Abstract
Tryptophan metabolites in the kynurenine pathway are up-regulated by pro-inflammatory cytokines or glucocorticoids, and are linked to anti-inflammatory and immunosuppressive activities. In addition, they are up-regulated in pathologies such as cancer, autoimmune diseases, and psychiatric disorders. The molecular mechanisms of how kynurenine pathway metabolites cause these effects are incompletely understood. On the other hand, pro-inflammatory cytokines also up-regulate the amounts of tetrahydrobiopterin (BH4), an enzyme cofactor essential for the synthesis of several neurotransmitter and nitric oxide species. Here we show that xanthurenic acid is a potent inhibitor of sepiapterin reductase (SPR), the final enzyme in de novo BH4 synthesis. The crystal structure of xanthurenic acid bound to the active site of SPR reveals why among all kynurenine pathway metabolites xanthurenic acid is the most potent SPR inhibitor. Our findings suggest that increased xanthurenic acid levels resulting from up-regulation of the kynurenine pathway could attenuate BH4 biosynthesis and BH4-dependent enzymatic reactions, linking two major metabolic pathways known to be highly up-regulated in inflammation.
Keywords: chemical biology, crystal structure, inflammation, inhibition mechanism, tetrahydrobiopterin (BH4), kynurenine pathway, sepiapterin reductase, xanthurenic acid
Introduction
Tetrahydrobiopterin (BH4)4 is a cofactor for the tyrosine, tryptophan, and phenylalanine hydroxylases and alkylglycerol monooxygenase as well as nitric oxide synthases (1). These BH4-dependent enzymes play essential roles in synthesis of signaling molecules such as dopamine, serotonin, and nitric oxide in animals.
BH4 levels are constitutively high in cells carrying out aromatic amino acid hydroxylation (for example, dopamine- and serotonin-producing cells in brain or intestine and phenylalanine-hydroxylating cells in liver), but also up-regulated in certain cell types by stimulation with pro-inflammatory cytokines (2). The latter observation suggests that BH4 plays a role in inflammatory processes. Furthermore, accumulating evidence shows that increased levels of BH4 intensify pain sensitivity, whereas reduction of BH4 biosynthesis is an efficient strategy to diminish neuropathic and inflammatory pain (3–5).
BH4 is synthesized de novo from GTP by the sequential action of three enzymes, of which sepiapterin reductase (SPR) is the last (see Fig. 1A) (1). The last step can also be catalyzed by alternative enzymes such as aldose reductase and carbonyl reductase (6). SPR furthermore reduces sepiapterin to dihydrobiopterin (BH2), which is subsequently reduced to BH4 by dihydrofolate reductase in the salvage pathway of BH4 synthesis.
FIGURE 1.
A, BH4 biosynthesis pathway. SPR is the last enzyme in the de novo BH4 synthesis (black arrow). SPR also catalyzes reduction of sepiapterin in the salvage pathway (gray arrow). Alternative enzymes that complement activity of SPR exist, depending on cell types. * indicates low activity in human brain. DHFR, dihydrofolate reductase. Dashed arrow, non-enzymatic reaction. B, tryptophan metabolites of the kynurenine pathway (black arrow) and the melatonin synthesis pathway (gray arrow). TPH, tryptophan hydroxylase; 5-HTP, 5-hydroxytryptophan.
We have reported that the anti-inflammatory/immunosuppressive drug sulfasalazine and its metabolite sulfapyridine are potent inhibitors of SPR, and we proposed that the mechanism of action of these two sulfonamides in rheumatoid arthritis involves SPR inhibition and interference with BH4 biosynthesis (7). Recently, we performed a screen for additional SPR inhibitors among clinically approved drugs, which revealed that various sulfa drugs are potent inhibitors of human SPR, both in vitro and in cell culture experiments (8). The decrease of BH4-dependent neurotransmitter biosynthesis in cell-based assays by sulfa drugs furthermore provides a rationale for some of their CNS-related side effects.
We now report that the kynurenine pathway metabolite xanthurenic acid is a potent SPR inhibitor, providing a link between two major metabolic pathways known to be highly up-regulated in inflammation.
Experimental Procedures
Small molecule screening and SPR enzymatic assays were performed as described (8). The natural products and bioactive compounds analyzed for SPR inhibition are part of a commercially available 1040-compound library (NINDS2, MicroSource). N-terminally hexahistidine-tagged SPR from human, mouse, and rat was used for enzymatic assays. The recombinant proteins were prepared as described (7). Human SPR was purified and cocrystallized with xanthurenic acid as described (5), except that SPR was eluted with xanthurenic acid in the presence of NADP+ and that 1.9 m ammonium sulfate was used for crystallization. The structure was solved by molecular replacement using 4HWK (8) as a search model and refined as described in Ref. 5. For isothermal titration calorimetry experiments, an N-terminal hexahistidine-tagged human SPR was expressed in BL21(DE3) Escherichia coli, purified to homogeneity by Ni2+-nitrilotriacetic acid chromatography (7), and subjected to chromatography on a HiTrap Blue HP resin (GE Healthcare) to obtain SPR devoid of any cofactor, as verified by the absence of absorbance bands at 260 and 340 nm. Isothermal titration calorimetry was performed using an ITC200 (GE Healthcare) in 20 mm sodium phosphate (pH 6.5) at 25 °C. Cofactor or xanthurenic acid dissolved in the same buffer was added by 15 sequential 2.49-μl injections.
Results and Discussion
To identify natural SPR inhibitors whose role might be a regulation of BH4 biosynthesis, we screened a collection of selected natural products and bioactive compounds for binding to human SPR. We identified two tryptophan metabolites as SPR inhibitors, xanthurenic acid and N-acetylserotonin, with potential physiological relevance (see below). N-Acetylserotonin is a metabolite in the pathway of synthesis of melatonin (Fig. 1B). In fact, the inhibitory activity of N-acetylserotonin toward SPR (Ki = 200 nm for rat SPR) was discovered by Katoh et al. (9) three decades ago, and it has been used as tool compound for controlling SPR activity (10). Furthermore, N-acetylserotonin has been used as the starting point for the recent development of the potent SPR inhibitor SPRi3 (Fig. 2), which has been shown to reduce neuropathic and inflammatory pain in mice through a reduction of BH4 levels (5). It has been speculated that the physiological role of inhibition of SPR by N-acetylserotonin is negative feedback regulation, if any, as the initial enzyme in the pathway is BH4-dependent tryptophan hydroxylase (11, 12).
FIGURE 2.
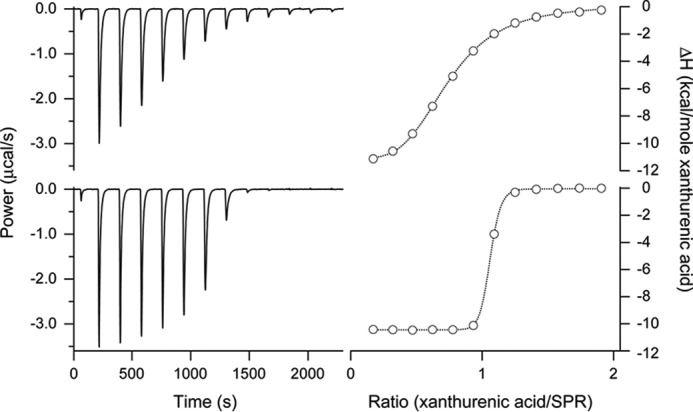
Thermodynamic analysis of the binding of xanthurenic acid to SPR by isothermal titration calorimetry. Representative experiments are shown. Aliquots of 2 mm xanthurenic acid were added to a 166 μm SPR solution containing either 1 mm NADPH (top) or 1 mm NADP+ (bottom), and the power difference relative to the reference cell was recorded at 25 °C (left). Integration of the power of the individual peaks resulted in titration curves as a function of the xanthurenic acid/SPR ratio (right), from which by curve fitting using a 1:1 interaction model the following thermodynamic parameters (mean ± S.E.) were determined for SPR saturated with NADPH or NADP+, respectively: Kd = 9.4 ± 0.4 μm or 68 ± 14 nm; ΔH = −12.4 ± 0.1 kcal/mol or −10.5 ± 0.1 kcal/mol; −TΔS = 5.6 ± 0.1 kcal/mol or 0.7 ± 0.1 kcal/mol; and the stoichiometry n = 0.69 ± 0.01 or 0.98 ± 0.01.
Xanthurenic acid on the other hand is a metabolite in the major tryptophan-catabolizing kynurenine pathway (Fig. 1B), in which all enzymes are BH4-independent. Inhibition of BH4 biosynthesis by any of the metabolites in the kynurenine pathway has not been reported. The physiological role of xanthurenic acid in mammals remains elusive. Xanthurenic acid has been frequently described as the inert end product of the detoxification of 3-hydroxykynurenine (13, 14). Depending on the conditions, xanthurenic acid has been shown to act in vitro as either an anti-oxidant or a pro-oxidant, scavenger or generator of reactive oxygen species, and as a photosensitizer (15). A role for xanthurenic acid in neurotransmission and as a neuromodulator has been proposed based on the study of distribution and transport of xanthurenic acid in rat brain (16, 17). Xanthurenic acid possesses competitive inhibitory activity of l-glutamate transport with a Ki value of 190 μm (18).
The initial and rate-limiting enzyme (tryptophan dioxygenase or indoleamine-2,3-dioxygenase) in the kynurenine pathway is up-regulated by stress or female sex glucocorticoid hormones (i.e. cortisol or estrogen) or highly induced by infection or pro-inflammatory cytokines (19–22). Up-regulation of the kynurenine pathway is linked to anti-inflammatory and immunosuppressive activities but also plays a role in pathologies such as cancer, autoimmune diseases, and neurodegenerative and psychiatric disorders (23–25). Given that BH4 is an essential cofactor for synthesis of various signaling molecules, it is tempting to speculate that increased xanthurenic acid induces some of these activities by inhibition of BH4 synthesis by targeting SPR.
To evaluate the inhibitory activity of xanthurenic acid and N-acetylserotonin toward human SPR, we measured their effect on the reduction of sepiapterin by SPR in an enzymatic assay (Table 1). Xanthurenic acid is a potent inhibitor of human SPR with an IC50 value of 150 nm. By comparison, the IC50 value of N-acetylserotonin under these conditions was measured to be 3.8 μm, i.e. 25-fold higher.
TABLE 1.
IC50 values (μm) in SPR enzymatic assays
Assays were performed using human, mouse and rat SPR.
| Human | Mouse | Rat | |
|---|---|---|---|
| Metabolitesa | |||
| Xanthurenic acid | 0.15 ± 0.03 | 0.053 ± 0.005 | 0.045 ± 0.005 |
| N-Acetylserotonin | 3.8 ± 1.2 | 35 ± 11 | 1.2 ± 0.1 |
| Kynurenic acid | 8.9 ± 0.9 | 5.3 ± 0.5 | 8.1 ± 1.5 |
| 8-Hydroxyquinaldic acid | 18 ± 2 | 40 ± 1 | 12 ± 1 |
| Picolinic acid | 27 ± 0.4 | NDb | ND |
| 3-Hydroxyanthranilic acid | 39 ± 0.4 | ND | ND |
| Kynurenine | >100 | ND | ND |
| 3-Hydroxykynurenine | >100 | ND | ND |
| Quinolinic acid | >100 | ND | ND |
| Synthetic inhibitorsc | |||
| Sulfasalazine | 0.0070 ± 0.0004d | 0.078 ± 0.007 | 0.043 ± 0.003 |
| SPRi3 | 0.053 ± 0.011 | ND | |
a Values are mean ± S.D. (at least 3 independent experiments).
b ND, not determined.
c Values are mean ± S.E. (n = 3–6).
d From Ref. 8.
The dissociation constant Kd for the binding of xanthurenic acid to human SPR was determined with isothermal titration calorimetry (Fig. 2). In the absence of cofactor, no binding curve could be obtained, indicating that Kd ≥ 1 mm for the interaction of xanthurenic acid with apo-SPR (data not shown). Xanthurenic acid bound strongly to the NADP+-saturated SPR with a Kd = 51 ± 10 nm (n = 2), and much bound much more weakly to NADPH-bound SPR with a Kd = 9.5 ± 0.4 μm (n = 2). These data suggest that xanthurenic acid inhibits the enzymatic activity of SPR mainly by binding to the catalytic site of NADP+-bound SPR and to a minor extent by binding to NADPH-bound SPR. This mode of inhibition is different from that of N-acetylserotonin, which is shown to be a competitive inhibitor for pterin substrate that binds to NADPH-bound SPR (9, 26).
Is it only xanthurenic acid among various metabolites in the kynurenine pathway that inhibits SPR? To address this question, we measured IC50 values for human SPR of kynurenine, kynurenic acid, 8-hydroxyquinaldic acid, 3-hydroxykynurenine, anthranilic acid, 3-hydroxyanthranilic acid, quinolinic acid, and picolinic acid in addition to xanthurenic acid (Fig. 1B and Table 1). The results showed that only xanthurenic acid is a potent SPR inhibitor. Finally, we measured IC50 values of xanthurenic acid and its analogues for mouse and rat SPR (Table 1). Xanthurenic acid also displayed high inhibitory activity against mouse and rat SPR, with IC50 values below 100 nm.
How do physiologically relevant concentrations of xanthurenic acid compare with the IC50 values measured here for SPR? It was reported that distribution of xanthurenic acid is heterogeneous in the brain of healthy rat and that its concentrations reach up to 1 μm in specific brain regions (16). It is not known whether xanthurenic acid distributes evenly or is concentrated in specific cell types in these brain regions; thus local concentrations of xanthurenic acid could be higher than 1 μm. Up-regulation of the kynurenine pathway can also result in increased xanthurenic acid concentrations. Indeed, increased metabolism of kynurenine to xanthurenic acid was demonstrated in quinolinic acid-lesioned rat striata (27). In summary, the inhibitory activity of xanthurenic acid presented here for SPR and its reported concentrations in vivo suggest that it could inhibit BH4 synthesis in vivo.
To gain further insights into the interaction of xanthurenic acid with human SPR, we solved the crystal structure of the enzyme with bound metabolite and NADP+ at 2.35 Å (Protein Data Bank (PDB) ID 4Z3K). The electron density clearly defines the structure and orientation of xanthurenic acid within human SPR, as demonstrated by the omit map shown at 3.5σ (Fig. 3A). Crystallographic parameters for data collection and refinement are listed in Table 2. Xanthurenic acid is bound through an extensive network of hydrogen bonds (Fig. 3B), most importantly to the catalytic residues Ser-154 and Tyr-167, and via two water molecules to Asp-254. This latter residue has been shown to be involved in substrate binding in mouse SPR (28). Further hydrogen bonds are formed between the carboxylic group and the backbone N–H of Leu-155, and between the 4-hydroxyl group of xanthurenic acid and both Gln-203 and a water molecule. In addition, apolar interactions with the side chains of Trp-164, Pro-197, and Leu-219 (Fig. 3B), as well as with the nicotinamide moiety of NADP+, contribute to inhibitor binding (Fig. 3B).
FIGURE 3.
A, left, overview of the crystal structure of SPR (ribbon) with xanthurenic acid (carbons in green) and NADP+ (carbons in gray). Right, stereo view of a zoom of the catalytic site of SPR showing xanthurenic acid enclosed by the omit map contoured at 3.5σ (0.1825 e/Å3), the side chains (cyan) of the catalytically important residues Ser-154 and Tyr-167, and NADP+ (gray). Refmac5 was used to calculate phases for the SPR structure without xanthurenic acid, and the omit map was calculated by Coot using these phases and the experimentally measured reflections. B, comparison of the binding within SPR of (top) xanthurenic acid, (middle) sulfasalazine, and (bottom) SPRi3. The structures of the three SPR inhibitors are shown on the left. The crystal structures of SPR with xanthurenic acid (PDB ID 4Z3K; 2.35 Å), sulfasalazine (PDB ID 4J7X; 2.60 Å), and the N-acetylserotonin analogue SPRi3 (PDB ID 4XWY; 2.35 Å) were superimposed, and are presented as stereo views showing the residues' interactions with the ligands (carbons in green) and the cofactor NADPH or NADP+. Extensive hydrogen bonding (dotted red lines) links the three ligands to SPR, in particular to Ser-154 and Tyr-167, the key residues of the catalytic site, and to Asp-254, which is important for substrate binding, either directly or via water molecules (red dots). Graphics were generated with PyMOL (Schrödinger, LLC).
TABLE 2.
Data collection and refinement statistics
| Data collection and processing | |
| Beamline | SLS–X06DA |
| Space group | P61 |
| Unit cell parameters | |
| a, b, c (Å) | 143.96, 143.96, 180.74 |
| α, β, γ (°) | 90, 90, 120 |
| Wavelength (Å) | 1.00000 |
| Resolution (Å) | 47.12–2.35 (2.48–2.35) |
| Unique reflections | 88,165 (6227) |
| Redundancy | 12.9 (13.3) |
| Completeness (%) | 99.5 (100.0) |
| Rmeas (%) | 11.0 (112.8) |
| I/σI | 20.0 (0.7) |
| CC½ | 0.999 (0.816) |
| Refinement and model composition | |
| Rwork/Rfree | 0.18 (0.28) / 0.21 (0.31) |
| No. of atoms | |
| Protein | 7768 |
| NADP | 192 |
| 4KLa | 60 |
| H2O | 160 |
| SO4 | 30 |
| EDOb | 4 |
| Average B-factor | 49 |
| Protein | 50 |
| Water | 44 |
| 4KLa | 37 |
| Wilson B-factor | 57.30 |
| r.m.s.c deviations | |
| Bond angle (°) | 1.4458 |
| Bond length (Å) | 0.0197 |
| PDB ID | 4Z3K |
a 4KL, xanthurenic acid.
b EDO, 1,2-dihydroxyethane.
c r.m.s., root mean square.
The mode of binding of xanthurenic acid to human SPR shows similarities with that of other inhibitors of this enzyme, i.e. sulfasalazine (PDB ID 4J7X) and the N-acetylserotonin analogue SPRi3 (PDB ID 4XWY) (Fig. 3B). In all cases, the inhibitors block the substrate-binding site of SPR. All three inhibitors do form hydrogen bonds with the catalytic residues Ser-154 and Tyr-167. They interact with active site residue Asp-254, although there are some noticeable differences; although the 5-hydroxyl group of SPRi3 interacts directly with Asp-254 (see also Ref. 5), sulfasalazine and xanthurenic acid interact with this residue via bridging water molecules. The backbone N–H of Gly-196 plays an interesting role; in the case of xanthurenic acid and sulfasalazine, it forms a hydrogen bond with the water molecule bridging the ligand and Asp-254, whereas with SPRi3, a hydrogen bond with the 5-hydroxy group of the ligand is formed.
Hydrophobic interactions further stabilize the binding of the ligands, although to a different extent. All three inhibitors interact with Trp-164 and Pro-197. Additionally, xanthurenic acid and sulfasalazine interact with Leu-219 and Gln-203, respectively. SPRi3 interacts with all mentioned residues and additionally with Leu-155. The nicotinamide moiety of the cofactor stacks against the pyridine ring of sulfasalazine and one of the rings of xanthurenic acid but shows only some overlap with the terminal part of the side chain of SPRi3.
The structure of SPR with xanthurenic acid allows one to rationalize the rank order of SPR inhibitory potencies of the tryptophan metabolites. Xanthurenic acid is a rigid planar molecule where all hetero-atoms are ideally positioned for hydrogen bond formation directly, or in the case of the carboxyl group via water molecules, with multiple residues of SPR. In addition, it engages in hydrophobic interactions with numerous active site residues and stacks against the nicotinamide ring of NADP+. Kynurenic acid and 8-hydroxyquinaldic acid lack one of the hydroxyl groups of xanthurenic acid; their reduced capacity to form hydrogen bonds thus explains their reduced potency. Picolinic acid and 3-hydroxyanthranilic acid are planar and retain the carboxylic acid, but are monocyclic and thus will less efficiently fill the active site of SPR. The kynurenine and 3-hydroxykynurenine are monocyclic and have a long, non-rigid side chain, placing the carboxyl group far from the aromatic ring; these structural changes allow rationalizing their absence of measurable inhibitory activity toward SPR.
Are there any observations in the literature suggesting that increased concentrations of xanthurenic acid, resulting from up-regulation of the kynurenine pathway, cause an inhibition of BH4 biosynthesis? Characteristic biochemical markers for the diagnosis of human genetic SPR deficiency include decreased dopamine and homovanillic acid and increased BH2 and sepiapterin levels in the cerebrospinal fluid (CSF) (29). Symptoms of the patients are characteristic of catecholamine and serotonin deficiency with cognitive and motor dysfunction (29), and resemble those observed upon administration of high doses of synthetic SPR inhibitors penetrating into the CNS (8). Patients also have impaired phenylalanine hydroxylation, as demonstrated by phenylalanine loading tests. Mild hyperphenylalaninemia was induced in healthy subjects treated with sulfamethoxazole (30, 31), which we have shown to be a potent SPR inhibitor (8). The kynurenine pathway is up-regulated upon IFN-α treatment, as evidenced by increased peripheral levels of kynurenine and increased levels of kynurenic acid and quinolinic acid in the CSF (xanthurenic acid levels in the CSF upon IFN-α treatment have not been reported) (32). Long-term IFN-α treatment is used for certain cancers and viral infections, but has adverse psychiatric effects including mental depression, anxiety, and psychomotor retardation in a significant number of patients (33). It has been demonstrated that chronic IFN-α treatment causes decreased dopamine and homovanillic acid and increased BH2 levels in the CSF, hallmarks of human SPR deficiency (34, 35). Furthermore, diminished conversion of phenylalanine to tyrosine in IFN-α treatment has been reported, indicating hepatic BH4 deficiency (34, 36). This higher phenylalanine/tyrosine ratio and this higher serum phenylalanine level are described in other diseases with immune activation and inflammation such as infectious diseases of HIV-1, dengue, and severe malaria, as well as trauma, sepsis, and cancer, indicating a common mechanism of action (37–39). Our findings raise the possibility that the biochemical and psychiatric/behavioral symptoms observed during IFN-α treatment are at least partially due to an up-regulation of the kynurenine pathway and inhibition of BH4 synthesis by xanthurenic acid. Measuring xanthurenic acid and sepiapterin levels in CSF of patients undergoing chronic IFN-α treatment would allow testing this hypothesis.
In summary, we report that xanthurenic acid is a potent inhibitor of SPR and propose that increased levels of xanthurenic acid resulting from up-regulation of the kynurenine pathway affect BH4 biosynthesis and BH4-dependent pathways.
Author Contributions
H. H. and K. J. conceived the study. H. H. and M. G. P. conducted screening and activity assays. R. H. crystallized the complex of SPR-xanthurenic acid-NADP+, determined its structure, and performed the calorimetry assays. All authors analyzed the results and contributed to the writing of the manuscript.
Acknowledgments
We thank G. Turcatti, M. Chambon, M. Busquets, and F. Pojer for technical assistance. We also thank K. I. Gorska and O. Sallin for the activity assay of SPRi3 and helpful comments, respectively.
This work was supported by the EPFL, the Swiss National Science Foundation, and the NCCR in Chemical Biology. The authors declare that they have no conflicts of interest with the contents of this article.
The atomic coordinates and structure factors (code 4Z3K) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- BH4
- tetrahydrobiopterin
- BH2
- dihydrobiopterin
- SPR
- sepiapterin reductase
- CSF
- cerebrospinal fluid.
References
- 1.Werner E. R., Blau N., and Thöny B. (2011) Tetrahydrobiopterin: biochemistry and pathophysiology. Biochem. J. 438, 397–414 [DOI] [PubMed] [Google Scholar]
- 2.Werner E. R., Werner-Felmayer G., and Mayer B. (1998) Tetrahydrobiopterin, cytokines, and nitric oxide synthesis. Proc. Soc. Exp. Biol. Med. 219, 171–182 [DOI] [PubMed] [Google Scholar]
- 3.Costigan M., Latremoliere A., and Woolf C. J. (2012) Analgesia by inhibiting tetrahydrobiopterin synthesis. Curr. Opin. Pharmacol. 12, 92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nasser A., Ali S., Wilsbech S., Bjerrum O. J., and Møller L. B. (2015) Intraplantar injection of tetrahydrobiopterin induces nociception in mice. Neurosci. Lett. 584, 247–252 [DOI] [PubMed] [Google Scholar]
- 5.Latremoliere A., Latini A., Andrews N., Cronin S. J., Fujita M., Gorska K., Hovius R., Romero C., Chuaiphichai S., Painter M., Miracca G., Babaniyi O., Remor A. P., Duong K., Riva P., Barrett L. B., Ferreirós N., Naylor A., Penninger J. M., Tegeder I., Zhong J., Blagg J., Channon K. M., Johnsson K., Costigan M., and Woolf C. J. (2015) Reduction of neuropathic and inflammatory pain through inhibition of the tetrahydrobiopterin pathway. Neuron 86, 1393–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirakawa H., Sawada H., Yamahama Y., Takikawa S.-I., Shintaku H., Hara A., Mase K., Kondo T., and Iino T. (2009) Expression analysis of the aldo-keto reductases involved in the novel biosynthetic pathway of tetrahydrobiopterin in human and mouse tissues. J. Biochem. 146, 51–60 [DOI] [PubMed] [Google Scholar]
- 7.Chidley C., Haruki H., Pedersen M. G., Muller E., and Johnsson K. (2011) A yeast-based screen reveals that sulfasalazine inhibits tetrahydrobiopterin biosynthesis. Nat. Chem. Biol. 7, 375–383 [DOI] [PubMed] [Google Scholar]
- 8.Haruki H., Pedersen M. G., Gorska K. I., Pojer F., and Johnsson K. (2013) Tetrahydrobiopterin biosynthesis as an off-target of sulfa drugs. Science 340, 987–991 [DOI] [PubMed] [Google Scholar]
- 9.Katoh S., Sueoka T., and Yamada S. (1982) Direct inhibition of brain sepiapterin reductase by a catecholamine and an indoleamine. Biochem. Biophys. Res. Comm. 105, 75–81 [DOI] [PubMed] [Google Scholar]
- 10.Smith G. K., Duch D. S., Edelstein M. P., and Bigham E. C. (1992) New inhibitors of sepiapterin reductase. Lack of an effect of intracellular tetrahydrobiopterin depletion upon in vitro proliferation of two human cell lines. J. Biol. Chem. 267, 5599–5607 [PubMed] [Google Scholar]
- 11.Jéquier E., Lovenberg W., and Sjoerdsma A. (1967) Tryptophan hydroxylase inhibition: the mechanism by which p-chlorophenylalanine depletes rat brain serotonin. Mol. Pharmacol. 3, 274–278 [PubMed] [Google Scholar]
- 12.Tong J. H., and Kaufman S. (1975) Tryptophan hydroxylase. Purification and some properties of the enzyme from rabbit hindbrain. J. Biol. Chem. 250, 4152–4158 [PubMed] [Google Scholar]
- 13.Han Q., Beerntsen B. T., and Li J. (2007) The tryptophan oxidation pathway in mosquitoes with emphasis on xanthurenic acid biosynthesis. J. Insect Physiol. 53, 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colín-González A. L., Maldonado P. D., and Santamaría A. (2013) 3-Hydroxykynurenine: an intriguing molecule exerting dual actions in the central nervous system. Neurotoxicology 34, 189–204 [DOI] [PubMed] [Google Scholar]
- 15.Reyes Ocampo J., Lugo Huitrón R., González-Esquivel D., Ugalde-Muñiz P., Jiménez-Anguiano A., Pineda B., Pedraza-Chaverri J., Ríos C., and Pérez de la Cruz V. (2014) Kynurenines with neuroactive and redox properties: relevance to aging and brain diseases. Oxid. Med. Cell. Longev. 2014, 646909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gobaille S., Kemmel V., Brumaru D., Dugave C., Aunis D., and Maitre M. (2008) Xanthurenic acid distribution, transport, accumulation and release in the rat brain. J. Neurochem. 105, 982–993 [DOI] [PubMed] [Google Scholar]
- 17.Taleb O., Maammar M., Brumaru D., Bourguignon J.-J., Schmitt M., Klein C., Kemmel V., Maitre M., and Mensah-Nyagan A. G. (2012) Xanthurenic acid binds to neuronal G-protein-coupled receptors that secondarily activate cationic channels in the cell line NCB-20. PLoS ONE 7, e48553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartlett R. D., Esslinger C. S., Thompson C. M., and Bridges R. J. (1998) Substituted quinolines as inhibitors of l-glutamate transport into synaptic vesicles. Neuropharmacology 37, 839–846 [DOI] [PubMed] [Google Scholar]
- 19.Schimke R. T., Sweeney E. W., and Berlin C. M. (1965) The roles of synthesis and degradation in the control of rat liver tryptophan pyrrolase. J. Biol. Chem. 240, 322–331 [PubMed] [Google Scholar]
- 20.Patnaik S. K., and Sarangi S. (1980) Age-related response of tryptophan pyrrolase to 17β-estradiol in the liver of female Rats. J. Biochem. 87, 1249–1252 [PubMed] [Google Scholar]
- 21.Yamazaki F., Kuroiwa T., Takikawa O., and Kido R. (1985) Human indolylamine 2,3-dioxygenase: its tissue distribution, and characterization of the placental enzyme. Biochem. J. 230, 635–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takikawa O., Yoshida R., Kido R., and Hayaishi O. (1986) Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J. Biol. Chem. 261, 3648–3653 [PubMed] [Google Scholar]
- 23.Munn D. H., and Mellor A. L. (2013) Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends in Immunology 34, 137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Platten M., Wick W., and Van den Eynde B. J. (2012) Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. 72, 5435–5440 [DOI] [PubMed] [Google Scholar]
- 25.Schwarcz R., Bruno J. P., Muchowski P. J., Wu H.-Q. (2012) Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 13, 465–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang S., Jan Y.-H., Gray J. P., Mishin V., Heck D. E., Laskin D. L., and Laskin J. D. (2013) Sepiapterin reductase mediates chemical redox cycling in lung epithelial cells. J. Biol. Chem. 288, 19221–19237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guidetti P., Eastman C. L., and Schwarcz R. (1995) Metabolism of [5-3H]kynurenine in the rat brain in vivo: evidence for the existence of a functional kynurenine pathway. J. Neurochem. 65, 2621–2632 [DOI] [PubMed] [Google Scholar]
- 28.Auerbach G., Herrmann A., Gütlich M., Fischer M., Jacob U., Bacher A., and Huber R. (1997) The 1.25 Å crystal structure of sepiapterin reductase reveals its binding mode to pterins and brain neurotransmitters. EMBO J. 16, 7219–7230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman J., Roze E., Abdenur J. E., Chang R., Gasperini S., Saletti V., Wali G. M., Eiroa H., Neville B., Felice A., Parascandalo R., Zafeiriou D. I., Arrabal-Fernandez L., Dill P., Eichler F. S., Echenne B., Gutierrez-Solana L. G., Hoffmann G. F., Hyland K., Kusmierska K., Tijssen M. A. J., Lutz T., Mazzuca M., Penzien J., Poll-The B. T., Sykut-Cegielska J., Szymanska K., Thöny B., and Blau N. (2012) Sepiapterin reductase deficiency: a treatable mimic of cerebral palsy. Ann. Neurol. 71, 520–530 [DOI] [PubMed] [Google Scholar]
- 30.England J. M., and Coles M. (1972) Effect of co-trimoxazole on phenylalanine metabolism in man. Lancet 2, 1341–1343 [DOI] [PubMed] [Google Scholar]
- 31.Andrews T. M., Purkiss P., Chalmers R. A., and Watts R. W. E. (1976) Effect of cotrimoxazole on the response to phenylalanine loading in man. Clin. Chim. Acta 68, 17–30 [PubMed] [Google Scholar]
- 32.Raison C. L., Dantzer R., Kelley K. W., Lawson M. A., Woolwine B. J., Vogt G., Spivey J. R., Saito K., and Miller A. H. (2010) CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-α: relationship to CNS immune responses and depression. Mol. Psychiatry 15, 393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dantzer R., O'Connor J. C., Lawson M. A., and Kelley K. W. (2011) Inflammation-associated depression: From serotonin to kynurenine. Psychoneuroendocrinology 36, 426–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Felger J. C., Li L., Marvar P. J., Woolwine B. J., Harrison D. G., Raison C. L., and Miller A. H. (2013) Tyrosine metabolism during interferon-α administration: association with fatigue and CSF dopamine concentrations. Brain Behav. Immun. 31, 153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Felger J. C., Hernandez C. R., and Miller A. H. (2015) Levodopa reverses cytokine-induced reductions in striatal dopamine release. Int. J. Neuropsychopharmacol. 18, pyu084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zoller H., Schloegl A., Schroecksnadel S., Vogel W., and Fuchs D. (2012) Interferon-α therapy in patients with hepatitis C virus infection increases plasma phenylalanine and the phenylalanine to tyrosine ratio. J. Interferon Cytokine Res. 32, 216–220 [DOI] [PubMed] [Google Scholar]
- 37.Klassen P., Fürst P., Schulz C., Mazariegos M., and Solomons N. W. (2001) Plasma free amino acid concentrations in healthy Guatemalan adults and in patients with classic dengue. Am. J. Clin. Nutr. 73, 647–652 [DOI] [PubMed] [Google Scholar]
- 38.Lopansri B. K., Anstey N. M., Stoddard G. J., Mwaikambo E. D., Boutlis C. S., Tjitra E., Maniboey H., Hobbs M. R., Levesque M. C., Weinberg J. B., and Granger D. L. (2006) Elevated plasma phenylalanine in severe malaria and implications for pathophysiology of neurological complications. Infect. Immun. 74, 3355–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sperner-Unterweger B., Kohl C., and Fuchs D. (2014) Immune changes and neurotransmitters: Possible interactions in depression? Progress in Neuro-Psychopharmacology and Biological Psychiatry 48, 268–276 [DOI] [PubMed] [Google Scholar]




