = 0.60 s−1 (40%) and k2 = 0.011 s−1 (60%) for AdoMet-bound CBS (t½ = 5.5 s) and k1 = 0.24 s−1 (20%) and k2 = 0.007 s−1 (80%) for AdoMet-free CBS (t1/2 = 55 s). As discussed in the text, k1 displayed a larger preparation-to-preparation variability compared with k2. Inset, optical transitions obtained by global fit analysis of the spectral data acquired in the absence (dotted line) or presence of AdoMet (solid line). C, CO concentration dependence of the observed rate constant (major kinetic phase) measured for CO binding to AdoMet-bound and AdoMet-free CBS ([AdoMet] = 500 μm before mixing). Data were fitted to a hyperbola yielding klim′ = 0.024 and 0.008 s−1 for AdoMet-bound and AdoMet-free CBS, respectively. D, observed rate constants (major kinetic phase) measured at 500 μm CO at the indicated AdoMet or AdoHcy concentration. Data for AdoMet were fitted to a hyperbola yielding C50 = 18.3 ± 2.7 μm AdoMet and klim′ = 0.017 s−1.
Both in the presence and absence of AdoMet, the observed rate constant relative to the major slow phase (k2′) followed a hyperbolic CO concentration dependence, with a markedly higher plateau value (k2(lim)′) for AdoMet-bound CBS (0.024 s−1 versus 0.008 s−1, Fig. 4C). In the absence of AdoMet, the rate constant of the minor fast phase (k1′) displayed a similar hyperbolic dependence on CO concentration yielding a plateau k1(lim)′ value of 0.05 s−1. Conversely, in the presence of AdoMet, independently of [CO], k1′ was ∼0.07–0.08 s−1, i.e. ∼50% higher than the k1(lim)′ value estimated in the absence of AdoMet. It should be noted, however, that among the different protein preparations tested, this minor kinetic phase displayed a larger variability compared with the major kinetic phase. Indeed, it can be appreciated that the traces in Fig. 4B are best fitted with k1′ values (0.24 and 0.60 s−1, in the absence or presence of AdoMet, respectively) significantly higher than those reported above (0.05 and ∼0.07–0.08 s−1, respectively), which were obtained from the three independent datasets that originated Fig. 4, C and D. We then analyzed the kinetics of the reaction between reduced CBS and 1 mm CO at varying AdoMet concentrations (from 0 to 500 μm before mixing). As shown in Fig. 4D, data analysis revealed a hyperbolic dependence of the rate constant of the major phase (k2′) on [AdoMet], best fitted with a C50 value of 18.3 ± 2.7 μm, and showing a nearly saturating effect at physiological concentrations (50–80 μm (39)) of AdoMet. Within the limits mentioned above, a similar hyperbolic dependence on AdoMet concentration was observed also for k1′, with the rate constant increasing from 0.03 s−1 in the absence of AdoMet to 0.08 s−1 at saturating [AdoMet] and an apparent C50 value of 12.0 ± 4.0 μm. The effect of AdoMet on the koff of CO was then evaluated by anaerobically mixing the Fe(II)-CO CBS adduct with authentic NO• (900 μm after mixing) and monitoring the decay of the 422 nm CO-bound adduct. In these experiments, CO dissociation proved to be slightly faster in the presence of AdoMet (500 μm before mixing) than in its absence (Fig. 7A). Importantly, no effect of AdoMet on the affinity or kinetics of CO binding was observed in control experiments carried out on a truncated form of the enzyme lacking the AdoMet-binding C-terminal domain (Fig. 5).
FIGURE 7.
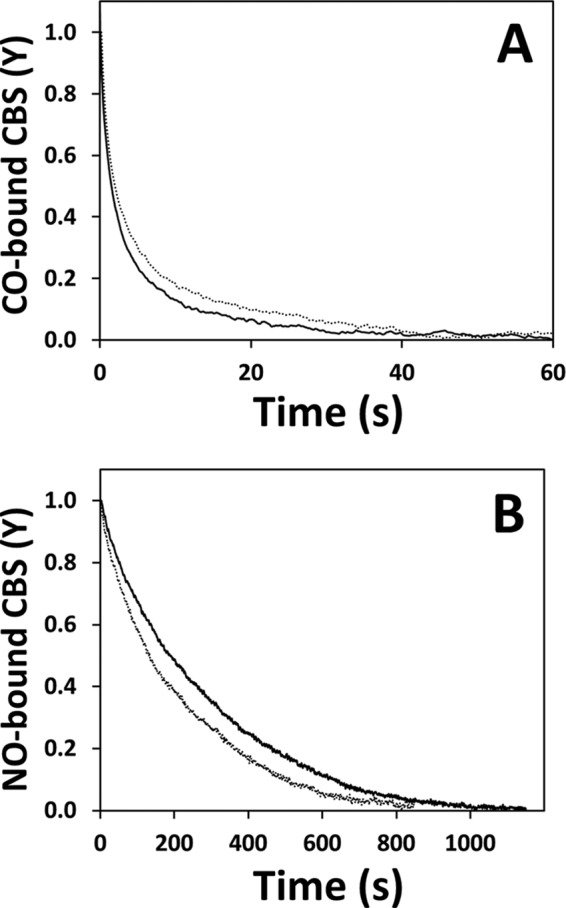
Effect of AdoMet on CO and NO• displacement from reduced CBS. A, time courses of CO displacement from ferrous CBS by 900 μm NO•, acquired in the absence (dotted line) or presence of AdoMet (solid line; 500 μm before mixing). λ = 422 nm. Traces were best fitted with the following rate constants (% reaction amplitude): k1 = 0.55 s−1 (60%) and k2 = 0.04 s−1 (40%) for AdoMet-free CBS; k1 = 0.91 s−1 (60%) and k2 = 0.11 s−1 (40%) for AdoMet-bound CBS. B, time courses of NO• displacement from ferrous CBS by CO and excess dithionite (1 and 200 mm before mixing, respectively) acquired in the absence (dotted line) or presence of AdoMet (solid line; 500 μm before mixing). λ = 422 nm. Traces were best fitted with the rate constants kobs = 0.004 s−1 for AdoMet-free CBS and kobs = 0.003 s−1 for AdoMet-bound CBS.
FIGURE 5.
AdoMet has no effect on CO binding to truncated CBS. A, CO titration profiles of reduced truncated CBS in the absence (solid symbols) or presence (open symbols) of 500 μm AdoMet. The two data sets were fitted to Equation 1 yielding Kd1 = 0.5 ± 0.4 μm (10%) and Kd2 = 16 ± 2 μm (90%), consistent with a C50 of 12 ± 1 μm. Experimental conditions are as in Fig. 3. B, reaction time courses acquired after stopped-flow mixing reduced truncated CBS (1.0 μm in heme) with 1 mm CO in the absence (dotted line) or presence of AdoMet (500 μm before mixing; solid line). Regardless of AdoMet, data fitting yielded the following rate constant (% reaction amplitude): k1 = 0.22 s−1 (20%) and k2 = 0.025 s−1 (80%). Inset, optical transitions obtained by global fit analysis of the spectral data acquired in the absence (dotted line) or presence of AdoMet (solid line).
AdoMet Enhances the Affinity and Binding Kinetics of NO• to CBS
We previously reported tighter and faster binding of NO• to human CBS with respect to CO (19). Here, we investigated the effect of AdoMet on NO• binding. By anaerobic NO• titrations of ferrous CBS in the presence of 45 μm dithionite, we observed ∼2-fold tighter binding in the presence of AdoMet (Kd = 0.5 ± 0.1 μm) than in its absence (Kd = 1.0 ± 0.2 μm) (Fig. 6A). It should be noted that the Kd value determined here for the AdoMet-free enzyme (1.0 ± 0.2 μm) is in good agreement with the value (1.84 μm) previously determined at the same dithionite concentration (19).
FIGURE 6.
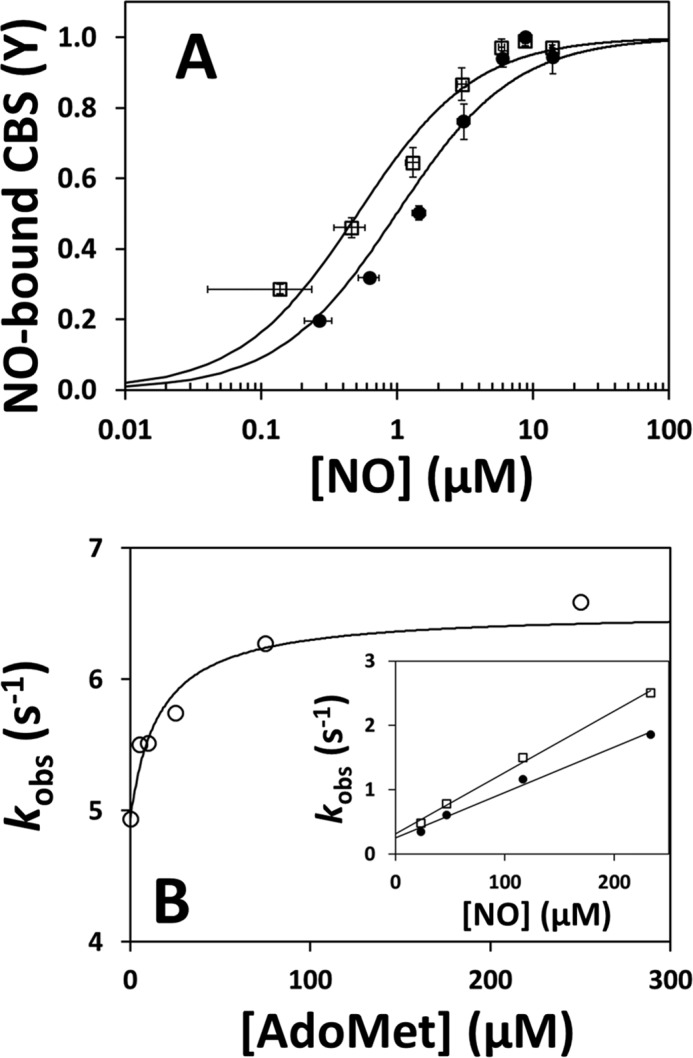
AdoMet elicits a tighter and faster binding of NO• to CBS. A, NO• titration profiles of reduced CBS (1.4 μm in heme, reduced with 45 μm dithionite) in the absence (solid symbols) or presence (open symbols) of 500 μm AdoMet. The two data sets were fitted to Equation 2 yielding Kd = 0.5 ± 0.1 μm and Kd = 1.0 ± 0.2 μm, for AdoMet-free and AdoMet-bound CBS, respectively. B, observed rate constants (major kinetic phase) measured upon mixing reduced CBS with 900 μm NO• at the indicated AdoMet concentration. Data were fitted to a hyperbola yielding C50 = 16.0 ± 2.4 μm AdoMet and klim′ =6.5 s−1. Inset, linear dependence of kobs on NO• concentration, as measured in the absence (solid symbols) or presence (open symbols) of 500 μm AdoMet.
Next, we analyzed by time-resolved absorption spectroscopy the effect of AdoMet on the kinetics of NO• binding, monitoring the conversion of reduced CBS into the penta-coordinate NO•-bound adduct. Analyzing the reaction with NO• (900 μm) at varying AdoMet concentrations (up to 500 μm before mixing), we observed a maximal ∼1.5-fold increase in the observed rate constant and a hyperbolic dependence on [AdoMet] (C50 =16.0 ± 2.4 μm), showing close to saturation effect at physiological AdoMet concentrations (Fig. 6B). At saturating AdoMet concentrations (500 μm before mixing), the kobs displayed a linear dependence on NO• concentration (up to 466 μm before mixing), yielding second-order rate constants kon of 9.5 × 103 and 7.1 × 103 m−1 s−1 for AdoMet-bound and AdoMet-free CBS, respectively (Fig. 6B, inset). In control experiments, we observed no significant effect of AdoHcy (500 μm before mixing) on the NO• association rate (5.2 and 5.6 s−1, respectively, in the absence or presence of AdoHcy). In addition, we investigated the effect of AdoMet on the koff of NO• from ferrous CBS employing the method of Moore and Gibson (40), as described previously (19), and we observed a slightly faster (1.3-fold) NO• dissociation in the absence of AdoMet (0.004 s−1) than in its presence (0.003 s−1) (Fig. 7B).
Relevantly, the kinetics of NO• binding to the truncated CBS proved to be unchanged by AdoMet, as observed for CO binding. Accordingly, by stopped-flow mixing truncated ferrous CBS with NO• (900 μm after mixing), we measured nearly identical observed rate constants in the absence (k′ = 7.0 s−1) or presence of AdoMet (k′ = 7.1 s−1; 500 μm AdoMet before mixing).
AdoMet Has No Effect on the Reduction and Oxidation Kinetics of CBS
To assess whether AdoMet binding to CBS, in addition to modulating the ligand binding properties of the heme, changes the redox properties of this cofactor, we analyzed by stopped-flow spectroscopy the kinetics of CBS reduction and its reoxidation by oxygen, in the AdoMet-bound and AdoMet-free enzyme. Upon mixing oxidized CBS with freshly prepared sodium dithionite (1 mm before mixing), the Soret band shifted from 428 (oxidized CBS) to 449 nm (reduced CBS) (data not shown). As revealed by global fit analysis, no differences were observed in the reduction rate in the presence or absence of AdoMet (Fig. 8A).
FIGURE 8.
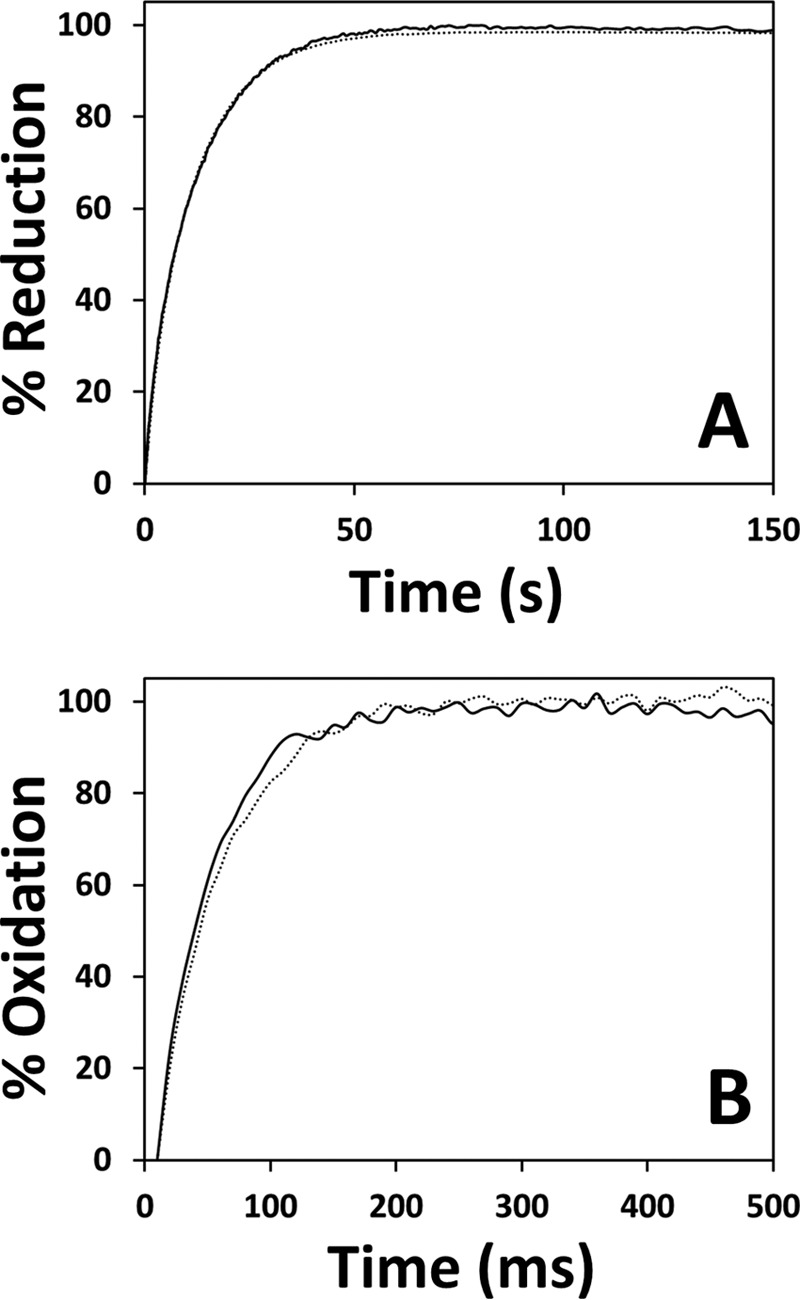
AdoMet has no effect on the reduction or oxidation kinetics of the CBS heme. A, time courses of ferric CBS (1.4–1.5 μm in heme content, before mixing) reduction by sodium dithionite (1 mm before mixing) in the absence (dotted line) or presence (solid line) of AdoMet (500 μm before mixing). T = 25 °C. Fitted rate constants (% reaction amplitude) are as follows: k1 =0.33 s−1 (10%) and k2 = 0.09 s−1 (90%). B, time courses of CBS oxidation by oxygen (240 μm before mixing) in the absence (dotted line) and presence (solid line) of AdoMet (500 μm before mixing). T = 25 °C. Buffer consisted of 50 mm potassium phosphate, 300 mm KCl, 10% glycerol, pH 7.0, containing 2 mm glucose, 4 units/ml glucose oxidase, 13 μg/ml catalase, and 60 units/ml SOD. Traces were best fitted with k = 19.9 ± 1.4 s−1 and k = 23.2 ± 2.2 s−1 in the absence (dotted line) and presence (solid line) of AdoMet, respectively.
To evaluate the effect of AdoMet on the kinetics of CBS oxidation, the AdoMet-free or AdoMet-bound protein was pre-reduced with 90 μm sodium dithionite and then mixed with air-equilibrated buffer supplemented with catalase (13 μg/ml) and SOD (6 units/ml). The spectral changes consisted of a blue-shift of the Soret band from 449 to 428 nm, yielding identical single optical transitions for both the AdoMet-free and AdoMet-bound protein (data not shown). As observed for the reduction process, no differences in the oxidation rate were observed following the incubation of CBS with AdoMet (Fig. 8B).
Discussion
The activity of the human H2S-synthesizing enzyme CBS is tightly regulated via two domains flanking the PLP-containing central catalytic core. The N-terminal domain harbors a heme redox sensor that in the reduced state upon binding CO or NO• leads to CBS inhibition. Conversely, binding of AdoMet to the C-terminal domain results in a 2–5-fold activity stimulation (Fig. 1). In this study, we provided experimental evidence for a functional communication between the two “peripheral” domains of CBS.
As a novel finding, we report that AdoMet binding, by lowering the apparent Ki value for CO by more than 10-fold (from 9.5 ± 1.0 to 0.7 ± 0.1 μm), markedly enhances the ability of the gasotransmitter CO to inhibit H2S production by CBS (Fig. 2). Using AdoHcy as a control, the same modulation of CO inhibition was not observed. To our knowledge, this is the first report of a Ki value for CO inhibition of CBS-mediated H2S production. These findings led us to assume that AdoMet, besides acting as a physiological allosteric activator of CBS, is also able to modulate the ligand binding properties of the CBS heme sensor, thereby sensitizing the enzyme toward heme-mediated inhibition. This hypothesis, fully consistent with the recent observation that AdoMet binding stimulates nitrite reduction by the ferrous CBS heme (20), was herein investigated from a mechanistic viewpoint.
The effect of AdoMet on the ligand binding properties of the CBS heme was explored by measuring the affinity of the protein for CO in the absence or presence of AdoMet. To this end, we performed anaerobic CO titrations of ferrous CBS. In agreement with previous studies (15, 17, 19, 37), conversion of ferrous CBS to the Fe(II)-CO adduct resulted in the characteristic absorption changes (Fig. 3A). Both in the absence and presence of AdoMet, the titration profiles were satisfactorily fitted with two dissociation constants, as reported previously (17, 19, 37). The existence of two binding constants for CO (Kd1 0.8–3.9 μm and Kd2 50–68 μm) has been attributed to differences in the heme micro-environment (37) or to heme anti-cooperativity within a CBS dimer (17). Here, we found that AdoMet binding significantly increases the affinity of CBS for CO, markedly decreasing the C50 by ∼5-fold (Fig. 3B). Next, we analyzed the effect of AdoMet on the kinetics of CO binding to the ferrous heme by stopped-flow absorption spectroscopy. As reported previously (15, 17, 19), in the absence of AdoMet, formation of the Fe(II)-CO adduct was found to proceed with biphasic kinetics, with most (80%) of the reaction occurring very slowly (k = 0.007 s−1), consistently with the rate limitation imposed by the slow dissociation of Cys-52 from the heme iron (klim = 0.012–0.017 s−1 (17, 19)). Notably, we found both CO binding and dissociation to be faster (although to a different extent) in the AdoMet-bound than in the AdoMet-free enzyme. At saturating AdoMet concentrations, the enhancing kinetic effect was more pronounced on CO binding (∼10-fold based on the reaction half-time, Fig. 4B) than on CO dissociation (<2-fold, Fig. 7A), consistently with the ∼5-fold higher CO affinity displayed by AdoMet-bound CBS, compared with the AdoMet-free enzyme. In the presence of AdoMet, the kobs determined for the major kinetic phase of CO binding retained the characteristic hyperbolic dependence on CO concentration, although the estimated plateau value (klim′) was significantly higher than observed for the AdoMet-free enzyme (0.024 s−1 versus 0.008 s−1, Fig. 4C). In line with previous work (17, 19), we envisage that such a difference in the klim′ value for CO binding originates from a weakening of the Fe–Cys-52 bond in response to AdoMet binding. Moreover, based on the observed dependence of the reaction kinetics on AdoMet concentration, we anticipate that physiological AdoMet concentrations are able to induce the effects documented herein (Fig. 4C). Taken together, these data neatly match the observation that AdoMet makes CBS more prone to CO inhibition.
Prompted by the effect of AdoMet on CO binding, we investigated the impact of this effector on NO• binding. Affinity measurements by anaerobic NO• titrations of reduced CBS revealed a 2-fold increase in affinity (Fig. 6A) in the presence of AdoMet. This result is fully consistent with the ∼1.5-fold increase in the observed rate constant for NO• binding (Fig. 6B) and the 1.3-fold decrease in the NO• koff measured in the presence of AdoMet (Fig. 7B). Moreover, and like CO, enhanced NO• binding is also expected to occur at physiological AdoMet concentrations (Fig. 6B).
Importantly, we found that the CO affinity and CO and NO• binding kinetics are unchanged by AdoMet (at 500 μm) in a truncated form of CBS lacking the AdoMet-binding C-terminal domain. While ruling out a direct effect of AdoMet on the heme moiety, this observation demonstrates that the effects produced by AdoMet on the full-length CBS heme sensor are mediated by the C-terminal regulatory domain. Moreover, these effects proved to be specific for AdoMet in control experiments carried out with AdoHcy.
The possible effects of AdoMet on the redox properties of the heme were also tested by stopped-flow absorption spectroscopy. We investigated in the presence and absence of AdoMet the kinetics of dithionite reduction of the CBS heme and its oxidation by oxygen. Similarly to other heme proteins with thiolate ligands (41–43), CBS reduction was slow (Fig. 8A). It required several tens of seconds to be complete, thus being remarkably slower than observed previously (15), where dithionite reduction of the protein was reported to be over within hundreds of milliseconds. Such a notable difference does not arise from the presence of contaminant oxygen in our experiments or the slightly different experimental conditions used in this study as compared with Ref. 15. Identical low reduction rates were indeed invariantly measured here in control experiments carried out either in the presence of deoxy-Fe(II) horse heart myoglobin acting as an oxygen trap or reproducing the experimental conditions described in Ref. 15. Despite this difference in the reduction rates, both the enzyme reduction and oxidation proceeded with identical kinetics in both experimental conditions (presence or absence of AdoMet), ruling out an effect of AdoMet on the heme redox properties.
From a mechanistic viewpoint, it is notable that AdoMet binding to CBS produces more profound effects on CO binding than on NO• binding. Whereas CO binding has been shown to markedly depend on the dissociation of the endogenous Cys-52 ligand, NO• binding has been suggested to initially displace the His-65 heme ligand, ultimately resulting in a pentacoordinate Fe(II)-NO species with both endogenous ligands displaced (19). Our observation that AdoMet affects the binding of CO and NO• differently is in line with the proposal that the two ligands initially “attack” the heme at opposite sides.
In summary, this study provides experimental evidence for a functional communication between the two regulatory domains of the H2S-synthesizing human cystathionine β-synthase. Our data demonstrate that binding of the allosteric activator AdoMet to the C-terminal domain facilitates CBS inhibition by CO. This observation is neatly matched by the observed impact of AdoMet on the thermodynamic and kinetic properties of CO and NO• binding to the heme at the N-terminal domain, previously shown to inhibit CBS with (patho)physiological implications. Based on the structural information and within the resolution limitations of the available crystallographic structures, it remains to be established at a molecular level how AdoMet binding at the C-terminal regulatory domain produces effects on the heme sensor distantly located at the N-terminal domain. The PLP and heme sites have been proposed to functionally communicate through α-helix 8 (44–46). At one end of this helix, Arg-266 establishes electrostatic interactions with the heme Cys-52 thiolate ligand, whereas at the opposite end Thr-257 and Thr-260 participate in a hydrogen bond network with the PLP phosphate moiety. The role played by Arg-266, Thr-257, and Thr-260 in the allosteric communication between the heme and PLP sites was established by characterizing both functionally and spectroscopically site-directed variants of these residues (44–46). Relevant to this work, these variants display different responsiveness to AdoMet. This led to the proposal that the PLP/heme communication extends to the AdoMet-binding site (46), which ultimately is in line with the findings presented herein.
Overall, the data obtained in this study further highlight the intricacy of the regulation of CBS, which is a key enzyme in human pathophysiology sitting at the crossroad between gasotransmitter signaling pathways.
Author Contributions
A. G. and J. B. V. conceived and coordinated the study, designed, performed, and analyzed the experiments, and wrote the paper. H. G. C. produced the protein samples and contributed to the setup and optimization of the experimental protocols. H. G. C., P. S., and P. L. contributed to data interpretation and critical revision of the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgment
We are grateful to Liliana Pinto for support with protein sample preparation.
This work was supported in part by Fundação para a Ciência e Tecnologia Grants PEst-OE/SAU/UI4013/2011 and PTDC/SAU-MIC/111447/2009 (to J. B. V.), Ministero dell'Istruzione, dell'Università e della Ricerca of Italy PNR-CNR Aging Program 2012-2014 (to A. G.), Fondo per gli Investimenti della Ricerca di Base RBIN06E9Z8 and Progetti di Rilevante Interesse Nazionale 20107Z8XBW_005 (to P. S.), and a bilateral grant award by Consiglio Nazionale delle Ricerche of Italy and Fundação para a Ciência e Tecnologia of Portugal (to A. G. and J. B. V.). The authors declare that they have no conflicts of interest with the contents of this article.
- CBS
- cystathionine β-synthase
- PLP
- pyridoxal 5′-phosphate
- AdoMet
- S-adenosyl-l-methionine
- AdoHcy
- S-adenosyl-l-homocysteine
- SOD
- superoxide dismutase
- Hcy
- homocysteine.
References
- 1.Stipanuk M. H. (2004) Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu. Rev. Nutr. 24, 539–577 [DOI] [PubMed] [Google Scholar]
- 2.Singh S., Padovani D., Leslie R. A., Chiku T., and Banerjee R. (2009) Relative contributions of cystathionine β-synthase and γ-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 284, 22457–22466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee R. (2011) Hydrogen sulfide: redox metabolism and signaling. Antioxid. Redox Signal. 15, 339–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabil O., and Banerjee R. (2010) Redox biochemistry of hydrogen sulfide. J. Biol. Chem. 285, 21903–21907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kabil O., and Banerjee R. (2014) Enzymology of H2S biogenesis, decay and signaling. Antioxid. Redox Signal. 20, 770–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kajimura M., Fukuda R., Bateman R. M., Yamamoto T., and Suematsu M. (2010) Interactions of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid Redox. Signal. 13, 157–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mustafa A. K., Gadalla M. M., and Snyder S. H. (2009) Signaling by gasotransmitters. Sci. Signal. 2, re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharyya S., Saha S., Giri K., Lanza I. R., Nair K. S., Jennings N. B., Rodriguez-Aguayo C., Lopez-Berestein G., Basal E., Weaver A. L., Visscher D. W., Cliby W., Sood A. K., Bhattacharya R., and Mukherjee P. (2013) Cystathionine β-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS ONE 8, e79167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szabo C., Coletta C., Chao C., Módis K., Szczesny B., Papapetropoulos A., and Hellmich M. R. (2013) Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. U.S.A. 110, 12474–12479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hellmich M. R., Coletta C., Chao C., and Szabo C. (2015) The therapeutic potential of cystathionine β-synthetase/hydrogen sulfide inhibition in cancer. Antioxid Redox Signal 22, 424–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ereño-Orbea J., Majtan T., Oyenarte I., Kraus J. P., and Martínez-Cruz L. A. (2013) Structural basis of regulation and oligomerization of human cystathionine β-synthase, the central enzyme of trans-sulfuration. Proc. Natl. Acad. Sci. U.S.A. 110, E3790–E3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ereño-Orbea J., Majtan T., Oyenarte I., Kraus J. P., and Martínez-Cruz L. A. (2014) Structural insight into the molecular mechanism of allosteric activation of human cystathionine β-synthase by S-adenosylmethionine. Proc. Natl. Acad. Sci. U.S.A. 111, E3845–E3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majtan T., Pey A. L., Fernández R., Fernández J. A., Martínez-Cruz L. A., and Kraus J. P. (2014) Domain organization, catalysis and regulation of eukaryotic cystathionine β-synthases. PLoS ONE 9, e105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherney M. M., Pazicni S., Frank N., Marvin K. A., Kraus J. P., and Burstyn J. N. (2007) Ferrous human cystathionine β-synthase loses activity during enzyme assay due to a ligand switch process. Biochemistry 46, 13199–13210 [DOI] [PubMed] [Google Scholar]
- 15.Carballal S., Cuevasanta E., Marmisolle I., Kabil O., Gherasim C., Ballou D. P., Banerjee R., and Alvarez B. (2013) Kinetics of reversible reductive carbonylation of heme in human cystathionine β-synthase. Biochemistry 52, 4553–4562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabil O., Weeks C. L., Carballal S., Gherasim C., Alvarez B., Spiro T. G., and Banerjee R. (2011) Reversible heme-dependent regulation of human cystathionine β-synthase by a flavoprotein oxidoreductase. Biochemistry 50, 8261–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puranik M., Weeks C. L., Lahaye D., Kabil O., Taoka S., Nielsen S. B., Groves J. T., Banerjee R., and Spiro T. G. (2006) Dynamics of carbon monoxide binding to cystathionine β-synthase. J. Biol. Chem. 281, 13433–13438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taoka S., and Banerjee R. (2001) Characterization of NO binding to human cystathionine β-synthase: possible implications of the effects of CO and NO binding to the human enzyme. J. Inorg. Biochem. 87, 245–251 [DOI] [PubMed] [Google Scholar]
- 19.Vicente J. B., Colaço H. G., Mendes M. I., Sarti P., Leandro P., and Giuffrè A. (2014) NO* binds human cystathionine β-synthase quickly and tightly. J. Biol. Chem. 289, 8579–8587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gherasim C., Yadav P. K., Kabil O., Niu W. N., and Banerjee R. (2014) Nitrite reductase activity and inhibition of H(2)S biogenesis by human cystathionine β-synthase. PLoS ONE 9, e85544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hishiki T., Yamamoto T., Morikawa T., Kubo A., Kajimura M., and Suematsu M. (2012) Carbon monoxide: impact on remethylation/transsulfuration metabolism and its pathophysiologic implications. J. Mol. Med. 90, 245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morikawa T., Kajimura M., Nakamura T., Hishiki T., Nakanishi T., Yukutake Y., Nagahata Y., Ishikawa M., Hattori K., Takenouchi T., Takahashi T., Ishii I., Matsubara K., Kabe Y., Uchiyama S., et al. (2012) Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway. Proc. Natl. Acad. Sci. U.S.A. 109, 1293–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prathapasinghe G. A., Siow Y. L., Xu Z., and O K. (2008) Inhibition of cystathionine-β-synthase activity during renal ischemia-reperfusion: role of pH and nitric oxide. Am. J. Physiol. Renal Physiol. 295, F912–F922 [DOI] [PubMed] [Google Scholar]
- 24.Janosík M., Kery V., Gaustadnes M., Maclean K. N., and Kraus J. P. (2001) Regulation of human cystathionine β-synthase by S-adenosyl-l-methionine: evidence for two catalytically active conformations involving an autoinhibitory domain in the C-terminal region. Biochemistry 40, 10625–10633 [DOI] [PubMed] [Google Scholar]
- 25.Prudova A., Bauman Z., Braun A., Vitvitsky V., Lu S. C., and Banerjee R. (2006) S-Adenosylmethionine stabilizes cystathionine β-synthase and modulates redox capacity. Proc. Natl. Acad. Sci. U.S.A. 103, 6489–6494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pey A. L., Majtan T., Sanchez-Ruiz J. M., and Kraus J. P. (2013) Human cystathionine β-synthase (CBS) contains two classes of binding sites for S-adenosylmethionine (SAM): complex regulation of CBS activity and stability by SAM. Biochem. J. 449, 109–121 [DOI] [PubMed] [Google Scholar]
- 27.Kluijtmans L. A., Boers G. H., Stevens E. M., Renier W. O., Kraus J. P., Trijbels F. J., van den Heuvel L. P., and Blom H. J. (1996) Defective cystathionine β-synthase regulation by S-adenosylmethionine in a partially pyridoxine responsive homocystinuria patient. J. Clin. Invest. 98, 285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendes M. I., Colaço H. G., Smith D. E., Ramos R. J., Pop A., van Dooren S. J., Tavares de Almeida I., Kluijtmans L. A., Janssen M. C., Rivera I., Salomons G. S., Leandro P., and Blom H. J. (2014) Reduced response of cystathionine β-synthase (CBS) to S-adenosylmethionine (SAM): identification and functional analysis of CBS gene mutations in Homocystinuria patients. J. Inherit. Metab. Dis. 37, 245–254 [DOI] [PubMed] [Google Scholar]
- 29.Módis K., Coletta C., Asimakopoulou A., Szczesny B., Chao C., Papapetropoulos A., Hellmich M. R., and Szabo C. (2014) Effect of S-adenosyl-l-methionine (SAM), an allosteric activator of cystathionine-β-synthase (CBS) on colorectal cancer cell proliferation and bioenergetics in vitro. Nitric Oxide 41, 146–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendes M. I., Santos A. S., Smith D. E., Lino P. R., Colaço H. G., de Almeida I. T., Vicente J. B., Salomons G. S., Rivera I., Blom H. J., and Leandro P. (2014) Insights into the regulatory domain of cystathionine β-synthase: characterization of six variant proteins. Hum. Mutat. 35, 1195–1202 [DOI] [PubMed] [Google Scholar]
- 31.McCorvie T. J., Kopec J., Hyung S. J., Fitzpatrick F., Feng X., Termine D., Strain-Damerell C., Vollmar M., Fleming J., Janz J. M., Bulawa C., and Yue W. W. (2014) Inter-domain communication of human cystathionine β-synthase: structural basis of S-adenosyl-l-methionine activation. J. Biol. Chem. 289, 36018–36030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 33.Carballal S., Madzelan P., Zinola C. F., Graña M., Radi R., Banerjee R., and Alvarez B. (2008) Dioxygen reactivity and heme redox potential of truncated human cystathionine β-synthase. Biochemistry 47, 3194–3201 [DOI] [PubMed] [Google Scholar]
- 34.Chiku T., Padovani D., Zhu W., Singh S., Vitvitsky V., and Banerjee R. (2009) H2S biogenesis by human cystathionine γ-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 284, 11601–11612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel L. M. (1965) A direct microdetermination for sulfide. Anal. Biochem. 11, 126–132 [DOI] [PubMed] [Google Scholar]
- 36.Dixon M. (1971) The acceptor specificity of flavins and flavoproteins. I. Techniques for anaerobic spectrophotometry. Biochim. Biophys. Acta 226, 241–258 [DOI] [PubMed] [Google Scholar]
- 37.Taoka S., West M., and Banerjee R. (1999) Characterization of the heme and pyridoxal phosphate cofactors of human cystathionine β-synthase reveals nonequivalent active sites. Biochemistry 38, 2738–2744 [DOI] [PubMed] [Google Scholar]
- 38.Henry E. R., and Hofrichter J. (1992) Singular value decomposition- application to analysis of experimental data. Methods Enzymol. 210, 129–192 [Google Scholar]
- 39.Finkelstein J. D., Kyle W. E., Harris B. J., and Martin J. J. (1982) Methionine metabolism in mammals: concentration of metabolites in rat tissues. J. Nutr. 112, 1011–1018 [DOI] [PubMed] [Google Scholar]
- 40.Moore E. G., and Gibson Q. H. (1976) Cooperativity in the dissociation of nitric oxide from hemoglobin. J. Biol. Chem. 251, 2788–2794 [PubMed] [Google Scholar]
- 41.Davydov D. R., Fernando H., Baas B. J., Sligar S. G., and Halpert J. R. (2005) Kinetics of dithionite-dependent reduction of cytochrome P450 3A4: heterogeneity of the enzyme caused by its oligomerization. Biochemistry 44, 13902–13913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hintz M. J., and Peterson J. A. (1980) The kinetics of reduction of cytochrome P-450cam by the dithionite anion monomer. J. Biol. Chem. 255, 7317–7325 [PubMed] [Google Scholar]
- 43.Quaroni L. G., Seward H. E., McLean K. J., Girvan H. M., Ost T. W., Noble M. A., Kelly S. M., Price N. C., Cheesman M. R., Smith W. E., and Munro A. W. (2004) Interaction of nitric oxide with cytochrome P450 BM3. Biochemistry 43, 16416–16431 [DOI] [PubMed] [Google Scholar]
- 44.Singh S., Madzelan P., Stasser J., Weeks C. L., Becker D., Spiro T. G., Penner-Hahn J., and Banerjee R. (2009) Modulation of the heme electronic structure and cystathionine β-synthase activity by second coordination sphere ligands: The role of heme ligand switching in redox regulation. J. Inorg. Biochem. 103, 689–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith A. T., Su Y., Stevens D. J., Majtan T., Kraus J. P., and Burstyn J. N. (2012) Effect of the disease-causing R266K mutation on the heme and PLP environments of human cystathionine β-synthase. Biochemistry 51, 6360–6370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yadav P. K., Xie P., and Banerjee R. (2012) Allosteric communication between the pyridoxal 5′-phosphate (PLP) and heme sites in the H2S generator human cystathionine β-synthase. J. Biol. Chem. 287, 37611–37620 [DOI] [PMC free article] [PubMed] [Google Scholar]