Abstract
Listeria monocytogenes is a bacterial parasite that uses host proteins to assemble an Arp2/3-dependent actin comet tail to power its movement through the host cell. Initiation of comet tail assembly is more efficient in cytosol than it is under defined conditions, indicating that unknown factors contribute to the reaction. We therefore fractionated cytosol and identified CRMP-1 as a factor that facilitates Arp2/3-dependent Listeria actin cloud formation in the presence of Arp2/3 and actin alone. It also scored as an important factor for Listeria actin comet tail formation in brain cytosol. CRMP-1 does not nucleate actin assembly on its own, nor does it directly activate the Arp2/3 complex. Rather, CRMP-1 scored as an auxiliary factor that promoted the ability of Listeria ActA protein to activate the Arp2/3 complex to trigger actin assembly. CRMP-1 is one member of a family of five related proteins that modulate cell motility in response to extracellular signals. Our results demonstrate an important role for CRMP-1 in Listeria actin comet tail formation and open the possibility that CRMP-1 controls cell motility by modulating Arp2/3 activation.
Keywords: actin, Arp2/3 complex, bacterial pathogenesis, cell motility, protein purification, CRMP, biochemical reconstitution
Introduction
A number of bacterial and viral pathogens use eukaryotic host proteins to assemble an actin cytoskeleton to propel themselves through the host's cytoplasm, a process that helps spread the pathogen from cell to cell (1). Details of how host components are hijacked to form these pathogen-associated cytoskeletal networks are important for understanding the molecular mechanisms underlying host-pathogen interactions. The relative simplicity of these actin networks also allows detailed dissection of the biochemical mechanisms underlying their assembly and dissection of the molecular mechanisms controlling their morphogenesis (2). Furthermore, understanding how parasites build an actin cytoskeleton frequently provides new insight in the functioning of the more complicated actin networks that drive eukaryotic cell motility.
The actin comet tail of Listeria monocytogenes (hereafter referred to as Listeria) is the most extensively characterized, physiologically relevant actin network. Listeria propulsion is driven by actin polymerization, which is assembled by frequent, Arp2/3-dependent actin nucleation reactions to form a Listeria actin comet tail (3). Listeria propulsion and comet tail formation are preceded by the formation of an actin cloud that assembles at the bacterial surface (3–5). Arp2/3 and actin alone are sufficient to initiate formation of the actin cloud, but other host factors are necessary for propulsion and formation of the comet tail (2, 3, 5).
Arp2/3 is a protein complex that nucleates the de novo formation of new actin polymer from monomeric actin subunits (6). However, Arp2/3 itself is largely inactive, displaying only weak actin nucleation activity on its own (7, 8). This complex requires the activity of a nucleation-promoting factor to activate the complex to trigger actin assembly (7–12). In the case of Listeria, ActA, which is expressed by Listeria on the bacterial surface, recruits and activates the Arp2/3 complex to locally stimulate actin polymerization (3, 7, 13). Arp2/3 and actin alone are therefore sufficient to form an actin cloud on the surface of Listeria.
Actin cloud formation is more efficient in cytosol than with Arp2/3 and actin alone, implying the existence of unknown cytosolic factors that promote the reaction (3, 5). In this study, we identified collapsin response mediator protein-1 (CRMP-1)2 as one factor that enhances Listeria actin cloud assembly and characterized its basic biochemistry.
Experimental Procedures
Plasmids and Protein Purification
Human CRMP-1 was cloned into pET30a. Listeria ActA was a gift from the Mullins lab (14). Rosetta Escherichia coli cells (EMD Millipore) were used for expressing recombinant His-tagged CRMP-1 and His-tagged ActA. Arp2/3 complex was purified from calf thymus as described (3). Expression of His-tagged CRMP-1 and His-tagged ActA was induced at room temperature with 0.2 mm isopropyl-1-thio-β-d-galactopyranoside for 6 h and then purified according to manufacturer's instructions (Qiagen). In brief, the bacterially expressed His-tagged CRMP-1 was pelleted and lysed with lysozyme in lysis buffer (150 mm NaCl and 50 mm Tris, pH 8.0). For His-tagged ActA, we used denaturing condition, in which 3 m guanidine hydrochloride was added to the lysis buffer. We applied the supernatant to a nickel-nitrilotriacetic acid column. The column was then washed with lysis buffer and eluted with increasing concentrations of imidazole. The final eluted fraction of CRMP-1 was dialyzed against a different buffer according to the experimental need. Final elution of His-tagged ActA was dialyzed into 1 mm EGTA, 50 mm KCl, 1 mm MgCl2, 10 mm Tris, or Hepes, pH 8.0. Filamin was purified from chicken gizzard as described previously (15).
Listeria Preincubation Assay
Listeria actin assembly reactions were performed in perfusion chambers as described previously (5). Briefly, Listeria absorbed to glass coverslips in perfusion chambers were incubated for 5 min with brain cytosol, column fractions, or recombinant protein at concentrations listed below. Chambers were washed three times with buffer A (1 mm EGTA, 50 mm KCl, 1 mm MgCl2, 10 mm Tris, pH 7.8). Chambers were then filled with a solution containing 100 nm Arp2/3 complex and 2 μm G-actin labeled with Oregon Green (10% labeled). After 10 min, this solution was washed out of the chamber, and actin cloud and comet tail assembly was imaged with a 20× (NA 0.7) objective attached to a 1,000 × 1,000 charge-coupled device camera (ORCA-ER; Hamamatsu Photonics) on a Zeiss Axio Imager with the Colibri illumination system using the Zeiss acquisition software (Carl Zeiss).
Purification of CRMP-1 from Brain Cytosol
All chromatographic media were purchased from GE Healthcare. 150 grams of frozen bovine calf brain (Animal Technologies, Tyler, TX) was homogenized in 2 volumes of buffer B (20 mm sodium phosphate, pH 7.5, 25 mm NaCl, 2 mm EGTA, 10 mm β-mercaptoethanol, and 0.1 mm PMSF). The homogenate was first centrifuged at 15,000 × g for 30 min. The pellet was discarded and the supernatant centrifuged at 100,000 × g for 2 h. The supernatant was applied to a 60-ml DE-52 column equilibrated in buffer A. The flow-through, which contained the activity, was applied to a 70-ml S HP column equilibrated in buffer A. The column was eluted with a 500-ml gradient to 400 mm NaCl in buffer A. Active fractions were pooled, and solid ammonium sulfate was added slowly to a final concentration of 1.25 m. Insoluble material was removed by centrifugation at 20,000 × g for 30 min at 4 °C. The supernatant was applied to a 70-ml Phenyl HP column equilibrated in 1.25 m ammonium sulfate in buffer A. The column was eluted with a 1-liter gradient to buffer A. Active fractions were concentrated in a Centricon with a 100-kDa nominal cutoff, and the retentate was applied to a Superdex 200 gel filtration column equilibrated in 20 mm MES, pH 6.5, 100 mm NaCl. Active fractions were pooled, diluted with an equal volume of water, and applied to a Mono S column equilibrated in 20 mm MES, pH 6.5, 20 mm NaCl. The column was eluted with a 25 column volume gradient to 300 mm NaCl in the same buffer.
Mass Spectrometry
Gel slices were destained in 50% acetonitrile + 25 mm ammonium bicarbonate, crushed using a plastic pestle, and dried. The dried gel was suspended in 25 mm ammonium bicarbonate and digested with MSG-Trypsin (G-Biosciences, St. Louis, MO) at a ratio of 1:10 to 1:50 using a CEM Discover microwave digester (Mathews, SC) at 55 °C and maximum power of 60 watts for 15 min. Digested peptides were extracted using 50% acetonitrile + 5% formic acid twice and lyophilized. The digested peptides were dissolved in 5% acetonitrile + 0.1% formic acid for LC/MS. LC/MS was performed using a Thermo Dionex Ultimate RSLC3000 operating in nano mode at 300 μl/min with a gradient from 0.1% formic acid to 100% acetonitrile + 0.1% formic acid in 120 min. The trap column used was a Thermo Acclaim PepMap 100 (100 μm × 2 cm), and the analytical column was a Thermo Acclaim PepMap RSLC (75 μm × 15 cm). The Xcalibur raw file was converted by Mascot Distiller into peak lists that were submitted to an in-house Mascot Server and searched against specific NCBI-NR protein databases.
Immunodepletion of CRMP-1 and Rescue Experiments
Polyclonal rabbit anti-CRMP antibodies were raised against purified recombinant human CRMP-1. The CRMP-1 antiserum itself was specific and sufficient for all Western blotting procedures. For immunodepletion experiments, however, the antibodies were affinity-purified using recombinant CRMP-1 coupled to Affi-Gel 10 gel according to the manufacturer's instructions (Bio-Rad). The affinity-purified antibodies were coupled to Affi-Gel 10 beads at a ratio of 1 mg of protein to 1 ml of beads. For immunodepletion of CRMP-1 from brain cytosol, 100 μl of affinity-purified anti-CRMP-1-antibody-coated beads or beads coupled with non-immune rabbit IgG was added to 200 μl of brain cytosol and incubated at 4 °C for 1 h with constant tumbling. Beads were then pelleted by centrifugation. The supernatant was diluted 1:3 into buffer A supplemented with 0.2 mm ATP and with additional 62.5 nm Arp2/3 complex and 2 μm 25% fluorophore-labeled actin. This mixture was applied to a perfusion chamber, containing L. monocytogenes, for 5 min at room temperature. In rescue experiments, recombinant CRMP was added in the same amount as endogenous CRMP-1 (0.01 mg/ml), which was determined by Western blot, into the perfusion chamber to preincubate with Listeria. Comet tail assembly was analyzed by fluorescence imaging using a 63× objective lens (NA 1.4) under a Hamamatsu camera described above. The number of Listeria with actin tail and the actin tail length were quantified using Fiji software (16). Depletion of CRMP-1 was confirmed by Western blotting.
Actin Polymerization Assays
Pyrene actin was prepared as described (17). Actin polymerization was monitored by the increase in fluorescence of pyrenyl-actin with excitation at 365 nm and emission at 410 nm. The reaction contains 2.5 μm actin (25% pyrene-labeled), 50 nm Arp2/3, 17.5 nm ActA, and various concentrations of CRMP-1 protein in buffer B (1 mm EGTA, 50 mm KCl, 1 mm MgCl2, 10 mm imidazole, pH 7.0) with 2 mm ATP.
Filament Branching Assay
Prepolymerized filaments were first prepared by incubating 2.5 μm Alexa Fluor 647-labeled actin (10% labeled) in buffer B for 30 min at room temperature. These filaments were then diluted 10-fold into solutions containing 2.5 μm Alexa Fluor 647-labeled G-actin with or without 0.6 μm Arp2/3, 0.4 μm ActA, and/or 0.125 μm CRMP-1, for 10 min. The filaments from this reaction were then diluted 1:50 into Buffer B with 15 mm glucose, 20 μg/ml catalase, 100 μg/ml glucose oxidase, and 1 mm TROLOX and then immediately applied into the chambers, which had been precoated with 40 μg/ml filamin. Filaments were allowed to attach to the chamber for 10 min and then detected by fluorescence imaging using a 63× objective lens as described above.
F-actin Co-sedimentation
Four sets of co-sedimentation experiments were conducted. Two sets used CRMP-1 concentrations of 0.125, 0.25, 0.5, 0.75, and 2.5 μm. Two other sets used CRMP-1 concentrations of 0.75, 1.25, 2.5, and 4 μm. Actin was provided at a constant 2 μm with various concentrations of CRMP-1 in different tubes. Actin was polymerized at 4 °C overnight in buffer B and 2 mm ATP in the absence or presence of various concentrations of CRMP-1. The tubes were then centrifuged in a Beckman TLA 100 rotor at 350,000 × g (k factor, 8.1) for 20 min at 4 °C to separate supernatant and pellet fractions. The fractions were separated using SDS-PAGE. The gel was stained with Coomassie Blue and analyzed using Fiji software. Curve fitting was done using OriginLab software.
ActA-CRMP Binding Interactions
The affinity of ActA for CRMP-1 was determined using the approach described by Pollard (18). Recombinant ActA was immobilized onto Affi-Gel 10 gel (Bio-Rad) according to the manufacturer's manual. Briefly, after the Affi-Gel 10 gel was activated, recombinant ActA protein was added to the gel solution containing 50 mm KCl, 1 mm MgCl2, and 10 mm Hepes, pH 7.8. ActA was allowed to couple onto the gel overnight at 4 °C. The final coupling density was 1.5 mg of ActA per ml of gel. The gel was blocked in casein solution for 30 min and then washed with buffer A before use. The control gel was coated only with casein.
12.5 μl of the ActA-coated gel was used to incubate with various concentrations of CRMP-1 to a final volume of 112.5 μl. For control reactions, the blank gel was used. After 1 h of incubation at 4 °C, the gel was pelleted using centrifugation. The supernatant was carefully separated from the gel, and then separated on SDS-PAGE followed by Western blotting using our custom polyclonal antisera (not affinity-purified) against CRMP-1. HRP-conjugated secondary antibodies (Bio-Rad, catalogue number: 1706515) and chemiluminescence were used to visualize the amount of CRMP-1 in the supernatants by exposing the blots to autoradiography film. Multiple exposures were obtained to ensure that the signals were in the linear range. The amount of CRMP-1 bound to ActA was determined by subtracting the total amount of CRMP-1 in the control condition from the amount of CRMP-1 left in the supernatant. This approach permitted the estimation of binding affinity from the concentration of CRMP-1 required for half-maximal binding to ActA beads. The results from three independent Western blots were analyzed using Fiji software, and the curve was fitted using OriginLab software.
Results
Identification of CRMP-1 as a New Factor for Listeria Actin Tail Formation
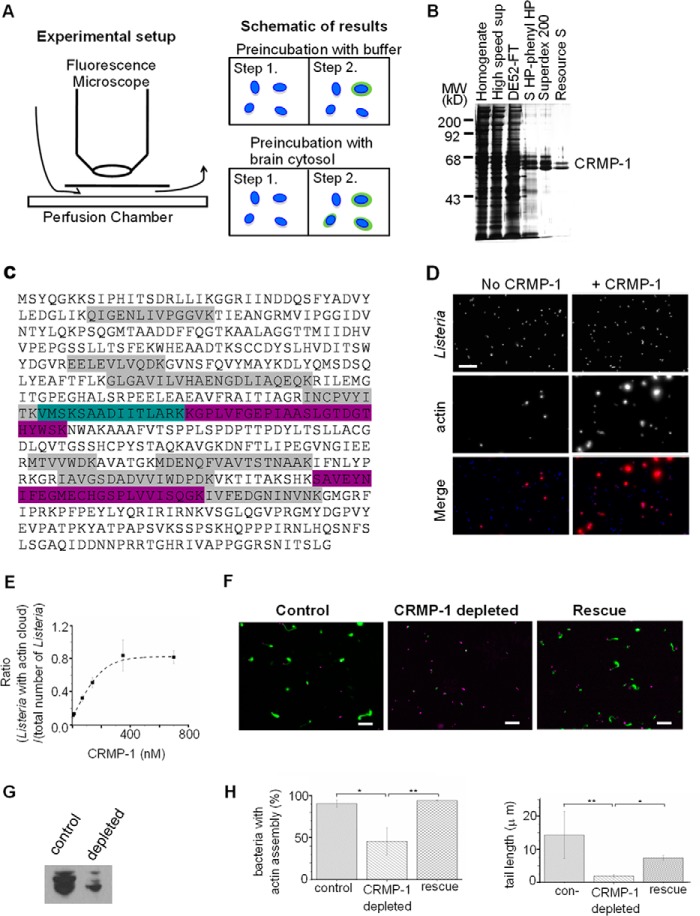
Previous work demonstrated that brain cytosol contains factors that associate with the Listeria cell surface and promote Arp2/3-dependent actin cloud formation (5). We used a visual assay to score for the unknown factors that promote actin cloud formation (Fig. 1A). In this assay, Listeria are introduced into a perfusion chamber, where they passively adhere to glass coverslips. Brain cytosol or chromatographic fractions of cytosol are then introduced into the chamber and allowed to incubate for 5 min. No actin assembly occurs under these conditions in this amount of time. The cytosol within the perfusion chamber is washed out with buffer and then replaced with a solution containing pure Arp2/3 and fluorescently labeled actin to initiate actin cloud formation, which is then assessed by fluorescence imaging. Using this assay, we confirmed previous results showing that a greater fraction of Listeria forms actin clouds when the Listeria are preincubated with brain cytosol than when the Listeria are preincubated with buffer alone (5).
FIGURE 1.
CRMP-1 enhances Arp2/3-mediated Listeria actin cloud formation. A, schematic representation of the method to identify factors that associate with Listeria and facilitate Arp2/3-dependent actin cloud formation. B, silver-stained gel summarizing the purification of CRMP-1 from brain cytosol. MW, molecular weight markers. C, mass spectrometry result of the last duplet band in B. Peptides highlighted in gray were identified in both bands. Peptides highlighted in purple were only identified in the upper band. The peptide highlighted in teal was only found in the lower band. D, recombinant CRMP-1 potentiates Arp2/3-dependent cloud formation. Top and bottom, field of Listeria preincubated with buffer (top) or with recombinant CRMP-1 (bottom), using the method described in A. Listeria were labeled with DAPI and pseudo-colored in blue. FITC-labeled actin was pseudo-colored in red. E, dose-dependent effect of CRMP-1 on Listeria actin cloud formation. Quantifications were done by at least three independent experiments. Column bars represent the mean values ± S.D. F, immunodepleting CRMP-1 from brain cytosol decreases actin tail formation. DAPI-labeled Listeria are pseudo-colored in magenta; fluorescent-labeled actin are in green. G, Western blot against CRMP-1 indicating the efficiency of the immunodepletion. H, quantifications of F. Quantifications were done by three independent experiments (n = 3). Column bars represent the mean values ± S.D. *, p < 0.05. **, p < 0.01. Scale bars are 20 μm.
To purify these factors, we used conventional chromatography and the visual assay described above to score column fractions of brain cytosol for the cloud-enhancing activity. Using this approach, we were able to track an activity across several different columns, resulting in the isolation of two polypeptides of ∼65 kDa that were both identified as CRMP-1 by mass spectrometry (Fig. 1B). Twelve peptides were identified in the upper band, representing 33% coverage of CRMP-1 by mass and 34% coverage by amino acid count. 11 peptides were identified in the lower band, corresponding to 28% coverage of CRMP-1 by mass and 28% coverage by amino acid count (Fig. 1C). We do not know why the protein runs as a doublet. CRMP proteins are known to be phosphorylated (19–22), which could alter the mobility of the protein on SDS gels. However, others have commented that the positively charged C terminus of CRMP family members is highly susceptible to proteolysis (23, 24). Recombinant CRMP-1 purified from E. coli also runs as a doublet, which is most likely explained by proteolysis as reported by others.
Recombinant CRMP-1 expressed and purified from E. coli also scored in the assay, confirming that we had purified the right factor (Fig. 1D). Quantification demonstrated that increasing concentrations of CRMP-1 induced a greater fraction of Listeria to form actin clouds. Using an equal concentration of Arp2/3, 80% of Listeria formed a detectable actin cloud when preincubated with 350 nm CRMP-1 as compared with only 15% of Listeria with clouds in the absence of CRMP-1 (Fig. 1E).
CRMP-1 Contributes to Listeria Actin Comet Tail Formation in Brain Cytosol
To address the importance of CRMP-1 for Listeria actin tail formation in a complex system, we immunodepleted it from brain cytosol. We were able to deplete 50–70% of CRMP-1 from the extract (Fig. 1G). Listeria actin comet tail formation was diminished in cytosol depleted of CRMP-1 but not in mock-depleted controls (Fig. 1, F and H). Only 46% of Listeria formed a detectable actin signal around the bacteria in depleted cytosol as compared with 90% in mock-depleted controls. Comet tails that did manage to form in CRMP-1-depleted cytosol were shorter than controls (2 μm for depleted cytosol and 14 μm for mock-depleted controls). These phenotypes could be rescued by adding pure, recombinant CRMP back to the extracts. These results reveal an important role for CRMP-1 in boosting the efficiency and robustness of Listeria actin cloud and comet tail assembly in complex cytosolic extracts.
CRMP-1 Facilitates ActA-mediated Arp2/3-dependent Actin Polymerization
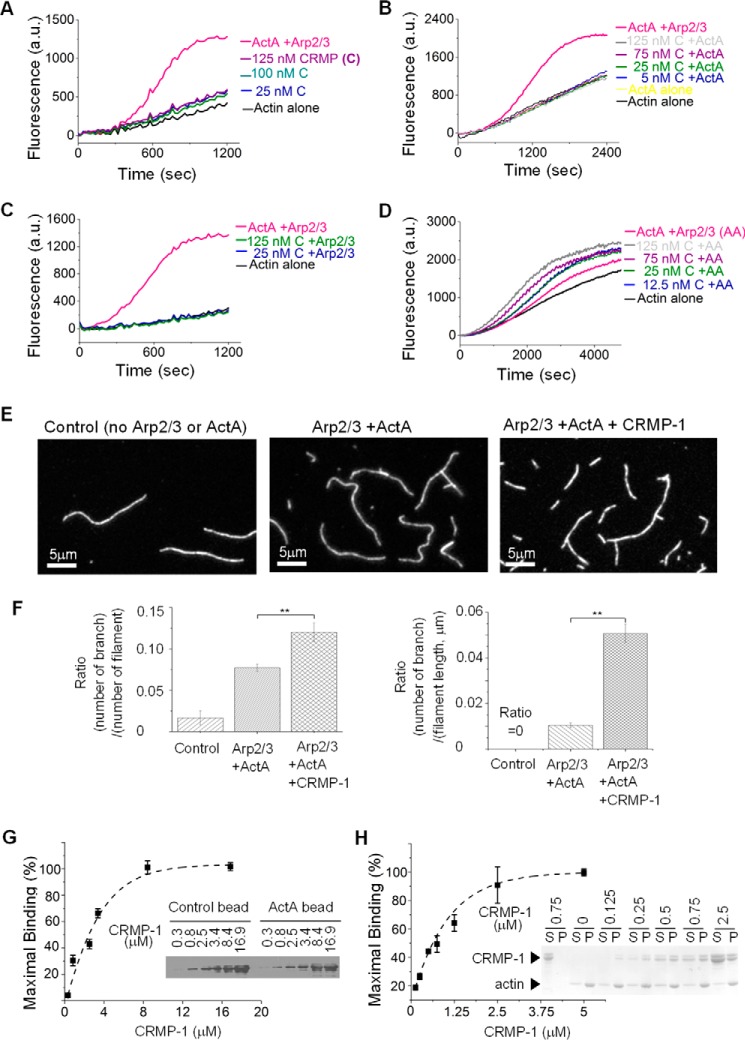
We considered several alternative mechanisms through which CRMP-1 might possibly promote Arp2/3-dependent Listeria actin cloud and comet tail formation. CRMP-1 could itself be a novel actin nucleation factor. Alternatively, CRMP-1 could function as a nucleation-promoting factor that directly activates the Arp2/3 complex. Finally, CRMP-1 could act as a coactivator for ActA, which is the only factor expressed by Listeria that is known to activate the Arp2/3 complex (13, 25–28). We used pyrene actin polymerization assays along with pure proteins to distinguish among these possibilities. We did not detect any stimulation of actin assembly by CRMP-1 alone (Fig. 2A) or by CRMP-1 in combination with ActA (Fig. 2B), nor could we detect any activation of the Arp2/3 complex by CRMP-1 alone (Fig. 2C). These negative results demonstrate that CRMP-1 is not itself an actin nucleation factor, nor is it a direct activator of the Arp2/3 complex. In contrast, CRMP-1 was able to stimulate ActA-activated, Arp2/3-dependent actin polymerization in a dose-dependent manner (Fig. 2D). Thus CRMP-1 is a new factor that works in concert with ActA to enhance Arp2/3-dependent actin nucleation.
FIGURE 2.
CRMP-1 facilitates ActA-Arp2/3-mediated actin polymerization. A–D, pyrene assembly assay testing the role of CRMP-1 in actin polymerization: CRMP-1 alone (A); CRMP-1 and ActA (B); CRMP-1 and Arp2/3 (C); and CRMP-1 with Arp2/3 and ActA (D). In different testing conditions, we used the curve of ActA-Arp2/3 polymerization as a positive control. The curve of actin alone is the negative control. a. u., arbitrary units. E, actin branching assay with CRMP-1, ActA, and Arp2/3. F, quantification results of E demonstrate that more branched filaments are detected in the presence CRMP-1. Quantifications were done by three independent experiments (n = 3). Bars show the mean values ± S.D. *, p < 0.05. **, p < 0.01. G, CRMP-1 binds to ActA. The image is a representative result from Western blotting against CRMP-1. It shows the amount of CRMP-1 remaining in the supernatants after co-sedimentation with control or ActA beads. The initial amount of CRMP-1 provided to each reaction was labeled on the top of each panel. The binding curve was generated from the average of three experiments. Error bars are the mean values ± S.D. The affinity of CRMP-1 for ActA is 2.5 μm. The gel is a representative Western blot of the binding assay. H, co-sedimentation assay and a representative Coomassie Blue-stained gel demonstrating that CRMP-1 binds F-actin. The value above the lanes indicates the initial amount of CRMP-1 provided in each reaction. The pellets after centrifugation are labeled as P on top of each panel; the supernatants are labeled as S. Points on the graph are the mean values ± S.D.
CRMP-1 Increases Arp2/3-dependent Branching, Binds to F-actin, and Binds to ActA
Arp2/3 produces branched actin filaments, yet not all Arp2/3 activators increase the density of filament branches (6, 29). To determine whether CRMP-1 increases Arp2/3-dependent branching, we imaged single actin filaments to compare the density of filament branches in the presence of ActA and Arp2/3 alone with that in the presence of added CRMP-1. Our results indicated a greater number of branches in the presence of CRMP-1 than in its absence (Fig. 2, E and F).
The purification method that we used to identify CRMP-1 implies that it binds to the Listeria surface. Because ActA is the only factor expressed by Listeria that is known to activate the Arp2/3 complex (13, 25–28), we tested whether CRMP-1 bound to pure ActA using a method described by Pollard (18). Solutions of CRMP-1 were incubated with ActA-coated beads. The amount of CRMP-1 remaining in solution following incubation with the ActA-coated beads was determined by Western blotting for CRMP-1 as described under “Experimental Procedures.” This allowed us to estimate the amount of CRMP-1 bound to the ActA-coated beads and the affinity constant from the amount of CRMP-1 necessary for half-maximal binding. Using this approach, we estimate that CRMP-1 binds to ActA with an affinity of 2.5 μm (Fig. 2G).
Many factors that facilitate Arp2/3-dependent actin nucleation and branching are also able to bind to F-actin (6, 30). Previous work has shown that CRMP-4, a member of the CRMP family proteins, can bind to F-actin (31). We used co-sedimentation to show that CRMP-1 can also bind F-actin with an apparent affinity of 0.7 μm (Fig. 2H). CRMP-1 is therefore an F-actin-binding protein that is capable of increasing Arp2/3-dependent actin nucleation and Arp2/3-dependent actin filament branching.
Discussion
We identified CRMP-1 as a factor that promotes Arp2/3-dependent Listeria actin cloud and comet tail formation (Fig. 1). Although CRMP-1 did not directly nucleate actin assembly or directly activate the Arp2/3 complex, it facilitated that ability of Listeria ActA to activate Arp2/3 to nucleate actin assembly (Fig. 2, A–D). Cytosolic extracts depleted of CRMP-1 were less likely to generate Listeria actin clouds and actin comet tails (Fig. 1, F and G). CRMP-1 therefore might help explain why Listeria actin cloud-forming activity decreases over the course of purification of Arp2/3 (32) and why Listeria actin cloud formation is more robust in the presence of brain cytosol than with Arp2/3 alone (5).
Listeria actin comet tail formation has been reconstituted in the absence of CRMP-1 using only seven purified factors (2). Here we showed that comet tail formation was reduced in brain cytosol depleted of CRMP-1. Auxiliary factors that promote comet tail formation are probably more important in cytosol than under defined conditions because cytosol contains other factors that inhibit actin assembly (33). Only a small subset of these inhibitory factors is present in the defined reconstitution system and then only at low concentrations.
Our results here showing CRMP-1 binding to F-actin and ActA provide a potential mechanism of how CRMP-1 might contribute to Arp2/3-dependent actin dynamics. Activation of the Arp2/3 complex involves not only association with a nucleation-promoting factor, but also association of Arp2/3 with an existing “mother” filament (6). ActA serves as the nucleation-promoting factor for Listeria actin comet tail assembly. We found that CRMP-1 binds to both ActA and F-actin. We therefore hypothesize that CRMP-1 promotes actin comet tail formation by helping to recruit the F-actin mother filament to the bacterial surface to position this filament in close proximity to both ActA and Arp2/3.
CRMP-1 is a member of the CRMP family, which consists of five related proteins (CRMPs1–5) that are also related to the enzyme dihydropyrimidinase (34–36). CRMP proteins are therefore also known as dihydropyrimidinase-like proteins 1–5. The CRMP proteins regulate cell movements in response to extracellular cues such as Semaphorin 3A (37), but they do not hydrolyze pyrimidine (38). The majority of work on CRMP proteins thus far has focused on CRMP-2 and its ability to modulate microtubule dynamics by binding to tubulin dimers (19, 20, 39, 40), but evidence has been accumulating that CRMP proteins also regulate the actin cytoskeleton (31, 41–45). Our demonstration here that CRMP-1 promotes Arp2/3-dependent assembly of Listeria actin comet tails opens the possibility that CRMP-1 might also contribute to the assembly of Arp2/3-dependent actin networks in uninfected cells. If true, CRMP-1 could provide a novel pathway connecting Semaphorin signaling to Arp2/3-dependent actin assembly.
Author Contributions
H.-C. Y. and W. M. B. conceived and performed experiments and wrote the paper.
Acknowledgments
We thank Dyche Mullins at the University of California-San Francisco for the ActA construct. We also thank Ruiqi Liao for the help on Fig. 1D.
This work was supported by National Institutes of Health Grant R01-GM106106-01 (to W. M. B.) and by American Heart Association Scientist Development Grant 0930282G (to W. M. B.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
- CRMP
- collapsin response mediator protein.
References
- 1.Welch M. D., and Way M. (2013) Arp2/3-mediated actin-based motility: a tail of pathogen abuse. Cell Host Microbe 14, 242–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loisel T. P., Boujemaa R., Pantaloni D., and Carlier M. F. (1999) Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature 401, 613–616 [DOI] [PubMed] [Google Scholar]
- 3.Welch M. D., Iwamatsu A., and Mitchison T. J. (1997) Actin polymerization is induced by Arp2/3 protein complex at the surface of Listeria monocytogenes. Nature 385, 265–269 [DOI] [PubMed] [Google Scholar]
- 4.Tilney L. G., and Portnoy D. A. (1989) Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109, 1597–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brieher W. M., Coughlin M., and Mitchison T. J. (2004) Fascin-mediated propulsion of Listeria monocytogenes independent of frequent nucleation by the Arp2/3 complex. J. Cell Biol. 165, 233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goley E. D., and Welch M. D. (2006) The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 7, 713–726 [DOI] [PubMed] [Google Scholar]
- 7.Welch M. D., Rosenblatt J., Skoble J., Portnoy D. A., and Mitchison T. J. (1998) Interaction of human Arp2/3 complex and the Listeria monocytogenes ActA protein in actin filament nucleation. Science 281, 105–108 [DOI] [PubMed] [Google Scholar]
- 8.Mullins R. D., Heuser J. A., and Pollard T. D. (1998) The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl. Acad. Sci. U.S.A. 95, 6181–6186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohatgi R., Ma L., Miki H., Lopez M., Kirchhausen T., Takenawa T., and Kirschner M. W. (1999) The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221–231 [DOI] [PubMed] [Google Scholar]
- 10.Machesky L. M., and Insall R. H. (1998) Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol. 8, 1347–1356 [DOI] [PubMed] [Google Scholar]
- 11.Linardopoulou E. V., Parghi S. S., Friedman C., Osborn G. E., Parkhurst S. M., and Trask B. J. (2007) Human subtelomeric WASH genes encode a new subclass of the WASP family. PLoS Genet. 3, e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machesky L. M., Mullins R. D., Higgs H. N., Kaiser D. A., Blanchoin L., May R. C., Hall M. E., and Pollard T. D. (1999) Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc. Natl. Acad. Sci. U.S.A. 96, 3739–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kocks C., Gouin E., Tabouret M., Berche P., Ohayon H., and Cossart P. (1992) L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68, 521–531 [DOI] [PubMed] [Google Scholar]
- 14.Akin O., and Mullins R. D. (2008) Capping protein increases the rate of actin-based motility by promoting filament nucleation by the Arp2/3 complex. Cell 133, 841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shizuta Y., Shizuta H., Gallo M., Davies P., and Pastan I. (1976) Purification and properties of filamin, and actin binding protein from chicken gizzard. J. Biol. Chem. 251, 6562–6567 [PubMed] [Google Scholar]
- 16.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J. Y., White D. J., Hartenstein V., Eliceiri K., Tomancak P., and Cardona A. (2012) Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryan J., and Coluccio L. M. (1985) Kinetic analysis of F-actin depolymerization in the presence of platelet gelsolin and gelsolin-actin complexes. J. Cell Biol. 101, 1236–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollard T. D. (2010) A guide to simple and informative binding assays. Mol. Biol. Cell 21, 4061–4067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arimura N., Inagaki N., Chihara K., Ménager C., Nakamura N., Amano M., Iwamatsu A., Goshima Y., and Kaibuchi K. (2000) Phosphorylation of collapsin response mediator protein-2 by Rho-kinase: evidence for two separate signaling pathways for growth cone collapse. J. Biol. Chem. 275, 23973–23980 [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura T., Kawano Y., Arimura N., Kawabata S., Kikuchi A., and Kaibuchi K. (2005) GSK-3β regulates phosphorylation of CRMP-2 and neuronal polarity. Cell 120, 137–149 [DOI] [PubMed] [Google Scholar]
- 21.Cole A. R., Soutar M. P., Rembutsu M., van Aalten L., Hastie C. J., McLauchlan H., Peggie M., Balastik M., Lu K. P., and Sutherland C. (2008) Relative resistance of Cdk5-phosphorylated CRMP2 to dephosphorylation. J. Biol. Chem. 283, 18227–18237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buel G. R., Rush J., and Ballif B. A. (2010) Fyn promotes phosphorylation of collapsin response mediator protein 1 at tyrosine 504, a novel, isoform-specific regulatory site. J. Cell. Biochem. 111, 20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deo R. C., Schmidt E. F., Elhabazi A., Togashi H., Burley S. K., and Strittmatter S. M. (2004) Structural bases for CRMP function in plexin-dependent semaphorin3A signaling. EMBO J. 23, 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponnusamy R., and Lohkamp B. (2013) Insights into the oligomerization of CRMPs: crystal structure of human collapsin response mediator protein 5. J. Neurochem. 125, 855–868 [DOI] [PubMed] [Google Scholar]
- 25.Domann E., Wehland J., Rohde M., Pistor S., Hartl M., Goebel W., Leimeister-Wächter M., Wuenscher M., and Chakraborty T. (1992) A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 11, 1981–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kocks C., Marchand J. B., Gouin E., d'Hauteville H., Sansonetti P. J., Carlier M. F., and Cossart P. (1995) The unrelated surface proteins ActA of Listeria monocytogenes and IcsA of Shigella flexneri are sufficient to confer actin-based motility on Listeria innocua and Escherichia coli respectively. Mol. Microbiol. 18, 413–423 [DOI] [PubMed] [Google Scholar]
- 27.Smith G. A., Portnoy D. A., and Theriot J. A. (1995) Asymmetric distribution of the Listeria monocytogenes ActA protein is required and sufficient to direct actin-based motility. Mol. Microbiol. 17, 945–951 [DOI] [PubMed] [Google Scholar]
- 28.Pistor S., Chakraborty T., Niebuhr K., Domann E., and Wehland J. (1994) The ActA protein of Listeria monocytogenes acts as a nucleator inducing reorganization of the actin cytoskeleton. EMBO J. 13, 758–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner A. R., Luan Q., Liu S. L., and Nolen B. J. (2013) Dip1 defines a class of Arp2/3 complex activators that function without preformed actin filaments. Curr. Biol. 23, 1990–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C., Ni Y., Wang T., Gao Y., Haudenschild C. C., and Zhan X. (1997) Down-regulation of the filamentous actin cross-linking activity of cortactin by Src-mediated tyrosine phosphorylation. J. Biol. Chem. 272, 13911–13915 [DOI] [PubMed] [Google Scholar]
- 31.Rosslenbroich V., Dai L., Baader S. L., Noegel A. A., Gieselmann V., and Kappler J. (2005) Collapsin response mediator protein-4 regulates F-actin bundling. Exp. Cell Res. 310, 434–444 [DOI] [PubMed] [Google Scholar]
- 32.Welch M. D., and Mitchison T. J. (1998) Purification and assay of the platelet Arp2/3 complex. Methods Enzymol. 298, 52–61 [DOI] [PubMed] [Google Scholar]
- 33.Pollard T. D., and Borisy G. G. (2003) Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 [DOI] [PubMed] [Google Scholar]
- 34.Fukada M., Watakabe I., Yuasa-Kawada J., Kawachi H., Kuroiwa A., Matsuda Y., and Noda M. (2000) Molecular characterization of CRMP5, a novel member of the collapsin response mediator protein family. J. Biol. Chem. 275, 37957–37965 [DOI] [PubMed] [Google Scholar]
- 35.Hamajima N., Matsuda K., Sakata S., Tamaki N., Sasaki M., and Nonaka M. (1996) A novel gene family defined by human dihydropyrimidinase and three related proteins with differential tissue distribution. Gene 180, 157–163 [DOI] [PubMed] [Google Scholar]
- 36.Wang L. H., and Strittmatter S. M. (1996) A family of rat CRMP genes is differentially expressed in the nervous system. J. Neurosci. 16, 6197–6207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goshima Y., Nakamura F., Strittmatter P., and Strittmatter S. M. (1995) Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature 376, 509–514 [DOI] [PubMed] [Google Scholar]
- 38.Schmidt E. F., and Strittmatter S. M. (2007) The CRMP family of proteins and their role in Sema3A signaling. Adv. Exp. Med. Biol. 600, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukata Y., Itoh T. J., Kimura T., Ménager C., Nishimura T., Shiromizu T., Watanabe H., Inagaki N., Iwamatsu A., Hotani H., and Kaibuchi K. (2002) CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat. Cell Biol. 4, 583–591 [DOI] [PubMed] [Google Scholar]
- 40.Arimura N., Ménager C., Kawano Y., Yoshimura T., Kawabata S., Hattori A., Fukata Y., Amano M., Goshima Y., Inagaki M., Morone N., Usukura J., and Kaibuchi K. (2005) Phosphorylation by Rho kinase regulates CRMP-2 activity in growth cones. Mol. Cell. Biol. 25, 9973–9984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ong Tone S., Dayanandan B., Fournier A. E., and Mandato C. A. (2010) GSK3 regulates mitotic chromosomal alignment through CRMP4. PLoS ONE 5, e14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan S. H., Chao Y. C., Hung P. F., Chen H. Y., Yang S. C., Chang Y. L., Wu C. T., Chang C. C., Wang W. L., Chan W. K., Wu Y. Y., Che T. F., Wang L. K., Lin C. Y., Lee Y. C., Kuo M. L., Lee C. H., Chen J. J., Hong T. M., and Yang P. C. (2011) The ability of LCRMP-1 to promote cancer invasion by enhancing filopodia formation is antagonized by CRMP-1. J. Clin. Invest. 121, 3189–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khazaei M. R., Girouard M. P., Alchini R., Ong Tone S., Shimada T., Bechstedt S., Cowan M., Guillet D., Wiseman P. W., Brouhard G., Cloutier J. F., and Fournier A. E. (2014) Collapsin response mediator protein 4 regulates growth cone dynamics through the actin and microtubule cytoskeleton. J. Biol. Chem. 289, 30133–30143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alabed Y. Z., Pool M., Ong Tone S., and Fournier A. E. (2007) Identification of CRMP4 as a convergent regulator of axon outgrowth inhibition. J. Neurosci. 27, 1702–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hotta A., Inatome R., Yuasa-Kawada J., Qin Q., Yamamura H., and Yanagi S. (2005) Critical role of collapsin response mediator protein-associated molecule CRAM for filopodia and growth cone development in neurons. Mol. Biol. Cell 16, 32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]